Abstract
The nucleus of the solitary tract (NST) processes substantial visceral afferent input and sends divergent projections to a wide array of CNS targets. The NST is essential to the maintenance of behavioural and autonomic homeostasis and is the source, as well as the recipient, of considerable noradrenergic (NE) projections. The significance of NE projections from the NST to other CNS regions has long been appreciated, but the nature of NE action on NST neurones themselves, especially on the α-1 receptor subtype, is controversial. We used a combination of methodologies to establish, systematically, the effects and cellular basis of action of the α-1 agonist, phenylephrine (PHE), to control NST neurones responsible for vago-vagal reflex regulation of the stomach. Immunocytochemical and retrograde tracing studies verified that the area postrema, A2, A5, ventrolateral medulla and locus coeruleus regions are sources of catecholaminergic input to the NST. In vivo electrophysiological recordings showed that PHE activates physiologically identified, second-order gastric sensory NST neurones. In vivo microinjection of PHE onto NST neurones caused a significant reduction in gastric tone. Finally, in vitro calcium imaging studies revealed that PHE caused dramatic cytosolic calcium oscillations in NST neurones. These oscillations are probably the result of an interplay between agonist-induced and inositol 1,4,5-trisphosphate (IP3)-mediated intracellular calcium release and Ca2+-ATPase control of intracellular calcium storage pumps. The oscillations persisted even in perfusions of zero calcium–EGTA Krebs solution suggesting that the calcium oscillation is mediated principally by intracellular calcium release–reuptake mechanisms. Cyclical activation of the NST may function to increase the responsiveness of these neurones to incoming afferent input (i.e., elevate the ‘gain’). An increase in gain of afferent input may cause an amplification of the response part of the reflex and help explain the powerful effects that α-1 agonists have in suppressing gastric motility and producing anorexia.
The nucleus of the solitary tract (NST) is the principal CNS recipient of general and special visceral afferent signals from the periphery. In turn, the NST projects to a number of brainstem, diencephalic and forebrain sites implicated in the regulation of a wide variety of behavioural and autonomic functions (Blessing, 1997a). The NST forms the neural conduit through which visceral afferent events affect homeostasis. Consequently, other sources of control input to the NST can have dramatic effects on the functions regulated by the NST. Descending neural and circulating chemical inputs to the NST can modulate systematic responses or even mimic the effect of visceral afferent input itself (Butcher & Rogers, 1978; Rogers et al. 1996; Bertolino et al. 1997; Blessing, 1997a; Emch et al. 2000; Browning & Travagli, 2001; Travagli & Rogers, 2001; Browning et al. 2002).
The medial portion of the NST (mNST) receives a large volume of visceral afferent input from the vagus nerve relating to the control of gastrointestinal function. The medial NST integrates this input and uses it to control the process of digestion through connections with the source of vagal efferent control of the gut, the dorsal motor nucleus of the vagus (DMV) (Rogers & McCann, 1993; Blessing, 1997b; Travagli & Rogers, 2001). Ascending projections from the NST carrying copies of the processed visceral afferent data regulate a diverse range of physiological functions and behaviours that impact on the maintenance of the internal milieu (Sawchenko, 1983; Gillis et al. 1989; Berthoud & Neuhuber, 2000). While glutamatergic input from primary afferents provides the NST with much of its excitation (Talman et al. 1980; McCann & Rogers, 1994; Lin et al. 2000), these neurones are also the recipients of modulatory inputs arising from all levels of the neuraxis (Blessing, 1997a). Activation of the NST by visceral afferent input (Gillis et al. 1989; Travagli & Rogers, 2001), afferent input from other CNS regions (McCann et al. 1989; McCann & Rogers, 1990; Lewis et al. 2002; Travagli et al. 2003), circulating hormones (McTigue et al. 1993; Chen et al. 1997) and cytokines (Hermann et al. 1999; Emch et al. 2000; Hermann et al. 2004), regulates gastrointestinal motility by controlling vagal efferent projections to the stomach. Furthermore, agents that cause activation of the NST can produce gastric stasis, anorexia, nausea and emesis (Fukuda et al. 1998; Emch et al. 2000; Andrews & Sanger, 2002; Rinaman & Rothe, 2002; Rogers et al. 2003; Travagli et al. 2003).
The NST is both the source and recipient of CNS noradrenergic (NE) projections (Sawchenko & Swanson, 1982; Kalia et al. 1985; Blessing, 1997a). The anatomical description and physiological role of NE projections from the NST to other levels of the neuraxis including vagal motor neurones controlling gastric function (Rinaman, 2003; Rogers et al. 2003) and the hypothalamus is well accepted (Sawchenko & Swanson, 1982; Blessing, 1997a). In contrast, the role of NE input from other areas of the CNS to the NST is not as certain. For example, catecholaminergic (CA) projections from the locus coeruleus to the NST are reported to be relatively few (Cunningham & Sawchenko, 1989); however, the locus coeruleus is able to regulate NST responses to chemosensory afferents (Perez & Ruiz, 1995; Perez et al. 1998). The functional significance of input from the A2 and area postrema CA groups (Blessing, 1997a) to the NST has been provided by Rinaman (2003), who showed that elimination of this input significantly reduced the anorexia induced by peptides known to activate CA neurones.
There have been very few studies of the role of α-1 adrenoceptors in the control of processes regulated by the NST (Hayward, 2001; Hayward et al. 2002). Much of the interest in CA–NST mechanisms has centred on the role of α-2 receptors in the control of cardiovascular reflex transmission from NST to its projection targets (Dashwood et al. 1985; Feldman & Felder, 1989b; Hayward, 2001; Hayward et al. 2002); the presence and functionality of α-1 adrenoceptors is not as clear. Autoradiographic binding studies using relatively specific radiolabelled α-1 agonists (Young & Kuhar, 1980) and in situ hybridization studies (Dashwood et al. 1985; Day et al. 1997) provided conflicting evidence for the presence of the receptor in the solitary nucleus. Regarding the α-1 receptor, these different in vitro histochemical detection methods often produce results that are at odds with one another. Additionally, histochemical results are frequently at odds with results obtained using physiological and pharmacological techniques (Pieribone et al. 1994). This sort of gross mismatch between the demonstration of functionality and the demonstration of receptor localization suggests that the mere performance of the in vitro histochemical methods may alter either the RNA message for the α-1 receptor or the receptor itself such that it is rendered undetectable (Dashwood et al. 1985; Pieribone et al. 1994; Day et al. 1997). This situation is comparable to one we have encountered regarding the demonstration of nervous system receptors for the cytokine tumour necrosis factor (TNF)-α. Multiple physiological studies demonstrated the role of TNFα to modulate gastric function via actions within the dorsal vagal complex (e.g. Hermann & Rogers, 1995; Emch et al. 2001, 2002). Initial immunohistochemical (IHC) studies were unable to demonstrate the presence of TNFα receptors (also referred to as p55 receptors). However, the employment of a heat-induced antigen retrieval technique (Shi et al. 2001) allowed the IHC demonstration of TNF receptors on vagal afferents (Hermann et al. 2004). This heat process enables the exposure of receptors for antibody tagging by restoring the electrostatic forces of the antigen that may have been disrupted during the exposure to formalin-fixation (Shi et al. 2001). Omission of the heat-induced antigen-exposure step resulted in no antibody (TNF-α)-specific binding. Perhaps similar conditions are interfering with the demonstration of the α-1 receptor and/or its message.
Physiological studies suggest a role for α-1 receptors on NST neurones and the functions that they may serve (Feldman & Moises, 1988; Feldman & Felder, 1989b, a). Some studies have shown evidence for α-1 adrenoceptor inhibition of NST neurones (Feldman & Moises, 1988; Feldman & Felder 1989b, a). However, results recorded from a variety of other neurones and excitable cells implicate the α-1 adrenoceptor as a neuronal excitant (Fukuda et al. 1987; Berretta et al. 2000; Hayar et al. 2001; Ohliger-Frerking et al. 2002; Martinez-Pena et al. 2004). In vitro experiments (Fukuda et al. 1987; Martinez-Pena et al. 2004) show that α-1 agonists strongly and specifically activate neurones in the dorsal motor nucleus of the vagus. This mismatch of histochemical and physiological results suggests that neurones of the dorsal vagal complex may express a unique subtype of α-1 adrenoceptor that has not yet been identified histochemically by current methods.
The role of α-1 adrenergic effects in the NST and their importance to the control of gastric function through the vago-vagal reflex has not been investigated directly. However, a good circumstantial case could be made implicating NE projections to the NST utilizing excitatory α-1 receptors. α-1 adrenergic agonists such as phenylephrine (PHE) are potent anorectic drugs that may also cause emesis (Lora-Vilchis et al. 1988; Hikasa et al. 1992; Yeh, 1999; Berretta et al. 2000; Wellman et al. 2003). If centrally acting α-1 agonists can excite NST neurones, then the resulting excitation could produce gastric stasis, anorexia and nausea through mechanisms involving the NST (Hornby, 2001; Rinaman, 2003; Saito et al. 2003). Multiple CNS noradrenergic pathways with terminal fields in the NST have long been associated with the production of the behavioural and autonomic responses to stressors (Koob, 1999; Greenwood et al. 2003). Some of these responses to stress include gastric stasis, nausea, emesis and anorexia (Forbes et al. 1999; Li & Balint, 2000). Elimination of noradrenergic neurones providing input to the dorsal vagal complex blocks these effects (Rinaman, 2003). At present, there is no mechanistic connection between CA input to the NST, the activation of α-1 receptors, and changes in functions related to gastrointestinal control.
The aims of this study were to investigate: (1) the likely sources of catecholaminergic input to the medial NST; (2) the effects of brainstem microinjections of α-1 agonists on gastric function; and (3) the cellular basis of α-1 adrenergic agonist effects on NST neurones.
Methods
All rats (Long-Evans, Charles River Laboratories, Wilmington, MA, USA) used in these studies were maintained in a room with a 12–12 h light–dark cycle and constant temperature and humidity, and given food and water ad libitum. All experimental protocols were performed according to the guidelines set forth by the National Institutes of Health and were approved by the Institutional Animal Care and Use Committees at the Pennington Biomedical Research Center.
