Abstract
The Na+–HCO3− cotransporter (NBC) is an important sarcolemmal acid extruder in cardiac muscle. The characteristics of NBC expressed functionally in heart are controversial, with reports suggesting electroneutral (NBCn; 1HCO3− : 1Na+; coupling coefficient n = 1) or electrogenic forms of the transporter (NBCe; equivalent to 2HCO3− : 1Na+; n = 2). We have used voltage-clamp and epifluorescence techniques to compare NBC activity in isolated ventricular myocytes from rabbit, rat and guinea pig. Depolarization (by voltage clamp or hyperkalaemia) reversibly increased steady-state pHi while hyperpolarization decreased it, effects seen only in CO2/HCO3−-buffered solutions, and blocked by S0859 (cardiac NBC inhibitor). Species differences in amplitude of these pHi changes were rat > guinea pig ≈ rabbit. Tonic depolarization (−140 mV to −0 mV) accelerated NBC-mediated pHi recovery from an intracellular acid load. At 0 mV, NBC-mediated outward current at resting pHi was +0.52 ± 0.05 pA pF−1 (rat, n = 5), +0.26 ± 0.05 pA pF−1 (guinea pig, n = 5) and +0.10 ± 0.03 pA pF−1 (rabbit, n = 9), with reversal potentials near −100 mV, consistent with n = 2. The above results indicate a functionally active voltage-sensitive NBCe in these species. Voltage-clamp hyperpolarization negative to the reversal potential for NBCe failed, however, to terminate or reverse NBC-mediated pHi-recovery from an acid load although it was slowed significantly, suggesting electroneutral NBC may also be operational. NBC-mediated pHi recovery was associated with a rise of [Na+]i at a rate ∼25% of that mediated via NHE, and consistent with an apparent NBC stoichiometry between n = 1 and n = 2. In conclusion, NBCe in the ventricular myocyte displays considerable functional variation among the three species tested (greatest in rat, least in rabbit) and may coexist with some NBCn activity.
Cardiac contractile and electrical activity is sensitive to changes of intracellular pH (Orchard & Kentish, 1990; Orchard & Cingolani, 1994). This sensitivity accounts for part of the depression of ventricular function during myocardial ischaemia, a condition associated with low pHi (see e.g. Allen & Orchard, 1987; Gettes & Casio, 1992). Sarcolemmal ion transporters regulate pHi in cardiac cells by importing or exporting excess acid or base. The major transporters responsible for acid extrusion are Na+–HCO3− cotransport (NBC) and Na+–H+ exchange (NHE) (Lagadic-Gossmann et al. 1992; Leem et al. 1999), although a monocarboxylic acid transporter (MCT) also extrudes lactic acid during periods of high glycolytic activity (Poole & Halestrap, 1993). There is universal agreement that cardiac NHE and MCT are electroneutral transporters.
In contrast to NHE and MCT, expression studies of the gene products of cardiac NBCs have identified at least two electrogenic (Choi et al. 1999; Pushkin et al. 2000; Sassani et al. 2002; Virkki et al. 2002) as well as one electroneutral (Pushkin et al. 1999; Choi et al. 2000) isoform (defined by Boron and coworkers as NBCe1-B, NBCe2-c (NBC4c) and NBCn1, respectively; see Romero et al. 2004, for a review of NBC classification). Evidence, however, that electrogenic and electroneutral NBCs are functional in intact heart tissue is less clear. For example, while NBC was proposed to be electrogenic in cat papillary muscle (Camilión de Hurtado et al. 1995, 1996) and rat ventricular myocytes (Aiello et al. 1998), earlier work on guinea pig ventricular myocytes (Lagadic-Gossmann et al. 1992) and sheep Purkinje strands (Dart & Vaughan-Jones, 1992) was suggestive of electroneutral transport. This raises the possibility of species and/or cell-type differences in the functional expression of NBCe and NBCn isoforms.
The presence of NBCe in heart cells (e.g. with an equivalent stoichiometric coupling, n HCO3− : 1 Na+, of n = 2 or 3) means that, providing the membrane potential is positive to the transporter's equilibrium potential (ENBC), acid extrusion (i.e. HCO3− influx) is favoured thermodynamically. The resulting outward ionic current (INBC) has been suggested to hyperpolarize the resting membrane potential in cat and rat ventricular tissue, and to shorten the evoked action potential in rat (Camilión de Hurtado et al. 1995; Aiello et al. 1998). NBCe activity may therefore affect cardiac excitation and contraction. Electrogenicity of NBC also implies that the transporter's activity may be sensitive to changes in the membrane potential (Aiello et al. 1998) and hence to sarcolemmal electrical activity (Camilión de Hurtado et al. 1996). Interest in the functional consequences of NBC activity has been heightened by reports that stimulation of an NBCe transporter (NBCe1-B, otherwise known as hhNBC) contributes to myocardial reperfusion injury and perhaps to the generation of postischaemic related arrhythmias (Khandoudi et al. 2001).
In view of the uncertainty regarding the functional expression of NBCe in heart, we have compared evidence for its operation in ventricular myocytes isolated enzymatically from three commonly used species (rabbit, guinea pig, rat). Voltage-clamp and epifluorescence measurements of membrane current, intracellular pH and intracellular Na+ have been used to assess NBC activity. We have examined the voltage sensitivity of acid efflux through NBC during intracellular acidosis and determined whether NBC activity results in significant Na+ ion influx. We consider the possibility that NBCe and NBCn isoforms may be coactive within a single ventricular myocyte and that the flux density of ion transport through NBC may differ among species.
Methods
Myocyte isolation
The experiments were performed on adult ventricular myocytes isolated from rabbit, guinea pig and rat by enzymatic digestion. The isolation procedures for rabbit and guinea pig myocytes have been previously described (e.g. Xu & Spitzer, 1994; Skolnick et al. 1998) and were also used for rats. All procedures involving animals conformed to the UK Animals (Scientific Procedures) Act 1986/local named Committee guidelines. In brief, animals were anaesthetized with sodium pentobarbital (50 mg kg−1, i.v. rabbit; i.p. guinea pig, rat) then the excised heart was attached to an aortic cannula and perfused with solutions gassed with 100% O2 and held at 37°C, pH 7.3. Perfusion with a 0 mm Ca2+ solution for 5 min was followed by 15 min of perfusion with the same solution containing 1 mg ml−1 collagenase (type II, Worthington Biochemical, Freehold, NJ, USA), 0.1 mg ml−1 protease (type XIV, Sigma Chemical, St Louis, MO, USA), and 0.1 mm CaCl2. The heart was then perfused for 5 min with the same solution containing no enzymes. The left ventricle was minced and shaken for 10 min, and then filtered through a nylon mesh. Cells were stored at room temperature in normal Hepes-buffered solution. All myocytes used in this study were rod-shaped, had well-defined striations, and did not spontaneously contract. Experiments were performed within 10 h of isolation.
Cell superfusion chamber
Bathing solutions were held at 37 ± 0.1°C in glass reservoir bottles that were sealed except for a small vent at the top and an exit port at the bottom. Solutions were delivered by gravity from the bottles to the cell bath through water-jacketed, gas-impermeable stainless steel tubing. The temperature of the solutions in the superfusion chamber was 36 ± 0.3°C. The 1-ml Plexiglas cell bath had a clear glass bottom and was mounted on the stage of an inverted microscope (Diaphot, Nikon, Japan). Bathing solutions flowed continuously through the bath at 4–6 ml min−1, and solution depth was held at approximately 3 mm. The bottom of the bath was coated with laminin (BDBiosciences, Bedford, MA, USA) to improve cell adhesion.
Solutions
Two systems were used to buffer H+ in the myocyte bathing solutions: 20–24 mm Hepes with no added CO2/HCO3−, and 5% CO2/HCO3− buffer with no added Hepes. The pH of all myocyte bathing solutions was 7.4, and adjusted to that value using 1 m NaOH (Hepes buffer) or NaHCO3 (CO2/HCO3− buffer). In the latter case, final NaHCO3 concentration was 22 mm (Oxford, UK) or 18.5 mm (Salt Lake City, USA). The lower value reflects the fact that barometric pressure in Salt Lake City is lower (typically 649 mmHg), yielding a PCO2 of approximately 30 mmHg in solutions saturated with 5% CO2–95% O2.
In addition to the buffer itself, Hepes-buffered solution contained (mm): 126 NaCl, 11 dextrose, 4.4 KCl, 1.0 MgCl2, 1.08 CaCl2. CO2/HCO3−-buffered solution was continuously gassed with 5.0% CO2–95% O2 and, in addition to the NaHCO3 buffer, contained (mm): 120 NaCl, 11 dextrose, 4.4 KCl, 1.0 MgCl2, 1.08 CaCl2.
For Na+-free CO2/HCO3−-buffered solution, NaCl and NaHCO3 were replaced completely with an equimolar concentration of n-methyl-d-glucamine (NMDG) and the pH was adjusted with HCl. Chloride-free CO2/HCO3−-buffered solution contained (mm): 120 sodium glucuronate (or sodium gluconate), 11 dextrose, 1.0 MgSO4, 3.0 calcium gluconate, 4.4 potassium gluconate and 18.5–22 NaHCO3 (5.0% CO2–95.0% O2).
When using osmotically compensated hyperkalaemic solutions, the control CO2/HCO3−-buffered solution contained (mm): 100 NaCl, 11 dextrose, 4.4 KCl, 1.0 MgCl2, 40 NMDG, 1.08 CaCl2 and 18.5–22 NaHCO3 (gassed with 5.0% CO2–95.0% O2, pH adjusted to 7.4 with HCl). Hepes-buffered solution contained (mm): 100 NaCl, 11 dextrose, 4.4 KCl, 1.0 MgCl2, 40 NMDG, 1.08 CaCl2, 24 Hepes and 12.9 NaOH (pH adjusted to 7.4 with HCl). For high (44.4 mm)-K+ solution, 40 mm NMDG was replaced with the same concentration of KCl.
The normal pipette filling solution contained (mm): 123 potassium glutamate, 15 NaCl, 10 KCl, 10 Hepes, 12 KOH and 4 HCl (pH 7.1). In the Cl−-free experiments, the pipette solution contained (mm): 132 potassium glutamate, 1.0 KCl, 15 sodium glucuronate, 10 Hepes and 6 KOH.
Measurement of pHi
The pHi was measured in single myocytes with an epifluorescence system coupled to the Nikon inverted microscope. Carboxy-seminaphthorhodafluor-1 (carboxy-SNARF-1), a pH-sensitive fluorophore, was used as the fluorescent pH indicator as previously described in detail (e.g. Spitzer & Bridge, 1992; Skolnick et al. 1998; Leem et al. 1999). In brief, myocytes were equilibrated at 37°C for 10 min in the normal Hepes-buffered solution containing 10–13 μm of the acetoxymethyl ester of SNARF-1, SNARF-AM (Molecular Probes, Eugene, OR, USA). They were then placed in the cell bath, where they were bathed in the normal solution (Hepes or CO2/HCO3−-buffered) for at least 20 min before pHi measurements began. Excitation at 515 nm was provided by a mercury arc lamp, and was directed to the bath via a 40× oil-immersion objective lens (NA 1.3). Emitted fluorescence was simultaneously collected by two photomultiplier tubes equipped with band-pass filters centred at 640 ± 20 nm and 580 ± 20 nm. The fluorescence emission ratio (640/580) was digitized at 5–10 kHz (Digidata 1322A, Axon Instruments, Union City, CA, USA). The emission ratio was calibrated as previously described (Buckler & Vaughan-Jones, 1990; Spitzer & Bridge, 1992; Leem et al. 1999) using solutions of varying pH that also contained 10 μm nigericin.
