Abstract
To investigate the effect of initial conditions on the modulation of motor unit discharge during fast voluntary contractions, we compared ballistic isometric contractions of the ankle dorsiflexor muscles that were produced from either a resting state or superimposed on a sustained contraction. The torque of the dorsiflexors and the surface and intramuscular EMGs from the tibialis anterior were recorded. The results showed that the performance of a ballistic contraction from a sustained contraction (∼25% maximal voluntary contraction (MVC)) had a negative effect on the maximal rate of torque development. Although the electromechanical delay was shortened, the EMG activity during the ballistic contraction was less synchronized. These observations were associated with a significant decline in the average discharge rate of single motor units (89.8 ± 3.8 versus 115 ± 5.8 Hz) and in the percentage of units (6.2 versus 15.5% of the whole sample) that exhibited double discharges at brief intervals (= 5 ms). High-threshold units that were not recruited during the sustained contraction displayed the same activation pattern, which indicates that the mechanisms responsible for the decline in discharge rate were not restricted to previously activated units, but appear to influence the entire motor unit pool. When a premotor silent period (SP) was observed at the transition from the sustained muscular activity to the ballistic contraction (19% of the trials), these adjustments in motor unit activity were not present, and the ballistic contractions were similar to those performed from a resting state. Together, these results indicate that initial conditions can influence the capacity for motor unit discharge rate and hence the performance of a fast voluntary contraction.
A contraction performed as fast as possible, often called a ballistic contraction, is characterized by a brief contraction time and high rate of force development that is followed rapidly by complete muscle relaxation (Desmedt & Godaux, 1977, 1978; Bawa & Calancie, 1983; Zehr & Sale, 1994; Palmer et al. 1994; Van Cutsem et al. 1998). The pattern of motor unit activation during fast contractions differs from that observed during gradual increases in force. Fast contractions involve high instantaneous discharge rates that decline during successive firings (Desmedt & Godaux, 1977; Bawa & Calancie, 1983; Ivanova et al. 1997; Van Cutsem et al. 1998; Garland & Griffin, 1999). For contraction that involves a gradual increase in muscle force, there is a progressive increase in discharge rate (Milner-Brown et al. 1973; Romaiguere et al. 1989; Erim et al. 1996; Enoka & Fuglevand, 2001).
When a ballistic contraction is superimposed on sustained submaximal muscular activity, a brief reduction or cessation of agonist activity sometimes occurs before the ballistic contraction (Gatev, 1972; Yabe, 1976; Conrad et al. 1983; Mortimer et al. 1987; Aoki et al. 1989; Wierzbicka et al. 1993; Tsukahara et al. 1995). This premotor silent period (SP) is usually associated with an increase in the peak acceleration of the ballistic movement and the rate of torque development (Conrad et al. 1983; Mortimer et al. 1987; Aoki et al. 1989). Because a SP can occur during isometric ballistic contractions (Wierzbicka et al. 1993; Tsukahara et al. 1995), the intention to make a fast contraction, rather than the nature of the mechanical action itself, determines the inclusion of a SP. The frequency of occurrence and duration of the premotor SP exhibits large variability across subjects and trials (for a review, see Zehr & Sale, 1994). In limb muscles, for example, the duration of the premotor SP usually ranges from 40 to 100 ms (Gatev, 1972; Yabe, 1976; Zehr et al. 1997) and it occurs in 5–60% of the trials (Gatev, 1972; Mortimer et al. 1987; Walter, 1988; Aoki et al. 1989). One consequence of the premotor SP could be to impose a non-refractory state on the motoneurones immediately before a rapid contraction, which could synchronize the motor unit discharges at the onset of phasic activity and thereby enhance the rate of torque development (Conrad et al. 1983; Mortimer et al. 1987; Aoki et al. 1989).
The current experiment was designed to examine the influence of preceding muscle activity on motor unit discharge and the rate of torque development during a ballistic action performed as fast as possible with the dorsiflexor muscles. To this end, we compared motor unit activity in the tibialis anterior muscle when ballistic contractions were performed with and without prior activation.
Methods
Seven subjects (1 woman and 6 men) aged 22–44 years (mean: 27.7 ± 3.1 years) took part in this investigation and were tested on at least three different occasions. All subjects were familiar with the experimental procedures. The local ethics committee approved this study and the subjects gave informed consent prior to participation in the investigation. All the experimental procedures were performed in accordance with the Declaration of Helsinki.
The subject sat on a chair in a slightly reclined position during the experiment, with the foot of the non-dominant leg strapped to a footplate. The plate was inclined at an angle of 45 deg to the floor and the subject's position was adjusted to obtain ankle and knee angles of about 90 deg and 110 deg, respectively. The foot was tightly attached to the plate by means of two straps and held in place by a heel block. One strap was placed around the ankle and the other around the foot 1–2 cm proximal to the metatarsophalangeal joint.
Force and EMG recordings
The isometric force developed by the dorsiflexor muscles was measured by connecting the footplate to a strain gauge transducer (TC 2000–500; Kulite, Basingstoke, UK) and the signal was amplified (AM 502, Tektronix, Bearverton, OR, USA; bandwidth DC –300 Hz). The transducer was attached at the level of the metatarsophalangeal joint of the big toe (lever arm, 17 cm). The sensitivity of the force transducer was 30 mV N−1 (linear range, 0–500 N).
