Abstract
We identified the ON–OFF direction-selective ganglion cells (DSGCs) in the mouse retina and characterized their physiological, morphological and pharmacological properties. These cells showed transient responses to the onset and termination of a stationary flashing spot, and strong directional selectivity to a moving rectangle. Application of various pharmacological reagents demonstrated that the ON–OFF DSGCs in the mouse retina utilize a similar array of transmitters and receptors to compute motion direction to their counterparts in the rabbit retina. Voltage clamp recording showed that ON–OFF DSGCs in the mouse retina receive a larger inhibitory input when the stimulus is moving in the null direction and a larger excitatory input when the stimulus is moving in the preferred direction. Finally, intracellular infusion of neurobiotin revealed a bistratified dendritic field with recursive dendrites forming loop-like structures, previously classified as RGD2 by morphology. Overall, the ON–OFF DSGCs in the mouse retina exhibit almost identical properties to their counterparts in the rabbit retina, indicating that the mechanisms for computing motion direction are conserved from mouse to rabbit, and probably also to higher mammals. This first detailed characterization of ON–OFF DSGCs in the mouse retina provides fundamental information for further study of maturation and regulation of the neuronal circuitry underlying computation of direction.
A classic example of neurocomputation in the mammalian retina is represented by two types of retinal ganglion cells responding selectively to visual stimuli moving in a few cardinal directions. The phenomenon is particularly well documented in the rabbit retina, where it was first discovered (Barlow & Hill, 1963). Four decades of intensive research have revealed the physiological, morphological, and pharmacological properties of rabbit ON–OFF direction-selective ganglion cells (DSGCs). Recently, important advances have been made, revealing mechanisms for computing motion direction (see recent reviews: Vaney et al. 2001; Vaney & Taylor, 2002; He et al. 2003; Taylor & Vaney, 2003). Cholinergic amacrine cells (a.k.a. starburst amacrine cells, SAs) have been shown to play a critical role in retinal direction selectivity. Ablating SAs abolishes retinal direction selectivity (Yoshida et al. 2001; Amthor et al. 2002). Double patch experiments have shown that current injection into SAs on the null side induces inhibition in the DSGCs, whereas current injection into SAs on the preferred side has no effect (Fried et al. 2002). Imaging calcium activity at the tips of SAs has revealed a direction-selective calcium signal (Euler et al. 2002). It appears that the mechanism for computing direction involves SAs generating inhibition in the outer rim and feeding the inhibition selectively to appropriate DSGCs in a spatially asymmetrical fashion. However, the report of simple geometrical asymmetry between DSGC dendrites and the SA plexus (Fried et al. 2002) has not been confirmed, and further, no geometrical asymmetry has been detected between processes of DSGCs and SAs, indicating that direction selectivity results from selective connections (Dong et al. 2004). How selective connections between DSGCs and SAs are formed, and what regulates the formation of selective connections, are intriguing but unanswered questions. To address these questions, genetic manipulation will be a key approach. The importance of the mouse model for such studies means that a better understanding of the mouse retina is a crucial step in pursuing the answers to these questions.
One previous study, using extracellular recording, reported DSGCs in the mouse retina (Yoshida et al. 2001), but the morphology and pharmacology of the DSGCs and their synaptic inputs, and hence the mechanism for computing motion direction, remain unclear. Our previous study examining morphological features of mouse RGCs discovered a subtype of bistratified cells morphologically similar to the ON–OFF DSGCs in the rabbit retina, which we classified as RGD2 (Sun et al. 2002). We suggested that RGD2 cells may be the mouse counterparts of rabbit ON–OFF DSGCs.
In this study, we demonstrate for the first time that mouse RGD2 cells are indeed ON–OFF DSGCs. Furthermore, the light responses, pharmacological characteristics, morphological features and synaptic inputs are almost identical to those that have been reported for the rabbit retina. These results indicate that the mechanisms for computing motion direction in the RGCs are conserved from mouse to rabbit, and probably throughout mammalian species.
Methods
Whole-mount retina preparation
C57BL/6N mice were used in this study. Use and handling of animals were strictly in accordance to the institutional guidelines and the Society for Neuroscience's policies on the use of animals and human subjects in neuroscience research. All the experimental procedures were carried out under very dim red light. Mice were dark adapted for at least 1 h before experiments. The animals were deeply anaesthetized with an i.p. injection of a mixture of ketamine (50 mg kg−1) and xylazine (10 mg kg−1), decapitated and the eyes immediately enucleated. A small cut was made in the sclera close to the cornea and the eyeball was submerged in Ames medium (Sigma) equilibrated with 95% O2 and 5% CO2. The front part (the cornea, lens and vitreous body) was removed and the retina carefully dissected from the pigment epithelium. Three to four cuts were made to flatten the retina. The retina was then attached, ganglion cell side up, to a piece of black Millipore filter paper (AABP02500) with a 2 mm diameter hole in the centre to allow adequate infrared illumination and visual stimulation during the electrophysiological recording. The whole-mount retinal preparation was then transferred into a recording chamber (0.5 ml in volume) on the fixed stage of an upright microscope (E600FN, Nikon) equipped with epifluorescence and a 40 × water-immersion objective lens configured for DIC. The preparation was continuously superfused with oxygenated bicarbonate-buffered Ames medium at 35°C.
