Abstract
Two experiments were performed to identify whether nitric oxide (NO) inhibits sympathetically mediated vasoconstriction in human skin. In eight subjects increasing doses of sodium nitroprusside (SNP; 8.4 × 10−6–8.4 × 10−3 m) were administered via intradermal microdialysis. At each dose of SNP, cutaneous vasoconstrictor responsiveness was assessed during a 3 min whole-body cold stress. The relative reduction in forearm cutaneous vascular conductance (CVC) during the cold stress was significantly attenuated for SNP doses greater than 8.4 × 10−4 m (control: 63.0 ± 4.1%, SNP 8.4 × 10−6 m: 57.1 ± 4.7%, SNP 8.4 × 10−5 m: 57.0 ± 3.6%, SNP 8.4 × 10−4m: 44.5 ± 5.4% and SNP 8.4 × 10−3m: 28.8 ± 7.9%). The second experiment was performed to identify whether this response was due to NO attenuating sympathetically mediated vasoconstriction or due to a non-specific effect of an elevated CVC secondary to SNP administration. In seven subjects forearm CVC during a whole-body cold stress was assessed at two sites: at a site dilated via microdialysis administration of SNP and at a site dilated with isoproterenol (ISO). CVC was not different between sites prior to (SNP: 0.42 ± 0.11; ISO: 0.46 ± 0.11 AU mmHg−1 (AU, arbitrary units), P > 0.05) or following drug infusion (SNP: 1.36 ± 0.21; ISO: 1.27 ± 0.23 AU mmHg−1, P > 0.05). The reduction in CVC during the subsequent cold stress was significantly less at the SNP site (38.1 ± 6.2%) relative to the ISO site (65.0 ± 5.5%; P = 0.007). These data suggest NO is capable of inhibiting sympathetically mediated vasoconstriction in the cutaneous vasculature.
First known as the endothelial derived relaxing factor, nitric oxide (NO) is a potent physiological mediator of vasodilatation that can be released from a variety of physiological sources such as skeletal muscle, vascular endothelium and nitroxidergic nerves (Joyner & Dietz, 1997). NO has been found to be important in local and systemic control of blood flow. Studies suggest that part of the vasodilator effect of NO may be attributed to NO attenuation of sympathetically mediated vasoconstriction (Greenberg et al. 1990; Zanzinger et al. 1994; Häbler et al. 1997; Costa et al. 2001; Chavoshan et al. 2002; Kolo et al. 2004). For example, Zanzinger et al. (1994) observed that the pressor effect of adrenaline was diminished after NO-donor administration, but was enhanced if NO synthase was inhibited. In the study of Häbler et al. (1997), the vasoconstrictor effect of electrical stimulation of the lumbar trunk in rats was enhanced in skeletal muscle after NO synthase inhibition. Finally, larger decreases in an index of muscle blood flow were observed during an orthostatic challenge when NO synthase was inhibited (Chavoshan et al. 2002). Among others, these studies suggest that NO has the capability of attenuating vasoconstrictor responses to adrenergic stimuli.
NO exerts an important physiological effect on the cutaneous microcirculation. NO has been shown to contribute to cutaneous vasodilator effects during local heating (Kellogg et al. 1999; Minson et al. 2001), during indirect whole-body heating (Shastry et al. 1998; Kellogg et al. 1998), and may contribute to the initial vasodilator response during the onset of whole-body heating (Shibasaki et al. 2002). Importantly, the cutaneous microcirculation is under significant control from the sympathetic vasoconstrictor system especially during cold stress (Kellogg et al. 1989) and perhaps during orthostatic challenges (Tripathi & Nadel, 1986; Kellogg et al. 1990; Crandall et al. 1996). The effect of NO on sympathetically mediated cutaneous vasoconstriction remains unknown. Thus, the purpose of this study was to test the hypothesis that NO is capable of attenuating sympathetically mediated vasoconstriction in human skin.
