Abstract
The bladder urothelium exhibits dynamic sensory properties that adapt to changes in the local environment. These studies investigated the localization and function of bradykinin receptor subtypes B1 and B2 in the normal and inflamed (cyclophosphamide (CYP)-induced cystitis) bladder urothelium and their contribution to lower urinary tract function in the rat. Our findings indicate that the bradykinin 2 receptor (B2R) but not the bradykinin 1 receptor (B1R) is expressed in control bladder urothelium. B2R immunoreactivity was localized throughout the bladder, including the urothelium and detrusor smooth muscle. Bradykinin-evoked activation of this receptor elevated intracellular calcium (EC50 = 8.4 nm) in a concentration-related manner and evoked ATP release from control cultured rat urothelial cells. In contrast, B1R mRNA was not detected in control rat urinary bladder; however, following acute (24 h) and chronic (8 day) CYP-induced cystitis in the rat, B1R mRNA was detected throughout the bladder. Functional B1Rs were demonstrated by evoking ATP release and increases in [Ca2+]i in CYP (24 h)-treated cultured rat urothelial cells with a selective B1 receptor agonist (des-Arg9-bradykinin). Cystometry performed on control anaesthetized rats revealed that intravesical instillation of bradykinin activated the micturition pathway. Attenuation of this response by the P2 receptor antagonist PPADS suggests that bradykinin-induced micturition facilitation may be due in part to increased purinergic responsiveness. CYP (24 h)-treated rats demonstrated bladder hyperactivity that was significantly reduced by intravesical administration of either B1 (des-Arg10-Hoe-140) or B2 (Hoe-140) receptor antagonists. These studies demonstrate that urothelial expression of bradykinin receptors is plastic and is altered by pathology.
The bladder urothelium, which was initially perceived to act only as a passive barrier, is now known to exhibit dynamic sensory properties that convey information regarding the local environment to underlying afferent fibres (Ferguson, 1999; Cockayne et al. 2000; Birder et al. 2001). Urothelial cells express a number of receptors, including transient receptor potential vanilloid subtype 1 (TRPV1) (Birder et al. 2001), adrenoceptors (Birder et al. 1998), purinoceptors (Ferguson et al. 1997; Elneil et al. 2001) and prostanoids (Maggi, 1992), that can transduce mechanical, thermal and chemical stimuli (Birder et al. 2001). Activation of these receptors can evoke urothelial release of neurotransmitters such as nitric oxide (Birder et al. 1998) and/or ATP (Ferguson et al. 1997; Cockayne et al. 2000), which can act in a paracrine manner to sensitize/activate the afferent fibres.
Chronic inflammatory bladder conditions such as interstitial cystitis are in most cases characterized by acute pelvic pain and increased urinary frequency/urgency (Bouchelouche & Nordling, 2003). The underlying causes of many of these conditions are unclear but various factors are thought to be involved including infection (Keay & Warren, 2002), neural factors (Habler et al. 1990; Yoshimura & de Groat, 1999) and the presence of irritants within the urine (Parsons et al. 1998, 2000). Moreover, increased urothelial permeability resulting from disruption of the glycosaminoglycan layer may occur during inflammation, allowing constituents of urine to evoke irritation/inflammation within the bladder wall (Parsons, 1994; Lavelle et al. 2000; Apodaca et al. 2003). Indeed, urine analysis of patients with interstitial cystitis has revealed elevated levels of proinflammatory mediators such as kinins (Rosamilia et al. 1994), leukotriene E4 (Bouchelouche et al. 2001) and interleukin-6 (Erickson et al. 2001).
Protease activation at the site of inflammation/tissue damage cleaves tissue/plasma kininogen precursors to release the nonapeptide, bradykinin (Dray & Perkins, 1993; Calixto et al. 2000). The physiological actions of bradykinin are mediated by activation of the B1 and/or B2 receptors. Bradykinin B2 receptors are expressed in a wide range of tissues and block of this receptor reduces inflammatory hyperalgesia in animal models (Wirth et al. 1991; Asano et al. 1997). In contrast the B1 receptors are expressed at low levels under normal conditions but expression is up-regulated following tissue damage/inflammation (Ahluwalia & Perretti, 1999).
Bradykinin has been reported to play an important role in normal and pathological conditions of urinary bladder function (Lecci et al. 1995; Maggi et al. 1993). Activation of the bradykinin B2 receptor stimulates detrusor muscle contractility (Meini et al. 2000) and evokes bladder hyperreflexia (Lecci et al. 1995; Meini et al. 2000). These effects on bladder contractility may occur via direct activation of pelvic afferent fibres (Lecci et al. 1995). Previous studies have also reported that the B1 receptor is up-regulated in chemically induced cystitis of the urinary bladder (Marceau et al. 1980). However, the cellular localization and regulation of bradykinin receptor function in the urinary bladder remain unclear. The aims of the present study were to investigate the localization and function of bradykinin receptors in the normal and inflamed (cyclophosphamide-induced cystitis) bladder urothelium and their contribution to lower urinary tract function.
Methods
Animals and chemically induced cystitis
Adult female Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA, USA; 250–350 g) were used in all experiments. The animals were maintained in a 12 h: 12 h light–dark cycle. Cyclophosphamide (CYP) was injected i.p. to produce a model of chemically induced cystitis. CYP is hepatically metabolized to acrolein, which is excreted into the urine and causes urinary bladder inflammation (Cox, 1979). Two periods of CYP-induced chemical cystitis were evaluated, ranging from acute (24 h; 150 mg kg−1i.p.) to chronic (8 days; 75 mg kg−1i.p. every 3 days). Control animals received a corresponding volume of physiological saline (0.9%i.p.). Histological analysis of bladders excised from CYP-treated rats, displayed characteristics commonly associated with inflammation, macroscopically and microscopically, including an increase in bladder weight and infiltration of white blood cells, consistent with previous studies (Hu et al. 2003). Urodynamically, acute CYP treatment induced bladder irritation, resulting in bladder hyperreflexia (discussed below). All procedures were conducted in accordance with Institutional Animal Care and Use Committee policies for the University of Pittsburgh. Cystometry was performed on animals anaesthetized with a subcutaneous injection of urethane (1.2 g kg−1, Sigma-Aldrich, St Louis, MO, USA).
Reverse transcription-PCR
Adult female Sprague-Dawley rats (250–350 g) were killed by inhalation of medical grade CO2 followed by thorocotomy and cardiac puncture. Urinary bladders were removed and total RNA was extracted from control (untreated) and CYP-treated (24 h and 8 day) rat bladders (n = 3 each group) using TriZol reagent (Life Technologies, Burlington, Ontario, Canada). For each bladder set, the urothelium was rapidly microdissected from the underlying smooth muscle in Hank's balanced salt solution (HBSS) containing protease inhibitors (Roche Diagnostics, Indianapolis, IN, USA) and tissues were pooled, prior to RNA extraction. cDNA synthesis was performed with First Strand Synthesis kit (Roche Diagnostics) using random oligomers as primers. PCR was completed using PCR Master Mix kit (Qiagen, Valencia, CA, USA) using the following primers. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH): TGCCACTCAGAAGACTGTGG (left), GGATGCAGGGATGATGTTCT (right). B1: TTTTCTATCCGGACCACAG (left), CAAGAGGCCAAAGAAGCAG (right). B2: CGAGAGGTGTACCAGCAAT (left), CAGGCTGAGAAGCTCAGGTC (right). PCR was initially conducted with a temperature gradient to determine ideal annealing temperatures. Following temperature optimization, a second round of PCR was performed to determine the appropriate number of cycles in order to obtain product in the linear phase of amplification. The ideal conditions were as follows. B1: 60°C, 25 cycles; B2: 60°C, 20 cycles; GAPDH: 60°C, 20 cycles. Products were visualized on a 1.2% agarose gel stained with ethidium bromide. Bradykinin bands were normalized to the GAPDH standard using TotalLab (Nonlinear Dynamics Inc, Durham, NC, USA). Positive results were sent for confirmation of sequence (University of Pittsburgh Sequencing Core Facility).
