Abstract
The purpose of this investigation was to utilize 2,3-butanedione monoxime (BDM; an inhibitor of contractile activation) to dissociate cytosolic [Ca2+] ([Ca2+]c) from the putative respiratory regulators that arise from muscle contraction-induced ATP utilization in order to determine the relative contribution of [Ca2+]c on intracellular PO2 (PiO2) kinetics during the transition from rest to contractions in single skeletal myocytes isolated from Xenopus laevis lumbrical muscle. Myocytes were subjected to electrically induced isometric tetanic contractions (0.25 Hz; 2-min bouts) while peak tension and either [Ca2+]c (n = 7; ratiometric fluorescence microscopy) or PiO2 (n = 7; phosphorescence microscopy) was measured continuously. Cells were studied under both control and 3 mm BDM conditions in randomized order. Initial (control, 100 ± 0%; BDM, 72.6 ± 4.6%), midpoint (control, 86.7 ± 1.8%; BDM, 61.6 ± 4.1%) and end (control, 85.0 ± 2.8%; BDM, 57.5 ± 5.0%) peak tensions (normalized to initial control values) were significantly reduced (P < 0.05) with BDM compared with control (n = 14). Despite the reduced peak tension, peak [Ca2+]c was not altered (P > 0.05) between control and BDM trials. Thus, the peak tension-to-peak [Ca2+]c ratio was reduced with BDM compared with control. The absolute fall in PiO2 with contractions, which is proportional to the rise in ![]() , was significantly reduced with BDM (13.2 ± 1.3 mmHg) compared with control (22.0 ± 2.0 mmHg). However, PiO2 onset kinetics (i.e. mean response time (MRT)) was not altered between BDM (66.8 ± 8.0 s) and control (64.9 ± 6.3 s) trials. Therefore, the initial rate of change (defined as the fall in PiO2/MRT) was significantly slower in BDM fibres compared with control. These data demonstrate in these isolated single skeletal muscle fibres that unchanged peak [Ca2+]c in the face of reduced metabolic feedback from the contractile sites evoked with BDM did not alter PiO2 onset kinetics in isolated single frog myocytes, suggesting that metabolic signals arising from the contractile sites play a more substantial role than [Ca2+]c in the signalling pathway to oxidative phosphorylation during the transition from rest to repeated tetanic contractions.
, was significantly reduced with BDM (13.2 ± 1.3 mmHg) compared with control (22.0 ± 2.0 mmHg). However, PiO2 onset kinetics (i.e. mean response time (MRT)) was not altered between BDM (66.8 ± 8.0 s) and control (64.9 ± 6.3 s) trials. Therefore, the initial rate of change (defined as the fall in PiO2/MRT) was significantly slower in BDM fibres compared with control. These data demonstrate in these isolated single skeletal muscle fibres that unchanged peak [Ca2+]c in the face of reduced metabolic feedback from the contractile sites evoked with BDM did not alter PiO2 onset kinetics in isolated single frog myocytes, suggesting that metabolic signals arising from the contractile sites play a more substantial role than [Ca2+]c in the signalling pathway to oxidative phosphorylation during the transition from rest to repeated tetanic contractions.
At the transition to an elevated muscle metabolic demand, ATP demand increases in immediate, square-wave fashion whereas the ATP contribution from oxidative phosphorylation is less rapid. The rapidity with which the mitochondria respond to an elevated metabolic stress will be inversely related to the reliance placed upon glycolysis and substrate-level phosphorylation to meet energetic demands. This is important as slowed oxygen uptake ![]() onset kinetics, which results in an increased O2 deficit, culminates ultimately in a more rapid onset of fatigue. It has been suggested that the rate-limiting step to the rise in
onset kinetics, which results in an increased O2 deficit, culminates ultimately in a more rapid onset of fatigue. It has been suggested that the rate-limiting step to the rise in ![]() at exercise onset is located within the myocyte and is not contingent upon residing O2 availability (for review see Tschakovsky & Hughson, 1999; Grassi, 2000). However, O2 may become limiting under extreme stresses such as very heavy, maximal exercise (Grassi, 2000) and also in disease states such as chronic heart failure where O2 delivery is slowed (Meakins & Long, 1927; Hepple et al. 1999).
at exercise onset is located within the myocyte and is not contingent upon residing O2 availability (for review see Tschakovsky & Hughson, 1999; Grassi, 2000). However, O2 may become limiting under extreme stresses such as very heavy, maximal exercise (Grassi, 2000) and also in disease states such as chronic heart failure where O2 delivery is slowed (Meakins & Long, 1927; Hepple et al. 1999).