Patch clamp recording
Micropipettes were manufactured from thick-walled borosilicate filament glass tubing (1.5 mm outer diameter, 0.86 mm inner diameter; Sutter Instruments, San Rafael, CA, USA) using a Flaming-Brown P97 puller (Sutter). Under visual control with infrared illumination through a cooled CCD camera (Sensicam, Cooke, Auburn Hills, MI, USA), a pipette was advanced to the retina using a micromanipulator (MP 285, Sutter), and the inner limiting membrane was dissected to expose somas of several RGCs. RGCs with an elliptical soma were targeted as potential ON–OFF DSGCs with a pipette filled with Ames medium (2–4 MΩ). Gentle suction was applied to establish loose-patch configuration and spike activities were recorded. Using a flashing spot and a moving bar, the ON–OFF DSGC could be identified, its receptive field mapped and the preferred-null axis determined. In some cases, spike activity was also studied in whole-cell current clamp mode (intracellular solutions, mm: 120 potassium gluconate, 5 NaCl, 10 KCl, 1 MgCl2, 1 EGTA, 10 Hepes, 2 ATP, and 0.5 GTP, adjusted to pH 7.2 with 1 m KOH). For whole-cell voltage clamp recording, the extracellular pipette was replaced with a patch pipette with 4–7 MΩ tip resistance filled with intracellular solution (mm: 120 caesium methanesulphonate, 0.5 CaCl2, 5 EGTA, 10 Hepes, 4 ATP, 0.5 GTP, and 5 QX-314, adjusted to pH 7.2 with 1 m CsOH). Sometimes 0.5% neurobiotin (Molecular Probes, Eugene, OR, USA) and/or 0.1% Lucifer Yellow (Sigma) were added into the intracellular solution in order to reveal the dendritic morphology of the recorded cells. The whole-cell configuration was formed when the seal resistance was > 1 GΩ. Cell capacitance was always compensated. Serial resistance, which in most cases was < 20 MΩ, was not compensated. The liquid junction potential of 10 mV was corrected. Data acquired from the Axopatch 200B amplifier were low-pass filtered at 2 kHz, digitized simultaneously with an A/D converter (Digidata 1320A, Axon Instruments), and stored on a personal computer. Offline data analysis was performed using Clampfit (Axon Instruments) and Mini Analysis (Synaptosoft Inc, Leonia, NJ, USA), and plotted with OriginPro 7.0 (OriginLab Corp., Northampton, MA, USA).
Pharmacological studies
All drugs were freshly prepared from stock solutions stored at −20°C using Ames medium and applied through the perfusion system after control data were collected. These include: picrotoxin (100 μm), d-tubocurarine (50 μm), physostigmine (10 μm), bicuculline (10 μm), 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide (NBQX) (5 μm), and d-(-)2-amino-7-phosphonoheptanoic acid (AP-7) (200 μm). Picrotoxin, physostigmine, bicuculline and NBQX were predissolved in DMSO to form stock solutions. Picrotoxin, d-tubocurarine, and bicuculline were obtained from ICN Biomedicals Inc. (Aurora, OH, USA). All other reagents in this study were obtained from Sigma (St Louis, MO, USA). Only data from cells showing clear recovery were included in the analysis. In order to compare effects of various reagents on directionality, we evaluated the change of response (measured in spike number) to a rectangle drifting in the preferred and null directions, and used a DS index (DSI) defined as the difference of the preferred and null responses divided by the sum of the preferred and null responses.
Light stimulation
Stimuli were generated using a program written in VC++ and Directx8 SDK, displayed on a monitor (Sony E230) and focused onto the retina through microscope condenser. The brightness of the stimuli was about 0.35 × 1011 photons cm−2 s−1. Two types of light stimuli were generated: (1) a spot of 25–1000 μm in diameter flashing for 0.5–10 s, was used to determine the size of the receptive field and response polarity, and (2) a rectangular bar of 100 × 500 μm moving parallel to its long axis at 4–100 deg s−1 over 1500 μm and in 12 directions at 30° intervals was used to determine the directionality and velocity preference. The elongated bar allowed clear separation of leading edge and trailing edge responses.
Dendritic morphology
Retinas were fixed with 4% paraformaldehyde for 1 h, washed 3 times in 0.1 m phosphate buffer (pH 7.4) and incubated in streptavidin-FITC overnight to visualize recorded cells. Preparations were coverslipped with Vectorshield (Vector Laboratories, Inc., Burlingame, CA, USA), and sealed with nail polish. Images were collected using a Leica SP2 confocal microscope equipped with a 63 × PlanApo objective (N.A. 1.32). Contrast and brightness of images were adjusted using Photoshop 8.0 (Adobe).