Immunocytochemistry
Adult female Sprague-Dawley rats (250–350 g) were killed by inhalation of medical grade CO2 followed by thorocotomy and cardiac puncture. Urinary bladders were rapidly excised, embedded in OCT Tissue-Tek (Sakura Finetek, Torrance, CA, USA), frozen over liquid nitrogen and stored at –20°C prior to use. Frozen urinary bladder sections (10–12 μm) were cut using a cryostat (Hacker-Bright Instruments, Fairfield, NJ, USA), mounted onto microscope slides and air-dried. Tissue sections were then fixed using 2% paraformaldehyde and washed in phosphate-buffered saline (PBS). Tissue sections were placed in a tissue-permeabilizing solution (0.5% Triton X-100 and 10% donkey serum) and washed in PBS prior to incubation in primary antisera.
Tissue sections were incubated in a goat-polyclonal anti-rat bradykinin B2 receptor antibody (10% goat serum in PBS; 1: 500, Santa Cruz Biotechnology, CA, USA; 4°C overnight). Primary antibodies were removed and tissue sections washed in PBS prior to incubation in fluorescein isothiocyanate (FITC)-conjugated anti-goat IgG (1: 500, Jackson Imunochemicals, West Grove, PA, USA) for 2 h at room temperature. Tissue sections were then washed in PBS and mounted with glass coverslips using a glycerol-based aqueous antifade mountant, Citifluor (Ted Pella Inc., Redding, CA, USA). In control experiments, primary antisera were preincubated with the corresponding blocking peptide (10 μg ml−1 at 4°C overnight; Santa Cruz Biotechnology) before application. In all cases, the blocking peptide reduced B2 receptor antibody labelling to background levels.
Urothelial cell culture
The preparation of rat urothelial cultures has been previously described (Birder et al. 1998, 2001; Truschel et al. 1999). In brief, Sprague-Dawley rats were killed as described above and urinary bladders were rapidly excised, gently stretched (urothelial side up) and incubated overnight in Dulbecco's modified Eagle's medium (DMEM) containing penicillin–streptomycin–fungizone and dispase (2.5 mg ml−1; Invitrogen, Carlsbad, CA, USA). The urothelium was then gently scraped, treated with trypsin-EDTA (0.25%, Invitrogen) and following gentle trituration, resuspended in serum-free keratinocyte media (Invitrogen). The cell suspension was plated (100 μl, 50 000 cells ml−1) onto collagen-coated glass coverslips and media changed after 2 days. For each urothelial culture, at least three rat bladders were required to achieve the sufficient numbers of dissociated cells for subsequent in vitro assays described below. The majority of cultured urothelial cells were cytokeratin 17 positive (DAKO, Carpentaria, CA, USA) and regarded as from epithelial origin, as previously reported (Birder et al. 2002a).
Estimation of changes in intracellular calcium, [Ca2+]i
Cultured rat urothelial cells (18–72 h following plating) were incubated with the fluorescent Ca2+ indicator fura-2-acetoxymethyl ester (fura-2-AM) (5 μm; Molecular Probes, Eugene, OR, USA) in HBSS-containing bovine serum albumin (5 mg ml−1) for 30 min at 37°C in an atmosphere of 5% CO2. Cells were washed in HBSS (containing (mm): NaCl 138, KCl 5, KH2PO4 0.3, NaHCO3 4, CaCl2 2, MgCl2 1, Hepes 10, glucose 5.6; pH = 7.35, titrated with NaOH, 310 mosmol l−1), transferred to a perfusion chamber and mounted onto an epifluorescence microscope (Olympus IX70). In Ca2+-free HBSS, the Ca2+ was substituted with NaCl (2 mm) and EGTA (0.5 mm). Measurement of [Ca2+]i was performed by ratiometric imaging of fura-2-AM at 340 and 380 nm (100 Hz) and the emitted light monitored at 510 nm. The fluorescence ratio, F340/F380 was calculated and acquired by C-Imaging systems (Compix Inc., Cranberry, PA, USA) and background fluorescence subtracted. All test agents were bath applied (flow rate = 1.5 ml min−1); for antagonist studies, urothelial cells were pretreated for at least 10 min prior to further agonist application. Data were obtained from at least three independent urothelial cultures and from at least six sets of experiments from each culture. Data were analysed using Student's unpaired t test and expressed as a mean percentage of the maximum response (± s.e.m.) to ATP (10 μm). In order to normalize the changes in intracellular calcium observed following B1R and B2R agonist application, ATP (10 μm) was used as a control. ATP produced a consistent and reproducible change in [Ca2+]i in > 95% of urothelial cells tested.
Measurement of ATP release
Cultured rat urothelial cells were transferred into a perfusion chamber and superfused with an oxygenated Krebs solution (containing (mm): KCl 4.8, NaCl 120, MgCl2 1, CaCl2 2, glucose 11 and Hepes 10, pH 7.4) at room temperature (flow rate = 1 ml min−1), until stable basal baseline levels of ATP release were obtained. All test agents were bath applied for antagonist studies; urothelial cells were pretreated for at least 10 min prior to further agonist application. Perfusate was collected (100 μl) at 30 s intervals following agonist stimulation and ATP levels quantified using a luciferin–luciferase reagent (ATP assay, Sigma-Aldrich). All data were normalized with respect to the maximum ATP released following application of the calcium ionophore A23187 (3 μm) at the end of each experiment. Data were obtained from at least three independent cultures and at least n = 3 from each culture. Data were expressed as mean ± s.e.m., analysed using Student's unpaired t test and statistical significance accepted when P < 0.05.
Permeability measurements
Permeability studies were performed as previously described (Lavelle et al. 2002). Briefly, normal rat and CYP (24 h)-treated bladders were rapidly excised after the animals were killed (as described above). The bladders were bisected and placed immediately into Ringer solution (containing (mm): NaCl 111.2, NaHCO3 25, KCl 5.8, CaCl2 2, MgSO4 1.2, KH2PO4 1.2 and glucose 11). The solution was oxygenated and maintained at 37°C and pH 7.2–7.5. The bladders were placed on a rack with the epithelium facing downward, stretched on a 0.73 cm2 ring and held in place by a set of pins located away from the area of investigation. The tissue was mounted between two halves of an Ussing chamber as described (Lavelle et al. 2002) and the chamber filled with Ringer solution. The temperature of the chamber was maintained at 37°C and the hemichambers were constantly stirred. Electrical measurements of transepithelial resistance (TER) were performed throughout the experiments using a four-electrode current/voltage clamp (Warner Instruments, Hamsden, CT, USA) as previously described (Truschel et al. 1999) to determine epithelial integrity. The membranes were allowed to stabilize for 1 h prior to addition of isotopes and measurements.