Currently, the exact site(s) of ‘metabolic inertia’ within the muscle remains obscure. However, muscle oxidative capacity is considered an integral determinant of ![]() kinetics as it is well accepted that exercise training-induced mitogenesis results in faster
kinetics as it is well accepted that exercise training-induced mitogenesis results in faster ![]() kinetics (Hagberg et al. 1980). Within the mitochondria, specific putative sites of limitation include cytochrome c oxidase (Kindig et al. 2001, 2002; Jones et al. 2003), and more controversially, pyruvate dehydrogenase (PDH; Timmons et al. 1998; Howlett et al. 1999; Grassi et al. 2002; Rossiter et al. 2003; Howlett & Hogan, 2003). Important roles for the NADH/NAD+ ratio, phosphorylation potential, creatine (Cr) and phosphocreatine (PCr), ADP and inorganic phosphate (Pi) in a cytosolic signalling pathway between sites of ATP hydrolysis and production have been postulated (for review see Mahler, 1985; Balaban, 1990; Meyer & Foley, 1996). Mahler (1985) suggested that the mitochondrial creatine kinase (CK) reaction may be a rate-limiting step in this process. Recently, our laboratory demonstrated that the CK-catalysed breakdown of PCr at exercise onset does play an important role in regulating
kinetics (Hagberg et al. 1980). Within the mitochondria, specific putative sites of limitation include cytochrome c oxidase (Kindig et al. 2001, 2002; Jones et al. 2003), and more controversially, pyruvate dehydrogenase (PDH; Timmons et al. 1998; Howlett et al. 1999; Grassi et al. 2002; Rossiter et al. 2003; Howlett & Hogan, 2003). Important roles for the NADH/NAD+ ratio, phosphorylation potential, creatine (Cr) and phosphocreatine (PCr), ADP and inorganic phosphate (Pi) in a cytosolic signalling pathway between sites of ATP hydrolysis and production have been postulated (for review see Mahler, 1985; Balaban, 1990; Meyer & Foley, 1996). Mahler (1985) suggested that the mitochondrial creatine kinase (CK) reaction may be a rate-limiting step in this process. Recently, our laboratory demonstrated that the CK-catalysed breakdown of PCr at exercise onset does play an important role in regulating ![]() kinetics (Kindig et al. 2004). In addition, Ca2+ released from the sarcoplasmic reticulum (SR) during contractile activation has been implicated as a rapid modulator of mitochondrial ATP production and thus has been postulated to play a role in setting the
kinetics (Kindig et al. 2004). In addition, Ca2+ released from the sarcoplasmic reticulum (SR) during contractile activation has been implicated as a rapid modulator of mitochondrial ATP production and thus has been postulated to play a role in setting the ![]() in response to increased energy demand (for review see Hansford, 1994; Balaban, 2002). Indeed, Ca2+ has been shown to activate several calcium-sensitive dehyrogenases (Hansford, 1994) including PDH (Randle et al. 1974) which will act to maximize the NADH/NAD+ ratio. In mitochondria isolated from skeletal muscle, elevating [Ca2+] within the physiological range stimulated mitochondrial respiratory rate directly, regardless of the residing substrate (Kavanagh et al. 2000). Recently, Territo et al. (2001) demonstrated, in cardiac mitochondria, that raising extramitochondrial Ca2+ levels resulted in elevated mitochondrial [Ca2+] and ATP production concomitantly in < 200 ms
in response to increased energy demand (for review see Hansford, 1994; Balaban, 2002). Indeed, Ca2+ has been shown to activate several calcium-sensitive dehyrogenases (Hansford, 1994) including PDH (Randle et al. 1974) which will act to maximize the NADH/NAD+ ratio. In mitochondria isolated from skeletal muscle, elevating [Ca2+] within the physiological range stimulated mitochondrial respiratory rate directly, regardless of the residing substrate (Kavanagh et al. 2000). Recently, Territo et al. (2001) demonstrated, in cardiac mitochondria, that raising extramitochondrial Ca2+ levels resulted in elevated mitochondrial [Ca2+] and ATP production concomitantly in < 200 ms
In the current investigation, we utilized BDM in order to reduce peak tension (and ATP utilization) yet maintain peak cytosolic [Ca2+] ([Ca2+]c). In doing so, [Ca2+]c was dissociated from the metabolites ([ADP], [Pi], etc.) arising from the ATP utilization at the contractile sites such that [Ca2+]c will be increased relative to the rise in the other cytosolic signalling pathway components. The purpose of this investigation was to study this effect on intracellular PO2 (PiO2) kinetics at the onset of moderate intensity contractions in intact single myocytes isolated from frog muscle. We tested the hypothesis that if [Ca2+]c is the primary determinant for increased oxidative phosphorylation at the transition from rest to contractions in frog skeletal muscle, then a similar initial rate of change of oxidative phosphorylation in the face of reduced metabolic demand with BDM would result in overall faster PiO2 kinetics of individual fibres (mean response time (MRT)) compared with a matched control trial. Alternatively, if the oxidative phosphorylation activation rate is proportional to the metabolic signals arising directly from contractile site ATP utilization, then the initial rate of change of oxidative phosphorylation would be decreased with BDM, resulting in unchanged MRT between BDM and control trials.
Methods
Female adult Xenopus laevis were used in this investigation. All procedures were approved by the University of California–San Diego animal use and care committee and conform to National Institutes of Health standards.
Myocyte preparation
Single muscle cells (n = 14) were isolated and prepared as previously described (Hogan, 1999). Briefly, frogs were submerged in chilled water, then stunned, decapitated and pithed, and the lumbrical muscles (II–IV) were removed from the hind feet. Single myocytes were dissected out with tendons intact in a chamber of physiological Ringer solution at a pH = 7.0. Cells were injected via micropipette pressure injection (PV830 pneumatic picopump, World Precision Instruments, Sarasota, FL, USA) with either a solution consisting of 0.5 mm Pd-meso-tetra (4-carboxyphenyl) porphine bound to bovine serum albumin (for phosphorescence quenching) and the Ca2+ indicator dye fura 2 (10 mm; Molecular Probes, Eugene, OR, USA) or fura 2 alone (for fluorescence microscopy).
Experimental protocol
Platinum clips were attached to the tendons of each myocyte to facilitate fibre positioning within the Ringer solution-filled chamber. One tendon was fixed, whereas the contralateral was attached to an adjustable force transducer (model 400A, Aurora Scientific, Aurora, Ontario, Canada), allowing the muscle to be set at optimum muscle length. The analog signal from the force transducer was recorded via a data acquisition system (AcqKnowledge, Biopac Systems, Santa Barbara, CA, USA) for subsequent analysis. Fibres were perfused throughout the experiment with Ringer solution equilibrated with 5% CO2 and 4% O2 in N2 balance. Constant perfusion was maintained throughout the protocol to maintain the extracellular PO2 at ∼30 Torr and to reduce the occurrence of an appreciable unstirred layer surrounding the cell. Tetanic contractions were elicited using direct (8–10 V) stimulation of the muscle (model S48, Grass Instruments, Warwick, RI, USA). The stimulation protocol consisted of ∼250 ms trains of 70 Hz impulses of 1 ms duration. Myocytes were subjected to trials of ∼120 s at a ∼0.25 Hz stimulation frequency with a 15 min recovery period between trials.
Separate experiments were performed to obtain either the [Ca2+]c (n = 7) or PO2 (n = 7) response to contractions under control and matched 3 mm BDM conditions. The effects of BDM on force production in skeletal muscle are fully reversible (Sun et al. 2001), so all experiments were randomized. One trial per treatment per cell was obtained for the [Ca2+]c data whereas two trials per treatment per cell were acquired for all PO2 data. In the latter, the two control and two BDM trials were averaged for data analysis.
