Abstract
The details of behaviour are determined by the interplay of synaptic connectivity within neuronal circuitry and the intrinsic membrane properties of individual neurones. One particularly dramatic intrinsic property displayed by neurones in many regions of the nervous system is membrane potential bistability, in which transient excitation of a neurone results in a persistent depolarization outlasting the initial excitation. Here we characterize the contribution of such intrinsic bistability, also referred to as plateau properties and mediated by persistent inward currents (PICs), in spinal motor neurones to the production of withdrawal behaviours in the frog. We performed experiments on the isolated frog spinal cord with attached hindlimb. This preparation allowed the simultaneous monitoring of muscle activations during motor behaviour and intracellular neuronal recordings. We found that PICs, following their potentiation by serotonin (5-HT), are recruited and contribute to the production of withdrawal behaviours. These properties conferred a voltage-dependent prolongation to the duration of motor neuronal activity. Consistent with this potentiation of motor neuronal PICs, 5-HT also increased the duration of evoked muscle activations. This behavioural potentiation, as well as the expression of PICs in individual neurones, was reduced following antagonism of L-type Ca2+ channels. These results demonstrate that PICs in motor neurones can be recruited during the production of behaviour and play a role in specifying the temporal details of motor output.
All behaviour results from interactions amongst neuronal populations. The nature of these interactions is dictated by properties intrinsic to individual neurones and the synapses between them. Understanding how such intrinsic neuronal properties act to shape the function of neuronal circuits, ultimately determining the details of behaviour, is a central issue in systems neuroscience. Further, intrinsic neuronal properties are not fixed, but are strongly regulated. Properties responsible for basic features of neuronal processing, such as action potential threshold and afterhyperpolarization duration, once considered to be immutable characteristics of individual neurones are now known to be alterable. Knowledge of intrinsic properties of neurones, their modulation and consequent contribution to the production of behaviour is therefore critical to our understanding of neural function.
An important aspect of basic neuronal function determined by intrinsic properties is the conduction of dendritic synaptic inputs to the spike initiation region of a neurone. This conduction can range from being essentially passive, involving simple electrical conductance of synaptic potentials along the membrane, to being dramatically active, involving non-linear amplification of synaptic inputs. In many systems, voltage-dependent persistent inward currents (PICs) have been shown to contribute to such strong non-linear amplification, increasing the amplitude and duration of synaptic inputs transmitted from dendrites to the soma and spike initiation region (Russell & Hartline, 1978; Schwindt & Crill, 1980; Hounsgaard et al. 1984; Crone et al. 1988; Hounsgaard & Mintz, 1988; Egorov et al. 2002). At the extreme, activation of such PICs can transform a strong transient depolarization into a sustained neuronal depolarization with action potential output far outlasting the original depolarization. These properties are generally under strong modulatory control, often requiring the presence of neuromodulators such as 5-HT or noradrenaline for their expression (Hounsgaard et al. 1984; Conway et al. 1988; Hounsgaard & Kiehn, 1989; Lee & Heckman, 1999; Alaburda et al. 2002; Perrier et al. 2002; Egorov et al. 2002). Whether contributing to amplification of synaptic inputs or to more dramatic sustained action potential output, such properties, often broadly referred to as ‘plateau properties’ in reference to the sustained firing which they can cause, are clearly capable of profoundly altering the information processing performed by individual neurones, thereby potentially contributing to basic aspects of neural function and behaviour.
Many roles have been proposed for such PICs in the function of neural systems. In systems such as cortex, PICs have been proposed to mediate the dynamics of working memory, acting to transform a phasic stimulus presentation into persistent neuronal activity (Egorov et al. 2002; Koulakov et al. 2002; Brody et al. 2003). In motor systems, such properties have been suggested to be involved in the maintenance of limb posture, transforming a phasic movement signal into tonic muscle activations (Kiehn & Eken, 1998).
Consistent with a possible role of PICs in the production of movement, several studies have demonstrated the presence of these properties in spinal motor neurones, with their expression contingent on neuromodulatory agents (Hounsgaard & Kiehn, 1989; Delgado-Lezama et al. 1997; Svirskis & Hounsgaard, 1998; Del Negro & Chandler, 1998; Perrier et al. 2002). These experiments have shown that PICs in motor neurones can be recruited by synaptic inputs resulting from stimuli such as muscle stretch (Hounsgaard et al. 1988b; Bennett et al. 1998a; Lee & Heckman, 2000), pudendal nerve stimulation (Paroschy & Shefchyk, 2000; Cueva-Rolon et al. 2002) or stimulation of descending systems (Crone et al. 1988; Delgado-Lezama et al. 1997), and contribute to spasticity following chronic spinalization (Eken et al. 1989; Bennett et al. 2001; Hultborn et al. 2004). Other experiments have suggested that PICs might more generally be involved in amplifying synaptic input to motor neurones (Conway et al. 1988; Hultborn, 1999; Rose & Cushing, 1999; Lee & Heckman, 2000; Powers & Binder, 2001; Hultborn et al. 2003; Hultborn et al. 2004), contributing to a short-term, graded prolongation and intensification of synaptic input and, consequently, of motor neurone activity. Evidence for a role of such PICs during behaviour has come from many experiments in invertebrates (Nagy et al. 1988; Dickinson & Nagy, 1983; see Marder & Calabrese, 1996), showing that these properties are recruited during behaviour and help shape the resulting motor patterns. In vertebrates, demonstrations of a role of PICs in behaviour have been more indirect, with studies observing persistent or bistable activity in motor units during locomotion or posture in rats and humans consistent with the recruitment of PICs in motor neurones (Eken & Kiehn, 1989; Kiehn & Eken, 1997; Gorassini et al. 1999).
The aim of the present experiments was to examine the role of such intrinsic motor neuronal properties in the production of vertebrate behaviour. We use a preparation of the frog spinal cord with attached hindlimb, allowing intracellular recordings from motor neurones, pharmacological manipulations and the monitoring of muscle activations during motor behaviour to be performed simultaneously. We show that motor neuronal PICs mediated by L-type Ca2+ channels, after being potentiated by 5-HT, are recruited during movement and play a role in specifying the temporal details of muscle activations. This role does not appear to be to mediate indefinite persistent motor output but, rather, to shape the specific temporal patterning of muscle activations during behaviour.
Methods
Preparation
All procedures were approved by the Committee on Animal Care at Massachusetts Institute of Technology. Thirty-three adult frogs (Rana catesbiana) were spinalized at the level of the obex under tricaine anaesthesia. Eight hindlimb muscles were implanted with bipolar EMG electrodes (Giszter et al. 1993): semitendinosus (ST), sartorius (SA), rectus internus (RI), vastus internus (VI), semimembranosus (SM), vastus externus (VE), biceps femoris (BF) and iliopsoas (IP). Electrodes were tunnelled subcutaneously to an exit point at the pelvis. After spinalization, frogs were refrigerated overnight. The next day, frogs were perfused intracardially with ice-cold oxygenated (95% O2–5% CO2) Ringer solution (containing mm): NaCl 113, KCl 2, NaHCO3 20, glucose 5.5, MgCl2 1, CaCl2 2; pH adjusted to 7.4–7.6. Frogs were then eviscerated, forelimbs removed and the remaining trunk with hindlimbs was placed in a dissecting dish filled with ice-cold oxygenated Ringer solution which was replenished periodically throughout the remaining dissection. The spinal cord was exposed by ventral laminectomy and the pia mater overlying the right ventral spinal cord was removed with fine scissors. The 7–9th dorsal and ventral roots innervating the right hindlimb were dissected in continuity with the dorsal root ganglia and peripheral nerves in order to maintain the main sensory and motor innervation of the hindlimb. All other spinal nerves were cut, the left hindlimb excised, and the spinal cord removed from the spinal column. The remaining preparation, consisting of the spinal cord, the right hindlimb and the pelvis, was then transferred to a Plexiglass chamber. The spinal cord and hindlimb were placed in separated chambers (see Fig. 1A for schematic diagram), with the spinal nerves passing through a communicating channel filled with Vaseline to prevent mechanical or fluid coupling between the two chambers. The chamber containing the spinal cord was continuously perfused with oxygenated Ringer solution. The descending aorta was catheterized in order to continuously perfuse the hindlimb with oxygenated Ringer solution. The pelvis was secured in the chamber by a number of insect pins placed through the bone and muscle to the bottom of the chamber, so that the hindlimb was allowed to move freely without large movement of the pelvis. Both chambers were cooled with a thermocouple placed underneath and maintained at a temperature of 12–14°C. Under these conditions, this preparation could remain viable for periods of up to 16 h, typically 10–12 h.