Motor unit potentials were recorded by a selective electrode that comprised 50 µm diamel-coated nichrome wires glued into the lumen of a hypodermic needle (Duchateau & Hainaut, 1990). The electrode was inserted in the middle part of the tibialis anterior muscle, and during each experimental session the needle was inserted in at least three separate locations. At each location, the needle was manipulated to various depths and angles to record as many motor units as possible. The EMG signal was amplified by a custom-made differential amplifier and filtered (100 Hz to 10 kHz) before being displayed on a Tektronix TAS 455 oscilloscope. The surface EMG of the tibialis anterior was recorded by means of two silver disk electrodes (8 mm in diameter) placed 2–3 cm apart on either side of the needle electrode. The EMG activity of antagonist muscles (soleus and medial gastrocnemius) was recorded by surface electrodes. One electrode was placed over the motor point, and the second 2 cm distal to this point. The location of the motor point was determined by electrical stimulation and defined as the location at which the minimal stimulus evoked a barely perceptible muscle contraction. The ground electrodes were positioned over the tibia. All surface EMG signals were amplified (×1000–2000) and filtered (10 Hz to 5 kHz) by a custom-made differential amplifier.
Experimental procedure
Before the recording of single motor units, the maximal voluntary torque exerted by the dorsiflexor and plantarflexor muscles during a maximal voluntary contraction (MVC) was determined. The subject performed three MVCs of 4–5 s duration separated by 2–3 min rest. At each recording site, motor units were first identified during two or three isometric ramp contractions performed at 10% MVC s−1 and the recruitment threshold of each unit was measured. Once a motor unit action potential had been clearly identified (usually 2–4 units at each site), the subject performed voluntary ballistic (isometric) contractions, as fast as possible, to different torque levels ranging from 5–75% MVC. No target level was provided and the subject self-selected the torque reached during each contraction. The ballistic contractions were performed, in a random order, either with or without pre-activation of the dorsiflexor muscles (Fig. 1A and B). For the pre-activation condition, subjects sustained a baseline contraction at ∼25% of MVC force for 3–4 s prior to performing the ballistic contraction. The subjects received visual feedback of the torque and an auditory signal that indicated the moment to perform the contraction. Successive contractions were separated by at least 3–5 s and 3–5 min of rest was allowed between motor unit recordings from different electrode locations.
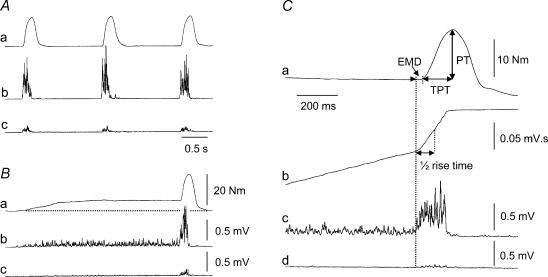
Figure 1. Contraction protocol and measurements.
Torque (a) and rectified surface EMG from the tibialis anterior (b) and soleus (c) during ballistic contractions of the ankle dorsiflexors performed from a resting state (A) or superimposed on a sustained muscle activation at 25% of MVC (B) in one subject. C, illustration of the different measurements on the torque (a) and EMG signals of the tibialis anterior (b and c) and soleus (d) during a ballistic contraction with pre-activation. The peak torque (PT) achieved during the ballistic contraction was measured as the difference between PT and baseline torque, which was zero without pre-activation and ∼25% PT with pre-activation. The time to peak torque (TPT) of the ballistic contraction was determined from its onset to the peak torque. The electromechanical delay (EMD) was defined as the time lag between the onset of the agonist EMG burst and of the torque. The time to reach 50% of the maximal EMG (½ rise time) during the ballistic burst was measured from the integrated EMG signal (b).
Data analysis
Data processing was performed off-line from taped records (Sony PCM-DAT, DTR 8000, Biologic, Claix, France). All signals were acquired on a personal computer at a sampling rate of 2 kHz (force and surface EMG) or 10 kHz (intramuscular EMG) by a MP150 data acquisition system (Biopac Systems Inc., Santa Barbara, CA, USA). The MVC force was determined from the trial that yielded the largest value. Each ballistic contraction was characterized by its peak torque relative to the baseline value, time to peak torque (Fig. 1Ca), and maximal rate of torque development, measured from the first derivative of the torque signal. The electromechanical delay (EMD) was determined as the time lag between the onset of the surface EMG activity of the tibialis anterior and the force (Fig. 1C). The mean EMG amplitude was measured during a 2-s epoch during the MVCs. The rate of rise of muscle activation during the ballistic contraction was quantified as the time to reach one-half of the maximal EMG value (½ rise time) as determined from the integrated signal (Fig. 1Cb). The occurrence of a SP prior to the performance of the ballistic contraction during the pre-activation condition was determined from visual inspection of the recordings. A premotor SP was defined as a reduction in EMG amplitude at the transition between the sustained and ballistic contractions that fell below 5% of that recorded during a MVC for at least 10 ms (Walter, 1988). Muscle co-activation was measured during the period of agonist activation and expressed as a percentage of maximal EMG recorded during the MVC. For ballistic contractions with pre-activation, antagonist EMG activity was also measured either during the 50 ms preceding the agonist burst or during the SP.
Motor unit discrimination was accomplished either with a window discriminator (Duchateau & Hainaut, 1990) or when necessary, by a computer-based, template-matching algorithm (Signal Processing Systems, SPS 8701, Malvern Victoria, Australia). Single motor unit action potentials were identified on the basis of amplitude, duration, and waveform shape. Only the motor units that were clearly identified were included in the analysis. Motor unit recruitment threshold was measured during the isometric ramp contractions and expressed as a percentage of the MVC torque when the action potential of the selected unit first appeared in the EMG trace. Motor unit discharge rate was determined at the onset of the ballistic contractions and the duration of the first three interspike intervals was measured for the fastest contraction (Van Cutsem et al. 1998). The analysis was limited to the first three interspike intervals because few motor units fired more than four times and because the recording was not contaminated by possible electrode movement. For ballistic contractions performed with pre-activation, the discharge rate was also measured for the last 10 discharges prior to the ballistic burst.
