Abstract
The major purpose of this study was to elucidate mechanisms by which decreasing enhanced physical activity induces decreased insulin sensitivity in skeletal muscle. Rats with access to voluntary running wheels for 3 weeks had their wheels locked for 5 h (WL5), 29 h (WL29), or 53 h (WL53); a separate group of rats never had wheel access (sedentary, SED). Relative to WL5, submaximal insulin-stimulated 2-deoxyglucose uptake into the epitrochlearis muscle was lower in WL53 and SED. Insulin binding, insulin receptor β-subunit (IRβ) protein level, submaximal insulin-stimulated IRβ tyrosine phosphorylation, and glucose transporter-4 protein level were each lower in both WL53 and SED than in WL5 and WL29. Akt/protein kinase B Ser473 phosphorylation was lower in WL53 and SED than in WL5. Protein levels of protein tyrosine phosphatase-1B, Src homology phosphatase-2, and protein kinase C-Θ did not vary among groups. The amount of protein tyrosine phosphatase-1B, Src homology phosphatase-2, and protein kinase C-Θ associated with IRβ in insulin-stimulated muscle also did not differ among the four groups. The mean of SED and WL53 had a significantly higher IRβ-associated protein tyrosine phosphatase-1B than the mean of WL5 and WL29. The enclosure of multiple changes (decreases in insulin binding, IRβ protein, IRβ tyrosine phosphorylation, and glucose transporter-4 protein) in the epitrochlearis muscle within the 29th to 53rd hour after cessation of voluntary wheel running raises the possibility that a single regulatory event could be responsible for the coordinated decrease.
There is a strong relationship between physical inactivity and insulin resistance (Kriska et al. 1993; Mayer-Davis et al. 1998; Gustat et al. 2002). This is supported by observations that insulin sensitivity falls off within days when physically active humans become sedentary, with the decreased physical activity decreasing insulin-mediated glucose uptake. For example, the area under the insulin curve during an oral glucose tolerance test increased 73, 30, or 93% when endurance-trained individuals did not exercise for 7–10, 14, or 10 days, respectively (Heath et al. 1983; Houmard et al. 1993; Arciero et al. 1998). Measured by submaximal hyperinsulinaemic, euglycaemic clamp techniques, glucose disposal rate decreased 23% after 10 days of no exercise in physically trained subjects (King et al. 1988), and the insulin concentration required for 50% of maximal glucose uptake increased 23% after 5 days of decreased physical activity in endurance-trained subjects (Mikines et al. 1989). Thus, it is clear that insulin sensitivity declines within days of decreasing physical activity in humans. However, little is known about the cellular basis for the effect.
Skeletal muscle accounts for 75–95% of insulin-stimulated glucose disposal in humans (Baron et al. 1988). We have shown that insulin resistance develops in the mouse soleus muscle after 1 day of hindlimb immobilization (Seider et al. 1982). Other studies have shown that following swim-training, insulin-stimulated glucose uptake into the isolated rat epitrochlearis muscle decreases to sedentary levels 40–90 h following the last swimming bout (Kawanaka et al. 1997; Host et al. 1998a; Reynolds et al. 2000). Taken together, the above reports suggest that skeletal muscle could be the source of the physical inactivity-induced decline in whole-body, insulin-stimulated glucose disposal in humans.
To gain a better understanding of the mechanisms by which decreased physical activity decreases insulin-stimulated glucose uptake, we wanted to employ an animal model that would mimick what happens when previously active subjects stop exercising. The use of voluntary running wheels more closely approximates the manner in which humans have historically engaged in physical activity in that rats both eat and engage in voluntary physical activity on an intermittent basis during their waking nocturnal hours (Rodnick et al. 1989). This enables the animals to be studied during a time when they are normally sleeping, not physically active, and not eating (Rodnick et al. 1989; Moraska & Fleshner, 2001). In addition, we chose to study rats in a postprandial state, rather than in a fasted state, as this, in our opinion, represents the more common condition in modern humans. Locking the running wheels so that the animals cannot run facilitates the study of events that occur with decreased physical activity (Tsai et al. 1981). One purpose of this study was to determine the time course of decline in glucose uptake into the epitrochlearis muscle with a return to normal cage activity following 3 weeks of voluntary wheel running. Based on these results, we next hypothesized that a reduction in measures of insulin receptor signalling would first occur at either 29 or 53 h after locking the running wheels. A major finding of this study was that the insulin receptor β-subunit (IRβ) protein level and two essential components of insulin receptor activation, insulin binding and tyrosine phosphorylation, decreased to sedentary levels in the rat epitrochlearis muscle between 29 and 53 h after cessation of voluntary running activity following 3 weeks of voluntary wheel running.
Methods
Materials
2-[1,2-3H(N)]Deoxyglucose and d-[1-14C]mannitol were from American Radiolabeled Chemicals. 125I-labelled insulin was from New England Nuclear. IRβ polyclonal, phosphotyrosine monoclonal, protein tyrosine phosphatase-1B (PTP1B) monoclonal, protein kinase C-Θ (PKC-Θ) monoclonal and Src homology phosphatase-2 (SHP2) monoclonal antibodies were from BD Biosciences. IRβ, Akt and Akt Ser473 phospho-specific polyclonal antibodies and protein A agarose were from Upstate Biotechnology. Glucose transporter-4 (GLUT4) polyclonal antibody was from Biogenesis. Horseradish peroxidase (HRP)-linked anti-mouse and anti-rabbit secondary antibodies and HRP-linked protein A were from Amersham. Vectastain ABC kit was from Vector Laboratories. All other chemicals were from Sigma.
Animal protocol
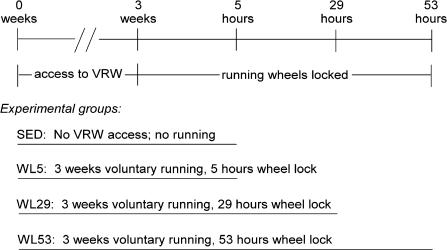
The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Missouri-Columbia. Fischer 344 × Brown Norway F1 hybrid rats (Harlan) were obtained at age 21–23 days and allowed to acclimatize for 1 week. The animals were housed in approved temperature-controlled animal quarters with a 04.00–16.00 h light : 16.00–04.00 h dark cycle that was maintained throughout the experimental period. After 1 week (day 0), the rats were randomly assigned to one of four groups as follows: wheel lock 5 (WL5), wheel lock 29 (WL29), or wheel lock 53 (WL53), representing 3 weeks of voluntary wheel access followed by 5, 29, or 53 h of wheel lock that prevented access to voluntary running, respectively, or SED, representing a sedentary group without running wheel access for the entire 3 weeks (Fig. 1). At this time, the animals were separated into individual cages of the same dimensions; cages for animals in the running groups (WL5, WL29, WL53) were equipped with a voluntary running wheel outfitted with a Sigma Sport BC 800 bicycle computer (Cherry Creek Cyclery, Foster Falls, VA, USA) for measuring running activity. After 3 weeks (on day 21), the wheels were locked for all running groups at 04.00 h. SED and WL5 animals were killed at 09.00 h. The WL29 group remained in their cages with locked wheels before being killed the following day (on day 22) at 09.00 h (after 29 h of wheel lock), and WL53 remained in their cages with locked wheels until day 23 when they were killed at 09.00 h (after 53 h of wheel lock). The animals had ad libitum access to food at all times until the day on which they were killed, when food was removed at 04.00 h. At 09.00 h, the animals were anaesthetized with 60 mg pentobarbital (kg body mass)−1. Epitrochlearis muscles were carefully dissected out, rinsed in preoxygenated Krebs-Henseleit buffer (KHB: 116 mm NaCl, 4.6 mm KCl, 1.16 mm KH2PO4, 25.3 mm NaHCO3, 2.5 mm CaCl2, 1.16 mm MgSO4, pH 7.4)−40 mm mannitol, and used for experiments as described below. The animals were exsanguinated by removal of the heart.
