Abstract
The contribution of endothelium-derived nitric oxide (NO) to exercise hyperaemia remains controversial. Disparate findings may, in part, be explained by different shear stress stimuli as a result of different types of exercise. We have directly compared forearm blood flow (FBF) responses to incremental handgrip and cycle ergometer exercise in 14 subjects (age ± s.e.m.) using a novel software system which calculates conduit artery blood flow continuously across the cardiac cycle by synchronising automated edge-detection and wall tracking of high resolution B-mode arterial ultrasound images and Doppler waveform envelope analysis. Monomethyl arginine (l-NMMA) was infused during repeat bouts of each incremental exercise test to assess the contribution of NO to hyperaemic responses. During handgrip, mean FBF increased with workload (P < 0.01) whereas FBF decreased at lower cycle workloads (P < 0.05), before increasing at 120 W (P < 0.001). Differences in these patterns of mean FBF response to different exercise modalities were due to the influence of retrograde diastolic flow during cycling, which had a relatively larger impact on mean flows at lower workloads. Retrograde diastolic flow was negligible during handgrip. Although mean FBF was lower in response to cycling than handgrip exercise, the impact of l–NMMA was significant during the cycle modality only (P < 0.05), possibly reflecting the importance of an oscillatory antegrade/retrograde flow pattern on shear stress-mediated release of NO from the endothelium. In conclusion, different types of exercise present different haemodynamic stimuli to the endothelium, which may result in differential effects of shear stress on the vasculature.
We recently published details of a method for assessment of blood flow across the cardiac cycle in real time using simultaneous assessment of arterial cross-sectional area and flow velocity with high temporal resolution (30 Hz) (Green et al. 2002b). Using this approach we observed that, during lower limb cycle ergometry, blood flow through the brachial artery of the resting upper limbs undergoes an oscillatory pattern of antegrade flow during systole, followed by substantial retrograde diastolic flow. We also demonstrated a significant contribution of endothelium-derived nitric oxide (NO) to forearm hyperaemia in the resting upper limbs during lower limb cycle exercise, suggesting that this form of exercise may present a ‘systemic’ stimulus to increase NO bioactivity (Green et al. 2002a). This finding may, in part, explain the consistent observation of improved upper limb NO function following predominantly lower limb exercise training programmes (Kingwell et al. 1997; Maiorana et al. 2000; Linke et al. 2001; Maiorana et al. 2001; Walsh et al. 2003a; Walsh et al. 2003b).
In contrast to the findings above, studies which have investigated the contribution of NO to forearm blood flow (FBF) during localised handgrip exercise have reported disparate results (Maiorana et al. 2003). Several studies have reported a significant contribution of NO to handgrip exercise hyperaemia (Gilligan et al. 1994; Dyke et al. 1995; Katz et al. 1996; Duffy et al. 1999b), while others report no greater contribution than that evident at rest (Wilson & Kapoor, 1993; Endo et al. 1994). Many of these studies utilised strain-gauge plethysmography (Maiorana et al. 2003), which reliably measures relative changes induced by pharmacological agents to a resting muscle bed (Joyner et al. 2001), but has several compelling limitations for measurement of exercise hyperaemia (Rowell, 1993); crucially, plethysmography does not provide data with good temporal resolution (Radegran, 1999). Those studies which have previously utilised ultrasound/Doppler modalities to measure FBF have relied upon estimated arterial diameter based on relative diastolic and systolic blood pressure phases, derived time-averaged mean blood velocities to calculate and reconstruct post hoc weighted composite flows, or determined velocity and diameter independently and then reassembled and time-aligned these measures post hoc to provide average beat-to-beat flow (Radegran, 1997; Radegran & Saltin, 1998, 1999; Hoetling et al. 2001). These approaches do not provide continuous assessment of blood flow changes across each cardiac cycle or have the resolution to assess the possible contribution of systolic antegrade versus diastolic retrograde flow components during exercise. To date, no studies have directly compared patterns of FBF which result from incremental localised handgrip exercise to those resulting from incremental cycle ergometry.
Since shear stress on the vessel wall is the probable physiological stimulus to endothelial NO production (Pohl et al. 1986; Rubanyi et al. 1986), we hypothesised that the pattern of blood flow through the vessel may have a bearing on NO bioactivity. We therefore also assessed, via intrabrachial infusion of a competitive NO antagonist (l-NMMA), the contribution of this substance to the hyperaemia observed following each exercise modality.
Methods
Subjects
Fourteen subjects (11 male, 3 female) aged 53 ± 3 years were recruited via public advertisement. Females were postmenopausal and not taking cyclical hormone therapy. Subjects were excluded if they were current smokers, diabetic, asthmatic, hypertensive (resting blood pressure (BP) > 160/90 mmHg) or being treated for hypertension, or if they displayed evidence of coronary or valvular heart disease from history, examination and exercise electrocardiography. On average, subjects had total cholesterol somewhat above the normal range (7.0 ± 0.2 mmol l−1; low density lipoprotein (LDL)–cholesterol 4.6 ± 0.2 mmol l−1), and for this reason they were included in a subsequent study of the effect of lipid-lowering drugs on vascular responses, data from which are being prepared for submission elsewhere (J. H. Walsh, W. Bilsborough, J. Wright, M. J. Joyner, G. O'Driscoll, R. R. Taylor & D. J. Green, unpublished observations). However, no subject was taking any medication or vitamin supplement. Baseline subject characteristics are shown in Table 1. All subjects provided informed consent according to the requirements of the Ethics Committee of Royal Perth Hospital, and all experiments were carried out in accordance with the Declaration of Helsinki.
Table 1.
Subject characteristics
| Age (years) | 53 ± 3 |
|---|---|
| Sex (male/female) | 11/3 |
| Body mass (kg) | 82.1 ± 2.5 |
| Height (cm) | 1.73 ± 0.02 |
| Waist:hip ratio | 0.93 ± 0.03 |
|
|
30.40 ± 1.78 |
| Resting SBP (mmHg) | 117 ± 3 |
| Resting DBP (mmHg) | 75 ± 2 |
Values are mean ± s.e.m.
Study design
Following screening and baseline assessment of peak oxygen uptake ![]() , all subjects underwent vascular function assessments during incremental handgrip and cycle ergometer exercise.
, all subjects underwent vascular function assessments during incremental handgrip and cycle ergometer exercise.
Assessment of vascular function and forearm blood flow
Subjects rested supine while, under local anaesthesia with <2 ml of 1% lignocaine, a 20-gauge cannula (Arrow, Reading, PA, USA) was inserted into the brachial artery of the non-dominant arm. The cannula allowed infusion of sterile saline or l-NMMA, and blood sampling. Following cannulation, subjects were moved to a comfortable chair where they rested for approximately 20 min to allow blood flow to return to normal prior to vascular function testing.