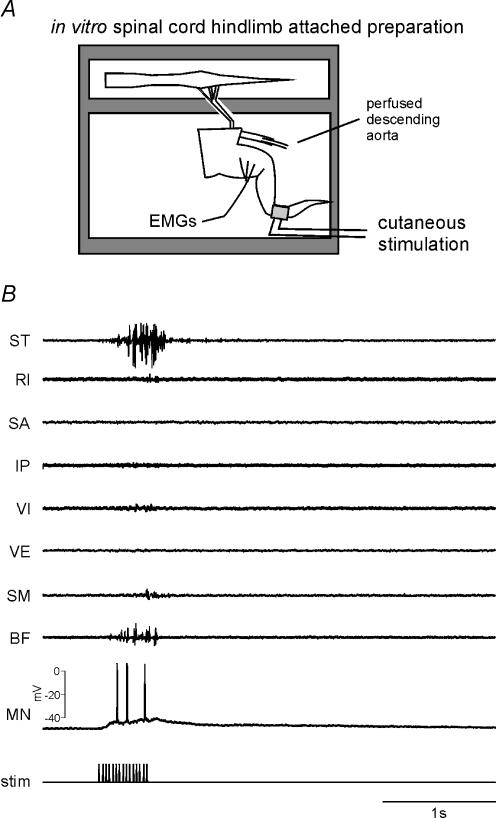
Figure 1. In vitro frog spinal cord with attached hindlimb allows intracellular motor neuronal recording with simultaneous monitoring of behaviour.
A, schematic diagram of the preparation. The spinal cord and innervated hindlimb were placed in separate chambers. The spinal cord was continuously perfused with oxygenated Ringer solution delivered to the bath. The hindlimb was perfused with oxygenated Ringer solution through a catheter inserted into the descending aorta. Withdrawal reflexes were evoked by electrical stimulation through a bipolar electrode placed on the foot. B, an example of a withdrawal behaviour evoked from electrical stimulation of the foot. The bottom trace shows the timing of biphasic stimulation applied to the foot via the stimulating electrodes. The stimulation caused activation of mainly ST and BF producing a withdrawal of the limb. The activity of a motor neurone recorded simultaneously during the behaviour is shown in the second from bottom trace. MN, motor neurone; stim, stimulation.
Withdrawal reflexes, characterized by predominant activation of ST and BF were evoked by electrical stimulation (500 to 1000-ms trains, 0.1 to 1.0-ms pulses, 5–20 V, 10–30 Hz) applied across bipolar electrodes placed on the skin of the foot. Stimulation was applied at long, constant intervals (30–120 s) to minimize habituation and potentiation of behavioural strength. In some experiments (not illustrated), the skin was also activated mechanically by pinching the same region of the skin as the one electrically stimulated. Electrical stimulation evoked essentially the same behaviour as the mechanical stimulation (see also Giszter et al. 1993; Kargo & Giszter, 2000). Evoked EMG activity recorded in implanted muscles was amplified and filtered (× 1000, 10 Hz high-pass filter, CyberAmp, Axon Instruments) and sampled at either 1000 or 10 000 Hz using custom Labview data acquisition software (National Instruments) and recorded for off-line analysis. EMG data sampled at 10 000 Hz were down-sampled to 1000 Hz off-line.
Effects of 5-HT (10 μm; Sigma) and nifedipine (10–40 μm; Sigma) on neuronal and behavioural responses were examined following superfusion of the spinal cord chamber.
Intracellular recordings
Intracellular recordings were made using glass pipettes (30–45 MΩ) filled with potassium acetate. Recordings were made in current-clamp mode using an Axoclamp 2B amplifier (Axon Instruments). Neurones were selected for study if they had a membrane potential of more than −50 mV (range −50 to −84 mV, mean ±s.d. −68.14 ± 8.24 mV). Electrodes were advanced from the ventral surface of the spinal cord in the lateral motor pools to depths of 800 μm. In initial experiments, neurones were recorded without antidromic ventral root stimulation and were presumed to be motor neurones based on the anatomical location of the recordings. However, in the majority of cases the ventral root of the segment in which neurones were recorded was placed en passant in a suction electrode to allow antidromic identification of motor neurones. With this technique, we confirmed that the large majority of neurones recorded in these regions were motor neurones: of 143 neurones tested, 129 could be antidromically activated by ventral root stimulation. Neuronal data were collected at 10 kHz using Labview software.
Data analysis
For quantification of behaviour, recorded EMG activity was rectified and digitally filtered (10 Hz low-pass Butterworth filter). Onsets and offsets of individual muscle activations following cutaneous stimulation were identified visually using custom analyses written in Matlab (Mathworks). Any responses with obvious multiple bursts of muscle activity were excluded from analysis. The duration of response for a muscle on a particular trial was taken as the time between its onset and offset. The activity of each muscle was also integrated from onset to offset to obtain a measure of the total intensity of the evoked response. For the analyses described below, we used the duration and intensity of ST activity to characterize the behaviour, because this muscle was most reliably activated between different frogs and between different trials. Durations of intracellular depolarizations following stimulation were also identified visually for each trial using custom software. For all statistical tests we used the conservative significance level of α= 0.01, in order to minimize type I errors.
Results
Motor neurone activity during behaviour
We recorded the activity of motor neurones during the production of withdrawal reflexes in the isolated frog spinal cord, hindlimb-attached preparation (Fig. 1A). In this preparation, the spinal cord and attached hindlimb were placed in separate chambers and peripheral nerves passed through a communicating channel, thereby maintaining the main sensory and motor innervation of the hindlimb. This separation allowed the examination of neuronal properties during the production of basic motor behaviours, as illustrated in Fig. 1B. This figure shows the pattern of muscle activations evoked from cutaneous stimulation of the foot, characterized by strong activation of ST and BF with occasional activation of IP, VE and VI. Activation of these muscles resulted in a withdrawal of the foot away from the site of stimulation. Figure 1B also shows the activity of a motor neurone recorded during the same behaviour. This neurone was strongly depolarized during the behaviour, sufficient to evoke a number of action potentials. Using this preparation, we examined the contribution of plateau properties mediated by PICs to the production of withdrawal behaviours.
Plateau potentials in frog motor neurones
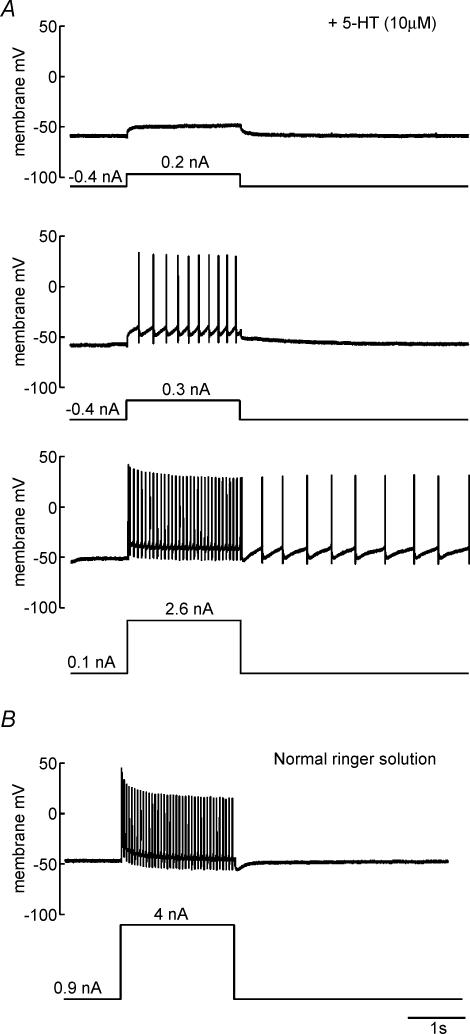
As described above, plateau properties are characterized by a persistent neuronal excitation following a transient depolarization, for instance from intracellular current injection. These properties and their underlying PICs are under strong modulatory control by neurotransmitters released by descending and segmental systems such as 5-HT, noradrenaline, acetylcholine or glutamate (Kiehn & Eken, 1998; Alaburda et al. 2002). In the present experiments, we found that 5-HT (10 μm; this concentration was used for all experiments) facilitated the expression of PICs in individual frog motor neurones. Figure 2A shows an example of a neurone which, in the presence of 5-HT, exhibited a plateau potential following intracellular current injection. With weak depolarization (top traces), the neurone essentially behaved passively, with no prolonged response other than that due to its intrinsic membrane time constant. With stronger depolarization, above threshold for action potential initiation (middle traces), there was an acceleration in the frequency of action potentials during the depolarizing pulse. With even stronger depolarization (bottom traces), the neurone produced a train of action potentials which continued following the offset of depolarization to the neurone. However Fig. 2B shows that this same neurone did not show such plateau properties in the absence of 5-HT, even with strong depolarization (plateau properties were absent with intermediate levels of depolarization as well in control Ringer solution). These observations were consistent across neurones: of 34 motor neurones tested in normal Ringer solution, none showed plateau properties following intracellular current injection (as reflected in prolonged action potential activity following transient depolarization, a continued depolarization following transient depolarization, or an acceleration in spike frequency during depolarization; Hounsgaard et al. 1988a). In contrast, following application of 5-HT, seven out of 17 motor neurones showed such plateau properties. These results demonstrate that frog motor neurones, similar to motor neurones in other animals, express PICs but that the expression of this intrinsic neuronal property is under strong modulatory control.