Cytosolic [Ca2+] measurement
[Ca2+]c was measured using an epifluorescent microscope system that consisted of a Nikon inverted microscope with a × 40 fluor objective and a DeltaScan illumination and detection system (Photon Technology International, South Brunswick, NJ, USA) as previously described (Stary & Hogan, 2000). Fibres injected with fura 2 were illuminated sequentially (20 Hz) with two excitation wavelengths of 340 and 380 nm, and the resulting fluorescence emission was measured at 510 nm. The ratio of 340/380 nm fluorescence was used to obtain the Ca2+-dependent signal (Grynkiewicz et al. 1985).
Assessment of PiO2
Each myocyte was observed with a Nikon × 40 fluor objective (0.70 numerical aperture). The phosphorescence quenching of the porphyrin compound within the myocyte was measured via a system consisting of a flash lamp (Oxygen Enterprises, Philadelphia, PA, USA), a 425 nm band-pass excitation filter, a 630 nm cut-on emission filter, and a photomultiplier tube for collection of the phosphorescence signal. To calculate phosphorescence lifetimes from the intracellular O2 probe, the phosphorescent decay curves from a series of 10 flashes (15 Hz) were averaged, and a mono-exponential function was fitted to the subsequent best-fit decay curve (analysis software from Medical Systems, Greenvale, NY, USA). The O2 dependence of phosphorescence quenching is described by the Stern-Volmer equation where:
thus
where τo and τ are the phosphorescence lifetimes at anoxia and a given PO2, respectively, and kq, the quenching constant (in Torr s−1), is a second-order rate constant that is related to the frequency of collisions between O2 and the excited triplet state of the porphyrin and the probability of energy transfer when collisions occur. The constants kq and τo were set at 690 mmHg s−1 and 100 µs for Pd-meso-tetra (4-carboxyphenyl) porphine bound to albumin in solution for this preparation as established previously (Hogan, 1999). Phosphorescent decay curves were recorded every 4 s from each cell throughout the experimental period.
Data and statistical analysis
Following experimental procedures, the mean response time (MRT) was calculated as the time to 63% of the fall in PiO2 with contractions. Both peak tension and [Ca2+]c data were normalized to the initial control point. Data are presented as mean ± s.e.m. Differences between trials in regard to the PiO2 fall and MRT were tested via a paired t test. Changes in peak tension and [Ca2+]c were tested via a repeated measures 2-way ANOVA. When significant F values were present, the Bonferroni post hoc test was employed for determination of between-group differences. Statistical significance was accepted at P < 0.05.
Results
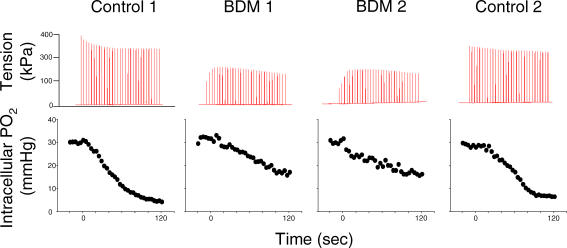
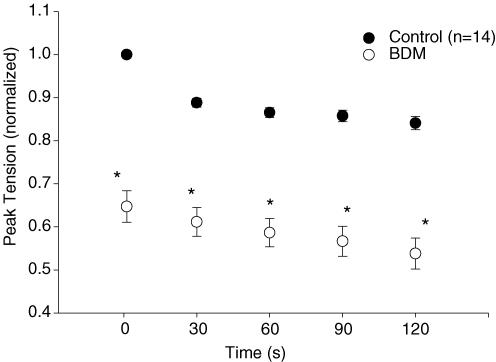
In Fig. 1, tension and PiO2 data for one representative cell over four contractions bouts (2 control and 2 BDM) are shown. BDM induced a significant reduction in peak tension (P < 0.05) compared with control over the duration of the contractions bout (n = 14; Fig. 2). Specifically, initial (control, 100 ± 0%; BDM, 72.6 ± 4.6%), midpoint (control, 86.7 ± 1.8%; BDM, 61.6 ± 4.1%) and end (control, 85.0 ± 2.8%; BDM, 57.5 ± 5.0%) peak tensions (normalized to initial control values) were significantly reduced with BDM compared with control.
Figure 1. Data for a representative myocyte.
Tension (upper panel) and intracellular PO2 (lower panel) for one representative single myocyte at rest and during a 2-min bout of isometric tetanic contractions under control and 3 mm BDM conditions.
Figure 2. Mean (± s.e.m.) peak tension for control and BDM trials.
Peak tension, normalized to initial control values, was significantly reduced (*P < 0.05) throughout duration of the isometric contractions bout in BDM compared with the control trial. Peak tension data were pooled from both the [Ca2+]c (n = 7) and PiO2 (n = 7) experiments.
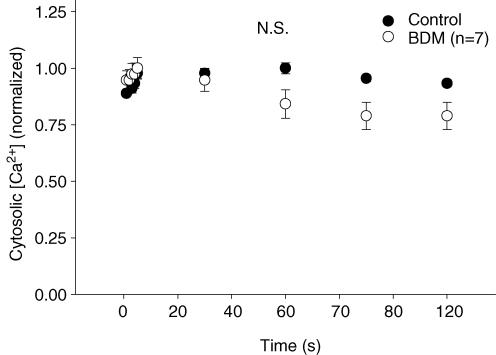
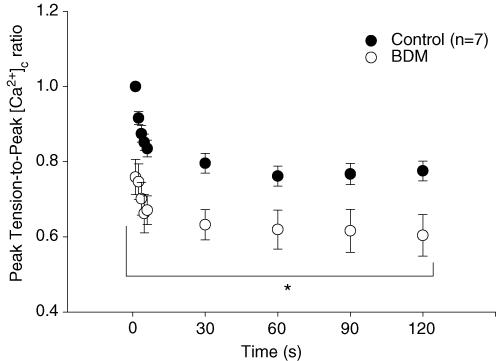
Despite the significant loss of peak tension from BDM administration, peak [Ca2+]c was not altered (P > 0.05) between control and BDM trials (n = 7; Fig. 3). Accordingly, the peak tension-to-peak [Ca2+]c was reduced (n = 7; P < 0.05) with BDM compared with control across the duration of the contractions bout (Fig. 4). Neither resting nor baseline [Ca2+]c values during contractions differed (P > 0.05) between control and BDM trials.
Figure 3. Peak cytosolic [Ca2+] for control and BDM trials.
Peak cytosolic [Ca2+] (mean ± s.e.m.), normalized to initial contraction values, was not different (N.S.; P > 0.05) between BDM and matched control across a 2-min bout of isometric tetanic contractions.