Methods
Subjects
Eight healthy subjects (3 men, 5 women) participated in protocol 1. These subjects' mean age, height and weight were 30.2 ± 2.5 years, 167.0 ± 3.9 cm and 62.8 ± 4.7 kg, respectively. Seven healthy subjects (3 men, 4 women) participated in protocol 2. These subjects' mean age, height and weight were 36.8 ± 3.1 years, 171.8 ± 4.1 cm and 67.2 ± 6.6 kg, respectively. Subjects were free of cardiovascular, metabolic and neurological disorders. Each subject signed an informed consent that was approved by the Institutional Review Boards of the University of Texas South-western Medical Center and Presbyterian Hospital of Dallas. All experiments were performed in accordance with the Declaration of Helsinki.
Measurements
Mean skin temperature was obtained from the average of six thermocouples attached to the skin (Taylor et al. 1989). Arterial blood pressure was measured throughout the protocol via R-wave gated brachial artery auscultation (Suntech, Raleigh, NC, USA). Mean arterial pressure was calculated as 1/3 pulse pressure + diastolic blood pressure. Heart rate was obtained from an electrocardiogram (Agilent Technologies, Palo Alto, CA, USA) with the signal interfaced with a cardiotachometer (CWE, Ardmore, PA, USA). Local forearm cutaneous blood flow was measured via laser Doppler flowmetry (Perimed, North Rayalton, OH, USA). Integrating flow probes were used because of the larger sampling area on the skin relative to single point flow probes. Skin blood flow is expressed in arbitrary units (AU). Cutaneous vascular conductance was calculated from the ratio of skin blood flow to mean arterial blood pressure.
Protocols
For both protocols, subjects dressed in a tube-lined suit perfusable with temperature-controlled water. Under the suit male subjects wore only shorts while female subjects wore shorts and a swimsuit top or sports bra. Each subject had one (protocol 1) or two (protocol 2) microdialysis probes (BAS, West Lafayette, IN, USA) placed in the dermal space of the dorsal aspect of a forearm. The membrane window for each probe was 10 mm in length. The probe was placed in the dermal space using a 25-gauge needle. The tip of the needle exited the skin ∼2 cm from the point of entry. The microdialysis probe was inserted through the lumen of the needle. The needle was then withdrawn, leaving the probe in place. After placement, the probes were perfused with lactated Ringer's solution at a rate of 2 μl min−1. This flow was used throughout the protocol. A laser Doppler probe (Perimed, North Rayalton, OH, USA) was placed over each membrane to monitor blood flow in this region. Sufficient time (minimum of 60 min) elapsed from probe placement prior to the start of the study to allow for the hyperaemic response associated with probe placement to subside.
Protocol 1
The purpose of this protocol was to identify the effects of increasing doses of NO on cutaneous vasoconstrictor responses during whole-body cold stress. Each subject underwent five 3 min cold stresses. The cold stress was imposed by changing the temperature of the water perfusing the suit from 34°C to 5°C. After each cold stress skin temperature and skin blood flow was returned to pre-cold stress levels by perfusing warm (34°C) water through the suit. Lactated Ringer solution was perfused through the microdialysis membrane for the first cold stress. Subsequent cold stresses were performed after varying degrees of cutaneous vasodilatation by perfusing sequentially greater concentrations of sodium nitroprusside (SNP; Abbott laboratories, North Chicago, IL, USA) in lactated Ringer's solution. Doses of SNP for each cold stress after the initial baseline cold stress exposure were 8.4 × 10−6, 8.4 × 10−5, 8.4 × 10−4 and 8.4 × 10−3m.
Protocol 2
The purpose of this protocol was to identify whether altered vasoconstrictor responses observed in protocol 1 were due to an effect associated with differing CVC baselines prior to the cold stress (i.e. higher baseline CVC resulting in less vasoconstriction) or whether NO impairs cutaneous vasoconstrictor responsiveness. This protocol was performed on a different day relative to the procedures outlined in protocol 1. For protocol 2 two microdialysis membranes were placed in dorsal forearm skin. One membrane was perfused with 80 μm of the beta receptor agonist isoproterenol (ISO; Crandall et al. 1997). The adjacent microdialysis membrane was perfused with a dose of SNP (typically 0.084 mm to 0.84 mm) that caused similar vasodilatation relative to the ISO-treated site. Once vasodilatation at both sites reached a plateau, the subject was exposed to a whole-body cold stress as outlined under protocol 1.