Statistics
The electromechanical characteristics of the ballistic contractions without and with pre-activation, including the presence or absence of a premotor SP, were compared by Student's paired t test. Data on motor unit discharge rate were statistically tested by an analysis of variance (ANOVA) with repeated measures on two factors (condition × time). When a significant main effect was obtained, the Tukey–Kraemer test was used to identify significant differences between the means. The best-fitting relations were tested by linear regressions using the least-squares method. The linear regressions recorded in the different experimental conditions (with and without pre-activation or premotor SP) were compared by an analysis of covariance (ancova). For all comparisons, the level of statistical significance was set at P < 0.05. Values are reported as means ± s.e.m. in the text.
Results
Characteristics of ballistic contractions with and without pre-activation
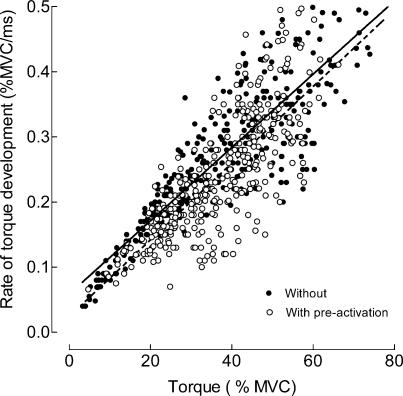
Ballistic contractions superimposed on a sustained contraction displayed a reduced rate of torque development, which was accompanied by a significant (P < 0.001) increase in the average time to peak torque (mean value for all trials: 157 ± 1.8 ms) compared with contractions performed from rest (136.3 ± 2.2 ms). The average maximal rate of torque development, which was the primary determinant of the performance, was 16% greater (0.27 ± 0.01 versus 0.23 ± 0.01% MVC ms−1; P < 0.001) when ballistic contractions were performed from a resting state compared with those superimposed on sustained activation. The rate was still 8% greater (0.35 ± 0.03 versus 0.32 ± 0.02% MVC ms−1; P < 0.05) when compared with the mean values for the five fastest ballistic contractions recorded for each subject. The rates of torque development were linearly related to the peak torque achieved during the ballistic contraction for both conditions (Fig. 2). However, pre-activation reduced the rate of torque development during the ballistic contractions, as indicated by a significant difference (ANCOVA; P < 0.001) between the linear regressions in Fig. 2.
Figure 2. Rate of torque development during ballistic contractions with and without pre-activation.
Relation between the rate of torque development and the peak torque during ballistic contractions performed with the ankle dorsiflexors. The linear regression lines are y = 0.0059x+ 0.024 (n = 361; r2 = 0.61; P < 0.001) for the contractions with pre-activation (solid line) and y = 0.0056x+ 0.059 (n = 347; r2 = 0.77; P < 0.001) for the contractions without pre-activation (broken line). The two relations are significantly different (ANCOVA; P < 0.001).
Although the EMD was briefer for ballistic contractions with pre-activation (18.4 ± 1.3 versus 30.7 ± 1.3 ms; P < 0.001; mean value for all trials), the time to reach 50% of the integrated surface EMG activity (½ rise time) occurred in a shorter time (63.2 ± 7.4 versus 94.4 ± 6.0 ms; P < 0.05) when there was no pre-activation. This difference could not be explained by co-activation of the antagonist muscles during the ballistic contraction, because this was similar (P > 0.05) in the absence (23.1 ± 2.3%versus 22.1 ± 1.1% of EMG during MVC, for soleus and medial gastrocnemius, respectively) and presence (22.0 ± 2.0%versus 19.3 ± 1.1%) of pre-activation.
Motor unit activation pattern during ballistic contractions
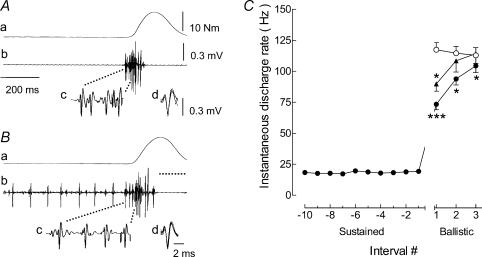
From the seven subjects, a total of 171 motor units were successfully discriminated in ballistic contractions without pre-activation and 222 units in the contractions with pre-activation. The recruitment threshold of these units ranged from 1.5 to 34% MVC (mean: 9.5 ± 1.7%). Figure 3 illustrates the typical behaviour of a single motor unit during the ballistic contractions. Although similar peak torques were achieved during the ballistic contractions performed in the two conditions, the maximal rate of torque development was greater without pre-activation (0.35% MVC ms−1) compared with pre-activation (0.29% MVC ms−1), and the motor unit activity differed. When the contraction was performed from a resting condition, the instantaneous discharge frequency of the first interspike interval was high and then decreased in successive intervals (Fig. 3A). In contrast, the instantaneous discharge frequency of the same unit increased progressively when the ballistic contraction was preceded by pre-activation. The average discharge rate for the first three interspike intervals of the unit shown in Fig. 3A and B was lower when the ballistic contraction was preceded by pre-activation (63.3 ± 5.3 Hz) compared with when there was no pre-activation (114.9 ± 13.8 Hz). This difference was observed for the entire population of motor units (Fig. 3C). The average discharge frequency for the first three interspike intervals was 115 ± 5.8 Hz without preaction and 89.8 ± 3.8 Hz with pre-activation. Furthermore, the percentage of all units that exhibited double discharges at brief intervals (two spikes separated by less than 5 ms; see Van Cutsem et al. 1998) at the onset of the ballistic contraction was significantly greater (P < 0.001) when performed from a resting state (15.5%), compared with from a sustained activation (6.2%). Additionally, the motor units (n = 51) that were recruited only during the ballistic contraction discharged at a slightly higher rate (P = 0.09) for the first interspike interval), but displayed a similar activation pattern compared with the units that were recruited before the onset of ballistic action (Fig. 3C).