Figure 1. Experimental design.
The length of the line for each experimental group corresponds to the time line at the top of the figure. See text for description. VRW, voluntary running wheel. n = 24 animals for each experimental group.
2-Deoxyglucose uptake
2-Deoxyglucose (2-DOG) uptake was performed essentially as described (Hokama et al. 1997) with minor modifications. Briefly, muscles were preincubated in KHB−0.1% bovine serum albumin (BSA)−38 mm mannitol–2 mm sodium acetate for 30 min. The muscles were then transferred to the same solution either without insulin (basal), or with 60 μU ml−1 (submaximal, 0.4 nm) or 2 mU ml−1 (maximal, 12.8 nm) insulin for 10 min. In all cases, the left epitrochlearis muscle was incubated without insulin and the contralateral right muscle was incubated with either submaximal or maximal insulin. The muscles were rinsed by incubation in KHB−0.1% BSA−40 mm mannitol with or without insulin for 10 min. The muscles were then incubated in KHB−0.1% BSA−38 mm mannitol (0.2 μCi ml−1)−2 mm 2-deoxyglucose (1.5 μCi ml−1) for 20 min after which they were blotted dry over ice on filter paper pre-wetted with incubation medium, rapidly cut cross-wise into two pieces (∼1/3 and 2/3 of the muscle size), placed in aluminium foil, and frozen in liquid nitrogen. During all incubation steps, the muscles were kept in stoppered vials under a constant stream of 95% O2−5% CO2 in a 37°C shaking water bath at 60 r.p.m. For determination of 2-DOG uptake, measurement of radiolabel in the muscle was performed as described by Hansen et al. (1994) using mannitol to determine the extracellular space. Briefly, the approximately 1/3 section of the muscle was weighed, boiled in 1 ml of water for 10 min, cooled on ice for 10 min, and centrifuged to remove the non-soluble material. Aliquots of the supernatant were counted in duplicate on a scintillation counter with preprogrammed windows for dual scintillation counting of 3H and 14C. As basal 2-DOG uptake did not differ within groups when the contralateral muscle was used for either submaximal or maximal 2-DOG uptake (data not shown), the results are combined for presentation of the data.
Insulin binding
Insulin binding experiments were performed essentially as described (Le Marchand-Brustel & Freychet, 1978; Bonen et al. 1986). Epitrochlearis muscles were preincubated in KHB−0.1% BSA−40 mm mannitol. After 15 min, the muscles were transferred to KHB−0.1% BSA−40 mm mannitol containing 125I-labelled insulin (0.12 μCi ml−1). The right epitrochlearis muscles were exposed to 0.4 nm insulin and the contralateral left muscles were incubated with 7.5 μm insulin. During these steps, the muscles were kept in stoppered vials under a constant stream of 95% O2−5% CO2 in a shaking water bath at 60 r.p.m. at 37°C. The muscles were then washed 8 times for 5 min each in 5 ml of ice-cold KHB−0.1% BSA−40 mm mannitol, dissolved in 1 n NaOH, and neutralized with 2 n HCl. Aliquots were counted in duplicate on a gamma counter. Non-specific binding was determined from samples incubated with 7.5 μm insulin and subtracted from the contralateral muscle to obtain values for specific insulin binding. Non-specific binding did not differ between groups (data not shown). An aliquot of the dissolved muscle was used for determining protein concentration using the Bradford method (Bradford, 1976).
Immunoblots and immunoprecipitations
Immunoanalysis was performed on the remaining 2/3 of the epitrochlearis muscles from animals where the right muscle was incubated with submaximal insulin. The left epitrochlearis muscles, which were incubated without insulin, were homogenized in 15 ml g−1 homogenization buffer (50 mm Hepes, pH 7.4, 4 mm EGTA, 10 mm Na4EDTA, 15 mm Na4P2O7, 100 mmβ-glycerophosphate, 25 mm NaF, 1% Igepal CA630, 5 mm activated Na3VO4, 50 μg ml−1 each of leupeptin, pepstatin, and aprotinin), rotated end-over-end at 4°C for 1 h, centrifuged at 16 000 g at 4°C for 20 min, and the supernatant frozen in aliquots at −80°C following a Bradford assay for protein determination (Bradford, 1976). These samples were used for immunoblot analysis and citrate synthase activity as described below. The right epitrochlearis muscles (incubated with submaximal insulin) were treated in the same manner, except that they were homogenized in immunoprecipitation buffer (50 mm Tris-HCl, pH 8.2, 150 mm NaCl, 1 mm Na4EDTA, 1 mm EGTA, 1% Triton X-100, 0.25% deoxycholic acid, 0.25% lauryl sulfobetaine, 0.25% caprylyl sulfobetaine, 0.5% 3-(1-pyridino)-1-propanesulphonate, 2.5 mm activated Na3VO4, 2.5 mm phenylmethanesulphonyl fluoride, 25 μg ml−1 each of leupeptin, pepstatin, and aprotinin); these samples were used for immunoprecipitation experiments as described below.
For immunoblot analysis, 20–40 μg of homogenate protein from the left muscles was subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The membranes were stained with Ponceau-S to verify equal loading and blocked for 1 h. Following an overnight incubation with primary antibodies at 4°C, the membranes were washed, incubated with secondary antibody for 1 h, washed again, treated with an enhanced chemiluminescent reagent, and exposed to film for visualization of the protein. Protein bands were quantified using a laser densitometer (Molecular Dynamics). Optimal blocking conditions, primary antibody dilutions and conditions, washing protocol and reagent, and secondary antibody concentration and conditions were determined separately for each protein assayed. All gels were loaded with an equal amount of a loading control which was used for normalization of data between blots. The loading control for PTP1B was SW13 cell extract; the loading control for all other antibodies was homogenate of an insulin-stimulated epitrochlearis muscle from a non-experimental rat. Immunoblots for Akt and Akt Ser473 phosphorylation were performed on muscles stimulated with submaximal insulin and homogenized in immunoprecipitation buffer.