Figure 2. 5-HT promotes PICs in lumbar motor neurones.
For all recordings, the upper trace shows the membrane potential of the recorded motor neurone, while the lower trace shows the current injected through the microelectrode. A, response of a motor neurone recorded in the presence of 5-HT to different levels depolarization. With small depolarization (top traces), no regenerative potentials were observed. With larger depolarizations, an acceleration in the frequency of action potentials was observed (middle traces). With even larger depolarizations, the neurone produced an afterdischarge (bottom traces). B, the same neurone in normal Ringer solution. Even with very large depolarization, this neurone showed no signs of regenerative plateau potentials, either in terms of spike frequency acceleration or afterdischarge.
Plateau potentials are recruited during the production of behaviour
We next examined whether such motor neuronal PICs were recruited during withdrawal behaviours. To test this possibility, we examined the voltage sensitivity of the duration of neuronal depolarization during this behaviour after application of 5-HT. In this analysis, the duration of synaptic input to a neurone on repeated trials of a particular behaviour is assumed to be similar from trial to trial. However if PICs were recruited in a neurone during behaviour, we would expect that the duration of neuronal depolarization should vary with the tonic membrane potential of the neurone. One consequence of this recruitment of PICs might be the persistent activation of the neurone, causing it to fire indefinitely following the transient synaptic input. On the other hand, several studies have shown that when PICs are not fully activated by stimulation, the duration increases with the strength of depolarization used to induce the potential (Hounsgaard & Kiehn, 1989; Hounsgaard & Kjaerulff, 1992; Russo & Hounsgaard, 1996; Lee & Heckman, 1998). This implies that the recruitment of PICs during behaviour might result in a graded prolongation of motor neuronal discharge, rather than a fully persistent activation. We examined these possibilities by manipulating the holding potential of motor neurones and observing any changes in the duration of neuronal depolarization following the evocation of behaviour.
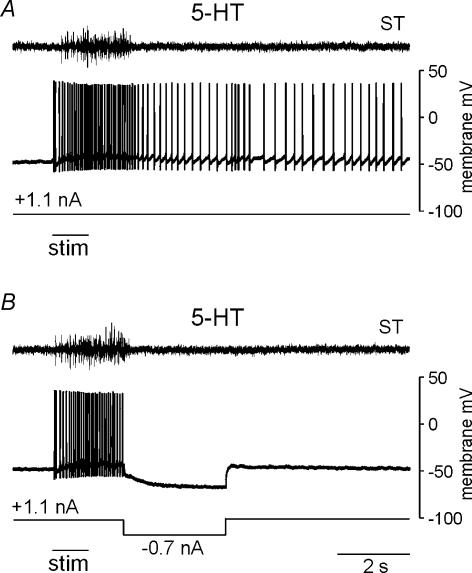
An example of persistent neuronal activity evoked during behaviour is shown in Fig. 3. In both trials illustrated in the figure, the neurone was depolarized by the same tonic current injection in the presence of 5-HT. After cutaneous stimulation was applied to evoke a withdrawal reflex, as indicated by the activation of ST in the figure, the action potential activity of this neurone continued for an extended period of time (Fig. 3A). However, following the same electrical cutaneous stimulation evoking a withdrawal reflex of similar duration and intensity as reflected in the activation of ST, transient hyperpolarization abolished this prolonged activity in the neurone (Fig. 3B). The fact that the duration of the behaviour was similar in the two cases while the duration of the neuronal response was abbreviated by transient hyperpolarization illustrates that PICs in this neurone were recruited during the behaviour.
Figure 3. Recruitment of bistable plateau potentials during behaviour.
A, a neurone depolarized with tonic current produced a prolonged train of action potentials during the production of a withdrawal reflex evoked in the presence of 5-HT. The duration of the reflex is indicated in the top trace showing the activation of ST during the behaviour. B, the same stimulation applied to the foot evoked a reflex of similar duration and intensity as the trial shown in A, as reflected by the activation of ST. However, hyperpolarization terminated the discharge of the neurone so that the prolonged activity seen in A was no longer observed. Bars underneath traces in A and B show the duration of electrical stimulation applied to the foot.
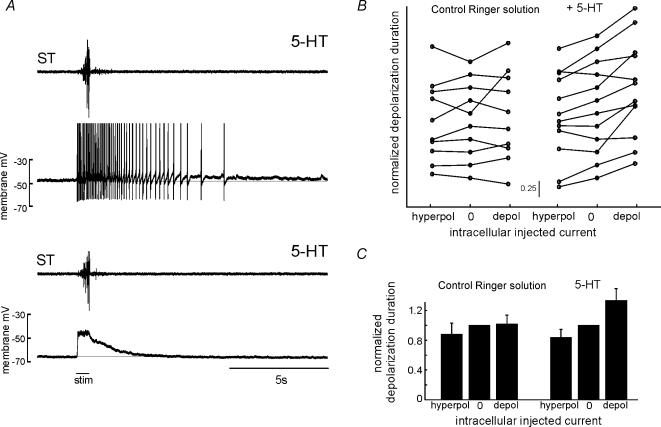
However, the persistent firing pattern of the neurone illustrated in Fig. 3 was not typical of most neurones examined here. Instead, the recruitment of PICs in most motor neurones could be observed as a graded voltage-dependent prolongation of the duration of depolarization following cutaneous stimulation. Figure 4A shows an example of a neurone in which such a graded increase of neuronal response duration was observed in the presence of 5-HT (see also Fig. 5A). With the neurone hyperpolarized with no injected current (lower traces), cutaneous stimulation evoked a depolarization insufficient to cause an action potential and the neurone returned to its prestimulation potential at approximately 4 s following the stimulation. With positive current injections (upper traces, action potentials truncated), the depolarization of this neurone was prolonged and the neurone produced action potentials for a period of approximately 7 s. Thus, the duration of depolarization of this neurone was strongly dependent on the amount of current injected into the neurone, even though the duration of the behaviour was similar between trials, as indicated by the activation of ST.