[3H]Water (1 μCi ml−1) and [14C]urea (0.25 μCi ml−1) were added to the apical side of the membrane and both hemichambers were sampled (2 × 100 μl per hemichamber) at 15 min intervals throughout the experiment. To determine the contribution of unstirred layers to the measured permeabilities, the apical membranes were destroyed by the addition of Triton X-100 (100 μl). In all experiments, Triton X-100 abolished the TER.
Experimental results for each rat were determined by averaging the four flux measurements during each experimental stage. Flux rates were obtained before and after permeabilization of the epithelium by Triton X-100. The electrical resistance values represent the means of at least five recordings taken during the course of each experiment. Results are expressed as mean ± s.e.m. and analysed using Student's t test for unpaired samples. Statistical significance was accepted when P < 0.05.
Continuous infusion cystometry
Female Sprague-Dawley rats (250–350 g) were anaesthetized with urethane (1.2 g kg−1 s.c.). The urinary bladder was exposed by a midline abdominal incision. A catheter (PE-50) was inserted through the apex of the bladder dome and connected with a T-stopcock to an infusion pump and pressure transducer. Continuous cystometry was performed by constant infusion of physiological saline (0.04 ml min−1) into the bladder to elicit repetitive voidings. Saline infusion was continued for at least 1 h to achieve a stable baseline prior to continuous infusion of bradykinin (100 μm) or the purinergic antagonist pyridoxal phosphate-6-azo(benzene-2,4,-disulphonic acid) tetrasodium salt (PPADS, 10 mm). Although lower concentrations of bradykinin were initially tested, the higher concentration resulted in the most reproducible findings and was used throughout the course of these studies. Various urodynamic parameters, including bladder contraction amplitude, frequency of non-voiding contractions (intravesical pressure waves whose amplitudes were less than 15 cmH2O and did not result in obvious voiding) as well as intercontraction intervals (ICIs) were recorded. All data are presented as mean ± s.e.m. and analysed using Student's t test for unpaired samples. Statistical significance was accepted when P < 0.05.
Materials
All standard chemicals were obtained from Sigma-Aldrich or Fisher, and were either analytical or laboratory grade. [14C]Urea and [3H]water were obtained from American Radiolabeled Chemicals (St Louis, MO, USA).
Results
Bradykinin B1 and B2 receptor mRNA expression in normal and CYP-inflamed rat urinary bladder
Two bradykinin receptor subtypes (B1 and B2) have been cloned (see Dray & Perkins, 1993, for review). RT-PCR was conducted to determine whether the mRNA for either of these receptors was expressed in normal and CYP-inflamed urinary bladders. No RNA signal was detected for B1R in tissue homogenates from the urothelium or detrusor smooth muscle of control animals (Fig. 1A, upper panel), whereas relatively high levels of B2R mRNA were present (Fig. 1A, lower panel). Following acute CYP-induced cystitis (24 h), the levels of B2R mRNA were unchanged in rat urothelium but a reduction was evident in detrusor smooth muscle (Fig. 1B). This is in contrast to chronic CYP (8 day)-induced cystitis where the levels of B2R were reduced in both the urothelium and detrusor smooth muscle. B1R mRNA was up-regulated in both urothelium and detrusor smooth muscle following acute CYP-induced cystitis. Following chronic CYP-induced cystitis, the expression of B1R mRNA in the urothelium was also elevated over control to approximately the same extent as in acute inflammation. However, the levels of B1R mRNA in the smooth muscle were undetectable following chronic inflammation (Fig. 1B).
Figure 1. Bradykinin B1 and B2 receptor mRNA expression in the rat urinary bladder.
A, time course of changes in bradykinin B1 and B2 receptor mRNA in the urinary bladder detrusor smooth muscle and urothelium in normal and cyclophosphamide (CYP)-treated rats (24 h and 8 day (8 d) treatments). GAPDH was used as a control. B, relative product strengths of B1 and B2 receptor mRNA determined by densitometry (density of product/density of GAPDH) in normal and CYP-treated rats. ND = none detected.
Bradykinin B2 receptor immunoreactivity in the rat urinary bladder
Immunocytochemical studies revealed the expression of the bradykinin B2 receptor in normal rat urinary bladder (Fig. 2). B2R expression was present in the bladder urothelium and detrusor smooth muscle. In the urothelium B2R expression was evident in the apical and basal layers and restricted primarily to the plasma membrane and cytoplasm (Fig. 2B and C). Preabsorption of the antibody with the antigenic C-terminal peptide completely abolished the labelling of the primary antibody (Fig. 2D).
Figure 2. Bradykinin B2 receptor immunocytochemistry in the rat urinary bladder.
A, bradykinin B2 receptor expression was distributed in the rat urinary bladder, with staining evident in the urothelium (white arrows). B, B2 receptor expression was present in both the underlying basal and apical cells of the urothelium (white arrows) and detrusor smooth muscle (asterisk). C, B2 receptor immunoreactivity was present predominantly on the plasma membrane and cytoplasmically. D, preabsorption of the B2 receptor antibody with the C-terminal antigenic peptide reduced staining to background levels.
Characterization of the bradykinin-evoked changes in [Ca2+]i in cultured rat urothelial cells
It is well recognized that bradykinin is one of the most potent algogenic compounds synthesized following tissue trauma, and has been strongly implicated in many events observed during inflammatory processes (see Dray & Perkins, 1993, for review). In this series of experiments, bath applied bradykinin produced a rapid increase in [Ca2+]i in control cultured rat urothelial cells (Fig. 3). The response typically reached a peak 1 min after bradykinin application and fully recovered to baseline 2–3 min after drug application. Approximately 30% of cells tested responded with an elevation in [Ca2+]i following application of bradykinin (30 nm, 40/139 cells tested). This response was present in the absence of extracellular calcium, suggesting that the release of intracellular calcium from intracellular stores was responsible for these effects (data not shown).
Figure 3. Bradykinin-evoked changes in [Ca2+]i in control cultured rat urothelial cells.
A, discontinuous representative traces of changes in F340/F380 in cultured rat urothelial cells following bath application of varying concentrations of bradykinin (1–100 nm) and ATP (10 μm). B, concentration–response curve for bradykinin-mediated changes in F340/F380 in cultured rat urothelial cells. Data are expressed as mean (± s.e.m.) change in F340/F380 as a percentage of the maximum ATP (10 μm) response.
The addition of varying concentrations of bradykinin (1–100 nm) produced a concentration-dependent increase in [Ca2+]i in cultured rat urothelial cells (EC50= 8.4 nm; Fig. 3). An EC75 concentration (30 nm) for bradykinin-evoked increase in [Ca2+]i was selected from the concentration–response curve as the test concentration for subsequent experiments. This concentration consistently and reproducibly evoked an increase in [Ca2+]i in responsive rat urothelial cells. However, due to marked tachyphylaxis, a wash period of 30 min was required between bradykinin (30 nm) applications, in order to obtain reproducible changes in [Ca2+]i. The mean responses, as a percentage of the ATP (10 μm) response following the first and second applications of bradykinin (30 nm), were 54.5 ± 5.5% and 64.4 ± 6.8%, respectively (n = 27, P = 0.29; Fig. 4A).
Figure 4. Bradykinin evoked changes in [Ca2+]i in control and CYP (24 h)-treated cultured rat urothelial cells.
