Abstract
The nucleus tractus solitarii (NTS) is essential for coordinating arterial baroreflex control of blood pressure. The primary baroreceptor afferent fibres make their first excitatory synaptic contact at second-order NTS neurones with glutamate as the major neurotransmitter. Glutamate regulates its own release by activating presynaptic metabotropic glutamate autoreceptors (mGluRs) on the baroreceptor central terminals to suppress its further release in frequency-dependent manner. γ-Aminobutyric acid (GABA) interneurones provide the major inhibitory synaptic input. It is the integration of excitatory and inhibitory inputs that shapes the NTS output of baroreceptor signals. We hypothesized that glutamate released from the primary central afferent terminals can spill over to presynaptic mGluRs on GABA interneurones to suppress GABA release at the second-order baroreceptor neurones. We assessed GABA transmission in second-order baroreceptor neurones identified by attached aortic depressor nerve (ADN) boutons. The medial NTS was stimulated to evoke GABA inhibitory postsynaptic currents (eIPSCs). Glutamate spillover, generated by brief 2 s, 25 Hz trains of stimuli applied to the tractus solitarius (TS), induced a small (10%) but significant reduction in the eIPSC amplitudes. The depression was enhanced to a 25% decrease by increasing glutamate in the cleft with a glutamate-uptake inhibitor (M-trans-pyrrolidine-2,4-dicarboxylic acid, 1 μm), blocked by a Group II mGluR antagonist (LY341495, 200 nm) and mimicked by a Group II agonist ((2S,3S,4S)-CCG/(2S,1′S,2′S)-2-carboxycyclopropyl; L-CCG-I). A presynaptic mGluR locus was established by the mGluR agonist-mediated increase in the paired-pulse ratio of two consecutive eIPSCs in conjunction with the decrease in the first eIPSC, and a decrease in the frequency (39–46% reduction at EC50 concentration), but not amplitude, of spontaneous and miniature GABA IPSCs. The data indicate that endogenous glutamate activation of Group II presynaptic mGluRs can decrease GABA release at the first central synapses, suggesting a heterosynaptic role for the Group II mGluRs in shaping baroreceptor signal transmission.
The nucleus tractus solitarii (NTS) is essential for coordinating baroreceptor reflex control of blood pressure. The NTS second-order neurones are the first site of synaptic contact of the primary baroreceptor afferent fibres. The processing of the blood pressure-related sensory information at these synapses can affect all downstream processing of baroreceptor signals and hence baroreflex function (Loewy & McKellar, 1980; Loewy, 1990; Spyer, 1990). The major excitatory neurotransmitter is glutamate, which binds to the ionotropic glutamate receptors on the second-order neurones to mediate the fast excitatory transmission (Talman et al. 1980; Perrone, 1981; Zhang & Mifflin, 1995; Gordon & Sved, 2002). The major inhibitory input is provided by GABA interneurones strategically positioned near the second-order baroreceptor neurones (Maqbool et al. 1991; Mifflin, 2001; Gordon & Sved, 2002). It is the balance of excitatory and inhibitory modulatory influences on the fast glutamatergic transmission that shapes the net NTS output of the baroreceptor signals to distal synapses in the central network. Ultimately, this NTS output orchestrates parasympathetic and sympathetic nervous system activities to restore blood pressure back towards normal (Loewy & McKellar, 1980; Loewy, 1990; Spyer, 1990; Mifflin, 2001).
The G-protein-coupled metabotropic glutamate receptors (mGluRs), which have been shown to modulate synaptic transmission throughout the centeral nervous system (CNS) (Conn & Pin, 1997; Cartmell & Schoepp, 2000; Doi et al. 2002), have been demonstrated in the NTS, but their precise role in shaping baroreceptor signal transmission has not been fully established (Pawloski-Dahm & Gordon, 1992; Foley et al. 1998, 1999; Liu et al. 1998; Hay & Hasser, 1998; Jones et al. 1999; Matsumura et al. 1999; Viard & Sapru, 2002; Chen et al. 2002). The mGluRs are classified into three groups based on sequence homology, agonist potency and signal transduction pathways (Suzdak et al. 1994; Conn & Pin, 1997). Throughout the CNS, the Group I mGluRs have been predominantly located on cell soma, and they increase neuronal excitability, essentially increasing the excitatory response to glutamate activation of the ionotropic glutamate receptors. The Group II and III mGluRs are located predominantly on presynaptic terminals to decrease glutamate release in a frequency-dependent manner, providing an inhibitory influence on glutamatergic transmission (Pin & Duvoisin, 1995; Conn & Pin, 1997; Scanziani et al. 1997; Cartmell & Schoepp, 2000).
In the NTS, the mGluRs were first indirectly implicated in modulating baroreflex function by microinjection studies. Glutamate-induced decreases in blood pressure and heart rate were shown to be prevented only when both ionotropic and metabotropic glutamate receptors were blocked (Foley et al. 1998). NTS microinjections of Group I, II and III mGluR agonists were shown to mimic baroreceptor activation by decreasing blood pressure, heart rate and sympathetic nerve activity, effects abolished by metabotropic but not ionotropic glutamate receptor antagonists (Pawloski-Dahm & Gordon, 1992; Foley et al. 1999; Viard & Sapru, 2002). On the other hand, injections of mGluR antagonists have resulted in variable responses: no effect on blood pressure, heart rate and sympathetic nerve activity; biphasic changes in blood pressure and sympathetic nerve activity; or decreases in heart rate and blood pressure (Foley et al. 1998, 1999; Jones et al. 1999; Matsumura et al. 1999; Viard & Sapru, 2002). Viewed together, the data suggest that the contribution of mGluRs to baroreceptor signalling is complex. While they seem to exhibit an overall excitatory effect in the NTS with exogenous agonist activation, the functional role of mGluRs in baroreceptor reflex remains to be resolved. More importantly, the physiological relevance of mGluRs in modulating baroreflex function by activation by endogenous glutamate release has not been resolved with the use of microinjections of mGluR antagonists. This may be explained in part by the temporal and spatial features of how endogenous glutamate release activates presynaptic mGluRs on glutamatergic terminals and postsynaptic mGluRs, which would have opposing effects on baroreceptor signalling and hence baroreflex function. Thus, without using approaches to isolate glutamate actions at presynaptic versus postsynaptic mGluRs, the physiological relevance of mGluRs in baroreceptor signalling may remain obscure. Using electrophysiological approaches in vivo and in vitro, we previously demonstrated that presynaptic Group II and III mGluRs are activated by endogenous glutamate to provide a frequency-dependent depression of baroreceptor or general primary sensory signal transmission to the second-order neurones (Liu et al. 1998; Chen et al. 2002). These findings suggest that presynaptic mGluRs are functionally important in limiting perhaps unnecessary glutamate release during high-frequency baroreceptor afferent traffic, but they do not explain the overall excitatory effects of mGluRs obtained by the microinjection studies in whole animals. Thus, the question remains as to how the mGluRs regulate baroreceptor signal transmission, and hence baroreflex function.
Recent studies have shown that in a few specific CNS regions, mGluRs are expressed on γ-aminobutyric acid (GABA) terminals where they can modulate GABA release. However, most studies have used mGluR agonists, so the extent to which endogenous glutamate activates mGluR on GABA neurones has only been demonstrated in the hippocampus, supraoptic nucleus and cerebellum (Mitchell & Silver, 2000; Semyanov & Kullmann, 2000; Piet et al. 2003). Moreover, the effect of mGluR activation on GABA transmission is not uniform in the CNS. Activation of mGluRs may induce an increase or decrease or have no effect on GABA release depending on the origin of the GABA input (Poncer et al. 2000; Woodhall et al. 2001), location of the receptors on the GABA neurones and the mGluR receptor subtypes (Poncer et al. 1995; Saransaari & Oja, 2001, 2004; Doi et al. 2002; Pampillo et al. 2002; Zheng & Johnson, 2003).
Studies of the ultrastructure of glutamate and GABA terminals in the NTS have demonstrated a close proximity of GABA and peripheral afferent glutamate terminals (Maqbool et al. 1991). These data indicate that neural network exists whereby glutamate can regulate GABA release at the second-order baroreceptor neurones through activation of presynaptic mGluRs. Whether and to what extent activation of mGluRs modulates GABA transmission to NTS baroreceptor neurones is not known. Given the profound inhibitory effect of GABA on baroreceptor signal transmission, if glutamate release activates mGluRs on GABA interneurones, the findings could help to explain the overall excitatory effect of mGluRs in the NTS. Of particular relevance to this possibility are the findings by Mitchell & Silver (2000) demonstrating the effectiveness of endogenous glutamate spillover from the mossy fibres in activating mGluRs on nearby GABA interneurones to decrease GABA release in the cerebellar glomerulus.
Against that background, we hypothesized that endogenous glutamate released from stimulation of sensory afferent fibres carried in the tractus solitarius (TS) can spill over to activate presynaptic mGluRs located on nearby GABA terminals to decrease GABA release onto the second-order baroreceptor neurones. Studies were performed using whole-cell voltage clamping on second-order baroreceptor neurones, which were anatomically identified by the presence of labelled central boutons of the baroreceptor afferent nerve fibres carried in the aortic depressor nerve (ADN). We focused on the Group II mGluRs because they have been most studied in the NTS, and because they have been shown to inhibit glutamate release at second-order baroreceptor neurones (Liu et al. 1998; Chen et al. 2002). If the hypothesis is true, then high- (but still physiologically relevant) frequency TS stimulation (endogenous glutamate release) should decrease the amplitude of the NTS-evoked GABA-mediated inhibitory postsynaptic currents (eIPSCs), an effect that should be enhanced further by increasing glutamate in the synaptic cleft (by inhibiting glutamate uptake), mimicked by a Group II mGluR agonist, and prevented by a Group II mGluR antagonist. In addition, an mGluR agonist-mediated decrease in the first NTS-evoked GABA IPSC, in conjunction with an increase in the paired-pulse ratio of two consecutive NTS-evoked GABA IPSCs, and a decrease in the frequency but not amplitude of spontaneous (sIPSCs) and miniature (mIPSCs) IPSCs, should provide further evidence for a presynaptic mechanism (Jang et al. 2001; Behr et al. 2002; Kerchner & Zhuo, 2002; Kirischuk et al. 2002; Sekizawa et al. 2003; Jeong et al. 2003).
