Abstract
Fifty-nanometre diameter, clear, synaptic-like vesicles (SLVs) are found in primary mechanosensory nerve terminals of vertebrate and invertebrate animals. We have investigated their role in mechanosensory function using the muscle spindle primary endings of rat Ia afferents as a model. Uptake and release of the synaptic vesicle marker FM1-43 indicated that SLVs recycle like synaptic vesicles and do so in a Ca2+-sensitive manner. Mechanical stimulation increased SLV recycling, increasing both dye uptake and release. Immunogold/electronmicroscopy showed that, like the central synaptic endings, Ia peripheral endings are enriched with glutamate. Moreover, exogenous glutamate enhanced stretch-induced Ia excitability. Enhanced excitability persisted in the presence of antagonists to the commonest ionotropic and metabotropic glutamate receptors (kynurenate, MCPG, CPPG and MAP4). However, excitation by glutamate was abolished by (R,S)-3,5-dihydroxyphenylglycine (DHPG), and rather more effectively by (2R,1′-S,2′-R,3′-S)-2-(2′-carboxy-3′-phenylcyclopropyl) glycine (PCCG-13). PCCG-13 also significantly reduced stretch-activated excitability in the absence of exogenous glutamate. These data indicate that SLVs recycle at rest, releasing glutamate, and that mechanical activity increases this process. The blockade with DHPG and PCCG-13 suggests that endogenous glutamate release acts, at least in part, through the recently described phospholipase D-linked metabotropic Glu receptor to maintain the excitability of the sensory endings.
Free-ending and encapsulated mechanoreceptive primary afferent neurones of vertebrates provide extero-, proprio- or enteroceptive information, depending principally on the site and form of their peripheral sensory terminals, but also on the specialized accessory structures that occur in the various distinct types of end-organ (Widdicombe, 1974; Moravec & Moravec, 1982; Munger & Ide, 1988; Zelená, 1994). The accessory structures may make important contributions to the specificity, receptive field properties, sensitivity and rate of adaptation of particular end-organs (Iggo, 1974; Zelená, 1994). However, the fundamental events of mechanotransduction seem to be integral to the sensory terminals of the afferent neurones themselves and probably depend on common cellular and molecular mechanisms (Hamill & Martinac, 2001). Candidates for a mechanically gated channel at these sites include amiloride-sensitive members of the degenerin/epithelial Na+ channel family (Welsh et al. 2002) and nompC, one of the transient receptor potential superfamily (Sidi et al. 2003).
Given this model of mechanotransduction, it is interesting that mechanosensory terminals of afferent neurones consistently have been demonstrated to contain a population of vesicles of which the majority are small (typically 50 nm diameter) and clear (Akoev et al. 1988; Zelená, 1994). Similar vesicles are, of course, a prominent feature of presynaptic terminals, a fact that was noted by Cauna as early as 1966 (Cauna, 1966), at a time when the role of the synaptic vesicles in quantal neurotransmission was still little more than a promising hypothesis. Now that the vesicular role in synaptic transmission is well established, the presence of synaptic-like vesicles (SLVs) in mechanosensory terminals is something of a puzzle because the terminals, as input devices with intrinsic mechanosensitivity, are clearly non-synaptic. These observations raise the question of whether the SLVs have a function in sensory terminals and, if so, to what extent this function resembles that of presynaptic vesicles. Using the primary sensory ending of the mammalian muscle spindle as a model mechanoreceptor, we present evidence in this paper that directly addresses this question.
Although there has been no detailed study of the role of SLVs in the muscle spindle primary ending, there are several observations that supplement the morphological similarity between SLVs and synaptic vesicles. These include: black widow spider venom-mediated depletion of vesicles in both spindle primary endings and motor nerve terminals (Queiroz & Duchen, 1982), indicating the presence of the synaptic proteins neurexin and/or latrophilin; tetanus neurotoxin-mediated blockage of the spindle sensory response as well as motor neuromuscular transmission, with similar time courses (Mizote & Takano, 1985), implying an important involvement of the synaptic vesicle protein synaptobrevin; immunocytochemical labelling for the synaptic proteins synapsin I and synaptophysin in the sensory endings (De Camilli et al. 1988); and frequent ‘Ω’ membrane profiles and coated vesicle formation, indicators of active vesicle recycling, along the sensory terminal membrane (R. W. Banks & R. Stewart, unpublished observations).
Collectively, these observations not only further highlight the similarity of SLVs to synaptic vesicles, but also indicate that SLVs play an important role in mechanosensory function. We have investigated this functional role using fluorescent marking of recycling vesicles, glutamate immunocytochemistry and electrophysiological recording of spindle output. Our results are consistent with the hypothesis that SLVs are part of an important mechanism for modulating mechanosensory excitability, involving a phospholipase D-coupled metabotropic glutamate receptor. Preliminary accounts of some aspects of this work have been published in the following communications: Banks et al. (2000, 2002); Bewick et al. (2000, 2004).
Methods
Animals and dissection
Adult rats (either sex, 200–450 g) were killed by cervical dislocation (approved under UK legislation as a method of humane killing under Schedule 1 of the Animals (Scientific Procedures) Act 1986). Lumbrical nerve–muscle preparations from hind paws were pinned out in silicon rubber-lined (Sylgard, Dow Corning, Stade, Germany) 35 or 50 mm tissue culture dishes under gassed (95% O2−5% CO2) physiological saline containing (mm): 138.8 NaCl, 4 KCl, 12 NaHCO3, 1 KH2PO4, 1 MgCl2, 2 CaCl2 and 11 glucose (Liley, 1956). All five chemicals were obtained from Sigma Chemical Company (Poole, UK) unless stated otherwise. All experiments were performed at ambient room temperature (18–21°C).
Fluorescence labelling
Spindle afferent terminals were labelled by immersion of the preparation in 5 μm FM1-43 (Molecular Probes, USA) for 2 h in gassed physiological saline, with the muscle held at either resting length (equivalent to the toes in neutral position with respect to plantar- versus dorsiflexion) or maximal stretch (equivalent to maximal dorsiflexion of the toes). Stretched preparations were periodically returned to resting length during incubation. In some experiments, FM1-43 uptake was tested for Ca2+ dependence by exposure to modified physiological saline, in which Ca2+ was increased in concentration to 10 mm, or substituted with 3 mm CoCl2. These preparations were soaked in dye-free modified physiological saline for 30 min before adding FM1-43 (5 μm final concentration) for 2 h. Time-matched normal [Ca2+] preparations from the same animals were run in parallel. Labelled preparations were then rinsed (3 changes) and bathed (30–60 min) in a large volume of dye-free, gassed physiological saline to remove dye from exposed membranes.
Fluorescence imaging
Wide-field epifluorescence microscopic images of live spindles (MicroInstruments M2B microscope, 100 W Hg arc Lamp, Zeiss 40× water-immersion objective, 0.75 NA, Nikon 2B filter set for FITC) were captured either on MonoCoolview (PhotonicScience, Cambridge, UK) or Orca ER (Hamamatsu, Welwyn Garden City, UK) digital cameras and saved on a Macintosh 8500/150AV computer running Openlab software (Improvision, Coventry, UK) to measure fluorescence intensity. For destaining, spindles were vibrated (200 Hz, ∼50 μm amplitude) at one pole with a parylene-coated stainless-steel microelectrode (World Precision Instruments Inc., Stevenage, UK) driven by an N-802 piezo-electric drive (The Vibrating Probe Company, Davis, CA, USA). In control experiments designed to ensure that destaining was specifically produced by direct mechanical stimulation of the spindles, muscle regions lacking spindles were vibrated under otherwise identical conditions, while monitoring the fluorescence intensity of sensory terminals in adjacent spindles of the same muscle. In experiments to examine the Ca2+ dependence of destaining, saline containing 0 Ca2+ and 500 μm EGTA was substituted 30 min before vibration began. For confocal microscopy, fixed FM1-43-labelled preparations (4% paraformaldehyde in 0.1 m phosphate-buffered 0.9% saline, pH 7.4, 30 min) were imaged on a Bio-Rad 1024 laser scanning confocal microscope (Nikon Diaphot 200, low-power images acquired using the Nikon fluor 40× (1.3 NA) objective, and high-power images acquired using a Nikon Planapo 60× (1.4 NA) oil-immersion objective.