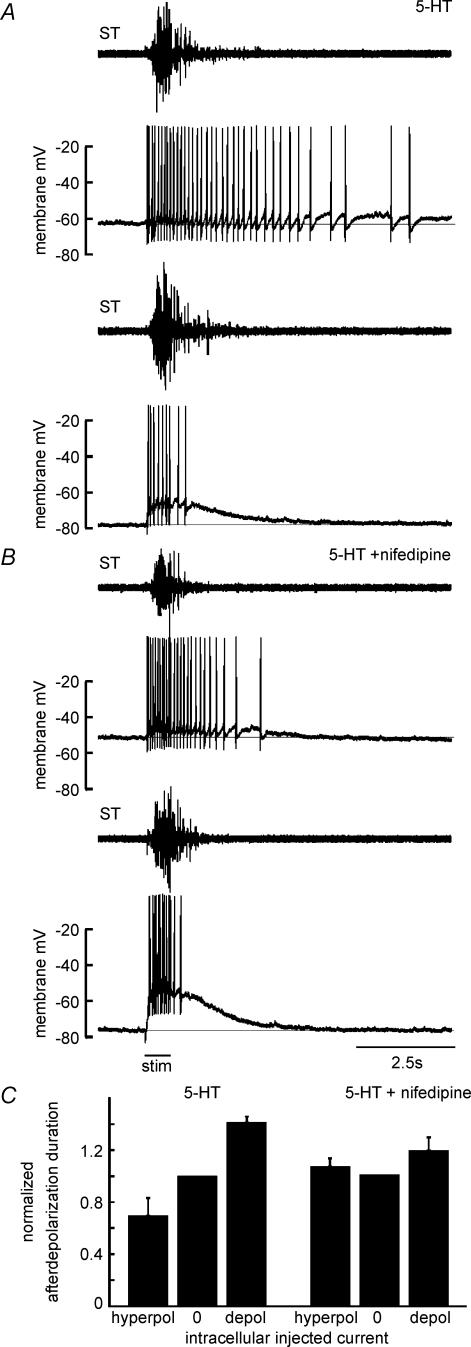
Figure 4. Duration of neuronal depolarization evoked during withdrawal reflexes is voltage dependent in the presence of 5-HT.
A, recording from a motor neurone during two trials of evoked withdrawal reflexes. Duration and intensity of the behaviour were similar in each trial as reflected in the ST traces. In the trial shown in the bottom two traces, the neurone was at resting potential with no injected current. In the trial shown in the top traces, the neurone was tonically depolarized. With the neurone depolarized, the duration of activity was longer than would be expected from the duration of the synaptic depolarization observed with the neurone hyperpolarized, suggesting a voltage-dependent prolongation in neuronal duration in this neurone. Action potentials were truncated to fit the figure. Bar beneath all traces indicates stimulus duration. B, the duration of neuronal depolarization observed in motor neurones for hyperpolarizing, depolarizing or no injected current, with and without application of 5-HT. Each connected line indicates data obtained from a single neurone. The vertical offsets between lines are arbitrary and are used to facilitate comparison between different neurones. The duration of depolarization in each neurone was normalized to the duration observed with zero injected current. A decrease of 0.25 from zero current injected indicates that the neuronal duration was reduced to three-quarters of the duration observed with zero current (see inset scale bar). Following application of 5-HT, there was an increased tendency for the duration of neuronal depolarization to depend on the injected current. C, the weighted means of normalized response durations averaged across cells. Each mean in B was weighted by its standard error. Error bars indicate one standard deviation from the weighted mean. The lack of standard deviation around the zero current average in each plot reflects the fact that this value was normalized to one for each neurone.
Figure 5. Motor neurone voltage dependence during behaviour is reduced by nifedipine.
In each panel, the upper trace represents the activity of the ST muscle and the lower trace represents the membrane potential of a motor neurone. All recordings are from the same motor neurone. A, in the presence of 5-HT (10 μm), the duration of the response evoked by cutaneous stimulation of the skin increased when the neurone was depolarized with positive current as compared to at resting potential. B, in the presence of nifedipine (10 μm) the duration of the response was not increased by positive current injection, even when the induced depolarization was stronger than in control conditions. Bar underneath traces in B indicates stimulus duration for both A and B. C, the weighted means (weighted by inverse squared standard error) of neuronal depolarization duration in the presence of 5-HT recorded in two neurones before and after application of nifedipine. The voltage sensitivity observed with 5-HT was not observed following application of nifedipine.
We quantified this voltage dependence of the duration of neuronal depolarization in two analyses. First, we anaylsed by regression the duration of neuronal depolarization to the amount of tonic current injected into the neurone. For this analysis we only included neurones for which responses were measured on at least 10 trials of behaviour. In normal Ringer solution, none of the 10 neurones which met this condition showed a significant positive correlation between neuronal depolarization duration and the amount of injected current (linear regression; P > 0.01 for each comparison). In the presence of 5-HT, seven out of 12 neurones showed such a significant positive correlation (P < 0.01).
To combine data from different neurones, we performed a second analysis. In this analysis, we grouped each stimulation trial according to the current injected into the neurone: either negative, zero or positive current. Each observed neuronal response duration was normalized to the mean response duration observed with zero injected current for that neurone. We then averaged the normalized duration of the neuronal depolarization for each of these groups. These averages are shown for each neurone in Fig. 4B in both normal Ringer solution and after application of 5-HT. In normal Ringer solution, none of 10 neurones showed a significant dependence of depolarization duration on the injected current (one-way ANOVA; P > 0.01). Following 5-HT application, five of 12 neurones showed a significant dependence of duration on the injected current (P < 0.01). These changes are similar to those described for the regression analyses above. We then combined these averages for each group across neurones, weighting each average by its inverse squared standard error to account for differences in the reliability of each measurement (Squires, 2001), as shown in Fig. 4C. In normal Ringer solution, there was no difference in response duration with either positive or negative current injection as compared to no injected current (t test; P > 0.01). After application of 5-HT, there was both a significant increase in the duration of neuronal depolarization following positive current injection and a significant decrease in duration following negative current injection as compared to no injected current (P < 0.01).
These results show that following enhancement of PICs by 5-HT, the response duration of individual motor neurones became dependent on the level of depolarization of that neurone. However, trial-to-trial variability in the evoked behaviour, for example in its intensity or duration, might have also contributed to the variability in the observed neuronal response. The correlation between injected current and neuronal response duration might therefore have reflected a more direct correlation of neuronal depolarization to the characteristics of the evoked behaviour. To assess this possibility we performed a multiple regression of neuronal response duration to the behavioural intensity and duration in addition to the amount of injected current. The significance of the relationship between each of these variables and the observed neuronal response duration was assessed by the significance of their respective regression coefficients. Duration and intensity of evoked behaviours were measured from the ST EMG with behavioural intensity measured as total integrated EMG. In two of the seven cases in the presence of 5-HT with significant regression to injected current, the evoked EMG activity was in many trials within the noise level, making determination of behavioural duration difficult and these cases were therefore not included in this analysis. In all five of the remaining cases, including these behavioural measures did not alter the significant relationship between neuronal response duration and the amount of injected current. This analysis suggests that there was a significant increase in the duration of neuronal depolarization with increasing levels of injected current, regardless of any trial-to-trial variability in the evoked behaviours.
Voltage dependence of response duration is due to activation of L-type Ca2+ channels
In many previous in vitro experiments, L-type Ca2+ channels have been shown to make a large contribution to PICs (Hounsgaard & Mintz, 1988; Russo & Hounsgaard, 1994; Morisset & Nagy, 1999; Carlin et al. 2000; Simon et al. 2003). We assessed whether these channels might contribute to the voltage-dependent responses observed during behaviour in the present experiments by applying the L-type Ca2+ channel antagonist nifedipine (10–40 μm). Figure 5A illustrates a neurone which showed a dependence of its behaviourally evoked depolarization duration on the injected current, assessed either by the regression analysis or the categorization analysis described in the previous section. After application of nifedipine for 1 h, this dependence was strongly reduced (Fig. 5B), with similar durations of neuronal depolarization when positive (upper traces) or negative bias currents (lower traces) were injected into the neurone. Figure 5C shows the summary data for the two neurones that showed a voltage dependence in the presence of 5-HT and which were recorded after 1 h application of nifedipine. As seen in the figure, the dependence of neuronal depolarization duration on injected current, which was observed in the presence of 5-HT, was strongly reduced following application of nifedipine. For each neurone, the regression analysis and the categorical analysis assessing the voltage dependence of depolarization duration were not significant following application of nifedipine, although in each case they had been significant prior to application of nifedipine. These observations suggest that L-type Ca2+ channels contribute to the voltage-dependent characteristics of the neuronal depolarizations observed here, although contributions from other currents to this voltage dependence are also possible (Di Prisco et al. 1997; Morisset & Nagy, 1999; Li & Bennett, 2003; see Discussion).