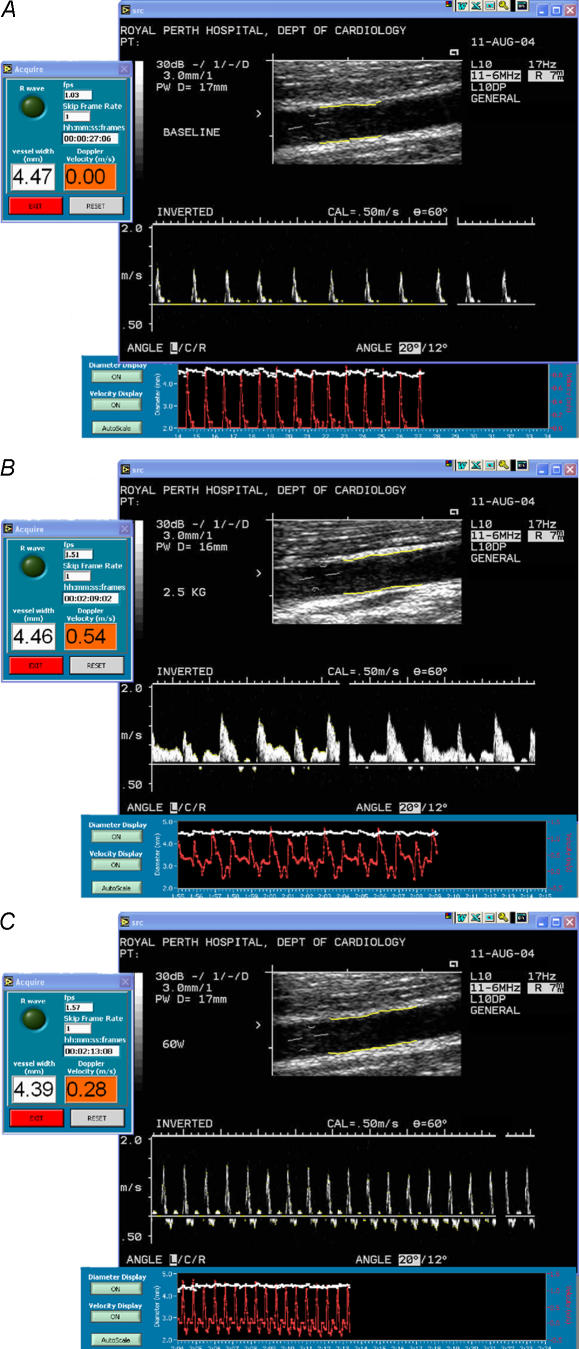
For all blood flow assessments, a 5 MHz multi frequency linear array probe attached to a high-resolution ultrasound machine (Aspen, Acuson; Mountain View, California) was used to image the brachial artery in the distal third of the upper arm, proximal to the infusion site. Ultrasound parameters were set to optimise longitudinal, B-mode images of the lumen/arterial wall interface (Fig. 1). Heart rate was continuously monitored with a three-lead electrocardiograph, and blood pressure was determined from the contralateral arm by the manual, auscultatory method. Images were initially recorded on a S-VHS videocassette recorder (SVO-9500 MDP, Sony; Tokyo, Japan) and transferred to DICOM.
Figure 1. Screen captures of B-mode ultrasound images at A, rest; B, during handgrip exercise and C, during cycle ergometer exercise for one subject.
Diameter and cross sectional area of the artery are calculated for each B-mode frame (at 25–30 Hz) using a rake algorithm which detects the edges of the near and far walls within the selected arterial region of interest. Then 200–400 individual measurements are taken and angle corrected along these edges with the median value being calculated as the final single composite diameter for that frame. Velocity is calculated via grey-scale filtering using an automatic thresholding algorithm with subsequent binary interrogation of each pixel column to detect the waveform envelope. The diameter and velocity output data, at 30 Hz, are displayed for visual feedback purposes (see Fig. 2). Note the appearance of substantial negative (retrograde) velocity during cycle exercise (C), not evident during handgrip (B).
Handgrip exercise protocol
Following an initial rest period of approximately 20 min, a handgrip device and custom-built adjustable arm support was moved into position to maintain a comfortable elbow position. The brachial artery image was then optimised and the ultrasound probe held in position by an experienced sonographer. A 1 min baseline was then recorded, following which subjects were requested to start handgripping to a timed auditory cue, at a rate of one isotonic contraction every 2 s. The weight was initially set at 1.25 kg and increased 1.25 kg every 3 min to a maximum of 3.75 kg for the final 3 min. Subjects paused for approximately 10 s at the start of each workload to allow the weight to be increased. The workload was kept constant for all subjects and for repeat assessments. Blood pressure was determined in the contralateral arm in the final minute of each workload. Ultrasound images for analysis were recorded during steady-state handgripping in the final minute of each workload (Fig. 1B).
Cycle protocol
Following the handgrip protocol, subjects were assisted onto an upright cycle ergometer and the arm was again positioned in the custom designed arm support, with the elbow and hand in a similar position to that in the handgrip protocol. The ultrasound probe was held in position and 20 min following the cessation of handgripping, another 1 min baseline was recorded. Subjects then began cycling at 60 W. The load increased every 3 min, initially to 80 W then 120 W. Workloads were kept constant for all subjects and repeat assessments. Blood pressure was determined in the contralateral arm in the final minute of each workload. Ultrasound images were recorded during cycling in the final minute of each workload (Fig. 1C).
At the end of the final cycle, workload subjects returned to the chair and rested for 30 min prior to repeating both the handgrip and cycle protocols during intrabrachial administration of l-NMMA. l-NMMA was infused at a loading dose of 16 µmol min−1 for 5 min prior to each baseline measurement. The dose was then reduced to 8 µmol min−1 for the duration of the handgrip and cycle protocols. This method of NO inhibition, particularly continuous blockade during exercise, emulates that recently shown by Schrage et al. to be optimal for the assessment of the impact of inhibitors during hyperaemia (Schrage et al. 2004). Saline was infused during the rest periods between the handgrip and cycle protocols. The order of saline and l-NMMA administration was not randomised due to the relatively long half-life of l-NMMA.