Methods
All experimental protocols in this work were reviewed and approved by the Institutional Animal Care and Use Committee in compliance with the Animal Welfare Act, and in accordance with Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Surgical preparation for labelling ADN boutons
Male Sprague-Dawley rats 11 weeks old (320–370 g) were anaesthetized with a combination of ketamine (50 mg kg−1) and xylazine (8 mg kg−1). A 4–5 mm segment of the ADN, between the superior laryngeal nerve and vagus nerve/sympathetic trunk, was carefully isolated and placed on a section of parafilm. The fluorescent dye crystals, 1,1′-dilinoleyl-3,3,3′,3′-tetra-methylindocarbocyanine, 4-chlorobenzenesulphonate (FAST DiI solid; DiIρ9,12-C18(3)), were gently placed on the ADN, and the area was embedded with polyvinylsiloxane gel. To allow for transport of the dye to the terminal boutons, the rats were allowed to recover for 2 weeks before the experimental protocols, as previously reported (Mendelowitz et al. 1992; Bonham & Chen, 2002).
Brainstem slice preparation
The rats were anaesthetized with a combination of ketamine (50 mg kg−1) and xylazine (8 mg kg−1), and then decapitated. The brain was rapidly exposed and submerged in ice-cold (<4°C), high-sucrose, artificial cerebrospinal fluid (aCSF) that contained (mm): 3 KCl, 2 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, 10 glucose, 220 sucrose and 2 CaCl2, pH 7.4 when continuously bubbled with 95% O2/5% CO2. Brainstem transverse slices (250 μm thick) were cut with the Vibratome 1000 (Technical Products International, St Louis, MO, USA). After incubation for 45 min at 37°C in high-sucrose aCSF, the slices were placed in normal aCSF that contained (mm): 125 NaCl, 2.5 KCl, 1 MgCl2, 1.25 NaH2PO4, 25 NaHCO3, 25 glucose and 2 CaCl2, pH 7.4 when continuously bubbled with 95% O2/5% CO2. During the experiments, a single slice was transferred to the recording chamber, held in place with a nylon mesh, and continuously perfused with oxygenated aCSF at a rate of approximately 3 ml min−1. All experiments were performed at 33–34°C.
Whole-cell voltage-clamp recordings
All whole-cell voltage-clamp recordings were performed on second-order NTS baroreceptor neurones with attached fluorescent ADN boutons. The neurones were visualized with infrared differential interference contrast (IR-DIC), and the fluorescent boutons were visualized with an optical filter set for DiI (XF108; Omega Optical Inc., Brattleboro, VT, USA) and an image integrating system (InvestiGater; Dage-MTI, Michigan City, IN, USA). All images were captured with a charge-coupled device (CCD) camera (CCD-100; Dage-MTI) displayed on a TV monitor and stored in a PC computer using Computer Eyes software (Digital Vision, Inc., Dedham, MA, USA). Borosilicate glass electrodes were filled with a KCl solution containing (mm): 130 KCl, 5 NaCl, 1 MgCl2, 3 Mg-ATP, 0.2 Na-GTP, 10 ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 10 N-2-hydroxy-ethylpiperazine-N′-2-ethanesulphonic acid (Hepes), and 5 QX314. The pH was adjusted to 7.3 with KOH. With this pipette solution, the reversal potential for Cl− calculated from Nernst equation is 0.7 mV. The junction potential was −3 mV and was corrected. The seal resistance was >1 GΩ. The pipette resistance ranged from 2 to 4 MΩ (2.7 ± 0.5 MΩ, mean ±s.d.), and the series resistance was no greater than 18 MΩ (10 ± 4 MΩ, mean ±s.d.). Recordings were made with the Axoclamp 1D patch-clamp amplifier (Axon Instruments). Whole-cell currents were filtered at 2 kHz, digitized at 10 kHz with the DigiData 1200 Interface (Axon Instruments) and stored in a PC computer.
Once the whole-cell configuration was established, the neurone was voltage-clamped at −50 mV. The neurone was then tested for TS input with five TS stimuli delivered at 0.2 Hz, and then for NTS input with five NTS stimuli delivered at 0.2 Hz. All experiments were performed in the presence of the ionotropic glutamate receptor antagonists 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulphonamide disodium salt (NBQX, 10 μm) and dl-2-amino-5-phosphonopentanoic acid (AP5, 50 μm). The rationale for NBQX and AP5 was threefold: (1) to isolate the mGluR-mediated effects from the fast synaptic transmission mediated by the ionotropic glutamate receptors; (2) to isolate the IPSCs from excitatory postsynaptic currents (EPSCs), since both GABA IPSCs and glutamate EPSCs are inward at the holding potential of −50 mV with a KCl-based pipette solution; and (3) to isolate the response to synapses in direct contact of the recorded second-order neurones to synapses from those of distal synapses origin by preventing activation over polysynaptic pathways (Grabauskas & Bradley, 1996; Smith et al. 1998; Butcher et al. 1999).
TS stimulation parameters
To generate endogenous glutamate release from the sensory afferent fibres, a stimulating electrode was positioned in the TS ipsilateral to the recording site. The stimulation voltage was limited to 10 V, 0.1 ms square-wave pulses to minimize the voltage spread outside the TS. The stimuli were delivered through a high impedence (10 MΩ) bipolar tungsten electrode (1 μm tips separated by 80 μm). The average distance between the stimulating electrode and the recorded neurone was 237 ± 53 μm (mean ±s.d.; ranging from 167 to 333 μm). The TS stimuli consisted of trains (25 Hz for 2 s) delivered at 50 ms prior to each eIPSC. In preliminary experiments, we tested different intervals (5, 20, 50 and 100 ms) between the TS stimulation and the eIPSC. The 50 ms interval provided the maximal depression of the eIPSC amplitude among all tested intervals (data not shown) and so was used for subsequent experiments.
Acquisition and analysis of GABA IPSCs
To stimulate GABA release from local inhibitory neurones in the NTS for the eIPSCs, we positioned a bipolar tungsten electrode (1 μm tips separated by 80 μm) in the intermediate NTS ipsilateral and medial to the recording site. We used the minimal intensity (2–20 V) required to consistently eIPSCs. The averaged distance between the stimulating electrode and the recorded neurones was 126 ± 25 μm (mean ±s.d.; ranging from 83 to 188 μm). The data were analysed off-line using the pClamp9 (Axon Instruments) software.
The frequency and amplitude of sIPSCs were recorded for determining presynaptic versus postsynaptic mechanisms. The sIPSCs could reflect inhibitory synaptic currents caused by both action potential-dependent and independent release of GABA. To further assess the presynaptic locus of mGluR activation, mIPSCs were recorded in the presence of the sodium channel blocker tetrodotoxin (TTX, 1 μm) in the same neurones. The mIPSCs recorded during TTX were assumed to be independent of invasion of action potentials in the presynaptic terminals (Edwards et al. 1990). A change in sIPSC but not mIPSC frequency would indicate the effect is on preterminal (soma or axon) locus. A decrease in mIPSC frequency would indicate an inhibition on GABA release machinery at the terminals (Seamans et al. 2001). The sIPSC and mIPSC events were detected with Mini Analysis software (Synaptosoft Inc., Decatur, GA, USA). The threshold for detection was set at six times the root mean square baseline noise. The accuracy of detection was confirmed by visual inspection.
To confirm that the IPSCs were GABAergic, both eIPSCs and sIPSCs were recorded: (1) at different holding potentials from −50 to +50 mV at 25 mV increments to determine the reversal potential; and (2) before, during and after perfusion with the GABAA receptor antagonist (bicuculline, 10 μm) at a holding potential of −50 mV.
Protocols
In protocol 1, we determined the extent to which endogenous glutamate on decreased the eIPSCs and whether the effect was mediated by activation of Group II mGluRs. After testing a neurone for TS and NTS inputs, the slice was perfused with aCSF containing NBQX and AP5. The NTS was continuously stimulated at 0.2 Hz, and the eIPSCs were recorded for 3 min during the control period, 3 min with endogenous glutamate release (with TS stimulation), and 5 min of recovery period. The protocol was performed under (1) control conditions, (2) in the presence of the glutamate uptake inhibitor l-trans-pyrrolidine-2,4-dicarboxylic acid (PDC, 1 μm) to enhance glutamate spillover, and (3) in the presence of PDC and the Group II mGluR antagonist (LY341495, 200 nm). LY341495 is a highly potent and selective Group II antagonist that has a 1000-fold higher affinity over that for Group I and III, and blocks both subtypes 2 and 3 of Group II mGluRs at nanomolar concentrations (Kingston et al. 1998).
Protocol 2 was implemented to determine whether the effect of glutamate spillover was mimicked by a Group II mGluR agonist (2S,3S,4S)-CCG/(2S,1′S,2′S)-2-carboxycyclopropyl (l-CCG-I) acting at presynaptic receptors. Pairs of NTS stimuli with an interpulse interval of 25 ms were continuously delivered at 0.2 Hz, and the eIPSCs were recorded for 3 min during the control period, 3 min during perfusion with one of the concentrations of the l-CCG-I (1, 3, 10, 30, 100 and 300 μm), and a washout period (4–14 min). The concentrations were applied in a random order. The paired-pulse depression of the NTS IPSCs evoked at this interval has been shown to be presynaptically mediated (Grabauskas & Bradley, 2003).