Retrograde tracing from the NST and tyrosine hydroxylase immunocytochemistry
Rats (n = 5) were anaesthetized with pentobarbital (50 mg kg−1; Nembutal, Abbott Laboratories, Chicago, IL, USA) and placed in a stereotaxic frame. Using aseptic technique, the scalp on the back of the head was opened and retracted. Opening the foramen magnum exposed the caudal portion of the floor of the fourth ventricle. Rhodamine-coated latex nanospheres (Lumafluor, Naples, FL, USA; 10 nl) were microinjected into the medial solitary nucleus (mNST; 300 μm lateral and anterior to calamus scriptorum and 350–400 μm ventral to the brainstem surface). Microinjections were made using tracer-filled glass micropipettes connected to a source of pulsed air pressure (Pneumatic Picopump, WPI, Sarasota, FL, USA). The volume of the injection was monitored by observing the meniscus of the fluid in the pipette with a 150 x microscope fitted with an eyepiece reticle calibrated in units of volume. The incision was closed with nylon sutures and the animal was returned to its home cage once it recovered from the anaesthesia. Time (5–7 days) was allowed for retrograde transport of the rhodamine-coated beads. At that time, animals were deeply anaesthetized with urethane (ethyl carbamate 1 g kg−1; i.p.; Sigma, St Louis, MO, USA) and transcardially perfused with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde in PBS. Brains were removed and stored in a solution of 4% paraformaldehyde and 20% sucrose overnight.
Brainstems were sectioned at 50 μm thickness on a freezing microtome. Tissue sections were processed for the fluorescence immunocytochemical (IHC) demonstration of tyrosine hydroxylase (TH). Briefly, primary antibody to rat TH (mouse anti-rat TH antibody; Immunostar, Hudson, WI, USA; dilution of 1 : 500) was visualized by the secondary, goat anti-mouse antibody (heavy chain of mouse IgG1; highly cross-absorbed) conjugated with Alexafluor 488 (Molecular Probes, Eugene, OR, USA; dilution of 1 : 200). Co-localization of retrograde rhodamine-coated dextran beads with CA cell groups (i.e., tyrosine hydroxylase-immunoreactive (TH-ir)) was evaluated with a Nikon E800 epifluorescence microscope or an Ultraview (Perkin Elmer, Wellesley, MA, USA) confocal illuminator fitted to a Nikon E600 microscope with a Hamamatsu Orca-ER CCD camera (Hamamatsu, Japan). Captured images were processed using Photoshop (Adobe) to adjust for uniform contrast and brightness. Occasionally, sections were also stained for the presence of glial fibrillary acidic protein (GFAP) to examine the morphology of astrocytes in the NST. Rabbit anti-rat GFAP polyclonal antibody (1 : 1000 dilution; Chemicon, Temecula, CA, USA) was visualized with Nova Red chromagen (Vector Laboratories, Burlingame, CA, USA). These immunohistochemical methods are described in detail in a previous paper (Hermann et al. 2001).
In vivo extracellular recordings
Rats were prepared for the electrophysiological recording and identification of gastric distension-related neurones in the NST as described in several previous reports (McCann & Rogers, 1992; Emch et al. 2000). Rats (n = 17) were deprived of food overnight and anaesthetized with thiobutabarbital (Inactin; 150 mg kg−1; Sigma). Via a midline laparotomy, a small latex balloon was placed in the gastric corpus through a purse-string suture in the pylorus. The balloon could be repeatedly inflated and deflated with 1–2 ml of air by syringe to produce mild gastric distensions. After the wound was closed, the animal was secured in a stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA). The scalp on the back of the head was opened, the musculature attached to the back of the occipital plate was retracted, the skull plate removed, and the floor of the fourth ventricle was exposed. A multibarrelled glass microinjection/carbon fibre electrode array (Carbostar, Kation Scientific, Minneapolis, MN, USA) was used to record extracellular potentials from the NST and inject drugs. The injection barrels, each with a tip diameter of 3–5 μm, were filled with either PBS or 10 μm PHE in PBS. This concentration of PHE induces increases in neuronal activation and calcium flux in other cell types (Tse & Tse, 1998; Martinez-Pena et al. 2004).
The pipette array was directed toward the NST with a hydraulic microdrive (Kopf 640) attached to the stereotaxic carrier. The recording barrel was connected to a Grass P15 AC-coupled headstage amplifier; signals were passed to a follower amplifier (DAM50, WPI). Thus, neuronal signals were subjected to a total amplification of 10 000 x and were band-pass filtered between 200 and 10 000 Hz. Pressure-injection pipettes were connected to Pneumatic Picopumps (PV820, WPI). Pipette solutions were micropressure-injected by applying six pulses (100 ms each, 5 p.s.i.) over 3 s to the pipette. Typically this protocol produced a total injection volume of 2 nl (Emch et al. 2000).
NST neurones were identified by their brisk excitatory response to mild gastric distension (McCann & Rogers, 1992). Identified NST cells were first exposed to control micropressure injections of PBS. If this resulted in any change in firing rate of the identified cell, this cell was discarded from further analysis. Five minutes after exposure to PBS microinjection, the same volume of 10 μm PHE (∼20 fmol) was applied using the same protocol. Neurones were considered responsive to PHE if their firing rate doubled within the first 10 s following drug injection. Neuronal activity was displayed on an oscilloscope and recorded on magnetic tape for later analysis with a PC-based waveform-analysis system (Datapac 2000; Run Technologies, Los Angeles, CA, USA). At the end of the experiment, the animal was killed with an overdose of urethane.
In vivo gastric strain-gauge recordings and NST phenylephrine injections
Activation of medial NST neurones (an area known to be involved in the vago-vagal reflex control of the stomach) can cause a dramatic reduction in gastric motility and tone (Rogers et al. 1996; Emch et al. 2000; Travagli & Rogers, 2001). Preliminary results suggested that α-1 agonists potently activate NST neurones in this area. To determine whether activation of NST neurones by PHE also results in decreased gastric tone and motility, food-deprived rats (n = 6; 200–400 g body weight) were anaesthetized with thiobutabarbital, as described above. A laparotomy was performed to expose the stomach; a 2-mm diameter purse-string suture opening was placed in the greater curvature of the antrum to allow placement of a small latex balloon (expanded volume, 2 ml; attached to a 1-mm i.d. silicone catheter) within the stomach as previously described (Rogers et al. 2003). An extralumenal strain gauge (RB Inc., Madison, WI, USA) was sewn to the antrum over the balloon. As we predicted that PHE would cause a relaxation of gastric tone and/or motility, the gastric balloon provided a mild preload distension of the antrum (load, ∼1 g). This arrangement produced a consistent distension against which the antrum, equipped with the strain gauge, could relax. The laparotomy incision was closed, the animal was placed in a stereotaxic frame, and the floor of the fourth ventricle was exposed as described above.
Gastric tone and motility was continuously monitored via a Model 7 Grass polygraph. Data were also stored for later analysis on a PC-based waveform-analysis system (DataPac 2000, Run Technologies). Glass micropipettes (30 μm bevelled tip) containing either PBS or 10 μm PHE in PBS were prepared for micropressure injection. With the gastric balloon slightly inflated to create a controlled distension, gastric motility and tone were monitored for a minimum of 10 min. A micropipette filled with PBS (i.e. control injections) was positioned in the medial NST. Injection volumes (40 nl) were monitored with a calibrated microscope focused on the fluid meniscus of the injection pipette. If microinjection of PBS into the NST had no effect on motility/tone, then 20 min later a similar volume of 10 μm PHE (∼400 fmol) was microinjected into the same site. At the end of the experiment, a pipette containing 1% rhodamine dextran conjugate (FluoroRuby, Molecular Probes) was positioned in the same spot and a marking injection was made. Each animal served as its own control. Thus, the effects of PHE versus PBS injections into the NST were evaluated by comparing the maximum change in tone elicited by either challenge using a paired Student's t test. Statistical significance was set at P < 0.05. At the end of the experiment, the animal was deeply anaesthetized with ure and transcardially perfused with PBS followed by 4% paraformaldehyde in PBS. Brains were postfixed overnight and subjected to routine histological methods to verify the location of the microinjection site.
Live cell calcium imaging of the NST
Medullary slices were prepared from 25 adult Long-Evans rats (body weight, 150–300 g). Animals were anaesthetized with urethane. After decapitation and swift removal of the brainstem to cold (∼4°C) carbogenated (95% O2−5% CO2) cutting solution (see below), 300-μm thick slices were cut coronally through the medulla with a sapphire knife on a Vibratome. Slices were incubated in a carbogenated normal Krebs solution (see recipe below) at 29°C for approximately 30 min.
Calcium-green acetoxymethyl ester (50 μg; Ca-green 1AM; Molecular Probes) was added to 40 μl of 10% pluronic acid in DMSO (F-127; Molecular Probes) to make a 1 mm solution of the fluorescent indicator (Zhang & Lipton, 1999). The Ca-green–pluronic–DMSO solution was added to a small plastic Petri dish containing 3 ml carbogenated normal Krebs solution (final Ca-green concentration, 13 μm). Medullary slices were incubated at room temperature in this Krebs–Ca-green solution for 20 min, rinsed in normal Krebs solution and returned to a holding chamber containing fresh, carbogenated, Krebs solution maintained at 29°C.
Pre-identification of NST neurones
A subset of animals (n = 5) received discrete injections of rhodamine dextran conjugate into the nodose ganglion specifically to reveal second-order NST neurones that receive vagal afferent input (Doyle et al. 2004). Thus, during in vitro recordings, Ca-green-loaded cells could be identified as NST neurones as indicated by rhodamine-filled vagal afferent input. These animals were anaesthetized with pentobarbital (50 mg kg−1, i.p.). Using aseptic technique, the nodose ganglion was exposed at the jugular foramen. A glass micropipette (bevelled tip; diameter, ∼70 μm) was filled with 20% rhodamine-coated dextran (in 0.1% Triton X-100 and distilled water). The micropipette was inserted into the nodose ganglion directly and rhodamine dextran conjugate (∼500–1000 nl) was injected using pressure pulses; the incision area was closed with nylon suture. Upon recovering from anaesthesia, the animals were returned to their home cages and the tracer dye was allowed to orthogradely transport for 1–3 weeks before medullary slices were harvested for the in vitro slice protocol described above.