Potentiation of plateau properties and L-type Ca2+ channels shapes motor behaviour
The previous sections have shown that PICs are present in frog spinal motor neurones, are unmasked by 5-HT, receive a large contribution from L-type Ca2+ channels, and can be recruited during the production of behaviour to shape the pattern of motor neuronal firing. Moreover, the results of Figs 4C and 5C, showing a decrease in neuronal response duration with hyperpolarizing current, show that these PICs are recruited in motor neurones during behaviour even without any additional depolarizing current. This observation suggests that such PICs might shape the temporal patterns of motor neuronal activity during the production of behaviour. In particular, we would expect that the potentiation of such motor neuronal PICs by 5-HT would lead to a potentiation of the observed behaviour. The upper traces in Fig. 6A show such an example in which the muscle activation was potentiated following 5-HT application. Of 16 cases examining the effects of 5-HT on the response duration of muscle activation (measured as from the onset to the offset of the ST EMG), eight showed a significant (one-way ANOVA; P < 0.01) increase in duration following 5-HT application, one showed a significant decrease, and seven showed no effect. The vertical scatter plot on the left-hand side of Fig. 6B shows the increase in the response duration after 5-HT application for each of the observed cases. Averaged across all preparations, the duration of the response observed in the presence of 5-HT increased by 0.56 ± 0.68 s (weighted mean ±s.d.) compared to that observed in control solution, which was a significant increase (t test; P < 0.01; this increase was also significant when the outlier on the left-hand side of Fig. 6B was excluded). The effect of 5-HT on the overall intensity of the behavioural responses, taken as the total integrated EMG, was more variable: in three cases there was a significant (P < 0.01) increase of integrated EMG amplitude, in four cases there was a significant decrease, and in nine cases no effect could be detected. In several cases, the decrease in amplitude could be substantial (in three out of four cases the decreases were > 50% of control). These more variable effects of 5-HT on response amplitude might reflect heterogeneous effects of 5-HT on spinal neuronal systems (Holohean et al. 1990; Schmidt & Jordan, 2000; see Discussion). In general, application of 5-HT did not dramatically alter the basic motor pattern observed following noxious cutaneous stimulation, in that the reflex involved the activation of the same muscles before and after 5-HT application. Also, we generally did not observe a dramatic change in the time course of muscle activations following application of 5-HT: as can be seen in the illustrated cases, 5-HT did not generally suppress responses evoked during the stimulation train and replace them with a later, delayed EMG burst, as has been described in the cat following systemic 5-hydroxytriptamine administration (Anden et al. 1964). Of the cases mentioned above in which there was a decrease in the intensity of EMG, in one animal the early response was replaced by a later more prolonged muscle activation, but this effect was not typical. Based on these observations, the most consistent effect of 5-HT application on withdrawal reflexes in these experiments was to increase the duration of evoked muscle activations.
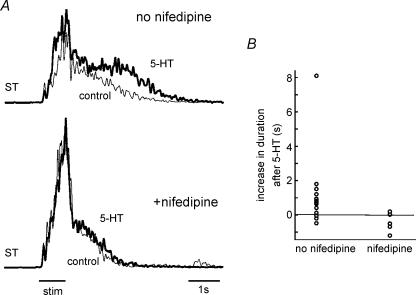
Figure 6. Serotonergic enhancement of L-type Ca2+ channels potentiates behaviour.
A, the mean EMG activity evoked during a withdrawal reflex and recorded in ST in control Ringer solution (light trace) and after application of 5-HT (dark trace). Top two traces show that 5-HT increased the EMG activity evoked by cutaneous stimulation. Bottom two traces show that after application of nifedipine, the potentiating effects of 5-HT were no longer observed. Bar underneath the figure indicates duration of stimulation train. B, mean increase in duration (in seconds) of ST EMG by 5-HT with and without application of nifedipine, shown for all observed cases.
To assess whether this potentiation of the duration of behavioural responses following application of 5-HT was due to potentiation of L-type Ca2+ channels, we repeated the same experiments in the presence of nifedipine. As illustrated in the lower traces in Fig. 6A, after application of nifedipine, 5-HT no longer caused a potentiation of the evoked motor response. Of five cases examining the effect of 5-HT in the presence of nifedipine, none showed a significant (P < 0.01) increase in behavioural duration (one showed a significant decrease, and four showed no effect). The vertical scatter plot on the right-hand side of Fig. 6B shows for each of these cases the mean fractional change after 5-HT application in the presence of nifedipine. After application of nifedipine, the duration of the response observed in the presence of 5-HT decreased by 0.39 ± 0.54 s (weighted mean ±s.d.) compared to that observed in control solution, which was a significant decrease in duration following 5-HT application as compared to the increase in duration observed without nifedipine (t test; P < 0.01; −0.39 vs. 0.56; this difference was also significant when the outlier on the left-hand side of Fig. 6B was excluded). The total intensity of responses was significantly (P < 0.01) decreased in one case, and did not significantly change in four cases. We also assessed whether nifedipine affected features of the behaviour evoked in control Ringer solution, independent of its effects on the serotonergic potentiation of behavioural duration. In two of five cases, there was a significant decrease in the duration of the evoked behaviour; in the other three cases there was no significant effect. In one case there was an increase in the intensity of the behaviour, in one case there was a decrease, and in three cases there was no effect. Taken together, these results suggest that PICs mediated by L-type Ca2+ channels, especially after their potentiation by 5-HT, contribute to the temporal pattern of muscle activations observed during withdrawal behaviours.
Discussion
The results presented here demonstrate a role for plateau properties and their underlying PICs in the production of a withdrawal behaviour in the frog. PICs mediated by L-type Ca2+ channels conferred a voltage-dependent prolongation to the duration of motor neuronal activity during withdrawal behaviours. This voltage dependence was observed following potentiation of PICs by 5-HT but was not observed in control Ringer solution. Consistent with this potentiation of motor neuronal activity, 5-HT also potentiated the duration of evoked behaviours. This behavioural potentiation was reduced by antagonism of L-type Ca2+ channels, showing that recruitment of PICs, following their potentiation by 5-HT, can contribute to the specification of muscle activation patterns underlying basic motor behaviours.
Plateau properties in frog motor neurones
Similar to results obtained in other species, we found that following application of 5-HT, approximately half of motor neurones showed evidence of PICs following intracellular current injection. These experiments are consistent with previous experiments demonstrating latent PICs in frog motor neurones (Alvarez-Leefmans & Miledi, 1980; Perrier & Hounsgaard, 2000). It is likely that the fraction of motor neurones possessing PICs is in reality higher than that observed here, given that dendritically located PICs might be difficult to recruit by somatic current injection (Hounsgaard & Kiehn, 1993; Bennett et al. 1998a; Carlin et al. 2000; Lee & Heckman, 2000; Perrier & Hounsgaard, 2003; Simon et al. 2003). It should be noted that although the present results suggest the contribution of L-type Ca2+ channels to these PICs, it is likely that other currents also contributed, as has been observed in other systems (Di Prisco et al. 1997; Morisset & Nagy, 1999; Li & Bennett, 2003). The fact that PICs were promoted in only a fraction of motor neurones could also reflect the fact that 5-HT modulates multiple currents in motor neurones, some of which might mask expression of PICs (Holohean et al. 1990; Rekling et al. 2000; Schmidt & Jordan, 2000). Alternatively, the variable expression of PICs seen here might reflect a heterogeneity in the expression of PICs in different types of motor neurones. For example, 5-HT might promote PICs only for some motor pools, such as extensor motor neurones, as suggested by previous findings (Conway et al. 1988; Hounsgaard et al. 1988a). Nevertheless, the demonstration of PICs in this preparation provides further evidence for the prevalence of PICs in vertebrate motor neurones.