Measurement and statistical analysis
Results (mean ± s.e.m.) are expressed either as CVC or as a percentage reduction of CVC from pre-cold stress baseline. Data were averaged during the 30 s period immediately preceding the cold stress (reported as ‘baseline’ values) and the last 30 s of the cold stress.
In protocol 1, the effects of different doses of SNP on vasoconstrictor responsiveness were assessed via a one-way repeated measure ANOVA. A Bonferonni post hoc test was performed when significant differences were identified in the ANOVA. In protocol 2, a paired t test was performed to compare the magnitude of the reduction in CVC between SNP and ISO sites. The alpha level for significance was set at P ≤ 0.05.
Results
Protocol 1
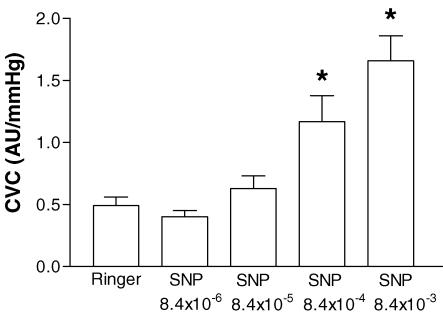
For the control condition (i.e. Ringer's solution through the microdialysis membrane), baseline CVC was 0.49 ± 0.07 AU mmHg−1. Cold stress decreased CVC by 63.0 ± 4.1%. Infusion of the lowest dose of SNP (i.e. 8.4 × 10−6 m) did not change CVC prior to the cold stress or the magnitude of vasoconstriction due to the cold stress (57.1 ± 4.7%). Incremental doses of SNP led to progressive increases in CVC prior to the cold stress (Fig. 1). Pre-cold stress CVCs for 8.4 × 10−4 m and 8.4 × 10−3 m SNP were significantly greater than CVC prior to SNP administration. There was no significant difference in the mean decrease in skin temperature between cold stresses (Table 1). In general, the higher the concentration of SNP the smaller the relative reduction in CVC during the cold stress (Table 1); with CVC decreasing 57.0 ± 3.6% (not significant) for 8.4 × 10−5m SNP, 44.5 ± 5.4%(P < 0.01) for 8.4 × 10−4m SNP, and 28.8 ± 7.9%(P < 0.001) for 8.4 × 10−3 m SNP.
Figure 1. Effect of increasing concentrations of sodium nitroprusside (SNP) on cutaneous vascular conductance (CVC) prior to the cold stress for protocol 1.
*P < 0.05 compared with Ringer solution (Ringer's).
Table 1.
Mean decreases in skin temperature and CVC during each whole-body cold stress in protocol 1
| Cutaneous vascular conductance (AU) | ||||
|---|---|---|---|---|
| Changes in skin temperature(°C) | Pre-cold stress | End cold stress | Relative reduction (End versus Pre-cold stress) | |
| Cold stress 1 (Ringer's) | − 4.5 ± 0.2 | 0.49 ± 0.20 | 0.17 ± 0.05 | 63.0 ± 4.1% |
| Cold stress 2 (SNP 8.4 × 10−6m) | − 4.6 ± 0.2 | 0.40 ± 0.13 | 0.16 ± 0.05 | 57.1 ± 4.7% |
| Cold stress 3 (SNP 8.4 × 10−5m) | − 4.4 ± 0.3 | 0.63 ± 0.28 | 0.28 ± 0.17 | 57.0 ± 3.6% |
| Cold stress 4 (SNP 8.4 × 10−4m) | − 4.6 ± 0.4 | 1.17 ± 0.60 | 0.69 ± 0.51 | 44.5 ± 5.4%* |
| Cold stress 5 (SNP 8.4 × 10−3m) | − 4.7 ± 0.6 | 1.66 ± 0.56 | 1.14 ± 0.42 | 28.8 ± 7.9%* |
The left column indicates the solution perfusing the microdialysis membrane throughout the cold stress.