For immunoprecipitation analysis, 500 μg of homogenate protein from muscles incubated with submaximal insulin was rotated end-over-end overnight at 4°C with 5 μg of polyclonal anti-IRβ antibody. A volume of 100 μl of a 50% protein A agarose bead slurry was added and the samples were rotated end-over-end at 4°C for 2 h, after which the beads were washed and boiled in 60 μl of sample buffer. Samples were loaded on a gel and subjected to immunoblot analysis for phosphotyrosine or IRβ using monoclonal antibodies as described above. The membranes were then stripped using Re-Blot Plus Mild (Chemicon) according to the manufacturer's instructions and re-probed for IRβ, PTP1B, SHP2, or PKC-Θ as indicated in the figure legends. Quantification of each blot with IRβ immunoprecipitate is expressed relative to the amount of IRβ detected in the same lane on the blot and normalized to the loading control. As a negative control, an equivalent amount of submaximal insulin-stimulated muscle was also subjected to the immunoprecipitation protocol using 5 μg of rabbit immunoglobulin G in place of anti-IRβ antibody.
Citrate synthase activity
Fifty micrograms of muscle homogenate from samples incubated without insulin were used for determination of citrate synthase activity (Srere, 1969). Briefly, samples were diluted into a final volume of 400 μl of 100 mm KH2PO4−100 mm K2HPO4, pH 7.4–50 μm EGTA−50 μm Na4EDTA and kept on ice. Duplicate samples of 100 μl and 200 μl each were mixed into a final volume of 900 μl of 100 mm Tris, pH 8.0–0.167 mm acetyl coenzyme A−0.111 mm 5,5′-dithiobis-(2-nitrobenzoic acid) and placed in a 30°C water bath for 5 min. Oxaloacetic acid, 100 μl at a concentration of 5 mm, was immediately added and the reaction measured in a spectrophotometer at 412 nm for 3 min.
Glycogen concentration
The approximately 2/3 sections of the left epitrochlearis muscles incubated without insulin (from animals where the right muscle was incubated with maximal insulin) were used for determination of glycogen concentration using the anthrone method (Hassid & Abraham, 1957). Briefly, frozen muscle samples were dissolved in 5 m KOH, and glycogen was precipitated with ethanol. The glycogen precipitate was then hydrolysed by boiling in 5 n HCl for 1 h, neutralized with 5 n NaOH, and an aliquot of sample was boiled in 95% H2SO4−0.1% anthrone for 15 min. The concentration of glucosyl units was then determined against glucose standards in a spectrophotometer at 620 nm.
Statistics
Groups were compared using analysis of variance (ANOVA), and the Student-Neuman-Keuls post hoc test was used to determine which groups were different. Total running distance, running distance during the third week and on day 21, initial and final body mass, total food intake, and food intake on the night before the animals were killed were each considered and eliminated as possible covariates for 2-DOG uptake. Experiments involving PTP1B, SHP2 and PKC-Θ were also analysed using ANOVA with a contrast statement (Neter et al. 1996) to compare the mean of WL5 and WL29 to the mean of WL53 and SED. Since an analysis of residuals showed some extreme values, the results were verified using the non-parametric Wilcoxon rank sum test. A one-sided alternative was used for both the contrasts and the rank sum test. Significance for all tests was defined at P≤ 0.05. All data are presented ± s.e.m. Statistics were performed using either SigmaStat (Systat Software, Inc., Point Richmond, CA, USA) or SAS (SAS Institute Inc., Cary, NC, USA).
Results
2-Deoxyglucose uptake
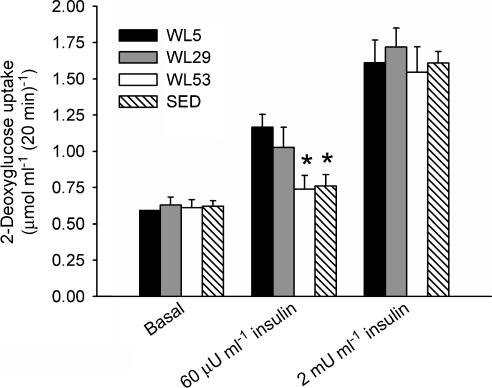
2-Deoxyglucose (2-DOG) is a glucose analogue whose uptake into isolated skeletal muscle preparations has been shown to accurately estimate glucose uptake (Hansen et al. 1994). 2-DOG uptake into the isolated epitrochlearis muscle in vitro was measured either without insulin (basal), or in the presence of a submaximally (60 μU ml−1) or maximally (2 mU ml−1) stimulating insulin concentration. Basal 2-DOG uptake did not differ among the four treatment groups, indicating that the postexercise increase in basal 2-DOG uptake had dissipated (Richter, 1996). Maximal insulin-stimulated 2-DOG uptake was also not different among the groups (Fig. 2). Submaximal 2-DOG uptake (μmol 2-DOG (ml intracellular space)−1 (20 min)−1) in WL53 (0.740 ± 0.093) was significantly lower relative to WL5 (1.166 ± 0.090); WL29 (1.027 ± 0.139) was intermediate between, but not different from, WL5 and WL53. SED (0.761 ± 0.079) was also lower than WL5 and not different from WL53 (see Fig. 1 for group descriptions). Thus, submaximal 2-DOG uptake declined from WL5 values to SED values after 53 h of wheel lock. For subsequent immunoanalyses, only muscle from animals used for the submaximal insulin concentrations was assayed as this was the only insulin treatment producing significant differences.
Figure 2. Effect of physical inactivity on 2-DOG uptake into isolated epitrochlearis muscle under basal (no added insulin), submaximal insulin-stimulated (60 μU ml−1), and maximal insulin-stimulated (2 mU ml−1) conditions.
Groups had wheels locked for 5 h (WL5), 29 h (WL29), or 53 h (WL53) after 3 weeks of voluntary wheel running, or were sedentary (SED) without running wheels. Columns are mean ± s.e.m.* Significantly different from groups without an asterisk within the same insulin concentration (ANOVA, P≤ 0.05). n = 15–16 in each group for basal and 7–8 in each group for both insulin concentrations.