Figure 4. The peak tension-to-peak cytosolic [Ca2+] ([Ca2+]c) ratio for control and matched BDM trials.
The peak tension-to-peak [Ca2+]c ratio was significantly reduced (*P < 0.05) across a 2-min bout of isometric tetanic contractions in BDM compared with control.
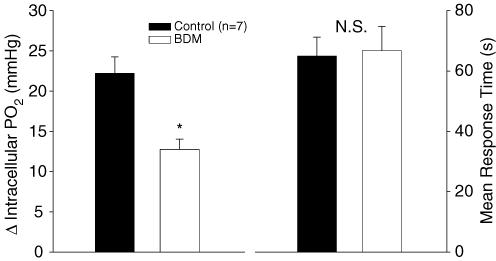
As shown in Fig. 5 (left panel), the fall in PiO2 from resting baseline to the end of contractions was significantly reduced (P < 0.05) with BDM (13.2 ± 1.3 mmHg) compared with control (22.0 ± 2.0 mmHg). There was a trend (P = 0.09) for the ratio of the fall in peak tension with BDM (0.63 ± 0.03) to be greater than the ratio of the fall in PiO2 (0.52 ± 0.06), indicative of a greater aerobic cost per contractions in the BDM trial compared with control. Additionally, PiO2 MRT was not altered (P > 0.05) between BDM (66.8 ± 8.0 s) and control (64.9 ± 6.3 s) trials (Fig. 5, right panel). A normalization of the MRT to PiO2 reductions yields an initial rate of change (fall in PiO2/MRT), which was significantly faster (P < 0.05) in control versus BDM as clearly seen in Fig. 1.
Figure 5. Intracellular PO2 (PiO2) data for BDM and control trials.
The fall in PiO2 with contractions was significantly greater (*P < 0.05) in control compared with the BDM trial (left panel). The mean response time for the fall in PiO2 with not different (N.S.; P > 0.05) between control and BDM trials (right panel).
Discussion
Cytosolic Ca2+ has been suggested to be a key mitochondrial signalling factor in muscle. In the current investigation, BDM was employed to reduce peak tension (and ATP utilization) yet maintain peak [Ca2+]c. Thus, peak [Ca2+]c was dissociated from the cytosolic signalling metabolites ([ADP], [Pi], etc.) arising from contraction-induced ATP utilization such that [Ca2+]c was greater relative to the rise in the other cytosolic signalling processes. The key and novel finding from the current investigation is that BDM administration did not alter the PiO2 MRT compared with control. In fact, the initial rate of change (i.e. fall in PiO2/MRT) was actually faster in control compared with the BDM trial in concert with apparent first-order metabolic control of oxidative phosphorylation by metabolic signals arising from the contractile sites (Mahler, 1985; Meyer, 1988; C.A. Kindig, R.A. Howlett & M.C. Hogan, unpublished observations). These data demonstrate that [Ca2+]c, in itself, does not exert control over the speed of the oxidative phosphorylation increase in response to repetitive tetanic contractions and suggest that [Ca2+]c may not be an integral metabolic signalling component for oxidative phosphorylation in this isolated single skeletal muscle model.
Methodological considerations
A direct relationship between ![]() and PiO2 for single myocytes lacking myoglobin, such as in Xenopus muscle, is described by Fick's law of diffusion where:
and PiO2 for single myocytes lacking myoglobin, such as in Xenopus muscle, is described by Fick's law of diffusion where:
where ![]() is the rate of diffusion of O2 and extracellular PO2 is the external partial pressure of oxygen.
is the rate of diffusion of O2 and extracellular PO2 is the external partial pressure of oxygen.
For our single myocyte preparation, this relationship has been verified previously (Howlett & Hogan, 2001). In intact muscle, the capillary (more specifically, red blood cell)-to-myocyte interface determines muscle-diffusing O2 capacity and thus ![]() is a dynamic variable (Federspiel & Popel, 1986; Mathieu-Costello et al. 1991). In this preparation,
is a dynamic variable (Federspiel & Popel, 1986; Mathieu-Costello et al. 1991). In this preparation, ![]() represents the muscle O2 diffusion coefficient, which at the temperature of our experimental set-up, is 1.01 × 105 cm2 s−1 (Mahler et al. 1985) and remains unchanged across the rest–contractions transition. Thus, in our single muscle fibre preparation, the fall in PiO2 reflects linearly the rise in
represents the muscle O2 diffusion coefficient, which at the temperature of our experimental set-up, is 1.01 × 105 cm2 s−1 (Mahler et al. 1985) and remains unchanged across the rest–contractions transition. Thus, in our single muscle fibre preparation, the fall in PiO2 reflects linearly the rise in ![]() . One consideration in the present study using the isolated single Xenopus skeletal muscle fibre preparation is that these large myoglobin-free fibres may yield slightly different
. One consideration in the present study using the isolated single Xenopus skeletal muscle fibre preparation is that these large myoglobin-free fibres may yield slightly different ![]() onset kinetics compared with mammalian skeletal muscle which have a much smaller diameter and contain myoglobin.
onset kinetics compared with mammalian skeletal muscle which have a much smaller diameter and contain myoglobin.
BDM, a drug with phosphatase-like activity, apparently affects anuran and mammalian muscle differently in terms of specific sites of action (Sellin & McArdle, 1994). In frog muscle, at concentrations greater than 2 mm, BDM has been shown to have a direct effect on the contractile apparatus where it inhibits cross-bridge cycling, whereas at concentrations greater than 5 mm BDM may impair SR Ca2+ release (Sun et al. 2001). In the current investigation, BDM was titrated at 3 mm in order to significantly reduce peak tension production (Figs 1 and 2) yet not affect SR Ca2+ handling (i.e. not alter peak [Ca2+]c with contractions; Fig. 3).
In the current investigation, there was a trend (P = 0.09) toward a greater specific aerobic cost of contractions (absolute fall in PiO2/force) in BDM compared with the control trial. This would be consistent with an increased (relative to control) energetic cost of Ca2+-ATPase activity with BDM in the face of reduced mechanical work which is consistent with a significant ATP cost of SR Ca2+ handling as has been demonstrated previously in dog muscle (Hogan et al. 1998).