Results
Light responses
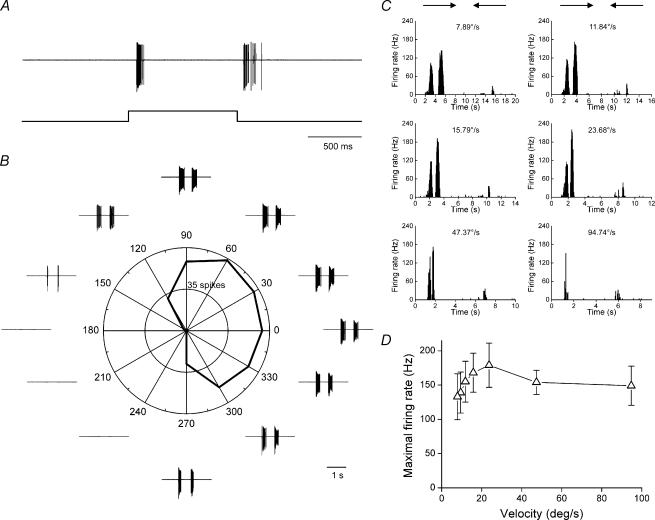
Electrophysiological recording in loose patch configuration demonstrated that ON–OFF DSGCs exist in the mouse retina. We recorded from 48 ON–OFF DSGCs in isolated, whole-mount mouse retina. Figure 1A shows the response of an ON–OFF DSGC to a stationary flashing spot: transient responses to both the onset and termination of the stimulus were clearly observable. A rectangular bar moving in 12 directions elicited clear directional responses in both polar plot and spike traces, as shown in Fig. 1B.
Figure 1. Light responses of an ON–OFF DSGC.
A, responses to a flashing spot, showing clear ON and OFF responses. B, responses to a rectangle moving in 12 directions; both the polar plot and spike traces demonstrate strong directional responses. C, responses to a rectangle moving back and forth in the preferred and null direction at different velocities. D, velocity tuning curve averaged over four ON–OFF DSGCs, revealing preference for medium velocities (means ± s.e.m.).
The velocity tuning properties of the ON–OFF DSGCs were also studied. Figure 1C shows responses of a DSGC to a rectangle drifting in the preferred and null directions at six different velocities, ranging from 8 to 95 deg s−1. Figure 1D shows the velocity-tuning curve, averaged over four DSGCs. As was found for the ON–OFF DSGCs in the rabbit retina (Wyatt & Daw, 1975), the ON–OFF DSGCs in the mouse retina responded to a wide range of velocities, and prefered medium velocity.
Overall, the ON–OFF DSGCs in the mouse retina exhibited almost identical physiology to the well-characterized ON–OFF DSGCs of the rabbit retina.
Pharmacological properties
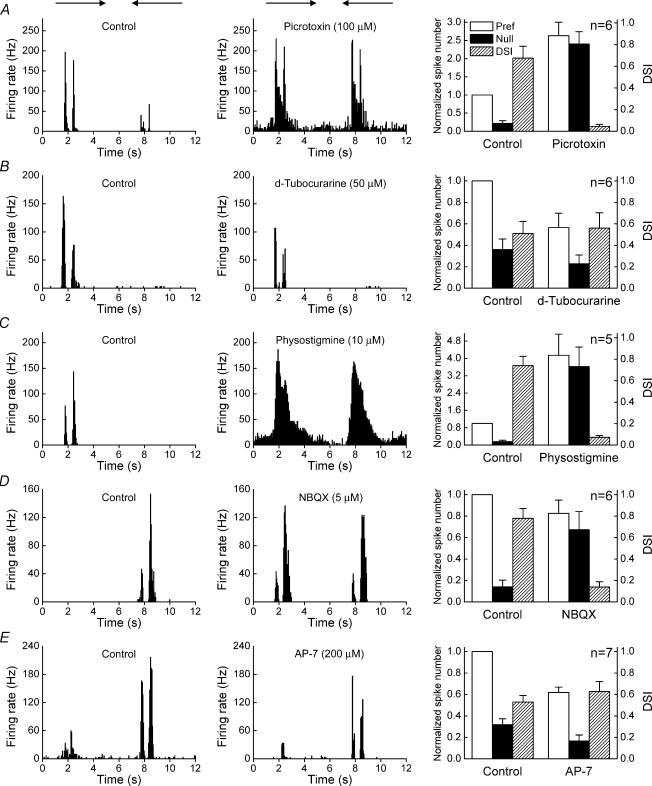
Previous studies on the rabbit retina have demonstrated that the asymmetrical inhibition critical for direction selectivity is mediated by GABA, via GABAA receptors (Wyatt & Daw, 1976; Caldwell et al. 1978; He & Masland, 1997; Kittila & Massey, 1997; Taylor et al. 2000). We found that picrotoxin, a GABA receptor antagonist, increased the amplitude of responses and completely abolished direction selectivity of the ON–OFF DSGCs of the mouse, reducing the direction-selective index (DSI) from 0.68 ± 0.11 to 0.05 ± 0.02 (Fig. 2A). Picrotoxin drove five cells to excitatory block (Supplementary material, on-line supplementary Fig. 1), and data from these cells were not included in the average shown on the right panel of Fig. 2A. The GABAA receptor specific antagonist, bicuculine, exhibited identical effects to picrotoxin (Supplementary material, on-line supplementary Fig. 2), confirming that the asymmetrical directional inhibition in mouse retina is mediated by GABAA receptors.