Electrophysiology
The second phalanx, constituting the distal insertion of 4th lumbrical nerve–muscle preparations, was securely pinned to the Sylgard-lined base of the organ bath and the other tendon hooked to a three-axis micromanipulator (Prior, Cambridge, UK). The electroneurogram was recorded en passant in paraffin oil using silver-wire electrodes elevated just above the saline surface. The muscle contains on average nine spindles but no Golgi tendon organs (R. W. Banks & Z. L. Rogers, unpublished observations), so the discharge recorded was exclusively from spindle endings. Signals were amplified (A103, Isleworth Electronics, Isleworth, UK and 8102, CF Palmer, High Wycombe UK preamplifiers in series), displayed on an oscilloscope (DSO 400, Gould, Diss, UK) and captured simultaneously on DAT tape (1204 DTR, Bio-Logic, Interacel, Royston, UK) and computer hard-drive (WCP software, John Dempster, University of Strathclyde, UK). Muscle length was adjusted so that afferent firing was minimal (<1 Hz) at the start of each experiment. Periodically, the muscle was stretched by 1 mm and held at the new length for 3–5 s, and total afferent firing (number of impulses) was compared during a defined period of the ‘hold’ phase within each stretch. To assess the effects of CoCl2 on afferent discharge and the pharmacological properties of the glutamate receptors, the electroneurogram was recorded for three consecutive 1 mm stretch-and-hold cycles, repeated every 15–30 min. At least two similar consecutive recordings were made before adding drugs to ensure consistency of baseline responses. Drugs were then applied for at least 2 h before washing with drug-free saline, during which the stretch-induced afferent discharges were recorded at 15–30 min intervals. Parallel control preparations, without drug addition, showed no run-down in response over the same time course.
EM immunocytochemistry
Extensor digitorum brevis muscle (EDB) and cerebellum samples were taken from two adult female albino rats (200 and 224 g) deeply anaesthetized with sodium pentobarbitone (Sagatal, (Merial Animal Health, Harlow, UK) 45 mg kg−1, i.p.) and transcardially perfused (2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 m sodium cacodylate buffer, pH 7.3). The cerebellum was used as a positive control for comparison with sensory terminals and with published data on glutamate-like immunoreactivity. Blocks were postfixed (cacodylate-buffered 1% OsO4), dehydrated and embedded in Araldite CY212 (Agar Scientific, Stanstead, UK). Ultrathin sections were collected on formvar-coated nickel grids and incubated with rabbit polyclonal antibody against conjugated glutamate (gift from Dr B. Leitch; derived by Storm-Mathisen et al. 1983), diluted 1:1000 in 20 mm Tris-buffered 0.9% saline containing 1% normal goat serum and 1% BSA, pH 7.4 for 2 h at ambient temperature. Secondary antibody incubation (goat-antirabbit IgG labelled with 10 nm gold particles, diluted 1:20 in Tris/BSA pH 8.4, Sigma) was for 1 h at ambient temperature. Primary antibody-negative controls were also prepared. Finally, all sections were stained with lead citrate and uranyl acetate to provide adequate contrast in the electron microscope.
Data analysis and statistics
For fluorescence brightness changes, the mean intensity above mean background for at least five labelled regions was determined for each image. In destaining experiments the changes in intensity of the same regions were monitored after subsequent stimulation and rest periods. For electrophysiology, WCP data files were assessed manually. The individual sweep duration depended on the sampling resolution of the WCP file. Initially, sweeps were 512 ms at 1 ms resolution. For most of the work, however, sweeps were 204.8 ms at 0.1 ms resolution. Sweeps were interrupted by brief data storage gaps in which data were not digitized. Spike counts were determined using electroneurograms from three consecutive 1 mm stretches at a bin width of either 100 ms (1 ms resolution) or 10 ms (0.1 ms resolution) over a standard duration at the extended length (e.g. between cursors in Fig. 5), excluding the transitory, high-frequency afferent burst during the length change. Electronmicrographs of immunogold-labelled sections were analysed using Scion Image (Scion Corporation, Frederick, MA, USA). Sampled areas were delineated on digitized micrographs (10 000 × magnification) and the immunostaining surface density expressed as number of particles per square micrometre. For electrophysiological data, the significance of differences between means of predrug and drug-present data was evaluated by Student's paired t test. For all other data, Student's t test (equal or unequal variance, according to a preceding F-test) was used to determine the significance of differences in mean values between appropriate comparative data sets. All differences were considered significant at P < 0.05.
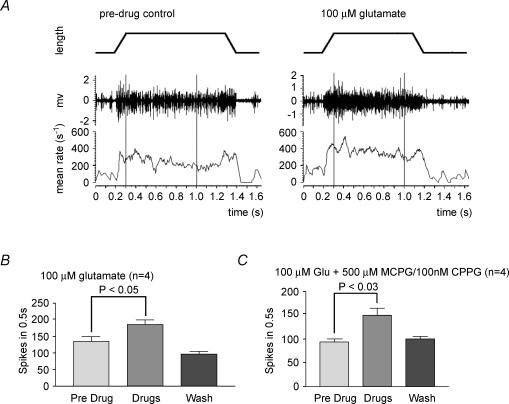
Figure 5. Glutamate-mediated increase in hold-phase afferent discharge frequency during stretch-and-hold cycles is not blocked by group I–III metabotropic Glu receptor antagonists.
A, representative recordings of muscle spindle activity in the absence and presence of exogenous glutamate. Electroneurogram (middle plot) and mean firing rate (lower plot; moving average, 50 ms bin width) of total afferent discharge recorded from the whole nerve during a 1 mm stretch-and-hold cycle (upper plot) of a rat 4th lumbrical nerve–muscle preparation. Lumbricals have no Golgi tendon organs and typically contain 9 spindles (see Methods). Pre-drug control, total number of action potentials in the 0.7 s period between the vertical cursors is 166; 100 μm glutamate, afferent discharge during the hold phase is increased in the same preparation on application of exogenous glutamate. The response shown here was recorded about 150 min after the application of glutamate; there are 256 action potentials in the 0.7 s period between the vertical cursors. B, mean data for 4 preparations treated with 100 μm glutamate, showing a repeatable significant increase in afferent discharge. This effect is reversible with washing in glutamate-free saline. C, this enhancement is not mediated by group I–III metabotropic Glu receptors, since broad-spectrum receptor antagonists MCPG (group I/II) and CPPG (group II/III) do not inhibit this effect (either applied alone or together, as shown). The enhancement is also unattenuated by kynurenate (1 mm; data not shown), a broad-spectrum ionotropic Glu receptor antagonist. Values in B and C are means ± s.e.m.; Student's paired t test, predrug versus drug.
Results
A series of experiments was undertaken to test the model that SLVs undergo rounds of endo- and exocytosis in an activity- and Ca2+-modulated manner, releasing a neuroactive substance which is important for mechanosensory function.
