Abstract
We used an anti-indole acetic acid (IAA or auxin) monoclonal antibody-based immunocytochemical procedure to monitor IAA level in Arabidopsis tissues. Using immunocytochemistry and the IAA-driven β-glucuronidase (GUS) activity of Aux/IAA promoter::GUS constructs to detect IAA distribution, we investigated the role of polar auxin transport in vascular differentiation during leaf development in Arabidopsis. We found that shoot apical cells contain high levels of IAA and that IAA decreases as leaf primordia expand. However, seedlings grown in the presence of IAA transport inhibitors showed very low IAA signal in the shoot apical meristem (SAM) and the youngest pair of leaf primordia. Older leaf primordia accumulate IAA in the leaf tip in the presence or absence of IAA transport inhibition. We propose that the IAA in the SAM and the youngest pair of leaf primordia is transported from outside sources, perhaps the cotyledons, which accumulate more IAA in the presence than in the absence of transport inhibition. The temporal and spatial pattern of IAA localization in the shoot apex indicates a change in IAA source during leaf ontogeny that would influence flow direction and, consequently, the direction of vascular differentiation. The IAA production and transport pattern suggested by our results could explain the venation pattern, and the vascular hypertrophy caused by IAA transport inhibition. An outside IAA source for the SAM supports the notion that IAA transport and procambium differentiation dictate phyllotaxy and organogenesis.
In 1880, Darwin stated: “Some influence moves from the tip of an oat coleoptile to the region below the tip where it controls elongation.” This moving influence—later shown to be indole acetic acid (IAA; Went, 1926; Kogl and Haagen-Smit, 1931)—is the first description of polar auxin transport. Polar auxin transport is ubiquitous among higher plants. Efficient transport of IAA modulates cell shape and differentiation and is necessary for normal organogenesis and vascular patterning (Sachs, 1989, 1991; Mattsson et al., 1999; Sieburth, 1999).
Vascular tissues are conduits for water and nutrients throughout the plant body. They are generated during embryogenesis and organogenesis, expanding along the growth axis of the organ. Vascular development begins with the differentiation of provascular tissue, the procambium (Esau, 1965), through periclinal cell division, cell elongation, and cell alignment. The procambium of dicotyledonous embryos such as Arabidopsis becomes evident at early heart stage as elongated cells in the center of the embryo distinct from the nearly isodiametric surrounding ground tissue cells (West and Harada, 1993). As the embryo matures, the procambial cells differentiate into phloem and xylene elements (Aloni, 1995). Vascular tissues connect the leaves and other parts of the shoot with the roots, enabling efficient long-distance transport between organs.
The vascular network is particularly extensive in leaves, with primary, secondary, tertiary (or 1°, 2°, and 3°, respectively), and higher order veins. The veins arise at different times and are arranged in a pattern, referred to as the venation pattern, reflecting the ontogeny and structural organization of the leaf. Arabidopsis leaves are pinnate with a single 1° vein (midvein) from which arise all the 2° veins that rejoin the 1° vein, forming a series of prominent arches (Hickey, 1979). The 3° veins form bridges between 2° veins, whereas quaternary veins extend from 3° veins and end blindly in areoles (Mattsson et al., 1999). The hierarchical differentiation of 1°, 2°, 3°, and higher order veins provides an excellent system to study the mechanism of vascular differentiation and pattern formation (Nelson and Dengler, 1997).
Vascular differentiation is related to auxin flux (Aloni, 1995). Auxin transport appears to be mediated by specific cellular influx and efflux proteins (Lomax et al., 1995; Estelle, 1998). The directionality of auxin flow is attributed to polar distribution of the efflux carrier molecules in the plant cell membrane (Galweiler et al., 1998). Two models, canalization of auxin flow and reaction-diffusion prepattern, have been proposed to explain the pattern of vascular differentiation (Nelson and Dengler, 1997). The canalization of signal flow hypothesis is based on a positive feedback mechanism: a proposed gradual restriction of IAA flow from a field to specialized files of cells, resulting in provascular, and later vascular, differentiation (Sachs, 1981). IAA-induced de novo vascular differentiation (Jacobs, 1952) and the effect of changing IAA flow on vascular pattern (Mattsson et al., 1999) support the IAA flow-dependent canalization hypothesis (Sachs, 1989, 1991). However, some investigators (Carland et al., 1999; Koizumi et al., 2000) have argued for the reaction diffusion theory based on observations such as the fragmented vascular strands in some vascular mutants. This theory emphasizes generation of stable patterns autonomously in an initially homogenous field by interacting substances with different diffusion rates (Meinhardt, 1996). Both theories predict vascular development based on a leaf autonomous signal source (Dengler and Kang, 2001).
When the polar auxin transport inhibitor 1-N-naphthylphtalamic acid (NPA) is used to block IAA flow, vascular development is impaired (Mattsson et al., 1999). NPA caused central and marginal vascular hypertrophy—a general increase in the number and size of veins. NPA treatment also interferes with organogenesis, inhibiting both lateral root development (Reed et al., 1998) and the formation of new leaf primordia (Reinhardt et al., 2000).
Although auxin transport is implicated in a variety of growth and differentiation processes (Aloni, 1995; Lomax et al., 1995), little is known about the site of IAA production or its route of transport. It has been generally believed that IAA is produced in the shoot apex (Avery, 1935; Bartel, 1997) and in the tips of older leaves (Aloni, 2001) and transported basipetally, but the site of IAA production and its distribution in plant tissue have not been characterized. Methods for detecting IAA in plant tissues are being developed. Constructs containing an IAA-inducible promoter (Aux/IAA) fused to the β-glucuronidase (GUS) reporter gene can detect IAA in situ (Oono et al., 1988; Gil and Green, 1997; Ulmasov et al., 1997; Yi et al., 1999). Monoclonal antibodies against IAA (Leverone et al., 1991; Caruso et al., 1995) have been used to localize IAA in maize (Zea mays; Shi et al., 1993; Kerk and Feldman, 1995) and peanut (Arachis hypogaea) tissues (Moctezuma, 1999).
In this work, we show that immunocytochemistry with a monoclonal anti-IAA antibody can detect free IAA in Arabidopsis tissues. While studying IAA distribution in growing organs, we found that NPA prevents accumulation of IAA in the shoot apical meristem (SAM) and the youngest pair of leaf primordia, but not in older leaf primordia. The implications of our findings for the direction of IAA flow and vascular differentiation are discussed below.