Figure 3. Behaviour of single motor units during ballistic contractions with and without pre-activation.
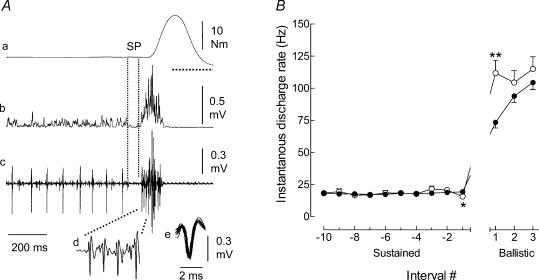
The discharge of the same motor unit during ballistic contractions performed from a resting state (A) or superimposed on a sustained muscle contraction (B) to similar torque levels (13.5 versus 15 N m, respectively). Traces correspond to the torque (a) and the intramuscular EMG of the tibialis anterior (b–d). The horizontal dotted line under the ballistic phase in B denotes zero torque for the pre-activation condition. The first four discharges of a motor unit at the onset of the ballistic contraction are shown with an extended time scale (c) and superimposed on one another (d). C, average (mean ± s.e.m.) discharge rates of single motor units during ballistic contractions without (○; n = 171) or with pre-activation (•; n = 222). Discharge rate was measured during the last 10 interspike intervals during the pre-activation phase (Sustained) and during the first three interspike intervals for ballistic contraction. The discharge of high threshold units (n = 51) that were activated during the ballistic contractions, but not during the preceding sustained activation, are also illustrated (▴). *P < 0.05 and ***P < 0.001 indicate a significant difference compared with the condition that did not involve pre-activation.
Characteristics of ballistic contractions with and without premotor SP
A careful inspection of the surface EMG recordings for the ballistic contractions superimposed on a sustained activation indicated that a premotor SP occurred in 52 out of 280 trials (19%; Fig. 4A). A premotor SP was present in all seven subjects and the occurrence for each subject ranged from 5.2 to 27.6% of the trials. The average duration of the premotor SP was 36.1 ± 3.4 ms (range 15–75 ms).
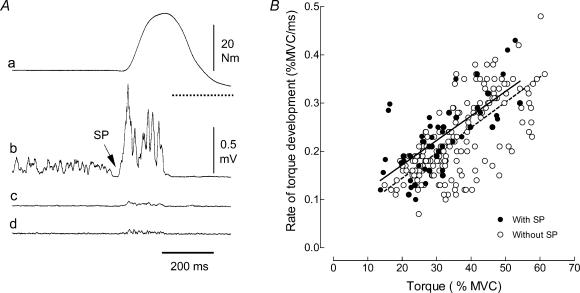
Figure 4. Effect of premotor SP on the rate of torque development during ballistic contractions with pre-activation.
Typical example of the torque (a) and rectified surface EMG from the tibialis anterior (b), soleus (c), and medial gastrocnemius (d) muscles during a ballistic contraction of the ankle dorsiflexors superimposed on a sustained pre-activation (A). The arrow indicates the presence of a premotor SP at the transition between the sustained and ballistic phases. The horizontal dotted line under the ballistic phase in A, trace a indicates zero torque. B, relations between the rate of torque development and peak torque for ballistic contractions with and without a premotor SP. The linear regression lines are y = 0.0051x+ 0.07 (n = 52; r2 = 0.49; P < 0.001) for the contractions with a premotor SP (solid line) and y = 0.0052x+ 0.039 (n = 201; r2 = 0.50; P < 0.001) for contractions without a premotor SP (broken line). The two relations are significantly different (ANCOVA; P < 0.05).
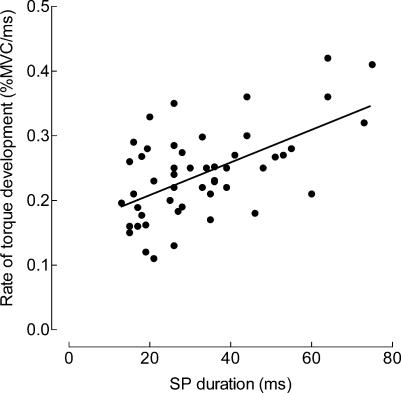
The relation between the rate of torque development and the peak torque achieved during the ballistic contractions indicated that those trials with a premotor SP involved a greater rate of torque development (ANCOVA; P < 0.05;), regardless of the peak torque reached during the ballistic contraction (Fig. 4B). There was a positive relation (r2 = 0.32; P < 0.001) between the rate of torque development and the duration of the SP (Fig. 5).
Figure 5. Relation between the rate of torque development and duration of the premotor SP.
The rate of torque development during ballistic contractions with pre-activation (expressed as percentage of MVC ms−1) increased with SP duration. The regression line (y = 0.0022x+ 0.17; n = 52) indicates a significant association between these two variables (r2 = 0.32; P < 0.001).