Software used for assessment of blood flow
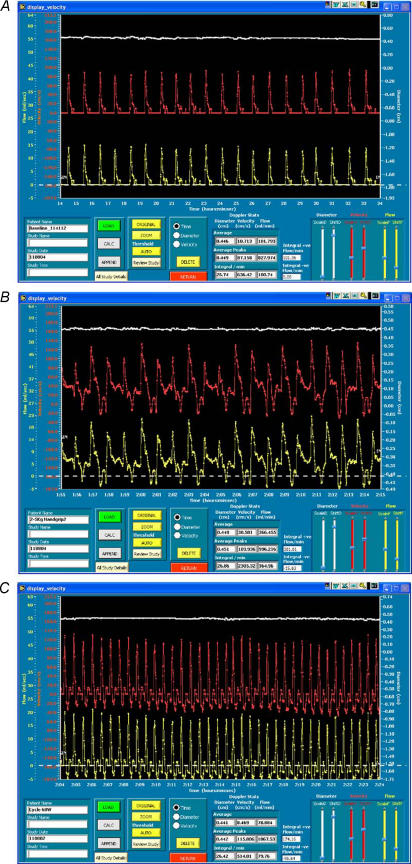
For post-test analysis, arterial diameter was stored and displayed with synchronised Doppler velocity assessment at a rate of 30 frames s−1. The analysis used custom-designed edge-detection and wall-tracking software which minimises investigator bias and has been validated against flows through a perspex phantom (Green et al. 2002b). Blood flow was calculated continuously from synchronised diameter and velocity measures using custom-designed software that has been described in detail previously (Green et al. 2002b). Briefly, B-mode images were viewed, and regions of interest selected for diameter and velocity data acquisition (Fig. 1A). Once acquired, diameter, velocity and flow (calculated as cross-sectional area × velocity) were viewed as continuous plots across the cardiac cycle (Fig. 2). A stable section of at least 10–20 s of data from the final minute of each workload was then demarcated and ‘zoomed’ for analysis (Fig. 2A). Note that because diameter and velocity data are continuously sampled and analysed across the cardiac cycle, blood flow values are calculated during both systole and diastole, so that ‘mean’ forearm blood flow may be influenced by the magnitude of retrograde, diastolic flow (Green et al. 2002b). Antegrade and retrograde components of flow were therefore also calculated as the area under all of the positive and negative blood flow data points between the cursors. This provides a measure of the volume of antegrade and retrograde blood flow per minute. We also calculated antegrade and retrograde vascular conductance by dividing blood flow data by arterial pressure. Here we followed the method of Tschakovsky and Hughson who recently suggested that the use of mean arterial pressure to assess forearm resistance vessel tone is inappropriate under conditions where diastolic retrograde flows are evident (Tschakovsky & Hughson, 2003). Antegrade vascular conductance was therefore calculated by dividing antegrade flow by systolic blood pressure, while retrograde conductance was derived from retrograde flow and diastolic pressure.
Figure 2. Screen captures demonstrating the ultrasound data ‘Display’ software with continuous traces of brachial artery diameter (upper), velocity (middle) and flow (lower) against time for one subject.
Frame A illustrates typical output at rest, B during handgrip exercise, and C during cycle ergometer exercise. Vertical ‘begin’ and ‘end’ cursors are placed to zoom in on selected data and calculate mean forearm blood flow (FBF) and the antegrade and retrograde areas under the curve. Note the appearance of substantial negative (retrograde) velocity and flow during cycle exercise, not evident at rest or during handgrip exercise.
Analysis of data
Results are expressed as means ± s.e.m. Differences in baseline FBF responses pre and post l-NMMA infusion were also compared using Student's paired t test. To determine the effect of exercise intensity and NO inhibition on blood flow variables (mean blood flow, retrograde and antegrade blood flows and conductance), a two-way ANOVA with repeated measures was performed. P < 0.05 was considered significant.
Results
Haemodynamic responses
No differences in heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP) or pulse pressure (PP) were evident in subjects between baseline and the rest periods preceding handgrip or cycle exercise, either before or after l-NMMA (Table 2). ANOVA revealed significant main effects for exercise workload on all haemodynamic variables, but not between saline and l-NMMA That is, HR and BPs were higher at each workload than during the preceding workload during handgrip and cycle exercise, but differences were not evident between saline and l-NMMA infusions within each modality.
Table 2.
Rest and exercise haemodynamic responses during saline and l-NMMA infusion
| Heart Rate | Systolic blood pressure | Diastolic blood pressure | Pulse pressure | |||||
|---|---|---|---|---|---|---|---|---|
| Saline | l-NMMA | Saline | l-NMMA | Saline | l-NMMA | Saline | l-NMMA | |
| Baseline | 66 ± 2 | 74 ± 2 | 117 ± 3 | 116 ± 3 | 75 ± 2 | 76 ± 2 | 42 ± 2 | 40 ± 2 |
| Handgrip | ||||||||
| Rest | 66 ± 2 | 72 ± 2 | 122 ± 3 | 120 ± 3 | 78 ± 2 | 82 ± 2 | 43 ± 2 | 38 ± 1 |
| 1.25 kg | 67 ± 2 | 71 ± 2 | 125 ± 4 | 123 ± 3 | 82 ± 2* | 85 ± 2* | 43 ± 3 | 38 ± 2 |
| 2.5 kg | 70 ± 3* | 73 ± 2 | 128 ± 4* | 127 ± 3* | 83 ± 2 | 84 ± 1 | 45 ± 3 | 43 ± 3 |
| 3.75 kg | 71 ± 2 | 75 ± 2* | 135 ± 4* | 133 ± 4* | 87 ± 2* | 90 ± 2* | 48 ± 3 | 44 ± 3 |
| Cycle | ||||||||
| Rest | 71 ± 2 | 76 ± 2 | 125 ± 4 | 126 ± 3 | 84 ± 3 | 86 ± 2 | 40 ± 2 | 40 ± 2 |
| 60 W | 108 ± 4* | 110 ± 4* | 156 ± 5* | 159 ± 5* | 83 ± 3 | 86 ± 3 | 73 ± 4* | 73 ± 4* |
| 80 W | 121 ± 5* | 124 ± 5* | 177 ± 6* | 181 ± 6* | 83 ± 3 | 87 ± 3 | 94 ± 5* | 94 ± 5* |
| 120 W | 138 ± 6* | 138 ± 6* | 196 ± 4* | 201 ± 5* | 81 ± 4 | 84 ± 4 | 123 ± 8* | 117 ± 5* |
Values are mean ± s.e.m. Cycle exercise had a significantly greater effect on the magnitude of change in HR, SBP and PP than handgrip exercise under both saline and l-NMMA conditions (all P < 0.001, ANOVA).
Significantly different from previous workload (P < 0.05).