RESULTS
IAA Immunolocalization in Arabidopsis Tissues
The production and isolation of monoclonal antibodies highly specific for IAA (Leverone et al., 1991; Caruso et al., 1995) provided the means to localize and evaluate in situ IAA levels in maize (Shi et al., 1993; Kerk and Feldman, 1995) and peanut tissues (Moctezuma, 1999). We prefixed Arabidopsis tissue samples with EDAC, which cross-links the carboxyl group of IAA to structural proteins in the plant tissues, creating the epitope recognized by this anti-IAA monoclonal antibody (Leverone et al., 1991; Caruso et al., 1995). The prefixed tissues were processed, sectioned, and reacted first with the monoclonal anti-IAA antibody then with the secondary antibody, anti-mouse IgG conjugated with alkaline phosphatase, before the enzymatic reaction was carried out to obtain the color signal.
IAA signal was low in pith but high in the epidermal and cortical tissues and vascular bundles of inflorescence stems (Fig. 1A). We verified the effectiveness of the immunolocalization technique and the specificity of the antibody with several controls. Because the monoclonal antibody was raised against free IAA cross-linked to bovine serum albumin (BSA) through its carboxyl group (Leverone et al., 1991), we tested unfixed tissues for color reaction. We observed no color comparable with the prefixed stem section (Fig. 1, A and B), indicating both that prefixation with EDAC is essential for free IAA detection by this antibody and that the antibody does not recognize other epitopes in these tissue sections. No signal was detected when the primary (Fig. 1C) or secondary antibody (Fig. 1D) was omitted, indicating that the color reaction is dependent on the presence of these antibodies on the tissue section and demonstrating again that the background color is very low.
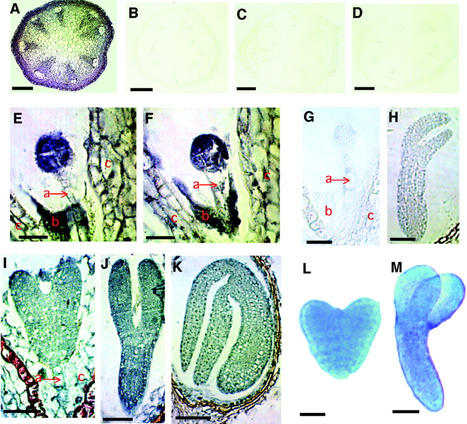
Figure 1.
IAA immunolocalization in Arabidopsis tissues. A through D, Cross sections of inflorescence stem. A, Stem tissues prefixed with ethyl-3(3-dimethylaminopropyl)carbodiimide hydrochloride (EDAC), embedded in paraffin, sectioned, and reacted with the anti-IAA antibody followed by anti-mouse IgG secondary antibody conjugated with alkaline phosphatase. There is a high level of IAA signal in the epidermal and cortical tissues and around vascular bundles: B through D, controls, showing very low levels of IAA signal; B, no EDAC prefixation; C, no primary antibody; D, no secondary antibody; E through K, longitudinal section of young siliques; E, eight-cell embryo; F, early globular stage embryo, showing high IAA signal in the embryo and endosperm cells (b) and a lower IAA level in the suspensor (a) and ovule cells (c); G, globular stage embryo with the omission of the primary anti-IAA antibody; and H, torpedo stage embryo with the omission of the secondary antibody. These controls show very low levels of the IAA signal: I, heart stage embryo; J, torpedo stage embryo; and K, walking stick stage embryo, showing high levels of IAA in the embryo. The suspensor (a), endosperm (b), and ovule cells (c) are indicated. L and M, GUS activity in embryos of DR5::GUS transgenic plants. L, Heart stage embryo; M, torpedo stage embryo showing high level of IAA in the embryos. Bar = 100 μm in A through D, 10 μm in E and F, 5 μm in G, 20 μm in H through J, 50 μm in K, 20 μm in L, and 50 μm in M.
We used the anti-IAA monoclonal antibody to study IAA distribution in growing tissues, embryos, leaf primordial, and SAMs (Figs. 1 and 2), where IAA is expected to be high. Immunocytochemistry on longitudinal sections of young siliques with different stages of embryo development is shown in Figure 1, E, F, and I through K. The signal is high in all embryo cells and lower in the suspensor and the ovule. Lower IAA signal may reflect more disperse cytoplasm in the more vacuolated suspensor and ovule cells. High signal is detected in the endosperm surrounding the suspensor in the early embryonic stages (Fig. 1, E and F).
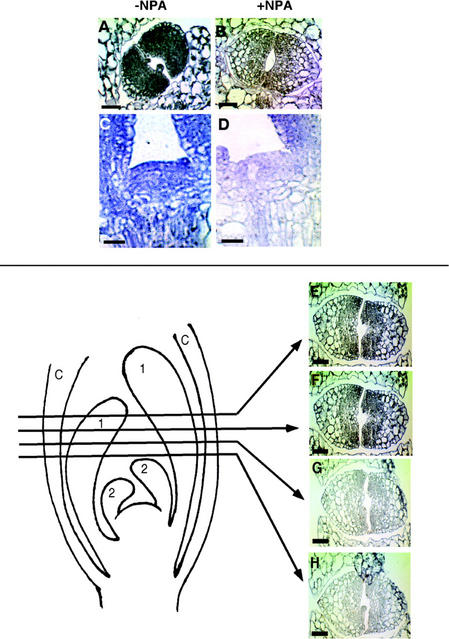
Figure 2.
IAA immunolocalization in leaf primordia and SAM. A and B, Cross sections of the shoot apex of 4-d-old seedlings were treated as described in the legend to Figure 1. A, Shoot apex of an untreated seedling, showing high IAA signal in the pair of first node leaf primordial. B, Shoot apex of a seedling grown in the presence of 40 μm NPA, showing little IAA signal in the leaf primordial. C and D, Longitudinal sections of 4-d-old seedlings. C, SAM of an untreated seedling, showing high IAA level. D, SAM of a seedling grown in the presence of 40 μm NPA, showing low IAA level. E through H, Serial cross sections of the shoot apex of 5-d-old seedlings that were treated as described in the legend to Figure 1. Drawing on the left depicts the sites where the four sections were made through the shoot apex including the first node (1) and second node (2) leaf primordia. Higher IAA levels are detected in the upper than in the lower sections. C, Petiole of the cotyledons. Bar = 20 μm in A and B, 10 μm in C and D, and 25 μm in E through H.
Embryonic stages in which procambium can be detected, i.e. heart stage (Fig. 1I), torpedo stage (Fig. 1J), and walking stick stage (Fig. 1K), have high IAA signal evenly distributed in all embryonic cells with no difference in signal level between procambium and ground tissue. No IAA signal was detected when the primary anti-IAA antibody (Fig. 1G) or the secondary antibody (Fig. 1H) was omitted, demonstrating again that the immunocytochemical reaction is specific and that background color is very low.