Insulin binding, insulin receptor protein level, and insulin receptor tyrosine phosphorylation
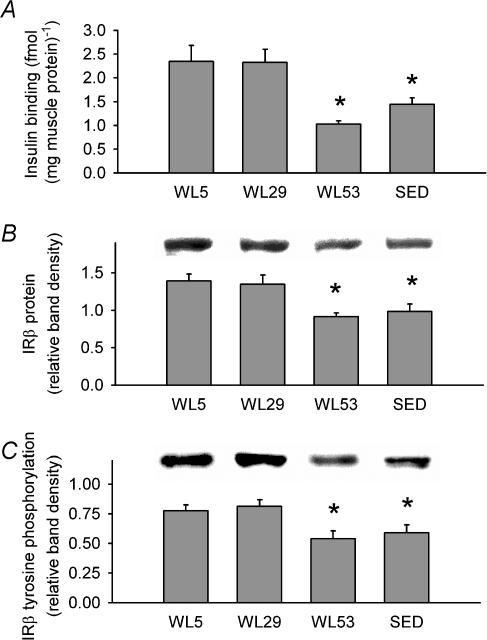
A change in submaximal response with no change in maximal response has been interpreted to suggest that there are alterations at the receptor level (Kahn, 1978; Goodman, 2001). As the results for 2-DOG uptake demonstrated this pattern, the next studies were directed toward the insulin receptor. Specific insulin binding (fmol (mg total protein)−1) at submaximal insulin in the epitrochlearis muscle was lower in WL53 (1.027 ± 0.069) compared to both WL5 and WL29 (2.346 ± 0.336 and 2.324 ± 0.279, respectively; Fig. 3A). WL53 and SED (1.446 ± 0.137) were not different. IRβ protein level in WL53 was decreased by 34% and 32% compared to WL5 and WL29, respectively, and was 29% and 27% lower in SED than in WL5 and WL29, respectively (Fig. 3B). Relative to the amount of IRβ on the same blot, submaximal insulin-stimulated IRβ tyrosine phosphorylation was decreased by 30% and 27% in WL53 compared to WL5 and WL29, respectively, and was 24% and 27% lower in SED than in WL5 and WL29, respectively (Fig. 3C). Thus, three descriptive indices of the insulin receptor (insulin binding, IRβ protein, and IRβ tyrosine phosphorylation) showed identical treatment patterns, being unchanged from WL5 to WL29, and decreasing in WL53 to SED levels.
Figure 3. Effect of decreased physical activity on descriptive indices of the insulin receptor and its activation in epitrochlearis muscle.
A, submaximal insulin binding to the epitrochlearis muscle. B, IRβ protein levels relative to loading control (see Methods) with a representative immunoblot (above graph). C, tyrosine phosphorylation of IRβ in response to 40 min of submaximal insulin stimulation with a representative immunoblot (above graph). IRβ immunoprecipitates were subjected to immunoblotting for phosphotyrosine, then stripped and re-probed for IRβ (see Methods). Data were normalized to a loading control (see Methods) and are expressed relative to the normalized band intensity for IRβ protein present in the same lane. Columns are mean ± s.e.m.* Signficantly different (ANOVA, P≤ 0.05) from groups without an asterisk. n = 6–8 in each group.
PTP1B, SHP2 and PKC-Θ
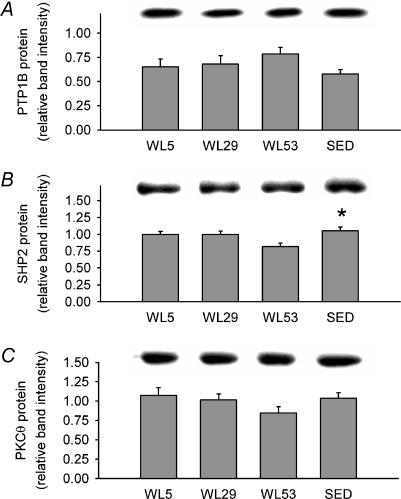
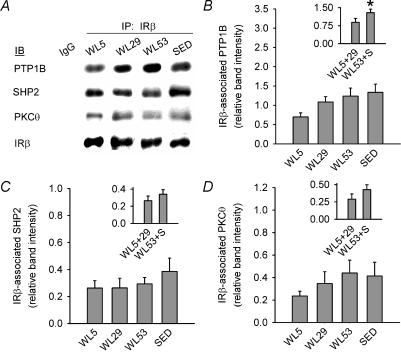
To obtain information on potential factors producing the change in IRβ tyrosine phosphorylation, levels of three proteins (PTP1B, SHP2 and PKC-Θ) that can negatively regulate IRβ tyrosine phosphorylation were measured in homogenates and in IRβ immunoprecipitates (Rocchi et al. 1996; Elchebly et al. 1999; Strack et al. 2000; Ouwens et al. 2001; Zabolotny et al. 2004). The pre-hoc hypothesis was that the level of each of these proteins and their association with IRβ upon submaximal insulin stimulation would show an inverse pattern of changes compared to the pattern of changes in IRβ tyrosine phosphorylation observed in the earlier part of the present study, i.e. in comparison to Fig. 3C where WL53 and SED were lower than WL5 and WL29 for IRβ tyrosine phosphorylation, WL53 and SED would be higher than WL5 and WL29 for PTP1B, SHP2 and PKC-Θ. Total protein level for PTP1B and PKC-Θ did not differ between groups (Fig. 4A and C), while the SHP2 protein level was 23% lower in WL53 than SED (Fig. 4B). The amount of PTP1B, SHP2, or PKC-Θ associated with IRβ, expressed per unit of IRβ on the same blot, was also not different between groups (P = 0.07 for PTP1B; Fig. 5). Because it had been hypothesized a priori that both WL53 and SED would each be greater than either WL5 or WL29, we further tested this hypothesis using ANOVA with a contrast statement (Neter et al. 1996). When this method was employed, it was found that the mean of SED and WL53 had a significantly higher IRβ-associated PTP1B than the mean of WL5 and WL29 (Fig. 5B, inset); PTP1B, SHP2 and PKC-Θ total protein levels (data not shown) and IRβ-associated SHP2 and PKC-Θ were not significantly different (Fig. 5C and D, insets). These results were confirmed using the Wilcoxon rank sum test.
Figure 4. Effect of decreased physical activity on negative regulators of insulin receptor activation in epitrochlearis muscle.
PTP1B (A), SHP2 (B) and PKC-Θ (C) protein levels normalized to a loading control (see Methods) with representative immunoblots (above graphs). Columns are mean ± s.e.m.* Significantly different from WL53 (ANOVA, P≤ 0.05). n = 6–8 in each group.
Figure 5. Representative immunoblots (A) and protein levels of PTP1B (B), SHP2 (C) and PKC-Θ (D) in IRβ immunoprecipitates from epitrochlearis homogenates from muscle incubated with submaximal insulin for 40 min.
IRβ immunoprecipitates were subjected to immunoblotting for IRβ, then stripped and re-probed for PTP1B, SHP2, or PKC-Θ (see Methods). Data in each panel were normalized to a loading control (see Methods) on the same blot and are expressed relative to the normalized band intensity for IRβ present in the same lane. IgG in the first lane of panel A was immunoprecipitated with rabbit immunoglobulin G and is a negative control with no detectable signal. Columns in the main graphs represent the means for each group ± s.e.m. The columns in the insets illustrate the means ± s.e.m. for the linear combination of the individual group means for WL5 and WL29 (WL5+29) and for WL53 and SED (WL53+S). See Results for more detail. IP, immunoprecipitation; IB, immunoblot. * Significantly different (ANOVA with contrast statement, P≤ 0.05). n = 6–8 in each group.