A, bradykinin (30 nm) consistently and reproducibly evoked an increase in [Ca2+]i in cultured rat urothelial cells. B, bradykinin (30 nm)-evoked increase in [Ca2+]i was significantly attenuated in the presence of the B2 selective antagonist, Hoe-140 (1 μm). C, the B1 selective agonist, des-Arg9-bradykinin (des-Arg9-BK; 10 μm) had a significantly smaller effect on [Ca2+]i in control rat urothelial cells compared to bradykinin (30 nm) alone. D, following CYP (24 h)-treatment, however, populations of cultured urothelial cells were responsive to des-Arg9-bradykinin (10 μm). **P≤ 0.01.
Bradykinin receptor-evoked changes in [Ca2+]i in normal and CYP (24 h)-treated rat urothelial cells
To determine which of the two bradykinin receptor subtypes was responsible for the changes in [Ca2+]i evoked by bradykinin in control rat urothelial cells, we tested the specific B2 receptor antagonist Hoe-140, a selective non-competitive bradykinin B2 receptor antagonist that has been used in several in vitro (Hock et al. 1991; Félétou et al. 1994; Kajekar & Myers, 2000) and in vivo (Wirth et al. 1991; Poole et al. 1999; Levy & Zochodne, 2000) studies.
In cultured rat urothelial cells, the increase in [Ca2+]i evoked by bradykinin (30 nm) was significantly inhibited, by 89.2 ± 0.1% (P < 0.01, n = 37), in the presence of Hoe-140 (1 μm; Fig. 4B). The mean bradykinin (30 nm)-evoked changes in [Ca2+]i in the absence and presence of Hoe-140 (1 μm) were 59.5 ± 5% and 4.3 ± 1.5% (n = 37, % of maximum ATP (10 μm) response), respectively.
The selective B1 receptor agonist, des-Arg9-bradykinin (10 μm) was tested to see if application of this drug could evoke changes in [Ca2+]i in normal cultured rat urothelial cells. Bath application of des-Arg9-bradykinin (10 μm) evoked only a small change in [Ca2+]i in control cultured rat urothelial cells. The mean bradykinin (30 nm) and des-Arg9-bradykinin (10 μm)-evoked changes in [Ca2+]i as a percentage of the ATP (10 μm) response were 45.7 ± 2.5% and 5.7 ± 0.8% (n = 37, P < 0.01), respectively (Fig. 4C). These findings indicate that bradykinin-mediated changes in [Ca2+]i in control urothelial cells occur primarily through the activation of constitutively expressed bradykinin B2 receptors.
In contrast, urothelial cells cultured from CYP (24 h)-treated rats were responsive to des-Arg9-bradykinin (10 μm) as indicated by an elevation in [Ca2+]i. These experiments were conducted in the presence of Hoe-140 (1 μm) which blocked the B2 receptor component. Approximately 25% of cells tested responded with an elevation in [Ca2+]i following application of des-Arg9-bradykinin (10 μm, 29/116 cells tested). Similar responses occurred in the absence of extracellular calcium (data not shown). The mean des-Arg9-bradykinin (10 μm)-evoked change in [Ca2+]i of responsive rat urothelial cells (CYP (24 h)-treated) was 47.3 ± 8% of the maximum ATP (10 μm) response (n = 29).
Bradykinin evokes ATP release from cultured rat urothelial cells
Bradykinin can stimulate the release of neuropeptides and other neurotransmitters from a number of cell types (Andreeva & Rang, 1993; Dray & Perkins, 1993; Hua & Yaksh, 1993; Schuligoi et al. 1998). Previous studies have also demonstrated that extracellular ATP, most likely of urothelial origin, can activate pelvic afferent fibres in a paracrine manner (Ferguson et al. 1997; Cockayne et al. 2000; Vlaskovska et al. 2001).
Bradykinin (30 nm) consistently and reproducibly evoked the release of ATP from control cultured rat urothelial cells (Fig. 5). Bradykinin (30 nm) was bath applied for 30 s and produced a rapid release of ATP, 51.3% of the A23187 (3 μm) response (n = 4). The response reached a peak 40–60 s after agonist application and returned to baseline levels 60–100 s post application (Fig. 5A). The B2 selective antagonist, Hoe-140 (1 μm, 10 min preincubation) almost completely inhibited bradykinin (30 nm)-evoked release of ATP by 98.7% (n = 4, P < 0.01). The selective B1 receptor agonist, des-Arg9-bradykinin (10 μm) only evoked minimal levels of ATP release from control cultured rat urothelial cells; the mean response was 2.9% of that evoked by A23187 (3 μm, n = 5, P < 0.01).
Figure 5. Bradykinin evoked the release of ATP from rat urothelial cells.
A, representative time course recordings of ATP release evoked from separate control cultured rat urothelial cells following either simulation with bradykinin (30 nm, BK), bradykinin (30 nm) in the presence of Hoe-140 (1 μm), des-Arg9-bradykinin (10 μm, des-Arg9-BK) and the effect of des-Arg9-bradykinin (10 μm) in the presence of Hoe-140 (1 μm) on urothelial cells cultured from CYP (24 h)-treated rats. All agonists were applied at time = 0. Cells were pretreated with antagonists 10 min prior to agonist application. B, histograms illustrating mean release of ATP from cultured rat urothelial cells following stimulation as described above. **P≤ 0.01.
Urothelial cells cultured from CYP (24 h)-treated rats were responsive to des-Arg9-bradykinin (10 μm). These experiments were conducted in the presence of Hoe-140 (1 μm) which antagonized the B2 receptor component. des-Arg9-Bradykinin (10 μm) evoked a large release of ATP (60% of the maximum A23187 (3 μm) response (n = 6) from cultured urothelial cells obtained from CYP (24 h)-treated rats (Fig. 5).
Transepithelial resistance (TER) and permeabilities of normal and CYP (24 h)-treated rat urothelium
Urinary bladder umbrella cells maintain an apical membrane of exceptionally low permeability and high resistance. B2R immunoreactivity was assessed in bladders excised from CYP (24 h)-treated rats. In contrast to a compact urothelial structure as observed in the control bladder (Fig. 2A–C), CYP (24 h)-treated bladders exhibited a loose cell–cell contact within the urothelium (Fig. 6A). We evaluated the TER and permeability to both water and urea in normal untreated and CYP (24 h)-treated rat bladders. TER for normal rat urothelium averaged 1926 ± 176 Ω cm−2(n = 23) and significantly decreased to 527 ± 64 Ω cm−2 (n = 5, P < 0.01) in animals pretreated with CYP (Fig. 6). The diffusive permeability of the apical membrane of the urothelium, PD(AM), to both water and urea was calculated as previously described (Lavelle et al. 2002). Significant differences in permeabilities to water and urea were evident in control compared to CYP (24 h)-treated rats. The water permeability of control rats was (2.6 ± 0.18) × 10−5 cm s−1 (n = 23) versus (15.4 ± 2.8) × 10−5 cm s−1 (n = 5, P = 0.01) in CYP (24 h)-treated rats. Urea permeability in control rats was (1.8 ± 0.2) × 10−6 cm s−1 (n = 23) versus (49.8 ± 12.2) × 10−6 cm s−1 (n = 5, P = 0.02) in CYP (24 h)-treated rats.
Figure 6. Transepithelial resistance (TER) and permeabilities of normal and CYP (24 h)-treated rat urothelium.
A, bradykinin B2 receptor expression was assessed in urinary bladder of CYP (24 h)-treated rats. In contrast to control urinary bladder (Fig. 2A), CYP-treated bladder urothelium exhibited a loss of compact urothelial structure (arrows). B, transepithelial resistance in CYP-treated bladders was significantly reduced compared to control. **P≤ 0.01.