The IAA signal pattern was consistent across embryos with only minor variation. All 42 pro-embryos and globular stage embryos examined showed high IAA signal in the embryo and surrounding endosperm and lower signal in the suspensor. Thirty-five of 39 heart stage embryos analyzed had the uniform immunostaining shown in Figure 1I, whereas four showed high signal only in the distal end of the cotyledons and not uniformly in all the embryonic cells. Seventeen of 18 torpedo stage embryos showed the signal pattern in Figure 1J. Figure 1K shows the high IAA signal seen in all 19 walking stick stage embryos examined.
To verify the anti-IAA antibody signal, we used the GUS activity of transgenic plants carrying the DR5::GUS construct, composed of the IAA-inducible promoter (Aux/IAA) fused to a GUS reporter gene (Ulmasov et al., 1997). Figure 1, L and M, show GUS activity throughout heart and torpedo stage embryos. The walking stick stage embryo has less GUS activity, which diminishes as the embryo matures and becomes dormant. The overall GUS pattern is consistent with the immunological signals during embryogenesis. For unknown reasons, there are a small percentage, about 10%, of the torpedo stage and walking stick stage embryos that show higher GUS activity in the root cap and the tips of the cotyledons (data not shown).
IAA Signal in the Shoot Apex
The immunocytochemistry technique was used to examine IAA distribution in shoot apices of Arabidopsis seedlings. The first node rosette leaves (Fig. 2A) and SAM (Fig. 2C) of 4-d-old seedlings had high levels of IAA signal. Figure 2A shows high levels of IAA signal in a cross section of the first node rosette leaves, and Figure 2C shows high levels of IAA in a longitudinal section of the SAM. The IAA signals in Figure 2 were representative of 48 leaf primordia and 17 SAMs examined.
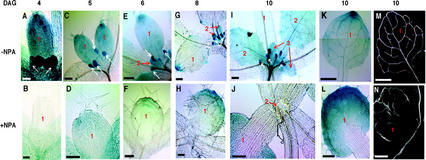
We also determined the GUS activity of transgenic seedlings carrying the DR5::GUS construct (Ulmasov et al., 1997). As shown in Figure 3A, high levels of GUS activity were detected in the first node leaf and stipule of 4-d-old seedlings. These results are similar to the IAA immunolocalization findings, demonstrating that the IAA inducibility of the DR5::GUS construct is a useful reporter of the endogenous IAA levels in the shoot apex. Figure 3, A, C, E, and G show the first true leaf at different ages. As the leaf primordium grows, only the distal end of the leaf primordium maintains high GUS activity (Fig. 3, A, C, E, and G). High GUS activity in the distal end of the leaf primordia was confirmed with the immunological assay for IAA. Figure 2, E through H, show a series of four cross sections of a 5-d-old shoot apex that has high anti-IAA antibody signal in the sections from the distal end of the first node leaf primordia, particular in the adaxial side of the leaf. Leaf primordia of subsequent nodes have a similar spatial and temporal pattern of GUS activity: high levels in the young leaf primordia that decline with growth (for example, see the second node leaf in Fig. 3, E, G, and I, and the third node leaf in Fig. 3I).
Figure 3.
IAA distribution and venation pattern in transgenic plants grown in the presence and absence of 40 μm NPA. DR5::GUS activity in transgenic plants: A, 4-d-old shoot apex, showing the GUS-positive first true leaf and the stipules (white arrows); B, 4-d-old shoot apex in a seedling grown in the presence of NPA, showing very low levels of IAA; C, 5-d-old shoot apex showing the GUS-positive true leaves and their stipules (white arrows); D, 5-d-old shoot apex in a seedling grown in the presence of NPA, showing some GUS signal in the distal end of the leaf; E, 6-d-old shoot apex showing first node leaf primordium with declining GUS activity and second node leaves (red arrow) and stipules (white arrows) with high level of IAA; F, 6-d-old shoot apex in seedling grown in the presence of NPA, showing more GUS signal in the leaf tip and the emerging marginal veins; G, 8-d-old shoot apex showing the GUS activity in the second node leaves (red arrows) and stipules (white arrows), whereas the signal in the first node true leaves decreased and is concentrated in the leaf tip; H, 8-d-old shoot apex in a seedling grown in the presence of NPA, showing increased GUS signal and the expanding veins along the leaf margin; I, IAA distribution in the subsequent leaf nodes of a 10-d-old seedling, showing high IAA signal in the third node leaves (red arrows) and the stipules (white arrows) and lower signals in the second node leaves; J, second rosette leaves of 10-d-old seedlings grown in the presence of NPA, showing no IAA signal; K and L, GUS activity in the first true leaf of a 10-d-old seedling (K) and a 10-d-old seedling grown in the presence of 40 μm NPA (L); M and N, venation pattern and IAA distribution in the first true leaf of 10-d-old seedling. Seedlings were fixed in 6:1 (v/v) ethanol:acetic acid for 4 h at room temperature and then rinsed and whole mounted as described in “Materials and Methods”; M, venation pattern of the first true leaf, showing 1o, 2o, and 3o veins; N, venation pattern of a first true leaf of seedling grown in the presence of 40 μm NPA, showing the marginal and central hypertrophy; 1, first node leaves; 2, second node leaves; and 3, third node leaves. Bar = 20 μm in A and B, 50 μm in C and D, 100 μm in E and F, 200 μm in G through J, and 400 μm in K through N.
Effect of NPA on IAA Distribution during Leaf Ontogeny
To study the effect of inhibition of IAA transport on IAA distribution, we germinated Arabidopsis seedlings in media supplemented with 40 μm NPA (concentration chosen based on studies of Mattsson et al., 1999) and sectioned shoot apices for IAA immunolocalization studies. The IAA signal in the leaf primordia (Fig. 2B) and in the SAM (Fig. 2D) of 4-d-old seedlings grown in the presence of NPA was very low relative to the untreated control (Fig. 2, A and C, respectively). (Three of 44 leaf primordia from seedlings grown on NPA showed a slightly higher IAA signal than in Fig. 2B.)
Similarly, DR5::GUS activity was barely detectable in shoot apices of seedlings grown in the presence of 40 μm NPA (Fig. 3, B and D). However, IAA signal was detectable in the apical area of the first node leaf primordium in 5- to 6-d-old NPA-grown seedlings (Fig. 3, D and F) and increased in intensity in leaf primordia of 8-d-old (Fig. 3H) and 10-d-old (Fig. 3L) seedlings, in the presence of NPA. No GUS activity was detected in the stipules or the SAM (Fig. 3, B, D, F, H, and J).