Akt, citrate synthase activity, glycogen concentration and GLUT4
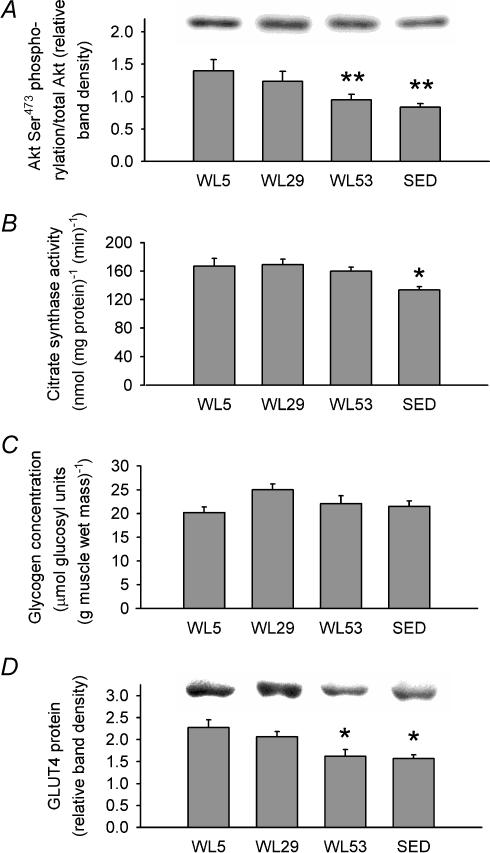
Akt total protein content did not differ between groups (data not shown). Akt Ser473 phosphorylation per unit of Akt on the same blot was 32% and 40% lower in WL53 and SED, respectively, than in WL5 (Fig. 6A), providing confirmation that signalling downstream of the insulin receptor is also decreased after 53 h of wheel lock. Citrate synthase activity (nmol (mg protein)−1 min−1) in the epitrochlearis was lower in SED compared to all other groups (169 ± 8 in WL5, 167 ± 11 in WL29, 160 ± 6 in WL53 and 134 ± 5 in SED; Fig. 6B), indicating that oxidative capacity (Holloszy et al. 1970) of the epitrochlearis muscle did not decline after 53 h of wheel lock. As other studies have shown a relationship between 2-DOG uptake and glycogen concentration (Henriksen & Halseth, 1994; Jensen et al. 1997; Host et al. 1998b) or GLUT4 protein level (Henriksen et al. 1990; Kawanaka et al. 1997; Host et al. 1998a; Reynolds et al. 2000), these were also measured in the present study. Consistent with other studies using voluntary wheel running (Rodnick et al. 1992; Hokama et al. 1997), epitrochlearis muscle glycogen levels in the present study were not significantly different between groups (Fig. 6C), suggesting that glycogen is not playing a regulatory role in the diminished submaximal insulin-stimulated 2-DOG uptake in the wheel lock period. The GLUT4 protein level in WL53 decreased 29% and 21% relative to WL5 and WL29, respectively (Fig. 6D). The level of GLUT4 was also less in SED by 31% and 24% relative to WL5 and WL29, respectively.
Figure 6. Biochemical adaptations to decreased physical activity in the epitrochlearis muscle.
A, Akt Ser473 phosphorylation relative to the total amount of Akt in the same lane and normalized to a loading control (see Methods) with a representative immunoblot (above graph). B, citrate synthase activity. C, glycogen concentration. D, GLUT4 protein levels normalized to a loading control (see Methods) with a representative immunoblot (above graph). Columns are mean ± s.e.m.* Signficantly different (ANOVA, P≤ 0.05) from groups without an asterisk. ** Significantly different (ANOVA, P≤ 0.05) from WL5. n = 6–8 in each group.
Running activity, body mass and food intake
Total running distance (103.6 ± 2.0 km), average daily running distance during the third week (5.7 ± 0.1 km), and distance run on the last day (day 21; 6.1 ± 0.4 km) did not differ among groups with wheel access. Initial body mass (71.9 ± 0.5 g) did not differ between groups. Final body mass was lower in SED (186.3 ± 3.9 g) than in WL5, WL29 and WL53 (199.0 ± 3.0, 208.9 ± 2.5 and 212.3 ± 3.3 g, respectively). Average daily food intake and food intake on the night before the animals were killed was also lower in SED (14.8 ± 0.3 and 16.0 ± 0.4 g) than in WL5 (17.9 ± 0.3 and 21.4 ± 0.5 g), WL29 (17.0 ± 0.4 and 19.5 ± 0.5 g), and WL53 (17.9 ± 0.3 and 20.9 ± 0.4 g). Data for running activity, body mass and food intake are not shown.
Discussion
In rats prohibited from voluntary running after 3 weeks of access to voluntary running wheels, submaximal insulin-stimulated 2-DOG uptake in the 53 h wheel lock group (WL53) exhibited a significant decline to values similar to those in the sedentary group (SED; Fig. 2). A novel observation of the current study is that insulin binding, IRβ protein level, and insulin-mediated IRβ tyrosine phosphorylation remained at 5 h wheel lock (WL5) values for 29 h of wheel lock (WL29), but declined to SED values after 53 h of wheel lock (Fig. 3). Moreover, GLUT4 protein levels followed this same pattern, declining to SED values between 29 and 53 h of wheel lock (Fig. 6D). These observations are important as they identify by association some of the potential factors that may be involved in diminished submaximal insulin-mediated glucose uptake into the epitrochlearis muscle upon decreased physical activity. Furthermore, the observations demonstrate that these reductions coordinately transpired between 29 and 53 h after the running wheels were locked (Table 1).
Table 1.
Directional changes in groups having wheels locked for 29 h (WL29) or 53 h (WL53), and in the sedentary (SED) group, relative to 5 h of wheel lock
| WL29 | WL53 | SED | |
|---|---|---|---|
| Insulin (60 μU ml−1)-stimulated 2-DOG uptake | ↔ | ↓ | ↓ |
| Insulin binding | ↔ | ↓ | ↓ |
| IRβ protein level | ↔ | ↓ | ↓ |
| IRβ tyrosine phosphorylation | ↔ | ↓ | ↓ |
| Akt Ser473 phosphorylation | ↔ | ↓ | ↓ |
| GLUT4 protein level | ↔ | ↓ | ↓ |
Arrows indicate the relative direction of significant changes (ANOVA, P≤ 0.05) for each of the measured variables. The table demonstrates the similar pattern of change observed in the listed variables.