Although there was no difference (P > 0.05) in the EMD for contractions with and without SP (19.7 ± 1.6 versus 18.4 ± 1.3 ms, respectively), the ½ EMG rise time tended to be slightly briefer when a premotor SP was present (84.5 ± 5.6 versus 94.4 ± 6 ms; P = 0.08). Co-activation of the antagonist muscle during the SP was low and did not differ significantly from the trials without a SP, both for soleus (0.8 ± 0.3% versus 0.7 ± 0.3% MVC) and medial gastrocnemius (1.4 ± 0.3% versus 1.7 ± 0.3% MVC).
Motor unit activation pattern during contractions with and without premotor SP
The discharge of 42 motor units recorded during ballistic contractions that were superimposed on a sustained activation with a premotor SP was compared with that of 180 units when there was no SP. The recruitment thresholds of the units were similar with (11.1 ± 1.7% of MVC) and without SP (9.5 ± 1.4% of MVC). Figure 6A illustrates the discharge of a single motor unit (same unit as in Fig. 3A and B) during a contraction with a premotor SP. The traces show clearly that a motor unit spike was absent at the time of SP occurrence. Furthermore, the discharge pattern of this unit during the ballistic part of the contraction was more similar to that recorded during ballistic contractions performed from a resting condition. When the firing rates of all motor units were averaged, the discharge rate decreased (−22.9%; P < 0.05) for the last interspike interval of the pre-activation phase (Sustained, Fig. 6B) and increased for the first interspike interval of the ballistic phase (52.3%; P < 0.01) for contractions with a premotor SP (Fig. 6B). Although slightly greater, there was no significant difference in the average discharge rate for the second and third interspike intervals of the ballistic contractions.
Figure 6. Behaviour of single motor units during ballistic contractions with a premotor SP.
Illustration of the discharge by a single motor unit (same unit as in Fig. 3) during a ballistic contraction that included a SP (A). The traces correspond to the torque (a), the rectified surface EMG (b), and the intramuscular EMG (c–e) of the tibialis anterior. The first four discharges of the motor unit at the onset of the ballistic contraction are shown with an extended time scale (d) and superimposed on one another (e). Note the increase of the interspike interval at the transition between the sustained and ballistic phases (c). The horizontal dotted line under the ballistic phase in A, trace a indicates zero torque. B, average (mean ± s.e.m.) discharge rates of single motor units during the ballistic contractions, without (•; n = 180) and with premotor SP (○; n = 42). Discharge rate was measured for the last 10 interspike intervals during the pre-activation phase (Sustained) and during the first three interspike intervals for the ballistic contraction. *P < 0.05 and **P < 0.01 indicate significant differences between the two conditions.
Discussion
The main finding of this study was that the discharge pattern of single motor units can be influenced by the conditions under which ballistic contractions are performed. The results showed that preceding a ballistic contraction with a sustained pre-activation decreased the instantaneous discharge rate of motor units, thereby reducing the maximal rate of torque development. Because high-threshold units that were not recruited during the sustained contraction displayed the same activation pattern, this observation indicates that the mechanisms responsible for the decline in discharge rate were not restricted to previously activated units, but appear to influence the entire motor unit pool. Consistent with previous studies (Mortimer et al. 1987; Walter, 1988; Aoki et al. 1989; Moritani, 1993), the data showed that the occurrence of a silent period in the pre-activation period increased the maximal rate of muscle torque development. Although the SP occurred infrequently in the current study, the observation that the instantaneous motor unit discharge rate was greater in the presence of a SP nevertheless emphasizes the potential role of brief inactivity in enhancing neuromuscular performance during fast contractions.
Effect of pre-activation on the characteristics of ballistic contractions
The maximal rate of torque development during a ballistic contraction that was superimposed on sustained muscle activity was lower, and the time to peak torque longer compared with a contraction performed from a resting condition. It could be argued that although we compared ballistic contractions of similar net amplitudes in the two conditions, the absolute torque achieved during the ballistic contraction superimposed on the sustained contraction was ∼25% greater than from a resting condition. However, if the maximal rate of torque development of the ballistic contraction was plotted against the absolute torque rather than the net change in torque, as illustrated in Fig. 2, the difference between the two conditions would be even greater. Furthermore, the average rate of torque development for the five fastest ballistic contractions (regardless of the peak torque achieved) performed by each subject was significantly greater for ballistic contractions that had no pre-activation, compared with those that did include pre-activation. Because pre-activation was expected to remove the slack in the musculo-tendinous structures and reduce the EMD, the greater rate of torque development during the ballistic contraction performed from a resting condition was unexpected. The reduced EMD was, however, counteracted by a prolongation of the ½ EMG rise time for ballistic contractions preceded by pre-activation. This difference between the two ballistic conditions was not associated with change in the level of co-activation in the antagonist muscles.
There was no evidence of a reversal in the pattern of motor unit recruitment in the present investigation; the units that were recruited during the sustained contraction were also activated during the ballistic contraction. Therefore, the slowing of the ballistic contractions in the presence of pre-activation was associated with reductions in maximal discharge rate of motor units. The instantaneous discharge rate for the first interspike interval when the task involved pre-activation was about half that recorded in ballistic contractions without pre-activation. In addition, the discharge pattern at the onset of the contraction decreased slightly during the successive firings, as classically described in untrained subjects for ballistic contraction performed from a resting state (Desmedt & Godaux, 1977; Bawa & Calancie, 1983; Van Cutsem et al. 1998; Garland & Griffin, 1999), whereas it increased progressively in ballistic contractions performed from a sustained contraction. Another difference between ballistic contractions performed with and without pre-activation was that in the former condition the number of double discharges at brief intervals (≤5 ms) was significantly reduced. Although such double discharges, associated with strong fast inputs to the motoneurones (Calvin, 1974; Baldissera et al. 1987), differ from those recorded at the onset of slow contractions (Calvin, 1974; Bawa & Calancie, 1983), they influence the contraction speed during ballistic contractions (Ivanova et al. 1997; Garland & Griffin, 1999). This is supported by one of our previous papers showing that the enhancement of the rate of torque development during ballistic contractions after 3 months of dynamic training was associated with an increase in maximal discharge rate and number of double discharges by the motor units (Van Cutsem et al. 1998).