Fluorescence imaging
Individual brain slices were transferred to the temperature-regulated perfusion chamber (Bioptechs Inc., Butler, PA, USA) and held in place with a harp-type pressor foot. Imaging was performed with a Nikon E600FN upright microscope equipped with a Perkin Elmer Ultraview spinning disc confocal illuminator and heated, water-immersion objectives. The recording chamber was continuously perfused at a rate of 2 ml min−1 with carbogenated Krebs solution warmed to 33°C. Identification of retrogradely labelled NST neurones (i.e. via rhodamine dextran conjugate) was performed using excitation at 568 nm and emission at 607 nm. Ca-green-preloaded cells were visualized using the excitation line at 488 nm. Ca-green-emissions at 509 nm were captured via a Hamamatsu-Orca-ER digital camera. Once NST neurones were identified (568/607 nm filter cube), relative cytoplasmic calcium levels in response to different agonists and antagonists were studied by switching to the 488 nm excitation/509 nm emission arrangement.
Solenoid valves were used to switch between the normal bathing solution and the challenge or experimental solutions (see below), without interruption to perfusion of the experimental slice. A concentration–response protocol was applied to establish the responsiveness to PHE. Slices were exposed to 1, 10 or 100 μm PHE for 50 s. Our results indicated that NST neurones responded strongly to the 10 μm concentration of PHE. Therefore, the studies examining the mechanisms of NST activation by PHE were performed using the 10 μm concentration.
Slices were first exposed to 10 μm PHE for 50 s to determine which cells responded to the α-1 agonist challenge. Responsive cells were defined as those producing a minimum increase in cytoplasmic calcium-dependent dye fluorescence (ΔF/F) of 30% relative to the unstimulated level (see analysis definitions below). In the ‘time control’ case, 10 min of washout elapsed after the first exposure to PHE, followed by an identical PHE challenge to determine whether calcium responses showed significant sensitization to repeated exposure to PHE. Preliminary results showed that desensitization to repeated agonist exposure was not a significant issue with this protocol. In some experimental groups, potential antagonists of intracellular calcium metabolism, α-1 receptor antagonist, or zero calcium–EGTA solutions were applied during the intervening 10 min period. In other instances, either glutamate or the α-2 adrenergic agonist (B-HT 933) was applied instead of the second exposure to 10 μm PHE.
In vitro drugs and solutions
The cutting solution contained (mm): choline chloride 110, NaHCO3 25, KCl 2.5, MgSO4.7H2O 7, NaH2PO4 1.25, glucose 10, CaCl2.2H2O 0.5; bubbled with 95% O2−5% CO2 during the entire cutting process. Calcium-free Krebs solution contained (mm): NaCl 124, NaHCO3 25, KCl 3.0, MgSO4.7H2O 2.5, NaH2PO41.5, glucose 10, EGTA 5; bubbled continuously with 95% O2−5% CO2.
Normal Krebs solution contained (mm): NaCl 124, NaHCO3 25, KCl 3.0, MgSO4.7H2O 1, NaH2PO4 1.5, glucose 10; CaCl2.2H2O 1.5; bubbled continuously with 95% O2−5% CO2; osmolality, 300 ± 10 mOsm; pH 7.3. Where specified in the experimental design sections above, one of the following drugs was added to the normal Krebs solution (references cited for each agonist/antagonist used specified concentrations of reagents in similar in vitro studies to investigate transduction mechanisms): phenylephrine (PHE, α1-adrenoceptor agonist; 1 μm, 10 μm, 100 μm; Martinez-Pena et al. 2004; Sigma); 2-aminoethoxydiphenylborane (2APB; IP3 antagonist; 100 μm; Bootman et al. 2002; Tocris); 1-[6-[[17β)-3-methoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione (U73122; phospholipase C inhibitor; 10 μm; e.g. Ishibashi et al. 2003; Tocris); chelerythrine (protein kinase C antagonist; 10 μm; e.g. Liu et al. 2003; Sigma); cadmium chloride (non-selective calcium channel blocker; 100 μm; Marinelli et al. 2003; Sigma); thapsigargin (inhibits endoplasmic reticular (ER) Ca2+-ATPase pump; 1 μm; Evans & Sanderson, 1999; Sigma); B-HT 933 dihydrochloride (selective α-2 agonist, 10 μm; Grass et al. 1998; Sigma); prazosin (α-1 adrenoceptor antagonist, 10 μm; Feldman & Felder, 1989a; Sigma); and glutamate (excitatory amino acid, 200 μm; Muyderman et al. 2001; Sigma).
In vitro calcium signal analysis
Relative changes in intracellular calcium concentration ([Ca2+]i), in response to agonist solutions, were quantified as changes in fluorescence (%): ΔF/F, where F is the fluorescence intensity within an area of interest (e.g. the outline of a NST neurone) prior to stimulation and ΔF is the change from this value during neuronal activity (Helmchen, 2000). Background fluorescence (i.e. from non-involved areas in same field) was subtracted from both ΔF and F. The following parameters were extracted from the calcium signal data from each cell examined: magnitude of response (peak ΔF/F), number of oscillations (if any), total duration of the agonist effect (if any), average duration of each oscillation (if any). The response to the first exposure to PHE was characterized for all cells. A one-way analysis of variance (ANOVA) for each parameter shows that all neurones responded similarly to PHE (see Table 1). For each parameter, the values of the responses to the second PHE challenge (i.e. after the 10 min drug treatment) across all groups were compared by ANOVA; Dunnett's post hoc comparisons were made to the ‘time control’ group that received PHE a second time. Statistical significance was assigned to values of P < 0.05.
Table 1.
Averaged NST neuronal population responses to 10 μm PHE exposure
| Response parameter | Mean | s.e.m. |
|---|---|---|
| Magnitude of response (ΔF/F) | 65% increase | 2.1 |
| Duration of response | 53 s | 1.0 |
| Number of oscillations | 4.8 | 0.2 |
| NO duration of each cycle | 13 s | 0.4 |
Results
Retrograde tracing from the NST and tyrosine hydroxylase immunocytochemistry
Punctate deposits of rhodamine-coated latex nanospheres into the medial NST yielded light retrograde label in TH-ir-neurones in the A2, A5, locus coeruleus and area postrema neurones (Fig. 1). TH-ir staining was extremely bright compared with the signal produced by a relatively small number of fluorescent nanospheres orthogradely transported to the NST. The relatively intense TH-ir signal essentially obstructed the nanosphere signal when observed with standard epifluorescence methods. However, laser confocal methods utilizing short exposure times for the TH-ir emissions versus long exposure times for the rhodamine signal yielded unambiguous TH (+)/rhodamine (+) double-label in the previously mentioned catecholaminergic nuclear groups. Typically, within a given 50-μm histological section, 2–3 double-labelled neurones per catecholamine nuclear group (i.e. A2, A5, etc.) were observed. Thus, these observations indicate that at least a portion of the catecholaminergic input to the medial NST originates from A2, A5, A6 and the area postrema.
Figure 1. Catecholaminergic projections to the medial solitary nucleus come from multiple sources.
Rhodamine-coated latex nanosphere injection site (A) is confined exclusively to the medial solitary nucleus. Retrograde transport of rhodamine-coated beads is traced to at least five brainstem sources: the A2 region of the NST (A), the locus coeruleus (B), ventrolateral medulla (C), the A5 area of the pons (D) and the area postrema (E). Histological sections were IHC stained for tyrosine hydroxylase (TH-ir). Open arrows, double-labelled neurones (rhodamine+/TH-ir +); filled arrow, retrogradely labelled AP cell not TH-ir. AP, area postrema; cc, central canal; DMN, dorsal motor nucleus of the vagus; LC, locus coeruleus; mNST, medial nucleus of the solitary tract; st, solitary tract; VLM, ventrolateral medulla.
In vivo extracellular recordings
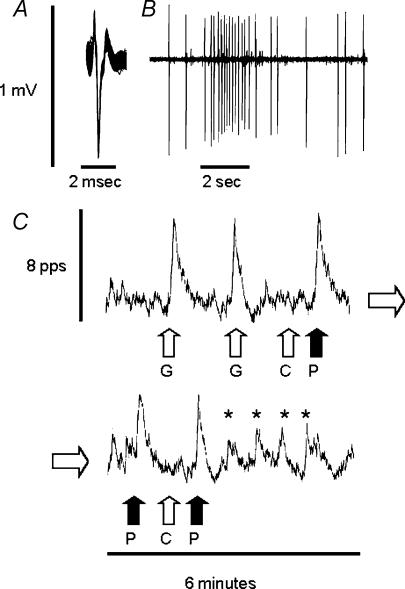
Neurones in the medial NST (n = 32) were identified by their characteristic responses to balloon distension of the antrum (McCann & Rogers, 1992; Emch et al. 2000). Unstimulated NST neurones produce an erratic, 1 to 3-Hz firing pattern. When activated by gastric distension, these cells respond with a brisk, stimulus-locked 500% increase in firing rate (Fig. 2). Of the neurones identified by gastric distension, 50% (n = 16) were also excited by PHE (2 nl of 10 μm), while none were inhibited. In five cases, repeated application of PHE initiated an oscillatory excitation pattern (marked by * in Fig. 2).
Figure 2. In vivo neurophysiological recording of an identified gastric–NST neurone: response to gastric distension and PHE micropressure injection.
A, 50 superimposed extracellular potentials showing the unitary nature of the record. B, cell characterized in A responds to gastric balloon distension (at the 2-s time scale bar). Action potential shape appears variable due to the slower rate of sampling necessary while recording long trains of spikes. C, ratemeter record of the activity of the same cell in response to: 2 ml gastric balloon distension (G); control microinjection of 2 nl PBS (C); or microinjection of 2 nl of 10 μm PHE (P) onto the recorded neurone. Lower panel is continuous with the upper panel and shows that repetitive exposure of the neurone to PHE is associated with the entrainment of an oscillatory activity pattern (marked by *).