Fluorescent staining with FM1-43: evidence for activity- and Ca2+-dependent recycling of SLVs
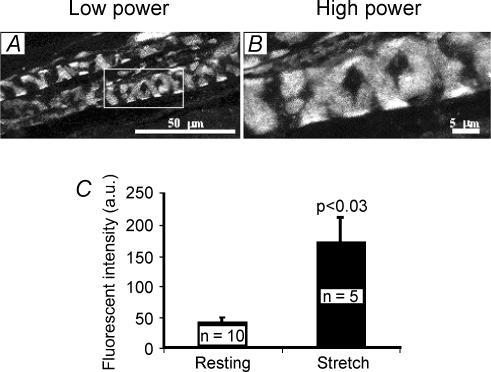
Styryl dyes such as FM1-43 have been introduced as fluorescent markers of recycling synaptic vesicles (Betz & Bewick, 1992; Betz et al. 1992). Spindle primary endings label brightly with FM1-43 (Fig. 1A and B; 5 μm FM1-43) when the muscles containing them are pinned at resting length. The phenomenon was first seen during the development of these dyes as synaptic vesicle markers (Betz et al. 1992). Dye uptake occurred even if muscles were not pinned out but left free-floating in the dye and in the presence of TTX to block spontaneous action potential generation (G. S. Bewick & W. J. Betz, unpublished observations). It was subsequently described in more detail by Chua & Hunt (1995). This labelling, together with the many similarities of SLVs to synaptic vesicles noted above, suggested that dye uptake might occur by SLV endocytosis. If SLV recycling was occurring and is important for mechanosensory function, mechanical activity might be expected to affect it. Consistent with this, we found that repeatedly stretching muscles to maximal in situ length during dye incubation increased the labelling intensity to 275.4 ± 91.0% (mean ± s.e.m., n = 5) above controls (n = 10; P < 0.03; Fig. 1C). Thus static muscle stretch increased dye uptake, consistent with a stretch-activated increase in endocytosis.
Figure 1. FM1-43 labelling in muscle spindle primary afferent terminals is enhanced by stretch.
Low- (A) and high-magnification fluorescence images (B) of rat lumbrical muscle spindles labelled by exposure to 5 μm FM1-43 for 2 h at resting in situ length. C, labelling by FM1-43 is approximately 4-fold brighter if preparations are repeatedly stretched to maximal in situ length during labelling (means ± s.e.m., Student's t test). a.u., arbitrary units of grey level.
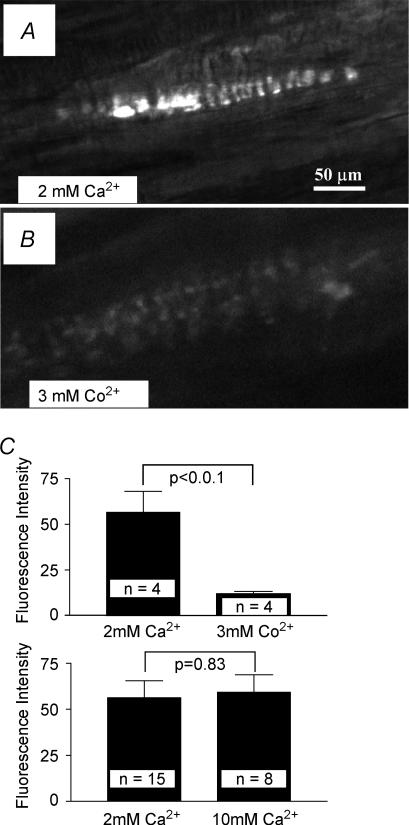
To test whether dye labelling, like synaptic vesicle recycling, is Ca2+ dependent, we examined the effects of cobalt, a voltage-gated Ca2+ channel blocker, on dye uptake. When 3 mm CoCl2 was substituted for 2 mm CaCl2 in the physiological saline, the amount of dye uptake was markedly reduced (n = 4, P < 0.01; Fig. 2A–C). In a previous study, Gale et al. (2001) reported that labelling of some mechanosensory cells (hair cells) occurs by styryl dye permeation of mechanically sensitive channels. This labelling is inhibited by 10 mm extracellular Ca2+ (Gale et al. 2001). In our preparations, 10 mm Ca2+ had no effect on fluorescence intensity (Fig. 2C), suggesting that little, if any, dye internalization occurs by channel permeation. Together, these observations indicate that FM1-43 uptake occurs tonically at rest, is by vesicle endocytosis in a Ca2+-dependent manner and that this endocytosis increases with mechanical activity.
Figure 2. Labelling is blocked by extracellular cobalt but not by high calcium concentrations, suggesting that labelling is by uptake into recycling SLVs rather than direct channel permeation.
A, B and C (upper panel), the voltage-gated Ca2+ channel blocker Co2+ substantially reduced FM1-43 uptake, indicating that Ca2+ influx through voltage-gated Ca2+ channels is important for dye internalization. C (lower panel), conversely, 10 mm Ca2+ extracellularly does not block labelling, suggesting that labelling is not due to dye permeation directly through mechanically sensitive channels.
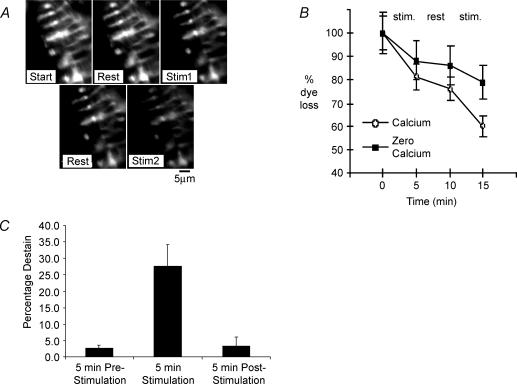
The uptake of FM1-43 by endocytosis implies a preceding exocytosis and local recycling. However, a more direct test would be the presence of dye loss, since dye internalized by endocytosis is released again during vesicle recycling. If such local SLV recycling was occurring tonically, as implied by the dye uptake at resting length, labelled spindle primary endings should destain spontaneously when transferred to normal saline. Conversely, if dye uptake is by channel permeation, labelling should be irreversible (Gale et al. 2001). When labelled spindles were repeatedly imaged, significant destaining occurred even at rest (2.7 ± 0.9% of initial intensity over 5 min, n = 6; Fig. 3A–C), after background subtraction for each image to correct for photobleaching and dye washout. This observation is consistent with an on-going tonic exocytosis, similar to that observed previously for the endocytosis-mediated labelling of SLVs at rest. In order to test whether the rate of destaining, and hence SLV exocytosis, was at all dependent on the mechanosensory transducing function of the primary ending, we made use of the well-known sensitivity of the primary ending to small-amplitude sinusoidal stretch. Over a range of frequencies from about 100 to 300 Hz, the primary ending of the cat spindle can be ‘driven’ or caused to fire an impulse for each stretch cycle (for review see Matthews, 1972). Similarly, in the rat soleus muscle, afferents identified as arising from the spindle primary endings can be driven up to at least 150 Hz by vibration of 0.25 mm amplitude applied to the muscle spindle (De-Doncker et al. 2003). If the proposed model is correct, such a strong mechanical stimulus should lead to significant SLV exocytosis and hence destaining. As predicted, when we applied a vibrating probe (200 Hz, ∼50 μm amplitude) to the spindle pole in the isolated lumbrical preparation, destaining increased 10-fold (27.6 ± 6.4% of initial intensity in 5 min, P < 0.01) during vibration. Importantly, on cessation of stimulation, destaining returned to prestimulation basal levels (3.4 ± 2.7%; P > 0.8 versus prestimulation destain rate; (Fig. 3B and C). Thus, dye uptake and release, consistent with SLV endo- and exocytosis, occur tonically at rest and both processes are markedly increased in parallel by mechanical activity.
Figure 3. Destaining of labelled spindles increases with mechanical activity and is Ca2+-modulated.
A, images of a living terminal acquired between alternating 5 min periods of rest and stimulation, beginning with a rest. Upper row, Start = initial image; Rest = 5 min from Start, no vibration applied; Stim1 = 5 min from Rest, during which 5 min vibration was applied. Lower row, Rest = 5 min from Stim1, no vibration applied; Stim2 = 5 min from Rest, during which a further 5 min of vibration was applied. B, comparison of dye loss in the presence, as seen in A, and absence of Ca2+ (0 Ca2+ and 500 μm EGTA). Destaining increased markedly during stimulation (5 min vibration) in each case but was significantly reduced in the absence of Ca2+. Data are means ± s.e.m. for 8 terminal regions. C, mean destain for 6 labelled preparations stimulated in the presence of 2 mm Ca2+. Spindles destained by only 2.7 ± 0.9 and 3.4 ± 2.7% in the 5 min before and after stimulation, but by 27.6 ± 6.4% during 5 min of stimulation (P < 0.01versus prestimulation and P < 0.005 versus poststimulation destain periods).