Protocol 3 was designed to further test whether the mGluR effect on GABA release was presynaptic, by measuring the effect of l-CCG-I on the frequency and amplitude of sIPSCs and mIPSCs. The sIPSCs were continuously recorded for 5 min during the control period, for 3 min during perfusion with l-CCG-I (30 μm), and for 7 min of washout period. For mIPSCs, the protocol was performed in the presence of TTX.
In protocol 4 we determined whether the Group II mGluR antagonist LY341495 blocked the effect of the Group II agonist l-CCG-I on both eIPSCs and sIPSCs. For eIPSCs and the paired-pulse ratio, pairs of NTS stimuli were continuously delivered at 0.2 Hz. The eIPSCs and sIPSCs were continuously recorded for (1) 3 min during the control period, (2) 3 min during perfusion with l-CCG-I (30 μm), (3) 7 min washout period, (4) 5 min perfusion with LY341495 (200 nm), (5) 3 min perfusion with l-CCG-I (30 μm) in the presence of LY341495, and (6) 7 min l-CCG-I washout period.
Data analysis
Data are expressed as means ± s.e.m. unless otherwise indicated. Differences were considered significant at P < 0.05. The statistical analyses were performed with SigmaStat software (SPSS Inc., Chicago, IL, USA). When appropriate, the ANOVA test was followed by the post-hoc test for pairwise comparisons.
For protocol 1, to determine the effect of the glutamate spillover on NTS-eIPSCs, the peak amplitudes of the eIPSCs were averaged 3 min before, during and after the TS train. The data were compared with a one-way repeated ANOVA (before versus during versus after). To determine the effect of the glutamate uptake inhibitor and mGluR blockade on the glutamate-induced depression of GABA transmission, the averaged peak eIPSC amplitude during the TS train was expressed as a percentage of the average peak eIPSC amplitude evoked before the TS train. The data were compared with a one-way repeated ANOVA (control versus PDC versus LY341495). To determine the reversal potential of the eIPSC, the peak eIPSC amplitudes were plotted against the holding potential, and the data were fitted with linear regression. To determine the effect of bicuculline on the eIPSCs, the peak eIPSC amplitudes were averaged over 3 min during the control period, the last minute of bicuculline perfusion, and the last minute of the washout period. The data were compared with a one-way repeated measure ANOVA (control versus bicuculline versus washout).
For protocol 2, to determine the effect of the Group II mGluR agonist on the eIPSCs, the peak eIPSC amplitude averaged during the last 2 min during l-CCG-I perfusion was expressed as a percentage of the peak eIPSC amplitude averaged over the 3 min control period. The agonist concentration–response curve was analysed with a one-way ANOVA. The paired-pulse ratio (the peak amplitude of the second eIPSC of the paired stimuli over the peak amplitude of the first eIPSC) was analysed in the same way.
The frequency and amplitude of the sIPSCs were averaged over the 3 min control period, during agonist perfusion, and the last 3 min during washout. The data were compared with a one-way repeated ANOVA (control versus agonist versus washout). The mIPSCs were analysed the same way.
For protocol 4, to determine the effect of the mGluR antagonist on the agonist induced depression, the peak eIPSC amplitude averaged over the last 2 min during agonist perfusion was expressed as percentage change from the control peak eIPSC amplitude averaged over the 3 min control period before the agonist perfusion. The data in the absence and presence of the antagonist were compared with a paired t test. The paired-pulse ratio was analysed in the same way. For sIPSCs, the frequency and amplitude averaged during the agonist perfusion were expressed as a percentage change from the control, which was averaged over the 3 min control period before the agonist perfusion. The data in the absence and presence of the antagonist were compared with a paired t test.
Drugs
Ketamine and xylazine were obtained from Vedco, Inc. (St Joseph, MO, USA). DiI was obtained from Molecular Probes (Eugene, OR, USA). Polyvinylsiloxane gel was obtained from Carlisle Laboratories Inc. (Rockville Centre, NY, USA). QX314, l-CCG-I, PDC and LY341495 were obtained from Tocris (Ballwin, MO, USA). Bicuculline, Mg-ATP, Na-GTP, EGTA and Hepes were obtained from Sigma (St Louis, MO, USA). All other chemicals were obtained from Fisher (Fairlawn, NJ, USA).
Results
All data were obtained in second-order baroreceptor neurones identified by their possession of fluorescently labelled attached boutons as shown in Fig. 1A and located in the dorsal and medial NTS between obex and calamus scriptorius (Fig. 1B).
Figure 1. Photographs and recording sites of second-order baroreceptor neurones.
A, a baroreceptor second-order NTS neurone. Aa, the neurone viewed with infrared differential interference contrast (IR-DIC). Ab, the labelled boutons of aortic depressor nerve viewed with fluorescence filter set. Ac, overlay of the IR-DIC and fluorescence images. Ad, neurone with patch electrode in whole-cell configuration. Bar, 10 μm. B, a composite of recording sites. TS, tractus solitarius; AP, area postrema; c, central canal; X, dorsal motor nucleus of vagus; XII, hypoglossal nucleus.
Glutamate spillover inhibited GABA transmission in second-order baroreceptor neurones
As shown in the example traces in Fig. 2A, NBQX (10 μm) and AP5 (50 μm) blocked the TS-evoked EPSC and spared the NTS-eIPSC, confirming that the TS-evoked EPSCs were glutamatergic. NTS-eIPSCs obtained in the presence of ionotropic glutamate receptor blockade with NBQX and AP5 were confirmed to be GABAergic by their reversal potential of 0.7 ± 3.9 mV, which is close to the calculated reversal potential for Cl− (0.7 mV) (n = 7, Fig. 2B), and by their elimination with bicuculline (n = 7, Fig. 2C). The peak amplitude of the NTS-eIPSCs in the control conditions across all protocols averaged 310 ± 157 pA (mean ±s.d.). The paired-pulse ratio of the eIPSCs averaged 0.85 ± 0.25 (mean ±s.d.).
Figure 2. The TS-evoked postsynaptic currents are glutamatergic and the NTS-evoked PSCs are GABAergic.
A, traces of TS-evoked EPSCs (left) and nucleus tractus solitarii (NTS)-eIPSCs (right) from one neurone before and during perfusion of 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulphonamide disodium salt (NBQX; 10 μm) and dl-2-amino-5-phosphonopentanoic acid (AP5; 50 μm) at a holding potential of −50 mV ▾, TS stimulus; •, NTS stimulus. B, an example (left) of traces of NTS-eIPSCs at different holding potentials (from −50 to +50 mV) in the present of NBQX and AP5. Vh, holding potential. Group data (right, n = 7) of the current–voltage relationship of the NTS-eIPSCs. The reversal potential approximated the predicted chloride equilibrium potential (ECl). C, an example of traces (left) of NTS-eIPSCs averaged over 1 min (12 IPSCs) during control, in the presence of the GABAA-receptor antagonist bicuculline (10 μm), and during washout (in the present of NBQX and AP5). Group data (right, n = 7) confirm that bicuculline blocked the NTS-eIPSCs.*P < 0.05.
Endogenous glutamate released by trains of TS stimuli depressed GABA transmission (Figs 3 and 4). The traces in Fig. 3A show that the NTS-eIPSC was reversibly depressed during the TS train in one neurone. The group data (n = 10, Fig. 3B) confirm that the eIPSC was significantly depressed during TS trains (one-way repeated ANOVA, P < 0.05). The depression observed under control conditions was enhanced by the glutamate uptake inhibitor PDC (1 μm), and was blocked by the addition of the Group II mGluR antagonist LY341495 (200 nm) (Fig. 4A). The group data (Fig. 4B) show that PDC significantly enhanced the TS train-induced depression of GABA transmission, and that LY341495 significantly attenuated the depression (one-way repeated ANOVA, P < 0.05). The response during mGluR antagonist in the presence of PDC was significantly lower than that of PDC alone and control (Fisher's LSD, P < 0.05). In a separate experiment, the antagonist also blocked the TS stimulation-induced reduction of the eIPSCs in the absence of PDC (from −16 ± 7 to −2.7 ± 2.6%, n = 5, P < 0.05).
Figure 3. Stimulation of sensory afferents in TS depressed GABA transmission onto baroreceptor second-order NTS neurones.
A, an example of traces of NTS-eIPSCs averaged over 3 min during control, with 2 s TS stimulation (25 Hz, 50 ms prior to the NTS eIPSC) and during recovery. All experiments were performed with NBQX and AP5 in the perfusate. •, NTS stimulus. B, group data (n = 10) confirm that trains of TS stimulations depressed the NTS-eIPSCs.*P < 0.05.
Figure 4. The TS stimulation-induced depression of GABA release to baroreceptor second-order NTS neurones is mediated by glutamate spillover.
A, an example of traces of NTS-eIPSCs before, with and after TS train in control (top), the presence of l-trans-pyrrolidine-2,4-dicarboxylic acid (PDC) (middle), and PDC + LY341495 (bottom), from one neurone. All experiments were performed with NBQX and AP5 in the perfusate. •, NTS stimulus. B, group data (n = 6) confirm endogenous glutamate spillover-induced depression of GABA release in baroreceptor second-order NTS neurones as it was enhanced by the glutamate uptake inhibitor PDC (1 μm), and attenuated by the Group II mGluR antagonist LY341495 (200 nm).*P < 0.05.
Group II mGluR agonist inhibited GABA release mimicking the glutamate spillover
Figure 5A shows example traces of an NTS-eIPSC before (control) and during perfusion with l-CCG-I (10 μm) and washout. The group data (Fig. 5B), fit to a sigmoid function, illustrate that the Group II mGluR agonist significantly depressed the first NTS-eIPSC in a concentration-dependent manner (one-way ANOVA, P < 0.05). Based on the curve fit result, the EC50 was 16 μm and the maximum depression was 17% of control.