Determination of efflux of acid equivalents via NBC and NHE
Intracellular acid loading was achieved with ammonium prepulses (10–30 mm NH4+) (see e.g. Leem et al. 1999). When NBC activity was examined, CO2/HCO3−-buffered solutions were superfused while NHE was inhibited using either 30 μm HOE 694 (Scholz et al. 1993) or HOE 642 (cariporide, Scholz et al. 1995) as previously described (Lagadic-Gossmann et al. 1992; Leem et al. 1999). Acid flux (JNBC) was estimated as −βtotdpHi/dt during pHi recovery from the acid load, where βtot is total intracellular buffering power. When NHE activity was examined, Hepes-buffered solutions were superfused, thus inactivating NBC (Leem et al. 1999); JNHE was estimated as −βintdpHi/dt during recovery from an acid load where βint is intrinsic intracellular buffering power.
Determination of intracellular buffering capacity
The intrinsic (non-CO2) buffering capacity (βint) of rabbit, rat and guinea pig myocytes over the pHi range 6.40–7.50 was taken from data published previously (Zaniboni et al. 2003). Intracellular buffering due to CO2 (βCO2) was calculated as, βCO2= 2.3[HCO3−]i, where [HCO3−]i=[HCO3−]o10pHi−pHo (Roos & Boron, 1981; Leem et al. 1999). Total buffering capacity (βtot) is equal to the sum of βint and βCO2. It was assumed that βtot = βint when myocytes were bathed in Hepes-buffered solutions containing no added CO2/HCO3−.
Electrophysiological techniques
All electrophysiological measurements were made with whole-cell perforated patch pipettes (amphotericin B-perforated, 240 μg ml−1) to minimize intracellular dialysis. Pipettes were constructed from borosilicate capillary glass (Corning 8250 glass) and had resistances of approximately 2 MΩ when filled. Resting membrane potential in unclamped cells was recorded with an Axoclamp-2A amplifier system (Axon Instruments) in bridge-mode and voltage-clamping was achieved with an Axopatch 200B clamp system using a CV203BU headstage. Membrane potential (Em) and membrane current (Im) were filtered at 5 kHz and digitized at 10–20 kHz with a 16-bit A/D converter (Digidata 1322A) and analysed using pCLAMP 8 software (Axon Instruments). The reference electrode was a flowing 3 m KCl bridge for experiments involving changes in bath Cl− and a Ag–AgCl pellet was used when [Cl−]o was held constant.
Current–voltage relationships for INBC were measured by voltage clamping myocytes with a ramp protocol. Cells were held initially at an Em of −80 mV and then clamped to +50 mV for 1 s followed by a linear ramp to −140 mV over 30 s. The ramp was applied first during superfusion with Hepes buffered and then after 4 min in CO2/HCO3−-buffered solution, with the difference current taken as INBC. The reversal potential (ENBC) was determined from the best fit of INBC. pHi was also measured during these experiments. In an earlier study, Aiello et al. (1998) used the same technique for measuring INBC in rat ventricular myocytes and obtained results very similar to ours. The previous observation that the pH buffer, Hepes, has no direct effect on cardiac action potential configuration (Spitzer & Hogan, 1979) suggests that Hepes per se did not affect our measurements of INBC.
All bathing solutions used in the voltage-clamp experiments (ramps and steps) contained 0.5 mm Ba2+ to block inward IK1 (e.g. Cordeiro et al. 1998) and 10 μm nifedipine to reduce Ca2+ entry that may subsequently affect pHi (e.g. Vaughan-Jones et al. 1983). Nifedipine did not alter pHi regulatory systems, as judged from its lack of effect on pHi recovery from an intracellular acid load under CO2/HCO3−-buffered conditions (rabbit ventricular myocytes, n = 14; data not shown).
Measurement of [Na+]i with SBFI fluorescence
Dye loading and ratiometric measurement
Intracellular Na+ concentration, [Na+]i, was measured in single myocytes using the fluorophore benzofuram isophthalate (SBFI). To load cells with the dye, 0.3 μl of 1 mm stock solution of the AM ester of the dye (in DMSO) was mixed with 2 μl of 20% (v/v) pluronic acid F127 (in DMSO) and added to 300 μl of ventricular myocytes, suspended in culture medium. The mixture was incubated in darkness for 2 h at room temperature. Cells were then transferred to the experimental chamber and allowed to settle for a few minutes whereupon they were superfused with normal Hepes or CO2/HCO3− solution at 37°C. It has been estimated that 70% of intracellular SBFI is cytoplasmic, the rest being located within intracellular organelles (Harootunian et al. 1989).
The SBFI signal was quantified ratiometrically in an epifluorescence system built around a Nikon Diaphot 200B inverted microscope. Excitation light was provided by a 75 W Xe UV lamp. As SBFI is a dual-excitation dye, excitation light was filtered alternately at 340 ± 5 nm and 380 ± 5 nm, using a rocking filter-wheel (see e.g. Donoso et al. 1992). Each filter was presented for 0.5 s at 1 Hz. The emission signal from a cell was directed through a 510 ± 10 nm interference filter and measured using a photomultiplier tube (Thorn EMI; Fact 50 MK III electron tubes). Current from the PMT was converted to voltage and digitized (Cambridge Electronic Design, CED 1401). The ratio of emission elicited at the two excitation wavelengths (F340/F380) was stored and converted later to a measure of [Na+]i using a ratiometric calibration.
Calibration of ratiometric signal
This was done by superfusing cells with solutions containing various known concentrations of sodium: 0, 5, 10, 15 and 20 mm (see e.g. Donoso et al. 1992). Solutions were obtained by appropriate mixing of two solutions containing 135 mm NaCl or 135 mm KCl. The other ingredients of calibration solutions were 2 mm EGTA, two ionophores to assist equilibration of intracellular with extracellular Na+ (2 μm gramicidin D and 40 μm monesin), 100 μm strophanthidin to inhibit Na+–K+ ATPase, and 10 mm Hepes; pH adjusted to 7.4 with known quantities of NaOH or KOH. Over the [Na+] range 0 mm to 20 mm, the relationship between fluorescence ratio and [Na+] was linear.
Correcting for pH sensitivity of ratiometric signal
The pH sensitivity of SBFI is modest (Minta & Tsien, 1989), typically leading to small artifactual excursions in the [Na+]i signal during large changes of pHi. These are characterized by a small step decrease in the [Na+]i signal when there is a large step decrease in pHi, and a step increase in response to a step rise of pHi (Harrison et al. 1992). Such artifacts are evident in the experiment illustrated in Fig. 8A. This shows a recording of [Na+]i in a guinea pig ventricular myocyte superfused with Hepes-buffered solution. The light-grey trace shows the uncorrected recording of [Na+]i. Note the small, initial step-changes in the trace upon addition and removal of extracellular NH4+, a procedure that induces large step-changes of pHi (typical pHi changes are plotted below the [Na+]i trace in Fig. 8A, derived from a computational model of pHi regulation in this cell type; Leem et al. 1999). The step artifacts were removed by applying a correction factor to the calibrated [Na+]i trace. This was estimated from the step change in the [Na+]i trace when extracellular ammonium concentration was reduced to zero in the presence of 30 μm cariporide (as shown in the latter part of Fig. 8A), using the following equation:
The best-fitting value of coefficient α for the data was 3. The corrected trace (black) is superimposed on the uncorrected trace in Fig. 8A, showing that the slower, physiological changes of [Na+]i were little affected by the correction. A similar approach was used to correct the [Na+]i signal on addition or removal of ammonium under CO2/HCO3−-buffered conditions. Again, the best-fit value for α was 3 (n = 14 cells).
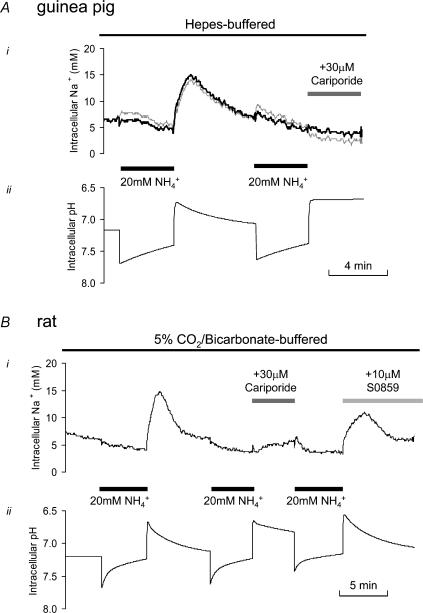
Figure 8. Change in intracellular sodium induced by activation of NBC and NHE.
A, response of [Na+]i in a guinea pig myocyte to intracellular acidosis imposed in Hepes-buffered bathing solution. The transient rise in [Na+]i was completely blocked by inhibition of NHE with cariporide. The [Na+]i signal shown in black was corrected for changes in pHi as described in the Methods. B, response of [Na+]i in a rat myocyte to intracellular acidosis imposed in CO2/HCO3−-buffered solution. Following the second and third prepulses, 30 μm cariporide and 10 μm S0859 were present in the superfusates, respectively. Both agents reduced the time course and magnitude of the transient rise in [Na+]i associated with pHi recovery. The bottom trace shows the simulated pHi time course necessary to correct the SBFI signal for its pHi sensitivity (see Methods). By relating the Na+ rise after the second or third prepulse to the control rise, the percentage of Na+ influx on NHE and NBC can be estimated.
Statistics
Summarized results are expressed as means ± s.e.m. A paired Student's t test was used to test significance between results obtained with each cell serving as its own control. An unpaired t test was used to test significance between results obtained on different cells. P < 0.05 was considered significant.
Results
Evidence for NBC in rabbit heart
Although NBC has been identified in cardiac tissue from guinea pig (Lagadic-Gossmann et al. 1992), sheep (Dart & Vaughan-Jones, 1992), rat (Ng et al. 1993; Kohout & Rogers, 1995; Le Prigent et al. 1997; Aiello et al. 1998; Khandoudi et al. 2001), cat (Camilión de Hurtado et al. 1995), ferret (Grace et al. 1993; Vandenberg et al. 1993) and human (Loh et al. 2002), its existence in rabbit ventricle is unresolved. Rabbit NBC was thus evaluated in the first series of experiments. Figure 1A shows that in the presence of a normal CO2/HCO3− buffer system, the potent NHE1 inhibitor, HOE 694 (30 μm), only partially blocked pHi recovery from intracellular acidosis. This contrasts with the essentially complete inhibition of pHi recovery by this agent or by cariporide (HOE 642) in cells bathed in Hepes-buffered, CO2/HCO3−-free solution (Fig. 1B). NHE is therefore the principal acid extrusion mechanism in the absence of carbonic buffer while, in its presence, a HCO3−-dependent acid extrusion mechanism is also evident. This latter mechanism requires extracellular Na+, as illustrated in Fig. 1C, where restoring [Na+]o to an acid-loaded myocyte exposed to cariporide activated pHi recovery. In contrast, HCO3−-dependent pHi recovery did not appear to require Cl− since there was no significant difference between acid extrusion estimated at a common pHi (6.85 units) in myocytes equilibrated with normal or zero Cl− solution (Fig. 1D). Collectively, these results are consistent with the presence of a functional NBC in rabbit ventricular myocytes.
Figure 1. Evidence for NBC in rabbit ventricular myocytes.