Significantly different from cold stress 1, P < 0.05.
Protocol 2
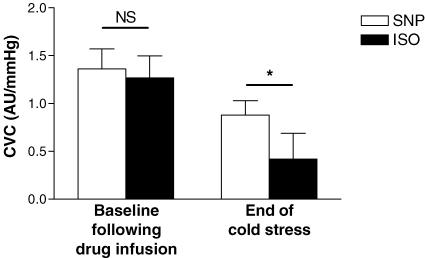
Prior to drug infusion, CVC was similar between sites (SNP site: 0.42 ± 0.11 AU mmHg−1; ISO site: 0.46 ± 0.11 AU mmHg−1). ISO and SNP caused equal increases in CVC at both sites (Fig. 2), resulting in CVC values that were not different between sites after drug administration (SNP site: 1.36 ± 0.21 AU mmHg−1; ISO site: 1.27 ± 0.23 AU mmHg−1; P > 0.05 between sites). Cold stress induced a significant decrease in CVC at both sites, but the reduction in CVC was greater at the ISO site (▵CVC = 0.84 ± 0.19 AU mmHg−1; relative reduction: 65.0 ± 5.5%) compared with the SNP site (▵CVC = 0.48 ± 0.08 AU mmHg−1; relative reduction: 38.1 ± 6.2%; P = 0.007).
Figure 2. Effect of sodium nitroprusside (SNP, NO donor) and isoproterenol (ISO, primarily a non-NO donor) on the magnitude of cutaneous vasoconstriction during the cold stress.
CVC was not different between SNP- and ISO-treated sites. However, the reduction in CVC due to the cold stress was significantly greater at the ISO-treated site (0.84 ± 0.19 AU mmHg−1) when compared with the SNP-treated site (0.48 ± 0.08 AU mmHg−1), resulting in CVC being significantly lower at the ISO-treated site at the end of the cold stress. NS, not significantly different; *P < 0.05.
Discussion
A number of studies proposed that a component of the vasodilator effect of NO could be due to inhibition of vasoconstriction through altered neurotransmission from adrenergic nerves and/or altered vasoconstrictor response to adrenergic agents (Greenberg et al. 1990; Zanzinger et al. 1994; Häbler et al. 1997; Costa et al. 2001; Chavoshan et al. 2002; Kolo et al. 2004). While this observation has been observed in human and animal studies in tissues such as skeletal and intestinal vasculature (Nase & Boegehold, 1996; Chavoshan et al. 2002), evidence is lacking concerning the possible existence of a NO-mediated attenuation of vasoconstriction in human skin. The primary finding of the present study supports the hypothesis that NO is capable of modulating cutaneous vasoconstrictor responses in humans.
Whole-body cold stress is a potent stimulator of the sympathetic nervous system that leads to cutaneous vasoconstriction. Thus, this was the ideal stimulus to assess the effects of NO on sympathetically mediated cutaneous vasoconstrictor responses. In general, sequentially higher doses of SNP attenuated the relative reduction in CVC during a cold stress (Table 1). Given that there was no difference in the decrease in skin temperature between these cold stresses (see Table 1), this observation suggests that NO may attenuate sympathetically mediated cutaneous vasoconstriction. However, prior to the cold stress CVC was progressively elevated with increasing doses of SNP. Thus, despite the relative reduction in CVC being attenuated at higher doses of SNP, absolute reduction in CVC during the cold stress was greater when higher doses of SNP were administered. These data therefore do not distinguish between an inhibitor effect of NO on sympathetically mediated cutaneous vasoconstriction from a non-specific effect of an elevated CVC. Protocol 2 was designed to address this limitation. In this protocol, the cutaneous vasculature was dilated via an NO-dependent method (SNP administration) and a method that is primarily NO independent (ISO administration). Both drugs caused similar absolute and relative increases in CVC. If the findings observed in protocol 1 were due to differences in baseline CVCs prior to the cold stress, then the magnitude of reduction in CVC between an NO-dependent vasodilator and primarily an NO-independent vasodilator would be similar. This was not the case as the magnitude of reduction in CVC at the ISO-treated site was almost twofold that of the SNP-treated site (Fig. 2), despite pre-cold stress CVCs not being significantly different between sites. These data strongly suggest that NO can attenuate cutaneous vasoconstrictor responses in human skin.