The second node leaves of NPA-grown seedlings showed the same spatial and temporal distribution of the GUS activity. No IAA signal was detected in newly emerged, 140-μm leaf primordia (Table I; Fig. 3J) until d 5 to 6, when IAA signal appeared at the leaf tip (data not shown). In contrast, control leaf primordia of the same length showed GUS signal throughout the entire leaf (second node leaf in Fig. 3E and third node leaf in Fig. 3I).
Table I.
Leaf lengths of Arabidopsis seedlings growing with or without NPA
| DAGa | First Node Leaves (−NPA + NPAb) | Second Node Leaves (−NPA + NPAb) | Third Node Leaves (−NPA + NPAb) | |||
|---|---|---|---|---|---|---|
| 4 | 120 ± 15 | 100 ± 10 | –c | – | – | – |
| 5 | 270 ± 30 | 165 ± 5 | – | – | – | – |
| 6 | 450 ± 15 | 370 ± 5 | 120 ± 30 | – | – | – |
| 8 | 1,450 ± 35 | 950 ± 55 | 400 ± 40 | – | 120 ± 20 | – |
| 10 | 4,500 ± 200 | 1,400 ± 50 | 1,400 ± 70 | 140 ± 15 | 400 ± 35 | – |
Data represent mean (±se) of 10 leaves. Measurements represent leaf blade + petiole. Lengths are in μm.
Days after germination.
40 μm NPA.
–, Not measurable on intact seedlings.
Effect of NPA on Organogenesis and Venation Pattern
NPA treatment altered the venation pattern and leaf and root shape. Leaf primordia grown in the presence of NPA (Fig. 3, D, F, H, and L) have broad leaf blades and broad, short petioles relative to leaves grown in the absence of the IAA transport inhibitor (Fig. 3, C, E, G, and I). Relative to the control root (Fig. 4N), the NPA-treated seedling root is shorter, lateral roots are inhibited, and the root tip is wider (Fig. 4O). The first node leaf primordia of untreated plants triple in length every 2 d. The leaves of NPA-treated seedlings grow much slower (Table I). NPA also slows the rate of emergence of leaves from subsequent nodes (Table I).
Figure 4.
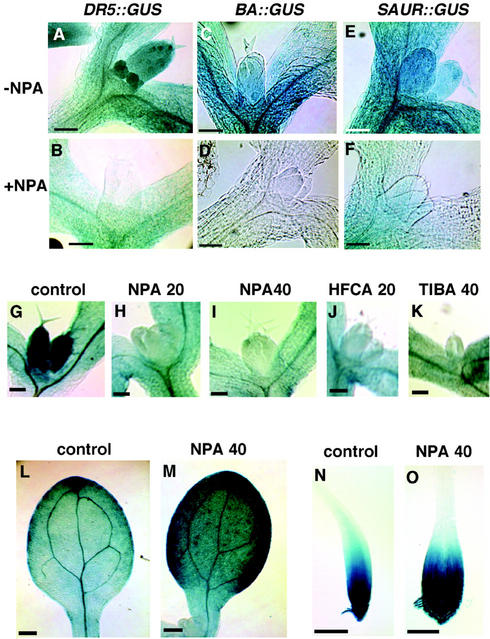
IAA distribution in seedlings containing a different IAA-inducible promoter::GUS and in a DR5::GUS-containing line treated with three different IAA transport inhibitors. A through F, GUS activity in 4-d-old transgenic seedlings containing different Aux/IAA promoter::GUS. A, DR5::GUS transgenic line; B, DR5::GUS transgenic line grown in the presence of 40 μm NPA; C, BA::GUS transgenic line; D, BA::GUS transgenic line grown in the presence of 40 μm NPA; E, SAUR-AC1::GUS transgenic line; and F, SAUR-AC1::GUS transgenic line grown in the presence of 40 μm NPA. G through K, GUS activity in a 5-d-old DR5::GUS transgenic line grown with a different IAA transport inhibitor; G, seedlings grown without any inhibitor, showing high level of GUS activity; H, seedlings grown in the presence of 20 μm NPA, showing low level of the GUS activity; I, seedlings grown in the presence of 40 μm NPA; J, seedlings grown in the presence of 20 μm 2-chloro-9-hydroxyfluorene-9-carboxylic acid (HFCA); K, seedlings grown in the presence of 40 μm 2,3,5-triiodobenzoic acid (TIBA); L, 5-d-old cotyledon, showing lower level of the GUS activity than a 5-d-old cotyledon of seedling grown in the presence of NPA (M); and N, 4-d-old root tip and O, 4-d-old root tip of seedling grown in the presence of 40 μm NPA, both showing high level of GUS activity. Bar = 50 μm in A through F, 100 μm in G through K, 200 μm in L and M, and 100 μm in N and O.
As reported in Mattsson et al. (1999), NPA treatment affects the venation pattern. For example, a 5-d-old leaf has a differentiated midvein, but leaves of seedlings grown in the presence of NPA do not (Fig. 3, F and H). Instead, the first veins form laterally along the margins of the leaf primordia at the distal end of the leaf in 6-d-old seedlings (Fig. 3F), then expand along the leaf margins to form a broad band of vascular tissue, called the marginal hypertrophy (Fig. 3H). By 8 d after germination, multiple files of veins, called the central hypertrophy (Fig. 3N), are formed in the center of the leaf blade, extending into the petiole but not connecting to the vascular system of the hypocotyl. This is consistent with the findings of Mattsson et al. (1999) and Sieburth (1999). It is worth noting that the area of IAA-driven DR5::GUS activity (Fig. 3, F and H) coincides with the site of NPA-induced vascular differentiation, especially in the marginal hypertrophy.
IAA Distribution in Different promoter::GUS- Containing Seedlings Grown in the Presence and Absence of NPA
Because the expression pattern of DR5::GUS depends on tissue-specific promoter activity as well as the presence of IAA, we tested two other Aux/IAA response promoter::GUS constructs: PSIAA4/5 BA::GUS (Oono et al., 1988) and SAUR-AC1::GUS (Gil and Green, 1997). BA is the PSIAA4/5 promoter region that contains two auxin-responsive domains (AuxRD A and AuxRD B). Domain A contains a highly conserved sequence found in various IAA-inducible genes that behaves as a major auxin-responsive element. Domain B functions as an enhancer element. The two domains, which act cooperatively to stimulate transcription (Ballas et al., 1995), were fused to the GUS reporter gene and introduced into Arabidopsis (Oono et al., 1988). The SAUR-AC-1 genes (Gee et al., 1991) encode auxin-inducible small RNAs. High promoter activity was reported by Gil and Green (1997) in vascular tissues of transgenic plants harboring the SAUR-AC-1:GUS construct.