Continuous infusion cystometry
To evaluate the role of bradykinin in reflex voiding, continuous infusion cystometry was conducted. Continuous slow (0.04 ml min−1) infusion of bradykinin (100 μm; n = 4) produced a consistent effect on the urodynamic parameters including a significant decrease of the intercontraction interval (ICI) between voiding episodes (57.5 ± 6.3%, P = 0.007, n = 4) and increased bladder contraction amplitude (98.1 ± 50.8%, P = 0.03, n = 4) in control rats (Fig. 7B). This bradykinin-evoked facilitation of the micturition reflex was blocked following 10 min pretreatment with either the B2 receptor antagonist Hoe-140 (10 μm, n = 4, Fig. 7C, E and F), or the non-selective P2 receptor antagonist PPADS (10 mm, n = 4; Fig. 7D, E and F). Pretreatment of the control urinary bladders with Hoe-140 (10 μm) or PPADS (10 mm) had no significant effect on urodynamic parameters measured prior to application of bradykinin (100 μm).
Figure 7. Bradykinin facilitates the micturition reflex in part by increased purinergic responsiveness.
Representative infusion cystometric recordings obtained from (separate) anaesthetized rats, illustrating urinary bladder contractions following infusion of physiological saline (A; 0.04 ml min−1) and then followed by infusion of bradykinin (B; 100 μm). In a separate series of experiments, bradykinin (100 μm) was infused in the presence of the B2 receptor antagonist Hoe-140 (C; 10 μm) or in the presence of the non-selective P2 receptor antagonist PPADS (D; 10 mm). E and F, bradykinin (BK) facilitates the micturition reflex as indicated by a significant decrease in intercontractile interval (ICI) between voiding episodes (n = 4, **P < 0.01) and increased bladder contraction amplitude (BCA; n = 4, *P = 0.03). These changes in urodynamic function were significantly attenuated following pretreatment with the B2 receptor antagonist Hoe-140 (10 μm) or the P2 purinergic receptor antagonist PPADS (10 mm).
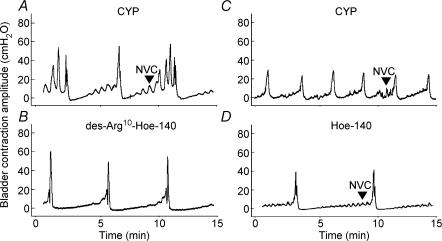
Acute cyclophosphamide treatment (24 h) induced bladder irritation, resulting in bladder hyperreflexia, as indicated by a decrease of ICI with saline infusion and an increase in non-voiding contractions (NVCs) as compared to untreated rats (Figs 7A and 8A). The contribution of either B1 or B2 receptors to this facilitation of bladder reflexes was evaluated by intravesical administration of Hoe-140 (10 μm) which significantly increased the saline-evoked ICI in CYP-treated rats (140 ± 37.8%, P = 0.008, n = 4), while having no significant effect on the bladder contraction amplitude or the frequency of NVCs. In contrast, the B1 receptor antagonist, des-Arg10-Hoe-140 (100 nm) significantly decreased the frequency of CYP-induced NVCs (68.1 ± 9.3%, P = 0.004, n = 4), while having no significant effect on the other urodynamic parameters assessed.
Figure 8. Cyclophosphamide (24 h)-induced bladder hyperreflexia involves B1 and B2 receptors.
A, rats pretreated with CYP (24 h) displayed bladder hyperreflexia as indicated by a significant decrease in ICI and increase in non-voiding contractions (NVCs). B, following instillation of B1 receptor antagonist, des-Arg10-Hoe-140 (100 nm), a significant attenuation of the frequency of NVCs was observable. C, discontinuous recording from a rat (CYP (24 h)-treated) prior to administration of Hoe-140 (10 μm). D, Hoe-140 (10 μm) had no significant effect on the frequency of NVCs but did significantly increase the ICI of CYP-treated rats.
Discussion
The data presented in this study demonstrate the expression and function (cellular and cystometric) of bradykinin B1 and B2 receptors in the normal rat urinary bladder urothelium and following chemically (cyclophosphamide) induced cystitis.
Previous studies have reported that bradykinin plays an important role in normal and pathophysiological conditions of the urinary bladder (Maggi et al. 1993; Lecci et al. 1995; Rosamilia et al. 1999; Meini et al. 2000). Using RT-PCR we found that B1R mRNA was not expressed in the normal rat urinary bladder. In contrast, B2R mRNA was constitutively expressed in the detrusor smooth muscle and urothelium. Closer examination using immunocytochemical studies revealed that B2R immunoreactivity was present in the detrusor smooth muscle and urothelium. Expression of B2R in the detrusor smooth muscle is consistent with previous findings which have reported that bradykinin-evoked bladder smooth muscle contractility occurs via activation of B2R (Andersson et al. 1992; Meini et al. 2000).
Recent studies have reported that the bladder urothelium exhibits sensory properties which allow urothelial cells to convey information regarding the local environment to underlying pelvic afferent nerves (Ferguson et al. 1997; Cockayne et al. 2000; Birder et al. 2001, 2002a, b). For example, adrenergic, cholinergic, prostanoid and vanilloid receptors may be involved in urothelial–pelvic afferent signalling. It has been demonstrated that a range of bioactive mediators released from the urothelium and suburothelial nerve plexus in response to bladder distension can regulate the micturition pathways (Ferguson et al. 1997; Cockayne et al. 2000; Vlaskovska et al. 2001; Birder et al. 1998, 2002b, 2003). In the present study, B2R immunoreactivity was present in the apical and underlying basal cells of the bladder urothelium. B2R activation in cultured rat urothelial cells resulted in increased cytosolic calcium within these cells. Such an elevation may trigger the release of a number of neurotransmitters including nitric oxide, which has been shown to cause urothelial damage in the cascade leading to the development of haemorrhagic cystitis (Ribeiro et al. 2002).
Furthermore, ATP was released from cultured rat urothelial cells following stimulation of the B2R. A number of studies have previously demonstrated that distension of hollow organs such as gut, lung, ureter and bladder releases ATP from the epithelial lining which can act in a paracrine manner to activate P2X2/3 receptors expressed on afferent fibres (Cockayne et al. 2000; Elneil et al. 2001; Vlaskovska et al. 2001). Indeed, in the urinary bladder, extracellular ATP released from urothelial cells following distension may act directly on purinergic P2X2/3 receptors expressed on submucosal pelvic afferents, altering micturition reflexes (Cockayne et al. 2000; Vlaskovska et al. 2001). The data obtained from the present study indicate that release of extracellular ATP from urothelial cells following bradykinin instillation within the normal or control urinary bladder altered micturition reflexes, as these effects were attenuated with the non-selective P2 receptor antagonist PPADS. These findings suggest that bradykinin evokes bladder hyperreflexia in part by the release of ATP from urothelial cells. Bladder hyperreflexia evoked by bradykinin in normal rats was also attenuated with the selective B2R antagonist Hoe-140. Consistent with these observations, Lecci et al. (1995) have reported that kinins may evoke bladder contractions by a direct action of bradykinin on pelvic afferent nerves. Taken together, these studies suggest that bradykinin can evoke bladder hyperreflexia by directly activating the constitutively expressed B2R on pelvic afferent nerve fibres and/or indirectly via the release of mediators such as ATP from the urothelium.