As also described for other systems, we found that PICs were predominantly observed following application of neuromodulators, such as 5-HT (Hounsgaard et al. 1988b; Conway et al. 1988; Hounsgaard & Kiehn, 1989; Alaburda et al. 2002; Perrier & Hounsgaard, 2003). In intact animals, 5-HT in the spinal cord arises mainly from raphé–spinal neurones projecting throughout the spinal grey matter including directly to motor neurones (Soller, 1977; Tan & Miletic, 1990; Jacobs & Azmitia, 1992). Most raphé-spinal neurones are tonically active in behaving animals (Jacobs et al. 2002), providing a background level of 5-HT in spinal systems. Removal of raphé–spinal influence on spinal systems has been shown to reduce motor neuronal persistent activity (Hounsgaard et al. 1988b; Kiehn et al. 1996). An understanding of the precise role of serotonergic modulation of PICs in the production of behaviour clearly requires experiments in behaving animals with intact raphé-spinal systems rather than the reduced preparations used here, so that consequences of normal patterns of 5-HT release can be assessed.
Recruitment of PICs potentiates motor neurone activation during behaviour
A main finding of the present study was that potentiation of PICs conferred a voltage dependence on the duration of motor neurone discharge during the production of withdrawal reflexes. Most commonly, this potentiation resulted in a prolongation of motor neuronal depolarization of the order of a few seconds. The present results also show that these properties were recruited at resting membrane potentials because the duration of neuronal discharge decreased when membrane potential was hyperpolarized from resting levels (Figs 4C and 5C). Consistent with this observation, we also found that the duration of muscle activation patterns was potentiated following 5-HT application and this potentiation was reduced by nifedipine. These results suggest that the potentiation of behavioural response by 5-HT previously described (Carlsson et al. 1963; Rekling et al. 2000; Schmidt & Jordan, 2000), might in part be due to the modulation of motor neuronal PICs. However, it is also clear that 5-HT can have multiple effects on motor neurone properties, including inhibition, depending on the receptor subtype activated. The more variable effects of 5-HT on response intensity observed here, for instance, might reflect such additional actions of 5-HT (Holohean et al. 1990; Rekling et al. 2000; Schmidt & Jordan, 2000).
Further, although we demonstrated here the serotonergic potentiation of PICs in motor neurones, it is also clear that 5-HT can have strong effects on spinal interneuronal function as well. Many studies have demonstrated that 5-HT can alter the properties of spinal interneurones and the patterns of synaptic connectivity between them (e.g. Jankowska et al. 2000; Jankowska, 2001; see Schmidt & Jordan, 2000), potentially resulting in a reorganization of spinal reflexes (e.g. Anden et al. 1964) and even the evocation of full behaviours such as locomotion (e.g. Cazalets et al. 1992). In the present study, we did not observe such a profound reorganization of spinal reflexes following 5-HT application, and in general, the pattern of EMGs in the presence of 5-HT was similar to that observed in control Ringer solution. In addition, several studies have shown that spinal interneurones also express PICs (Hounsgaard & Kjaerulff, 1992; Morisset & Nagy, 1999; Russo & Hounsgaard, 1996) and we cannot therefore exclude the possible contribution of interneuronal PICs to the potentiation of behavioural duration observed here, especially given the bath application of pharmacological agents used here. We also observed that the duration of motor neuronal depolarization was consistently considerably longer than the duration of the cutaneous stimulation train even when the neurone was strongly hyperpolarized, consistent with the possible presence of such interneuronal PICs even in the absence of neuromodulation (Bennett et al. 2001), although recurrent connections within interneuronal systems might also contribute. Thus, serotonergic actions at a premotorneuronal level might contribute to behavioural potentiation observed here. Nonetheless, the demonstration here that PICs are potentiated in motor neurones following 5-HT application and are recruited during behaviour strongly suggests that at least a portion of this potentiation is mediated by facilitation of PICs in motor neurones.
Roles for plateau properties in neuronal processing
The possible role of PICs in the production of behaviour has been examined previously, showing evidence both for (Eken & Kiehn, 1989; Di Prisco et al. 1997; Kiehn & Eken, 1997; Gorassini et al. 1999; Paroschy & Shefchyk, 2000; Cueva-Rolon et al. 2002) and against (Sanchez-Vives & McCormick, 2000; Aksay et al. 2001) contributions of PICs to nervous system function. As described in the Introduction, many studies have proposed a role for such PICs in specifying the activation pattern of motor neurones and have provided evidence for persistent activity of motor units in awake behaving animals consistent with the involvement of PICs in determining motor neurone activity patterns (Eken & Kiehn, 1989; Kiehn & Eken, 1997; Gorassini et al. 1999). In the present experiments, by exploiting the methodological advantages of the spinal cord with attached hindlimb preparation, we were able to pharmacologically manipulate the expression of PICs in intracellularly recorded spinal neurones while simultaneously monitoring the changes in the observed motor behaviour in order to test directly the contribution of PICs to the production of behaviour. The results described here show that PICs can make such a contribution, demonstrating both the recruitment of PICs during behaviour and the consequent potentiation of muscle activation patterns underlying that behaviour.
These results suggest that in withdrawal behaviours in the frog, PICs induced a voltage-dependent potentiation of the duration of motor neuronal depolarization, thereby prolonging the duration of muscle activations observed during behaviour, rather than producing a sustained, indefinite motor output. It therefore appeared that the role of PICs in these experiments was to amplify synaptic input to motor neurones, consistent with previous proposals (Conway et al. 1988; Hultborn, 1999; Rose & Cushing, 1999; Lee & Heckman, 2000; Powers & Binder, 2001; Hultborn et al. 2003, 2004). Of course, these results do not rule out the possibility that persistent activity might be utilized in different species or in behavioural contexts other than withdrawal reflexes. For example, PICs enhanced by 5-HT have been shown to be especially prevalent in extensor muscles in cats (Conway et al. 1988; Hounsgaard et al. 1988a) and the relative infrequency of persistent activity patterns during behaviour described here might reflect the fact that mainly flexors were activated in the withdrawal behaviours studied. Similarly, the frog hindlimb plays a relatively minor role in postural weight support and hindlimb muscles only rarely show sustained tonic activity during behaviour (A. d'Avella and E. Bizzi, unpublished observations). While it remains possible that PICs in other situations and behaviours might bring about a fully persistent activation pattern, it is also possible that the present results might indicate that persistent activity brought about by PICs simply does not make a substantial contribution to the production of motor behaviour. Although there remain several possible roles for intrinsically generated persistent activity in the production of normal behaviours, the present results show that PICs can nonetheless contribute to the specification of motor output, even without generating persistent activity. This contribution further emphasizes that motor neurones do not simply passively integrate activity from presynaptic networks, but instead actively transform synaptic input to shape patterns of muscle activations during behaviour.
Such a role for PICs in sculpting neuronal activation patterns by amplifying synaptic inputs is consistent with suggestions that PICs might play a range of roles in neural function. The present study suggests that one such role might be in adapting temporal patterns of muscle activations during behaviour. In other situations, this same voltage-dependent persistent current might contribute to functions such as the ‘wind-up’ potentiation of reflexes (Russo & Hounsgaard, 1994; Bennett et al. 1998b; Alaburda & Hounsgaard, 2003), gating of ascending information (Derjean et al. 2003), coincidence detection for synaptic plasticity (Grover & Teyler, 1990; Magee & Johnston, 1997), or to the stability of neuronal activity (Koulakov et al. 2002; Brody et al. 2003). Regardless of their precise role in neuronal processing, it seems likely that PICs, interacting with synaptic inputs within a highly interconnected and dynamic network, will make strong contributions to basic neuronal function throughout the nervous system.
Acknowledgments
We are grateful to Aidas Alaburda, Emilio Bizzi, Jørn Hounsgaard and Andrew Richardson for reading a previous version of the paper. Experiments were carried out in the laboratory of Emilio Bizzi at Massachusetts Institute of Technology. J.-F.P. was supported by a grant from the Danish Medical Research Council. M.C.T. was supported by National Institutes of Health – National Institute of Neurological Disorders and Stroke NS39865.