When haemodynamic responses were compared between cycle and handgrip exercise under the saline condition, ANOVA revealed significant main effects for HR, SBP and PP (all P < 0.001), indicating a significantly greater impact of cycle exercise on haemodynamics than handgrip exercise. Similar results were evident for comparisons under the l-NMMA infusion condition (all P < 0.001).
Pattern of forearm blood flow response to handgrip and cycle exercise protocols
Handgrip exercise
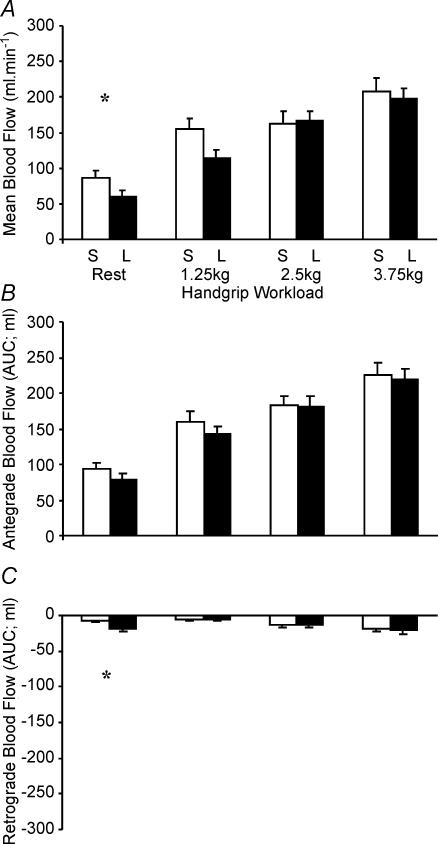
There was a significant main effect for handgrip exercise intensity in mean blood flow during infusion of saline (all P < 0.01, ANOVA; Fig. 3A). Post hoc tests showed mean blood flow during saline infusion was significantly greater at all workloads compared to rest (P < 0.01). In addition, mean FBF was greater at 3.75 kg than 2.5 kg (P < 0.02; Fig. 3A). A similar main effect for handgrip exercise intensity was observed during l-NMMA infusion (P < 0.001, ANOVA; Fig. 3A) with post hoc tests showing significantly greater mean blood flows at each workload than at rest and previous workloads (all P < 0.05). Hence, FBF increased incrementally in response to increases in handgrip exercise intensity.
Figure 3. Mean blood flow (A) and antegrade (B) and retrograde (C) blood flows at rest and during handgrip exercise during saline (S; open bars) and l-NMMA (L; filled bars) infusion.
*Significant difference (P < 0.05) in flows during saline and l-NMMA infusions.
Cycle ergometer exercise
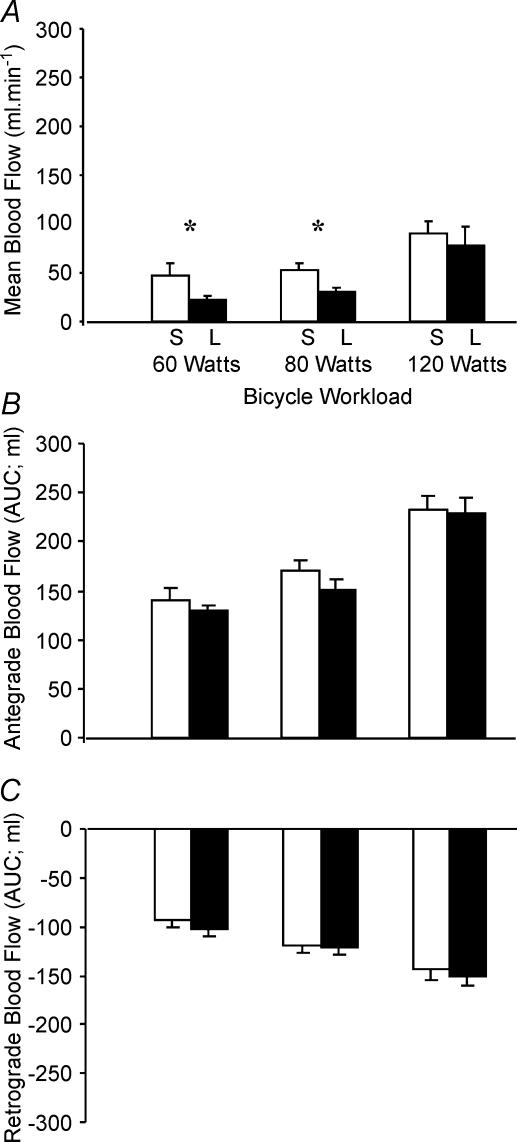
A significant relationship was also observed between cycle exercise intensity and mean blood flow (P < 0.01, ANOVA; Fig. 4A). Post hoc tests showed mean blood flow during saline infusion was significantly lower at 60 W than at rest (P < 0.02), remained unchanged between 60 and 80 W (P = 0.80) and significantly increased from 80 to 120 W (P < 0.03; Fig. 4A). This biphasic upper-limb mean blood flow response to cycle exercise is consistent with our previous finding (Green et al. 2002b) and differs fundamentally from that observed during handgrip exercise (Fig. 3A). The explanation for this relates to the relative impact of retrograde and antegrade flows in the different protocols (see below).
Figure 4. Mean blood flow (A) and antegrade (B) and retrograde (C) blood flows at rest and during bicycle exercise during saline (S; open bars) and l-NMMA (L; filled bars) infusion.
*Significant difference (P < 0.05) in flows during saline and l-NMMA infusions.
Comparison of antegrade and retrograde flows to handgrip and cycle exercise
Figures 3 and 4 illustrate the relative contributions of antegrade and retrograde flows to mean FBF during handgrip and cycle exercise, respectively. Consistent with the mean blood flow data above, the magnitude of antegrade flow during handgrip exercise increased significantly with increasing exercise intensity under both saline and l-NMMA conditions (all P < 0.001, Fig. 3B). The magnitude of retrograde flow was modest during handgrip exercise, but significantly increased with workload (P < 0.05, Fig. 3C). The magnitude of antegrade flow in response to cycle exercise also increased significantly with each increase in exercise intensity under both saline and l-NMMA conditions (all P < 0.01, Fig. 4B). However, in contrast to handgrip responses, retrograde flow increased substantially during cycle exercise, being significantly greater than resting values at all workloads under both saline and l-NMMA conditions (all P < 0.001, Fig. 4C).
When handgrip and cycle exercise were directly compared under either saline and l-NMMA conditions (two-way ANOVA), no difference existed between modalities in the magnitude of increase in antegrade flow, whereas retrograde flow was significantly greater during cycle exercise (P < 0.001). This significantly greater retrograde flow component during cycling explains the biphasic mean FBF response to cycling (Fig. 3A) and also represents the major difference in flow pattern between the two exercise modalities.