We next tested whether destaining, like dye uptake, was Ca2+ sensitive, consistent with SLV exocytosis being Ca2+ mediated. The mechanical enhancement of destaining was at least partly Ca2+ dependent, since vibration-induced destaining in Ca2+-free saline (0 CaCl2 and 500 μm EGTA, n = 4) was significantly reduced (P < 0.03, n = 4). A representative example is shown in Fig. 3B, where destaining is reduced by approximately 50% over the course of two stimulation periods (1 terminal analysed per spindle, fluorescence intensity of 5 regions analysed per terminal).
These observations therefore extend the parallels between synaptic vesicles and SLVs in four ways. First, significant amounts of dye are internalized via endocytosis (though a small amount of dye entry by channel permeation cannot be excluded, see Gale et al. 2001). Second, the re-release of previously internalized dye suggests that SLVs maintain their functional integrity throughout the cycle, consistent with endocytosis followed by exocytosis, i.e. localized SLV recycling. Third, they demonstrate that activity increases both dye internalization (endocytosis) and release (exocytosis), i.e. activity increases the total number of SLVs recycling. Finally, both internalization and release of dye exhibit a marked Ca2+ dependence.
Immunogold labelling for glutamate in spindle Ia afferent endings
This localized SLV recycling presumably releases vesicular contents during exocytosis, to perform a physiological role. In order to test what such a role might be, it was important to identify what the SLV contents might be. According to Dale's Principle (Dale, 1935), all terminals of a particular neurone release the same neurotransmitter(s). The similarity of SLVs and synaptic vesicles led us to postulate that SLVs might therefore contain the same neuroactive substance as the central presynaptic endings of the spindle primary-ending afferent (the Ia afferent). Since the central terminals of Ia afferents are glutamatergic (Engberg et al. 1993; Walmsley & Bolton, 1994), we used EM immunocytochemistry to test for glutamate-like immunoreactivity (glutamate-LI) in the sensory terminals (Fig. 4A). No attempt was made to determine absolute amounts of glutamate, but relative comparisons were made with components of the cerebellar cortex, treating this as a positive control. Negative controls, in which the primary antibody was omitted, showed very low particle densities. Nevertheless, this background labelling varied from one component to another, ranging from 0.23 gold particles μm−2 in sensory terminals to 1.46 gold particles μm−2 in axonal terminals of Golgi cells (Fig. 4B). In principle, these mean background values could be subtracted from each item of data from similar components in the positively stained sections. However, since the lowest value was that of the sensory terminals themselves, background subtraction would only serve to increase any observed differences between the sensory terminals and other components, and so was not carried out.
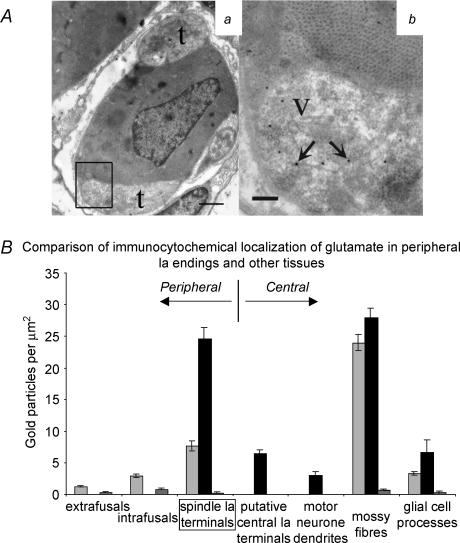
Figure 4. Elevated glutamate-like immunoreactivity in muscle spindle primary afferents terminals.
Aa, electron micrograph of muscle spindle primary afferent terminals (t) enclosing an intrafusal muscle fibre (note dark-staining nucleus in the centre) processed for glutamate-like immunogold labelling. Scale bar = 1 μm; box indicates region seen in higher power in b. Ab, higher power view, showing labelling (arrows) in a terminal region containing synaptic-like vesicles (V). Vesicular membranes are less clear than in conventionally prepared material. Note that particle density is much lower in the adjacent intrafusal muscle fibre. Scale = 0.2 μm B, quantitative comparison of gold particle density with other tissues. Data are expressed as means ± s.e.m., up to 3 columns per tissue. Left-hand (light grey) and centre (black) columns represent 2 samples treated with antiglutamate primary antibodies. The right-hand (dark grey) column represents the labelling density for control samples with no primary antibody, treated in parallel with left-hand or centre column specimens (mean for pooled data if 2 samples were processed).
Sections of muscle and cerebellum from one animal, and of muscle, cerebellum and lumbosacral spinal cord from a second, were processed using identical protocols but on different days. Reproducibility of the procedure is demonstrated both by the very similar labelling density data obtained from the cerebellar cortical components in each case (Fig. 4B) and also by their similarity to those found by Somogyi et al. (1986) in the same region of the cat brain. A spindle sensory ending from each muscle was sampled at multiple sites for glutamate-LI and though the average particle densities for the two endings differed (7.65 ± 0.15 gold particles μm−2, n = 22 and 24.58 ± 1.71 gold particles μm−2, n = 20), the densities of both sensory endings were at least 300% of the intrafusal (2.08 ± 0.22 gold particles μm−2, n = 22) or extrafusal (non-spindle) muscle fibre labelling (1.21 ± 0.16 gold particles μm−2, n = 9; P < 0.001 in each case). In one of the spindle sensory endings the particle density was similar to that shown by components of glutamatergic cerebellar neurones (parallel fibres, 26.29 ± 4.19 gold particles μm−2, n = 8; mossy fibres, 27.86 ± 1.55 gold particles μm−2, n = 20; and granule cell dendrites, 18.36 ± 1.87 gold particles μm−2, n = 20) and significantly higher than non-neuronal, non-synaptic, cerebellar glial cell processes (6.60 ± 1.95 gold particles μm−2, n = 8; P < 0.001).
In the sample of lumbosacral spinal cord, two components from lamina IX were quantitatively analysed: large, type S boutons in synaptic contact with proximal dendrites of motoneurones; and the dendrites themselves. The axonal source of the individual type S boutons could not be identified, but Ia afferents have been shown to have similar central terminals (Conradi et al. 1983), and only the largest dendritic profiles were selected to ensure that they were most probably of motoneuronal origin. While the postsynaptic dendrites themselves expressed very little glutamate-LI (3.01 ± 0.52 gold particles μm−2, n = 19), glutamate-LI was significantly greater in the synaptic boutons (6.48 ± 0.54 gold particles μm−2, n = 20; P < 0.001). Interestingly, these presumed glutamatergic terminals showed only as much glutamate LI as the cerebellar glial cell processes, which was much less than the (peripheral) Ia sensory terminals processed at the same time (Fig. 4B).
In summary, therefore, peripheral sensory terminals have elevated levels of glutamate-LI. Furthermore, the level of glutamate-LI in spindle sensory endings is at least comparable to that in central Ia synaptic terminals and is significantly elevated above that in either adjacent muscle fibres or central non-glutamatergic cellular components. Thus, these data indicate that there is a high glutamate content in these endings.
Effect of exogenous glutamate on spindle afferent excitability
If SLVs contain glutamate, it is presumably released during exocytosis to elicit a physiological response. We next asked, therefore, what role glutamate release might have. To assess the effect of glutamate on spindle responsiveness, exogenous glutamate (0.01–1 mm) was added to the bathing solution and spindle discharge frequency monitored during repeated 1 mm stretch-and-hold cycles (resting muscle length, ∼10 mm). Glutamate at 0.1–1 mm significantly increased the discharge frequency during the ‘hold’ phase of such cycles (Fig. 5A and B), over a period of 30–60 min. For example, 100 μm glutamate increased the number of spikes to 137.4 ± 14.4% (mean ± s.e.m. of 4 preparations; P < 0.05) of the control value. There may also have been some increase in afferent discharge activity at resting length, but this was not quantified. These effects reversed fully upon washing, over a similar time scale.