A, inhibition of NHE1 with HOE 694 in CO2/HCO3−-buffered solution did not completely block recovery of pHi from the intracellular acidosis elicited by NH4Cl prepulses (10 mm). B, summary of acid extrusion in Hepes-buffered solution without HOE (n = 31 cells) and with HOE compounds (30 μm, 642, n = 12 cells; 694, n = 6 cells). The inset shows a representative experiment. C, example of the requirement for external sodium to induce pHi recovery in a rabbit myocyte bathed in CO2/HCO3−-buffered solution with NHE inhibited. One of four similar experiments. D, example experiment showing that acid extrusion, in the presence of CO2/HCO3− with NHE inhibited, persists in chloride-free solution. One of 11 similar experiments. The results are summarized in the inset, which shows mean net acid extrusion measured between pHi values of 6.8 and 6.9 (filled bars: control, n = 13; open bars: chloride free, n = 8). In this protocol cells were bathed in Cl−-free solution for at least 25 min prior to applying the ammonium prepulse, a protocol sufficient to remove [Cl−]i from rabbit myocytes.
Comparison of pHi dependence of NBC among three species
Intracellular acidosis is a major activator of acid extrusion via both NHE and NBC. The relationship between pHi and acid efflux through NBC (JNBC) in rat, rabbit and guinea pig ventricular myocytes has been plotted in Fig. 2. JNBC was measured from pHi recovery in CO2/HCO3−-buffered solution with NHE blocked using either 30 μm cariporide or HOE 694 (e.g. Fig. 1A). As individual cells were neither voltage clamped nor field stimulated, membrane potential would have been close to its normal resting value of approximately −85 mV. At any given pHi, mean values for JNBC were essentially identical in the presence of cariporide or HOE 694 (not shown) and so have been combined in the data plotted in Fig. 2A.
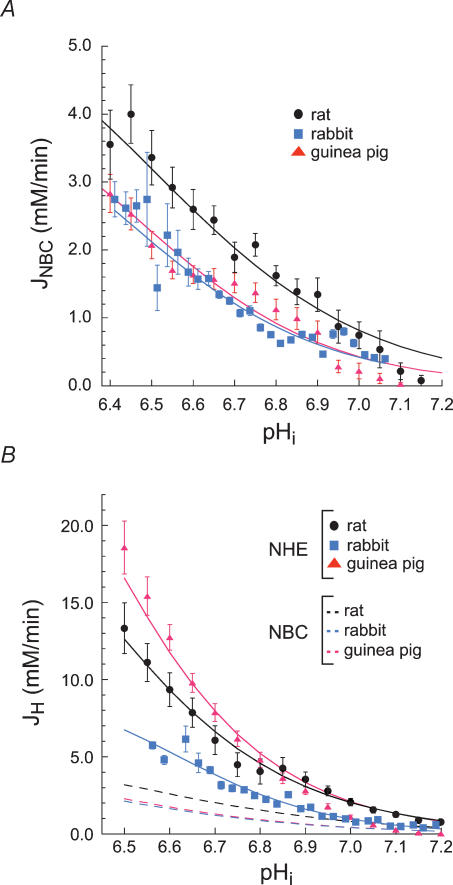
Figure 2. Effect of pHi on acid efflux.
A, summary of the relationship between JNBC and pHi in rat (n = 66, black), rabbit (n = 26, blue), and guinea pig (n = 73, red) myocytes. Continuous lines are best-fits of data to the equation: JNBC = Jmax·[H+]m/(Km+[H+]m), where Jmax is the maximum flux, K is the Km for protons, [H+] is proton concentration and m is the Hill coefficient. The respective values for Jmax, K and m for NBC in rat were: 6.315, 10−6.506 and 1.677. For guinea pig they were: 4.861, 10−6.47 and 1.913. For rabbit they were: 5.0, 10−6.427 and 1.816. B, summary of the relationship between JNHE and pHi in rat (n = 70, black), rabbit (n = 31, blue), and guinea pig (n = 118, red) myocytes. JNHE in guinea pig myocytes was greater than that in rat or rabbit cells at pHi values less than ∼6.8. The dashed lines are the best-fit results for JNBC given in panel A. Continuous lines are best-fits of data to the equation: JNHE = Jmax[H+]m/(Km+[H+]m). The respective values for Jmax, K and m for NHE in rat were: 34.188, 10−6.380 and 1.933. For guinea pig they were: 47.110, 10−6.376 and 2.134. For rabbit they were: 8.011, 10−6.667 and 2.544.
JNBC was similar in rabbit and guinea pig myocytes over the pHi range of 6.4–7.2, but higher in rat at pHi values below approximately 6.9 (Fig. 2A). For example, at pHi 6.6 the mean JNBC in rat myocytes was approximately 50% greater than that in rabbit and guinea pig cells. Figure 2B presents data for the pHi dependence of acid extrusion via NHE for all three species. Results were derived from pHi recovery in CO2/HCO3−-free solution (pHo 7.4) buffered with Hepes.
Voltage sensitivity of steady-state pHi
Studies of NBCe1 and NBCe2 gene products, when expressed in oocytes, have indicated that activity of both transporters is voltage sensitive (Choi et al. 1999; Virkki et al. 2002) with INBC displaying a reversal potential (ENBC, equilibrium potential) in accordance with equilibrium thermodynamics:
| (1) |
where R, T, and F have their usual meaning, and n is the coupling coefficient (n HCO3− ions cotransported with each Na+ ion). If NBCe1 and/or NBCe2 gene products are functionally active in ventricular myocytes, they should mediate an acid extrusion that is sensitive to membrane potential, with depolarization enhancing and hyperpolarization reducing or even reversing the extrusion, thereby affecting resting pHi. It is not known if acid efflux via NBCn1 is voltage sensitive, but the flux would not be expected to display a reversal potential, as the equilibrium condition for such a transporter is defined only by the sarcolemmal gradients of Na+ and HCO3− ions.
Changing membrane potential under voltage clamp conditions
Figure 3A illustrates the response of resting pHi (rat, carbonic buffer) to a voltage-clamp hyperpolarization from a holding potential of −80 mV to −140 mV followed by a step to 0 mV. At −80 mV the calculated ENBC (eqn (1)) was −96 mV, assuming equality of the pipette and intracellular sodium concentrations. Hyperpolarization decreased pHi while depolarization increased it. Similar changes in pHi occurred in rabbit and guinea pig myocytes subjected to the same clamp protocol, but the changes were smaller in magnitude (Fig. 3B). When voltage steps were applied to rabbit myocytes superfused with Hepes solution, nominally free of CO2/HCO3−, there were no significant changes in pHi (Fig. 3C), indicating that they are bicarbonate dependent.
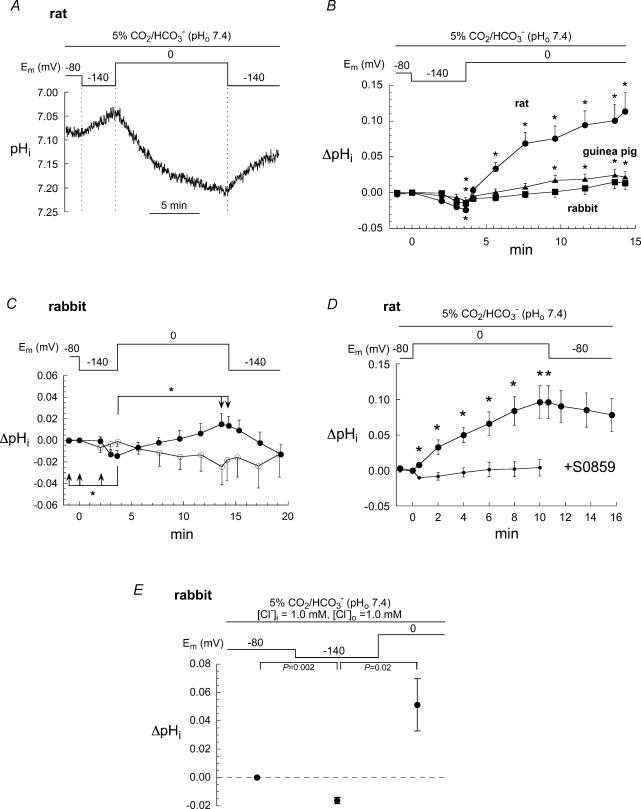
Figure 3. Response of pHi to changing Em with voltage clamp.
A, example of the large changes in pHi elicited by the clamp protocol when applied to a rat myocyte bathed in CO2/HCO3− solution. Cells were initially held at −80 mV, then clamped to −140 mV for 3 min followed by 10 min at 0 mV, then back to −140 mV. B, summary of Em-induced changes in pHi in rat (n = 5), guinea pig (n = 8) and rabbit (n = 12). *P-values (paired) ranged between 0.01 and 0.004. C, summary of the lack of response of pHi to changing Em in rabbit myocytes (n = 7) bathed in Hepes-buffered solution containing no added CO2/HCO3−. In contrast, when cells (n = 12) were bathed in CO2/HCO3−-buffered solution, hyperpolarization caused a significant fall in pHi (*P < 0.05, paired) while depolarization induced a small increase. D, in the absence of the NBC blocker, S0859, rat myocytes (n = 6) displayed a large rapid increase in pHi in response to a 10 min step voltage change from −80 mV to 0 mV. *P-values ranged from 0.02 to 0.009, paired. This increase was completely blocked in myocytes (n = 4) bathed for 5 min in S0859 (10 μm). E, the pattern of Em-induced changes in rabbit pHi (n = 5) was not altered by lowering [Cl−]o to 1 mm for at least 25 min (paired P-values).
The voltage sensitivity of pHi was inhibited by 10 μm S0859, a selective cardiac NBC inhibitor (Ch'en & Vaughan-Jones, 2001b), as shown in Fig. 3D which pools data gathered from rat myocytes. The voltage-dependent changes of pHi may therefore be attributed to a modulation of NBC activity. An alternative explanation is that the changes in pHi were secondary to voltage-dependent changes in [Cl−]i (Vaughan-Jones, 1979). Alterations in [Cl−]i could, in principle, change pHi by varying net membrane fluxes of HCO3− and OH− anions via Cl−–HCO3− exchange (Vaughan-Jones, 1979) and Cl−–OH− exchange (Sun et al. 1996), respectively. To test this hypothesis, the clamp protocol was applied to rabbit cells bathed in very low [Cl−]o solution (present as 0.5 mm BaCl2) using a pipette filling solution that also contained 1 mm Cl− (Fig. 3E). The depolarization-induced changes in pHi were very similar to those in normal [Cl−]o, strongly suggesting that the anion exchangers were not involved.
Changing membrane potential by varying [K+]o (constant osmolarity)
In myocytes not subjected to voltage clamp, increasing [K+]o from 4.4 to 44.4 mm in CO2/HCO3−-buffered solution (pHo 7.4) depolarized Em from approximately −85 mV to −25 mV (rabbit, n = 2). Superfusate osmolarity, [Na+]o and [Cl−]o were held constant at 317 mosmol l−1, 118.5 mm and 148 mm, respectively, by replacing NMDG-Cl with KCl (see Methods). Figure 4A shows that, in rat myocytes, pHi increased under these conditions. In contrast, as summarized in Fig. 4B, the same changes of [K+]o made in Hepes-buffered solution produced no change of pHi. The hyperkalaemia-induced increase of pHi was inhibited by 10 μm S0859, indicating that it was mediated by acid extrusion on NBC (Fig. 4C). Figure 4D summarizes data from all three species, showing that similar, but smaller, effects were also evident in rabbit and guinea pig myocytes. The rank order for effects on pHi was rat > rabbit ≈ guinea pig, paralleling the effect on pHi of voltage-clamp steps (Fig. 3B), and confirming that pHi in the presence of CO2/HCO3− is voltage sensitive.