In vivo gastric strain gauge recordings and NST phenylephrine injections
As we predicted that PHE would cause a relaxation of gastric tone and/or motility, we installed a gastric balloon that could provide a mild preload distension of the antrum (load, ∼1 g). Each animal served as its own control therefore the effects of PHE versus PBS injections into the NST were compared using a paired Student's t tests. PHE microinjected into the NST caused a significant reduction in gastric tone (−0.61 ± 0.1 g; t= 4.7; degrees of freedom = 5; P < 0.05). The reduction in tone lasted 21 ± 7 min (Fig. 3).
Figure 3. Microinjection of PHE into the NST decreases gastric tone.
Upper panel, raw strain gauge records of gastric tone. Microinjection into NST of 40 nl PBS (black trace) has no effect on gastric tone, whereas the same volume of 10 μm PHE (blue trace; total dose, 400 fmol) causes a marked drop in tone. Statistical representation of data is shown below. Lower panel, magnitude and duration of effects on fundic tone as a consequence of NST microinjection of PBS or PHE. Each animal served as its own control; Student's paired t test, *P < 0.05.
Live cell calcium imaging of the NST in response to PHE
A total of 225 NST cells responded to the exposure to PHE with our minimum criterion of a 30% change in calcium dye fluorescence. NST neurones were faintly labelled at rest and exhibited no spontaneous activity. A 50 s exposure to 10 μm PHE induced dramatic cytosolic calcium oscillations in presumptive neuronal NST cells (Figs 4, 5, 6 and Table 1). The increase in cytoplasmic calcium began within seconds of the entry of PHE into the recording chamber. We further characterized the initial response to PHE across four parameters.
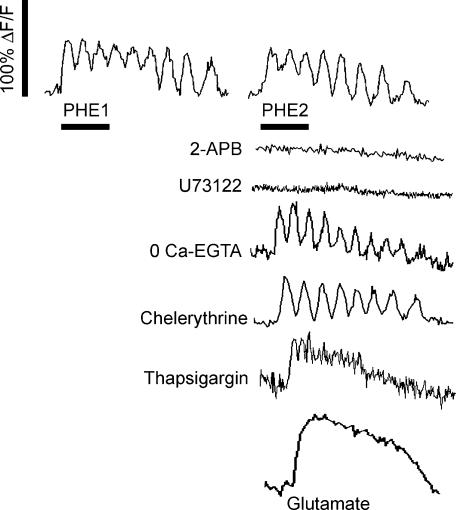
Figure 4. Effects of PHE on relative cytoplasmic calcium levels in NST neurones.
In all cases, an initial exposure to PHE was used to identify responsive cells, followed by a 10-min washout period of either no treatment (time/desensitization control) or various drug/medium treatments designed to determine the mechanistic basis of the PHE effect. The right side of this figure shows cytoplasmic calcium responses to the second exposure to PHE following the 10-min drug treatment period. Second exposure to PHE (time control) showed little diminution or desensitization of the cytoplasmic Ca2+ response; 2-APB (IP3 antagonist) completely blocked the increase in Ca2+; U73122 (phospholipase C inhibitor) completely blocked the increase in Ca2+; zero calcium–EGTA or chelerythrine (PKC antagonist) did not affect Ca2+ oscillations; thapsigargin (blocker of calcium ATPase ER pumps) converted the PHE cytoplasmic Ca2+ response to a non-oscillatory increase. Glutamate evoked a non-oscillatory increase in cytoplasmic Ca2+. Scale bar for all traces, 50 s.
Figure 5. Concentration-dependent effects of PHE on cytoplasmic Ca2+ in NST neurones.
NST neurones demonstrated concentration-dependent responses to 1 μm, 10 μm, and 100 μm PHE. All cells that responded to 10 μm PHE also responded to 100 μm but with a greater magnitude (F2,33 = 14.0; Newman–Keul's post hoc test, P < 0.05), longer duration (F2,33 = 161.7; P < 0.05), higher numbers of oscillations per stimulation period (F2,33 = 64.3; P < 0.05) and shorter duration per oscillation cycle (F2,33 = 9.1; P < 0.05). Neurones responded to 1 μm PHE at significantly lower levels across all parameters measured except the duration per oscillation.
Figure 6. Overall effects of PHE and potential antagonists of PHE action on NST neuronal cytoplasmic calcium.
Our results show that NST neurones produced consistent, repeatable and significant responses to PHE that did not demonstrate diminution over time (compare 1st PHE and 2nd PHE). The effects of PHE were completely blocked by the IP3 antagonist (2-APB), the phospholipase C inhibitor (U73122), and by the α-1 adrenoceptor antagonist (prazosin). Thapsigargin (blocker of ER calcium ATPase pumps) converted an oscillatory response to a single, non-oscillating wave (see also Fig. 4); this is also reflected in the increased duration per cycle. The fact that chelerythrine, Cd+ and zero calcium–EGTA solution had no impact on PHE-driven calcium oscillations in the NST suggests that the effect is based completely on the interplay between agonist-induced ER calcium release and ER calcium ATPase pumping. Lastly, the α-2 agonist, B-HT 933, had no effect on cytoplasmic calcium.
Repetitive PHE
PHE was applied to 30 neurones a second time, that is following wash out for 10 min, to evaluate the possibility that multiple exposures could produce desensitization to the agonist. The second exposure to PHE caused a peak increase in cytoplasmic calcium-induced Ca-green fluorescence (ΔF/F) of 63.0 ± 6.0%; this response was not significantly different from that observed after the first exposure to PHE (Student's t test; P = 0.24). The total duration of elevated fluorescence, number of oscillations and cycle period were also not statistically different between the first and second exposure of the NST to PHE (Figs 4 and 6).
Concentration–response to PHE
Sixteen neurones responsive to 10 μm PHE were randomly exposed to 1 μm or 100 μm PHE. All cells that responded to 10 μm PHE also responded to 100 μm but with a greater magnitude (F2,33= 14.0; Newman–Keul's post hoc test, P < 0.05), longer duration (F2,33= 161.7; P < 0.05), higher numbers of oscillations per stimulation period (F2,33= 64.3; P < 0.05) and shorter duration per oscillation cycle (F2,33= 9.1; P < 0.05, see Fig. 5). In contrast, only four neurones also responded to 1 μm PHE; with lower resposes for all parameters measured. These decreases in responsiveness were statistically significant for magnitude and duration, only.
NST response to PHE after different pretreatments
NST neurones responding to PHE exposure displayed response characteristics as listed in Table 1. NST neurones were exposed to PHE following different pretreatment conditions described above. The overall ANOVA of responses to this second exposure of PHE, revealed significant between-group differences in the NST response across the four parameters that were quantified. The overall ANOVA (F8,193) of each of these parameters was: magnitude of the response (ΔF/F), F = 46; duration of response, F = 58; number of oscillations, F = 27; and duration per oscillation, F = 20. These F-values are associated with overall P < 0.0001 (Fig. 6).
Dunnett's post hoc comparisons were made against the responses observed in the ‘2nd PHE’ group, i.e. those NST neurones that received no treatment during the 10-min interval. (Statistical significance was assigned to values of P < 0.05.) The effect of PHE to induce an increase in cytoplasmic calcium flux was eliminated by exposure to the soluble intracellular IP3 antagonist (2APB), the selective phospholipase C antagonist (U73122) or the α-1 adrenergic antagonist (prazosin). Thapsigargin (ER calcium ATPase pump antagonist) eliminated the oscillatory NST response to PHE by converting it to a constant (dome-like) response and extending its duration. In contrast, chelerythrine (protein kinase C antagonist), cadmium (transmembrane calcium flux blocker) or zero calcium–EGTA Krebs solution did not significantly affect the magnitude (ΔF/F%), duration of response, or the number of oscillations evoked by PHE (Fig. 6).
NST neurones responded to glutamate with a large, but non-oscillatory increase in Ca-green fluorescence (Figs 4 and 7). The α-2 specific agonist (B-HT 933) produced no discernable effects on cytoplasmic calcium (Fig. 6). A subset of glutamate-responsive NST neurones (n = 23) was exposed to glutamate stimulation a second time under conditions of modified extracellular Ca2+ availability. Specifically, the bathing solution was switched to either zero calcium–EGTA Krebs solution or the Ca2+ was replaced by Cd2+. Predictably (Ciardo & Meldolesi, 1991; Guiramand et al. 1991), both of these conditions essentially blocked the effects of glutamate to elevate cytoplasmic Ca2+ in NST neurones (data not shown).
Figure 7. In vitro slice preparation with NST neurone identified by closely apposed rhodamine-labelled vagal afferent endings.
A, double-exposure image demonstrating Ca-green-labelled NST neurone with closely apposed pre-labelled vagal fibres (rhodamine-coated beads appear orange in colour). B, Ca-green-signal only, showing NST cell at rest before exposure to glutamate. C, same cell at the peak of the response to glutamate (image taken at arrow in GLU response curve; D). Scale bar, 20 μm. D, plot of calcium-dependent fluorescence of the same NST neurone in response to glutamate (GLU). E, response of the same cell to 10 μm PHE, note oscillatory response as opposed to monotonic increases in cytoplasmic calcium evoked by glutamate.
To verify that the induced oscillatory behaviour observed in the NST was a property of second-order viscero-sensory neurones, discrete injections of rhodamine dextran conjugate were made into the nodose ganglion of five additional rats. In this way, we could visually identify second-order NST neurones that receive vagal afferent input (Doyle et al. 2004). During in vitro recordings, Ca-green-loaded cells could be specifically identified as NST neurones as indicated by their closely apposed rhodamine-labelled vagal afferent input. These pre-identified cells (n = 30) in the NST elicited calcium oscillations in response to PHE and a sustained, monotonic increase in calcium in response to glutamate (Fig. 7).
Infrequently (n = 5), we observed Ca-green-labelled cells in the NST that demonstrated a distinct glial morphology (i.e. relatively flattened and stellate appearance; Kang & Nedergaard, 2000). These cells behaved quite differently from NST neurones in their response to PHE. In addition to their basal fluorescence being brighter than neurones, they produced a slow, ‘devil's staircase'-like augmenting response (Chay et al. 1995) to PHE that did not return to baseline in the timeframe used to study NST neuronal responses; Fig. 8 illustrates one such response. This effect of PHE to initiate a step increase in glial calcium levels was not further investigated.