B1R mRNA was undetectable in the control rat urinary detrusor smooth muscle and urothelium. These findings are consistent with binding studies conducted on control urinary bladder homogenates (Lecci et al. 1999), although there have been previous reports suggesting that B1R mRNA is constitutively present in the mouse bladder (Trevisani et al. 1999). Application of the selective B1R agonist, des-Arg9-bradykinin evoked only minimal increases in [Ca2+]i and ATP release from control cultured rat urothelial cells, suggesting no or very low constitutive B1 receptor expression.
Bradykinin B1 receptors are normally only expressed at very low levels under normal conditions in a number of tissues, but expression is significantly up-regulated following tissue damage/inflammation. Inflammatory urinary bladder conditions are commonly investigated using a cyclophosphamide (CYP)-induced model of chemical cystitis. There is increasing evidence to suggest that bradykinin is involved in the processes underlying cystitis. For example there is an up-regulation of B1R in biopsies obtained from patients with interstitial cystitis (Ruggieri et al. 1997) and in CYP-treated animals B1R-mediated bladder responses are significantly increased (Meini et al. 1998; Lecci et al. 1999).
CYP is metabolically broken down to the toxin acrolein, and storage within the bladder causes irritation of the bladder urothelium leading to severe inflammation of the bladder, characterized by bladder hyperreflexia (Cox, 1979). CYP treatment causes a multisystem dysfunction of the micturition pathway which affects both neural and non-neural pathways (Maggi et al. 1993; Lecci et al. 1995; Vizzard et al. 1996; Yoshimura & de Groat, 1999; Vizzard, 2000a, b). CYP-treated bladders exhibited significantly reduced transepithelial resistance (TER) and increased permeabilities to urea and water. Under these conditions, constituents of urine, particularly kinins (which can also be produced by various cells at the site of inflammation), may activate submucosal afferent fibres, urothelial cells and detrusor smooth muscle cells, contributing to increased urgency and pain, symptoms commonly associated with cystitis.
In the present study, B1R mRNA was significantly up-regulated in the detrusor smooth muscle and the urothelium following acute CYP (24 h)-induced cystitis. Consistent with this increased expression, the selective B1 receptor agonist, des-Arg9-bradykinin evoked the release of ATP and elevated intracellular calcium levels in cultured rat urothelial cells obtained from CYP (24 h)-treated rats. Increased release of ATP has previously been reported following distension from urothelial cells obtained from patients with interstitial cystitis (Sun & Chai, 2004) and also in the naturally occurring model of feline interstitial cystitis (Birder et al. 2003). These findings raise the possibility that augmented release of mediators, including ATP, from urothelial cells could sensitize/activate submucosal afferent fibres leading to changes in sensory input and bladder function.
Cystometry performed on CYP (24 h)-treated rats revealed that instillation of the B1 receptor antagonist, des-Arg10-Hoe-140 significantly reduced the frequency of non-voiding contractions. The reduction of the non-voiding contractions by a B1 receptor antagonist suggests that B1 receptors may regulate the release of urothelial-derived factors or alter smooth muscle tone. In contrast, instillation of the B2 receptor antagonist Hoe-140 decreased the frequency of voiding contractions (increased the intercontraction interval). These findings might be explained by the possibility that B1-sensitive mechanisms and/or afferent pathways could trigger the emergence of non-voiding contractions and B2-sensitive mechanisms could trigger voiding contractions. Alternatively, it is equally likely that other mediators (released from urothelial or other cells) following CYP inflammation may also play a role in these changes. Regardless, these results demonstrate a role for both B1 and B2 receptors in CYP-induced bladder hyperactivity and indicate that bradykinin is active in this model of cystitis and may, in part, induce bladder hyperreflexia by the extracellular release of urothelial-derived factors (such as ATP) acting on pelvic nerve afferents.
In the chronic model of CYP (8 day)-induced cystitis, PCR studies revealed that B1R mRNA levels declined to undetectable levels in the detrusor smooth muscle but remained at similar levels in the urothelium as observed with acute CYP-treated rats. The maintained expression of B1R in the urothelium following chronic inflammation suggests a role for urothelially derived neurotransmitters in chronic bladder syndromes. In addition, it has been previously demonstrated that the up-regulation of B1R following CYP-induced cystitis in the urinary bladder was prevented by pretreatment with the corticosteroid dexamethasone (Lecci et al. 1999).
CYP-induced cystitis is characterized by gross histological changes in bladder structure, such as increased bladder weight, oedema, up-regulation of proteins/enzymes and electrophysiologically by the recruitment of previously silent C-fibres and sensitization of mechanosensitive Aδ-fibres (Maggi et al. 1992; Ahluwalia et al. 1994; Lecci et al. 1994; Vizzard et al. 1996). Infiltration of inflammatory cells such as mast cells and macrophages into the bladder submucosa following CYP-induced cystitis and interstitial cystitis has been reported (Theoharides et al. 2001; Wein & Hanno, 2002). Mast cells lie in close proximity to both urothelial cells as well as afferent fibres in the submucosa of the urinary bladder, and degranulation of these cells has been shown to release a wide range of neurotransmitters and cytokines. Liberation of such mediators (including kinins) could directly activate/sensitize the afferent fibres to further release neurogenic mediators and may also stimulate bradykinin (B2) receptors expressed on nearby urothelial cells to release ATP and other neurotransmitters. This sequence of events would lead to the further inflammation and alteration of the urothelium and bladder reflex pathways. The selective B2 receptor antagonist Hoe-140 has been shown to reduce oedema generation and bladder hyperreflexia in CYP-induced cystitis (Lecci et al. 1999). The results from the present study suggest that in addition to a direct effect on afferent fibres, pharmacological modulation of urothelially expressed bradykinin receptors may prevent bladder hyperreflexia by also inhibiting the release of pro-inflammatory mediators (e.g. ATP, prostanoids and NO).
In summary, we provide further evidence that the bladder urothelium has sensory properties that adapt rapidly to changes in the local environment. The constitutive expression of B2R in the urothelium suggests that bradykinin may play a role in normal bladder function. The up-regulation of the B1R in the CYP model of chemically induced cystitis suggests that a complex interplay between urothelial cells, afferent nerves, immune cell signalling and detrusor smooth muscle may contribute to alterations in bladder reflex pathways in disease states.
Acknowledgments
This work was supported by NIH grants DK R0154824 and DKR0157284 and a grant from Roche Palo Alto (to L.A.B.).