Cytosolic Ca2+
Cytosolic Ca2+ is believed to control the two major ATPase reactions in the myocyte, myosin-ATPase, through its interaction with troponin, and Ca2+-ATPase of the SR. There are many targets for Ca2+-induced activation of oxidative phosphorylation. It is well-established that Ca2+ activates several calcium-sensitive dehydrogenases located within the permeability barrier of the inner mitochondrial membrane including pyruvate, isocitrate and 2-oxogluturate (fro review see Hansford, 1994). Additionally, it is thought that glycerol 3-phosphate dehydrogenase, which is located on the outer surface of the inner mitochondrial membrane and thus exposed to the cytosolic environment, is also sensitive to [Ca2+] (Hansford & Chappell, 1967). Furthermore, it has been demonstrated that Ca2+ directly activates oxidative phosphorylation in mitochondria isolated from skeletal muscle (Kavanaugh et al. 2000). These studies have made Ca2+ an attractive candidate as a putative controlling signal of oxidative phosphorylation in cardiac and skeletal muscle (for review see Hansford, 1994; Balaban, 2002).
The time delay prior to a discernible rise in ![]() at the transition to an elevation in metabolic demand is thought to occur within a matter of seconds, if not almost immediately (Bangsbo et al. 2000; Behnke et al. 2002; Kindig et al. 2003b). Thus, from the above, it would appear most likely, although not necessary, that for Ca2+ to modulate
at the transition to an elevation in metabolic demand is thought to occur within a matter of seconds, if not almost immediately (Bangsbo et al. 2000; Behnke et al. 2002; Kindig et al. 2003b). Thus, from the above, it would appear most likely, although not necessary, that for Ca2+ to modulate ![]() onset kinetics, Ca2+ would have to move from the cytosol into the mitochondria in a similar time course and, also, over the brief contractile transient when cytosolic levels are elevated. Mitochondria have been shown to take up Ca2+ within the time scale of physiological Ca2+ pulses (Sparagna et al. 1995; Duchen et al. 1998). This Ca2+ uptake is mediated by a uniporter and is driven by a large potential difference across the inner mitochondrial membrane (Duchen et al. 1998). In mitochondria isolated from cardiac muscle, Territo et al. (2001) demonstrated that mitochondrial [Ca2+] and ATP production increases to step increases in extramitochondrial [Ca2+] occurred within 150 ms. In muscle fibres isolated from rat soleus, Bruton et al. (2003) demonstrated that Ca2+ uptake into the mitochondria increased after a single tetanic contraction. Most pertinent to the current investigation, Lannergren et al. (2001) demonstrated that, in contracting single Xenopus myocytes, mitochondrial [Ca2+] had increased markedly within the first 10 tetanic contractions. However, how rapidly Ca2+ within the mitochondria was detected and the time course of that rise was not reported (Lannergren et al. 2001). Thus, as a whole, these data suggest that mitochondrial [Ca2+] increases in a manner such that it theoretically might play a role in modulating the rise in
onset kinetics, Ca2+ would have to move from the cytosol into the mitochondria in a similar time course and, also, over the brief contractile transient when cytosolic levels are elevated. Mitochondria have been shown to take up Ca2+ within the time scale of physiological Ca2+ pulses (Sparagna et al. 1995; Duchen et al. 1998). This Ca2+ uptake is mediated by a uniporter and is driven by a large potential difference across the inner mitochondrial membrane (Duchen et al. 1998). In mitochondria isolated from cardiac muscle, Territo et al. (2001) demonstrated that mitochondrial [Ca2+] and ATP production increases to step increases in extramitochondrial [Ca2+] occurred within 150 ms. In muscle fibres isolated from rat soleus, Bruton et al. (2003) demonstrated that Ca2+ uptake into the mitochondria increased after a single tetanic contraction. Most pertinent to the current investigation, Lannergren et al. (2001) demonstrated that, in contracting single Xenopus myocytes, mitochondrial [Ca2+] had increased markedly within the first 10 tetanic contractions. However, how rapidly Ca2+ within the mitochondria was detected and the time course of that rise was not reported (Lannergren et al. 2001). Thus, as a whole, these data suggest that mitochondrial [Ca2+] increases in a manner such that it theoretically might play a role in modulating the rise in ![]() at the transition to elevated metabolic demand.
at the transition to elevated metabolic demand.
In the present investigation, mitochondrial [Ca2+] was not measured. However, as BDM has no known effect on mitochondrial Ca2+ uptake, if it is assumed that mitochondrial [Ca2+] is largely contingent upon residing cytosolic values (as discussed above), then in the current investigation, both would be similar under control and BDM conditions (see Fig. 3). Despite the elevated [Ca2+]c-to-metabolic demand ratio with BDM application, the PiO2 MRT did not differ from control values (Fig. 5). This would initially appear to be in conflict with investigations of metabolic control by Ca2+ in cardiac muscle (for review see Balaban, 2002). However, in heart muscle, unlike skeletal muscle (as discussed below), key regulatory feedback metabolites including [Cr], [ADP], [Pi] and [NADH] remain nearly constant over a wide range of metabolic rates (for review see Balaban, 2002).
Putative controllers of oxidative phosphorylation in skeletal muscle
The data presented herein demonstrate that unchanged [Ca2+]c, in the face of reduced cytosolic metabolite signals commensurate with the reduced peak tension and ATP utilization, does not alter the PiO2 MRT, at least in frog skeletal muscle. What singular component is responsible for the ‘metabolic inertia’ seen at the transition to an elevated metabolic demand is not clear. Unlike that reported in cardiac muscle, alterations in key skeletal muscle cytosolic signals such as ADP, Pi, NADH and PCr occur almost instantaneously at the onset of exercise and tend to change in linear/quasi-linear fashion with increases in exercise intensity. Thus putative mechanisms for metabolic control include: kinetic limitation by cytosolic [ADP] and/or [Pi] in accordance with Michaelis-Menten kinetics (Chance & Williams, 1955), non-equilibrium thermodynamic control via the phosphorylation potential (i.e. [ATP]/[ADP][Pi]) and electron transport chain redox potential (i.e. [NADH]/[NAD]), and alterations in Gibbs free energy of cytoplasmic ATP hydrolysis (for review see Balaban, 1990; Meyer & Foley, 1996). Additionally, it was demonstrated recently that [PCr] and the [PCr]/[Cr] ratio may play an important role in the regulation of mitochondrial ADP-stimulated respiration (Walsh et al. 2001). An attractive hypothesis is that mitochondrial respiration may be controlled by local production of ADP via CK-catalysed breakdown of PCr (e.g. Mahler, 1985). Additionally, the regulatory effect of [O2] on oxidative phosphorylation during exercise is an issue that remains currently unresolved due, in part, to differences associated with work intensity and the organ/system studied (for review see Tschakovsky & Hughson, 1999; Grassi, 2000). Within isolated mitochondria, as PO2 falls across a physiological range, cytochrome c is reduced, whereas ![]() remains uniform to levels near 1 mmHg (Wilson et al. 1983; Wilson & Rumsey, 1988). In intact, exercising muscle, the microcirculatory PO2 considered rate limiting is dependent upon the capillary-to-myocyte interface, fibre type and mitochondrial capacity. For the purposes of the current study, extracellular PO2 was tightly controlled at 30 mmHg which is well above that considered ‘rate limiting’ at the onset of contractions in this preparation (Hogan, 2001; Kindig et al. 2003a).