Figure 8. Small cell in the NST with an astrocyte-like morphology responds to PHE.
These small, astrocyte-like cells responded to PHE by generating a staircase cytoplasmic calcium response rather than oscillations as seen in identified NST neurones (see Fig. 7). A–C show the same small cell at different time points in the response to PHE. Each photograph corresponds to the numbered segment of the relative fluorescence graph. D is a photograph of a small cell immunostained for glial fibrillary acidic protein (GFAP) from another experiment, illustrating the morphology of an astrocyte in the NST.
Discussion
These studies reveal that the medial NST (i.e. the portion implicated in the regulation of gastrointestinal function) is the recipient of CA input from, at least, the area postrema, A2, A5 and locus coeruleus (A6) areas. The α-1 agonist, PHE, strongly activates identified gastric–NST neurones. Microinjection of PHE into the NST produces an immediate and potent relaxation of the gastric wall, i.e. drop in tone. The in vitro calcium-imaging studies revealed that this agonist also causes dramatic calcium oscillations in NST neurones. The mechanism producing oscillations in the NST is qualitatively similar to that reported for other cells (Somogyi & Stucki, 1991; Verkhratsky et al. 1998). These oscillations may be important to the entrainment of burst activation or the gating of other afferent inputs. This rhythmic pattern of excitation may help explain the basis for the powerful central autonomic and behavioural effects mediated by α-1 adrenoceptors. Lastly, to our knowledge, this is the first such in vitro calcium-imaging study performed in the brainstem of the adult rat.
Microinjections for our retrograde tracing studies were tightly restricted to regions containing only the medial NST, yet immunostaining of these projections showed that CA neurones in the area postrema, A2, A5, ventrolateral medulla and locus coeruleus (A6) all provide input to this highly circumscribed part of the NST. All of these CA cell groups have been implicated in managing the autonomic or behavioural responses to different stressors including circulating toxins, cardiorespiratory emergencies and inescapable pain (Blessing, 1997a; Greenwood et al. 2003; Rinaman, 2003). Some of the consequences of severe stress that are referred to the gastrointestinal tract include stasis, nausea, emesis and anorexia (Forbes et al. 1999; Koob, 1999; Li & Balint, 2000; Greenwood et al. 2003). Agents that activate gastric-related NST circuitry (e.g. TNF, cholecystokinin, nicotine) can also elicit these effects (Rinaman et al. 1995; Emch et al. 2000; Ferreira et al. 2002; Rinaman, 2003). Some of these agents are known to mediate their effects through CA circuitry in the dorsal medulla (Ferreira et al. 2002; Rinaman, 2003) and α-1 agonists, such as PHE, produce the same gastrointestinal malaise and anorexia (Lora-Vilchis et al. 1988; Wellman et al. 2003). These disparate data suggest that activation of α-1 receptors on NST neurones could be responsible for the stasis and other symptoms of gastrointestinal distress produced by severe stress or by some adrenergic agonists.
The present study provides evidence that medial NST neurones responsive to gastric distension are activated by PHE. Microinjection of PHE (∼400 fmol) into the NST induced a significant and relatively long-lasting (>15 min) reduction in gastric tone. This response would be expected if the primary action of α-1 agonists is to excite NST neurones (McCann & Rogers, 1992; Rogers et al. 1996; Emch et al. 2000; Travagli & Rogers, 2001).
Studies have shown that α-1 agonist action on neurones is predominantly excitatory and, in some systems, produces a burst-type activation (Wang & Hatton, 2004) or oscillations (Kogo et al. 2000; Morin et al. 2000). There are several potential means by which PHE can regulate cellular excitability including inhibition of potassium currents (Fukuda et al. 1987; Martinez-Pena et al. 2004) and the initiation of waves of intracellular calcium release from the ER. These changes in the dynamics of intracellular calcium are highly correlated with oscillatory variations in brainstem neuronal excitability (Koshiya & Smith, 1999; Johnson et al. 2001).
Our in vitro calcium-imaging results show that PHE initiates calcium oscillations in the NST. The mechanism generating the oscillation appears common to a number of cell types (Somogyi & Stucki, 1991; Tse & Tse, 1998; Verkhratsky et al. 1998); (see Fig. 9). The increase in cytoplasmic calcium in the NST, as well as in other cell types, is apparently caused by the action of the α-1 adrenoceptor to activate a Gq-protein dependent increase in phospholipase-C (PLC) which, in turn, catalyses the formation of IP3 and diacylglycerol (DAG) from phosphatidyl inositol (Somogyi & Stucki, 1991; Tse & Tse, 1998; Verkhratsky et al. 1998). IP3 then acts at the ER to release stored calcium. Our studies showed that 2-APB (a membrane permeant inhibitor of IP3 action in neurones) completely inhibited the ability of PHE to increase cytoplasmic calcium in NST neurones. (It should be acknowledged that the effect of 2-APB in some cells or systems is not restricted to IP3 blockade but extends to include blockade of intracellular store-operated calcium channels. However, at the concentrations used in these experiments, 2-APB was selective for interruption of IP3 action in intact neurones (Bootman et al. 2002).) Additionally, the 2-APB results were supported by our observations of the effects of U73122, a potent and selective phospholipase C inhibitor. Like 2-APB, U73122 also eliminated the excitatory and oscillatory effects of PHE.
Figure 9. Model of a cellular calcium oscillator adapted from Somogyi & Stucki (1991) showing a minimal composition of the potential components.
Our data suggest that PHE activation of NST neurones produces cytoplasmic calcium oscillations using a relatively simple mechanism in which agonist binding activates IP3 production and ER calcium release. Calcium ATPase pumps replace the released cytoplasmic calcium into the ER. The interplay between these factors (filled arrows) is the cause of the oscillation. Other factors (open arrows) do not appear significant to the PHE-induced oscillation for these cells.
The PHE-elicited increase in intracellular calcium was not affected by treatment with Cd+ or a zero calcium–EGTA solution (in contrast to the increase in intracellular calcium evoked by glutamate). Thus, the source of cytoplasmic calcium released by PHE was intracellular stores. While the initiation (i.e. upstroke) of the oscillation of cytoplasmic calcium is almost always dependent on PLC activation, the down-stroke in the oscillations may be caused by one of several mechanisms including the oscillatory production of IP3 induced by the cyclical activation of PLC, feedback inhibition of IP3 formation, or by the rapid uptake of calcium by the ER. In the former case, protein kinase C (PKC) activated by DAG can feedback-inhibit PLC, and thus reduce IP3 formation. In the latter, the action of the calcium ATP-ase pump reduces cytoplasmic calcium by restoring it into the ER (Somogyi & Stucki, 1991; Bird et al. 1993; Tse & Tse, 1998; Verkhratsky et al. 1998; Dale et al. 2001). The downstroke is often a mix of the effects of PKC inhibition of PLC and activation of Ca+-ATPase pumping. The mix is highly variable, ranging from no PKC involvement (e.g. Dale et al. 2001) to PKC inhibition late in the process (e.g. Muyderman et al. 1998) to critical involvement (e.g. Sanchez-Bueno et al. 1990). Our experiments on the effects of PHE on NST neurones demonstrated that the oscillation downstroke was entirely explained by blockade of Ca+-ATPase pumping in the ER via thapsigargin. That is, thapsigargin converted the Ca2+ oscillations induced by PHE to a monotonic increase. This effect of thapsigargin to eliminate oscillatory behaviour has been observed in several cell systems (Evans & Sanderson, 1999).
Although α-1 adrenergic agonists produce wave-like increases in cytoplasmic calcium in astrocytes (Verkhratsky et al. 1998), it is unlikely that the in vitro recordings of PHE-induced calcium oscillations we report here were produced by glia. Astrocyte morphology, even recorded as dim calcium dye images, is distinctly different from neurones. Calcium-imaged astrocytes appear small, flattened and stellate; while imaged neurones, even the smallest interneurones, have larger, ovoid cell bodies with occasional primary dendrites visible (Kang & Nedergaard, 2000) (compare Figs 7 and 8). Furthermore, we observed the oscillation-inducing effects of PHE in NST neurones that received closely apposing vagal afferent terminations (Doyle et al. 2004). There are functional differences between glia and neurones that further reinforce our conclusions. For example, astrocytes produce oscillations in response to glutamate (Codazzi et al. 2001), while NST neurones produce only monotonic increases in cytoplasmic calcium in responses to this agonist (Figs 4 and 7). On the other hand, astrocytes produce heterogeneous slow responses to α-1 agonists (Venance et al. 1998), and, as seen here, a PHE-induced devil's staircase-like augmentation in cytoplasmic calcium (Chay et al. 1995; Gracheva & Gunton, 2003). This contrasts with the regular oscillations that PHE produced in NST cells.
The value to the organism of cytoplasmic calcium oscillators in inexcitable cells has been debated extensively (Somogyi & Stucki, 1991; Codazzi et al. 2001). However, there is little argument that calcium waves are extremely important to the function of neurones. Aside from the potential role of calcium oscillators as regulators of pacemaking and burst-type activation patterns (Butera et al. 1999; Koshiya & Smith, 1999; Johnson et al. 2001; Wang & Hatton, 2004), oscillatory calcium activation provides a mechanism by which high levels of cellular excitability can be maintained without incurring excitotoxicity (Sapolsky, 2001). Furthermore, calcium oscillations have been shown to encode information via their amplitude, duration and frequency that are decoded into the pattern of gene expression and phosphorylation of target proteins (Prank et al. 2001); functional consequences include effects on synaptic plasticity and neural outgrowth (Fields et al. 2001). Although these issues have not been specifically addressed for the solitary nucleus, the concept of postsynaptic modulation of NST responsiveness to vagal afferent stimulation has been modelled using an extensive database obtained from in vitro neurophysiological data (Schild et al. 1995). This model showed that as afferent synaptic drive increased, intrinsic metabolic properties of NST neurones should significantly modify, indeed entrain, the response to afferent input. Calcium oscillations could represent another class of ‘intrinsic metabolic property’ that serves to modulate responsiveness to afferent input. Calcium levels and changing concentration of other transduction intermediates can have dramatic neurotransmission ‘gating’ effects on neurones in the dorsal vagal complex (Paton et al. 2001; Travagli et al. 2003).