When elicited during resting conditions, the Hoffmann reflex and motor evoked potentials in response to transcranial stimulation are facilitated prior to a fast voluntary contraction (Eichenberger & Ruegg, 1984; MacKinnon & Rothwell, 2000) and, accordingly, motor units discharge with a high initial instantaneous rate (Desmedt & Godaux, 1977; Bawa & Calancie, 1983; Van Cutsem et al. 1998; Garland & Griffin, 1999). Such high discharge frequencies were also observed at the onset of motoneurone firing induced by intense current injection (Kernell, 1965; Baldissera et al. 1987; Hsiao et al. 1997; Beaumont et al. 2004) and correspond to the ‘secondary’ range of motoneurone discharges (Kernell, 1965). One mechanism that might account for the differential behaviour in motor unit activity between ballistic contractions performed from rest and superimposed on a sustained contraction is adaptation of motoneurone discharge. It is well known that repetitive activation causes a progressive increase in the duration of the motoneurone afterhyperpolarization and thereby reduces discharge rate for a given current intensity (Kernell, 1965; Baldissera et al. 1987; Sawzuck et al. 1995; Jones & Bawa, 1995). Albeit at a slightly greater rate, the discharge pattern recorded during ballistic contraction performed with pre-activation was also observed for higher threshold units that were recruited during the ballistic contraction and not during the sustained contraction. This observation indicates that the mechanisms responsible for the decline of the instantaneous discharge rate at the onset of a ballistic contraction superimposed on a sustained contraction did not act exclusively on previously activated motor units, but seemed to influence the entire pool of units. Therefore, a neural mechanism related to a change from a static to a ballistic motor programme should modulate the excitability of the motoneurone pool (Hufschmidt & Hufschmidt, 1954; Wierzbicka et al. 1993). However, we cannot rule out the possibility that motoneurones, not activated during the sustained contraction because of their subthreshold depolarization, have engaged the processes responsible for adaptation (Alaburda et al. 2002) and that this mechanism has also contributed to the overall decrease in motor unit discharge rate.
Functional significance of premotor SP
In prior studies, a premotor SP has been observed to occur when a rapid movement was superimposed on a sustained contraction. A premotor SP has been observed during both movements (Conrad et al. 1983; Mortimer et al. 1987; Aoki et al. 1989; Zehr et al. 1997) and isometric contractions (Moritani, 1993; Wierzbicka et al. 1993; Tsukahara et al. 1995). In the current investigation, the premotor SP was observed in all subjects with a mean range of 5–28% (average: 19% of the trials), which is consistent with the range reported by Wierzbicka et al. (1993) for ballistic isometric contractions performed with the elbow flexor muscles. There was also marked variability in the SP duration (15–75 ms), both within subjects and across trials performed by each subject, as reported previously (Conrad et al. 1983; Mortimer et al. 1987; Aoki et al. 1989; Zehr et al. 1997). The low incidence of the premotor SP can be partly attributed to the type of contraction used in this study. It seems that the SP occurs more frequently during fast and self-paced ballistic movements that require coordinated actions, rather than during isometric conditions (Wierzbicka et al. 1993; Zehr & Sale, 1994). Although not always accepted (Zehr et al. 1997), the observation that some subjects appear to be more capable of producing a premotor SP than others and with a variable duration from trial to trial, suggests that the SP may be a learned behaviour rather than an automatic component of the movement programme (Mortimer et al. 1987; Walter, 1989; Moritani, 1993).
The relation between the maximal rate of torque development and the peak torque achieved during the ballistic contractions superimposed on a sustained contraction showed that trials with a SP were faster (see also Conrad et al. 1983). This change, obtained regardless of the peak torque reached during the ballistic contractions, led to a similar rate of torque development compared with ballistic contraction performed from a resting state. Although the contractions that included a SP were not the fastest, there was a significant correlation between premotor SP duration and rate of torque development. The faster contractions in the presence of a SP were accompanied by a trend toward a briefer ½ EMG rise time in the agonist burst. Consistent with the study of Tsukahara et al. (1995), the discharge intervals that preceded the ballistic burst were slightly prolonged when a premotor SP was present, compared with when it was absent. In addition, the current study showed that this adjustment was associated with an increased average discharge rate at the onset of the ballistic burst (110.4 ± 3.1 Hz versus 90.6 ± 9.1 Hz, with and without SP, respectively). The difference was largely limited to the first interspike interval (Fig. 6B) and subsequently the discharge pattern and maximal discharge rate were similar to those recorded during ballistic contractions performed from a resting state.