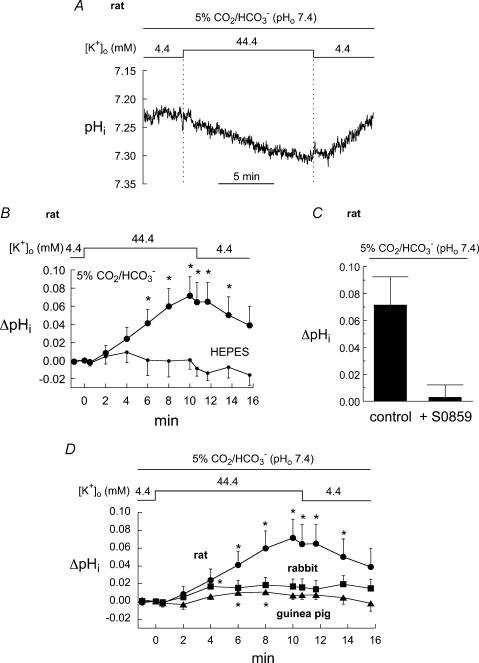
Figure 4. Effect of potassium-induced depolarization on pHi with bathing solution osmolarity held constant.
A, example of the rise in pHi that occurred in rat myocytes bathed in CO2/HCO3−-buffered solution and exposed to high [K+]o for 10 min. B, summary of results from rat myocytes bathed in CO2/HCO3−-buffered (n = 7) and Hepes-buffered (n = 5) solution. C, mean change in rat pHi following 10 min exposure to high [K+]o (CO2/HCO3− buffer) in the absence (n = 7) and presence of S0859 (n = 4). The NBC inhibitor completely blocked the rise in pHi. D, summary of the rise in pHi elicited by 10 min exposure to high [K+]o in CO2/HCO3−-buffered solution (rat, n = 7; rabbit, n = 13; guinea pig, n = 13). *P < 0.05 (paired).
Changing membrane potential by varying [K+]o (uncompensated osmolarity)
Much larger effects of hyperkalaemia on resting pHi (0.1–0.2 pHi units) have been reported previously for cat papillary muscle (Camilión de Hurtado et al. 1995, 1996) but in those cases [K+]o was elevated (4.5 mm to 45 mm) without osmotic compensation. We therefore examined the effect of [K+]o-induced depolarization on pHi in myocytes under conditions in which additional KCl was added to the bathing solution without osmotic compensation. In contrast to the small alkalosis shown in Fig. 4, uncompensated hyperkalaemia elicited much larger increases of pHi in all three species (Fig. 5).
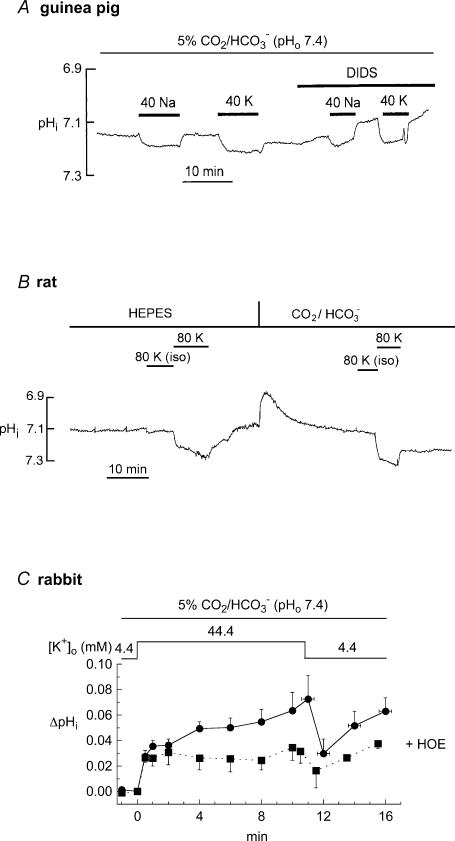
Figure 5. Response of pHi to potassium-induced depolarization without osmotic compensation.
A, guinea pig myocyte: response of pHi to superfusion with hypertonic solution (generated by directly adding either 40 mm NaCl or 40 mm KCl to Tyrode solution, denoted as 40 Na and 40 K, respectively) in the absence and then in the presence of 250 μm DIDS. B, rat myocyte: response of pHi to isotonic hyperkalaemia (80 mm) or hypertonic hyperkalaemia (80 mm), denoted by 80K(iso) and 80K, respectively. These solutions were first applied in the absence of CO2/HCO3− (Hepes-buffered), then in CO2/HCO3−-buffered solution. C, rabbit myocytes: pooled data showing effect on pHi of hypertonic hyperkalaemia (44.4 mm) without (n = 5) and with (n = 7) 30 μm HOE 642. The absence of full pHi recovery after removal of hyperkalaemia was evident in all cells tested.
For example, as shown in Fig. 5A, an 8 min exposure of guinea pig myocytes to osmotically uncompensated high K+ solution (44.5 mm) increased pHi by 0.09 ± 0.02 units (n = 9), instead of the 0.01 unit alkalosis seen with osmotically compensated hyperkalaemia in this species (Fig. 4D). This figure also shows that a similar alkalosis occurred (0.06 ± 0.01 units; n = 12) when an equivalent rise of extracellular tonicity was induced at constant 4.5 mm [K+]o (achieved in this case by adding 40 mm NaCl to the bathing solution). These hypertonically induced increases in pHi persisted in the presence of 250 μm DIDS (a non-selective inhibitor of NBC; Lagadic-Gossmann et al. 1992) ruling out any major role for NBC activation. In the presence of DIDS, pHi increased by 0.09 ± 0.02 (n = 9) during hypertonic addition of 40 mm KCl, and by 0.05 ± 0.01 (n = 9) during hypertonic addition of 40 mm NaCl.
Similar results were observed in experiments on rat ventricular myocytes (n = 6). Figure 5B illustrates one such experiment. The myocyte was exposed to hyperkalaemia (80 mm) under conditions where the solution osmolarity was kept constant by an equivalent reduction of [Na+]o, or under hypertonic conditions where 75.5 mm KCl was added to the Tyrode solution. Once again, a large alkalosis was only evident during exposure to hypertonic solution. In rabbit myocytes, osmotically uncompensated hyperkalaemia (4.4 mm to 44.4 mm) elicited an increase of pHi that was inhibited markedly by cariporide, suggesting that much of the alkalosis was secondary to an activation of NHE (Fig. 5C). In addition, exposure of rabbit myocytes (n = 7) to an equivalent osmotic step (288 to 347 mosmol l−1) by adding sucrose, while keeping [K+]o and [Na+]o constant, elicited an alkalosis similar to that shown in Fig. 5C in the absence of cariporide (data not shown). These results demonstrate that the use of hypertonic hyperkalaemia to explore the voltage dependence of NBC induces significant pHi changes that are unrelated to the transporter's activity.
Voltage sensitivity of pHi recovery from intracellular acidosis
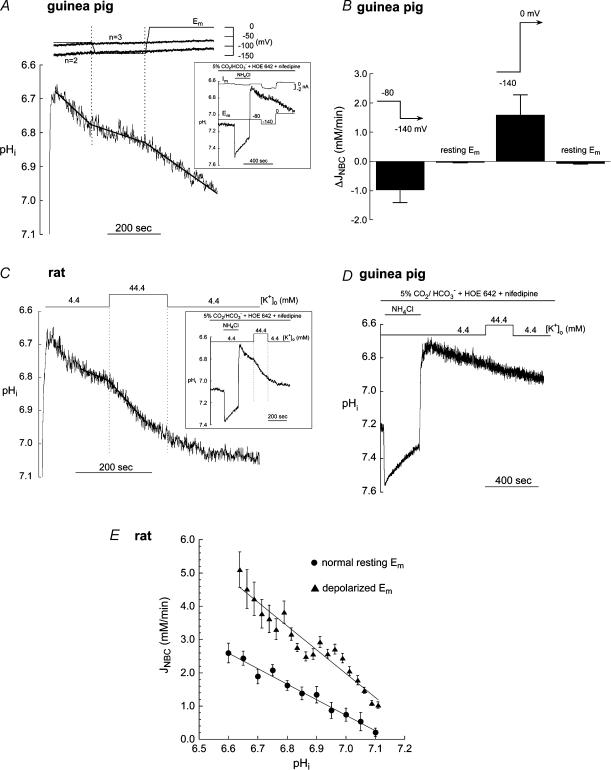
We assessed NBC's voltage sensitivity during its activation by intracellular acidosis. Figure 6A illustrates a typical experiment on a guinea pig ventricular myocyte. Em was clamped initially at −80 mV in the presence of 30 μm cariporide, while an acid load was imposed by prepulsing the cell with extracellular ammonium. During the ensuing pHi recovery, Em was hyperpolarized to −140 mV for 200 s, and then depolarized to 0 mV. Hyperpolarization roughly halved the rate of pHi recovery while the subsequent depolarization restored it. These findings are consistent with hyperpolarization decreasing and depolarization increasing HCO3− influx via a voltage-sensitive NBC. Similar results were obtained in six other experiments.
Figure 6. Effect of Em on NBC during recovery from intracellular acidosis.
A, hyperpolarization slows and depolarization speeds the rate of pHi recovery mediated by NBC in a guinea pig ventricular myocyte. Only the expanded recovery phase of pHi is illustrated while the inset shows the entire experimental protocol. The calculated values of ENBC for n values of 2 and 3 are superimposed on the voltage-clamped Em signal. B, mean changes in JNBC (▵JNBC) in guinea pig myocytes (n = 7) elicited by the two clamp steps (−80 to −140 mV; −140−0 mV). The ▵JNBC that would have occurred due to pHi recovery alone over this time interval, with Em kept at its normal resting value, is also shown. C, response of pHi recovery rate (following an ammonium prepulse) to K+-induced depolarization in a rat myocyte. D, response of pHi recovery rate to K+-induced depolarization in a guinea pig myocyte. E, comparison of JNBC–pHi curves from rat myocytes at normal resting Em (•, same data as Fig. 2A) and in cells depolarized with 44.4 mm [K+]o (▴, n = 11) during pHi recovery from an acid-load (induced by ammonium prepulse). All bathing solutions used in this figure were buffered with CO2/HCO3− and contained 30 μm HOE 642. Nifedipine (10 μm) was also included during the voltage clamp and high [K+]o experiments.
At pHi 6.80 during the acid load shown in Fig. 6A, ENBC (estimated from eqn (1)) would have been −85 mV and −130 mV, for an ionic coupling coefficient of n = 3 and 2, respectively (assuming [Na+]pip=[Na+]i= 15 mm). Thus, at an Em of −140 mV, acid efflux via an electrogenic NBC (n = 2 or 3) would have been terminated or even reversed (thus mediating HCO3− ion efflux, equivalent to acid influx into the cell). In none of the seven experiments, however, was pHi recovery prevented, although in all cases it was slowed significantly. The magnitude of the change in net acid efflux (▵JNBC) elicited by the switch between the two holding potentials is summarized in Fig. 6B. ▵JNBC was calculated as the difference in JNBC measured 30 s before and after the voltage step. For purposes of comparison, the small decrease in JNBC (▵JNBC) that may be attributed simply to the pHi change during this 1 min period (independent of the voltage jump), is also shown. At pHi∼6.80, all acid extrusion is mediated via NBC (with NHE blocked by cariporide, pHi recovery is dependent on extracellular HCO3− and Na+; Lagadic-Gossmann et al. 1992, see also Fig. 1C). The failure of a large hyperpolarization to terminate or reverse pHi recovery therefore suggests that, in addition to NBCe, an NBCn may be operating across the sarcolemma, as the thermodynamic energy source for this would be voltage independent and would still have favoured acid extrusion.