To test whether the enhancement was receptor mediated and identify the type of receptor involved, a range of glutamate (Glu) receptor antagonists was applied. Kynurenic acid (1 mm), a non-selective ionotropic Glu receptor antagonist, had little effect on the glutamate-mediated enhancement (data not shown). Somewhat surprisingly, neither did broad-spectrum antagonists of the group I–III metabotropic Glu receptors (MCPG, 0.5–1.0 mm, groups I and II; CPPG, 10–100 nm, groups II and III; and MAP4, 1 mm, groups II and III), even when applied in combination, with or without kynurenate. For example, the presence of 500 μm MCPG and 100 nm CPPG failed to prevent enhancement in the response to stretch by 100 μm glutamate, the number of spikes increasing to 156.8 ± 13.1% (mean ± s.e.m. of 4 preparations; P < 0.03, Student's paired t test) of the predrug control value (Fig. 5C). Thus, exogenous glutamate increased spindle excitability, but this effect was not mediated through the best-characterized Glu receptors.
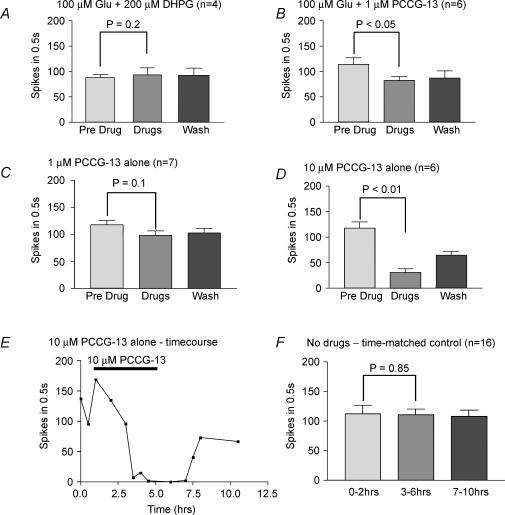
Recently, however, a metabotropic Glu receptor coupled to phospholipase D (PLD) has been described (Pellegrini-Giampietro et al. 1996) that is currently designated outside the standard group I–III metabotropic Glu receptor categories. This receptor is inhibited by (R,S)3,5-dihydroxyphenylglycine (DHPG), a group I agonist (Ito et al. 1992), and rather more effectively by (2R,1′-S,2′-R,3′-S)-2-(2′-carboxy-3′-phenylcyclopropyl) glycine (PCCG-13; Albani-Torregrossa et al. 1999). Accordingly, when recording in the presence of 200 μm DHPG, 100 μm glutamate produced only a slight, insignificant enhancement of the mean discharge rate. The number of spikes increased to 111.3 ± 10.0% of the control value (mean ± s.e.m. of 4 preparations; P > 0.2, Student's paired t test), indicating that DHPG effectively blocked the expected enhancement by glutamate (Fig. 6A). Further experiments applying the selective antagonist PCCG-13 (1 μm) confirmed the involvement of PLD-coupled receptors (Fig. 6B). Thus, when glutamate (100 μm) was applied in the presence of PCCG-13 (1 μm) the mean number of spikes fell significantly below (71.8 ± 8.7%) that of the control value (mean ± s.e.m. of 6 preparations; P < 0.05, Student's paired t test). Spindle endings were therefore sensitive to the application of exogenous glutamate and only application of antagonists of PLD-coupled metabotropic Glu receptors effectively blocked this action.
Figure 6. Antagonists of PLD-coupled metabotropic Glu receptors inhibit glutamate-mediated increases in spindle excitability and reduce spindle excitability when applied alone.
A, the afferent discharge in the presence of both glutamate and DHPG (200 μm) was not significantly different from predrug levels. Thus, DHPG blocked the effect of exogenous glutamate, indicating that glutamate-mediated excitability requires a PLD-metabotropic Glu receptor. B, because DHPG can also act as an agonist at type I metabotropic Glu receptors, a more specific PLD-metabotropic Glu receptor antagonist, PCCG-13, was tested. It too abolished the effects of exogenous glutamate at 1 μm.C and D, PCCG-13 also reduced spindle discharge frequency in the absence of exogenous glutamate, but required higher concentrations (10 μm). This is consistent with PCCG-13 blocking receptor activation through tonic release of endogenous glutamate. E, representative experiment showing the time course and profound effect of PCCG-13 applied alone on the responsiveness of a spindle to stretch. F, time-matched controls showed that stretch-evoked responses are well maintained in the absence of drugs. Values in A–D and F are means ± s.e.m.; Student's paired t test comparison of predrug and with drug responses.
Effect of blocking endogenous glutamate on spindle afferent excitability
While the preceding experiments indicate that exogenous glutamate can activate metabotropic receptors, they do not test directly whether there is endogenous glutamate release or, if so, that it activates the same receptors. To test more directly for endogenous glutamate release (i.e. exocytosis from glutamatergic SLVs) activating this same pathway, PCCG-13 was applied in the absence of exogenous glutamate (Fig. 6C and D). At 1 μm, PCCG-13 did tend to reduce the mean number of spikes in the responses to stretch of seven preparations to 80.2 ± 12.1% of the control value, but this was not significant (P < 0.1, Student's paired t test). However, at 10 μm, PCCG-13 in the bathing medium profoundly inhibited the response, the number of spikes falling to just 24.7 ± 7.9% (mean ± s.e.m.) of the predrug control values, the effect being highly significant (P < 0.01, Student's paired t test, n = 6). An example of such an experiment is shown in Fig. 6E. Figure 6F shows that afferent discharges are otherwise well maintained in time-matched controls in the absence of PCCG-13. Thus, endogenous glutamate release is indeed occurring during normal stretch activity and, furthermore, it does act through the same receptors as exogenously applied glutamate.
Finally, we investigated the effect of blocking Ca2+-mediated SLV recycling on spindle function. Previous experiments showed that CoCl2 markedly inhibits vesicle recycling (see above). Cobalt chloride is also reported to inhibit spindle afferent discharge (Kruse & Poppele, 1991). We therefore tested the affect on afferent output of CoCl2 concentrations that profoundly reduce SLV recycling. We found that after 1 h of 3 mm CoCl2 application spindle activity was completely blocked in two preparations and was just detectable (<4% of control values) in three others (mean number of spikes in 3 mm CoCl2 was 2.4 ± 1.0% of predrug control values, P < 0.002, n = 5, Student's paired t test).
Discussion
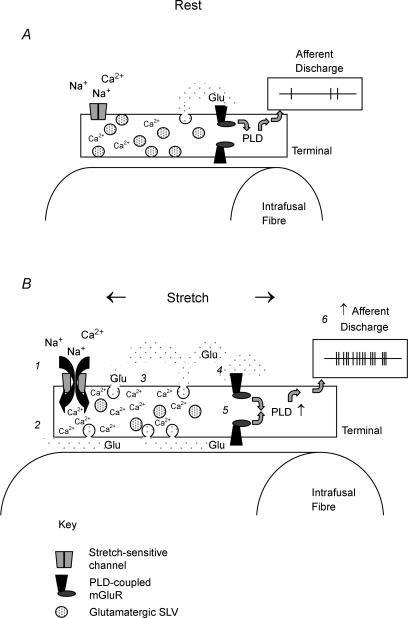
The primary mechanism of mechanical transduction in muscle spindle sensory endings is the activation of stretch-sensitive ionotropic channels. However, our data show that during this process rat spindle mechanosensory terminals take up and release exogenous markers of recycling vesicles, a process which is modulated by activity and external [Ca2+]. These peripheral terminals also have elevated levels of glutamate, similar to central glutamatergic neuronal processes. Exogenous glutamate in the range 0.1–1.0 mm enhances spindle excitability, an effect that can be blocked by PCCG-13, and spindle excitability is reduced by PCCG-13 acting alone. The data are therefore consistent with the following model (Fig. 7). SLVs in rat spindle sensory terminals tonically undergo rounds of Ca2+-mediated endo- and exocytosis (Fig. 7A). This tonic recycling is modulated by mechanical activity (Fig. 7B). During exocytosis, the SLVs release glutamate, autogenically enhancing spindle excitability via a metabotropic glutamate receptor that is linked to phospholipase D. Since SLVs have been reported to be present in many kinds of mechanosensory terminals, this system may be an important modulatory mechanism employed by most, if not all, mechanosensory endings.