Figure 2. Pharmacological properties of ON–OFF DSGCs.
Left-hand panels: spike histograms for the control. Middle panels: histograms of the effect of drug application. Right-hand panels: comparison of the changing response strength and directional index. Responses were normalized to the averaged control responses to stimuli moving in the preferred direction and plotted as means ± s.e.m.
In the rabbit retina, physostigmine, an inhibitor of acytelcholinesterase, increases the amplitude of responses and abolishes direction selectivity, whereas the nicotinic ACh receptor antagonist, d-tubocurarine, reduces the amplitude of responses, but does not block direction selectivity (Ariel & Daw, 1982; He & Masland, 1997; Kittila & Massey, 1997). Here we found that similar to rabbit, d-tubocurarine reduced the amplitude of DSGC responses, but did not block direction selectivity (Fig. 2B), and the DSI remained almost unchanged (from 0.51 ± 0.11 to 0.56 ± 0.14, P = 0.79). Physostigmine increased the amplitude of responses, and abolished direction selectivity (the DSI dropped from 0.74 ± 0.09 to 0.07 ± 0.02) (Fig. 2C). Occasionally, physostigmine also drove the cell to excitatory block.
The ON–OFF DSGCs in the rabbit retina mainly contain nicotinic and NMDA receptors (Cohen & Miller, 1995; Kittila & Massey, 1997), while AMPA/KA receptors affect ON–OFF DSGCs through regulating ACh release of SAs or activity of other amacrine cells (Linn et al. 1991; Linn & Massey, 1991). The AMPA/KA specific antagonist, NBQX, reduced the amplitude of the responses and blocked direction selectivity (Fig. 2D), the DSI decreased from 0.78 ± 0.09 to 0.14 ± 0.05, whereas the NMDA antagonist, AP-7, reduced the amplitude of responses but left direction selectivity intact (Fig. 2E), and the DSI remained almost unchanged (from 0.53 ± 0.06 to 0.63 ± 0.09, P = 0.39).
In summary, the pharmacological properties of mouse ON–OFF DSGCs again appear almost identical to those of the ON–OFF DSGCs in the rabbit retina.
Synaptic mechanisms of direction selectivity
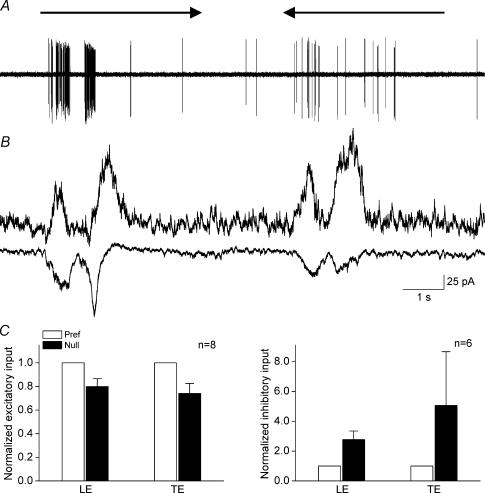
Voltage clamp experiments from eight ON–OFF DSGCs showed asymmetrical inputs elicited by a rectangle moving in the preferred and null directions. Spike responses of an ON–OFF DSGC to the preferred and null movement are shown in Fig. 3A. Holding the membrane potential at −65 mV to isolate excitatory inputs revealed a larger current when the stimulus was moving in the preferred direction, whereas holding the membrane potential at 0 mV to isolate inhibitory inputs revealed a larger current when the stimulus was moving in the null direction (Fig. 3B). Summarizing data from these cells reveals that inhibitory input was 3–5 times stronger when the stimulus was moving in the null direction, and the excitatory input was 20% weaker when the stimulus was moving in the null direction (Fig. 3C). These findings are also similar to what has been observed for the ON–OFF DSGCs in the rabbit retina (Taylor et al. 2000; Fried et al. 2002; Taylor & Vaney, 2002).
Figure 3. Spike and current responses of an ON–OFF DSGC to a rectangle moving in the preferred and null direction.
A, spike responses to movement in the preferred and null directions, showing clear direction selectivity. B, isolated excitatory inputs and inhibitory inputs when the membrane potential was held at −65 mV and 0 mV, respectively, showing larger excitatory inputs when the stimulus was moving in the preferred direction and larger inhibitory inputs when the stimulus was moving in the null direction. C, bar graphs comparing averaged input sizes measured as peak current induced by leading and trailing edges for movement in the preferred and null directions. Excitatory currents were about 20% stronger when the stimulus was moving in the preferred direction and inhibitory currents were 3–5 times stronger when the stimulus was moving in the null direction. Responses were normalized to the averaged peak responses to stimulus moving in the preferred direction and plotted as means ± s.e.m.