The decrease in IRβ tyrosine phosphorylation per unit of IRβ at 53 h of wheel lock suggests that, in addition to the decrease in IRβ protein level, there are also concurrent adaptations which diminish IRβ phosphorylation. An important step regulating signal transduction through the insulin receptor is the action of protein tyrosine phosphatases, which dephosphorylate tyrosine residues on the receptor, rendering it inactive (Cheng et al. 2002). To further investigate potential changes in the regulation of IRβ tyrosine phosphorylation, we examined two protein tyrosine phosphatases, PTP1B and SHP2 (Rocchi et al. 1996; Seely et al. 1996; Elchebly et al. 1999; Boute et al. 2003; Zabolotny et al. 2004). In addition, we examined PKC-Θ, which decreases insulin-stimulated tyrosine phosphorylation by inducing serine phosphorylation of IRβ, although it is unclear whether this is a direct or indirect effect (Strack et al. 2000). PTP1B, SHP2 and PKC-Θ protein levels did not differ among WL5, WL29 and WL53, while WL53 was higher than SED for SHP2 protein level (Fig. 4). Due to a scarcity of tissue, we were unable to determine the possibility that there is altered localization of PKC-Θ to the sarcolemma, which could affect its activity and association with the insulin receptor (Idris et al. 2002; Altman & Villalba, 2003). The amount of PTP1B, SHP2 and PKC-Θ associated with IRβ also did not differ between groups (Fig. 5). When ANOVA was used with a contrast statement, the mean of WL53 and SED was found to be significantly greater than the mean of WL5 and WL29 for IRβ-associated PTP1B, but not for IRβ-associated SHP2 or PKC-Θ (Fig. 5, insets). This provides some support for the hypothesis that IRβ-associated PTP1B would be higher in WL53 and SED than in WL5 and WL29. Based on evidence showing that PTP1B negatively regulates IRβ tyrosine phosphorylation and skeletal muscle glucose uptake (Elchebly et al. 1999; Klaman et al. 2000; Cheng et al. 2002; Zabolotny et al. 2004), this suggests that PTP1B might play a role in the down-regulation of submaximal insulin-stimulated tyrosine phosphorylation between 29 and 53 h of wheel lock, but further experiments are needed to test this hypothesis.
Insulin stimulates glucose uptake into skeletal muscle by inducing translocation of GLUT4 to the cell membrane (Watson & Pessin, 2001). Others have shown an association between decreases in epitrochlearis muscle GLUT4 protein level and insulin-stimulated glucose uptake 40–90 h following the cessation of swim- or treadmill-training (Kawanaka et al. 1997; Host et al. 1998a; Reynolds et al. 2000). In the current study, GLUT4 declined to SED values between 29 and 53 h of wheel lock, confirming the previous studies and extending them to include cessation of voluntary wheel running. It is noteworthy that GLUT4 exhibited the same time pattern of decrease as insulin binding, IRβ protein level, and IRβ tyrosine phosphorylation (Table 1), as it raises the intriguing possibility that a single regulatory event could be responsible for the synchronization.
An additional noteworthy finding in the present study is that the sedentary group, which was housed without voluntary running wheels, weighed 6% less after 3 weeks than animals with exposure to the running wheels. This is in agreement with a study using male Fischer 344 rats of a similar age (Yano et al. 1997), whereas studies using similarly aged male rats of other strains have shown either no difference (Hokama et al. 1997; Zachwieja et al. 1997) or an increase (Collier et al. 1969; Johnson et al. 1977) in body mass with normal cage activity relative to those with access to running wheels. The lower body mass in the sedentary group in this study is at least partly explained by their lower food intake; this is consistent with other voluntary wheel running studies in which male rats of a similar age without access to running wheels consume less food than do those with access (Yano et al. 1997; Zachwieja et al. 1997), although some studies show that similarly aged male rats with normal cage activity consume more food (Collier et al. 1969; Johnson et al. 1977).
A potential translational significance of the current study to human physiology is that many studies have shown that decreased physical activity produces lower insulin sensitivity in healthy human subjects in as little as 5 days following the cessation of habitual exercise (see Introduction). Despite the clear relationships between physical inactivity and insulin resistance and between insulin resistance and chronic disease (Booth et al. 2002), there have been few studies examining the initial cellular changes by which a reduction of voluntary physical activity to sedentary levels decreases insulin-stimulated glucose uptake. A unique aspect of the present study is the emphasis on cessation of voluntary running activity in freely fed rats, and the results demonstrate that submaximal 2-DOG uptake into the epitrochlearis muscle declines to sedentary levels between 5 and 53 h after cessation of voluntary wheel running (Fig. 2). Moreover, this decline is accompanied by a concomitant decrease in insulin binding, IRβ protein level, submaximal insulin-stimulated IRβ tyrosine phosphorylation, Akt Ser473 phosphorylation, and GLUT4 protein level (Table 1). Others have reported the decline in GLUT4 protein following cessation of swim- or treadmill-training (Kawanaka et al. 1997; Host et al. 1998a; Reynolds et al. 2000), but this is the first study to demonstrate a rapid reduction in IRβ protein and essential components of insulin receptor activation upon the cessation of voluntary wheel running, although our interpretations must be tempered by not knowing whether there is a difference in these variables in the non-insulin-stimulated condition.
In conclusion, decreased physical activity precipitated a rapid and synchronous decline in submaximal insulin-stimulated glucose uptake, descriptive indices of the insulin receptor and its activation, and GLUT4 protein. This suggests that in rats accustomed to daily voluntary wheel running, multiple adaptations regulating insulin-mediated glucose uptake into the epitrochlearis muscle take place between 29 and 53 h of decreased physical activity, and it raises the possibility that a single regulatory event could be responsible for the coordinated decrease.
Acknowledgments
We thank Dr Richard Madsen for statistical advice on ANOVA with contrasts, Dr Craig S. Stump for helpful comments on the manuscript, Dr John Holloszy for helpful comments on the experimental design and for assistance with dissection of the epitrochlearis muscle and the assay for 2-DOG transport, and Tsghe Abraha for her expert technical assistance. The research was supported by the following grants: American Heart Association 0135202Z (D.S.K.) and NIH R21AR48368 and RO1AR19393 (F.W.B.).