References
- Aksay E, Gamkrelidze G, Seung HS, Baker R, Tank DW. In vivo intracellular recording and perturbation of persistent activity in a neural integrator. Nat Neurosci. 2001;4:184–193. doi: 10.1038/84023. 10.1038/84023. [DOI] [PubMed] [Google Scholar]
- Alaburda A, Hounsgaard J. Metabotropic modulation of motoneurons by scratch-like spinal network activity. J Neurosci. 2003;23:8625–8629. doi: 10.1523/JNEUROSCI.23-25-08625.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaburda A, Perrier JF, Hounsgaard J. Mechanisms causing plateau potentials in spinal motoneurones. Adv Exp Med Biol. 2002;508:219–226. doi: 10.1007/978-1-4615-0713-0_27. [DOI] [PubMed] [Google Scholar]
- Alvarez-Leefmans FJ, Miledi R. Voltage sensitive calcium entry in frog motoneurones. J Physiol. 1980;308:241–257. doi: 10.1113/jphysiol.1980.sp013470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anden N-E, Jukes MGM, Lundberg A, Vyklicky L. A new spinal flexor reflex. Nature. 1964;202:1344–1345. doi: 10.1038/2021344b0. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Synaptic activation of plateaus in hindlimb motoneurons of decerebrate cats. J Neurophysiol. 1998a;80:2023–2037. doi: 10.1152/jn.1998.80.4.2023. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Hultborn H, Fedirchuk B, Gorassini M. Short-term plasticity in hindlimb motoneurons of decerebrate cats. J Neurophysiol. 1998b;80:2038–2045. doi: 10.1152/jn.1998.80.4.2038. [DOI] [PubMed] [Google Scholar]
- Bennett DJ, Li Y, Siu M. Plateau potentials in sacrocaudal motoneurons of chronic spinal rats, recorded in vitro. J Neurophysiol. 2001;86:1955–1971. doi: 10.1152/jn.2001.86.4.1955. [DOI] [PubMed] [Google Scholar]
- Brody CD, Romo R, Kepecs A. Basic mechanisms for graded persistent activity: discrete attractors, continuous attractors, and dynamic representations. Curr Opin Neurobiol. 2003;13:204–211. doi: 10.1016/s0959-4388(03)00050-3. [DOI] [PubMed] [Google Scholar]
- Carlin KP, Jones KE, Jiang Z, Jordan LM, Brownstone RM. Dendritic L-type calcium currents in mouse spinal motoneurons: implications for bistability. Eur J Neurosci. 2000;12:1635–1646. doi: 10.1046/j.1460-9568.2000.00055.x. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Magnusson T, Rosengren E. 5-Hydroxytramine of the spinal cord normally and after transection. Experientia. 1963;19:359. doi: 10.1007/BF02152316. [DOI] [PubMed] [Google Scholar]
- Cazalets JR, Sqalli-Houssaini Y, Clarac F. Activation of the central pattern generators for locomotion by serotonin and excitatory amino acids in neonatal rat. J Physiol. 1992;455:187–204. doi: 10.1113/jphysiol.1992.sp019296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O, Mintz I. Plateau potentials in alpha-motoneurones induced by intravenous injection of L-dopa and clonidine in the spinal cat. J Physiol. 1988;405:369–384. doi: 10.1113/jphysiol.1988.sp017337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Kiehn O, Mazieres L, Wigstrom H. Maintained changes in motoneuronal excitability by short-lasting synaptic inputs in the decerebrate cat. J Physiol. 1988;405:321–343. doi: 10.1113/jphysiol.1988.sp017335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cueva-Rolon R, Delgado-Lezama R, Raya JG, Raya M, Tecuanhuey R, Munoz-Martinez EJ. Sustained firing of alpha and gamma hind limb motoneurons induced by stimulation of the pudendal nerve. J Neurophysiol. 2002;88:3232–3242. doi: 10.1152/jn.00157.2002. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Chandler SH. Regulation of intrinsic and synaptic properties of neonatal rat trigeminal motoneurons by metabotropic glutamate receptors. J Neurosci. 1998;18:9216–9226. doi: 10.1523/JNEUROSCI.18-22-09216.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Lezama R, Perrier JF, Nedergaard S, Svirskis G, Hounsgaard J. Metabotropic synaptic regulation of intrinsic response properties of turtle spinal motoneurones. J Physiol. 1997;504:97–102. doi: 10.1111/j.1469-7793.1997.097bf.x. 10.1111/j.1469-7793.1997.097bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derjean D, Bertrand S, Le Masson G, Landry M, Morisset V, Nagy F. Dynamic balance of metabotropic inputs causes dorsal horn neurons to switch functional states. Nat Neurosci. 2003;6:274–281. doi: 10.1038/nn1016. 10.1038/nn1016. [DOI] [PubMed] [Google Scholar]
- Di Prisco GV, Pearlstein E, Robitaille R, Dubuc R. Role of sensory-evoked NMDA plateau potentials in the initiation of locomotion. Science. 1997;278:1122–1125. doi: 10.1126/science.278.5340.1122. 10.1126/science.278.5340.1122. [DOI] [PubMed] [Google Scholar]
- Dickinson PS, Nagy F. Control of a central pattern generator by an identified modulatory interneurone in crustacea. II. Induction and modification of plateau properties in pyloric neurones. J Exp Biol. 1983;105:59–82. doi: 10.1242/jeb.105.1.59. [DOI] [PubMed] [Google Scholar]
- Egorov AV, Hamam BN, Fransen E, Hasselmo ME, Alonso AA. Graded persistent activity in entorhinal cortex neurons. Nature. 2002;420:173–178. doi: 10.1038/nature01171. 10.1038/nature01171. [DOI] [PubMed] [Google Scholar]
- Eken T, Hultborn H, Kiehn O. Possible functions of transmitter-controlled plateau potentials in alpha motoneurones. Prog Brain Res. 1989;80:257–267. doi: 10.1016/s0079-6123(08)62219-0. [DOI] [PubMed] [Google Scholar]
- Eken T, Kiehn O. Bistable firing properties of soleus motor units in unrestrained rats. Acta Physiol Scand. 1989;136:383–394. doi: 10.1111/j.1748-1716.1989.tb08679.x. [DOI] [PubMed] [Google Scholar]
- Giszter SF, Mussa-Ivaldi FA, Bizzi E. Convergent force fields organized in the frog's spinal cord. J Neurosci. 1993;13:467–491. doi: 10.1523/JNEUROSCI.13-02-00467.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorassini M, Bennett DJ, Kiehn O, Eken T, Hultborn H. Activation patterns of hindlimb motor units in the awake rat and their relation to motoneuron intrinsic properties. J Neurophysiol. 1999;82:709–717. doi: 10.1152/jn.1999.82.2.709. [DOI] [PubMed] [Google Scholar]
- Grover LM, Teyler TJ. Two components of long-term potentiation induced by different patterns of afferent activation. Nature. 1990;347:477–479. doi: 10.1038/347477a0. 10.1038/347477a0. [DOI] [PubMed] [Google Scholar]
- Holohean AM, Hackman JC, Davidoff RA. Changes in membrane potential of frog motoneurons induced by activation of serotonin receptor subtypes. Neuroscience. 1990;34:555–564. doi: 10.1016/0306-4522(90)90164-y. 10.1016/0306-4522(90)90164-Y. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Intrinsic membrane properties causing a bistable behaviour of alpha-motoneurones. Exp Brain Res. 1984;55:391–394. doi: 10.1007/BF00237290. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol. 1988a;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Serotonin-induced bistability of turtle motoneurones caused by a nifedipine-sensitive calcium plateau potential. J Physiol. 1989;414:265–282. doi: 10.1113/jphysiol.1989.sp017687. 10.1007/BF00584625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Calcium spikes and calcium plateaux evoked by differential polarization in dendrites of turtle motoneurones in vitro. J Physiol. 1993;468:245–259. doi: 10.1113/jphysiol.1993.sp019769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O, Mintz I. Response properties of motoneurones in a slice preparation of the turtle spinal cord. J Physiol. 1988a;398:575–589. doi: 10.1113/jphysiol.1988.sp017058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Kjaerulff O. Ca2+-mediated plateau potentials in a subpopulation of interneurons in the ventral horn of the turtle spinal cord. Eur J Neurosci. 1992;4:183–188. doi: 10.1111/j.1460-9568.1992.tb00865.x. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Mintz I. Calcium conductance and firing properties of spinal motoneurones in the turtle. J Physiol. 