Figure 7. Proposed model of the role of SLVs in Glu receptor-mediated increase of mechanosensory afferent excitability.
A, at resting length, relatively few mechanosensitive channels are open, and there is a small level of tonic SLV turnover, producing a basal level of glutamate release. Given the effectiveness of specific antagonists, the glutamate activates phospholipase D (PLD)-coupled metabotropic Glu receptors, maintaining a basal level of PLD activity and a basal afferent discharge. B, upon stretch, increasing numbers of mechanosensitive channels open, depolarizing the terminal by cationic influx. The latter is mainly carried by Na+, but with a small Ca2+ component (1). This depolarization is presumed to be the proximate cause of afferent discharge (not shown). However, in addition, the small level of Ca2+ conductance through the mechanosensitive channels elevates the intracellular Ca2+ level (2). (3) The increase in intracellular Ca2+ increases SLV exocytosis (and subsequent recapture by endocytosis, omitted from the diagram in the interests of clarity), which enhances glutamate release. (4) The increase in extracellular glutamate, within the confines of the spindle capsule, activates additional PLD-coupled metabotropic Glu receptors (mGluR) (5), increasing the afferent excitability even further and allowing maintained discharges during stretch (6). While the present study provides evidence for each of the steps numbered in this model, there are many outstanding questions regarding the intervening steps.
FM1-43 labels recycling SLVs
Styryl dyes, especially FM1-43, have now been used to study membrane trafficking in various neuronal and non-neuronal cells, including both central and peripheral (particularly neuromuscular) synapses, where photoconversion studies have shown that the dye is internalized on synaptic vesicle membranes (Cochilla et al. 1999). Unlike the neuromuscular junction, uptake occurs spontaneously in the spindle sensory ending (Betz et al. 1992; Chua & Hunt, 1995 and Fig. 1), which thus resemble hair cells from Xenopus lateral line and from guinea-pig cochlea, where FM1-43 is rapidly taken up into the region of the apical plate (Nishikawa & Sasaki, 1996; Kilner & Ashmore, 1997).
Despite their very different mechanical arrangements, both hair cells and spindle sensory endings may use a similar mechanosensitive channel (Hamill & Martinac, 2001). It has been claimed by Gale et al. (2001) that FM1-43 enters the hair cell by direct permeation through the mechanosensitive channel, irreversibly blocking the channel, and that labelling is blocked by 10 mm extracellular Ca2+. Against this, Griesinger et al. (2002) have also reported that FM1-43 labelling of the hair cell in the adult mammalian cochlea is due to a rapid, Ca2+-dependent endocytosis, which is consistent with our findings in the spindle; and we have found no effect of FM1-43 on spindle discharge at the concentrations used in our experiments (G. S. Bewick & C.-L. Aryiku, unpublished observations).
The close similarity of the molecular composition of SLVs and synaptic vesicles is indicated by the occurrence in spindle sensory endings of the synaptic vesicle proteins synapsin I, synaptophysin (De Camilli et al. 1988) and NAP-22 (Iino et al. 2004) as well as the docking proteins syntaxin IB (Aguado et al. 1999) and VAMP/synaptobrevin, both isoforms of which (I and II) are present in spindle sensory endings (Li et al. 1996).
In the spindle, the stretch-activated receptor current is carried almost entirely by Na+ but, in the absence of extracellular Na+, a small Ca2+ current is discernible (Hunt et al. 1978). Probably the Ca2+ component couples mechanosensory transduction to SLV turnover in an activity-dependent fashion. This interpretation is supported by neurotoxicological evidence, since black widow spider venom (BSWV) depletes both SLVs and synaptic vesicles (Queiroz & Duchen, 1982), whereas tetanus toxin (TeTx) inhibits spindle discharges and nerve-evoked extrafusal twitch responses over similar time courses (Mizote & Takano, 1985). α-Latrotoxin, the neuroactive component of BWSV, is a pore-forming protein permeable to mono- and divalent cations, thus promoting exocytosis (Ushkaryov et al. 2004). Conversely, TeTx acts to block vesicular release by selective cleavage of the v-SNARE protein VAMP/synaptobrevin (reviewed by Pellizari et al. 1999). An important role for Ca2+ in vertebrate spindle sensory endings is further suggested by the presence in them of various Ca2+-binding proteins of the EF-hand superfamily (Hietanen-Peltola et al. 1992; Duc et al. 1994; El-Tarhouni & Banks, 1995; Iino et al. 1998; Werle et al. 2000). Endocytosis and exocytosis of SLVs both appear to be Ca2+ dependent, since we have shown that blocking voltage-dependent Ca2+ channels with Co2+ markedly reduces the amount of FM1-43 labelling of sensory terminals, and the rate of dye loss is reduced in Ca2+-free Ringer solution. Compelling evidence for a central role for Ca2+ in sensory terminal function, possibly through its influence on SLV recycling, is the observation that blocking Ca2+ channels using diltiazem or CoCl2 (this study; Kruse & Poppele, 1991) or NiCl2/CdCl2 (G. S. Bewick, unpublished observations) results in the rapid abolition of spiking activity.
SLVs contain glutamate
Our immunocytochemical results indicate that SLVs contain glutamate, a classical excitatory neurotransmitter at many central synapses, further extending the parallels with synaptic vesicles. This observation does not exclude the possibility that other neuroactive substances also occur in these sensory terminals. While we did not investigate this in detail, the large dense-core vesicles seen in some micrographs (Adal, 1969) indicate the presence of neuropeptides. The level of glutamate that appears to be present in spindle sensory terminals is at least as high as that in the glutamatergic central terminals of probable Ia afferents, though only in some cases are they as high as the glutamatergic mossy fibres and granule cells of cerebellar cortex. We have tried to ensure comparability and reproducibility of the results obtained at different times by using cerebellar cortex as a positive control, so it is also worth re-emphasizing that the relative levels of glutamate-LI that we found in various components of rat cerebellum are very similar to those reported by Somogyi et al. (1986) from the cat. The presence of glutamate in SLVs implies its release during membrane cycling and, subsequently, a requirement for a replenishment mechanism. It is interesting, therefore, that a candidate glutamate transporter protein has recently been reported in spindle sensory endings that is likely to fulfil this role, the vesicular glutamate transporter 1 (VGLUT1), as demonstrated by Wu et al. (2004) using both light microscope and electron microscope immunocytochemistry.
Glutamate enhances spindle excitability
Exogenous glutamate markedly and reversibly increased spindle afferent discharge frequency, by as much as 143% above untreated preparations (mean 87.8 ± 27.6%). The protracted time course for enhancement and washout (>60 min) probably reflects, in large part, the time for agents to penetrate the preparation. Muscle spindle sensory endings often lie deep within the muscle and are surrounded by a selectively permeable capsule. Both constitute significant barriers to diffusion of exogenous agents (Dow et al. 1980), to the extent that pharmacological experiments on spindles often employ partially dissected (‘decapsulated’) preparations. In fact, this type of preparation first implicated the importance of glutamate for mechanosensory function, since Poppele et al. (1979) reported that in order to maintain the responses of decapsulated spindles it was necessary to add amino acids, including ∼1 mm glutamate, to the bathing saline.