Dendritic morphology
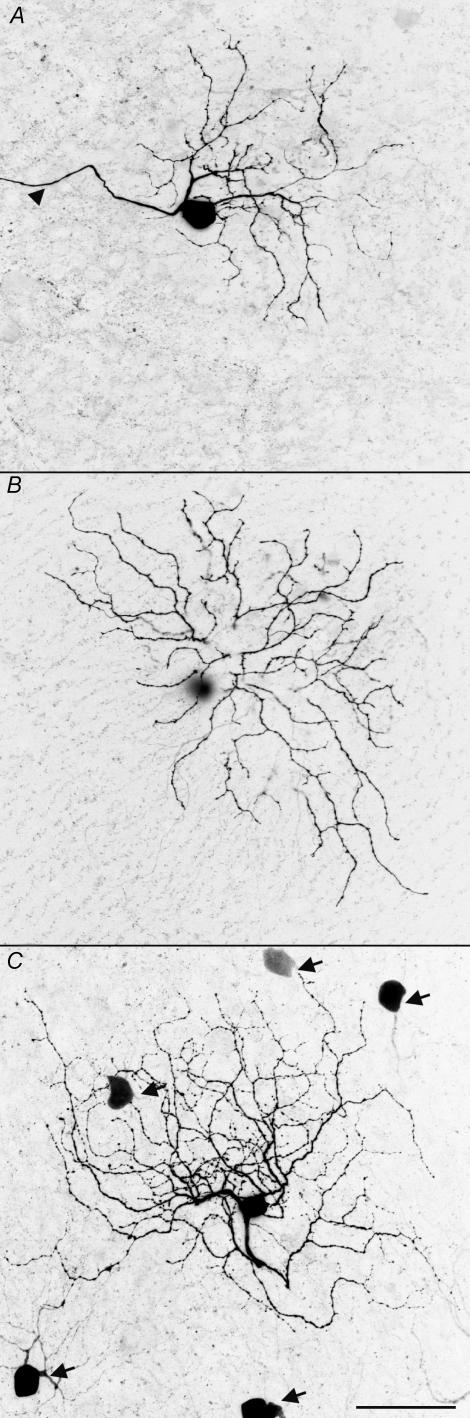
Visualization of tracer neurobiotin infused intracellularly during recording, revealed a bistratified dendritic morphology identical to that previously classified as RGD2 (Sun et al. 2002). As shown in Fig. 4A and B, dendritic ramifications were observed in the inner and outer part of the inner plexiform layer (IPL). This recursive and loop-forming pattern is strikingly similar to the dendritic morphology of rabbit ON–OFF DSGCs (Amthor et al. 1984; Amthor et al. 1989).
Figure 4. Dendritic morphology and coupling pattern.
The dendritic ramifications in the ON and OFF sublaminae are illustrated in A and B; arrowhead points to the axon. The somas coupled via gap junctions revealed by neurobiotin is shown in C, indicated with arrows. Scale bar: 50 μm.
A proportion of ON–OFF DSGCs in the rabbit retina have been shown to be coupled to neighbouring cells with gap junction (Vaney, 1991, 1994; Vaney & Pow, 2000). For 19 cells whose dendritic morphology was recovered, four showed clear tracer coupling revealed by visualization of neurobiotin (Fig. 4C). A ring of coupled somas was clearly visible surrounding the dendritic field of the recorded ON–OFF DSGC.
The dendritic morphology and coupling pattern are very similar to what has been documented for rabbit ON–OFF DSGCs.
Discussion
In this study, we investigated the physiological, morphological and pharmacological properties of ON–OFF DSGCs in the mouse retina. In every aspect, mouse retinal ON–OFF DSGCs behave almost identically to the ON–OFF DSGCs characterized in the rabbit retina. This first detailed characterization of ON–OFF DSGCs in the mouse retina provides fundamental information for further study of maturation and regulation of the neuronal circuitry underlying computation of direction.
Positive identification of ON–OFF DSGCs
Very little is known about the mouse retina: the literature contains only one paper describing the properties of ON–OFF DSGCs before and after toxin treatment to ablate SAs (Yoshida et al. 2001). Because that study used the extracellular recording technique, many properties of the ON–OFF DSGCs in the mouse retina, such as dendritic morphology and synaptic inputs, could not be determined.
When we surveyed mouse RGCs (Sun et al. 2002), we encountered two subtypes of bistratified RGCs. One of these, classified as RGD2, exhibits a striking resemblance to the denditic morphology of rabbit ON–OFF DSGCs in dendritic morphology (Amthor et al. 1984, 1989). We postulated that this subtype might have a directional response to moving stimuli (Sun et al. 2002). The present study confirms our proposal, and shows that the RGD2 cells indeed function as ON–OFF DSGCs in the mouse retina.