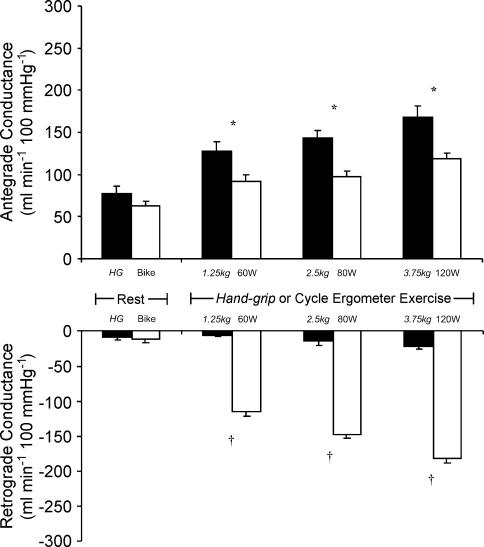
Comparison of antegrade and retrograde conductance to handgrip and cycle exercise
Figure 5 presents a comparison of antegrade and retrograde conductance data under the saline infusion condition between the exercise modalities as workload increased. Antegrade conductance was significantly greater during handgrip ergometer exercise at all workloads (P < 0.01), but the magnitude of difference between the exercise modalities was even more striking in terms of retrograde flows (all workloads P < 0.0001).
Figure 5. Antegrade (above) and retrograde (below) brachial artery conductance at rest and during incremental handgrip (filled bars) and bicycle ergometer (open bars) exercise.
Handgrip workloads are denoted in italics. *P < 0.01 and †P < 0.0001 for significant difference in conductance between exercise modalities.
Effect of l-NMMA on forearm blood flow responses to handgrip and cycle exercise
Paired t tests revealed that l-NMMA infusion significantly attenuated baseline FBF responses (P < 0.05; Table 3), confirming the well established role for NO in mediating forearm blood flow at rest.
Table 3.
Mean, antegrade and retrograde forearm blood flows during saline and l-NMMA infusion
| Mean FBF | Antegrade FBF | Retrograde FBF | ||||
|---|---|---|---|---|---|---|
| Saline | l-NMMA | Saline | l-NMMA | Saline | l-NMMA | |
| Handgrip | ||||||
| Rest | 86 ± 11 | 61 ± 9† | 93 ± 10 | 79 ± 9 | −7 ± 3 | −19 ± 4† |
| 1.25 kg | 155 ± 15* | 114 ± 12* | 159 ± 15* | 142 ± 12* | −5 ± 2 | −6 ± 2* |
| 2.5 kg | 163 ± 16 | 167 ± 13* | 182 ± 12* | 181 ± 14* | −12 ± 4* | − 14 ± 3* |
| 3.75 kg | 207 ± 19* | 198 ± 14* | 225 ± 18* | 219 ± 16* | −18 ± 4 | − 21 ± 4* |
| Cycle | ||||||
| Rest | 69 ± 7 | 42 ± 4† | 78 ± 7 | 61 ± 5† | −10 ± 3 | −19 ± 3† |
| 60 W | 48 ± 12* | 22 ± 5*† | 141 ± 11* | 129 ± 6* | −93 ± 8* | −102 ± 8* |
| 80 W | 52 ± 7 | 31 ± 4† | 170 ± 10* | 151 ± 9* | −119 ± 7* | −121 ± 9* |
| 120 W | 90 ± 12* | 78 ± 19* | 232 ± 14* | 229 ± 15* | −143 ± 12* | −152 ± 8* |
Values are mean ± s.e.m. There was a significant main effect for l-NMMA on mean blood flow during cycle exercise (P < 0.05; ANOVA), but no such significant effect for handgrip exercise (P = 0.2; ANOVA). Exercise intensity significantly increased mean, antegrade and retrograde blood flows during cycle and handgrip exercise (all P < 0.01; ANOVA). No difference existed between bike and handgrip modalities in the increase in antegrade flow, whereas retrograde flow was significantly greater during cycle exercise (P < 0.001).
Significantly different from previous workload (P < 0.05; paired t test).
Significantly different from saline (P < 0.05; paired t test).
Analysis of variance revealed no significant effect of l-NMMA on mean blood flow during handgrip exercise (P < 0.2, Fig. 3A). In contrast, a significant main effect was observed for NO inhibition on mean blood flow responses during cycle exercise (P < 0.05 ANOVA, Fig. 4A). Post hoc t tests revealed that compared to flows during saline infusion, l-NMMA significantly reduced mean blood flow at 60 and 80 W (both P < 0.05; Fig. 4A).
Discussion
The principal outcome of the present study is the descriptive comparison between blood flow responses to cycle and handgrip exercise. We utilised a novel software analysis system to calculate blood flow across the cardiac cycle with high temporal resolution, by synchronising conduit artery diameter and blood velocity assessments of B-mode ultrasound and Doppler images. This approach has allowed us to observe, for the first time in vivo, that although blood flow to the forearm changes as a result of both localised handgrip and lower limb cycle exercise, the nature of the hyperaemic response observed fundamentally differs between these forms of exercise. Furthermore, this difference in the pattern of hyperaemic response may contribute to the second major finding; that NO contributes significantly to blood flow responses in the forearm during lower limb cycle exercise, but not to the hyperaemia associated with handgrip exercise.
The present study confirms our recent published observation, in young healthy subjects, that mean FBF responses in the resting upper limb exhibit a biphasic response pattern during cycle ergometer exercise; an initial decline relative to baseline flow, followed by a significant increase at higher workloads (Green et al. 2002b). The explanation for this unusual pattern is that, while systolic flow becomes progressively more positive with increasing intensities of cycle exercise, negative velocities indicative of retrograde blood flow through the brachial artery are observed during all exercise intensities. The impact of this retrograde diastolic flow is relatively great at lower intensities of exercise, when positive flows are modest, hence a biphasic mean FBF response is observed. In contrast with this unusual flow pattern in the resting upper limb during leg exercise, incremental handgrip exercise was associated with progressive increases in antegrade flow alone; retrograde flow was minimal during handgrip exercise.