References
- Altman A, Villalba M. Protein kinase C-theta (PKCtheta): it's all about location, location, location. Immunol Rev. 2003;192:53–63. doi: 10.1034/j.1600-065x.2003.00027.x. 10.1034/j.1600-065X.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- Arciero PJ, Smith DL, Calles-Escandon J. Effects of short-term inactivity on glucose tolerance, energy expenditure, and blood flow in trained subjects. J Appl Physiol. 1998;84:1365–1373. doi: 10.1152/jappl.1998.84.4.1365. [DOI] [PubMed] [Google Scholar]
- Baron AD, Brechtel G, Wallace P, Edelman SV. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am J Physiol. 1988;255:E769–E774. doi: 10.1152/ajpendo.1988.255.6.E769. [DOI] [PubMed] [Google Scholar]
- Bonen A, Clune PA, Tan MH. Chronic exercise increases insulin binding in muscles but not liver. Am J Physiol. 1986;251:E196–E203. doi: 10.1152/ajpendo.1986.251.2.E196. [DOI] [PubMed] [Google Scholar]
- Booth FW, Chakravarthy MV, Gordon SE, Spangenburg EE. Waging war on physical inactivity: using modern molecular ammunition against an ancient enemy. J Appl Physiol. 2002;93:3–30. doi: 10.1152/japplphysiol.00073.2002. [DOI] [PubMed] [Google Scholar]
- Boute N, Boubekeur S, Lacasa D, Issad T. Dynamics of the interaction between the insulin receptor and protein tyrosine-phosphatase 1B in living cells. EMBO Rep. 2003;4:313–319. doi: 10.1038/sj.embor.embor767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cheng A, Dube N, Gu F, Tremblay ML. Coordinated action of protein tyrosine phosphatases in insulin signal transduction. Eur J Biochem. 2002;269:1050–1059. doi: 10.1046/j.0014-2956.2002.02756.x. [DOI] [PubMed] [Google Scholar]
- Collier G, Leshner AI, Squibb RL. Dietary self-selection in active and non-active rats. Physiol Behav. 1969;4:79–82. [Google Scholar]
- Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- Goodman HM. Endocrinology concepts for medical students. Adv Physiol Educ. 2001;25:213–224. doi: 10.1152/advances.2001.25.4.213. [DOI] [PubMed] [Google Scholar]
- Gustat J, Srinivasan SR, Elkasabany A, Berenson GS. Relation of self-rated measures of physical activity to multiple risk factors of insulin resistance syndrome in young adults: the Bogalusa Heart Study. J Clin Epidemiol. 2002;55:997–1006. doi: 10.1016/s0895-4356(02)00427-4. 10.1016/S0895-4356(02)00427-4. [DOI] [PubMed] [Google Scholar]
- Hansen PA, Gulve EA, Holloszy JO. Suitability of 2-deoxyglucose for in vitro measurement of glucose transport activity in skeletal muscle. J Appl Physiol. 1994;76:979–985. doi: 10.1152/jappl.1994.76.2.979. [DOI] [PubMed] [Google Scholar]
- Hassid WZ, Abraham S. Chemical procedures for analysis of polysaccharides. Meth Enzymol. 1957;3:34–50. [Google Scholar]
- Heath GW, Gavin JR, 3rd, Hinderliter JM, Hagberg JM, Bloomfield SA, Holloszy JO. Effects of exercise and lack of exercise on glucose tolerance and insulin sensitivity. J Appl Physiol. 1983;55:512–517. doi: 10.1152/jappl.1983.55.2.512. [DOI] [PubMed] [Google Scholar]
- Henriksen EJ, Bourey RE, Rodnick KJ, Koranyi L, Permutt MA, Holloszy JO. Glucose transporter protein content and glucose transport capacity in rat skeletal muscles. Am J Physiol. 1990;259:593–598. doi: 10.1152/ajpendo.1990.259.4.E593. [DOI] [PubMed] [Google Scholar]
- Henriksen EJ, Halseth AE. Early alterations in soleus GLUT-4, glucose transport, and glycogen in voluntary running rats. J Appl Physiol. 1994;76:1862–1867. doi: 10.1152/jappl.1994.76.5.1862. [DOI] [PubMed] [Google Scholar]
- Hokama JY, Streeper RS, Henriksen EJ. Voluntary exercise training enhances glucose transport in muscle stimulated by insulin-like growth factor I. J Appl Physiol. 1997;82:508–512. doi: 10.1152/jappl.1997.82.2.508. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Oscai LB, Don IJ, Mole PA. Mitochondrial citric acid cycle and related enzymes: adaptive response to exercise. Biochem Biophys Res Commun. 1970;40:1368–1373. doi: 10.1016/0006-291x(70)90017-3. [DOI] [PubMed] [Google Scholar]
- Host HH, Hansen PA, Nolte LA, Chen MM, Holloszy JO. Rapid reversal of adaptive increases in muscle GLUT-4 and glucose transport capacity after training cessation. J Appl Physiol. 1998a;84:798–802. doi: 10.1152/jappl.1998.84.3.798. [DOI] [PubMed] [Google Scholar]
- Host HH, Hansen PA, Nolte LA, Chen MM, Holloszy JO. Glycogen supercompensation masks the effect of a training-induced increase in GLUT-4 on muscle glucose transport. J Appl Physiol. 1998b;85:133–138. doi: 10.1152/jappl.1998.85.1.133. [DOI] [PubMed] [Google Scholar]
- Houmard JA, Shinebarger MH, Dolan PL, Leggett-Frazier N, Bruner RK, McCammon MR, Israel RG, Dohm GL. Exercise training increases GLUT-4 protein concentration in previously sedentary middle-aged men. Am J Physiol. 1993;264:896–901. doi: 10.1152/ajpendo.1993.264.6.E896. [DOI] [PubMed] [Google Scholar]
- Idris I, Gray S, Donnelly R. Insulin action in skeletal muscle: isozyme-specific effects of protein kinase C. Ann N Y Acad Sci. 2002;967:176–182. [PubMed] [Google Scholar]
- Jensen J, Aslesen R, Ivy JL, Brors O. Role of glycogen concentration and epinephrine on glucose uptake in rat epitrochlearis muscle. Am J Physiol. 1997;272:E649–E655. doi: 10.1152/ajpendo.1997.272.4.E649. [DOI] [PubMed] [Google Scholar]
- Johnson PB, Kochan R, Graves D. Voluntary exercise, food intake pattern, and early growth in young male albino rats. Med Sci Sports. 1977;9:54. [Google Scholar]
- Kahn CR. Insulin resistance, insulin insensitivity, and insulin unresponsiveness: a necessary distinction. Metabolism. 1978;27:1893–1902. doi: 10.1016/s0026-0495(78)80007-9. [DOI] [PubMed] [Google Scholar]
- Kawanaka K, Tabata I, Katsuta S, Higuchi M. Changes in insulin-stimulated glucose transport and GLUT-4 protein in rat skeletal muscle after training. J Appl Physiol. 1997;83:2043–2047. doi: 10.1152/jappl.1997.83.6.2043. [DOI] [PubMed] [Google Scholar]