Conserved mechanisms for computing motion direction
The mechanism for retinal direction selectivity has been a subject of intensive research since its discovery in the rabbit retina four decades ago (Barlow & Hill, 1963). Barlow & Levick (1965) elegantly demonstrated that the fundamental mechanism for direction selectivity is a spatially asymmetrical inhibition propagating in the null direction. This finding was further substantiated by demonstration of a cardioid-shaped inhibitory zone displaced to the null side (Wyatt & Daw, 1975). This directional inhibition was shown to be mediated by GABA through GABAA receptors (Wyatt & Daw, 1976; Caldwell et al. 1978; Kittila & Massey, 1997). Here we showed that GABAA receptor antagonists, picrotoxin and bicuculline, completely abolish the directionality of ON–OFF DSGCs in the mouse retina, indicating that directional inhibition also mediated by GABA through GABAA receptors.
In order to generate directional responses, the inhibition modulates excitatory inputs to the DSGCs. It has been shown that the excitatory inputs to DSGCs are mediated by two neurotransmitters: ACh, via nicotinic receptors, and glutamate, via NMDA receptors. Nicotinic receptors have been shown to be located directly on the DSGCs and to mediate about 30–50% of excitatory inputs (Masland & Ames, 1976; Ariel & Daw, 1982; Kittila & Massey, 1997), while the rest of excitatory inputs have been shown to be mediated by NMDA receptors (Cohen & Miller, 1995; Kittila & Massey, 1997). Asymmetry in excitatory input to DSGCs was only recently demonstrated, by voltage clamp experiments (Fried et al. 2002; Taylor & Vaney, 2002).
Despite some structural differences compared with other mammals, such as possessing only one type of horizontal cell (Peichl & Gonzalez-Soriano, 1993; He et al. 2000), the mouse retina appears to utilize exactly the same strategy to compute motion direction. This observation indicates the dendritic architecture and connection within the IPL is important for DS computation. The ON–OFF DSGCs in the cat retina (Cleland & Levick, 1974) are morphologically very similar to those in rabbit and mouse (Berson et al. 1997). We therefore expect that the mechanisms for computing motion direction are similar in cat, and are probably conserved throughout mammalian species.
Development of circuitry for motion computation
Morphologically distinguishable RGD2 cells are among the first cells to display adult morphological characteristics in early postnatal development. Among 215 ganglion cells we examined at postnatal day 3 (P3), four cells already exhibited clear characteristics resembling adult RGD2 cells (Diao et al. 2004). Examining the relationship between RGC dendrites and the cholinergic plexus reveals that large bistratified cells (equivalent to our RGD2 cells) begin to contact the cholinergic plexus as early as P3, and the number of contacts increases with development, whereas the number of contacts between large monostrifitied cells (equivalent to our RGA cells) and the cholinergic plexus decreases with development (Stacy & Wong, 2003).
It is conceivable that spontaneous wave activity in the retina mediated by ACh (Meister et al. 1991; Wong, 1999; Zhou & Zhao, 2000; Zhou, 2001) may play a role in regulation of circuitry formation because of the close relationship between DSGC and SA processes during early development. It has been shown that in mice lacking nicotinic receptors, the pattern of spontaneous activity is dramatically changed, with more diffuse dendritic fields of the RGCs at P7/8, which then returned to normal at P14 (Bansal et al. 2000). If ACh does play a role in regulating maturation of retinal circuitry, DSGCs are a good candidate to allow detection of cholinergic regulation, and the influence is likely to be most obvious early in development.
Acknowledgments
This project was supported by a MOST Major State Basic Research Program Grant to the Institute of Neuroscience (G2000077800), an NSFC Outstanding Young Researcher Award 39925010 and NSFC project grants 30170305 and 30270460 to S.H. We thank Dr Sarah Perrett for helpful comments on style and grammar and Yingye Zhang and Weiqi Xu for technical support.
Supplementary material
The online version of this paper can be accessed at: 10.1113/jphysiol.2004.076695 http://jp.physoc.org/cgi/content/full/jphysiol.2004.076695/DC1 and contains two supplementary figures showing that picrotoxin drives ON-OFF OSGC to excitatory block (Fig. 1) and that the GABA specific antagonist, bicucalline, blocks direction selectivity.