In another series of experiments, a smaller depolarization to approximately −25 mV was induced in unclamped rat (n = 11) and guinea pig (n = 3) myocytes by applying 44.4 mm [K+]o (with solution osmolarity and [Na+]o held constant; Fig. 6C, rat myocyte; Fig. 6D, guinea pig myocyte). In this case, the driving force for acid efflux through both electrogenic and electroneutral NBCs would remain outward throughout. In the rat myocyte there was a stimulatory effect on NBC-mediated pHi recovery, but in the guinea pig myocyte (Fig. 6D) no effect was detected. This species difference in the responsiveness of pHi recovery to modest hyperkalaemic depolarization is consistent with the findings for steady-state pHi shown in Fig. 4D. Figure 6E plots the relationship between JNBC and pHi in rat myocytes exposed to a [K+]o of 4.4 mm (normal Em) and 44.4 mm (depolarized Em). Depolarization induced a large upward shift in the JNBCversus pHi curve, and increased its slope. This indicates that the fractional increase in JNBC in 44.4 mm [K+]o (about 100%) was the same over the range of pHi values tested.
In summary, depolarization can enhance the ability of intracellular acidosis to activate NBC activity.
Voltage dependence of INBC
Given that the voltage-dependent changes in pHi observed in CO2/HCO3−-buffered solution appeared to be mediated by NBC, it was of interest to determine the voltage dependence of INBC (Fig. 7). Our approach was first to apply a voltage-clamp ramp while measuring membrane current in Hepes-buffered solution. NBC was then activated by switching to a CO2/HCO3−-buffered solution and, after 4 min, the ramp was reapplied. The difference current on switching to CO2/HCO3− was taken as INBC (see Methods, and see Aiello et al. 1998). After 4 min, the intracellular acidosis produced initially by the switch to CO2/HCO3− had been attenuated by sarcolemmal acid extrusion and was relatively modest (pHi 7.0–7.1; n = 19). By abbreviating the time between the first and second voltage ramps, the likelihood of INBC rundown or time-dependent changes in other currents activated by the ramp was minimized. In control experiments using ammonium prepulses to induce intracellular acidification, we found no effect of changing pHiper se (over the pHi range from 7.0 to 7.3) on I–V curves from myocytes (rabbit, n = 6–13) bathed continuously in Hepes-buffered solution. Similarly, successive ramps applied to myocytes (rabbit, n = 5) bathed in Hepes-buffered solution over a 4 min interval produced virtually identical I–V curves.
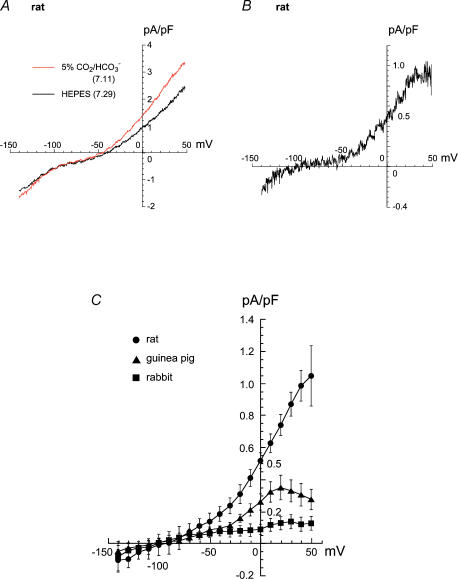
Figure 7. Em dependence of INBC in all species.
A, example of I–V curves recorded in a rat myocyte bathed first in Hepes (pHi 7.29, t= 0) then in CO2/HCO3−(pHi 7.11, t= 4 min). B, difference current (INBC) calculated from the I–V curves in panel A. C, summary of difference currents (INBC) from all species: (•, rat, n = 5) (▴, guinea pig, n = 9) (▪, rabbit, n = 5). The mean pHi values measured during the ramps, first in Hepes- and then 4 min later in CO2/HCO3−-buffered solution were: rat 7.25 ± 0.01 (Hepes), 7.05 ± 0.02 (CO2/HCO3−); rabbit 7.19 ± 0.02 (Hepes), 7.00 ± 0.03 (CO2/HCO3−); guinea pig 7.23 ± 0.02 (Hepes), 7.07 ± 0.02 (CO2/HCO3−).
Current–voltage relationships for INBC are summarized in Fig. 7. A representative rat myocyte experiment is shown in Fig. 7A and includes I–V curves recorded first in Hepes- and then after 4 min in CO2/HCO3−-buffered solution, as well as the difference current (Fig. 7B). A voltage-sensitive, bicarbonate-dependent outward current was clearly evident in the voltage range positive to about −100 mV, reversing at more negative voltages. This is similar to that previously attributed to electrogenic NBC in this cell-type (Aiello et al. 1998). The current cannot be ascribed to HCO3− flowing through an anion channel as, at the pHi for the measurement (about 7.05), any outward HCO3− current would have reversed at about −40 mV (e.g. Spitzer & Hogan, 1979), similar to that for Cl− (Harvey & Hume, 1989). Current reversal at −100 mV, however, is consistent with INBC for a coupling coefficient of n = 2 (estimated from eqn (1)). Results from similar experiments are summarized in Fig. 7C. In all cases, there was a significant HCO3−-activated current, displaying a similar reversal potential (ENBC) of −98 ± 12 mV for rat (n = 5), −103 ± 20 mV for rabbit (n = 5), and −106 ± 14 mV for guinea pig (n = 9). There were, however, marked species differences in INBC density, with rat > guinea pig > rabbit. For example, at an Em of 0 mV, INBC at resting pHi was +0.52 ± 0.05 pA pF−1 (rat), +0.26 ± 0.05 pA pF−1 (guinea pig) and +0.10 ± 0.03 pA pF−1 (rabbit).
Effect of NBC on the resting membrane potential
Activation of electrogenic NBC when membrane potential is positive to ENBC will be associated with outward current and may therefore hyperpolarize the resting membrane potential. We evaluated this prediction in quiescent myocytes using patch-pipettes filled with normal solution, containing either 5 mm or 15 mm[Na+]. Em and pHi were recorded simultaneously as the superfusate was switched from Hepes-buffered to CO2/HCO3−-buffered solution (pHo 7.4) for 10 min. In all three species, the switch to CO2/HCO3−-buffered solution elicited a small depolarization rather than the predicted hyperpolarization. This ranged from approximately 0.4 mV to 2 mV (rat n = 5; guinea pig, n = 9; rabbit, n = 11) and was significant only in rabbit and rat myocytes at 1 min, suggesting that resting membrane potential is little affected by NBCe activity.
Changes in [Na+]i associated with NBC activation
Acid efflux via NBC should be associated with Na+ influx. Figure 8 shows representative examples of changes in [Na+]i recorded from guinea pig and rat myocytes during activation of NBC and NHE. In Fig. 8A, a guinea pig myocyte bathed in Hepes-buffered solution was subjected to an intracellular acid load (induced by ammonium prepulse). The subsequent pHi recovery was accompanied by a transient rise of [Na+]i that was blocked by cariporide, as shown in the latter part of the trace, indicating that it was mediated by a stimulation of NHE. Plotted below the [Na+]i traces in Fig. 8 are the computed pHi changes expected during the protocols (see Methods).
Figure 8B shows a recording of [Na+]i in a rat myocyte superfused with carbonic buffered solution. In this case cariporide substantially reduced but did not abolish the transient rise of [Na+]i following an ammonium prepulse, suggesting that the remaining rise was mediated by Na+ influx on NBC. This was supported by the observation that, later in the same experiment, 10 μm S0859 also attenuated the post-ammonium rise of [Na+]i. The predicted time course for pHi is plotted beneath the [Na+]i trace and was calculated assuming that cariporide selectively blocks NHE (Scholz et al. 1995) and that S0859 blocks NBC activity (Ch'en & Vaughan-Jones, 2001b). In guinea pig myocytes subjected to the same experimental protocol as shown in Fig. 8B, similar changes of [Na+]i were seen.
The rate of rise of [Na+]i 0.5–2 min after ammonium removal (dNa+i/dt) has been averaged for several cells in Fig. 9A (for guinea pig) and Fig. 9B (for rat), normalized to the rate seen initially in the same cell after ammonium-removal in the absence of cariporide or S0859. There was roughly a 20–30% reduction of dNa+i/dt in the presence of S0859, while an 80–90% reduction occurred with cariporide. This indicates that, at the pHi at which the measurements were made (∼6.80, see Fig. 8B), about 20% of total Na+ influx associated with pHi regulation was trafficked on the NBC transport system. Reference to the acid-efflux curves determined for NBC and NHE (Fig. 2) permits an estimate of the fraction of total acid efflux through each transporter under these conditions. These fractions have been plotted in Fig. 9C (guinea pig) and Fig. 9D (rat), along with the corresponding fractions for total Na+ influx. It is apparent that fractional Na+ influx is similar to fractional acid efflux. The apparent coupling coefficient for total NBC, n, was estimated in two separate ways, using experimental data for NHE and NBC kinetics (Figs 9C and D):
and where FHNBC and FNaNBC are, respectively, the fraction of total acid efflux and Na+ influx carried on NBC (ratio of flux in the presence and absence of cariporide), and where FHNHE and FNaNHE are, respectively, the fraction of acid efflux and Na+ influx carried on NHE (ratio of flux in presence and absence of S0859). The data shown in Fig. 9C and D yield values for n of 1.53 ± 0.37 (n = 17) for guinea pig and 1.40 ± 0.42 (n = 23) for rat. These are non-integer numbers although neither was significantly different statistically from n = 1 or n = 2 (P > 0.05).
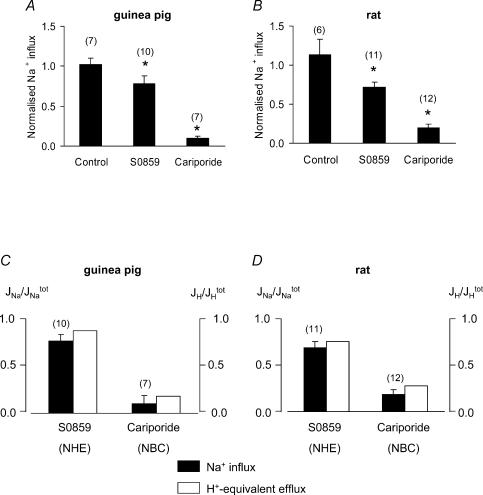
Figure 9. Sodium influx associated with NBC and NHE activation.
A and B, guinea pig and rat, respectively: data for net Na+ influx (estimated from dNa+i/dt measured following 20 mm ammonium removal) obtained in the absence of drug or in the presence of S0859 or cariporide. Data have been normalized to a control dNa+i/dt obtained following NH4+ removal in the absence of drug. In the case of the control Na+ influx, two successive ammonium prepulses were performed, and dNa+i/dt after the second was normalized to that after the first. Figure 8B illustrates one experimental protocol. C, flux of Na+, JNa (filled bars) or acid, JH (open bars, estimated from Fig. 2) mediated by NHE or NBC, expressed as a fraction of total flux (through both NHE and NBC). D, analogous data for rat myocytes. Sample sizes (n) in panels C and D shown above bars.
Discussion
Functional diversity of electrogenic NBC
A major aim of this study was to establish whether electrogenicity is a general property of Na+–HCO3− cotransport in commonly used ventricular myocyte preparations (rat, rabbit and guinea pig). Using electrophysiological and fluorescence techniques we have obtained evidence for NBCe in all three species. However, INBC density (Fig. 7) and the responsiveness of resting pHi to changes in Em (Figs 3 and 4) varied considerably among species, in the order: rat > guinea pig ≃ rabbit. This sequence is also similar to that for the pHi dependence of acid efflux via total NBC (all isoforms) shown in Fig. 2A and may reflect a lower level of NBCe expression in guinea pig and rabbit compared with rat.
Our identification of NBCe in rat ventricular myocytes corroborates a previous report (Aiello et al. 1998). Thus, it seems likely that NBCe is a common pHi regulatory system in mammalian ventricles. This is consistent with mRNA, protein measurement, and oocyte expression studies of NBCe1 and NBCe2 isoforms from human, rat and guinea pig heart (Choi et al. 1999; Choi et al. 2000; Virkki et al. 2002).