Figure 5. The Group II mGluR agonist L-CCG-I depressed the first NTS-eIPSC and increased the paired-pulse ratio in baroreceptor second-order NTS neurones.
A, an example of traces of NTS-eIPSCs before (control), during, and after (washout) (2S,3S,4S)-CCG/(2S,1′S,2′S)-2-carboxycyclopropyl (l-CCG-I) perfusion. •, NTS stimulus. B, g roup data showing the peak amplitude of first NTS-eIPSCs averaged over the last 2 min of l-CCG-I perfusion, and expressed as percentages of the control IPSC. C, group data showing that l-CCG-I produced a concentration-dependent increase in the paired-pulse ratio. All experiments were performed with NBQX and AP5 in the perfusate. Numbers in parentheses indicate number of neurones.*P < 0.05.
Evidence for presynaptic sites of l-CCG-I effects: paired pulse ratio
As shown in the example traces from one neurone (Fig. 5A), l-CCG-I (10 μm) depressed the peak amplitude of the first NTS-eIPSC to a greater extent than the second IPSC of the pair, resulting in an increased paired-pulse ratio. The group data (Fig. 5C) demonstrate that l-CCG-I increased the paired-pulse ratio in a concentration-dependent manner (one-way ANOVA, P < 0.05).
Evidence for presynaptic sites of l-CCG-I effects: sIPSCs and mIPSCs
The sIPSCs were confirmed to be GABAergic by their reversal potential of 3 ± 5 mV (n = 3) which is close to the calculated reversal potential for Cl−, and by their elimination with perfusion with bicuculline (n = 6, data not shown).
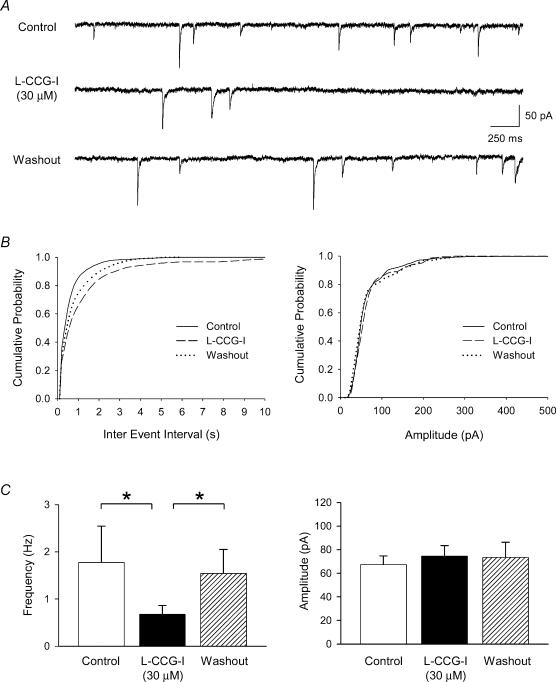
The Group II mGluR agonist l-CCG-I (30 μm) decreased the frequency but not the amplitude of sIPSCs (Fig. 6). Recordings of sIPSCs before, during and after washout of l-CCG-I in one neurone are shown in Fig. 6A. For the same neurone, Fig. 6B shows a rightward shift in the cumulative probability of the sIPSC interevent intervals during l-CCG-I perfusion, and no change in the cumulative probability of the sIPSC amplitude (Fig. 6B). The group data (n = 9, Fig. 6C) confirm that l-CCG-I significantly and reversibly decreased the sIPSC frequency (one-way repeated ANOVA, P < 0.05) with no change in the amplitude (one-way repeated ANOVA, P > 0.05). There was no change in the holding current during l-CCG-I (delta change = −0.2 ± 3 pA).
Figure 6. The Group II mGluR agonist l-CCG-I decreased the frequency but not the amplitude of sIPSCs in baroreceptor second-order NTS neurones.
A, an example of traces of recordings of sIPSCs before (control), during and after (washout) l-CCG-I perfusion. B, cumulative probability of the interevent interval (left) and amplitude (right) before, during and after l-CCG-I perfusion in the same neurone recorded in A. C, group data (n = 9) confirm that l-CCG-I decreased the sIPSC frequency (left), but not the amplitude, suggesting a presynaptic mechanism. All experiments were performed with NBQX and AP5 in the perfusate.*P < 0.05.
Since the decrease in sIPSC frequency could be the result of l-CCG-I activation of mGluRs on the axon or cell body of the GABA neurones, we tested the effect of l-CCG-I on mIPSCs in seven of the nine neurones. Blockade of the TTX-sensitive Na+ channel significantly decreased the sIPSCs frequency from 2.1 ± 0.96 to 0.5 ± 0.04 Hz (paired t test, P < 0.05) with no significant change in the amplitude (67 ± 7 and 75 ± 7 pA, respectively). In the presence of TTX, l-CCG-I also decreased the frequency of action potential-independent mIPSCs, with no change in their amplitude. Figure 7A shows example recordings from one neurone. The cumulative probability of the mIPSC interevent intervals for this neurone was shifted to the right during l-CCG-I, without changes in the cumulative probability of the mIPSC amplitude (Fig. 7B). The group data (n = 7, Fig. 7C) confirm that l-CCG-I significantly and reversibly decreased the mIPSC frequency (one-way repeated ANOVA, P < 0.05), with no change in the amplitude (one-way repeated ANOVA, P > 0.05; l-CCG-I similarly depressed the sIPSC (by 39 ± 7%) and mIPSC (by 46 ± 10%) frequency in these seven neurones (paired t test, P > 0.05). There was no change in the holding current during l-CCG-I (delta change =−0.9 ± 7 pA).
Figure 7. The Group II mGluR agonist l-CCG-I decreased the frequency but not the amplitude of mIPSCs in baroreceptor second-order NTS neurones.
A, an example of traces of recordings of sIPSCs before (control), during and after (washout) l-CCG-I perfusion. B, cumulative probability of the interevent interval (left) and amplitude (right) before, during and after l-CCG-I perfusion in the same neurone as recorded in A. C, group data (n = 7) confirm that l-CCG-I decreased the mIPSC frequency (left), but not the amplitude, suggesting a presynaptic mechanism. All experiments were performed with NBQX and AP5 in the perfusate.*P < 0.05.
Antagonist blockade of agonist effects
The effects of l-CCG-I on the eIPSCs and sIPSCs were prevented by the Group II antagonist LY341495 (200 nm) (Fig. 8). As shown by the group data (n = 5) in Fig. 8A, LY341495 blocked the l-CCG-I induced reduction in eIPSC amplitude (paired t test, P < 0.05), and the increase in paired-pulse ratio (paired t test, P < 0.05). Figure 8B shows that the Group II antagonist also significantly blocked the decrease in sIPSC frequency during l-CCG-I perfusion (n = 6, paired t test, P < 0.05).
Figure 8. The effects of Group II mGluR agonist l-CCG-I (30 μM) was blocked by the Group II mGluR antagonist LY341495 (200 nM).
A, group data (n = 5) of the first NTS-eIPSC (left) and the paired-pulse ratio (right) expressed as percentage changes from control. B, group data (n = 6) of the sIPSC frequency (left) and amplitude (right) during l-CCG-I perfusion expressed as percentage changes from control.*P < 0.05, l-CCG-I versusl-CCG-I + LY341495.
Discussion
The data presented in this study suggest a new role for Group II mGluRs in regulating baroreceptor signal transmission through inhibition of GABA release at second-order baroreceptor neurones. First, endogenous glutamate released by stimulation of sensory afferent fibres depressed the amplitude of NTS-evoked GABA IPSCs at second-order baroreceptor neurones. The depression was enhanced by increasing glutamate in the synaptic cleft, abolished by a Group II mGluR antagonist, and mimicked by a Group II mGluR agonist in a concentration-dependent manner. Evidence that glutamate activated presynaptic mGluRs located on GABA terminals to decrease GABA release emerged from the following findings. The Group II mGluR agonist decreased the first of the NTS-evoked GABA IPSCs in conjunction with an increase in the paired-pulse ratio and decreased the frequency, but not amplitude, of the sIPSCs and mIPSCs. These findings are consistent with a presynaptic mechanism for decreasing GABA release.
The physiological relevance of Group II mGluRs on the GABA terminals turns on the extent to which the receptors are activated by endogenous glutamate. The close proximity of GABA interneurones and peripheral afferent terminals (Maqbool et al. 1991) provides the neuronal configuration for endogenous glutamate released from the peripheral afferent terminals to activate mGluRs on GABA terminals to modulate both evoked and spontaneous GABA release at the second-order baroreceptor neurones. We generated endogenous glutamate release by electrically stimulating the sensory afferent fibres in the TS at a frequency (25 Hz) to approximate a physiologically relevant frequency of baroreceptor afferent fibre firing. The frequency was based on data from rats showing that nonmyelinated aortic baroreceptor afferent fibres in the ADN discharge with a resting frequency of ∼2–5 Hz, and a maximal frequency of 20–23 Hz, and that the Aδ fibres have a mean frequency of ∼28–34 Hz, and a maximal frequency of 65–90 Hz (Thoren & Jones, 1977; Thoren et al. 1999). In the whole animal, ADN stimulation at 25 Hz results in a ∼20 mmHg decrease in blood pressure, which is about 66% of the maximal ADN stimulation-induced decrease in blood pressure (De Paula et al. 1999; Kobayashi et al. 1999). An inevitable limitation of TS stimulation is that it does not exclusively activate baroreceptor afferent fibres. So, the release of glutamate from other sensory afferent fibres in addition to the baroreceptor fibres undoubtedly contributed to the endogenous glutamate activation of the presynaptic mGluRs on the GABA terminals. However, multiple visceral afferent fibres of different modalities converge onto NTS neurones (Mifflin et al. 1988; Felder & Mifflin, 1988; Mifflin, 1993, 1996; Hines et al. 1994; Toney & Mifflin, 1994; Takagi et al. 1995; Silva-Carvalho et al. 1998), so it seems likely that in the whole animal, endogenous glutamate could also be released from baroreceptor afferent fibres either alone or in combination with glutamate released from other peripheral afferent fibres to activate the mGluRs on the GABA interneurones. In this regard, inhibition of GABA release via activation of mGluRs by glutamate spillover from afferent fibres may not be limited to baroreceptor second-order neurones.