This material can also be found at: http://www.blackwellpublishing.com/products/journals/suppmat/tjp/tjp676/tjp676sm.htm
References
- Amthor FR, Keyser KT, Dmitrieva NA. Effects of the destruction of starburst-cholinergic amacrine cells by the toxin af64a on rabbit retinal directional selectivity. Vis Neurosci. 2002;19:495–509. doi: 10.1017/s0952523802194119. 10.1017/S0952523802194119. [DOI] [PubMed] [Google Scholar]
- Amthor FR, Oyster CW, Takahashi ES. Morphology of on-off direction-selective ganglion cells in the rabbit retina. Brain Res. 1984;298:187–190. doi: 10.1016/0006-8993(84)91167-3. 10.1016/0006-8993(84)91167-3. [DOI] [PubMed] [Google Scholar]
- Amthor FR, Takahashi ES, Oyster CW. Morphologies of rabbit retinal ganglion cells with complex receptive fields. J Comp Neurol. 1989;280:97–121. doi: 10.1002/cne.902800108. 10.1002/cne.902800108. [DOI] [PubMed] [Google Scholar]
- Ariel M, Daw NW. Pharmacological analysis of directionally sensitive rabbit retinal ganglion cells. J Physiol. 1982;324:161–185. doi: 10.1113/jphysiol.1982.sp014105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal A, Singer JH, Hwang BJ, Xu W, Beaudet A, Feller MB. Mice lacking specific nicotinic acetylcholine receptor subunits exhibit dramatically altered spontaneous activity patterns and reveal a limited role for retinal waves in forming on and off circuits in the inner retina. J Neurosci. 2000;20:7672–7681. doi: 10.1523/JNEUROSCI.20-20-07672.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow HB, Hill RM. Selective sensitivity to direction of motion in ganglion cells of the rabbit's retina. Science. 1963;139:412–414. doi: 10.1126/science.139.3553.412. [DOI] [PubMed] [Google Scholar]
- Barlow HB, Levick WR. The mechanism of directionally selective units in rabbit's retina. J Physiol. 1965;178:477–504. doi: 10.1113/jphysiol.1965.sp007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson DM, Isayama T, Pu M. Morphology of presumed on-off direction selective ganglion cell of cat retina. Soc Neurosci Abstr. 1997;23:730. [Google Scholar]
- Caldwell JH, Daw NW, Wyatt HJ. Effects of picrotoxin and strychnine on rabbit retinal ganglion cells: Lateral interactions for cells with more complex receptive fields. J Physiol. 1978;276:277–298. doi: 10.1113/jphysiol.1978.sp012233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland BG, Levick WR. Properties of rarely encountered types of ganglion cells in the cat's retina and an overall classification. J Physiol. 1974;240:457–492. doi: 10.1113/jphysiol.1974.sp010618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ED, Miller RF. Quinoxalines block the mechanism of directional selectivity in ganglion cells of the rabbit retina. Proc Natl Acad Sci U S A. 1995;92:1127–1131. doi: 10.1073/pnas.92.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao L, Sun W, Deng Q, He S. Development of the mouse retina: Emerging morphological diversity of the ganglion cells. J Neurobiol. 2004;61:236–249. doi: 10.1002/neu.20041. 10.1002/neu.20041. [DOI] [PubMed] [Google Scholar]
- Dong W, Sun W, Zhang Y, Chen X, He S. Dendritic relationship between starburst amacrine cells and direction selective ganglion cells in the rabbit retina. J Physiol. 2004;556:11–17. doi: 10.1113/jphysiol.2004.060715. 10.1113/jphysiol.2004.060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Detwiler PB, Denk W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature. 2002;418:845–852. doi: 10.1038/nature00931. 10.1038/nature00931. [DOI] [PubMed] [Google Scholar]
- Fried SI, Munch TA, Werblin FS. Mechanisms and circuitry underlying directional selectivity in the retina. Nature. 2002;420:411–414. doi: 10.1038/nature01179. 10.1038/nature01179. [DOI] [PubMed] [Google Scholar]
- He S, Dong W, Deng Q, Weng S, Sun W. Seeing more clearly: Recent advances in understanding retinal circuitry. Science. 2003;302:408–411. doi: 10.1126/science.1085457. 10.1126/science.1085457. [DOI] [PubMed] [Google Scholar]
- He S, Masland RH. Retinal direction selectivity after targeted laser ablation of starburst amacrine cells. Nature. 1997;389:378–382. doi: 10.1038/38723. 10.1038/38723. [DOI] [PubMed] [Google Scholar]
- He S, Weiler R, Vaney DI. Endogenous dopaminergic regulation of horizontal cell coupling in the mammalian retina. J Comp Neurol. 2000;418:33–40. doi: 10.1002/(sici)1096-9861(20000228)418:1<33::aid-cne3>3.0.co;2-j. 10.1002/(SICI)1096-9861(20000228)418:1<33::AID-CNE3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Kittila CA, Massey SC. Pharmacology of directionally selective ganglion cells in the rabbit retina. J Neurophysiol. 1997;77:675–689. doi: 10.1152/jn.1997.77.2.675. [DOI] [PubMed] [Google Scholar]