References
- Ahluwalia A, Maggi CA, Santicioli P, Lecci A, Giuliani S. Characterization of the capsaicin-sensitive component of cyclophosphamide-induced inflammation in the rat urinary bladder. Br J Pharmacol. 1994;111:1017–1022. doi: 10.1111/j.1476-5381.1994.tb14845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahluwalia A, Perretti M. B1 receptors as a new inflammatory target. Could this B the 1? Trends Pharmacol Sci. 1999;20:100–104. doi: 10.1016/s0165-6147(99)01321-8. 10.1016/S0165-6147(99)01321-8. [DOI] [PubMed] [Google Scholar]
- Andersson KE, Hedlund H, Stahl M. Contractions induced by angiotensin I, angiotensin II and bradykinin in isolated smooth muscle from the human detrusor. Acta Physiol Scand. 1992;145:253–259. doi: 10.1111/j.1748-1716.1992.tb09362.x. [DOI] [PubMed] [Google Scholar]
- Andreeva L, Rang HP. Effect of bradykinin and prostaglandins on the release of calcitonin gene-related peptide-like immunoreactivity from the rat spinal cord in vitro. Br J Pharmacol. 1993;108:185–190. doi: 10.1111/j.1476-5381.1993.tb13460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apodaca G, Kiss S, Ruiz W, Meyers S, Zeidel M, Birder L. Disruption of bladder epithelium barrier function after spinal cord injury. Am J Physiol Renal Physiol. 2003;284:F966–F976. doi: 10.1152/ajprenal.00359.2002. [DOI] [PubMed] [Google Scholar]
- Asano M, Hatori C, Inamura N, Sawai H, Hirosumi J, Fujiwara T, Nakahara K. Effects of a nonpeptide bradykinin B2 receptor antagonist, FR167344, on different in vivo animal models of inflammation. Br J Pharmacol. 1997;122:1436–1440. doi: 10.1038/sj.bjp.0701534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, Apodaca G, de Groat WC, Kanai AJ. Adrenergic- and capsaicin-evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am J Physiol. 1998;275:F226–F229. doi: 10.1152/ajprenal.1998.275.2.F226. [DOI] [PubMed] [Google Scholar]
- Birder LA, Barrick SR, Roppolo JR, Kanai AJ, de Groat WC, Kiss S, Buffington CA. Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. Am J Physiol Renal Physiol. 2003;285:F423–F429. doi: 10.1152/ajprenal.00056.2003. [DOI] [PubMed] [Google Scholar]
- Birder LA, Kanai AJ, de Groat WC, Kiss S, Nealen ML, Burke NE, Dineley KE, Watkins S, Reynolds IJ, Caterina MJ. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci U S A. 2001;98:13396–13401. doi: 10.1073/pnas.231243698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002a;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- Birder LA, Nealen ML, Kiss S, de Groat WC, Caterina MJ, Wang E, Apodaca G, Kanai AJ. Beta-adrenoceptor agonists stimulate endothelial nitric oxide synthase in rat urinary bladder urothelial cells. J Neurosci. 2002b;22:8063–8070. doi: 10.1523/JNEUROSCI.22-18-08063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchelouche K, Nordling J. Recent developments in the management of interstitial cystitis. Curr Opin Urol. 2003;13:309–313. doi: 10.1097/00042307-200307000-00007. [DOI] [PubMed] [Google Scholar]
- Bouchelouche K, Kristensen B, Nordling J, Horn T, Bouchelouche P. Increased urinary leukotriene E4 and eosinophil protein X excretion in patients with interstitial cystitis. J Urol. 2001;166:2121–2125. [PubMed] [Google Scholar]
- Calixto JB, Cabrini DA, Ferreira J, Campos MM. Kinins in pain and inflammation. Pain. 2000;87:1–5. doi: 10.1016/S0304-3959(00)00335-3. [DOI] [PubMed] [Google Scholar]
- Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- Cox PJ. Cyclophosphamide cystitis – identification of acrolein as the causative agent. Biochem Pharmacol. 1979;28:2045–2049. doi: 10.1016/0006-2952(79)90222-3. [DOI] [PubMed] [Google Scholar]
- Dray A, Perkins M. Bradykinin and inflammatory pain. Trends Neurosci. 1993;16:99–104. doi: 10.1016/0166-2236(93)90133-7. 10.1016/0166-2236(93)90133-7. [DOI] [PubMed] [Google Scholar]
- Elneil S, Skepper JN, Kidd EJ, Williamson JG, Ferguson DR. Distribution of P2X(1) and P2X(3) receptors in the rat and human urinary bladder. Pharmacology. 2001;63:120–128. doi: 10.1159/000056122. 10.1159/000056122. [DOI] [PubMed] [Google Scholar]
- Erickson DR. Urine markers of interstitial cystitis. Urology. 2001;57:15–21. doi: 10.1016/s0090-4295(01)01128-1. 10.1016/S0090-4295(01)01128-1. [DOI] [PubMed] [Google Scholar]
- Félétou M, Germain M, Thurieau C, Fauchere JL, Canat E. Agonistic and antagonist properties of the bradykinin B2 receptor antagonist, HOE-140, in isolated blood vessels from different species. Br J Pharmacol. 1994;112:683–689. doi: 10.1111/j.1476-5381.1994.tb13130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson DR. Urothelial function. BJU Int. 1999;84:235–242. doi: 10.1046/j.1464-410x.1999.00187.x. 10.1046/j.1464-410x.1999.00187.x. [DOI] [PubMed] [Google Scholar]
- Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes – a possible sensory mechanism? J Physiol. 1997;505:503–511. doi: 10.1111/j.1469-7793.1997.503bb.x. 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habler HJ, Janig W, Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol. 1990;425:545–562. doi: 10.1113/jphysiol.1990.sp018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock FJ, Wirth K, Albus U, Linz HJ, Gerhards G, Wiemer G, Henke ST, Breipohl G, Konig W, Knolle J, Scholkens BA. HOE-140, a new potent and long acting bradykinin-antagonist: in vitro studies. Br J Pharmacol. 1991;102:769–773. doi: 10.1111/j.1476-5381.1991.tb12248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu VY, Malley S, Dattilio A, Folsom JB, Zvara P, Vizzard MA. COX-2 and prostanoid expression in micturition pathways after cyclophosphamide-induced cystitis in the rat. Am J Physiol Regul Integr Comp Physiol. 2003;284:R574–R585. doi: 10.1152/ajpregu.00465.2002. [DOI] [PubMed] [Google Scholar]
- Hua XY, Yaksh TL. Pharmacology of the effects of bradykinin, serotonin, and histamine on the release of calcitonin gene-related peptide from C-fiber terminals in the rat trachea. J Neurosci. 1993;13:1947–1953. doi: 10.1523/JNEUROSCI.13-05-01947.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajekar R, Myers AC. Effect of bradykinin on membrane properties of guinea pig bronchial parasympathetic ganglion neurones. Am J Physiol Lung Cell Mol Physiol. 2000;278:L485–L491. doi: 10.1152/ajplung.2000.278.3.L485. [DOI] [PubMed] [Google Scholar]
- Keay SK, Warren JW. Is interstitial cystitis an infectious disease? Int J Antimicrob Agents. 2002;19:480–483. doi: 10.1016/s0924-8579(02)00089-4. 10.1016/S0924-8579(02)00089-4. [DOI] [PubMed] [Google Scholar]
- Lavelle J, Meyers S, Ramage R, Bastacky S, Doty D, Apodaca G, Zeidel ML. Bladder permeability barrier: recovery from selective injury of surface epithelial cells. Am J Physiol Renal Physiol. 2002;283:F242–F253. doi: 10.1152/ajprenal.00307.2001. [DOI] [PubMed] [Google Scholar]
- Lavelle JP, Meyers SA, Ruiz WG, Buffington CA, Zeidel ML, Apodaca G. Urothelial pathophysiological changes in feline interstitial cystitis: a human model. Am J Physiol Renal Physiol. 2000;278:F540–F553. doi: 10.1152/ajprenal.2000.278.4.F540. [DOI] [PubMed] [Google Scholar]