Acknowledgments
Excellent technical services were provided by Ms Montina VanMeter. The authors would like to thank Dr Gregory Holmes for his invaluable input and Dr Alberto Travagli for his critical reading of this manuscript. This work was supported by National Institutes of Health grants DK56373, DK52142 and the Metabolife settlement fund. Parts of this manuscript were presented at the Annual Meeting of Experimental Biology in Washington DC, April 2004.
References
- Andrews PL, Sanger GJ. Abdominal vagal afferent neurones: an important target for the treatment of gastrointestinal dysfunction. Curr Opin Pharmacol. 2002;2:650–656. doi: 10.1016/s1471-4892(02)00227-8. 10.1016/S1471-4892(02)00227-8. [DOI] [PubMed] [Google Scholar]
- Berretta N, Bernardi G, Mercuri NB. Alpha(1)-adrenoceptor-mediated excitation of substantia nigra pars reticulata neurons. Neuroscience. 2000;98:599–604. doi: 10.1016/s0306-4522(00)00135-4. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- Bertolino M, Vicini S, Gillis R, Travagli A. Presynaptic alpha2-adrenoceptors inhibit excitatory synaptic transmission in rat brain stem. Am J Physiol. 1997;272:G654–G661. doi: 10.1152/ajpgi.1997.272.3.G654. [DOI] [PubMed] [Google Scholar]
- Bird GS, Rossier MF, Obie JF, Putney JW., Jr Sinusoidal oscillations in intracellular calcium requiring negative feedback by protein kinase C. J Biol Chem. 1993;268:8425–8428. [PubMed] [Google Scholar]
- Blessing WW. The Lower Brainstem and Bodily Homeostasis. Oxford: Oxford University Press; 1997a. Anatomy of the lower brainstem; pp. 29–100. [Google Scholar]
- Blessing WW. The Lower Brainstem and Bodily Homeostasis. Oxford: Oxford University Press; 1997b. Eating and metabolism; pp. 323–372. [Google Scholar]
- Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-Aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002;16:1145–1150. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- Browning KN, Kalyuzhny AE, Travagli RA. Opioid peptides inhibit excitatory but not inhibitory synaptic transmission in the rat dorsal motor nucleus of the vagus. J Neurosci. 2002;22:2998–3004. doi: 10.1523/JNEUROSCI.22-08-02998.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Travagli RA. The peptide TRH uncovers the presence of presynaptic 5-HT1A receptors via activation of a second messenger pathway in the rat dorsal vagal complex. J Physiol. 2001;531:425–435. doi: 10.1111/j.1469-7793.2001.0425i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher LL, Rogers RC. Histochemical effects of kainic acid on neostriatal dopamine and acetylcholinesterase. Eur J Pharmacol. 1978;50:287–289. doi: 10.1016/0014-2999(78)90365-5. [DOI] [PubMed] [Google Scholar]
- Butera RJ, Jr, Rinzel J, Smith JC. Models of respiratory rhythm generation in the pre-Botzinger complex. I. Bursting pacemaker neurons. J Neurophysiol. 1999;82:382–397. doi: 10.1152/jn.1999.82.1.382. [DOI] [PubMed] [Google Scholar]
- Chay TR, Lee YS, Fan YS. Appearance of phase-locked Wenckebach-like rhythms, devil's staircase and universality in intracellular calcium spikes in non-excitable cell models. J Theor Biol. 1995;174:21–44. doi: 10.1006/jtbi.1995.0077. [DOI] [PubMed] [Google Scholar]
- Chen CH, Stephens RL, Jr, Rogers RC. PYY and NPY: control of gastric motility via action on Y1 and Y2 receptors in the DVC. Neurogastroenterol Motil. 1997;9:109–116. doi: 10.1046/j.1365-2982.1997.d01-26.x. [DOI] [PubMed] [Google Scholar]
- Ciardo A, Meldolesi J. Regulation of intracellular calcium in cerebellar granule neurons: effects of depolarization and of glutamatergic and cholinergic stimulation. J Neurochem. 1991;56:184–191. doi: 10.1111/j.1471-4159.1991.tb02579.x. [DOI] [PubMed] [Google Scholar]
- Codazzi F, Teruel MN, Meyer T. Control of astrocyte Ca2+ oscillations and waves by oscillating translocation and activation of protein kinase C. Curr Biol. 2001;11:1089–1097. doi: 10.1016/s0960-9822(01)00326-8. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Jr, Sawchenko PE. A circumscribed projection from the nucleus of the solitary tract to the nucleus ambiguus in the rat: anatomical evidence for somatostatin- 28-immunoreactive interneurons subserving reflex control of esophageal motility. J Neurosci. 1989;9:1668–1682. doi: 10.1523/JNEUROSCI.09-05-01668.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale LB, Babwah AV, Bhattacharya M, Kelvin DJ, Ferguson SS. Spatial-temporal patterning of metabotropic glutamate receptor-mediated inositol 1,4,5-triphosphate, calcium, and protein kinase C oscillations: protein kinase C-dependent receptor phosphorylation is not required. J Biol Chem. 2001;276:35900–35908. doi: 10.1074/jbc.M103847200. 10.1074/jbc.M103847200. [DOI] [PubMed] [Google Scholar]
- Dashwood MR, Gilbey MP, Spyer KM. The localization of adrenoceptors and opiate receptors in regions of the cat central nervous system involved in cardiovascular control. Neuroscience. 1985;15:537–551. doi: 10.1016/0306-4522(85)90232-5. 10.1016/0306-4522(85)90232-5. [DOI] [PubMed] [Google Scholar]
- Day HE, Campeau S, Watson SJ, Jr, Akil H. Distribution of alpha 1a-, alpha 1b- and alpha 1d-adrenergic receptor mRNA in the rat brain and spinal cord. J Chem Neuroanat. 1997;13:115–139. doi: 10.1016/s0891-0618(97)00042-2. 10.1016/S0891-0618(97)00042-2. [DOI] [PubMed] [Google Scholar]
- Doyle MW, Bailey TW, Jin YH, Appleyard SM, Low MJ, Andresen MC. Strategies for cellular identification in nucleus tractus solitarius slices. J Neurosci Methods. 2004;137:37–48. doi: 10.1016/j.jneumeth.2004.02.007. 10.1016/j.jneumeth.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Emch GS, Hermann GE, Rogers RC. TNF-alpha activates solitary nucleus neurons responsive to gastric distension. Am J Physiol Gastrointest Liver Physiol. 2000;279:G582–G586. doi: 10.1152/ajpgi.2000.279.3.G582. [DOI] [PubMed] [Google Scholar]
- Emch GS, Hermann GE, Rogers RC. TNF-alpha-induced c-Fos generation in the nucleus of the solitary tract is blocked by NBQX and MK-801. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1394–R1400. doi: 10.1152/ajpregu.2001.281.5.R1394. [DOI] [PubMed] [Google Scholar]
- Emch GS, Hermann GE, Rogers RC. Tumor necrosis factor-alpha inhibits physiologically identified dorsal motor nucleus neurons in vivo. Brain Res. 2002;951:311–315. doi: 10.1016/s0006-8993(02)03178-5. 10.1016/S0006-8993(02)03178-5. [DOI] [PubMed] [Google Scholar]
- Evans JH, Sanderson MJ. Intracellular calcium oscillations induced by ATP in airway epithelial cells. Am J Physiol. 1999;277:L30–L41. doi: 10.1152/ajplung.1999.277.1.L30. [DOI] [PubMed] [Google Scholar]
- Feldman PD, Felder RB. Alpha 2-adrenergic modulation of synaptic excitability in the rat nucleus tractus solitarius. Brain Res. 1989a;480:190–197. doi: 10.1016/0006-8993(89)91582-5. 10.1016/0006-8993(89)91582-5. [DOI] [PubMed] [Google Scholar]
- Feldman PD, Felder RB. Alpha-adrenergic influences on neuronal responses to visceral afferent input in the nucleus tractus solitarius. Neuropharmacology. 1989b;28:1081–1087. doi: 10.1016/0028-3908(89)90121-4. 10.1016/0028-3908(89)90121-4. [DOI] [PubMed] [Google Scholar]
- Feldman PD, Moises HC. Electrophysiological evidence for alpha 1- and alpha 2-adrenoceptors in solitary tract nucleus. Am J Physiol. 1988;254:H756–H762. doi: 10.1152/ajpheart.1988.254.4.H756. [DOI] [PubMed] [Google Scholar]
- Ferreira M, Jr, Sahibzada N, Shi M, Panico W, Niedringhaus M, Wasserman A, Kellar KJ, Verbalis J, Gillis RA. CNS site of action and brainstem circuitry responsible for the intravenous effects of nicotine on gastric tone. J Neurosci. 2002;22:2764–2779. doi: 10.1523/JNEUROSCI.22-07-02764.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD, Eshete F, Dudek S, Ozsarac N, Stevens B. Regulation of gene expression by action potentials: dependence on complexity in cellular information processing. Novartis Found Symp. 2001;239:160–172. doi: 10.1002/0470846674.ch13. Discussion 172–166, 234–140. [DOI] [PubMed] [Google Scholar]
- Forbes D, Withers G, Silburn S, McKelvey R. Psychological and social characteristics and precipitants of vomiting in children with cyclic vomiting syndrome. Dig Dis Sci. 1999;44:19S–22S. [PubMed] [Google Scholar]
- Fukuda A, Minami T, Nabekura J, Oomura Y. The effects of noradrenaline on neurones in the rat dorsal motor nucleus of the vagus, in vitro. J Physiol. 1987;393:213–231. doi: 10.1113/jphysiol.1987.sp016820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H, Koga T, Furukawa N, Nakamura E, Shiroshita Y. The tachykinin NK1 receptor antagonist GR205171 prevents vagal stimulation-induced retching but not neuronal transmission from emetic vagal afferents to solitary nucleus neurons in dogs. Brain Res. 1998;802:221–231. doi: 10.1016/s0006-8993(98)00630-1. 10.1016/S0006-8993(98)00630-1. [DOI] [PubMed] [Google Scholar]