The GUS expression patterns in seedling shoot apex of the BA::GUS (Fig. 4, C and D) and the SAUR-AC-1::GUS (Fig. 4, E and F) transgenic plants are similar to that of the DR5::GUS plants (Fig. 4, A and B), with high GUS activities in the shoot apex of 4-d-old seedlings and reduced GUS activities in the shoot apex of seedlings grown in the presence of NPA. Little GUS activity is detected in the SAM, leaf primordial, or stipules of NPA-grown seedlings. The faint GUS activity in the shoot apex of the NPA-grown SAUR-AC-1::GUS transgenic plants (Fig. 4F) is much lower than in non-treated SAUR-AC-1::GUS transgenic plants (Fig. 4E). In general, these results confirm the IAA distribution pattern during Arabidopsis leaf ontogeny determined by DR5::GUS activity and by IAA immunocytochemistry. It is presumed that NPA prevents IAA transport to the shoot apex, resulting in the absence of the IAA necessary for inducing GUS activity.
IAA Distribution in DR5::GUS Seedlings Grown in the Presence of Three Different IAA Transport Inhibitors
To confirm that NPA affects IAA transport to the shoot apex, we grew DR5::GUS transgenic plants in the presence of two other IAA transport inhibitors, TIBA and HFCA. Seedlings grown in the presence of these two inhibitors had reduced IAA signal in their shoot apices (Fig. 4, J and K) relative to the untreated control (Fig. 4G), as did the NPA-grown seedlings (Fig. 4, H and I). The congruence of results with these three IAA transport inhibitors confirms the conclusion that the reduced IAA signal in the shoot apex is due to lack of auxin transport. Thus, the IAA in the untreated shoot apex comes from an outside source. In an attempt to identify this outside source, we examined GUS activity in the cotyledons and root tip of seedlings grown in the presence of NPA. The signal detected in root tips of NPA-grown seedlings was similar to the control (Fig. 4, N and O). The cotyledons of seedlings grown in the presence of NPA (Fig. 4M), TIBA, and HFCA (data not shown) have higher GUS activity than those grown in the absence of inhibitors (Fig. 4L). This result implicates the cotyledons as a probable source of the shoot apex IAA of germinating seedlings.
DISCUSSION
Because polar auxin transport plays a major role in plant development, characterization of the IAA distribution pattern in relation to the processes that are regulated by IAA will contribute to our overall understanding of how organs communicate with each other to coordinate the growth of the whole plant. To elucidate the role of polar auxin transport in vascular differentiation, we investigated the temporal and spatial pattern of IAA localization in the shoot apex of Arabidopsis seedlings. We found that inhibition of auxin transport prevented IAA accumulation in the SAM and the youngest pair of leaves, but not in older leaf primordia. The temporal pattern of IAA localization implies a switch in IAA transport direction that is consistent with the mechanism of vascular differentiation proposed in the canalization of IAA flux hypothesis.
IAA Immunocytochemistry
IAA distribution in Arabidopsis was determined using the anti-IAA monoclonal antibody employed to detect IAA in maize (Kerk and Feldman, 1995) and peanut (Moctezuma, 1999) tissues. This monoclonal antibody, raised in mice (Mus musculus) against IAA conjugated to albumin through its carboxyl group (Leverone et al., 1991), shows maximal cross-reactivity to the methyl ester of IAA in radioimmuno assay and ELISA (Caruso et al., 1995; Gao et al., 1999). The antibody does recognize IAA conjugates (Gao et al., 1999), but Arabidopsis has little IAA-Glu conjugate, IAA-Asp conjugate, or IAA-Glc conjugate (Tam et al., 2000).
We used EDAC to cross-link the exposed carboxyl group of free IAA to the free amino groups of structural proteins in Arabidopsis cells. IAA conjugates lack a free carboxyl and cannot be cross-linked to structural proteins by EDAC, which precludes antibody reaction with any conjugates that might be present in the tissues. The free carboxyl group of Trp can be cross-linked by EDAC, but this monoclonal anti-IAA antibody has a very low cross-reactivity with Trp (Pence and Caruso, 1988). Positive controls, including adding exogenous IAA to tissues and radioactive IAA blotting assay to test the EDAC cross-linking of IAA, verified the specificity of the antibody for free IAA (Moctezuma, 1999). The antibody failed to detect any signal in tissues not prefixed with EDAC (Fig. 1B), further confirming the specificity of the antibody to cross-linked IAA. Moreover, the immunological signal was absent from shoot apical tissues when IAA transport was inhibited, indicating that the signal is detecting IAA and not other compounds, such as Trp. Finally, the anti-IAA monoclonal antibody and the three different Aux/IAA promoter::GUS constructs produced similar patterns, indicating that immunocytochemistry is a reliable method for identifying IAA in Arabidopsis tissues. IAA immunocytochemistry will be an important tool for detecting IAA in very small tissues such as the SAM, in tissues where the Aux/IAA promoters are not expressed, and in plant species such as monocots in which promoter::reporter constructs are not yet available.
IAA Production and Transport in Shoot Apex
Using in situ IAA determination, we have found that Arabidopsis rosette shoot apex, i.e. the SAM and the youngest set of leaf primordia, do not accumulate free IAA if IAA transport is inhibited with NPA. We observed similar depletion of IAA in seedlings treated with two other IAA transport inhibitors, confirming that the reduced IAA in the NPA-treated seedlings results from lack of IAA transport. Thus, IAA is likely transported into the shoot apex, not produced there. However, as leaf primordia grow and mature, IAA is found at the distal end of the leaf regardless of IAA transport inhibition. The presence of IAA in older leaf primordia of NPA-grown seedlings indicates that IAA can be produced in the presence of NPA. Elevated IAA in cotyledons of seedlings grown in the presence of NPA (Fig. 4, L and M) suggests that IAA is normally transported out of the cotyledons and that they might be the source of the IAA in the first pair of true leaves. IAA accumulation at the distal end of transport-blocked leaves implies IAA production at the leaf tip. IAA produced in the leaf tip must drain out of the leaf (Mattsson et al., 1999). Based on these results, we propose the “IAA flow model” to describe the temporal and spatial pattern of IAA flow in the shoot apex (Fig. 5). During leaf development, the IAA source changes from extrinsic to intrinsic, which would change IAA flow direction and affect the pattern of vascular differentiation (see below) and probably cell and organ differentiation as well. The correspondence of the patterns of IAA flux and the leaf vascular formation, e.g. as seen in Figure 3, E and H, supports Sachs' canalization hypothesis (1991) and is in good agreement with the leaf venation hypothesis recently proposed by Aloni (2001).