The torque level at the transition between the sustained and ballistic contractions was constant and therefore the change in firing rate cannot be explained by a transient reduction in voluntary activation (Fig. 6A). In addition, the absence of a difference in the level of co-activation for the two pre-activation conditions discounts a role for reciprocal inhibition. Consequently, other physiological mechanisms must account for the premotor SP that occurs at the transition from a sustained to a ballistic contraction. The potential mechanisms include disfacilitation of tonically active motoneurones caused by supraspinal inhibition, postsynaptic inhibition induced by spinal interneurones, and presynaptic inhibition of Ia terminals (Mortimer et al. 1987; Tsukahara et al. 1995). Because SP latencies are too brief for postsynaptic inhibition via spinal interneuronal networks operating in parallel with activation of alpha motoneurones (Moritani, 1993), disfacilitation of tonically active motoneurones and presynaptic inhibition by the supraspinal centres (Hultborn et al. 1987; Nielsen & Petersen, 1994) are the most likely candidates. This hypothesis is consistent with the observations of Fetz et al. (2000) who found that global inhibition can be present at spinal and supraspinal levels during the preparatory events for quick motor actions performed by monkeys. Despite the uncertainty regarding the mechanism, the functional significance of the premotor SP is to bring motoneurones into a non-refractory state and thereby enable them to discharge more synchronously and at a higher rate during the subsequent ballistic contraction.
In conclusion, these results indicate that the performance of a ballistic contraction from a sustained activation decreases the instantaneous discharge rate of motor units and consequently the maximal rate of the muscle contraction. The inclusion of a SP between the sustained and ballistic contractions, however, enables recovery of the high instantaneous discharge rate, the synchronous discharge of motor units, and the contraction speed. Although the SP occurred infrequently in the current study, these observations nevertheless underscore the potential significance of a brief period of motoneurone inactivity.
Acknowledgments
The authors are particularly grateful to Professor R. Enoka, Professor K. Hainaut and Dr L. de Montigny for comments on the manuscript. The assistance of Mrs A. Deisser in the preparation of the manuscript and Mr M. Lévénez in the collection of some data is also acknowledged. This study was supported by the Fonds National de la Recherche Scientifique of Belgium and the Conseil de la Recherche of the Université Libre de Bruxelles.
References
- Alaburda A, Perrier JF, Hounsgaard J. An M-like outward current regulates the excitability of spinal motoneurones in the adult turtle. J Physiol. 2002;540:875–881. doi: 10.1113/jphysiol.2001.015982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki H, Tsukahara R, Yabe K. Effects of pre-motion electromyographic silent period on dynamic exertion during a rapid ballistic movement in man. Eur J Appl Physiol. 1989;58:426–432. doi: 10.1007/BF00643520. 10.1007/BF00643520. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Campadelli P, Piccinelli L. The dynamic response of cat gastrocnemius motor units investigated by ramp-current injection into their motoneurones. J Physiol. 1987;387:317–330. doi: 10.1113/jphysiol.1987.sp016575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawa P, Calancie B. Repetitive doublets in human flexor carpi radialis muscle. J Physiol. 1983;339:123–132. doi: 10.1113/jphysiol.1983.sp014707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont E, Houle JD, Peterson CA, Gardiner PF. Passive exercise and fetal spinal cord transplant both help to restore motoneuronal properties after spinal cord transection in rats. Muscle Nerve. 2004;29:234–242. doi: 10.1002/mus.10539. [DOI] [PubMed] [Google Scholar]
- Calvin WH. Three mode of repetitive firing and the role of threshold time course. Brain Res. 1974;69:341–346. doi: 10.1016/0006-8993(74)90012-2. [DOI] [PubMed] [Google Scholar]
- Conrad B, Benecke R, Goehmann M. Premovement silent period in fast movement initiation. Exp Brain Res. 1983;51:310–313. doi: 10.1007/BF00237208. [DOI] [PubMed] [Google Scholar]
- Desmedt JE, Godaux E. Ballistic contractions in man: characteristic recruitment pattern of single motor units of the tibialis anterior muscle. J Physiol. 1977;264:673–693. doi: 10.1113/jphysiol.1977.sp011689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmedt JE, Godaux E. Ballistic contractions in fast or slow human muscle, discharge patterns of single motor units. J Physiol. 1978;285:185–196. doi: 10.1113/jphysiol.1978.sp012566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchateau J, Hainaut K. Effects of immobilization on contractile properties, recruitment and firing rates of human motor units. J Physiol. 1990;422:55–65. doi: 10.1113/jphysiol.1990.sp017972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenberger A, Ruegg DG. Relation between the specific H reflex facilitation preceding a voluntary movement and movement parameters in man. J Physiol. 1984;347:545–559. doi: 10.1113/jphysiol.1984.sp015082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoka RM, Fuglevand AJ. Motor unit physiology, some unresolved issues. Muscle Nerve. 2001;24:4–17. doi: 10.1002/1097-4598(200101)24:1<4::aid-mus13>3.0.co;2-f. 10.1002/1097-4598(200101)24:1<4::AID-MUS13>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Erim Z, De Luca CJ, Mineo K, Aoki T. Rank-ordered regulation of motor units. Muscle Nerve. 1996;19:563–573. doi: 10.1002/(SICI)1097-4598(199605)19:5<563::AID-MUS3>3.0.CO;2-9. 10.1002/(SICI)1097-4598(199605)19:5<563::AID-MUS3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Fetz EE, Perlmutter SI, Prut Y. Functions of mammalian spinal interneurons during movement. Curr Opin Neurobiol. 2000;10:699–707. doi: 10.1016/s0959-4388(00)00160-4. 10.1016/S0959-4388(00)00160-4. [DOI] [PubMed] [Google Scholar]