- Gillis RA, Quest JA, Pagani FD, Norman WP. Control centers in the central nervous system for regulating gastrointestinal motility. In: Wood JD, editor. Handbook of Physiology, section 6, The Gastrointestinal System, vol. I, Motility and circulation. Bethesda, MD: American Physiological Society; 1989. pp. 621–683. [Google Scholar]
- Gracheva ME, Gunton JD. Intercellular communication via intracellular calcium oscillations. J Theor Biol. 2003;221:513–518. doi: 10.1006/jtbi.2003.3201. 10.1006/jtbi.2003.3201. [DOI] [PubMed] [Google Scholar]
- Grass K, Prast H, Philippu A. Influence of catecholamine receptor agonists and antagonists on the ultradian rhythm of the EEG in the posterior hypothalamus. Naunyn Schmiedebergs Arch Pharmacol. 1998;357:169–175. doi: 10.1007/pl00005151. [DOI] [PubMed] [Google Scholar]
- Greenwood BN, Kennedy S, Smith TP, Campeau S, Day HE, Fleshner M. Voluntary freewheel running selectively modulates catecholamine content in peripheral tissue and c-Fos expression in the central sympathetic circuit following exposure to uncontrollable stress in rats. Neuroscience. 2003;120:269–281. doi: 10.1016/s0306-4522(03)00047-2. 10.1016/S0306-4522(03)00047-2. [DOI] [PubMed] [Google Scholar]
- Guiramand J, Vignes M, Recasens M. A specific transduction mechanism for the glutamate action on phosphoinositide metabolism via the quisqualate metabotropic receptor in rat brain synaptoneurosomes. II. Calcium dependency, cadmium inhibition. J Neurochem. 1991;57:1501–1509. doi: 10.1111/j.1471-4159.1991.tb06344.x. [DOI] [PubMed] [Google Scholar]
- Hayar A, Heyward PM, Heinbockel T, Shipley MT, Ennis M. Direct excitation of mitral cells via activation of alpha1-noradrenergic receptors in rat olfactory bulb slices. J Neurophysiol. 2001;86:2173–2182. doi: 10.1152/jn.2001.86.5.2173. [DOI] [PubMed] [Google Scholar]
- Hayward LF. Evidence for alpha-2 adrenoreceptor modulation of arterial chemoreflexes in the caudal solitary nucleus of the rat. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1464–R1473. doi: 10.1152/ajpregu.2001.281.5.R1464. [DOI] [PubMed] [Google Scholar]
- Hayward LF, Riley AP, Felder RB. alpha (2)-Adrenergic receptors in NTS facilitate baroreflex function in adult spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2002;282:H2336–H2345. doi: 10.1152/ajpheart.00167.2001. [DOI] [PubMed] [Google Scholar]
- Helmchen F. Calibration of fluorescent calcium indicators. In: Yuste R, Lanni F, Konnerth A, editors. Imaging Neurons: a Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. pp. 31–39. [Google Scholar]
- Hermann GE, Hebert SL, Van Meter MJ, Holmes GM, Rogers RC. TNF(alpha)-p55 receptors: medullary brainstem immunocytochemical localization in normal and vagus nerve-transected rats. Brain Res. 2004;1004:156–166. doi: 10.1016/j.brainres.2003.11.078. 10.1016/j.brainres.2003.11.078. [DOI] [PubMed] [Google Scholar]
- Hermann G, Rogers RC. Tumor necrosis factor-alpha in the dorsal vagal complex suppresses gastric motility. Neuroimmunomodulation. 1995;2:74–81. doi: 10.1159/000096874. [DOI] [PubMed] [Google Scholar]
- Hermann GE, Rogers RC, Bresnahan JC, Beattie MS. Tumor necrosis factor-alpha induces cFOS and strongly potentiates glutamate-mediated cell death in the rat spinal cord. Neurobiol Dis. 2001;8:590–599. doi: 10.1006/nbdi.2001.0414. 10.1006/nbdi.2001.0414. [DOI] [PubMed] [Google Scholar]
- Hermann GE, Tovar CA, Rogers RC. Induction of endogenous tumor necrosis factor-alpha: suppression of centrally stimulated gastric motility. Am J Physiol. 1999;276:R59–R68. doi: 10.1152/ajpregu.1999.276.1.R59. [DOI] [PubMed] [Google Scholar]
- Hikasa Y, Akiba T, Iino Y, Matsukura M, Takase K, Ogasawara S. Central alpha-adrenoceptor subtypes involved in the emetic pathway in cats. Eur J Pharmacol. 1992;229:241–251. doi: 10.1016/0014-2999(92)90562-i. 10.1016/0014-2999(92)90562-I. [DOI] [PubMed] [Google Scholar]
- Hornby PJ. Central neurocircuitry associated with emesis. Am J Med. 2001;111(Suppl. 8A):106S–112S. doi: 10.1016/s0002-9343(01)00849-x. 10.1016/S0002-9343(01)00849-X. [DOI] [PubMed] [Google Scholar]
- Ishibashi H, Umezu M, Jang IS, Ito Y, Akaike N. Alpha 1-adrenoceptor-activated cation currents in neurones acutely isolated from rat cardiac parasympathetic ganglia. J Physiol. 2003;548:111–120. doi: 10.1113/jphysiol.2002.033100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SM, Koshiya N, Smith JC. Isolation of the kernel for respiratory rhythm generation in a novel preparation: the pre-Botzinger complex ‘island’. J Neurophysiol. 2001;85:1772–1776. doi: 10.1152/jn.2001.85.4.1772. [DOI] [PubMed] [Google Scholar]
- Kalia M, Fuxe K, Goldstein M. Rat medulla oblongata. III. Adrenergic (C1 and C2) neurons, nerve fibers and presumptive terminal processes. J Comp Neurol. 1985;233:333–349. doi: 10.1002/cne.902330304. 10.1002/cne.902330304. [DOI] [PubMed] [Google Scholar]
- Kang J, Nedergaard M. Calcium imaging of identified astrocytes in hippocampal slices. In: Yuste R, Lanni F, Konnerth A, editors. Imaging Neurons: a Laboratory Manual. Cold Springs Harbor, NY: Cold Springs Harbor Laboratory Press; 2000. pp. 42.1–42.11. [Google Scholar]
- Kogo M, Mori A, Koizumi H, Ishihama K, Iida S, Tanaka S, Matsuya T. Effect of norepinephrine receptors on trigeminal rhythm generation in newborn rats. Brain Res Bull. 2000;53:171–174. doi: 10.1016/s0361-9230(00)00322-1. 10.1016/S0361-9230(00)00322-1. [DOI] [PubMed] [Google Scholar]
- Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. 10.1016/S0006-3223(99)00164-X. [DOI] [PubMed] [Google Scholar]
- Koshiya N, Smith JC. Neuronal pacemaker for breathing visualized in vitro. Nature. 1999;400:360–363. doi: 10.1038/22540. 10.1038/22540. [DOI] [PubMed] [Google Scholar]
- Lewis MW, Hermann GE, Rogers RC, Travagli RA. In vitro and in vivo analysis of the effects of corticotropin releasing factor on rat dorsal vagal complex. J Physiol. 2002;543:135–146. doi: 10.1113/jphysiol.2002.019281. 10.1113/jphysiol.2002.019281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BU, Balint JP. Cyclic vomiting syndrome: evolution in our understanding of a brain-gut disorder. Adv Pediatr. 2000;47:117–160. [PubMed] [Google Scholar]
- Lin LH, Emson PC, Talman WT. Apposition of neuronal elements containing nitric oxide synthase and glutamate in the nucleus tractus solitarii of rat: a confocal microscopic analysis. Neuroscience. 2000;96:341–350. doi: 10.1016/s0306-4522(99)00560-6. 10.1016/S0306-4522(99)00560-6. [DOI] [PubMed] [Google Scholar]
- Liu Z, Bunney EB, Appel SB, Brodie MS. Serotonin reduces the hyperpolarization-activated current (Ih) in ventral tegmental area dopamine neurons: involvement of 5-HT2 receptors and protein kinase C. J Neurophysiol. 2003;90:3201–3212. doi: 10.1152/jn.00281.2003. [DOI] [PubMed] [Google Scholar]
- Lora-Vilchis MC, Chambert G, Rodriguez-Zendejas AM, Soto-Mora LM, Russek M, Epstein AN. Ontogeny of alpha- and beta-adrenergic anorexia in rats. Am J Physiol. 1988;255:R908–R913. doi: 10.1152/ajpregu.1988.255.6.R908. [DOI] [PubMed] [Google Scholar]
- Marinelli S, Di Marzo V, Berretta N, Matias I, Maccarrone M, Bernardi G, Mercuri NB. Presynaptic facilitation of glutamatergic synapses to dopaminergic neurons of the rat substantia nigra by endogenous stimulation of vanilloid receptors. J Neurosci. 2003;23:3136–3144. doi: 10.1523/JNEUROSCI.23-08-03136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann MJ, Hermann GE, Rogers RC. Thyrotropin-releasing hormone: effects on identified neurons of the dorsal vagal complex. J Auton Nerv Syst. 1989;26:107–112. doi: 10.1016/0165-1838(89)90158-6. 10.1016/0165-1838(89)90158-6. [DOI] [PubMed] [Google Scholar]
- McCann MJ, Rogers RC. Oxytocin excites gastric-related neurones in rat dorsal vagal complex. J Physiol. 1990;428:95–108. doi: 10.1113/jphysiol.1990.sp018202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann MJ, Rogers RC. Impact of antral mechanoreceptor activation on the vago-vagal reflex in the rat: functional zonation of responses. J Physiol. 1992;453:401–411. doi: 10.1113/jphysiol.1992.sp019235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann MJ, Rogers RC. Functional and chemical neuroanatomy of a gastric vago-vagal reflex. In: Tache Y, Wingate DL, Burks TF, editors. Innervation of the Gut: Pathophysiological Implications. Boca Raton, FL: CRC Press; 1994. pp. 81–92. [Google Scholar]
- McTigue DM, Edwards NK, Rogers RC. Pancreatic polypeptide in dorsal vagal complex stimulates gastric acid secretion and motility in rats. Am J Physiol. 1993;265:G1169–G1176. doi: 10.1152/ajpgi.1993.265.6.G1169. [DOI] [PubMed] [Google Scholar]