Glutamate receptor characterization
Inhibition of the expected glutamate-induced enhancement of the afferent response by DHPG and PCCG-13 is consistent with antagonism of a PLD-coupled metabotropic glutamate (mGlu) receptor (Pellegrini-Giampietro et al. 1996; Albani-Torregrossa et al. 1999). DHPG is most commonly used as a group I mGlu receptor agonist (Ito et al. 1992), so its lack of a glutamate-mimicking action is further confirmation of the absence of mGlu I receptor involvement in the spindle sensory response. PCCG-13 was a more potent antagonist, since it not only abolished the glutamate-mediated enhancement, but also reduced or abolished the stretch-induced afferent discharge in the absence of exogenous glutamate. In our proposed model, SLVs recycle even with the spindle at rest, as indicated by spontaneous uptake of FM1-43, and therefore will constitutively release glutamate to stimulate PLD-coupled mGlu receptors present in the sensory terminals. Thus, spindles even at rest are excited above a glutamate-free baseline. The addition of PCCG-13 in the absence of exogenous glutamate would therefore produce a total glutamate receptor blockade, antagonizing this elevated basal tone, and would reduce excitability below non-glutamate controls (Fig. 6). Quite how the mechanosensory channels are linked to this glutamatergic system, and the broader significance of the linkage, remain to be determined. Finally, these data also indicate that the PLD-coupled receptor is the predominant, and possibly sole, mGlu receptor at the spindle primary ending. Again, the functional significance of this observation and the potential of these receptors as selective targets for peripherally acting drugs to modulate mechanosensory function are matters for further investigation.
Conclusions
In the first instance, these data extend previous evidence that SLVs resemble synaptic vesicles in chemically transmitting synapses in their morphology, association with synaptic vesicle proteins and FM1-43 uptake. More crucially, however, we have also provided the first evidence that SLVs undergo recycling in an activity- and Ca2+-modulated manner, releasing their content (glutamate), which then acts via a PLD-coupled mGlu receptor to enhance terminal excitability during maintained stretches. The precise function of this enhancement is not clear at present but, whatever its specific role is eventually found to be, the dramatic reduction of spiking activity induced by perturbing the system suggests that it is crucial for regulating mechanosensory transduction by stretch-sensitive channels.
Acknowledgments
We wish to thank Dr B. Leitch for the generous gift of antiglutamate antibody and also Professor Neil Gow, Dr Rod Scott, Dr Stephen Davies and Dr Tony Ridge for their critical reading of the manuscript and helpful discussions during the course of this work. We wish to acknowledge Cheryl-Lee Aryiku, Courtney Maguire, Rona Duncan and Wayne Eddie who, as undergraduate students, generated some of the pharmacological data as part of their undergraduate project work. Finally, we are grateful to the Nuffield Foundation for provided funding for an undergraduate summer research bursary to Cheryl-Lee Aryiku.
References
- Adal MN. The fine structure of the sensory region of cat muscle spindles. J Ultrastruct Res. 1969;26:332–354. doi: 10.1016/s0022-5320(69)80011-0. [DOI] [PubMed] [Google Scholar]
- Aguado F, Majó G, Ruiz-Montasell B, Llorens J, Marsal J, Blasi J. Syntaxin 1A and 1B display distinct distribution patterns in the rat peripheral nervous system. Neuroscience. 1999;88:437–446. doi: 10.1016/s0306-4522(98)00247-4. [DOI] [PubMed] [Google Scholar]
- Akoev GN, Alekseev NP, Krylov BV. Mechanoreceptors: Their Functional Organization. Berlin: Springer-Verlag; 1988. [Google Scholar]
- Albani-Torregrossa S, Attucci S, Marinozzi M, Pellicciari R, Moroni F, PellegriniGiampietro DE. Antagonist pharmacology of metabotropic glutamate receptors coupled to phospholipase D activation in adult rat hippocampus: focus on (2R,1′S,2′R,3′S)-2-(2′-carboxy-3′-phenylcyclopropyl) glycine versus 3,5-dihydroxyphenylglycine. Mol Pharmacol. 1999;55:699–707. [PubMed] [Google Scholar]
- Banks RW, Bewick GS, Reid B, Richardson AC. Evidence for activity-dependent modulation of sensory terminal excitability in spindles by glutamate release from synaptic-like vesicles. Adv Exp Med Biol. 2002;508:13–18. doi: 10.1007/978-1-4615-0713-0_2. [DOI] [PubMed] [Google Scholar]
- Banks RW, Richardson C, Bewick GS. Immunocytochemical demonstration of glutamate in the sensory terminals of rat muscle spindles. J Physiol. 2000;528.P:62. [Google Scholar]
- Betz WJ, Bewick GS. Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science. 1992;255:200–203. doi: 10.1126/science.1553547. [DOI] [PubMed] [Google Scholar]
- Betz WJ, Mao F, Bewick GS. Activity-dependent fluorescent staining and destaining of living vertebrate motor nerve terminals. J Neurosci. 1992;12:363–375. doi: 10.1523/JNEUROSCI.12-02-00363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewick GS, Duncan R, Eddie W, Maguire C, Banks RW. Glutamate-enhanced muscle spindle excitability – inhibition by PLD-coupled metabotropic glutamate receptor antagonists. J Physiol. 2004;557.P:65. [Google Scholar]
- Bewick GS, Reid B, Banks RW. Investigating the role of small clear vesicles in vertebrate mechanosensory endings using rat muscle spindles. J Physiol. 2000;528.P:62, 63. [Google Scholar]
- Cauna N. Fine structure of the receptor organs and its probable functional significance. In: De Reuck AVS, Knight J, editors. Touch, Heat and Pain. London: Churchill; 1966. pp. 117–136. [Google Scholar]
- Chua M, Hunt CC. Sensory endings of living isolated mammalian muscle spindles. In: Taylor A, Gladden MH, Durbaba R, editors. Alpha and Gamma Motor Systems. New York: Plenum Press; 1995. pp. 251–254. [Google Scholar]
- Cochilla AJ, Angelson JK, Betz WJ. Monitoring secretory membrane with FM1-43 fluorescence. Annu Rev Neurosci. 1999;22:1–10. doi: 10.1146/annurev.neuro.22.1.1. [DOI] [PubMed] [Google Scholar]
- Conradi S, Cullheim S, Gollvik L, Kellerth J-O. Electron microscopic observations on the synaptic contacts of group Ia muscle spindle afferents in the cat lumbosacral spinal cord. Brain Res. 1983;265:31–39. doi: 10.1016/0006-8993(83)91330-6. [DOI] [PubMed] [Google Scholar]
- Dale HH. Pharmacology and nerve endings. Proc R Soc Med. 1935;28:319–332. doi: 10.1177/003591573502800330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P, Vitadello M, Canevini MP, Zanoni R, Jahn R, Gorio A. The synaptic vesicle proteins synapsin-I and synaptophysin (protein-p38) are concentrated both in efferent and afferent nerve-endings of the skeletal-muscle. J Neurosci. 1988;8:1625–1631. doi: 10.1523/JNEUROSCI.08-05-01625.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De-Doncker L, Picquet F, Petit J, Falempin M. Characterization of spindle afferents in rat soleus muscle using ramp-and-hold and sinusoidal stretches. J Neurophysiol. 2003;89:442–449. doi: 10.1152/jn.00153.2002. [DOI] [PubMed] [Google Scholar]
- Dow PR, Shinn SL, Ovalle WK. Ultrastructural study of a blood–muscle spindle barrier after systemic administration of horseradish peroxidase. Am J Anat. 1980;157:375–388. doi: 10.1002/aja.1001570406. [DOI] [PubMed] [Google Scholar]