Comparison of acid efflux through NHE and NBC (cf. Fig. 2A with Fig. 2B) reveals that, over much of the pHi range, flux through NHE is usually larger in all three species. Nevertheless, the different pHi sensitivity of the two types of transporter means that fractional acid extrusion through NBC (FHNBC) in the three species increases from 15 to 20% at pHi 6.50 to about 50% at pHi 7.1. This confirms a previous report (Leem et al. 1999) that NBC is an important controller of steady-state pHi in heart.
The NBC substrates
While one HCO3− anion may accompany the influx of one Na+ ion on electroneutral NBC in heart (i.e. n = 1), it is not certain that HCO3− is the transported substrate on electrogenic NBC. Alternative models involve coinflux of Na+ with CO32− anions, or the uncoupled inward transport of a NaCO3− ion pair (see e.g. Romero et al. 2004). These alternative models are equivalent to conventional electrogenic Na+–HCO3− co-influx, with an apparent coupling coefficient (n·HCO3− : Na+) equal to or greater than 2. For simplicity, we have expressed our analysis of cardiac NBC in terms of equivalent movements of HCO3− and Na+ ions.
Voltage sensitivity of pHi
The presence of NBCe in non-cardiac tissues is characterized by voltage dependence of both pHi and INBC (Siebens & Boron, 1989; Deitmer & Szatkowski, 1990; Grichtchenko & Chesler, 1994; Munsch & Deitmer, 1994; Shumaker et al. 1999; Gross et al. 2001). Depolarization positive to ENBC favours HCO3− influx, outward INBC and an increase in pHi. Conversely, hyperpolarization negative to ENBC favours HCO3− efflux, inward INBC and a reduction of pHi. In all three species, we detected Em sensitivity of pHi attributable to NBC both under steady-state conditions and during pHi recovery from intracellular acidosis. Indeed, our work using the perforated patch technique is the first to show a direct Em dependence of pHi in heart. Previous work utilized either hyperkalaemia or electrical pacing to modulate Em (Camilión de Hurtado et al. 1995, 1996).
Voltage-dependent changes of resting pHi were observed under voltage-clamp conditions and during manipulation of [K+]o. While our results point to NBCe activity in the heart, it is important to stress that Em sensitivity alone is not proof of electrogenicity of a transporter. However, the fall of resting pHi observed in all three species upon hyperpolarization to −140 mV (Fig. 3B) is consistent with reversed NBCe since it also occurred in Cl−-free conditions (Fig. 3E). In this setting the other acid-loading transporters, Cl−–HCO3− exchange and Cl−–OH− exchange, cannot function (Leem et al. 1999). NBCe reversal is also in agreement with the reversal of INBC observed in all three species at potentials negative to −100 mV (Fig. 7C) and would indicate that, over the voltage range tested, the changes of resting pHi are due to both forward and backward modes of NBCe activity.
Two previous reports have shown that hyperkalaemia when applied with an accompanying rise of solution tonicity elicits a large alkalosis (∼0.2 units for a 10-fold rise in [K+]o) in cat papillary muscle in vitro (Camilión de Hurtado et al. 1995, 1996). This change of pHi is much greater than that observed in isolated ventricular myocytes exposed to hyperkalaemia under isotonic conditions (Fig. 4, see also Aiello et al. 1998). In addition, we found that NHE blockade markedly attenuated the alkalosis elicited by hypertonic hyperkalaemia (Fig. 5C). This finding is consistent with earlier work showing that exposure of guinea pig ventricular muscle to hypertonic solutions activates NHE (Whalley et al. 1991). Thus it seems possible that much of the hyperkalaemia-induced modulation of pHi reported previously in cat papillary muscle was secondary to osmotic rather than voltage-dependent effects.
Voltage dependence of pHi recovery
The present work provides the first demonstration that NBC-mediated pHi recovery from intracellular acidosis in cardiac tissue is voltage sensitive, with depolarization speeding and hyperpolarization slowing acid extrusion (Fig. 6). Human pancreatic duct cells also display depolarization-induced stimulation of electrogenic NBC during acid load recovery (Shumaker et al. 1999).
In rat myocytes, depolarization from approximately −85 mV to −25 mV with 44.4 mm [K+]o nearly doubled NBC-mediated acid extrusion (Fig. 6). This demonstrates dramatically that, in this species, in addition to pHi and pHo (Ch'en & Vaughan-Jones, 2001a), membrane potential is a key regulator of NBC activity. In contrast, although pHi recovery from acidosis in guinea pig myocytes was responsive to relatively large depolarizing voltage-clamp steps (140 mV), it did not appear to be affected by the smaller depolarization induced by 44.4 mm [K+]o (∼60 mV). Lagadic-Gossmann et al. (1992) also failed to detect depolarization-induced changes of pHi recovery in guinea pig ventricular myocytes subjected to smaller voltage-clamp steps than used here, and to 45 mm [K+]o (constant osmolarity). Similarly, pHi recovery in sheep cardiac Purkinje fibres (Dart & Vaughan-Jones, 1992) and in cat papillary muscle (Camilión de Hurtado et al. 1995) was unresponsive to depolarization induced by hyperkalaemia. A low voltage sensitivity of pHi recovery in certain species is similar to the reduced responsiveness of steady-state pHi to depolarization in guinea pig and rabbit myocytes (Figs 3 and 4), and may reflect lower levels of INBC (Fig. 7C). It may also reflect the coexpression of a functionally active electroneutral NBC (see section on NBC stoichiometry).
Effect of NBC activation on resting membrane potential
Hyperpolarization of resting Em is a frequently observed response of non-cardiac cells to activation of NBCe using application of extracellular CO2/HCO3−-buffered solution (Deitmer & Schlue, 1989; Bevensee et al. 1997; Choi et al. 1999) Similarly, a switch from Hepes to CO2/HCO3−-buffered solution (pHo 7.4) was reported to induce a small (1–4 mV) hyperpolarization in canine cardiac Purkinje strands (Spitzer & Hogan, 1979; Dart & Vaughan-Jones, 1992), cat papillary muscle (Camilión de Hurtado et al. 1995), and rat ventricular myocytes (Aiello et al. 1998). In contrast, we found that resting Em either depolarized slightly or did not change in any of the three species following the switch to CO2/HCO3−-buffered solution. Examination of the I–V curves (Fig. 7C) indicates the predicted hyperpolarization is likely to be small. For example, the average outward INBC at normal resting values of Em was approximately 0.03 pA pF−1, which for a 150 pF cell yields 4.5 pA. Assuming a normal resting input resistance of between 30 and 50 MΩ (Brown et al. 1981; Hume & Uehara, 1985; Huelsing et al. 1998) a hyperpolarization of less than 1 mV would be predicted. Such a small hyperpolarization may easily be obscured by pHi-induced changes in other membrane currents, for example, by an acidosis-induced decrease in inward rectifier K+ current, as occurs in guinea pig ventricular myocytes (Ito et al. 1992).
Stoichiometry of NBC
Electrogenic NBC
Since NBC clearly supports acid extrusion under normal conditions, its coupling coefficient, n, must be ≤ 2 (Lagadic-Gossmann et al. 1992; Dart & Vaughan-Jones, 1992; Leem et al. 1999). In addition, ENBC, measured as the reversal potential for electrogenic NBC-current, matches closely the value predicted from equilibrium thermodynamics for n =2. Thus ENBC was measured to be −103 mV (calculated −108 mV) for rabbit, −106 mV (calculated −99 mV) for guinea pig, and −98 mV (calculated −102 mV) for rat. Aiello et al. (1998) also concluded that the stoichiometry of NBCe in rat ventricular myocytes was n = 2. The present work, however, gives no clue as to the NBCe isoform (e.g. NBCe1-B or NBCe2-c) responsible for INBC in heart. Indeed mRNA for both isoforms has been identified in mammalian cardiac tissue (Choi et al. 1999; Pushkin et al. 2000).
Electroneutral NBC
Recently, a novel NBC has been cloned from human heart (Pushkin et al. 1999; Choi et al. 2000) that, when expressed in Xenopus oocytes, mediates electroneutral NBC (NBCn1, known variously as NBC2 or NBC3; see Romero et al. 2004 for a review of NBC classification). The mRNA and protein product for NBCn1 has been detected in human and rat heart (Pushkin et al. 1999; Choi et al. 2000; Khandoudi et al. 2001).
Although the voltage-dependent changes in pHi and membrane current reported here are consistent with expression of one or more NBC electrogenic gene products, the experiments do not provide direct evidence concerning the operation of NBCn. However, the following three observations suggest that NBCn may operate in parallel with NBCe in ventricular myocytes.
Voltage clamping Em negative to ENBC (calculated for n≥ 2) in a guinea pig ventricular myocyte reduced but did not terminate or reverse NBC-mediated pHi recovery from an intracellular acid load. This suggests that, although NBCe was expected to have reversed, as predicted from the voltage dependence of INBC (Fig. 7), NBCn (whose thermodynamic energy source is independent of membrane potential) may have continued to operate in the forward mode, producing acid extrusion. Thus, the effect on pHi recovery under these conditions would reflect the net activities of NBCe and NBCn.
From our SBFI-fluorescence studies, the fraction of Na+ influx attributable to NBC during pHi regulation suggests an apparent NBC coupling coefficient of n≈ 1.4, i.e. a non-integer number (note that a range of values between n = 1 and n = 2 was statistically feasible). While this may be consistent with an ionic coupling of 3HCO3− : 2Na+, our values of ENBC indicated n = 2 in all three species (Fig. 7C). The alternative interpretation of apparently non-integer values for ‘n’ is that a fraction of the myocyte's NBC population may operate with n = 1 while the rest operates with n = 2.
We have compared total acid efflux through NBC (JtotNBC) derived from the acid-efflux curves in Fig. 2A, with acid-efflux derived from INBC (i.e. electrogenic acid efflux, JNBCe; Fig. 7C). Results for each species are summarized in Table 1. Values derived for JNBCe are greatly influenced by the value assumed for the surface area to volume ratio (S/V) of the myocyte. Nevertheless, irrespective of various assumed S/V values, the results in all three species indicate that JtotNBC > JNBCe, suggesting that both NBCe and NBCn transporters may be coactive within a ventricular myocyte. One may criticize these particular calculations on the grounds that they are based on measurement of low levels of NBC flux and INBC estimated at resting pHi. Nevertheless, the resting value for total JNBC presented in Fig. 2 is very similar to that reported previously in guinea pig myocytes (Leem et al. 1999), while the INBC value reported here for rat is in line with that of Aiello et al. (1998).
Table 1.
Estimate of JNBCe/JtotNBC in resting myocytes under normal conditions
| INBC (pA pF−1) | volume (pl) | JNBCe (mm min−1) | JtotNBC (mm min−1) | JNBCe/JtotNBC | |
|---|---|---|---|---|---|
| Rat | 0.04 | 18a | 0.40 | 0.68 | 0.6 |
| (pHi 7.05) | 32b | 0.22 | 0.68 | 0.3 | |
| 44c | 0.16 | 0.68 | 0.2 | ||
| Rabbit | 0.04 | 29d | 0.28 | 0.42 | 0.7 |
| (pHi 7.00) | 31e | 0.26 | 0.42 | 0.6 | |
| 38f | 0.22 | 0.42 | 0.5 | ||
| Guinea pig | 0.02 | 18a | 0.18 | 0.32 | 0.6 |
| (pHi 7.07) | 46c | 0.08 | 0.32 | 0.3 | |
| 28g | 0.12 | 0.32 | 0.4 |
It was assumed that electrogenic NBC had a 1 : 2 stoichiometry and JNBCe (mm min−1 l−1) was given as: JNBCe= 2(INBC× Cm)/(F× cell volume), where Cm is membrane capacitance (pF) and F is the Faraday constant. Values for Cm were 149 pF (rat), 175 pF (rabbit) and 155 pF (guinea pig). Values of INBC at −85 mV were taken from Fig. 7C and those for JtotNBC at the same pHi were taken from Fig. 2A. Surface/volume ratios (μm2μm−3) were:
0.84 (Satoh et al. 1996),
0.46 (Page, 1978),
0.34 (Page et al. 1971),
0.60 (Page & Surdyk-Droske, 1979),
0.56 (Page, 1978),
0.46 (Satoh et al. 1996).