- King DS, Dalsky GP, Clutter WE, Young DA, Staten MA, Cryer PE, Holloszy JO. Effects of exercise and lack of exercise on insulin sensitivity and responsiveness. J Appl Physiol. 1988;64:1942–1946. doi: 10.1152/jappl.1988.64.5.1942. [DOI] [PubMed] [Google Scholar]
- Klaman LD, Boss O, Peroni OD, Kim JK, Martino JL, Zabolotny JM, Moghal N, Lubkin M, Kim YB, Sharpe AH, Stricker-Krongrad A, Shulman GI, Neel BG, Kahn BB. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol. 2000;20:5479–5489. doi: 10.1128/mcb.20.15.5479-5489.2000. 10.1128/MCB.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriska AM, LaPorte RE, Pettitt DJ, Charles MA, Nelson RG, Kuller LH, Bennett PH, Knowler WC. The association of physical activity with obesity, fat distribution and glucose intolerance in Pima Indians. Diabetologia. 1993;36:863–869. doi: 10.1007/BF00400363. 10.1007/BF00400363. [DOI] [PubMed] [Google Scholar]
- Le Marchand-Brustel Y, Freychet P. Studies of insulin insensitivity in soleus muscles of obese mice. Metabolism. 1978;27:1982–1993. doi: 10.1016/s0026-0495(78)80014-6. [DOI] [PubMed] [Google Scholar]
- Mayer-Davis EJ, D'Agostino R, Jr, Karter AJ, Haffner SM, Rewers MJ, Saad M, Bergman RN. Intensity and amount of physical activity in relation to insulin sensitivity: the Insulin Resistance Atherosclerosis Study. JAMA. 1998;279:669–674. doi: 10.1001/jama.279.9.669. 10.1001/jama.279.9.669. [DOI] [PubMed] [Google Scholar]
- Mikines KJ, Sonne B, Tronier B, Galbo H. Effects of acute exercise and detraining on insulin action in trained men. J Appl Physiol. 1989;66:704–711. doi: 10.1152/jappl.1989.66.2.704. 10.1063/1.343541. [DOI] [PubMed] [Google Scholar]
- Moraska A, Fleshner M. Voluntary physical activity prevents stress-induced behavioral depression and anti-KLH antibody suppression. Am J Physiol Regul Integr Comp Physiol. 2001;281:R484–R489. doi: 10.1152/ajpregu.2001.281.2.R484. [DOI] [PubMed] [Google Scholar]
- Neter J, Wasserman W, Kutner MH, Nachtscheim CJ. Applied Linear Statistical Models. 4th edn. Boston: Richard D. Irwin, Inc; 1996. 10.1016/S0303-7207(01)00389-6. [Google Scholar]
- Ouwens DM, van der Zon GC, Maassen JA. Modulation of insulin-stimulated glycogen synthesis by Src Homology Phosphatase 2. Mol Cell Endocrinol. 2001;175:131–140. doi: 10.1016/s0303-7207(01)00389-6. [DOI] [PubMed] [Google Scholar]
- Reynolds TH, Brozinick JT, Jr, Larkin LM, Cushman SW. Transient enhancement of GLUT-4 levels in rat epitrochlearis muscle after exercise training. J Appl Physiol. 2000;88:2240–2245. doi: 10.1152/jappl.2000.88.6.2240. 10.1063/1.1287758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter EA. Glucose utilization. In: Rowell LB, Shepherd LT, editors. In Handbook of Physiology, section 12, Exercise. Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 870–911. 10.1210/en.137.11.4944. [Google Scholar]
- Rocchi S, Tartare-Deckert S, Sawka-Verhelle D, Gamha A, van Obberghen E. Interaction of SH2-containing protein tyrosine phosphatase 2 with the insulin receptor and the insulin-like growth factor-I receptor: studies of the domains involved using the yeast two-hybrid system. Endocrinology. 1996;137:4944–4952. doi: 10.1210/endo.137.11.8895367. [DOI] [PubMed] [Google Scholar]
- Rodnick KJ, Henriksen EJ, James DE, Holloszy JO. Exercise training, glucose transporters, and glucose transport in rat skeletal muscles. Am J Physiol. 1992;262:9–14. doi: 10.1152/ajpcell.1992.262.1.C9. [DOI] [PubMed] [Google Scholar]
- Rodnick KJ, Reaven GM, Haskell WL, Sims CR, Mondon CE. Variations in running activity and enzymatic adaptations in voluntary running rats. J Appl Physiol. 1989;66:1250–1257. doi: 10.1152/jappl.1989.66.3.1250. [DOI] [PubMed] [Google Scholar]
- Seely BL, Staubs PA, Reichart DR, Berhanu P, Milarski KL, Saltiel AR, Kusari J, Olefsky JM. Protein tyrosine phosphatase 1B interacts with the activated insulin receptor. Diabetes. 1996;45:1379–1385. doi: 10.2337/diab.45.10.1379. [DOI] [PubMed] [Google Scholar]
- Seider MJ, Nicholson WF, Booth FW. Insulin resistance for glucose metabolism in disused soleus muscle of mice. Am J Physiol. 1982;242:12–18. doi: 10.1152/ajpendo.1982.242.1.E12. [DOI] [PubMed] [Google Scholar]
- Srere PA. Citrate synthase. Meth Enzymol. 1969;13:3–5. [Google Scholar]
- Strack V, Hennige AM, Krutzfeldt J, Bossenmaier B, Klein HH, Kellerer M, Lammers R, Haring HU. Serine residues 994 and 1023/25 are important for insulin receptor kinase inhibition by protein kinase C isoforms beta2 and theta. Diabetologia. 2000;43:443–449. doi: 10.1007/s001250051327. 10.1007/s001250051327. [DOI] [PubMed] [Google Scholar]
- Tsai AC, Bach J, Borer KT. Somatic, endocrine, and serum lipid changes during detraining in adult hamsters. Am J Clin Nutr. 1981;34:373–376. doi: 10.1093/ajcn/34.3.373. [DOI] [PubMed] [Google Scholar]
- Watson RT, Pessin JE. Intracellular organization of insulin signaling and GLUT4 translocation. Recent Prog Horm Res. 2001;56:175–193. doi: 10.1210/rp.56.1.175. 10.1210/rp.56.1.175. [DOI] [PubMed] [Google Scholar]
- Yano H, Yano L, Kinoshita S, Tsuji E. Effect of voluntary exercise on maximal oxygen uptake in young female Fischer 344 rats. Jpn J Physiol. 1997;47:139–141. doi: 10.2170/jjphysiol.47.139. [DOI] [PubMed] [Google Scholar]
- Zabolotny JM, Haj FG, Kim YB, Kim HJ, Shulman GI, Kim JK, Neel BG, Kahn BB. Transgenic overexpression of protein-tyrosine phosphatase 1B in muscle causes insulin resistance, but overexpression with leukocyte antigen-related phosphatase does not additively impair insulin action. J Biol Chem. 2004;279:24844–24851. doi: 10.1074/jbc.M310688200. 10.1074/jbc.M310688200. [DOI] [PubMed] [Google Scholar]
- Zachwieja JJ, Hendry SL, Smith SR, Harris RB. Voluntary wheel running decreases adipose tissue mass and expression of leptin mRNA in Osborne-Mendel rats. Diabetes. 1997;46:1159–1166. doi: 10.2337/diab.46.7.1159. [DOI] [PubMed] [Google Scholar]