The explanation for the distinct patterns of flow response observed in the present study must ultimately relate to differences between exercise modalities in pressure gradients, that is, differences in upstream arterial driving pressure relative to downstream pressure in the resistance vessels, the latter being dependent, in turn, upon resistance vessel tone. Handgrip exercise was associated with only small changes in central haemodynamics, exemplified by the small increases in heart rate and systolic pressure, relative to the cycle protocol. At the same time, vasodilatation of the resistance vessels in the forearm muscle clearly increased during handgrip exercise as illustrated in the systolic conductance data (Fig. 5), calculated according to the method of Tschakosky & Hughson (2003). This vasodilatation probably occurs as a result of increased concentrations of vasodilator byproducts of the local metabolism (Laughlin et al. 1996). In essence therefore, handgrip exercise was associated with a small increase in the upstream driving force for flow, and decreased downstream resistance in the forearm, a combination which might be expected to increase the pulsatile antegrade flow of blood into the active vessel bed, as was indeed observed. In contrast, cycle exercise was associated with a large change in central haemodynamics, specifically systolic function exemplified by increased HR and SBP. Simultaneously, sympathetic vasoconstriction probably occurred in inactive vessel beds, including the forearm (Rowell, 1993). In the absence of local vasodilator metabolites to counteract this sympathetic vasoconstriction in the resting forearm, blood flow during cycle exercise obeys the observed oscillatory pattern of systolic antegrade movement, followed by retrograde flow during diastole when the systolic driving force diminishes and resistance in the forearm is elevated. The presence of greater sympathetic constriction in the forearm during cycle exercise relative to handgrip exercise is exemplified in Fig. 5, which indicates lower systolic antegrade conductance and exaggerated retrograde conductance during diastole. In addition, diastolic pressure did not substantially change during incremental cycling, despite significant increases in retrograde flow, suggesting that the large increase in retrograde diastolic flow observed must be due to increased downstream pressure due to resistance vessel constriction. The precise reason for the actual retrograde movement of blood in the upper limb during cycling, rather than simply an exaggerated decrease in antegrade movement or perhaps stasis, remains unknown.
Our observation of different blood flow responses in the upper limb during local and systemic exercise is not merely a novel descriptive finding; its significance becomes apparent when the relative impact of these flow patterns on the vessel wall is considered. Shear stress on the endothelium is acknowledged as the principal physiological stimulus for production of NO by the constitutive NOS isoform, and increases in shear stress induce vasodilatation in vivo (Neibauer & Cooke, 1996). Furthermore, shear stress may be a homeostatically regulated variable, with paracrine hormonal vasodilatation counteracting localised changes in wall stress (Kamiya & Togawa, 1980; Hutcheson & Griffith, 1991). In the present study we observed lower mean FBF responses to incremental cycle exercise than those evident during handgripping (Figs 3 and 4A), but the contribution of NO to hyperaemia, presumably mediated by resistance vessel vasodilatation secondary to increased blood flow and endothelial shear stress, was significant only in response to cycle exercise. This apparent paradox – attenuated mean flows during cycling yet greater shear stress-mediated NO bioactivity – may be explained by differences in the relative pattern of flow between the modes of exercise. That is, while mean flows were greater during handgrip exercise, the magnitude of antegrade systolic flow was similar in response to both forms of exercise (Figs 3B and 4B), and retrograde flow was significantly higher during cycling. We therefore speculate that, in response to cycling, the repeated drawing of flow across the surface of the endothelium as a result of substantial antegrade/retrograde oscillation may present a more potent stimulus to endothelial cell membrane deformation and consequent signalling events favouring NO production (Oleson et al. 1988; Cooke et al. 1991; Dimmeler & Zeiher, 2003), than the stimulus presented by handgrip exercise which might be characterised as pulsatile increases in antegrade flow alone.
The above results suggest that, from the perspective of the endothelial cell responsiveness to shear stress, exercise is a complex stimulus. While handgrip exercise theoretically represents a useful surrogate for the effects of metabolic vasodilatation in a small exercising vessel bed, it probably does not validly reflect the in vivo effects, in either the active or inactive vasculature, of large muscle group (particularly leg) exercise, which generates a haemodynamic stimulus to hyperaemia in addition to the metabolic component present in the active tissue. This observation may resolve another conundrum in the literature, that exercise training studies involving localised handgrip training have not always produced significant improvement in NO bioactivity (Green et al. 1994, 1996; Franke et al. 1998), while studies which have utilised typical ‘whole body’ exercise training regimes, predominantly involving lower limb exercise (cycling, running, etc.) have observed improvements in NO-mediated vasodilator capacity, even in the untrained upper limbs (Kingwell et al. 1997; Clarkson et al. 1999; Higashi et al. 1999; DeSouza et al. 2000; Goto et al. 2003). This is not surprising in the context of the present study, since our data suggest that an acute bout of cycle exercise may be a more potent stimulus to endothelial NO production in the forearm vasculature that a bout of localised handgrip exercise.
There are several limitations to the present study. It is possible that the pattern of blood flow, and associated shear stress we observed in the brachial artery may not be representative of what is occurring at the resistance vessel level, where NO bioavailability was assessed. However, the principal locus of flow control during exercise lies in the feed arteries and arterioles upstream from the muscle interstitium (Segal, 1992; Lash, 1994; Segal, 2000), and we believe that, given the magnitude of difference in retrograde flows we observed between exercise modalities and the fact that it is the passage through these resistance vessels which is responsible for the conversion of pulsatile into continuous low pressure flow into the microvasculature, these vessels will be exposed to at least some differential shear stimulus during cycle versus handgrip exercise. A second potential limitation relates to the redundancy that exists in control mechanisms during exercise hyperaemia; other vasodilators may compensate for NO blockade during exercise, masking the real magnitude of NO contribution. However, Schrage et al. recently concluded that NO blockade during exercise, as undertaken in the present study, minimises this possibility and provides a valid approach to test the importance of vasodilator mechanisms (Schrage et al. 2004). Furthermore, the dose of l-NMMA we used was comparable to that previously used in exercise studies (Gilligan et al. 1994; Dyke et al. 1995; Duffy et al. 1999a, b) and 2–4 times higher than the highest dose typically used at rest (Vallance et al. 1989). We did not use a higher dose because we thought the prolonged infusion time might cause accumulation of l-NMMA and systemic overflow, leading to reflex complications (Sheriff et al. 2000). Finally the subjects we studied, though healthy with no evidence of coronary or peripheral vascular disease, were middle-aged and possessed mildly elevated plasma cholesterol concentrations. This raises a valid concern regarding impaired contribution of NO to hyperaemia, but the major outcome of the study, the comparison of flow patterns between modalities, was not predicated on the l-NMMA data and is not likely to be substantially affected by this limitation. Furthermore, since the study involved within-subjects comparisons, findings related to the magnitude of difference in NO contribution between exercise modalities are not compromised. Finally, in our previous study of young healthy subjects devoid of risk factors (Green et al. 2002a), we observed similar brachial antegrade and retrograde flows, and l-NMMA effects on these, during cycle exercise as those observed in the present subjects.