remains uniform to levels near 1 mmHg (Wilson et al. 1983; Wilson & Rumsey, 1988). In intact, exercising muscle, the microcirculatory PO2 considered rate limiting is dependent upon the capillary-to-myocyte interface, fibre type and mitochondrial capacity. For the purposes of the current study, extracellular PO2 was tightly controlled at 30 mmHg which is well above that considered ‘rate limiting’ at the onset of contractions in this preparation (Hogan, 2001; Kindig et al. 2003a).
Regardless of the exact mechanism(s) that ultimately determines the speed of the rise in oxidative phosphorylation at exercise onset, the data in the current investigation support the proposition that muscle respiration at the transition to an altered metabolic demand is under first-order control (Mahler, 1985; Meyer, 1988). First-order respiratory control requires a similar time course for ![]() onset kinetics to step changes in metabolism and a time course (i.e. MRT) independent of submaximal ATP demand. Recently, our laboratory demonstrated that altering metabolic demand in single contracting frog fibres by altering the contraction duration did not affect the PiO2 MRT (C.A. Kindig, R.A. Howlett & M.C. Hogan, unpublished observations). However, the initial rate of PiO2 change was greater in the trial with the augmented contraction duration, demonstrating that, in accordance with a greater initial impulse (i.e. larger signal for augmented respiration) with increased contractile duration, the available mitochondria were able to respond more rapidly to the higher metabolic rate (C.A. Kindig, R.A. Howlett & M.C. Hogan, unpublished observations). Similarly (and as clearly seen in Fig. 1) the MRT was not different between control and BDM trials in the current investigation, whereas the initial rate of change was more rapid in the trial with the greater metabolic demand (i.e. control > BDM). Although the potential for compensatory mechanisms affecting the MRT cannot be dismissed, the present study suggests that the respiratory control signals that originate from myosin-ATPase activity are a greater determinant of PiO2 onset kinetics than is a relative increase in [Ca2+]c in these isolated single skeletal muscle fibres.
onset kinetics to step changes in metabolism and a time course (i.e. MRT) independent of submaximal ATP demand. Recently, our laboratory demonstrated that altering metabolic demand in single contracting frog fibres by altering the contraction duration did not affect the PiO2 MRT (C.A. Kindig, R.A. Howlett & M.C. Hogan, unpublished observations). However, the initial rate of PiO2 change was greater in the trial with the augmented contraction duration, demonstrating that, in accordance with a greater initial impulse (i.e. larger signal for augmented respiration) with increased contractile duration, the available mitochondria were able to respond more rapidly to the higher metabolic rate (C.A. Kindig, R.A. Howlett & M.C. Hogan, unpublished observations). Similarly (and as clearly seen in Fig. 1) the MRT was not different between control and BDM trials in the current investigation, whereas the initial rate of change was more rapid in the trial with the greater metabolic demand (i.e. control > BDM). Although the potential for compensatory mechanisms affecting the MRT cannot be dismissed, the present study suggests that the respiratory control signals that originate from myosin-ATPase activity are a greater determinant of PiO2 onset kinetics than is a relative increase in [Ca2+]c in these isolated single skeletal muscle fibres.
Summary
The pharmacologic agent BDM reduced peak tension and thus ATP utilization yet did not affect peak [Ca2+]c in contracting single frog myocytes. Hence, BDM peak [Ca2+]c was dissociated from the cytosolic signalling metabolites (i.e. reduced [ADP], [Pi], etc. commensurate with the reduced peak tension) such that [Ca2+]c was greater relative to the rise in the other cytosolic signalling processes. While BDM reduced the fall in PiO2 with contractions in a manner similar to the loss of peak tension, this manipulation did not alter the PiO2 MRT. With invariant MRTs and different metabolic rates, the initial rate of PiO2 change was faster in control compared with the BDM trial, suggesting that ![]() on-kinetics in isolated frog skeletal muscle fibres are significantly more contingent upon metabolic signals from the contractile sites compared with [Ca2+]c.
on-kinetics in isolated frog skeletal muscle fibres are significantly more contingent upon metabolic signals from the contractile sites compared with [Ca2+]c.
Acknowledgments
This work was supported, in part, by grants from the NIH: NIAMSD AR-40155 (M.C.H.) and AR-48461. C.A.K. was a Parker B. Francis pulmonary fellow.