This important observation raises new considerations about previous studies investigating mechanisms of control of cutaneous blood in conditions of local or whole-body heating when NO production may be elevated (Kellogg et al. 1998; Kellogg et al. 1999; Minson et al. 2001). For example, Wilson et al. (2002) observed that vasoconstrictor responses to exogenous administration of noradrenaline (norepinephrine) were diminished when local skin temperature was greater than 37°C or when whole-body heating was performed. The aforementioned observation may then be at least partially explained by NO-mediated attenuation of vascular responsiveness to noradrenaline. The study of Wilson et al. (2002), when combined with the present findings, suggests that NO alters noradrenaline-induced cutaneous vasoconstriction at the postsynaptic level. Secondly, Kellogg et al. (1990) and Crandall et al. (1996) reported that in heat-stressed subjects reductions in CVC during an orthostatic challenge are due entirely to withdrawal of active vasodilator activity, with no influence from the cutaneous vasoconstrictor system. One possible explanation for the absence of an increase in cutaneous vasoconstrictor responses during combination of heat and orthostatic stress, even at the point of pre-syncope, may be due to NO attenuation of neurotransmitter release from adrenergic neurones and/or attenuated cutaneous vasoconstrictor responsiveness to those neurotransmitters.
Study limitations
In the first protocol, the effect of SNP on cold-induced vasoconstriction was evaluated using repeated cold stress. The subjects underwent five consecutive cold stresses throughout the protocol. Subjects were warmed between each cold stress until skin temperature and CVC returned to pre-cold stress levels. Although unlikely, repeated cold stresses may affect cutaneous microvascular responses leading to a misinterpretation of the data in protocol 1. To address this issue, in a pilot study two subjects were exposed to five repeated cold stresses while forearm CVC was continuously measured at a site in which a microdialysis membrane was placed and was perfused with Ringer's solution. There was no difference in the magnitude of the vasoconstriction between these repeated trials (reduction in CVC for cold stress 1: −53.4%, cold stress 2: −57.2%, cold stress 3: −54.3%, cold stress 4: −48.4%, cold stress 5: −57.1%). Given these findings, it is unlikely that repeated cooling affected the magnitude of vasoconstriction at the SNP treated site. Regardless, this potentially confounding variable did not affect the key finding in protocol 2 since repeated cold stresses were not performed during that trial.
Forearm vasodilator responses to intra-arterial infusions of ISO can be partially (∼30%) accounted for by NO-dependent mechanisms (Cardillo et al. 1997; Eisenach et al. 2002; Garovic et al. 2003). On the contrary, in the human hand vein ISO-mediated vasodilation does not occur via NO-dependent mechanisms (Chalon et al. 1999; Schindler et al. 2004). It remains unknown whether a component of ISO-mediated vasodilation in human skin is NO dependent. Thus, in the present protocol we cannot exclude the possibility that some of the cutaneous vasodilator responses observed during ISO administration may have been NO mediated. Despite this possibility, the magnitude of vasoconstriction to the cold stress was significantly greater at the ISO site relative to the SNP site (Fig. 2). Thus, this possible limitation does not negatively impact on the interpretation of the findings that, in human skin, NO has the capability of impairing vasoconstrictor responses.