- Garland SJ, Griffin L. Motor unit double discharges: statistical anomaly or functional entity? Can J Appl Physiol. 1999;24:113–130. doi: 10.1139/h99-010. [DOI] [PubMed] [Google Scholar]
- Gatev V. Role of inhibition in the development of motor co-ordination in early childhood. Dev Med Child Neurol. 1972;14:336–341. doi: 10.1111/j.1469-8749.1972.tb02599.x. [DOI] [PubMed] [Google Scholar]
- Hsiao CF, Trueblood PR, Levine MS, Chandler SH. Multiple effects of serotonin on membrane properties of trigeminal motoneurons in vitro. J Neurophysiol. 1997;77:2910–2924. doi: 10.1152/jn.1997.77.6.2910. [DOI] [PubMed] [Google Scholar]
- Hufschmidt HJ, Hufschmidt T. Antagonist inhibition as the earliest sign of a sensory-motor reaction. Nature. 1954;174:607. doi: 10.1038/174607a0. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Pierrot-Deseilligny E, Shindo M. Changes in presynaptic inhibition of Ia fibres at the onset of voluntary contraction in man. J Physiol. 1987;389:757–772. doi: 10.1113/jphysiol.1987.sp016681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova T, Garland SJ, Miller KJ. Motor unit recruitment and discharge behavior in movement and isometric contractions. Muscle Nerve. 1997;20:867–874. doi: 10.1002/(sici)1097-4598(199707)20:7<867::aid-mus11>3.0.co;2-p. 10.1002/(SICI)1097-4598(199707)20:7<867::AID-MUS11>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Jones KE, Bawa P. Responses of human motoneurons to Ia inputs: effects of background firing rate. Can J Physiol Pharmacol. 1995;73:1224–1234. doi: 10.1139/y95-174. [DOI] [PubMed] [Google Scholar]
- Kernell D. High-repetitive firing of cat lumbosacral motoneurons stimulated by long-lasting injected currents. Acta Physiol Scand. 1965;65:74–86. doi: 10.1111/j.1748-1716.1965.tb04081.x. [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Rothwell JC. Time-varying changes in corticospinal excitability accompanying the triphasic EMG pattern in humans. J Physiol. 2000;528:663–645. doi: 10.1111/j.1469-7793.2000.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner-Brown HS, Stein RB, Yemm R. Changes in firing rate of human motor units during linearly changing voluntary contractions. J Physiol. 1973;230:371–390. doi: 10.1113/jphysiol.1973.sp010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritani T. Neuromuscular adaptations during the acquisition of muscle strength, power and motor tasks. J Biomech. 1993;26:95–107. doi: 10.1016/0021-9290(93)90082-p. 10.1016/0021-9290(93)90082-P. [DOI] [PubMed] [Google Scholar]
- Mortimer JA, Eisenberg P, Palmer SS. Premovement silence in agonist muscles preceding maximum efforts. Exp Neurol. 1987;98:542–554. doi: 10.1016/0014-4886(87)90263-9. 10.1016/0014-4886(87)90263-9. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Petersen N. Is presynaptic inhibition distributed to cortical fibres in man? J Physiol. 1994;477:47–58. doi: 10.1113/jphysiol.1994.sp020170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer E, Cafarelli E, Ashby P. The processing of human ballistic movements explored by stimulation over the cortex. J Physiol. 1994;481:509–520. doi: 10.1113/jphysiol.1994.sp020459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaiguere P, Vedel JP, Pagni S, Zenatti A. Physiological properties of the motor units of the wrist extensor muscles in man. Exp Brain Res. 1989;78:51–61. doi: 10.1007/BF00230686. [DOI] [PubMed] [Google Scholar]
- Sawzuck A, Powers RK, Binder MD. Contribution of outward currents to spike-frequency adaptation in hypoglossal motoneurons of the rats. J Neurophysiol. 1995;78:2246–2253. doi: 10.1152/jn.1997.78.5.2246. [DOI] [PubMed] [Google Scholar]
- Tsukahara R, Aoki H, Yabe K, Mano T. Effects of premotion silent period on single motor unit firing at initiation of a rapid contraction. Electroencephalogr Clin Neurophysiol. 1995;97:223–230. doi: 10.1016/0013-4694(94)00327-h. 10.1016/0924-980X(94)00327-0. [DOI] [PubMed] [Google Scholar]
- Van Cutsem M, Duchateau J, Hainaut K. Changes in single motor unit behaviour contribute to the increase speed after dynamic training in humans. J Physiol. 1998;513:295–305. doi: 10.1111/j.1469-7793.1998.295by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter CB. The influence of agonist premotor silence and the stretch-shortening cycle on contractile rate in active skeletal muscle. Eur J Appl Physiol. 1988;57:577–582. doi: 10.1007/BF00418465. 10.1007/BF00418465. [DOI] [PubMed] [Google Scholar]
- Walter CB. Voluntary control of agonist premotor silence preceding limb movements of maximal effort. Percept Motor Skills. 1989;69:819–826. doi: 10.1177/00315125890693-119. [DOI] [PubMed] [Google Scholar]
- Wierzbicka MM, Wolf W, Staude G, Donstanzer A, Dengler R. Inhibition of EMG activity in isometrically loaded agonist muscle preceding a rapid contraction. Electromyogr Clin Neurophysiol. 1993;33:271–278. [PubMed] [Google Scholar]
- Yabe K. Premotion silent period in rapid voluntary movement. J Appl Physiol. 1976;41:470–473. doi: 10.1152/jappl.1976.41.4.470. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Sale DG. Ballistic movement, muscle activation and neuromuscular adaptation. Can J Appl Physiol. 1994;19:363–378. doi: 10.1139/h94-030. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Sale DG, Dowling JJ. Ballistic movement performance in karate athletes. Med Sci Sports Exerc. 1997;29:1366–1373. doi: 10.1097/00005768-199710000-00014. [DOI] [PubMed] [Google Scholar]