The glutamate released at the 25 Hz TS input frequency had a modest inhibitory effect on the amplitude of the GABA eIPSCs measured at a single neurone. While a 10% inhibition at one synapse may seem trivial, the culmination of this inhibition of GABA release at many synapses from glutamate spillover in the whole animal would have a pronounced effect on baroreceptor signalling. In a previous study, we examined the possible consequences of a 10% inhibition of NTS output of baroreceptor signal transmission due to frequency-dependent depression in the NTS (Liu et al. 2000). We simultaneously recorded NTS single neurone and lumbar sympathetic nerve activity (LSNA) during ADN stimulation. From curve fitting to determine by how much the decrease in LSNA would have been enhanced (if there were no frequency-dependent depression in the NTS), the data predicted that the baroreflex inhibition of sympathetic nerve activity was reduced by 20% in the presence of a subtle (10%) reduction in glutamatergic synaptic transmission between the ADN and the NTS neurone due to the frequency-dependent depression. Although extrapolating the data from measuring glutamatergic synaptic transmission (previous study) to the present study measuring IPSCs is speculative, it provides an example of the potential impact of a subtle reduction in IPSC amplitude recorded in an NTS baroreceptor neurone in the whole animal.
In order to maximize the detection of the effect of the mGluRs, we used the glutamate uptake inhibitor PDC to increase the amount of glutamate in the synaptic cleft. The degree of inhibition of the GABA eIPSC was increased from 10 to 25%. Regardless of whether PDC was used, the mGluR antagonist abolished the inhibition of the GABA eIPSC following TS stimulation.
The amount of glutamate in the cleft available to spill over to activate mGluR on GABA terminals might be expected to be regulated by surrounding glia. The extent to which the glia mop up the glutamate depends on the frequency of neuronal activity. Studies in other networks indicate that glutamate transporters located on glia can clear glutamate diffused from synaptic cleft during low neuronal activity (Bergles & Jahr, 1997; Lehre & Danbolt, 1998; Ventura & Harris, 1999). However, at the slightly higher frequencies, such as observed in the present study, the transporters are not sufficient to clear the glutamate, therefore resulting in ‘glutamate spillover’ (Mitchell & Silver, 2000; Semyanov & Kullmann, 2000; Arnth-Jensen et al. 2002; Piet et al. 2003, 2004). In the present study, the finding that the mGluR antagonist prevented the effect of endogenous glutamate on the GABA eIPSCs is consistent with the hypothesis that there was sufficient glutamate to activate the mGluRs.
To establish whether activation of the Group II mGluRs depressed GABA transmission by a presynaptic mechanism, we determined the effect of the mGluR agonist l-CCG-I on the amplitude of the NTS-evoked GABA IPSCs in conjunction with the paired-pulse ratio of two consecutively evoked IPSCs, and on the frequency and amplitude of sIPSCs and mIPSCs. l-CCG-I produced a concentration-dependent inhibition of the peak amplitude of the GABA eIPSC in conjunction with an increase in the paired-pulse ratio, evidence that the inhibition was mediated by activation of presynaptic mGluRs (Debanne et al. 1996; Saitow et al. 2000; Jang et al. 2001; Morisset & Urban, 2001; Kato & Shigetomi, 2001; Behr et al. 2002; Dietrich et al. 2002; Kerchner & Zhuo, 2002; Kirischuk et al. 2002; Kline et al. 2002; Saviane et al. 2002; Hjelmstad & Fields, 2003; Jeong et al. 2003; Kombian et al. 2003; Sekizawa et al. 2003). Had the mGluR agonist acted postsynaptically to decrease GABA responsiveness of the baroreceptor neurone, then the amplitudes of the first and second eIPSCs would have decreased to the same extent resulting in an unchanged paired-pulse ratio (Henneberger et al. 2002; Chang et al. 2003; Scheiderer et al. 2004).
The presynaptic locus was further confirmed by the findings that the frequencies of the sIPSCs and mIPSCs were decreased, while the amplitudes remained unchanged (Koyama et al. 1999, 2000, 2002; Glitsch & Marty, 1999; Cooper & Stanford, 2001; Iyadomi et al. 2000; Kishimoto et al. 2001; Doi et al. 2002; Kerchner & Zhuo, 2002; Jeong et al. 2003; Lim et al. 2003; Mtchedlishvili & Kapur, 2003; Ziskind-Conhaim et al. 2003; Huang & Bordey, 2004). Had the agonist acted at postsynaptic mGluRs to decrease GABA transmission, then the amplitudes rather than frequencies of the sIPSCs and mIPSCs would have decreased (Henneberger et al. 2002), and the holding current would have changed (Brussaard et al. 1996; Wardle & Poo, 2003). The demonstration that mIPSC frequency decreased the same as the sIPSC frequency suggests that the agonist depressed the GABA release mainly by activation of the mGluRs located on the terminals rather than preterminal axons or cell bodies (Banks et al. 2002).
Taken together, the findings are consistent with the hypothesis that glutamate spillover from sensory afferent fibres activates presynaptic Group II mGluRs on nearby GABA terminals to suppress GABA release at second-order baroreceptor neurones. A number of mechanisms have been shown to fine-tune baroreceptor signal output of NTS neurones (Chiba & Kato, 1978; Glaum & Miller, 1993; Liu et al. 1998; Zhang & Mifflin, 1998; Kato & Shigetomi, 2001; Chen et al. 2002; Kline et al. 2002; Seagard et al. 2003). The distinctive feature of the glutamate–mGluR–GABA interaction is that the glutamate, through activation of mGluRs, can regulate not only its own release, but also that of a major inhibitory input. The question is how are these opposing effects integrated to shape the NTS output of the baroreceptor signal. At the very least, the opposing effects provide a flexible mechanism for glutamate regulation of baroreceptor signalling, such that depending on other synaptic inputs or intrinsic excitability of the neurone, the excitability could be enhanced (by greater inhibition of GABA) or blunted (by greater inhibition of glutamate). The opposing mechanisms might also provide a means of enhancing the storage capacity of the network and preventing neurones from becoming overactive and energy depleted (Vogt & Nicoll, 1999; Paton et al. 2001). Finally, they could boost transmission of excitatory information while increasing the signal-to-noise (depression of glutamate release) during higher baroreceptor inputs (Chen et al. 1999; Piet et al. 2004). In any event, these opposing effects appear to be an important characteristic in glutamate transmission, inasmuch as they have been demonstrated in other neural networks. (Bonci et al. 1997; Schrader & Tasker, 1997; Wittmann et al. 2001; Matsui & Kita, 2003).
In conclusion, the findings present new evidence suggesting that glutamate released at the first central baroreceptor synapses can not only regulate its own signalling, but can further shape baroreceptor signal transmission by suppressing GABA release. While regulation of glutamate release by activation of presynaptic autoreceptors on glutamatergic terminals has been demonstrated throughout the CNS, the heterosynaptic regulation of GABA release appears to be much less common, possibly because of the requirement for an appropriate configuration of GABA and glutamate terminals. The results underscore the complexity and flexibility of mGluR modulation of baroreceptor signalling in the NTS, and may help to explain the excitatory contribution of mGluRs as determined by in vivo microinjection studies. The challenge will be to build on these studies to determine when and where suppression of glutamate or GABA dominates the NTS output.
Acknowledgments
This study was supported by National Heart, Lung, and Blood Institute grant HL-60560. The authors gratefully acknowledge Dr John Horowitz for helpful criticisms in preparing the manuscript.