In both protocols NO concentrations were elevated via exogenous SNP administration. Relative to endogenous NO release, it is possible that the concentration of NO was greater in the experimental protocols. Although the present study clearly documents that NO is capable of attenuating sympathetically induced cutaneous vasoconstriction, it is recognized that further research needs to be performed to identify whether endogenous concentrations of NO can reach a level such that cutaneous vasoconstrictor responses are similarly attenuated in humans.
In conclusion, the present study supports the hypothesis that NO is capable of attenuating cutaneous vasoconstriction in humans during sympathetic activation. These finding extend to human skin observations from others of an inhibitory effect of NO on sympathetic vasoconstrictor responsiveness. The role of NO-induced inhibition of sympathetically mediated vasoconstriction in the regulation of skin blood flow warrants further investigation.
Acknowledgments
The authors would like to express their appreciation to all the subjects for their participation in this project. This research project was funded in part by grants from the National Institutes of Health – National Heart, Lung, and Blood Institute (HL-61388, HL-67422), National Institute of General Medical Sciences (GM-68865), and the American Heart Association (0225036Y).
References
- Cardillo C, Kilcoyne CM, Quyyumi AA, Cannon RO, Panza JA. Decreased vasodilator response to isoproterenol during nitric oxide inhibition in humans. Hypertension. 1997;30:918–921. doi: 10.1161/01.hyp.30.4.918. [DOI] [PubMed] [Google Scholar]
- Chalon S, Tejura B, Moreno H, Urae A, Blaschke TF, Hoffman BB. Role of nitric oxide in isoprenaline and sodium nitroprusside-induced relaxation in human hand veins. Br J Clin Pharmacol. 1999;47:91–98. doi: 10.1046/j.1365-2125.1999.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavoshan B, Sander M, Sybert TE, Hansen J, Victor RG, Thomas GD. Nitric oxide-dependent modulation of sympathetic neural control of oxygenation in exercising human skeletal muscle. J Physiol. 2002;540:377–386. doi: 10.1113/jphysiol.2001.013153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa F, Christensen NJ, Farley G, Biaggoni I. NO modulates norepinephrine release in human skeletal muscle: implications for neural preconditioning. Am J Physiol Reg Int Comp Phys. 2001;280:R1494–1498. doi: 10.1152/ajpregu.2001.280.5.R1494. [DOI] [PubMed] [Google Scholar]
- Crandall CG, Etzel RA, Johnson JM. Evidence of functional beta-adrenoceptors in the cutaneous vasculature. Am J Physiol. 1997;273:H1038–1043. doi: 10.1152/ajpheart.1997.273.2.H1038. [DOI] [PubMed] [Google Scholar]
- Crandall CG, Johnson JM, Kosiba WA, Kellogg DL., Jr Baroreceptor control of the cutaneous active vasodilator system. J Appl Physiol. 1996;81:2192–2198. doi: 10.1152/jappl.1996.81.5.2192. [DOI] [PubMed] [Google Scholar]
- Eisenach JH, Clark ES, Charkoudian N, Dinenno FA, Atkinson JL, Fealey RD, Dietz NM, Joyner MJ. Effects of chronic sympathectomy on vascular function in the human forearm. J Appl Physiol. 2002;92:2019–2025. doi: 10.1152/japplphysiol.01025.2001. [DOI] [PubMed] [Google Scholar]
- Garovic V, Joyner MJ, Dietz NM, Boerwinkle E, Turner ST. β2-adrenergic receptor polymorphism and nitric oxide-dependent forearm blood flow responses to isoproterenol in humans. J Physiol. 2003;546:583–589. doi: 10.1113/jphysiol.2002.031138. 10.1113/jphysiol.2002.031138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg SS, Diecke FP, Peevy K, Tanaka TP. Release of norepinephrine from adrenergic nerve endings of blood vessels is modulated by endothelium-derived relaxing factor. Am Hypertens. 1990;3:211–218. doi: 10.1093/ajh/3.3.211. [DOI] [PubMed] [Google Scholar]