- Duc C, Barakat-Walter I, Droz B. Innervation of putative rapidly adapting mechanoreceptors by calbindin- and calretinin-immunoreactive primary sensory neurons in the rat. Eur J Neurosci. 1994;6:264–271. doi: 10.1111/j.1460-9568.1994.tb00269.x. [DOI] [PubMed] [Google Scholar]
- El-Tarhouni A, Banks RW. The distribution of calretinin in muscle receptors of the cat. J Physiol. 1995;487.P:77. [Google Scholar]
- Engberg I, Tarnawa I, Durand J, Ouardouz M. An analysis of synaptic transmission to motoneurons in the cat spinal-cord using a new selective receptor blocker. Acta Physiol Scand. 1993;148:97–100. doi: 10.1111/j.1748-1716.1993.tb09537.x. [DOI] [PubMed] [Google Scholar]
- Gale JE, Marcotti W, Kennedy HJ, Kros CJ, Richardson GP. FM1-43 dye behaves as a permeant blocker of the hair-cell mechanotransducer channel. J Neurosci. 2001;21:7013–7025. doi: 10.1523/JNEUROSCI.21-18-07013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesinger CB, Richards CD, Ashmore JF. FM1-43 reveals membrane recycling in adult inner hair cells of the mammalian cochlea. J Neurosci. 2002;22:3939–3952. doi: 10.1523/JNEUROSCI.22-10-03939.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiol Rev. 2001;81:685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- Hietanen-Peltola M, Pelto-Huikko M, Rechardt L, Emson P, Hökfelt T. Calbindin-D-28k-immunoreactivity in rat muscle spindle; a light and electron microscopic study. Brain Res. 1992;579:327–332. doi: 10.1016/0006-8993(92)90069-l. [DOI] [PubMed] [Google Scholar]
- Hunt CC, Wilkinson RS, Fukami Y. Ionic basis of the receptor potential in primary endings of mammalian muscle spindles. J General Physiol. 1978;71:683–698. doi: 10.1085/jgp.71.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A. Cutaneous receptors. In: Hubbard JI, editor. The Peripheral Nervous System. New York: Plenum Press; 1974. pp. 347–404. [Google Scholar]
- Iino S, Kobayashi S, Hidaka H. Neurocalcin immunopositive nerve terminals in the muscle spindle, Golgi tendon organ and motor endplate. Brain Res. 1998;808:294–299. doi: 10.1016/s0006-8993(98)00750-1. [DOI] [PubMed] [Google Scholar]
- Iino S, Taguchi K, Maekawa Si, Nojyo Y. Motor, sensory and autonomic nerve terminals containing NAP-22 immunoreactivity in the rat muscle. Brain Res. 2004;1002:142–150. doi: 10.1016/j.brainres.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Ito I, Kohda A, Tanabe S, Hirose E, Hayashi M, et al. 3,5-Dihydroxyphenylglycine – a potent agonist of metabotropic glutamate receptors. Neuroreport. 1992;3:1013–1016. [PubMed] [Google Scholar]
- Kilner JM, Ashmore JF. Staining pattern of isolated inner hair cells of the guinea-pig cochlea using the fluorescent probe FM1-43. J Physiol. 1997;504.P:30, 31. [Google Scholar]
- Kruse MN, Poppele RE. Components of the dynamic response of mammalian muscle spindles that originate in the sensory terminals. Exp Brain Res. 1991;86:359–366. doi: 10.1007/BF00228959. [DOI] [PubMed] [Google Scholar]
- Li J-Y, Edelmann L, Jahn R, Dahlström A. Axonal transport and distribution of synaptobrevin I and II in the rat peripheral nervous system. J Neurosci. 1996;16:137–147. doi: 10.1523/JNEUROSCI.16-01-00137.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liley AW. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956;132:650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PBC. Mammalian Muscle Receptors and Their Central Actions. London: Edward Arnold; 1972. [Google Scholar]
- Mizote M, Takano K. The response of cat muscle spindle primary endings to FM muscle vibration during fusimotor stimulation or following local injection of tetanus toxin. In: Boyd IA, Gladden MH, editors. The Muscle Spindle. London: Macmillan Press; 1985. pp. 365–369. [Google Scholar]
- Moravec M, Moravec J. Presence of mechanoreceptors in the atrioventricular junction of the rat-heart – microanatomical and ultrastructural evidences. J Ultrastruct Res. 1982;81:47–65. doi: 10.1016/s0022-5320(82)90040-5. [DOI] [PubMed] [Google Scholar]
- Munger BL, Ide C. The structure and function of cutaneous sensory receptors. Arch Histol Cytol. 1988;51:1–34. doi: 10.1679/aohc.51.1. [DOI] [PubMed] [Google Scholar]
- Nishikawa S, Sasaki F. Internalization of styryl dye FM1-43 in the hair cells of lateral line organs in Xenopus larvae. J Histochem Cytochem. 1996;44:733–741. doi: 10.1177/44.7.8675994. [DOI] [PubMed] [Google Scholar]
- Pellegrini-Giampietro DE, Torregrossa SA, Moroni F. Pharmacological characterization of metabotropic glutamate receptors coupled to phospholipase D in the rat hippocampus. Br J Pharmacol. 1996;118:1035–1043. doi: 10.1111/j.1476-5381.1996.tb15503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellizari R, Rossetto O, Schiavo G, Montecucco C. Tetanus and botulinum neurotoxins: mechanism of action and therapeutic uses. Phil Trans R Soc LondB. 1999;354:259–268. doi: 10.1098/rstb.1999.0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppele RE, Kennedy WR, Quick DC. A determination of static mechanical properties of intrafusal muscle in isolated cat spindles. Neuroscience. 1979;4:401–411. doi: 10.1016/0306-4522(79)90103-9. [DOI] [PubMed] [Google Scholar]
- Queiroz LS, Duchen LW. Effects of Latrodectus spider venoms on sensory and motor-nerve terminals of muscle-spindles. Proc R Soc LondB. 1982;216:103–110. doi: 10.1098/rspb.1982.0063. [DOI] [PubMed] [Google Scholar]
- Sidi S, Friedrich RW, Nicolson T. NompC TRP channel required for vertebrate sensory hair cell mechanotransduction. Science. 2003;301:96–99. doi: 10.1126/science.1084370. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Halasy K, Somogyi J, Storm-Mathisen J, Ottersen OP. Quantification of immunogold labelling reveals enrichment of glutamate in mossy and parallel fibre terminals in cat cerebellum. Neuroscience. 1986;19:1045–1050. doi: 10.1016/0306-4522(86)90121-1. [DOI] [PubMed] [Google Scholar]
- Storm-Mathisen J, Leknes AK, Bore AT, Vaaland JL, Edminson P, et al. First visualization of glutamate and GABA in neurons by immunocytochemistry. Nature. 1983;301:517–520. doi: 10.1038/301517a0. [DOI] [PubMed] [Google Scholar]
- Ushkaryov YA, Volynski KE, Ashton AC. The multiple actions of black widow spider toxins and their selective use in neurosecretion studies. Toxicon. 2004;43:527–542. doi: 10.1016/j.toxicon.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Walmsley B, Bolton PS. An in-vivo pharmacological study of single group-Ia fiber contacts with motoneurons in the cat spinal-cord. J Physiol. 1994;481:731–741. doi: 10.1113/jphysiol.1994.sp020477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh MJ, Price MP, Xie J. Biochemical basis of touch perception: mechanosensory function of degenerin/epithelial Na+ channels. J Biol Chem. 2002;277:2369–2372. doi: 10.1074/jbc.R100060200. [DOI] [PubMed] [Google Scholar]
- Werle MJ, Roder J, Jeromin A. Expression of frequenin at the frog (Rana) neuromuscular junction, muscle spindle and nerve. Neurosci Lett. 2000;284:33–36. doi: 10.1016/s0304-3940(00)01004-1. [DOI] [PubMed] [Google Scholar]
- Widdicombe JG. Enteroceptors. In: Hubbard JI, editor. The Peripheral Nervous System. New York: Plenum Press; 1974. pp. 455–485. [Google Scholar]
- Wu S-X, Koshimizu Y, Feng Y-P, Okamoto K, Fujiyama F, et al. Vesicular glutamate transporter immunoreactivity in the central and peripheral endings of muscle-spindle afferents. Brain Res. 2004;1011:247–251. doi: 10.1016/j.brainres.2004.03.047. [DOI] [PubMed] [Google Scholar]
- Zelená J. Nerves and Mechanoreceptors. London: Chapman & Hall; 1994. [Google Scholar]