References
- Arnth-Jensen N, Jabaudon D, Scanziani M. Cooperation between independent hippocampal synapses is controlled by glutamate uptake. Nat Neurosci. 2002;5:325–331. doi: 10.1038/nn825. 10.1038/nn825. [DOI] [PubMed] [Google Scholar]
- Banks MI, Hardie JB, Pearce RA. Development of GABA(A) receptor-mediated inhibitory postsynaptic currents in hippocampus. J Neurophysiol. 2002;88:3097–3107. doi: 10.1152/jn.00026.2002. [DOI] [PubMed] [Google Scholar]
- Behr J, Gebhardt C, Heinemann U, Mody I. Kindling enhances kainate receptor-mediated depression of GABAergic inhibition in rat granule cells. Eur J Neurosci. 2002;16:861–867. doi: 10.1046/j.1460-9568.2002.02152.x. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Jahr CE. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron. 1997;19:1297–1308. doi: 10.1016/s0896-6273(00)80420-1. [DOI] [PubMed] [Google Scholar]
- Bonci A, Grillner P, Siniscalchi A, Mercuri NB, Bernardi G. Glutamate metabotropic receptor agonists depress excitatory and inhibitory transmission on rat mesencephalic principal neurons. Eur J Neurosci. 1997;9:2359–2369. doi: 10.1111/j.1460-9568.1997.tb01653.x. [DOI] [PubMed] [Google Scholar]
- Bonham AC, Chen CY. Glutamatergic neural transmission in the nucleus tractus solitarius: N-methyl-d-aspartate receptors. Clin Exp Pharmacol Physiol. 2002;29:497–502. doi: 10.1046/j.1440-1681.2002.03662.x. [DOI] [PubMed] [Google Scholar]
- Brussaard AB, Kits KS, de Vlieger TA. Postsynaptic mechanism of depression of GABAergic synapses by oxytocin in the supraoptic nucleus of immature rat. J Physiol. 1996;497:495–507. doi: 10.1113/jphysiol.1996.sp021783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher WJ, Kasparov S, Paton FJ. Differential effects of apamin on neuronal excitability in the nucleus tractus solitarii of rats studied in vitro. J Auton Nerv Syst. 1999;77:90–97. [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Chang EH, Kotak VC, Sanes DH. Long-term depression of synaptic inhibition is expressed postsynaptically in the developing auditory system. J Neurophysiol. 2003;90:1479–1488. doi: 10.1152/jn.00386.2003. [DOI] [PubMed] [Google Scholar]
- Chen C-Y, Horowitz JM, Bonham AC. A presynaptic mechanism contributes to depression of autonomic signal transmission in NTS. Am J Physiol Heart Circ Physiol. 1999;277:H1350–1360. doi: 10.1152/ajpheart.1999.277.4.H1350. [DOI] [PubMed] [Google Scholar]
- Chen C-Y, Ling E, Horowitz JM, Bonham AC. Synaptic transmission in nucleus tractus solitarius is depressed by Group II and III but not Group I presynaptic metabotropic glutamate receptors in rats. J Physiol. 2002;538:773–786. doi: 10.1113/jphysiol.2001.012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T, Kato M. Synaptic structures and quantification of catecholaminergic axons in the nucleus tractus solitarius of the rat: possible modulatory roles of catecholamines in baroreceptor reflexes. Brain Res. 1978;151:323–338. doi: 10.1016/0006-8993(78)90888-0. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin J-P. Pharmacology and functions of metabotropic glutamate receptors. Ann Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Cooper AJ, Stanford IM. Dopamine D2 receptor mediated presynaptic inhibition of striatopallidal GABA(A) IPSCs in vitro. Neuropharmacol. 2001;41:62–71. doi: 10.1016/s0028-3908(01)00038-7. [DOI] [PubMed] [Google Scholar]
- De Paula PM, Castania JA, Bonagamba LG, Salgado HC, Machado BH. Hemodynamic responses to electrical stimulation of the aortic depressor nerve in awake rats. Am J Physiol Regul Integr Comp Physiol. 1999;277:R31–38. doi: 10.1152/ajpregu.1999.277.1.R31. [DOI] [PubMed] [Google Scholar]
- Debanne D, Guérineau NC, Gähwiler BH, Thompson SM. Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release. J Physiol. 1996;491:163–176. doi: 10.1113/jphysiol.1996.sp021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich D, Kral T, Clusmann H, Friedl M, Schramm J. Presynaptic group II metabotropic glutamate receptors reduce stimulated and spontaneous transmitter release in human dentate gyrus. Neuropharmacology. 2002;42:297–305. doi: 10.1016/s0028-3908(01)00193-9. 10.1016/S0028-3908(01)00193-9. [DOI] [PubMed] [Google Scholar]
- Doi A, Ishibashi H, Jinno S, Kosaka T, Akaike N. Presynaptic inhibition of GABAergic miniature currents by metabotropic glutamate receptor in the rat CNS. Neuroscience. 2002;109:299–311. doi: 10.1016/s0306-4522(01)00484-5. 10.1016/S0306-4522(01)00484-5. [DOI] [PubMed] [Google Scholar]
- Edwards FA, Konnerth A, Sakmann B. Quantal analysis of inhibitory synaptic transmission in the dentate gyrus of rat hippocampal slices: a patch-clamp study. J Physiol. 1990;430:213–249. doi: 10.1113/jphysiol.1990.sp018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder RB, Mifflin SW. Modulation of carotid sinus afferent input to nucleus tractus solitarius by parabrachial nucleus stimulation. Circ Res. 1988;63:35–49. doi: 10.1161/01.res.63.1.35. [DOI] [PubMed] [Google Scholar]
- Foley CM, Moffitt JA, Hay M, Hasser EM. Glutamate in the nucleus of the solitary tract activates both ionotropic and metabotropic glutamate receptors. Am J Physiol Regul Integr Comp Physiol. 1998;275:R1858–1866. doi: 10.1152/ajpregu.1998.275.6.R1858. [DOI] [PubMed] [Google Scholar]
- Foley CM, Vogl HW, Mueller PJ, Hay M, Hasser EM. Cardiovascular response to group I metabotropic glutamate receptor activation in NTS. Am J Physiol Regul Integr Comp Physiol. 1999;276:R1469–1478. doi: 10.1152/ajpregu.1999.276.5.R1469. [DOI] [PubMed] [Google Scholar]
- Glaum SR, Miller RJ. Metabotropic glutamate receptors depress afferent excitatory transmission in the rat nucleus tractus solitarii. J Neurophysiol. 1993;70:2669–2672. doi: 10.1152/jn.1993.70.6.2669. [DOI] [PubMed] [Google Scholar]
- Glitsch M, Marty A. Presynaptic effects of NMDA in cerebellar Purkinje cells and interneurons. J Neurosci. 1999;19:511–519. doi: 10.1523/JNEUROSCI.19-02-00511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon FJ, Sved AF. Neurotransmitters in central cardiovascular regulation: glutamate and GABA. Clin Exp Pharmacol Physiol. 2002;29:522–524. doi: 10.1046/j.1440-1681.2002.03666.x. 10.1046/j.1440-1681.2002.03666.x. [DOI] [PubMed] [Google Scholar]
- Grabauskas G, Bradley RM. Synaptic interactions due to convergent input from gustatory afferent fibers in the rostral nucleus of the solitary tract. J Neurophysiol. 1996;76:2919–2927. doi: 10.1152/jn.1996.76.5.2919. [DOI] [PubMed] [Google Scholar]
- Grabauskas G, Bradley RM. Frequency-dependent properties of inhibitory synapses in the rostral nucleus of the solitary tract. J Neurophysiol. 2003;89:199–211. doi: 10.1152/jn.00963.2001. [DOI] [PubMed] [Google Scholar]
- Hay M, Hasser EM. Measurement of synaptic vesicle exocytosis in aortic baroreceptor neurons. Am J Physiol Heart Circ Physiol. 1998;275:H710–716. doi: 10.1152/ajpheart.1998.275.2.H710. [DOI] [PubMed] [Google Scholar]
- Henneberger C, Juttner R, Rothe T, Grantyn R. Postsynaptic action of BDNF on GABAergic synaptic transmission in the superficial layers of the mouse superior colliculus. J Neurophysiol. 2002;88:595–603. doi: 10.1152/jn.2002.88.2.595. [DOI] [PubMed] [Google Scholar]
- Hines T, Toney GM, Mifflin SW. Responses of neurons in the nucleus tractus solitarius to stimulation of heart and lung receptors in the rat. Circ Res. 1994;74:1188–1196. doi: 10.1161/01.res.74.6.1188. [DOI] [PubMed] [Google Scholar]
- Hjelmstad GO, Fields HL. Kappa opioid receptor activation in the nucleus accumbens inhibits glutamate and GABA release through different mechanisms. J Neurophysiol. 2003;89:2389–2395. doi: 10.1152/jn.01115.2002. [DOI] [PubMed] [Google Scholar]
- Huang H, Bordey A. Glial glutamate transporters limit spillover activation of presynaptic NMDA receptors and influence synaptic inhibition of Purkinje neurons. J Neurosci. 2004;24:5659–5669. doi: 10.1523/JNEUROSCI.1338-04.2004. 10.1523/JNEUROSCI.1338-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyadomi M, Iyadomi I, Kumamoto E, Tomokuni K, Yoshimura M. Presynaptic inhibition by baclofen of miniature EPSCs and IPSCs in substantia gelatinosa neurons of the adult rat spinal dorsal horn. Pain. 2000;85:385–393. doi: 10.1016/S0304-3959(99)00285-7. 10.1016/S0304-3959(99)00285-7. [DOI] [PubMed] [Google Scholar]
- Jang IS, Rhee JS, Watanabe T, Akaike N, Akaike N. Histaminergic modulation of GABAergic transmission in rat ventromedial hypothalamic neurones. J Physiol. 2001;534:791–803. doi: 10.1111/j.1469-7793.2001.00791.x. 10.1111/j.1469-7793.2001.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong HJ, Jang IS, Nabekura J, Akaike N. Adenosine A1 receptor-mediated presynaptic inhibition of GABAergic transmission in immature rat hippocampal CA1 neurons. J Neurophysiol. 2003;89:1214–1222. doi: 10.1152/jn.00516.2002. [DOI] [PubMed] [Google Scholar]