- Lecci A, Giuliani S, Meini S, Maggi CA. Pharmacological analysis of the local and reflex responses to bradykinin on rat urinary bladder motility in vivo. Br J Pharmacol. 1995;114:708–714. doi: 10.1111/j.1476-5381.1995.tb17196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecci A, Meini S, Patacchini R, Tramontana M, Giuliani S, Criscuoli M, Maggi CA. Effect of dexamethasone on cyclophosphamide-induced cystitis in rats: lack of relation with bradykinin B1 receptor-mediated motor responses. Eur J Pharmacol. 1999;369:99–106. doi: 10.1016/s0014-2999(99)00052-7. 10.1016/S0014-2999(99)00052-7. [DOI] [PubMed] [Google Scholar]
- Levy D, Zochodne D. Increased mRNA expression of B1 and B2 bradykinin receptors and antinociceptive effects of their antagonists in an animal model of neuropathic pain. Pain. 2000;86:265–271. doi: 10.1016/S0304-3959(00)00256-6. 10.1016/S0304-3959(00)00256-6. [DOI] [PubMed] [Google Scholar]
- Maggi CA. Prostanoids as local modulators of reflex micturition. Pharmacol Res. 1992;25:13–20. doi: 10.1016/s1043-6618(05)80059-3. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Santicioli P, Del Bianco E, Lecci A, Guliani S. Evidence for the involvement of bradykinin in chemically-evoked cystitis in anaesthetized rats. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:432–437. doi: 10.1007/BF00165395. 10.1007/BF00165395. [DOI] [PubMed] [Google Scholar]
- Marceau F, Barabe J, St-Pierre S, Regoli D. Kinin receptors in experimental inflammation. Can J Physiol Pharmacol. 1980;58:536–542. doi: 10.1139/y80-088. [DOI] [PubMed] [Google Scholar]
- Meini S, Lecci A, Cucchi P, Catalioto RM, Criscuoli M, Maggi CA. Inflammation modifies the role of cyclooxygenases in the contractile responses of the rat detrusor smooth muscle to kinin agonists. J Pharmacol Exp Ther. 1998;287:137–143. [PubMed] [Google Scholar]
- Meini S, Patacchini R, Giuliani S, Lazzeri M, Turini D, Maggi CA, Lecci A. Characterization of bradykinin B(2) receptor antagonists in human and rat urinary bladder. Eur J Pharmacol. 2000;388:177–182. doi: 10.1016/s0014-2999(99)00882-1. 10.1016/S0014-2999(99)00882-1. [DOI] [PubMed] [Google Scholar]
- Parsons CL. The therapeutic role of sulfated polysaccharides in the urinary bladder. Urol Clin North Am. 1994;21:93–100. [PubMed] [Google Scholar]
- Parsons CL, Bautista SL, Stein PC, Zupkas P. Cyto-injury factors in urine: a possible mechanism for the development of interstitial cystitis. J Urol. 2000;164:1381–1384. 10.1097/00005392-200010000-00078. [PubMed] [Google Scholar]
- Parsons CL, Greenberger M, Gabal L, Bidair M, Barme G. The role of urinary potassium in the pathogenesis and diagnosis of interstitial cystitis. J Urol. 1998;159:1862–1866. doi: 10.1016/S0022-5347(01)63178-1. 10.1097/00005392-199806000-00021. [DOI] [PubMed] [Google Scholar]
- Poole S, Lorenzetti BB, Cunha JM, Cunha FQ, Ferreira SH. Bradykinin B1 and B2 receptors, tumour necrosis factor alpha and inflammatory hyperalgesia. Br J Pharmacol. 1999;126:649–656. doi: 10.1038/sj.bjp.0702347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro RA, Freitas HC, Campos MC, Santos CC, Figueiredo FC, Brito GA, Cunha FQ. Tumor necrosis factor-alpha and interleukin-1beta mediate the production of nitric oxide involved in the pathogenesis of ifosfamide induced hemorrhagic cystitis in mice. J Urol. 2002;167:2229–2234. 10.1097/00005392-200205000-00077. [PubMed] [Google Scholar]
- Rosamilia A, Clements JA, Dwyer PL, Kende M, Campbell DJ. Activation of the kallikrein kinin system in interstitial cystitis. J Urol. 1999;162:129–134. doi: 10.1097/00005392-199907000-00030. 10.1097/00005392-199907000-00030. [DOI] [PubMed] [Google Scholar]
- Ruggieri MR, Wang J, Whitmore KE, Pontari MA, Hanno PM. Expression of bradykinin 1 receptor subtype in interstitial cystitis bladder biopsies. J Urol. 1997;157:131–136. [Google Scholar]
- Schuligoi R, Peskar BA, Donnerer J, Amann R. Bradykinin-evoked sensitization of neuropeptide release from afferent neurons in the guinea-pig lung. Br J Pharmacol. 1998;125:388–392. doi: 10.1038/sj.bjp.0702079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Chai TC. Upregulation of P2X3 receptors during stretch of bladder urothelial cells from patients with interstitial oystitis. J Urol. 2004;171:448–452. doi: 10.1097/01.ju.0000099660.46774.3c. 10.1097/01.ju.0000099660.46774.3c. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Kempuraj D, Sant GR. Mast cell involvement in interstitial cystitis: a review of human and experimental evidence. Urology. 2001;57:47–55. doi: 10.1016/s0090-4295(01)01129-3. 10.1016/S0090-4295(01)01129-3. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Schmidlin F, Tognetto M, Nijkamp FP, Gies JP, Frossard N, Amadesi S, Folkerts G, Geppetti P. Evidence for in vitro expression of B1 receptor in mouse trachea and urinary bladder. Br J Pharmacol. 1999;126:1293–1300. doi: 10.1038/sj.bjp.0702410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truschel ST, Ruiz WG, Shulman T, Pilewski J, Sun TT, Zeidel ML, Apodaca G. Primary uroepithelial cultures. A model system to analyze umbrella cell barrier function. J Biol Chem. 1999;274:15020–15029. doi: 10.1074/jbc.274.21.15020. 10.1074/jbc.274.21.15020. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Alterations in spinal cord Fos protein expression induced by bladder stimulation following cystitis. Am J Physiol Regul Integr Comp Physiol. 2000a;278:R1027–R1039. doi: 10.1152/ajpregu.2000.278.4.R1027. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol. 2000b;161:273–284. doi: 10.1006/exnr.1999.7254. 10.1006/exnr.1999.7254. [DOI] [PubMed] [Google Scholar]
- Vizzard MA, Erdman SL, de Groat WC. Increased expression of neuronal nitric oxide synthase in bladder afferent pathways following chronic bladder irritation. J Comp Neurol. 1996;370:191–202. doi: 10.1002/(SICI)1096-9861(19960624)370:2<191::AID-CNE5>3.0.CO;2-Y. 10.1002/(SICI)1096-9861(19960624)370:2<191::AID-CNE5>3.3.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, Ford AP, Burnstock G. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci. 2001;21:5670–5677. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wein AJ, Hanno PM. Targets for therapy of the painful bladder. Urology. 2002;59:68–73. doi: 10.1016/s0090-4295(01)01640-5. 10.1016/S0090-4295(01)01640-5. [DOI] [PubMed] [Google Scholar]
- Wirth K, Hock FJ, Albus U, Linz W, Alpermann HG, Anagnostopoulous H, Henke ST, Breipohl G, Konig W, Knolle J, Scholkens BA. HOE-140, a new potent and long acting bradykinin antagonist: in vivo studies. Br J Pharmacol. 1991;102:774–777. doi: 10.1111/j.1476-5381.1991.tb12249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci. 1999;19:4644–4653. doi: 10.1523/JNEUROSCI.19-11-04644.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]