Figure 5.
“IAA flow model” in the shoot apex of Arabidopsis seedling. The youngest leaf primordia (marked “2” for second node leaves) and SAM do not produce IAA. Rather, IAA is being acropetally transported into this region. IAA is produced at the tip and marginal regions of the older leaf primordia (marked “1” for first node leaves) and basipetally drained, primarily through the midvein. Arrows represent flow direction of IAA. The IAA source for the youngest pair of leaf primordia and SAM may be the cotyledons (C), older leaves, and shoot tissues underneath the SAM. The acropetal flow of the IAA into the leaf primordia can explain the acropetal formation of the midvein. The time of IAA appearance in the distal end of the leaf corresponds to the time of secondary vein differentiation along the leaf margin, consistent with the notion that inability to drain IAA from the leaf tip basipetally through the midvein would cause marginal hypertrophy (Mattsson et al., 1999).
Role of IAA Flux on Venation Pattern
There are at least three orders of veins in the Arabidopsis rosette leaf: 1o (or midvein), 2o, and 3o, which form in a hierarchical fashion (Esau, 1965). The midvein is usually seen growing acropetally from the hypocotyl vasculature into the emerging leaf primordium (Sieburth, 1999). The extrinsic IAA source and acropetal flow pattern suggested by our results is consistent with the acropetal differentiation of midvein procambium at the onset of leaf ontogeny. If the 5- to 6-d-old leaf begins to produce IAA at the distal end, IAA could then flow basipetally into the plant. Seedlings germinated on NPA do not form the prominent midvein. Instead, files of veins appear, first along the leaf margins (Fig. 3, F and H), and afterward toward the leaf base (Fig. 3N), that are not connected to the hypocotyl vascular system (Sieburth, 1999). This is again consistent with the canalization hypothesis: Without the midvein, there is no constant basipetal flow of IAA from the leaf tip. The IAA produced at the leaf tip, the presumed IAA source of 6-d and older leaves, cannot itself be cannalized into specific cell files and cause differentiation of a vascular bundle connected to the plant vascular system. Prior differentiation of the midvein by flow of auxin into the leaf appears to be required for the later flow from the leaf tip to generate the normal venation pattern.
The three pairs of secondary veins in the young rosette leaves appear as arches (Mattsson et al., 1999). As the leaf primordium grows laterally, the first pair forms as a loop from the tip of the midvein along the leaf margin that rejoins the midvein at a basal location. The secondary veins may join the midvein again because IAA flows toward the midvein, which drains the IAA out of the leaf. In the absence of a midvein in NPA-treated leaves, IAA flows along the growing leaf margin rather than toward the center of the leaf. Thus, the pair of arches is replaced by a band of vascular tissues along the leaf margin forming the marginal hypertrophy (Mattsson et al., 1999). The mechanism of IAA flows along the leaf margin is unknown, but flow is apparently independent of the IAA transport mechanism that can be disrupted by NPA, TIBA, or HFCA.
Role of Localized IAA Production and IAA Transport in Organogenesis
Chemical inhibition of polar auxin transport alters venation pattern and leaf shape. The accumulation of IAA and vasculature in the upper one-half of the leaf may promote lateral leaf growth and inhibit longitudinal growth, resulting in broad leaves with short petioles (Fig. 3, C, D, G, and H).
It has been generally believed that IAA, which plays a major role in regulating tissue differentiation and organogenesis, is produced in the SAM (Avery, 1935; Bartel, 1997). Recently, a very different concept was proposed—IAA is transported to, not produced in, the shoot meristem proper. It was further proposed that, because of the acropetally advancing procambial strand from the stem, IAA from an extrinsic source might dictate the position of new leaf primordia, phyllotaxis, and initiate organogenesis (Kuhlemeier and Reinhardt, 2001). Our results provide the first evidence in support of the concept that IAA flows from the plant to the SAM. Import of IAA into the SAM would explain impairment of organogenesis when IAA flow is blocked. Growth observed in NPA-treated, IAA-depleted shoot apices may be supported by very low levels of IAA or by other forms of auxin such as indole butyric acid (Bartel, 1997).
In conclusion, finding both extrinsic and intrinsic sources of IAA in leaf primordia is novel, providing both the first evidence for changing IAA flow pattern and insights into the mechanism of IAA flux-mediated venation pattern. Our results establish a foundation from which to pursue the IAA signaling pathways of phyllotaxis and organogenesis.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis ecotype Columbia-0 was used in the study. Transgenic seeds containing the promoter::GUS constructs were kindly provided by: DR5::GUS, Dr. Tom Guilfoyle (University of Missouri, Columbia); PSIAA4/5 BA::GUS, Dr. Anastasios Theologis (The Plant Gene Expression Center, U.S. Department of Agriculture, Albany, CA); and SAUR-AC1::GUS, Dr. Pamela Green (Michigan State University, East Lansing).
Seeds were surface sterilized in 70% (v/v) ethanol for 1 min, followed by 10% (v/v) commercial bleach for 15 min, washed three times in sterile distilled water, and plated in 0.4% (w/v) molten agar on top of a solid germinating medium in 9-cm petri dishes. Germinating medium contained 0.5× Murashige and Skoog basal salts (Murashige and Skoog, 1962), 1.5% (w/v) Suc, and 0.8% (w/v) agar. Plates were sealed with parafilm, incubated at 6°C in the dark for 2 d, and then transferred to a growth chamber set at 22°C for a 16-h-light (120–150 μmol m−2 s−1), 8-h-dark cycle (long-day conditions). The time of transfer to growth chamber was considered the starting point of all the experiments. Plants were transplanted into soil 2 weeks later and were grown under long-day conditions until seeds were harvested. For the inflorescence stem tissues, seedlings were germinated as described above and were grown for 2 weeks at 22°C in an 8-h-light (120–150 μmol m−2 s−1), 16-h-dark cycle (short-day conditions), then transferred to soil and grown in the greenhouse under long-day conditions (16-h-light, 8-h-dark cycle). Four- to 10-cm inflorescence stems were collected for the IAA immunocytochemical studies.
Auxin transport inhibitors NPA (Chem Service, West Chester, PA), TIBA (Sigma, St. Louis), and HFCA (Sigma) were dissolved in dimethyl sulfoxide (Sigma). The concentration of the dimethyl sulfoxide in the growth media never exceeded 0.1% (v/v).
IAA Immunocytochemical Localization
The monoclonal anti-IAA antibody used in the immunolocalization studies was kindly provided to us by Dr. John L. Caruso (Department of Biological Sciences, University of Cincinnati). The antibody was raised against free IAA that was cross-linked to BSA at the carboxyl group in mice (Mus musculus) (Leverone et al., 1991).