- Linn DM, Blazynski C, Redburn DA, Massey SC. Acetylcholine release from the rabbit retina mediated by kainate receptors. J Neurosci. 1991;11:111–122. doi: 10.1523/JNEUROSCI.11-01-00111.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn DM, Massey SC. Acetylcholine release from the rabbit retina mediated by NMDA receptors. J Neurosci. 1991;11:123–133. doi: 10.1523/JNEUROSCI.11-01-00123.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland RH, Ames A., 3rd Responses to acetylcholine of ganglion cells in an isolated mammalian retina. J Neurophysiol. 1976;39:1220–1235. doi: 10.1152/jn.1976.39.6.1220. [DOI] [PubMed] [Google Scholar]
- Meister M, Wong RO, Baylor DA, Shatz CJ. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991;252:939–943. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- Peichl L, Gonzalez-Soriano J. Unexpected presence of neurofilaments in axon-bearing horizontal cells of the mammalian retina. J Neurosci. 1993;13:4091–4100. doi: 10.1523/JNEUROSCI.13-09-04091.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacy RC, Wong RO. Developmental relationship between cholinergic amacrine cell processes and ganglion cell dendrites of the mouse retina. J Comp Neurol. 2003;456:154–166. doi: 10.1002/cne.10509. 10.1002/cne.10509. [DOI] [PubMed] [Google Scholar]
- Sun W, Li N, He S. Large-scale morphological survey of mouse retinal ganglion cells. J Comp Neurol. 2002;451:115–126. doi: 10.1002/cne.10323. 10.1002/cne.10323. [DOI] [PubMed] [Google Scholar]
- Taylor WR, He S, Levick WR, Vaney DI. Dendritic computation of direction selectivity by retinal ganglion cells. Science. 2000;289:2347–2350. doi: 10.1126/science.289.5488.2347. 10.1126/science.289.5488.2347. [DOI] [PubMed] [Google Scholar]
- Taylor WR, Vaney DI. Diverse synaptic mechanisms generate direction selectivity in the rabbit retina. J Neurosci. 2002;22:7712–7720. doi: 10.1523/JNEUROSCI.22-17-07712.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WR, Vaney DI. New directions in retinal research. Trends Neurosci. 2003;26:379–385. doi: 10.1016/S0166-2236(03)00167-X. 10.1016/S0166-2236(03)00167-X. [DOI] [PubMed] [Google Scholar]
- Vaney DI. Many diverse types of retinal neurons show tracer coupling when injected with biocytin or neurobiotin. Neurosci Lett. 1991;125:187–190. doi: 10.1016/0304-3940(91)90024-n. 10.1016/0304-3940(91)90024-N. [DOI] [PubMed] [Google Scholar]
- Vaney DI. Territorial organization of direction-selective ganglion cells in rabbit retina. J Neurosci. 1994;14:6301–6316. doi: 10.1523/JNEUROSCI.14-11-06301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaney DI, He S, Taylor WR, Levick WR. Direction selective ganglion cells in the retina. In. In: Zanker JM, Zeil J, editors. Motion Vision. Berlin: Springer; 2001. pp. 13–56. [Google Scholar]
- Vaney DI, Pow DV. The dendritic architecture of the cholinergic plexus in the rabbit retina: Selective labeling by glycine accumulation in the presence of sarcosine. J Comp Neurol. 2000;421:1–13. 10.1002/(SICI)1096-9861(20000522)421:1<1::AID-CNE1>3.3.CO;2-8. [PubMed] [Google Scholar]
- Vaney DI, Taylor WR. Direction selectivity in the retina. Curr Opin Neurobiol. 2002;12:405–410. doi: 10.1016/s0959-4388(02)00337-9. 10.1016/S0959-4388(02)00337-9. [DOI] [PubMed] [Google Scholar]
- Wong RO. Retinal waves and visual system development. Annu Rev Neurosci. 1999;22:29–47. doi: 10.1146/annurev.neuro.22.1.29. 10.1146/annurev.neuro.22.1.29. [DOI] [PubMed] [Google Scholar]
- Wyatt HJ, Daw NW. Directionally sensitive ganglion cells in the rabbit retina: Specificity for stimulus direction, size, and speed. J Neurophysiol. 1975;38:613–626. doi: 10.1152/jn.1975.38.3.613. [DOI] [PubMed] [Google Scholar]
- Wyatt HJ, Daw N. Specific effects of neurotransmitter antagonists on ganglion cells in rabbit retina. Science. 1976;191:204–205. doi: 10.1126/science.1857. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Watanabe D, Ishikane H, Tachibana M, Pastan I, Nakanishi S. A key role of starburst amacrine cells in originating retinal directional selectivity and optokinetic eye movement. Neuron. 2001;30:771–780. doi: 10.1016/s0896-6273(01)00316-6. 10.1016/S0896-6273(01)00316-6. [DOI] [PubMed] [Google Scholar]
- Zhou ZJ. The function of the cholinergic system in the developing mammalian retina. Prog Brain Res. 2001;131:599–613. doi: 10.1016/s0079-6123(01)31047-6. [DOI] [PubMed] [Google Scholar]
- Zhou ZJ, Zhao D. Coordinated transitions in neurotransmitter systems for the initiation and propagation of spontaneous retinal waves. J Neurosci. 2000;20:6570–6577. doi: 10.1523/JNEUROSCI.20-17-06570.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.