Fixed volume of 28 pl (Campbell et al. 1987).
Functional significance of Na+ influx through cardiac NBC
In addition to restoring pHi following an acute intracellular acid load, activation of NBC and NHE induces Na+ influx (Fig. 8). The increase in [Na+]i that accompanies NHE activation increases [Ca2+]i (via effects on sarcolemmal Na+–Ca2+ exchange) and this contributes to the recovery of contraction during a sustained acidosis (Bountra & Vaughan-Jones, 1989; Harrison et al. 1992). It is well documented, however, that an overdrive of NHE, such as that accompanying postischaemic reperfusion in the heart, can cause [Ca2+]i overload resulting in arrhythmia and myocardial injury (Dennis et al. 1990; Karmazyn et al. 1999). Inhibition of NHE reduces these effects by prolonging acidosis and by attenuating the [Ca2+]i overload, through blockade of the NHE-induced rise in [Na+]i (Scholz et al. 1993; An et al. 2001).
In addition to NHE, NBC activation may also significantly contribute to reperfusion injury. For example, inhibition of the transporter is reported to be cardioprotective in rat myocytes subjected to anoxic acidosis (Schäfer et al. 2000) and during postischaemic reperfusion of ferret (Vandenberg et al. 1993) and rat heart (Khandoudi et al. 2001). The latter study suggested a specific role for NBCe in promoting injury. The authors also reported significant up-regulation of NBCe in human cardiomyopathy. The present work is the first to show a net Na+ influx associated with NBC activity in a cardiac cell. Figure 8 shows that, while this is less than that mediated through NHE activity, it nevertheless drives a significant rise of [Na+]i in the order of 1 mm min−1 at pHi 6.70. Low pHi activation of NBC during reperfusion events in the heart may therefore promote [Ca2+]i loading by inducing a rise of [Na+]i, as occurs with NHE.
Significant NBCe activity in tissue exposed to the hyperkalaemia that typically accompanies ischaemia (e.g. Allen & Orchard, 1987) may serve to exacerbate [Ca2+]i overload, as elevating [K+]o considerably enhances activation of NBCe by intracellular acidosis (Fig. 6E). The Ca2+-overloading properties of NBCe activation will, however, be mitigated by the transporter's Na+-sparing properties. On NBCe (n = 2), an average of 0.5 Na+ ions will accompany the influx of one equivalent HCO3− ion, compared with 1.0 Na+ ion on NBCn, or 1.0 Na+ ion on NHE (in exchange for one H+ ion). Because of this Na+-sparing property, selective NBCe inhibitor drugs may be less cardioprotective than NBCn inhibitors or NHE inhibitors (like cariporide) during episodes of ischaemia–reperfusion. Exploration of the pharmacological properties and overall expression of different cardiac NBC gene products may form a useful approach to exploring the cardioprotective potential of NBC inhibition.
Acknowledgments
This study was supported by a MERIT grant from the National Heart, Lung, and Blood Institute (5R37HLO42873) and Nora Eccles Treadwell Foundation (to K.W.S.) and a Programme Grant from the British Heart Foundation (to R.D.V.J.). P.S. was funded by a Wellcome Prize Studentship, and an ORS award (UK Overseas Research Studentship). We gratefully acknowledge the excellent technical assistance of Ms Neelam Banger. HOE (694/642) and S0859, were kindly provided by Dr J. Puenter (Aventis Pharma, Germany).
References
- Aiello EA, Petroff MG, Mattiazzi AR, Cingolani HE. Evidence for an electrogenic Na+–HCO3− symport in rat cardiac myocytes. J Physiol. 1998;512:137–148. doi: 10.1111/j.1469-7793.1998.137bf.x. 10.1111/j.1469-7793.1998.137bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DG, Orchard CH. Myocardial contractile function during ischemia and hypoxia. Circ Res. 1987;60:153–168. doi: 10.1161/01.res.60.2.153. [DOI] [PubMed] [Google Scholar]
- An J, Varadarajan SG, Camara A, Chen Q, Novalija E, Gross GJ, et al. Blocking Na+/H+ exchange reduces [Na+]i and [Ca+]i load after ischemia and improves function in intact hearts. Am J Physiol. 2001;281:H2398–H2409. doi: 10.1152/ajpheart.2001.281.6.H2398. [DOI] [PubMed] [Google Scholar]
- Bevensee MO, Apkon M, Boron WF. Intracellular pH regulation in cultured astrocytes from rat hippocampus. II. Electrogenic Na/HCO3 cotransport. J General Physiol. 1997;110:467–483. doi: 10.1085/jgp.110.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bountra C, Vaughan-Jones RD. Effect of intracellular and extracellular pH on contraction in isolated, mammalian cardiac muscle. J Physiol. 1989;418:163–187. doi: 10.1113/jphysiol.1989.sp017833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM, Lee KS, Powell T. Voltage clamp and internal perfusion of single rat heart muscle cells. J Physiol. 1981;318:455–477. doi: 10.1113/jphysiol.1981.sp013878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler KJ, Vaughan-Jones RD. Application of a new pH-sensitive fluoroprobe (carboxy-SNARF-1) for intracellular pH measurement in small, isolated cells. Pflugers Arch. 1990;417:234–239. doi: 10.1007/BF00370705. [DOI] [PubMed] [Google Scholar]
- Camilión de Hurtado MC, Alvarez BV, Pérez NG, Cingolani HE. Role of an electrogenic Na+-HCO3− cotransport in determining myocardial pHi after an increase in heart rate. Circ Res. 1996;79:698–704. doi: 10.1161/01.res.79.4.698. [DOI] [PubMed] [Google Scholar]
- Camilión de Hurtado MC, Pérez NG, Cingolani HE. An electrogenic sodium-bicarbonate cotransport in the regulation of myocardial intracellular pH. J Mol Cell Cardiol. 1995;27:231–242. [PubMed] [Google Scholar]
- Campbell SE, Gerdes AM, Smith TD. Comparison of regional differences in cardiac myocyte dimenstions in rats, hamsters, and guinea pigs. Anat Rec. 1987;219:53–59. doi: 10.1002/ar.1092190110. [DOI] [PubMed] [Google Scholar]
- Ch'en FF, Vaughan-Jones RD. Na+-HCO3− co-transport is instructed by pH and not bicarbonate or Na+ Biophys J. 2001a;80:74. (abstract) [Google Scholar]
- Ch'en FF, Vaughan-Jones RD. Effect of S0859, a putative Na+-HCO3− co-transport inhibitor, on intracellular pH regulation in the guinea-pig ventricular myocyte. Proceedings of the 34th International Congress of Physiological Sciences ID no. 1495; 2001b. (abstract) [Google Scholar]
- Choi I, Aalkjaer C, Boulpaep EL, Boron WF. An electroneutral sodium/bicarbonate cotransporter NBCn1 and associated sodium channel. Nature. 2000;405:571–575. doi: 10.1038/35014615. [DOI] [PubMed] [Google Scholar]
- Choi I, Romero MF, Khandoudi N, Bril A, Boron WF. Cloning and characterization of a human electrogenic Na+-HCO3− cotransporter isoform (hhNBC) Am J Physiol. 1999;276:C576–C584. doi: 10.1152/ajpcell.1999.276.3.C576. [DOI] [PubMed] [Google Scholar]
- Cordeiro JM, Spitzer KW, Giles WR. Repolarizing K+ currents in rabbit heart Purkinje cells. J Physiol. 1998;508:811–823. doi: 10.1111/j.1469-7793.1998.811bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart C, Vaughan-Jones RD. Na+-HCO3− symport in the sheep cardiac Purkinje fibre. J Physiol. 1992;451:365–385. doi: 10.1113/jphysiol.1992.sp019169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer JW, Sehlue WR. An inwardly directed electrogenic sodium-bicarbonate co-transport in leech glial cells. J Physiol. 1988;411:179–194. doi: 10.1113/jphysiol.1989.sp017567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer JW, Szatkowski M. Membrane potential dependence of intracellular pH regulation by identified glial cells in the leech central nervous system. J Physiol. 1990;421:617–631. doi: 10.1113/jphysiol.1990.sp017965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis SC, Coetzee WA, Cragoe EJ, Jr, Opie LH. Effects of proton buffering and of amiloride derivatives on reperfusion arrhythmias in isolated rat hearts. Possible evidence for an arrhythmogenic role of Na+-H+ exchange. Circ Res. 1990;66:1156–1159. doi: 10.1161/01.res.66.4.1156. [DOI] [PubMed] [Google Scholar]
- Donoso P, Mill JG, O'Neill SC, Eisner DA. Fluorescence measurements of cytoplasmic and mitochondrial sodium concentration in rat ventricular myocytes. J Physiol. 1992;448:493–509. doi: 10.1113/jphysiol.1992.sp019053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gettes LS, Casio WE. Effect of acute ischemia on cardiac electrophysiology. In: Fozzard HA, Jennings RB, Haber E, Katz M, Morgan HE, editors. The Heart and Cardiovascular System. 2nd. New York: Raven Press; 1992. pp. 2021–2054. [Google Scholar]
- Grace AA, Kirschenlohr HL, Metcalfe JC, Smith GA, Weissberg PL, Cragoe EJ, Jr, Vandenberg JI. Regulation of intracellular pH in the perfused heart by external HCO3− and Na+-H+ exchange. Am J Physiol. 1993;265:H289–H298. doi: 10.1152/ajpheart.1993.265.1.H289. [DOI] [PubMed] [Google Scholar]
- Grichtchenko II, Chesler M. Depolarization-induced alkalinization of astrocytes in gliotic hippocampal slices. Neuroscience. 1994;62:1071–1078. doi: 10.1016/0306-4522(94)90344-1. 10.1016/0306-4522(94)90344-1. [DOI] [PubMed] [Google Scholar]
- Gross E, Abuladze N, Pushkin A, Kurtz I, Cotton CU. The stoichiometry of the electrogenic sodium bicarbonate cotransporter pNBC1 in mouse pancreatic duct cells is 2 HCO3− : 1 Na+ J Physiol. 2001;531:375–382. doi: 10.1111/j.1469-7793.2001.0375i.x. 10.1111/j.1469-7793.2001.0375i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harootunian AT, Kao JP, Eckert BK, Tsien RY. Fluorescence ratio imaging of cytosolic free Na+ in individual fibroblasts and lymphocytes. J Biol Chem. 1989;264:19458–19467. [PubMed] [Google Scholar]
- Harrison SM, Frampton JE, McCall E, Boyett MR, Orchard CH. Contraction and intracellular Ca2+, Na+, and H+ during acidosis in rat ventricular myocytes. Am J Physiol. 1992;262:C348–C357. doi: 10.1152/ajpcell.1992.262.2.C348. [DOI] [PubMed] [Google Scholar]
- Harvey RD, Hume JR. Autonomic regulation of a chloride current in heart. Science. 1989;244:983–985. doi: 10.1126/science.2543073. [DOI] [PubMed] [Google Scholar]