1988;398:591–603. doi: 10.1113/jphysiol.1988.sp017059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H. Plateau potentials and their role in regulating motoneuronal firing. Prog Brain Res. 1999;123:39–48. doi: 10.1016/s0079-6123(08)62842-3. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Brownstone RB, Toth TI, Gossard JP. Key mechanisms for setting the input-output gain across the motoneuron pool. Prog Brain Res. 2004;143:77–95. doi: 10.1016/s0079-6123(03)43008-2. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Denton ME, Wienecke J, Nielsen JB. Variable amplification of synaptic input to cat spinal motoneurones by dendritic persistent inward current. J Physiol. 2003;552:945–952. doi: 10.1113/jphysiol.2003.050971. 10.1113/jphysiol.2003.050971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Martin-Cora FJ, Fornal CA. Activity of medullary serotonergic neurons in freely moving animals. Brain Res Brain Res Rev. 2002;40:45–52. doi: 10.1016/s0165-0173(02)00187-x. 10.1016/S0165-0173(02)00187-X. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Spinal interneuronal systems: identification, multifunctional character and reconfigurations in mammals. J Physiol. 2001;533:31–40. doi: 10.1111/j.1469-7793.2001.0031b.x. 10.1111/j.1469-7793.2001.0031b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Chojnicka B, Heden CH. Effects of monoamines on interneurons in four spinal reflex pathways from group I and/or group II muscle afferents. Eur J Neurosci. 2000;12:701–714. doi: 10.1046/j.1460-9568.2000.00955.x. 10.1046/j.1460-9568.2000.00955.x. [DOI] [PubMed] [Google Scholar]
- Kargo WJ, Giszter SF. Rapid correction of aimed movements by summation of force-field primitives. J Neurosci. 2000;20:409–426. doi: 10.1523/JNEUROSCI.20-01-00409.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O, Eken T. Prolonged firing in motor units: evidence of plateau potentials in human motoneurons. J Neurophysiol. 1997;78:3061–3068. doi: 10.1152/jn.1997.78.6.3061. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Eken T. Functional role of plateau potentials in vertebrate motor neurons. Curr Opin Neurobiol. 1998;8:746–752. doi: 10.1016/s0959-4388(98)80117-7. 10.1016/S0959-4388(98)80117-7. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Erdal J, Eken T, Bruhn T. Selective depletion of spinal monoamines changes the rat soleus EMG from a tonic to a more phasic pattern. J Physiol. 1996;492:173–184. doi: 10.1113/jphysiol.1996.sp021299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulakov AA, Raghavachari S, Kepecs A, Lisman JE. Model for a robust neural integrator. Nat Neurosci. 2002;5:775–782. doi: 10.1038/nn893. 10.1038/nn893. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in rhythmic firing patterns. J Neurophysiol. 1998;80:572–582. doi: 10.1152/jn.1998.80.2.572. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Enhancement of bistability in spinal motoneurons in vivo by the noradrenergic alpha1 agonist methoxamine. J Neurophysiol. 1999;81:2164–2174. doi: 10.1152/jn.1999.81.5.2164. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Adjustable amplification of synaptic input in the dendrites of spinal motoneurons in vivo. J Neurosci. 2000;20:6734–6740. doi: 10.1523/JNEUROSCI.20-17-06734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol. 2003;90:857–869. doi: 10.1152/jn.00236.2003. [DOI] [PubMed] [Google Scholar]
- Magee JC, Johnston D. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science. 1997;275:209–213. doi: 10.1126/science.275.5297.209. 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. Physiol Rev. 1996;76:687–717. doi: 10.1152/physrev.1996.76.3.687. [DOI] [PubMed] [Google Scholar]
- Morisset V, Nagy F. Ionic basis for plateau potentials in deep dorsal horn neurons of the rat spinal cord. J Neurosci. 1999;19:7309–7316. doi: 10.1523/JNEUROSCI.19-17-07309.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy F, Dickinson PS, Moulins M. Control by an identified modulatory neuron of the sequential expression of plateau properties of, and synaptic inputs to, a neuron in a central pattern generator. J Neurosci. 1988;8:2875–2886. doi: 10.1523/JNEUROSCI.08-08-02875.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paroschy KL, Shefchyk SJ. Non-linear membrane properties of sacral sphincter motoneurones in the decerebrate cat. J Physiol. 2000;523:741–753. doi: 10.1111/j.1469-7793.2000.00741.x. 10.1111/j.1469-7793.2000.00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier JF, Alaburda A, Hounsgaard J. Spinal plasticity mediated by postsynaptic L-type Ca2+ channels. Brain. Res Brain Res Rev. 2002;40:223–229. doi: 10.1016/s0165-0173(02)00204-7. 10.1016/S0165-0173(02)00204-7. [DOI] [PubMed] [Google Scholar]
- Perrier JF, Hounsgaard J. Development and regulation of response properties in spinal cord motoneurons. Brain Res Bull. 2000;53:529–535. doi: 10.1016/s0361-9230(00)00386-5. 10.1016/S0361-9230(00)00386-5. [DOI] [PubMed] [Google Scholar]
- Perrier JF, Hounsgaard J. 5-HT2 receptors promote plateau potentials in turtle spinal motoneurons by facilitating an L-type calcium current. J Neurophysiol. 2003;89:954–959. doi: 10.1152/jn.00753.2002. [DOI] [PubMed] [Google Scholar]
- Powers RK, Binder MD. Input-output functions of mammalian motoneurons. Rev Physiol Biochem Pharmacol. 2001;143:137–263. doi: 10.1007/BFb0115594. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose PK, Cushing S. Non-linear summation of synaptic currents on spinal motoneurons: lessons from simulations of the behaviour of anatomically realistic models. Prog Brain Res. 1999;123:99–107. doi: 10.1016/s0079-6123(08)62847-2. [DOI] [PubMed] [Google Scholar]
- Russell DF, Hartline DK. Bursting neural networks: a reexamination. Science. 1978;200:453–456. doi: 10.1126/science.644309. [DOI] [PubMed] [Google Scholar]
- Russo RE, Hounsgaard J. Short-term plasticity in turtle dorsal horn neurons mediated by L-type Ca2+ channels. Neuroscience. 1994;61:191–197. doi: 10.1016/0306-4522(94)90222-4. 10.1016/0306-4522(94)90222-4. [DOI] [PubMed] [Google Scholar]
- Russo RE, Hounsgaard J. Plateau-generating neurones in the dorsal horn in an in vitro preparation of the turtle spinal cord. J Physiol. 1996;493:39–54. doi: 10.1113/jphysiol.1996.sp021363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci. 2000;3:1027–1034. doi: 10.1038/79848. 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull. 2000;53:689–710. doi: 10.1016/s0361-9230(00)00402-0. 10.1016/S0361-9230(00)00402-0. [DOI] [PubMed] [Google Scholar]
- Schwindt P, Crill W. Role of a persistent inward current in motoneuron bursting during spinal seizures. J Neurophysiol. 1980;43:1296–1318. doi: 10.1152/jn.1980.43.5.1296. [DOI] [PubMed] [Google Scholar]
- Simon M, Perrier J-F, Hounsgaard J. Subcellular distribution of L-type Ca2+ channels responsible for plateau potentials in motoneurons from the lumbar spinal cord of the turtle. Eur J Neurosci. 2003;18:258–266. doi: 10.1046/j.1460-9568.2003.02783.x. 10.1046/j.1460-9568.2003.02783.x. [DOI] [PubMed] [Google Scholar]
- Soller RW. Monoaminergic inputs to frog motoneurons: an anatomical study using fluorescence histochemical and silver degeneration techniques. Brain Res. 1977;122:445–458. doi: 10.1016/0006-8993(77)90456-5. 10.1016/0006-8993(77)90456-5. [DOI] [PubMed] [Google Scholar]
- Squires GL. Practical Physics. Cambridge, UK: Cambridge University Press; 2001. [Google Scholar]
- Svirskis G, Hounsgaard J. Transmitter regulation of plateau properties in turtle motoneurons. J Neurophysiol. 1998;79:45–50. doi: 10.1152/jn.1998.79.1.45. [DOI] [PubMed] [Google Scholar]
- Tan HJ, Miletic V. Bulbospinal serotoninergic pathways in the frog Rana pipiens. J Comp Neurol. 1990;292:291–302. doi: 10.1002/cne.902920211. 10.1002/cne.902920211. [DOI] [PubMed] [Google Scholar]