- Jones NM, Beart PM, Monn JA, Widdop RE. Type I and II metabotropic glutamate receptors mediate depressor and bradycardic actions in the nucleus of the solitary tract of anaesthetized rats. Eur J Pharmacol. 1999;380:129–135. doi: 10.1016/s0014-2999(99)00518-x. 10.1016/S0014-2999(99)00518-X. [DOI] [PubMed] [Google Scholar]
- Kato F, Shigetomi E. Distinct modulation of evoked and spontaneous EPSCs by purinoceptors in the nucleus tractus solitarii of the rat. J Physiol. 2001;530:469–486. doi: 10.1111/j.1469-7793.2001.0469k.x. 10.1111/j.1469-7793.2001.0469k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner GA, Zhuo M. Presynaptic suppression of dorsal horn inhibitory transmission by mu-opioid receptors. J Neurophysiol. 2002;88:520–522. doi: 10.1152/jn.2002.88.1.520. [DOI] [PubMed] [Google Scholar]
- Kingston AE, Ornstein PL, Wright RA, Johnson BG, Mayne NG, Burnett JP, Belagaje R, Wu S, Schoepp DD. LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors. Neuropharmacology. 1998;37:1–12. doi: 10.1016/s0028-3908(97)00191-3. 10.1016/S0028-3908(97)00191-3. [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Clements JD, Grantyn R. Presynaptic and postsynaptic mechanisms underlie paired pulse depression at single GABAergic boutons in rat collicular cultures. J Physiol. 2002;543:99–116. doi: 10.1113/jphysiol.2002.021576. 10.1113/jphysiol.2002.021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto K, Koyama S, Akaike N. Synergistic mu-opioid and 5-HT1A presynaptic inhibition of GABA release in rat periaqueductal gray neurons. Neuropharmacology. 2001;41:529–538. doi: 10.1016/s0028-3908(01)00100-9. 10.1016/S0028-3908(01)00100-9. [DOI] [PubMed] [Google Scholar]
- Kline DD, Takacs KN, Ficker E, Kunze DL. Dopamine modulates synaptic transmission in the nucleus of the solitary tract. J Neurophysiol. 2002;88:2736–2744. doi: 10.1152/jn.00224.2002. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Cheng ZB, Tanaka K, Nosaka S. Is the aortic depressor nerve involved in arterial chemoreflexes in rats? J Auton Nerv Syst. 1999;78:38–48. doi: 10.1016/s0165-1838(99)00054-5. 10.1016/S0165-1838(99)00054-5. [DOI] [PubMed] [Google Scholar]
- Kombian SB, Ananthalakshmi KV, Parvathy SS, Matowe WC. Dopamine and adenosine mediate substance P-induced depression of evoked IPSCs in the rat nucleus accumbens in vitro. Eur J Neurosci. 2003;18:303–311. doi: 10.1046/j.1460-9568.2003.02753.x. 10.1046/j.1460-9568.2003.02753.x. [DOI] [PubMed] [Google Scholar]
- Koyama S, Kubo C, Rhee JS, Akaike N. Presynaptic serotonergic inhibition of GABAergic synaptic transmission in mechanically dissociated rat basolateral amygdala neurons. J Physiol. 1999;518:525–538. doi: 10.1111/j.1469-7793.1999.0525p.x. 10.1111/j.1469-7793.1999.0525p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S, Matsumoto N, Kubo C, Akaike N. Presynaptic 5-HT3 receptor-mediated modulation of synaptic GABA release in the mechanically dissociated rat amygdala neurons. J Physiol. 2000;529:373–383. doi: 10.1111/j.1469-7793.2000.00373.x. 10.1111/j.1469-7793.2000.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S, Matsumoto N, Murakami N, Kubo C, Nabekura J, Akaike N. Role of presynaptic 5-HT1A and 5-HT3 receptors in modulation of synaptic GABA transmission in dissociated rat basolateral amygdala neurons. Life Sci. 2002;72:375–387. doi: 10.1016/s0024-3205(02)02280-4. 10.1016/S0024-3205(02)02280-4. [DOI] [PubMed] [Google Scholar]
- Lehre KP, Danbolt NC. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J Neurosci. 1998;18:8751–8757. doi: 10.1523/JNEUROSCI.18-21-08751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R, Oleskevich S, Few AP, Leao RN, Walmsley B. Glycinergic mIPSCs in mouse and rat brainstem auditory nuclei: modulation by ruthenium red and the role of calcium stores. J Physiol. 2003;546:691–699. doi: 10.1113/jphysiol.2002.035071. 10.1113/jphysiol.2002.035071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Chen C-Y, Bonham AC. Metabotropic glutamate receptors depress vagal and aortic baroreceptor signal transmission in the NTS. Am J Physiol Heart Circ Physiol. 1998;275:H1682–1694. doi: 10.1152/ajpheart.1998.275.5.H1682. [DOI] [PubMed] [Google Scholar]
- Liu Z, Chen C-Y, Bonham AC. Frequency limits on aortic baroreceptor input to nucleus tractus solitarii. Am J Physiol Heart Circ Physiol. 2000;278:H577–585. doi: 10.1152/ajpheart.2000.278.2.H577. [DOI] [PubMed] [Google Scholar]
- Loewy AD. Central autonomic pathways. In: Loewy AD, Spyer KM, editors. Central Regulation of Autonomic Functions. New York: Oxford University Press; 1990. pp. 88–103. [Google Scholar]
- Loewy AD, McKellar S. The neuroanatomical basis of central cardiovascular control. Fed Proc. 1980;39:2495–2503. [PubMed] [Google Scholar]
- Maqbool A, Batten TF, McWilliam PN. Ultrastructural relationships between GABAergic terminals and cardiac vagal preganglionic motoneurons and vagal afferents in the cat: a combined HRP tracing and immunogold labelling study. Eur J Neurosci. 1991;3:501–513. doi: 10.1111/j.1460-9568.1991.tb00837.x. [DOI] [PubMed] [Google Scholar]
- Matsui T, Kita H. Activation of group III metabotropic glutamate receptors presynaptically reduces both GABAergic and glutamatergic transmission in the rat globus pallidus. Neuroscience. 2003;122:727–737. doi: 10.1016/j.neuroscience.2003.08.032. 10.1016/j.neuroscience.2003.08.032. [DOI] [PubMed] [Google Scholar]
- Matsumura K, Tsuchihashi T, Kagiyama S, Abe I, Fujishima M. Subtypes of metabotropic glutamate receptors in the nucleus of the solitary tract of rats. Brain Res. 1999;842:461–468. doi: 10.1016/s0006-8993(99)01889-2. 10.1016/S0006-8993(99)01889-2. [DOI] [PubMed] [Google Scholar]
- Mendelowitz D, Yang M, Andresen MC, Kunze DL. Localization and retention in vitro of fluorescently labeled aortic baroreceptor terminals on neurons from the nucleus tractus solitarius. Brain Res. 1992;581:339–343. doi: 10.1016/0006-8993(92)90729-s. 10.1016/0006-8993(92)90729-S. [DOI] [PubMed] [Google Scholar]
- Mifflin SW. Inhibition of chemoreceptor inputs to nucleus of tractus solitarius neurons during baroreceptor stimulation. Am J Physiol Regul Integr Comp Physiol. 1993;265:R14–20. doi: 10.1152/ajpregu.1993.265.1.R14. [DOI] [PubMed] [Google Scholar]
- Mifflin SW. Convergent carotid sinus nerve and superior laryngeal nerve afferent inputs to neurons in the NTS. Am J Physiol Regul Integr Comp Physiol. 1996;271:R870–880. doi: 10.1152/ajpregu.1996.271.4.R870. [DOI] [PubMed] [Google Scholar]
- Mifflin SW. What does the brain know about blood pressure? News Physiol Sci. 2001;16:266–271. doi: 10.1152/physiologyonline.2001.16.6.266. ? [DOI] [PubMed] [Google Scholar]
- Mifflin SW, Spyer KM, Withington-Wray DJ. Baroreceptor inputs to the nucleus tractus solitarius in the cat: postsynaptic actions and the influence of respiration. J Physiol. 1988;399:349–367. doi: 10.1113/jphysiol.1988.sp017085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. Glutamate spillover suppresses inhibition by activating presynaptic mGluRs. Nature. 2000;404:498–502. doi: 10.1038/35006649. 10.1038/35006649. [DOI] [PubMed] [Google Scholar]
- Morisset V, Urban L. Cannabinoid-induced presynaptic inhibition of glutamatergic EPSCs in substantia gelatinosa neurons of the rat spinal cord. J Neurophysiol. 2001;86:40–48. doi: 10.1152/jn.2001.86.1.40. [DOI] [PubMed] [Google Scholar]
- Mtchedlishvili Z, Kapur J. A presynaptic action of the neurosteroid pregnenolone sulfate on GABAergic synaptic transmission. Mol Pharmacol. 2003;64:857–864. doi: 10.1124/mol.64.4.857. 10.1124/mol.64.4.857. [DOI] [PubMed] [Google Scholar]
- Pampillo M, Scimonelli T, Duvilanski BH, Celis ME, Seilicovich A, Lasaga M. The activation of metabotropic glutamate receptors differentially affects GABA and alpha-melanocyte stimulating hormone release from the hypothalamus and the posterior pituitary of male rats. Neurosci Lett. 2002;327:95–98. doi: 10.1016/s0304-3940(02)00386-5. 10.1016/S0304-3940(02)00386-5. [DOI] [PubMed] [Google Scholar]
- Paton JF, Li YW, Schwaber JS. Response properties of baroreceptive NTS neurons. Ann N Y Acad Sci. 2001;940:157–168. doi: 10.1111/j.1749-6632.2001.tb03674.x. [DOI] [PubMed] [Google Scholar]
- Pawloski-Dahm C, Gordon FJ. Evidence for a kynurenate-insensitive glutamate receptor in nucleus tractus solitarii. Am J Physiol Heart Circ Physiol. 1992;262:H1611–1615. doi: 10.1152/ajpheart.1992.262.5.H1611. [DOI] [PubMed] [Google Scholar]
- Perrone MH. Biochemical evidence that l-glutamate is a neurotransmitter of primary vagal afferent nerve fibers. Brain Res. 1981;230:283–293. doi: 10.1016/0006-8993(81)90407-8. 10.1016/0006-8993(81)90407-8. [DOI] [PubMed] [Google Scholar]