- Huelsing DJ, Spitzer KW, Cordeiro JM, Pollard AE. Conduction between isolated rabbit Purkinje and ventricular myocytes coupled by a variable resistance. Am J Physiol. 1998;274:H1163–H1173. doi: 10.1152/ajpheart.1998.274.4.H1163. [DOI] [PubMed] [Google Scholar]
- Hume JR, Uehara A. Ionic basis of the different action potential configurations of single guinea-pig atrial and ventricular myocytes. J Physiol. 1985;368:525–544. doi: 10.1113/jphysiol.1985.sp015874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Vereecke J, Carmeliet E. Intracellular protons inhibit inward rectifier K+ channel of guinea-pig ventricular cell membrane. Pflugers Arch. 1992;422:280–286. doi: 10.1007/BF00376214. 10.1007/BF00376214. [DOI] [PubMed] [Google Scholar]
- Karmazyn M, Gan XT, Humphreys RA, Yoshida H, Kusumoto K. The myocardial Na+-H+ exchange: structure, regulation, and its role in heart disease. Circ Res. 1999;85:777–786. doi: 10.1161/01.res.85.9.777. [DOI] [PubMed] [Google Scholar]
- Khandoudi N, Albadine J, Robert P, Krief S, Berrebi-Bertrand I, Martin X, Bevensee MO, Boron WF, Bril A. Inhibition of the cardiac electrogenic sodium bicarbonate cotransporter reduces ischemic injury. Cardiovasc Res. 2001;52:387–396. doi: 10.1016/s0008-6363(01)00430-8. 10.1016/S0008-6363(01)00430-8. [DOI] [PubMed] [Google Scholar]
- Kohout TA, Rogers TB. Angiotensin II activates the Na+/HCO3− symport through a phosphoinositide-independent mechanism in cardiac cells. J Biol Chem. 1995;270:20432–20438. doi: 10.1074/jbc.270.35.20432. 10.1074/jbc.270.35.20432. [DOI] [PubMed] [Google Scholar]
- Lagadic-Gossmann D, Buckler KJ, Vaughan-Jones RD. Role of bicarbonate in pH recovery from intracellular acidosis in the guinea-pig ventricular myocyte. J Physiol. 1992;458:361–384. doi: 10.1113/jphysiol.1992.sp019422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prigent K, Lagadic-Gossmann D, Mongodin E, Feuvray D. HCO3−-dependent alkalinizing transporter in adult rat ventricular myocytes: characterization and modulation. Am J Physiol. 1997;273:H2596–H2603. doi: 10.1152/ajpheart.1997.273.6.H2596. [DOI] [PubMed] [Google Scholar]
- Leem CH, Lagadic-Gossmann D, Vaughan-Jones RD. Characterization of intracellular pH regulation in the guinea-pig ventricular myocyte. J Physiol. 1999;517:159–180. doi: 10.1111/j.1469-7793.1999.0159z.x. 10.1111/j.1469-7793.1999.0159z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh SH, Jin JS, Tsai CS, Chao CM, Chiung CS, Chen WH, et al. Functional evidence for intracellular acid extruders in human ventricular myocardium. Jpn J Physiol. 2002;52:277–284. doi: 10.2170/jjphysiol.52.277. [DOI] [PubMed] [Google Scholar]
- Minta A, Tsien RY. Fluorescent indicators for cytosolic sodium. J Biol Chem. 1989;264:19449–19457. [PubMed] [Google Scholar]
- Munsch T, Deitmer JW. Sodium–bicarbonate cotransport current in identified leech glial cells. J Physiol. 1994;474:43–53. doi: 10.1113/jphysiol.1994.sp020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng LL, Davies JE, Quinn P. Intracellular pH regulation in isolated myocytes from adult rat heart in HCO3−-containing and HCO3−-free media. Clin Sci (Lond) 1993;84:133–139. doi: 10.1042/cs0840133. [DOI] [PubMed] [Google Scholar]
- Orchard CH, Cingolani HE. Acidosis and arrhythmias in cardiac muscle. Cardiovasc Res. 1994;28:1312–1319. doi: 10.1093/cvr/28.9.1312. [DOI] [PubMed] [Google Scholar]
- Orchard CH, Kentish JC. Effects of changes of pH on the contractile function of cardiac muscle. Am J Physiol. 1990;258:C967–C981. doi: 10.1152/ajpcell.1990.258.6.C967. [DOI] [PubMed] [Google Scholar]
- Page E. Quantitative ultrastructural analysis in cardiac membrane physiology. Am J Physiol. 1978;235:C147–C158. doi: 10.1152/ajpcell.1978.235.5.C147. [DOI] [PubMed] [Google Scholar]
- Page E, McCallister LP, Power B. Stereological measurements of cardiac ultrastructures implicated in excitation-contraction coupling. Proc Natl Acad Sci U S A. 1971;68:1465–1466. doi: 10.1073/pnas.68.7.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page E, Surdyk-Droske M. Distribution, surface density, and membrane area of diadic junctional contacts between plasma membrane and terminal cisterns in mammalian ventricle. Circ Res. 1979;45:260–267. doi: 10.1161/01.res.45.2.260. [DOI] [PubMed] [Google Scholar]
- Poole RC, Halestrap AP. Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am J Physiol. 1993;264:C761–C782. doi: 10.1152/ajpcell.1993.264.4.C761. [DOI] [PubMed] [Google Scholar]
- Pushkin A, Abuladze N, Lee I, Newman D, Hwang J, Kurtz I. Cloning, tissue distribution, genomic organization, and functional characterization of NBC3, a new member of the sodium bicarbonate cotransporter family. J Biol Chem. 1999;274:16569–16575. doi: 10.1074/jbc.274.23.16569. 10.1074/jbc.274.23.16569. [DOI] [PubMed] [Google Scholar]
- Pushkin A, Abuladze N, Newman D, Lee I, Xu G, Kurtz I. Cloning, characterization and chromosomal assignment of NBC4, a new member of the sodium bicarbonate cotransporter family. Biochim Biophys Acta. 2000;1493:215–218. doi: 10.1016/s0167-4781(00)00149-4. [DOI] [PubMed] [Google Scholar]
- Romero MF, Fulton CM, Boron WF. The SLC4 family of HCO3− transporters. Pflugers Arch. 2004;447:495–509. doi: 10.1007/s00424-003-1180-2. 10.1007/s00424-003-1180-2. [DOI] [PubMed] [Google Scholar]
- Roos A, Boron WF. Intracellular pH. Physiol Rev. 1981;61:296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Sassani P, Pushkin A, Gross E, Gomer A, Abuladze N, Dukkipati R, Carpenito G, Kurtz I. Functional characterization of NBC4: a new electrogenic sodium bicarbonate cotransporter. Am J Physiol. 2002;282:C408–C416. doi: 10.1152/ajpcell.00409.2001. [DOI] [PubMed] [Google Scholar]
- Satoh H, Delbridge LM, Blatter LA, Bers DM. Surface: Volume relationship in cardiac myocytes studied with confocal microscopy and membrane capacitance measurements: species-dependence and developmental effects. Biophys J. 1996;70:1494–1504. doi: 10.1016/S0006-3495(96)79711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer C, Ladilov YV, Siegmund B, Piper HM. Importance of bicarbonate transport for protection of cardiomyocytes against reoxygenation injury. Am J Physiol. 2000;278:H1457–H1463. doi: 10.1152/ajpheart.2000.278.5.H1457. [DOI] [PubMed] [Google Scholar]
- Scholz W, Albus U, Counillon L, Gogelein H, Lang HJ, Linz W, Weichert A, Scholkens BA. Protective effects of HOE642, a selective sodium-hydrogen exchange subtype 1 inhibitor, on cardiac ischaemia and reperfusion. Cardiovasc Res. 1995;29:260–268. 10.1016/0008-6363(96)88579-8. [PubMed] [Google Scholar]
- Scholz W, Albus U, Lang HJ, Linz W, Martorana PA, Englert HC, Scholkens BA. Hoe 694, a new Na+/H+ exchange inhibitor and its effects in cardiac ischaemia. Br J Pharmacol. 1993;109:562–568. doi: 10.1111/j.1476-5381.1993.tb13607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumaker H, Amlal H, Frizzell R, Ulrich CDII, Soleimani M. CFTR drives Na+-nHCO3− cotransport in pancreatic duct cells: a basis for defective HCO3− secretion in CF. Am J Physiol. 1999;276:C16–C25. doi: 10.1152/ajpcell.1999.276.1.C16. [DOI] [PubMed] [Google Scholar]
- Siebens AW, Boron WF. Depolarization-induced alkalinization in proximal tubules. II. Effects of lactate and SITS. Am J Physiol. 1989;256:F354–F365. doi: 10.1152/ajprenal.1989.256.2.F354. [DOI] [PubMed] [Google Scholar]
- Skolnick RL, Litwin SE, Barry WH, Spitzer KW. Effect of ANG II on pHi, [Ca2+]i, and contraction in rabbit ventricular myocytes from infarcted hearts. Am J Physiol. 1998;275:H1788–H1797. doi: 10.1152/ajpheart.1998.275.5.H1788. [DOI] [PubMed] [Google Scholar]
- Spitzer KW, Bridge JHB. Relationship between intracellular pH and tension development in resting ventricular muscle and myocytes. Am J Physiol. 1992;262:C316–C327. doi: 10.1152/ajpcell.1992.262.2.C316. [DOI] [PubMed] [Google Scholar]
- Spitzer KW, Hogan PM. The effects of acidosis and bicarbonate on action potential repolarization in canine cardiac Purkinje fibers. J General Physiol. 1979;73:199–218. doi: 10.1085/jgp.73.2.199. 10.1085/jgp.73.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Leem CH, Vaughan-Jones RD. Novel chloride-dependent acid loader in the guinea-pig ventricular myocyte: part of a dual acid-loading mechanism. J Physiol. 1996;495:65–82. doi: 10.1113/jphysiol.1996.sp021574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg JI, Metcalfe JC, Grace AA. Mechanisms of pHi recovery after global ischemia in the perfused heart. Circ Res. 1993;72:993–1003. doi: 10.1161/01.res.72.5.993. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones RD. Regulation of chloride in quiescent sheep-heart Purkinje fibres studied using intracellular chloride and pH-sensitive micro-electrodes. J Physiol. 1979;295:111–137. doi: 10.1113/jphysiol.1979.sp012957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan-Jones RD, Lederer WJ, Eisner DA. Ca2+ ions can affect intracellular pH in mammalian cardiac muscle. Nature. 1983;301:522–524. doi: 10.1038/301522a0. 10.1038/301522a0. [DOI] [PubMed] [Google Scholar]
- Virkki LV, Wilson DA, Vaughan-Jones RD, Boron WF. Functional characterization of human NBC4 as an electrogenic Na+-HCO3− cotransporter (NBCe2) Am J Physiol. 2002;282:C1278–C1289. doi: 10.1152/ajpcell.00589.2001. [DOI] [PubMed] [Google Scholar]
- Whalley DW, Hemsworth PD, Rasmussen HH. Sodium-hydrogen exchange in guinea-pig ventricular muscle during exposure to hyperosmolar solutions. J Physiol. 1991;444:193–212. doi: 10.1113/jphysiol.1991.sp018873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Spitzer KW. Na-independent Cl−-HCO3− exchange mediates recovery of pHi from alkalosis in guinea pig ventricular myocytes. Am J Physiol. 1994;267:H85–H91. doi: 10.1152/ajpheart.1994.267.1.H85. [DOI] [PubMed] [Google Scholar]
- Zaniboni M, Swietach P, Rossini A, Yamamoto T, Spitzer KW, Vaughan-Jones RD. Intracellular proton mobility and buffering power in cardiac ventricular myocytes from rat, rabbit, and guinea pig. Am J Physiol. 2003;285:H1246–H1236. doi: 10.1152/ajpheart.00277.2003. [DOI] [PubMed] [Google Scholar]