Excised tissue samples were immediately prefixed in 3% (w/v) aqueous solution of EDAC (Sigma) and postfixed in FAA (3.7% formaldehyde:50% ethanol:5% glacial acetic acid [v/v]) for 16 h at 4°C, dehydrated with a graded ethanol series, embedded in paraffin, and sectioned to 10-μm slices. Sections were affixed onto slides (Probe-On Plus, Fisher Scientific, Pittsburgh). After overnight drying at 42°C, sections were deparaffinized with xylene and hydrated in an ethanol-water series. Slides were processed as described in Moctezuma (1999) with some modifications: Slides were incubated in a blocking solution containing 10 mm phosphate-buffered saline (PBS; 2.68 mm KCl, 0.15 m Na2HPO4, and 0.086 m KH2PO4), 0.1% (v/v) Tween 20, 1.5% (v/v) Gly, and 5% (w/v) BSA, for 45 min at 22°C, then rinsed in a regular salt rinse solution (10 mm PBS, 0.88% [w/v] NaCl, 0.1% [v/v] Tween 20, and 0.8% [w/v] BSA), and washed briefly with 10 mm PBS and 0.8% (w/v) BSA solution to remove the Tween 20. Fifty microliters of 1:200 (w/v) anti-IAA antibody (1 mg mL−1) were placed on each slide, covered with coverslips, and incubated overnight in a humidity chamber at 22°C. Two 10-min vigorous washes with high-salt rinse solution, 10 mm PBS, 2.9% (w/v) NaCl, 0.1% (v/v) Tween 20, and 0.1% (v/v) BSA were followed by a 10-min wash with a regular salt rinse and a brief rinse with 10 mm PBS and 0.8% (v/v) BSA. Fifty microliters of 1:100 (w/v) dilution of the 1 mg mL−1 anti-mouse IgG-alkaline phosphatase-conjugate (Promega, Madison, WI) were added to each slide, which was covered with coverslip and incubated for 4 to 6 h in a humidity chamber at 22°C. Two 15-min washes with a regular salt rinse were followed by a 15-min wash with water. Two hundred microliters of ready-to-use Western Blue (Promega) were added to each slide; each slide was then covered with a coverslip and incubated in the dark for 15 to 30 min. When blue/purple color was observed, slides were rinsed with water and then dehydrated in a graded water-ethanol series, ethanol-xylene, xylene. Slides were mounted with Permount (Fisher Scientific), dried overnight in a 42°C oven, and observed under an Axiophot microscope (Zeiss, Jena, Germany). Photomicrographs were taken by a video camera attached to the microscope and processed with the Scion Image 1.60. The figures were arranged using Adobe Photoshop version 5.5 (Adobe, Mountain View, CA).
GUS Activity
To assay GUS activity, dissected samples were incubated with 5-bromo-4-chloro-3-indolyl-β-d-glucuronide (X-Gluc) solution as described by Cheng et al. (2000). Excised samples were vacuum infiltrated in the X-Gluc solution for 10 min at room temperature and then incubated at 37°C in the dark for 16 h. Samples were rinsed with 50 mm sodium phosphate buffer, pH 7.0, and then fixed in ethanol:acetic acid (9:1 [v/v]) for 4 h at room temperature.
X-Gluc-treated samples were rinsed with 95% (v/v) ethanol and transferred to 70% (v/v) ethanol. Tissue samples were whole mounted on microscope slides in a clearing solution of chloral-hydrate:glycerol:water (8:1:2 [v/v]) as described by Berleth and Jurgens (1993). The samples were covered and observed with a Zeiss Axiophot microscope. Photomicrographs were taken as described above.
For GUS staining of the zygotic embryos, ovules were dissected from siliques. To minimize wounding the embryo, a hole was punctured to allow the penetration of the X-Gluc solution into each ovule. After 16 h of incubation, embryos were dissected from the broken ovule for fixation, clearing, and photography. 35S::GUS embryos were used as control to ensure proper penetration of the X-Gluc solution through the damaged ovule.
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes.
ACKNOWLEDGMENTS
We thank Prof. Tsvi Sachs (Alexander Silberman Institute of Life Sciences, Hebrew University of Jerusalem, Israel) for helpful comments and discussion, and Dr. John Caruso (Department of Biological Sciences, University of Cincinnati) for the monoclonal anti-IAA antibodies and helpful information. We also thank Dr. Denise Schichnes and Dr. Steven Ruzin (Biological Imaging Facility, College of Natural Resources, University of California, Berkeley) for their help and support with the microscopy work.
Footnotes
This work was supported by the National Science Foundation (grant no. DBI–9813361 to Z.R.S.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.003228.