- Martinez-Pena Y, Valenzuela I, Rogers RC, Hermann GE, Travagli RA. Norepinephrine effects on identified neurons of the rat dorsal motor nucleus of the vagus. Am J Physiol Gastrointest Liver Physiol. 2004;286:G333–G339. doi: 10.1152/ajpgi.00289.2003. 10.1152/ajpgi.00289.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin D, Bonnot A, Ballion B, Viala D. alpha1-adrenergic receptor-induced slow rhythmicity in nonrespiratory cervical motoneurons of neonatal rat spinal cord. Eur J Neurosci. 2000;12:2950–2966. doi: 10.1046/j.1460-9568.2000.00154.x. 10.1046/j.1460-9568.2000.00154.x. [DOI] [PubMed] [Google Scholar]
- Muyderman H, Angehagen M, Sandberg M, Bjorklund U, Olsson T, Hansson E, Nilsson M. Alpha 1-adrenergic modulation of metabotropic glutamate receptor-induced calcium oscillations and glutamate release in astrocytes. J Biol Chem. 2001;276:46504–46514. doi: 10.1074/jbc.M103849200. 10.1074/jbc.M103849200. [DOI] [PubMed] [Google Scholar]
- Muyderman H, Nilsson M, Blomstrand F, Khatibi S, Olsson T, Hansson E, Ronnback L. Modulation of mechanically induced calcium waves in hippocampal astroglial cells. Inhibitory effects of alpha 1-adrenergic stimulation. Brain Res. 1998;793:127–135. doi: 10.1016/s0006-8993(98)00151-6. 10.1016/S0006-8993(98)00151-6. [DOI] [PubMed] [Google Scholar]
- Ohliger-Frerking P, Horowitz JM, Horwitz BA. Enhanced adrenergic excitation of serotonergic dorsal raphe neurons in genetically obese rats. Neurosci Lett. 2002;332:107–110. doi: 10.1016/s0304-3940(02)00931-x. 10.1016/S0304-3940(02)00931-X. [DOI] [PubMed] [Google Scholar]
- Paton JF, Li YW, Schwaber JS. Response properties of baroreceptive NTS neurons. Ann N Y Acad Sci. 2001;940:157–168. doi: 10.1111/j.1749-6632.2001.tb03674.x. [DOI] [PubMed] [Google Scholar]
- Perez H, Ruiz S. Medullary responses to chemoreceptor activation are inhibited by locus coeruleus and nucleus raphe magnus. Neuroreport. 1995;6:1373–1376. doi: 10.1097/00001756-199507100-00003. [DOI] [PubMed] [Google Scholar]
- Perez H, Ruiz S, Laurido C, Hernandez A. Locus coeruleus-mediated inhibition of chemosensory responses in the rat nucleus tractus solitarius is mediated by alpha2-adrenoreceptors. Neurosci Lett. 1998;249:37–40. doi: 10.1016/s0304-3940(98)00387-5. 10.1016/S0304-3940(98)00387-5. [DOI] [PubMed] [Google Scholar]
- Pieribone VA, Nicholas AP, Dagerlind A, Hokfelt T. Distribution of alpha 1 adrenoceptors in rat brain revealed by in situ hybridization experiments utilizing subtype specific probes. J Neurosci. 1994;14:4252–4268. doi: 10.1523/JNEUROSCI.14-07-04252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prank K, Kropp M, Brabant G. Humoral coding and decoding. Novartis Found Symp. 2001;239:96–107. doi: 10.1002/0470846674.ch9. Discussion pp 107–110, 150–159. [DOI] [PubMed] [Google Scholar]
- Rinaman L. Hindbrain noradrenergic lesions attenuate anorexia and alter central cFos expression in rats after gastric viscerosensory stimulation. J Neurosci. 2003;23:10084–10092. doi: 10.1523/JNEUROSCI.23-31-10084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L, Hoffman GE, Dohanics J, Le WW, Stricker EM, Verbalis JG. Cholecystokinin activates catecholaminergic neurons in the caudal medulla that innervate the paraventricular nucleus of the hypothalamus in rats. J Comp Neurol. 1995;360:246–256. doi: 10.1002/cne.903600204. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Rothe EE. GLP-1 receptor signaling contributes to anorexigenic effect of centrally administered oxytocin in rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R99–R106. doi: 10.1152/ajpregu.00008.2002. [DOI] [PubMed] [Google Scholar]
- Rogers RC, McCann MJ. Intramedullary connections of the gastric region in the solitary nucleus: a biocytin histochemical tracing study in the rat. J Auton Nerv Syst. 1993;42:119–130. doi: 10.1016/0165-1838(93)90043-t. 10.1016/0165-1838(93)90043-T. [DOI] [PubMed] [Google Scholar]
- Rogers RC, McTigue DM, Hermann GE. Vagal control of digestion: modulation by central neural and peripheral endocrine factors. Neurosci Biobehav Rev. 1996;20:57–66. doi: 10.1016/0149-7634(95)00040-l. 10.1016/0149-7634(95)00040-L. [DOI] [PubMed] [Google Scholar]
- Rogers RC, Travagli RA, Hermann GE. Noradrenergic neurons in the rat solitary nucleus participate in the esophageal-gastric relaxation reflex. Am J Physiol Regul Integr Comp Physiol. 2003;285:R479–R489. doi: 10.1152/ajpregu.00155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito R, Takano Y, Kamiya HO. Roles of substance P and NK(1) receptor in the brainstem in the development of emesis. J Pharmacol Sci. 2003;91:87–94. doi: 10.1254/jphs.91.87. 10.1254/jphs.91.87. [DOI] [PubMed] [Google Scholar]
- Sanchez-Bueno A, Dixon CJ, Woods NM, Cuthbertson KS, Cobbold PH. Inhibitors of protein kinase C prolong the falling phase of each free-calcium transient in a hormone-stimulated hepatocyte. Biochem J. 1990;268:627–632. doi: 10.1042/bj2680627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM. Cellular defenses against excitotoxic insults. J Neurochem. 2001;76:1601–1611. doi: 10.1046/j.1471-4159.2001.00203.x. 10.1046/j.1471-4159.2001.00203.x. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE. Central connections of the sensory and motor nuclei of the vagus nerve. J Auton Nerv Syst. 1983;9:13–26. doi: 10.1016/0165-1838(83)90129-7. 10.1016/0165-1838(83)90129-7. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res. 1982;257:275–325. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- Schild JH, Clark JW, Canavier CC, Kunze DL, Andresen MC. Afferent synaptic drive of rat medial nucleus tractus solitarius neurons: dynamic simulation of graded vesicular mobilization, release, and non-NMDA receptor kinetics. J Neurophysiol. 1995;74:1529–1548. doi: 10.1152/jn.1995.74.4.1529. [DOI] [PubMed] [Google Scholar]
- Shi SR, Cote RJ, Taylor CR. Antigen retrieval techniques: current perspectives. J Histochem Cytochem. 2001;49:931–937. doi: 10.1177/002215540104900801. [DOI] [PubMed] [Google Scholar]
- Somogyi R, Stucki JW. Hormone-induced calcium oscillations in liver cells can be explained by a simple one pool model. J Biol Chem. 1991;266:11068–11077. [PubMed] [Google Scholar]
- Talman WT, Perrone MH, Reis DJ. Evidence for 1-glutamate as the neurotransmitter of baroreceptor afferent nerve fibers. Science. 1980;209:813–815. doi: 10.1126/science.6105709. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Hermann GE, Browning KN, Rogers RC. Musings on the wanderer: what's new in our understanding of vago-vagal reflexes? III. Activity-dependent plasticity in vago-vagal reflexes controlling the stomach. Am J Physiol Gastrointest Liver Physiol. 2003;284:G180–G187. doi: 10.1152/ajpgi.00413.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travagli RA, Rogers RC. Receptors and transmission in the brain-gut axis: potential for novel therapies. V. Fast and slow extrinsic modulation of dorsal vagal complex circuits. Am J Physiol Gastrointest Liver Physiol. 2001;281:G595–G601. doi: 10.1152/ajpgi.2001.281.3.G595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse A, Tse FW. alpha-adrenergic stimulation of cytosolic Ca2+ oscillations and exocytosis in identified rat corticotrophs. J Physiol. 1998;512:385–393. doi: 10.1111/j.1469-7793.1998.385be.x. 10.1111/j.1469-7793.1998.385be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venance L, Premont J, Glowinski J, Giaume C. Gap junctional communication and pharmacological heterogeneity in astrocytes cultured from the rat striatum. J Physiol. 1998;510:429–440. doi: 10.1111/j.1469-7793.1998.429bk.x. 10.1111/j.1469-7793.1998.429bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Orkand RK, Kettenmann H. Glial calcium: homeostasis and signaling function. Physiol Rev. 1998;78:99–141. doi: 10.1152/physrev.1998.78.1.99. [DOI] [PubMed] [Google Scholar]
- Wang YF, Hatton GI. Milk ejection burst-like electrical activity evoked in supraoptic oxytocin neurons in slices from lactating rats. J Neurophysiol. 2004;91:2312–2321. doi: 10.1152/jn.00697.2003. 10.1152/jn.00697.2003. [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Miller DK, Ho DH. Noradrenergic modulation of ephedrine-induced hypophagia. Synapse. 2003;48:18–24. doi: 10.1002/syn.10182. 10.1002/syn.10182. [DOI] [PubMed] [Google Scholar]
- Yeh SY. Comparative anorectic effects of metaraminol and phenylephrine in rats. Physiol Behav. 1999;68:227–234. doi: 10.1016/s0031-9384(99)00180-8. 10.1016/S0031-9384(99)00180-8. [DOI] [PubMed] [Google Scholar]
- Young WS, III, Kuhar MJ. Noradrenergic alpha 1 and alpha 2 receptors: light microscopic autoradiographic localization. Proc Natl Acad Sci U S A. 1980;77:1696–1700. doi: 10.1073/pnas.77.3.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lipton P. Cytosolic Ca2+ changes during in vitro ischemia in rat hippocampal slices: major roles for glutamate and Na+-dependent Ca2+ release from mitochondria. J Neurosci. 1999;19:3307–3315. doi: 10.1523/JNEUROSCI.19-09-03307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]