In summary, this study suggests that forearm handgrip exercise should not be used as a surrogate for the effects of whole body exercise on the vasculature, as the latter constitutes both metabolic and haemodynamic stimuli to active and inactive vessel bed hyperaemia. Furthermore our data suggest that lower limb exercise may be a more potent stimulus to forearm shear stress and NO production than localised handgrip exercise, indicating that an oscillatory pattern of antegrade/retrograde flow may be a more potent stimulus to shear stress-mediated endothelial NO production than a pulsatile, albeit larger, though predominantly unidirectional, antegrade flow stimulus.
Acknowledgments
This study was supported by the National Health and Medical Research Council of Australia (NHMRC).
References
- Clarkson P, Montgomery HE, Mullen MJ, Donald AE, Powe AJ, Bull T, Jubb M, World M, Deanfield JE. Exercise training enhances endothelial function in young men. J Am Coll Cardiol. 1999;33:1379–1385. doi: 10.1016/s0735-1097(99)00036-4. [DOI] [PubMed] [Google Scholar]
- Cooke JP, Rossitch EJ, Andon NA, Loscalzo L, Dzau VJ. Flow activates an endothelial potassium channel to release an endogenous nitrovasodilator. J Clin Invest. 1991;88:1663–1671. doi: 10.1172/JCI115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSouza CA, Shapiro LF, Clevenger C, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores the age-related decile in endothelium-dependent vasodilation. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Zeiher AM. Exercise and cardiovascular health. Get active to AKTivate your endothelial nitric oxide synthase. Circulation. 2003;107:3118–3120. doi: 10.1161/01.CIR.0000074244.82874.A0. Editorial. [DOI] [PubMed] [Google Scholar]
- Duffy SJ, New G, Harper RW, Meredith IT. Metabolic vasodilation in the human forearm is preserved in hypercholesterolemia despite impairment of endothelium-dependent and independent vasodilation. Cardiovascular Res. 1999a;43:721–730. doi: 10.1016/s0008-6363(99)00082-6. [DOI] [PubMed] [Google Scholar]
- Duffy SJ, New G, Tran BT, Harper RW, Meredith IT. Relative contribution of vasodilator prostanoids and NO to metabolic vasodilation in the human forearm. Am J Physiol Heart Circ Physiol. 1999b;276:H663–H670. doi: 10.1152/ajpheart.1999.276.2.H663. [DOI] [PubMed] [Google Scholar]
- Dyke CK, Proctor DN, Deitz NM, Joyner MJ. Role of nitric oxide in exercise hyperemia during prolonged rhythmic handgripping in humans. J Physiol. 1995;488:259–265. doi: 10.1113/jphysiol.1995.sp020964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Imaizumi T, Tagawa T, Shiramoto M, Ando S, Takeshita A. Role of nitric oxide in exercise-induced vasodilation of the forearm. Circulation. 1994;90:2886–2890. doi: 10.1161/01.cir.90.6.2886. [DOI] [PubMed] [Google Scholar]
- Franke WD, Stephens GM, Schmid PG. Effects of intense exercise training on endothelium-dependent exercise induced vasodilatation. Clin Physiol. 1998;18:521–528. doi: 10.1046/j.1365-2281.1998.00122.x. [DOI] [PubMed] [Google Scholar]
- Gilligan DM, Panza JA, Kilcoyne CM, Waclawiw MA, Casino PR, Quyyumi AA. Contribution of endothelium-derived nitric oxide to exercise-induced vasodilation. Circulation. 1994;90:2853–2858. doi: 10.1161/01.cir.90.6.2853. [DOI] [PubMed] [Google Scholar]
- Goto C, Higashi Y, Kimura M, Noma K, Hara K, Nakagawa K, Kawamura M, Chayama K, Yoshizumi M, Nara I. Effect of different intensities of exercise on endothelium-dependent vasodilation in humans. Role of endothelium-dependent nitric oxide and oxidative stress. Circulation. 2003;108:530–535. doi: 10.1161/01.CIR.0000080893.55729.28. [DOI] [PubMed] [Google Scholar]
- Green DJ, Cable NT, Fox C, Rankin JM, Taylor RR. Modification of forearm resistance vessels by exercise training in young men. J Appl Physiol. 1994;77:1829–1833. doi: 10.1152/jappl.1994.77.4.1829. [DOI] [PubMed] [Google Scholar]
- Green DJ, Cheetham C, Mavaddat L, Watts K, Best M, Taylor RR, O'Driscoll G. Effect of lower limb blood flow on forearm vascular function: Contribution of nitric oxide. Am J Physiol Heart Circ Physiol. 2002a;283:H899–H907. doi: 10.1152/ajpheart.00049.2002. [DOI] [PubMed] [Google Scholar]
- Green DJ, Cheetham C, Reed C, O'Driscoll G. Assessment of brachial artery blood flow across the cardiac cycle: Retrograde flows during lower limb exercise. J Appl Physiol. 2002b;93:361–368. doi: 10.1152/japplphysiol.00051.2002. [DOI] [PubMed] [Google Scholar]
- Green DJ, Fowler DT, O'Driscoll JG, Blanksby BA, Taylor RR. Endothelium-derived nitric oxide activity in forearm vessels of tennis players. J Appl Physiol. 1996;81:943–948. doi: 10.1152/jappl.1996.81.2.943. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Sasaki S, Kurisu S, Yoshimizu A, Sasaki N, Matsuura H, Kajiyama G, Oshima T. Regular aerobic exercise augments endothelium-dependent vascular relaxation in normotensive as well as hypertensive subjects. Circulation. 1999;100:1194–1202. doi: 10.1161/01.cir.100.11.1194. [DOI] [PubMed] [Google Scholar]
- Hoetling BD, Scheuermann BW, Barstow TJ. Effect of contraction frequency on leg blood flow druing knee extension in humans. J Appl Physiol. 2001;91:671–679. doi: 10.1152/jappl.2001.91.2.671. [DOI] [PubMed] [Google Scholar]
- Hutcheson IR, Griffith TM. Release of endothelium-derived relaxing factor is modulated both by frequency and amplitude of pulsatile flow. Am J Physiol Heart Circ Physiol. 1991;261:H257–H262. doi: 10.1152/ajpheart.1991.261.1.H257. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Dietz NM, Shephard JT. From Belfast to Mayo and beyond: the use and future of plethysmography to study blood flow in humans limbs. J Appl Physiol. 2001;91:2431–2441. doi: 10.1152/jappl.2001.91.6.2431. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Togawa T. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am J Physiol Heart Circ Physiol. 1980;239:H14–H21. doi: 10.1152/ajpheart.1980.239.1.H14. [DOI] [PubMed] [Google Scholar]