LITERATURE CITED
- Aloni R. The induction of vascular tissues by auxin and cytokinin. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 531–546. [Google Scholar]
- Aloni R. Foliar and axial aspects of vascular differentiation: hypotheses and evidence. J Plant Growth Regul. 2001;20:22–34. [Google Scholar]
- Avery GS. Differential distribution of phytohormone in the developing leaf of Nicotiana, and its relation to polarized growth. Bull Toray Club. 1935;62:313–330. [Google Scholar]
- Ballas N, Wong LM, Ke M, Theologis A. Two auxin-responsive domains interact positively to induce expression of the early indolacetic acid-inducible gene PS-IAA4/5. Proc Natl Acad Sci. 1995;92:3483–3487. doi: 10.1073/pnas.92.8.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel B. Auxin biosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:51–66. doi: 10.1146/annurev.arplant.48.1.51. [DOI] [PubMed] [Google Scholar]
- Berleth T, Jurgens G. The role of monopteros gene in organizing the basal body region of the Arabidopsis embryo. Development. 1993;118:575–587. [Google Scholar]
- Carland FM, Berg BL, FitzGerald JN, Jinamornphongs S, Nelson T, Keith B. Genetic regulation of vascular tissue patterning in Arabidopsis. Plant Cell. 1999;11:2123–2137. doi: 10.1105/tpc.11.11.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso JL, Pence VC, Leverone LA. Immunoassay methods of hormone analysis. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 433–447. [Google Scholar]
- Cheng JC, Lertpiriyapong K, Wang S, Sung ZR. The role of the Arabidopsis ELD1 gene in cell development and photomorphogenesis in darkness. Plant Physiol. 2000;123:509–520. doi: 10.1104/pp.123.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin CR. The Power of Movement in Plants. J. London: Murray; 1880. [Google Scholar]
- Dengler N, Kang J. Vascular patterning and leaf shape. Curr Opin Plant Biol. 2001;4:50–56. doi: 10.1016/s1369-5266(00)00135-7. [DOI] [PubMed] [Google Scholar]
- Esau K. Vascular differentiation in plants. New York: Holt, Rinehart and Winston, Inc.; 1965. [Google Scholar]
- Estelle M. Polar auxin transport: new support for an old model. Plant Cell. 1998;10:1775–1777. doi: 10.1105/tpc.10.11.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galweiler L, Guan C, Muller A, Wisman E, Mendgen K, Yephemov A, Palme K. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science. 1998;282:2226–2230. doi: 10.1126/science.282.5397.2226. [DOI] [PubMed] [Google Scholar]
- Gao H, Jiang T, Heineman WR, Halsall HB, Caruso HL. Capillary enzyme immunoassay with electrochemical detection for determining indole-3-acetic acid in tomato embryos. Fresenius J Anal Chem. 1999;364:170–174. [Google Scholar]
- Gee MA, Hagen G, Guilfoyle TJ. Tissue specific expression of soybean auxin-responsive transcripts GH3 and SAURs. Plant Cell. 1991;3:419–430. doi: 10.1105/tpc.3.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil P, Green PJ. Regulatory activity exerted by the SAUR-AC1 promoter region in transgenic plants. Plant Mol Biol. 1997;34:803–808. doi: 10.1023/a:1005875300606. [DOI] [PubMed] [Google Scholar]
- Hickey LJ. A revised classification of the architecture of dicotyledons leaves. In: Metcalfe CR, Chalk L, editors. Anatomy of the Dicotyledons, Ed II. I. Oxford: Clarendon Press; 1979. pp. 25–39. [Google Scholar]
- Jacobs WP. The role of auxin in differentiation of xylene around a wound. Am J Bot. 1952;39:301–309. [Google Scholar]
- Kerk NM, Feldman LJ. A biochemical model for the initiation and maintenance of the quiescent center: implications for organization of root meristems. Development. 1995;121:2825–2833. [Google Scholar]
- Kogl F, Haagen-Smit AJ. “Uber die Chemie des Wuchsstoffs K. Akad. Wetenschap. Amsterdam.”. Proc Sect Sci. 1931;34:1411–1416. [Google Scholar]
- Koizumi K, Sugiyama M, Fukuda HA. Series of novel mutants of Arabdiopsis thaliana that are defective in the formation of continuous vascular network: calling the auxin signal flow canalization hypothesis into questions. Development. 2000;127:3179–3204. doi: 10.1242/dev.127.15.3197. [DOI] [PubMed] [Google Scholar]
- Kuhlemeier C, Reinhardt D. Auxin and phyllotaxis. Trends Plant Sci. 2001;6:187–189. doi: 10.1016/s1360-1385(01)01894-5. [DOI] [PubMed] [Google Scholar]
- Leverone LA, Stroup TL, Caruso JL. Western blot analysis of cereal grain prolamins using an antibody to carboxyl-linked indolacetic acid. Plant Physiol. 1991;96:1076–1078. doi: 10.1104/pp.96.4.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax TL, Muday GK, Rubery PH. Auxin transport. In: Davies PJ, editor. Plant Hormones: Physiology, Biochemistry and Molecular Biology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 509–530. [Google Scholar]
- Mattsson J, Sung ZR, Berleth T. Responses of the Arabidopsis vascular system to auxin transport inhibition. Development. 1999;126:2979–2991. doi: 10.1242/dev.126.13.2979. [DOI] [PubMed] [Google Scholar]
- Meinhardt H. Models of biological pattern formation: common mechanism in plant and animal development. Int J Dev Biol. 1996;40:123–134. [PubMed] [Google Scholar]
- Moctezuma E. Changes in auxin patterns in developing gynophores of the peanut plant (Arachis hypogaea L.) Ann Bot. 1999;83:235–242. doi: 10.1006/anbo.1998.0814. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Nelson T, Dengler N. Leaf vascular pattern formation. Plant Cell. 1997;9:1121–1135. doi: 10.1105/tpc.9.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oono Y, Chen QG, Overvoorde PJ, Koehler C, Theologis A. age Mutants of Arabidopsis exhibit altered auxin-regulated gene expression. Plant Cell. 1988;10:1649–1662. doi: 10.1105/tpc.10.10.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence VC, Caruso JL. Immunoassay methods of plant hormone analysis. In: Davies PJ, editor. Plant Hormones and Their Role in Plant Growth and Development. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1988. pp. 240–256. [Google Scholar]
- Reed RC, Brady SR, Muday GK. Inhibition of auxin movement from the shoot into the root development in Arabidopsis. Plant Physiol. 1998;118:1369–1378. doi: 10.1104/pp.118.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D, Mandel T, Kuhlemeier C. Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell. 2000;12:507–518. doi: 10.1105/tpc.12.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs T. The control of the patterned differentiation of vascular tissues. Adv Bot Res. 1981;9:151–262. [Google Scholar]
- Sachs T. The development of vascular networks during leaf development. Curr Top Plant Biochem Physiol. 1989;8:168–183. [Google Scholar]
- Sachs T. Cell polarity and tissue patterning in plants. Development Suppl. 1991;1:1179–1190. [Google Scholar]
- Shi L, Miller L, Moore R. Immunocytochemical localization of indole-3-acetic acid in primary roots of Zea mays. Plant Cell Environ. 1993;16:967–973. [Google Scholar]
- Sieburth LE. Auxin is required for leaf vein pattern in Arabidopsis. Plant Physiol. 1999;121:1179–1190. doi: 10.1104/pp.121.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam YY, Epstein E, Normanly J. Characterization of auxin conjugates in Arabidopsis. Low steady-state levels of indole-3-acetyl-aspartate, indole-3-acetyl-glutamate, and indole-3-acetyl-glucose. Plant Physiol. 2000;123:589–595. doi: 10.1104/pp.123.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. Aux-IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell. 1997;9:1963–1971. doi: 10.1105/tpc.9.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Went FW. On growth-accelerating substances in the coleoptile of Avena sativa. Proc Kon Ned Akad Wet. 1926;30:10–19. [Google Scholar]
- West MAL, Harada JJ. Embryogenesis in higher plants: an overview. Plant Cell. 1993;5:1361–1369. doi: 10.1105/tpc.5.10.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi HC, Joo S, Nam KH, Lee JS, Kang BG, Kim WT. Auxin and brassinosteroid differentially regulate the expression of three members of the 1-aminocyclopropane-1-carboxylate synthase gene family in mung bean (Vigna radiata L.) Plant Mol Biol. 1999;41:443–454. doi: 10.1023/a:1006372612574. [DOI] [PubMed] [Google Scholar]