- Häbler HJ, Wasner G, Janig W. Attenuation of neurogenic vasoconstriction by nitric oxide in hindlimb microvascular beds of the rat in vivo. Hypertension. 1997;30:957–961. doi: 10.1161/01.hyp.30.4.957. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Dietz NM. Nitric oxide and vasodilation in human limbs. J Appl Physiol. 1997;83:1785–1796. doi: 10.1152/jappl.1997.83.6.1785. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous vasodilation during heat stress in humans. J Appl Physiol. 1998;85:824–829. doi: 10.1152/jappl.1998.85.3.824. [DOI] [PubMed] [Google Scholar]
- Kellogg DJ, Jr, Johnson JM, Kosiba WA. Selective abolition of adrenergic vasoconstrictor responses in skin by local iontophoresis of bretylium. Am J Physiol. 1989;257:H1599–1606. doi: 10.1152/ajpheart.1989.257.5.H1599. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Johnson JM, Kosiba WA. Baroreflex control of the cutaneous active vasodilator system in humans. Circ Res. 1990;66:1420–1426. doi: 10.1161/01.res.66.5.1420. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Liu Y, Kosiba IF, O'Donnell D. Role of nitric oxide in the vascular effects of local warming of the skin in humans. J Appl Physiol. 1999;86:1185–1190. doi: 10.1152/jappl.1999.86.4.1185. [DOI] [PubMed] [Google Scholar]
- Kolo LL, Westfall TC, Macarthur H. Nitric oxide decreases the biological activity of norepinephrine resulting in altered vascular tone in the rat mesenteric arterial bed. Am J Physiol Heart Circ Physiol. 2004;286:H296–303. doi: 10.1152/ajpheart.00668.2003. 10.1152/ajpheart.00668.2003. [DOI] [PubMed] [Google Scholar]
- Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91:1619–1626. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- Nase GP, Boegehold MA. Nitric oxide modulates arteriolar responses to increased sympathetic nerve activity. Am J Physiol. 1996;271:H860–869. doi: 10.1152/ajpheart.1996.271.3.H860. [DOI] [PubMed] [Google Scholar]
- Schindler C, Dobrev D, Grossmann M, Francke K, Pittrow D, Kirch W. Mechanisms of beta-adrenergic receptor-mediated venodilation in humans. Clin Pharmacol Ther. 2004;75:49–59. doi: 10.1016/j.clpt.2003.09.009. 10.1016/j.clpt.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Shastry S, Dietz NM, Halliwill JR, Reed AS, Joyner MJ. Effects of nitric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. J Appl Physiol. 1998;85:830–834. doi: 10.1152/jappl.1998.85.3.830. [DOI] [PubMed] [Google Scholar]
- Shibasaki M, Wilson TE, Cui J, Crandall CG. Acetylcholine released from cholinergic nerves contributes to cutaneous vasodilation during heat stress. J Appl Physiol. 2002;93:1947–1951. doi: 10.1152/japplphysiol.00036.2002. [DOI] [PubMed] [Google Scholar]
- Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol. 1989;66:1586–1592. doi: 10.1152/jappl.1989.66.4.1586. [DOI] [PubMed] [Google Scholar]
- Tripathi A, Nadel ER. Forearm skin and muscle vasoconstriction during lower body negative pressure. J Appl Physiol. 1986;60:1535–1541. doi: 10.1152/jappl.1986.60.5.1535. 10.1063/1.337286. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Cui J, Crandall CG. Effect of whole-body and local heating on cutaneous vasoconstrictor responses in humans. Autonom Neurosci: Basic Clin. 2002;97:122–128. doi: 10.1016/s1566-0702(02)00046-2. 10.1016/S1566-0702(02)00046-2. [DOI] [PubMed] [Google Scholar]
- Zanzinger J, Czachurski J, Seller H. Inhibition of sympathetic vasoconstriction is a major principle of vasodilation by nitric oxide in vivo. Circ Res. 1994;75:1073–1077. doi: 10.1161/01.res.75.6.1073. [DOI] [PubMed] [Google Scholar]