- Katz SD, Krum H, Kahn T, Knecht M. Exercise-induced vasodilation in forearm circulation of normal subjects and patients with congestive heart failure: Role of endothlium-derived nitric oxide. J Am Coll Cardiol. 1996;28:585–590. doi: 10.1016/0735-1097(96)00204-5. [DOI] [PubMed] [Google Scholar]
- Kingwell BA, Sherrard B, Jennings GJ, Dart AM. Four weeks of cycle training increases basal production of nitric oxide from the forearm. Am J Physiol heart Circ Physiol. 1997;272:H1070–H1077. doi: 10.1152/ajpheart.1997.272.3.H1070. [DOI] [PubMed] [Google Scholar]
- Lash JM. Contribution of arterial feed vessels to skeletal muscle functional hyperemia. J Appl Physiol. 1994;76:1512–1519. doi: 10.1152/jappl.1994.76.4.1512. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Korthuis RJ, Duncker DJ, Bache RJ. Control of blood flow to cardiac and skeletal muscle during exercise. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. Bethesda: American Physiological Society; 1996. pp. 705–769. chapter 16. [Google Scholar]
- Linke A, Schoene N, Geilen S, Hofer J, Erbs S, Schuler G, Hambrecht R. Endothelial dysfunction in patients with chronic heart failure: systemic effects of lower-limb exercise training. J Am Coll Cardiol. 2001;37:392–397. doi: 10.1016/s0735-1097(00)01108-6. [DOI] [PubMed] [Google Scholar]
- Maiorana A, O'Driscoll G, Cheetham C, Dembo L, Stanton K, Goodman C, Taylor RR, Green D. The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetes. J Am Coll Cardiol. 2001;38:860–866. doi: 10.1016/s0735-1097(01)01439-5. [DOI] [PubMed] [Google Scholar]
- Maiorana A, O'Driscoll G, Dembo L, Cheetham C, Goodman C, Taylor R, Green D. Effect of aerobic and resistance exercise training on vascular function in heart failure. Am J Physiol Heart Circ Physiol. 2000;279:H1999–H2005. doi: 10.1152/ajpheart.2000.279.4.H1999. [DOI] [PubMed] [Google Scholar]
- Maiorana A, O'Driscoll GJ, Taylor RR, Green DJ. Exercise and the nitric oxide vasodilator system. Sports Med. 2003;33:1013–1035. doi: 10.2165/00007256-200333140-00001. [DOI] [PubMed] [Google Scholar]
- Neibauer J, Cooke JP. Cardiovascular effects of exercise: role of endothelial shear stress. J Am Coll Cardiol. 1996;28:1652–1660. doi: 10.1016/S0735-1097(96)00393-2. [DOI] [PubMed] [Google Scholar]
- Oleson SP, Clapham DE, Davies PF. Haemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 1988;331:168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension. 1986;8:37–44. doi: 10.1161/01.hyp.8.1.37. [DOI] [PubMed] [Google Scholar]
- Radegran G. Ultrasound Doppler estimates of femoral artery blood flow during dynamic knee extensor exercise in humans. J Appl Physiol. 1997;83:1383–1388. doi: 10.1152/jappl.1997.83.4.1383. [DOI] [PubMed] [Google Scholar]
- Radegran G. Limb and skeletal muscle blood flow measurements at rest and during exercise in human subjects. Proc Nutr Soc. 1999;58:887. doi: 10.1017/s0029665199001196. [DOI] [PubMed] [Google Scholar]
- Radegran G, Saltin B. Muscle blood flow at the onset of dynamic exercise in humans. Am J Physiol Heart Circ Physiol. 1998;274:H314–H322. doi: 10.1152/ajpheart.1998.274.1.H314. [DOI] [PubMed] [Google Scholar]
- Radegran G, Saltin B. Nitric oxide in the regulation of vasomotor tone in human skeletal muscle. Am J Physiol Heart Circ Physiol. 1999;276:H1951, H1960. doi: 10.1152/ajpheart.1999.276.6.H1951. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human Cardiovascular Control. New York: Oxford University Press; 1993. [Google Scholar]
- Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol Heart Circ Physiol. 1986;250:H1145–H1149. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol. 2004;557:599–611. doi: 10.1113/jphysiol.2004.061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal SS. Communication among endothelial and smooth muscle cells coordinates blood flow control during exercise. News Physiol Sci. 1992;7:152–156. [Google Scholar]
- Segal SS. Integration of blood flow control to skletal muscle: key role of feed arteries. Acta Physiol Scand. 2000;168:511–518. doi: 10.1046/j.1365-201x.2000.00703.x. [DOI] [PubMed] [Google Scholar]
- Sheriff DD, Nelson CD, Sundermann RK. Does autonomic blockade reveal a potent contribution of nitric oxide to locomotion-induced vasodilation? Am J Physiol Heart Circ Physiol. 2000;279:H726–H732. doi: 10.1152/ajpheart.2000.279.2.H726. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Hughson RL. Rapid blunting of sympathetic vasoconstriction in the human forearm at the onset of exercise. J Appl Physiol. 2003;94:1785–1792. doi: 10.1152/japplphysiol.00680.2002. [DOI] [PubMed] [Google Scholar]
- Vallance P, Collier J, Moncada S. Effects of endothelium-derived nitric oxide on peripheral arteriolar tone in man. Lancet. 1989;2:997–1000. doi: 10.1016/s0140-6736(89)91013-1. [DOI] [PubMed] [Google Scholar]
- Walsh JH, Best M, Maiorana AJ, Taylor RR, O'Driscoll GJ, Green DJ. Exercise improves conduit vessel endothelial function in CAD patients. J Appl Physiol. 2003a;285:20–25. doi: 10.1152/japplphysiol.00012.2003. [DOI] [PubMed] [Google Scholar]
- Walsh JH, Yong G, Cheetham C, Watts GF, O'Driscoll GJ, Taylor RR, Green DJ. Effect of exercise training on conduit and resistance vessel function in medicated and unmedicated hypercholesterolaemic patients. Eur Heart J. 2003b;24:1681–1689. doi: 10.1016/s0195-668x(03)00384-1. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Kapoor S. Contribution of endothelium-deriving relaxing factor to exercise-induced vasodilation in humans. J Appl Physiol. 1993;75:2740–2744. doi: 10.1152/jappl.1993.75.6.2740. [DOI] [PubMed] [Google Scholar]