- Piet R, Bonhomme R, Theodosis DT, Poulain DA, Oliet SH. Modulation of GABAergic transmission by endogenous glutamate in the rat supraoptic nucleus. Eur J Neurosci. 2003;17:1777–1785. doi: 10.1046/j.1460-9568.2003.02611.x. 10.1046/j.1460-9568.2003.02611.x. [DOI] [PubMed] [Google Scholar]
- Piet R, Vargova L, Sykova E, Poulain DA, Oliet SH. Physiological contribution of the astrocytic environment of neurons to intersynaptic crosstalk. Proc Natl Acad Sci U S A. 2004;101:2151–2155. doi: 10.1073/pnas.0308408100. 10.1073/pnas.0308408100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin J-P, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. 10.1016/0028-3908(94)00129-G. [DOI] [PubMed] [Google Scholar]
- Poncer JC, McKinney RA, Gahwiler BH, Thompson SM. Differential control of GABA release at synapses from distinct interneurons in rat hippocampus. J Physiol. 2000;528:123–130. doi: 10.1111/j.1469-7793.2000.00123.x. 10.1111/j.1469-7793.2000.00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncer JC, Shinozaki H, Miles R. Dual modulation of synaptic inhibition by distinct metabotropic glutamate receptors in the rat hippocampus. J Physiol. 1995;485:121–134. doi: 10.1113/jphysiol.1995.sp020717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitow F, Satake S, Yamada J, Konishi S. beta-adrenergic receptor-mediated presynaptic facilitation of inhibitory GABAergic transmission at cerebellar interneuron-Purkinje cell synapses. J Neurophysiol. 2000;84:2016–2025. doi: 10.1152/jn.2000.84.4.2016. [DOI] [PubMed] [Google Scholar]
- Saransaari P, Oja SS. Metabotropic glutamate receptors modulate GABA release from mouse hippocampal slices. Neurochem Res. 2001;26:175–180. doi: 10.1023/a:1011055014357. 10.1023/A:1011055014357. [DOI] [PubMed] [Google Scholar]
- Saransaari P, Oja SS. Metabotropic glutamate receptors modulate ischemia-induced GABA release in mouse hippocampal slices. Neurochem Res. 2004;29:1511–1518. doi: 10.1023/b:nere.0000029563.94579.f6. 10.1023/B:NERE.0000029563.94579.f6. [DOI] [PubMed] [Google Scholar]
- Saviane C, Savtchenko LP, Raffaelli G, Voronin LL, Cherubini E. Frequency-dependent shift from paired-pulse facilitation to paired-pulse depression at unitary CA3-CA3 synapses in the rat hippocampus. J Physiol. 2002;544:469–476. doi: 10.1113/jphysiol.2002.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanziani M, Salin PA, Vogt KE, Malenka RC, Nicoll RA. Use-dependent increases in glutamate concentration activate presynaptic metabotropic glutamate receptors. Nature. 1997;385:630–634. doi: 10.1038/385630a0. 10.1038/385630a0. [DOI] [PubMed] [Google Scholar]
- Scheiderer CL, Dobrunz LE, McMahon LL. Novel form of long-term synaptic depression in rat hippocampus induced by activation of alpha1 adrenergic receptors. J Neurophysiol. 2004;91:1071–1077. doi: 10.1152/jn.00420.2003. 10.1152/jn.00420.2003. [DOI] [PubMed] [Google Scholar]
- Schrader LA, Tasker JG. Presynaptic modulation by metabotropic glutamate receptors of excitatory and inhibitory synaptic inputs to hypothalamic magnocellular neurons. J Neurophysiol. 1997;77:527–536. doi: 10.1152/jn.1997.77.2.527. [DOI] [PubMed] [Google Scholar]
- Seagard JL, Dean C, Hopp FA. Activity-dependent role of NMDA receptors in transmission of cardiac mechanoreceptor input to the NTS. Am J Physiol Heart Circ Physiol. 2003;284:H884–891. doi: 10.1152/ajpheart.00601.2002. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Gorelova N, Durstewitz D, Yang CR. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J Neurosci. 2001;21:3628–3638. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekizawa S, Joad JP, Bonham AC. Substance P presynaptically depresses the transmission of sensory input to bronchopulmonary neurons in the guinea pig nucleus tractus solitarii. J Physiol. 2003;552:547–559. doi: 10.1113/jphysiol.2003.051326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Kullmann DM. Modulation of GABAergic signaling among interneurons by metabotropic glutamate receptors. Neuron. 2000;25:663–672. doi: 10.1016/s0896-6273(00)81068-5. 10.1016/S0896-6273(00)81068-5. [DOI] [PubMed] [Google Scholar]
- Silva-Carvalho L, Paton JFR, Rocha I, Goldsmith GE, Spyer KM. Convergence properties of solitary tract neurons responsive to cardiac receptor stimulation in the anesthetized cat. J Neurophysiol. 1998;79:2374–2382. doi: 10.1152/jn.1998.79.5.2374. [DOI] [PubMed] [Google Scholar]
- Smith BN, Dou P, Barber WD, Dudek FE. Vagally evoked synaptic currents in the immature rat nucleus tractus solitarii in an intact in vitro preparation. J Physiol. 1998;512:149–162. doi: 10.1111/j.1469-7793.1998.149bf.x. 10.1111/j.1469-7793.1998.149bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyer KM. The central nervous organization of reflex circulatory control. In: Loewy AD, Spyer KM, editors. Central Regulation of Autonomic Functions. New York: Oxford University Press; 1990. pp. 168–188. [Google Scholar]
- Suzdak PD, Thomsen C, Mulvihill E, Kristensen P. Molecular cloning, expression, and characterization of metabotropic glutamate receptor subtypes. In: Conn PJ, Patel J, editors. The Metabotropic Glutamate Receptors. Totowa, NJ, USA: Humana Press Inc; 1994. pp. 1–30. [Google Scholar]
- Takagi S, Umezaki T, Shin T. Convergence of laryngeal afferents with different natures upon cat NTS neurons. Brain Res Bull. 1995;38:261–268. doi: 10.1016/0361-9230(95)00098-y. 10.1016/0361-9230(95)00098-Y. [DOI] [PubMed] [Google Scholar]
- Talman WT, Perrone MH, Reis DJ. Evidence for l-glutamate as the neurotransmitter of primary baroreceptor afferent nerve fibers. Science. 1980;290:813–815. doi: 10.1126/science.6105709. [DOI] [PubMed] [Google Scholar]
- Thoren P, Jones JV. Characteristics of aortic baroreceptor C-fibres in the rabbit. Acta Physiol Scand. 1977;99:448–456. doi: 10.1111/j.1748-1716.1977.tb10397.x. [DOI] [PubMed] [Google Scholar]
- Thoren P, Munch PA, Brown AM. Mechanisms for activation of aortic baroreceptor C-fibres in rabbits and rats. Acta Physiol Scand. 1999;166:167–174. doi: 10.1046/j.1365-201x.1999.00556.x. 10.1046/j.1365-201x.1999.00556.x. [DOI] [PubMed] [Google Scholar]
- Toney GM, Mifflin SW. Time-dependent inhibition of hindlimb somatic afferent inputs to nucleus tractus solitarius. J Neurophysiol. 1994;72:63–71. doi: 10.1152/jn.1994.72.1.63. [DOI] [PubMed] [Google Scholar]
- Ventura R, Harris KM. Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci. 1999;19:6897–6906. doi: 10.1523/JNEUROSCI.19-16-06897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viard E, Sapru HN. Cardiovascular responses to activation of metabotropic glutamate receptors in the nTS of the rat. Brain Res. 2002;952:308–321. doi: 10.1016/s0006-8993(02)03260-2. 10.1016/S0006-8993(02)03260-2. [DOI] [PubMed] [Google Scholar]
- Vogt KE, Nicoll RA. Glutamate and gamma-aminobutyric acid mediate a heterosynaptic depression at mossy fiber synapses in the hippocampus. Proc Natl Acad Sci U S A. 1999;96:1118–1122. doi: 10.1073/pnas.96.3.1118. 10.1073/pnas.96.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle RA, Poo MM. Brain-derived neurotrophic factor modulation of GABAergic synapses by postsynaptic regulation of chloride transport. J Neurosci. 2003;23:8722–8732. doi: 10.1523/JNEUROSCI.23-25-08722.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Marino MJ, Bradley SR, Conn PJ. Activation of group III mGluRs inhibits GABAergic and glutamatergic transmission in the substantia nigra pars reticulata. J Neurophysiol. 2001;85:1960–1968. doi: 10.1152/jn.2001.85.5.1960. [DOI] [PubMed] [Google Scholar]
- Woodhall G, Evans DI, Jones RS. Activation of presynaptic group III metabotropic glutamate receptors depresses spontaneous inhibition in layer V of the rat entorhinal cortex. Neuroscience. 2001;105:71–78. doi: 10.1016/s0306-4522(01)00178-6. 10.1016/S0306-4522(01)00178-6. [DOI] [PubMed] [Google Scholar]
- Zhang W, Mifflin SW. Excitatory amino-acid receptors contribute to carotid sinus and vagus nerve evoked excitation of neurons in the nucleus of the tractus solitarius. J Auton Nerv Syst. 1995;55:50–56. doi: 10.1016/0165-1838(95)00027-u. 10.1016/0165-1838(95)00027-U. [DOI] [PubMed] [Google Scholar]
- Zhang J, Mifflin SW. Differential roles for NMDA and non-NMDA receptor subtypes in baroreceptor afferent integration in the nucleus of the solitary tract of the rat. J Physiol. 1998;511:733–745. doi: 10.1111/j.1469-7793.1998.733bg.x. 10.1111/j.1469-7793.1998.733bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng F, Johnson SW. Dual modulation of gabaergic transmission by metabotropic glutamate receptors in rat ventral tegmental area. Neuroscience. 2003;119:453–460. doi: 10.1016/s0306-4522(03)00190-8. 10.1016/S0306-4522(03)00190-8. [DOI] [PubMed] [Google Scholar]
- Ziskind-Conhaim L, Gao BX, Hinckley C. Ethanol dual modulatory actions on spontaneous postsynaptic currents in spinal motoneurons. J Neurophysiol. 2003;89:806–813. doi: 10.1152/jn.00614.2002. [DOI] [PubMed] [Google Scholar]