References
- Balaban RS. Regulation of oxidative phosphorylation in the mammalian cell. Am J Physiol. 1990;258:C377–389. doi: 10.1152/ajpcell.1990.258.3.C377. [DOI] [PubMed] [Google Scholar]
- Balaban RS. Cardiac energy metabolism homeostasis: role of cytosolic calcium. J Mol Cell Cardiol. 2002;34:1259–1271. doi: 10.1006/jmcc.2002.2082. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Krustrup P, Gonzalez-Alonso J, Boushel R, Saltin B. Muscle oxygen kinetics at onset of intense dynamic exercise in humans. Am J Physiol Regul Integr Comp Physiol. 2000;279:R899–906. doi: 10.1152/ajpregu.2000.279.3.R899. [DOI] [PubMed] [Google Scholar]
- Behnke BJ, Barstow TJ, Kindig CA, McDonough P, Musch TI, Poole DC. Dynamics of oxygen uptake following exercise onset in rat skeletal muscle. Respir Physiol Neurobiol. 2002;133:229–239. doi: 10.1016/s1569-9048(02)00183-0. [DOI] [PubMed] [Google Scholar]
- Bruton J, Tavi P, Aydin J, Westerblad H, Lannergren J. Mitochondrial and myoplasmic [Ca2+] in single fibres from mouse limb muscles during repeated tetanic contractions. J Physiol. 2003;551:179–190. doi: 10.1113/jphysiol.2003.043927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955;217:383–393. [PubMed] [Google Scholar]
- Duchen MR, Leyssens A, Crompton M. Transient mitochondrial depolarizations reflect focal sarcoplasmic reticular calcium release in single rat cardiomyocytes. J Cell Biol. 1998;142:975–988. doi: 10.1083/jcb.142.4.975. 10.1083/jcb.142.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federspiel WJ, Popel AS. A theoretical analysis of the effect of the particulate nature of blood on oxygen release in capillaries. Microvasc Res. 1986;32:164–189. doi: 10.1016/0026-2862(86)90052-x. 10.1016/0026-2862(86)90052-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi B. Skeletal muscle VO2 on-kinetics: set by O2 delivery or by O2 utilization? New insights into an old issue. Med Sci Sports Exerc. 2000;32:108–116. doi: 10.1097/00005768-200001000-00017. 10.1097/00005768-200001000-00017. [DOI] [PubMed] [Google Scholar]
- Grassi B, Hogan MC, Greenhaff PL, Hamann JJ, Kelley KM, Aschenbach WG, Constantin-Teodosiu D, Gladden LB. Oxygen uptake on-kinetics in dog gastrocnemius in situ following activation of pyruvate dehydrogenase by dichloroacetate. J Physiol. 2002;538:195–207. doi: 10.1113/jphysiol.2001.012984. 10.1113/jphysiol.2001.012984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hagberg JM, Hickson RC, Ehsani AA, Holloszy JO. Faster adjustment to and recovery from submaximal exercise in the trained state. J Appl Physiol. 1980;48:218–224. doi: 10.1152/jappl.1980.48.2.218. [DOI] [PubMed] [Google Scholar]
- Hansford RG. Role of calcium in respiratory control. Med Sci Sports Exerc. 1994;26:44–51. [PubMed] [Google Scholar]
- Hansford RG, Chappell JB. The effect of Ca2+ on the oxidation of glycerol phosphate by blowfly flight-muscle mitochondria. Biochem Biophys Res Commun. 1967;27:686–692. doi: 10.1016/s0006-291x(67)80090-1. [DOI] [PubMed] [Google Scholar]
- Hepple RT, Liu PP, Plyley MJ, Goodman JM. Oxygen uptake kinetics during exercise in chronic heart failure: influence of peripheral vascular reserve. Clin Sci (Lond) 1999;97:569–577. [PubMed] [Google Scholar]
- Hogan MC. Phosphorescence quenching method for measurement of intracellular PO2 in isolated skeletal muscle fibers. J Appl Physiol. 1999;86:720–724. doi: 10.1152/jappl.1999.86.2.720. [DOI] [PubMed] [Google Scholar]
- Hogan MC. Fall in intracellular PO2 at the onset of contractions in Xenopus single skeletal muscle fibers. J Appl Physiol. 2001;90:1871–1876. doi: 10.1152/jappl.2001.90.5.1871. [DOI] [PubMed] [Google Scholar]
- Hogan MC, Ingham E, Kurdak SS. Contraction duration affects metabolic energy cost and fatigue in skeletal muscle. Am J Physiol. 1998;274:E397–402. doi: 10.1152/ajpendo.1998.274.3.E397. [DOI] [PubMed] [Google Scholar]
- Howlett RA, Heigenhauser GJ, Hultman E, Hollidge-Horvat MG, Spriet LL. Effects of dichloroacetate infusion on human skeletal muscle metabolism at the onset of exercise. Am J Physiol. 1999;277:E18–25. doi: 10.1152/ajpendo.1999.277.1.E18. [DOI] [PubMed] [Google Scholar]
- Howlett RA, Hogan MC. Intracellular PO2 decreases with increasing stimulation frequency in contracting single Xenopus muscle fibers. J Appl Physiol. 2001;91:632–636. doi: 10.1152/jappl.2001.91.2.632. [DOI] [PubMed] [Google Scholar]
- Howlett RA, Hogan MC. Dichloroacetate accelerates the fall in intracellular PO2 at onset of contractions in Xenopus single muscle fibers. Am J Physiol Regul Integr Comp Physiol. 2003;284:R481–485. doi: 10.1152/ajpregu.00078.2002. [DOI] [PubMed] [Google Scholar]
- Jones AM, Wilkerson DP, Koppo K, Wilmshurst S, Campbell IT. Inhibition of nitric oxide synthase by l-NAME speeds phase II pulmonary PO2 kinetics in the transition to moderate-intensity exercise in man. J Physiol. 2003;552:265–272. doi: 10.1113/jphysiol.2003.045799. 10.1113/jphysiol.2003.045799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh NI, Ainscow EK, Brand MD. Calcium regulation of oxidative phosphorylation in rat skeletal muscle mitochondria. Biochim Biophys Acta. 2000;1457:57–70. doi: 10.1016/s0005-2728(00)00054-2. [DOI] [PubMed] [Google Scholar]
- Kindig CA, Howlett RA, Hogan MC. Effect of extracellular PO2 on the fall in intracellular PO2 in contracting single myocytes. J Appl Physiol. 2003a;94:1964–1970. doi: 10.1152/japplphysiol.00893.2002. [DOI] [PubMed] [Google Scholar]
- Kindig CA, Howlett RA, Stary CM, Walsh BJ, Hogan MC. Effect of acute creatine kinase inhibition on metabolism and contractility in isolated single myocytes. J Appl Phys. 2004 doi: 10.1152/japplphysiol.00354.2004. (in press) [DOI] [PubMed] [Google Scholar]
- Kindig CA, Kelley KM, Howlett RA, Stary CM, Hogan MC. Assessment of O2 uptake dynamics in isolated single skeletal myocytes. J Appl Physiol. 2003b;94:353–357. doi: 10.1152/japplphysiol.00559.2002. [DOI] [PubMed] [Google Scholar]
- Kindig CA, McDonough P, Erickson HH, Poole DC. Effect of L-NAME on oxygen uptake kinetics during heavy intensity exercise in the horse. J Appl Physiol. 2001;91:891–896. doi: 10.1152/jappl.2001.91.2.891. [DOI] [PubMed] [Google Scholar]
- Kindig CA, McDonough P, Erickson HH, Poole DC. Nitric oxide synthase inhibition speeds oxygen uptake kinetics in horses during moderate domain running. Respir Physiol Neurobiol. 2002;132:169–178. doi: 10.1016/s1569-9048(02)00068-x. 10.1016/S1569-9048(02)00068-X. [DOI] [PubMed] [Google Scholar]
- Lannergren J, Westerblad H, Bruton JD. Changes in mitochondrial Ca2+ detected with Rhod-2 in single frog and mouse skeletal muscle fibres during and after repeated tetanic contractions. J Muscle Res Cell Motil. 2001;22:265–275. doi: 10.1023/a:1012227009544. 10.1023/A:1012227009544. [DOI] [PubMed] [Google Scholar]
- Mahler M. First-order kinetics of muscle oxygen consumption, and an equivalent proportionality between QO2 and phosphorylcreatine level. Implications for the control of respiration. J General Physiol. 1985;86:135–165. doi: 10.1085/jgp.86.1.135. 10.1085/jgp.86.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler M, Louy C, Homsher E, Peskoff A. Reappraisal of diffusion, solubility, and consumption of oxygen in frog skeletal muscle, with applications to muscle energy balance. J General Physiol. 1985;86:105–134. doi: 10.1085/jgp.86.1.105. 10.1085/jgp.86.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu-Costello O, Ellis CG, Potter RF, MacDonald IC, Groom AC. Muscle capillary-to-fiber perimeter ratio: morphometry. Am J Physiol. 1991;261:H1617–1625. doi: 10.1152/ajpheart.1991.261.5.H1617. [DOI] [PubMed] [Google Scholar]
- Meakins J, Long CNH. Oxygen consumption, oxygen debt, and lactic acid in circulatory failure. J Clin Invest. 1927;4:273–293. doi: 10.1172/JCI100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RA. A linear model of muscle respiration explains monoexponential phosphocreatine changes. Am J Physiol. 1988;254:C548–553. doi: 10.1152/ajpcell.1988.254.4.C548. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Foley JM. Handbook of Physiology. Bethesda, MD: American Physiological Society; 1996. Cellular processes integrating the metabolic response to exercsise; pp. 841–869. In sect. 12, chap. 18 pp. [Google Scholar]
- Randle PJ, Denton RM, Pask HT, Severson DL. Calcium ions and the regulation of pyruvate dehydrogenase. Biochem Soc Symp. 1974;39:75–87. [PubMed] [Google Scholar]
- Rossiter HB, Ward SA, Howe FA, Wood DM, Kowalchuk JM, Griffiths JR, Whipp BJ. Effects of dichloroacetate on VO2 and intramuscular 31P metabolite kinetics during high-intensity exercise in humans. J Appl Physiol. 2003;95:1105–1115. doi: 10.1152/japplphysiol.00964.2002. [DOI] [PubMed] [Google Scholar]
- Sellin LC, McArdle JJ. Multiple effects of 2,3-butanedione monoxime. Pharmacol Toxicol. 1994;74:305–313. doi: 10.1111/j.1600-0773.1994.tb01365.x. [DOI] [PubMed] [Google Scholar]
- Sparagna GC, Gunter KK, Sheu SS, Gunter TE. Mitochondrial calcium uptake from physiological-type pulses of calcium: a description of the rapid uptake model. J Biol Chem. 1995;270:27510–27515. doi: 10.1074/jbc.270.46.27510. 10.1074/jbc.270.46.27510. [DOI] [PubMed] [Google Scholar]
- Stary CM, Hogan MC. Impairment of Ca2+ release in single Xenopus muscle fibers fatigued at varied extracellular PO2. J Appl Physiol. 2000;88:1743–1748. doi: 10.1152/jappl.2000.88.5.1743. [DOI] [PubMed] [Google Scholar]
- Sun Y-B, Lou F, Edman KAP. 2, 3-Butanedione monoxime increases speed of relaxation in single muscle fibers of frog. Acta Physiol Scand. 2001;172:53–61. doi: 10.1046/j.1365-201X.2001.00818.x. 10.1046/j.1365-201X.2001.00818.x. [DOI] [PubMed] [Google Scholar]
-
Territo PR, French SA, Dunleavy MC, Evans FJ, Balaban RS. Calcium activation of heart mitochondrial oxidative phosphorylation: rapid kinetics of
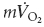 , NADH and light scattering. J Biol Chem. 2001;276:2586–2599. doi: 10.1074/jbc.M002923200. 10.1074/jbc.M002923200. [DOI] [PubMed] [Google Scholar]
, NADH and light scattering. J Biol Chem. 2001;276:2586–2599. doi: 10.1074/jbc.M002923200. 10.1074/jbc.M002923200. [DOI] [PubMed] [Google Scholar] - Timmons JA, Gustafsson T, Sundberg CJ, Jansson E, Greenhaff PL. Muscle acetyl group availability is a major determinant of oxygen deficit in humans during submaximal exercise. Am J Physiol. 1998;274:E377–380. doi: 10.1152/ajpendo.1998.274.2.E377. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Hughson RL. Interaction of factors determining oxygen uptake at the onset of exercise. J Appl Physiol. 1999;86:1101–1113. doi: 10.1152/jappl.1999.86.4.1101. [DOI] [PubMed] [Google Scholar]
- Walsh B, Tonkonogi M, Soderlund K, Hultman E, Saks V, Sahlin K. Role of phosphorylcreatine and creatine in the regulation of mitochondrial respiration in human skeletal muscle. J Physiol. 2001;537:971–978. doi: 10.1111/j.1469-7793.2001.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DF, Erecinska M, Silver IA. Metabolic effects of lowering oxygen tension in vivo. Adv Exp Med Biol. 1983;159:293–301. doi: 10.1007/978-1-4684-7790-0_26. [DOI] [PubMed] [Google Scholar]
- Wilson DF, Rumsey WL. Factors modulating the oxygen dependence of mitochondrial oxidative phosphorylation. Adv Exp Med Biol. 1988;222:121–131. doi: 10.1007/978-1-4615-9510-6_14. [DOI] [PubMed] [Google Scholar]