Abstract
We previously reported that sodium restriction during pregnancy reduces plasma volume expansion and promotes intra-uterine growth restriction (IUGR) in rats while it activates the renin–angiotensin–aldosterone system (RAAS). In the present study, we proceeded to determine whether expression of the two angiotensin II (ANGII) receptor subtypes (AT1 and AT2) change in relation to maternal water–electrolyte homeostasis and fetal growth. To this end, pregnant (gestation day 15) and non-pregnant Sprague-Dawley rats were randomly assigned to two groups fed either normal, or Na+-restricted diets for 7 days. At the end of the treatment period, plasma aldosterone and renin activity as well as plasma and urine electrolytes were measured. Determinations for AT1 and AT2 mRNA and protein were made by RNase protection assay and photoaffinity labelling, respectively, using a number of tissues implicated in volume regulation and fetal growth. In non-pregnant rats, Na+ restriction decreases Na+ excretion without altering plasma volume, plasma Na+ concentration or the expression of AT1 and AT2 mRNA or protein in the tissues examined. In normally fed pregnant rats when compared to non-pregnant controls, AT1 mRNA increases in the hypothalamus as well as pituitary and declines in uterine arteries, while AT1 protein decreases in the kidney and AT2 mRNA declines in the adrenal cortex. In pregnant rats, Na+ restriction induces a decrease in plasma Na+, an increase in plasma urea, as well as a decline in renal urea and creatinine clearance rates. Protein levels for both AT1 and AT2 in the pituitary and AT2 mRNA in the adrenal cortex are lower in the Na+-restricted pregnant group when compared to normally fed pregnant animals. Na+ restriction also induces a decrease in AT1 protein in the placenta. In conclusion, these results suggest that pregnancy may increase sensitivity to Na+ depletion by the tissue-specific modulation of ANGII receptors. Finally, these receptors may be implicated in the IUGR response to low Na+.
Maternal haemodynamic adaptation and marked physiological changes occur throughout pregnancy in both humans and rats to ensure the normal evolution of mother and fetus. During normal pregnancy in the rat, remodelling of the uterine vasculature results in longer, larger calibre, and more distensible vessels (Osol & Cipolla, 1993) that can then accommodate the increased blood flow to the uterine artery (Ahokas et al. 1983) that helps support fetal growth. Furthermore, maternal extracellular or plasma volume expansion occurs during pregnancy concomitant with the activation of the renin–angiotensin–aldosterone system (RAAS) (Verkeste et al. 1998). Despite the marked increase in plasma volume, blood pressure decreases significantly during gestation in the rat (St-Louis & Massicotte, 1985) due to a major reduction of peripheral vascular resistance (Dowell & Kauer, 1997). In addition, while the glomerular filtration rate and renal plasma flow are known to increase in the rat during pregnancy (Baylis, 1987), Na+ retention is markedly elevated, particularly during the last week of gestation (Barron, 1987), the time for maximum fetal growth (Beaudoin, 1980). However, decreasing dietary Na+ during the last week of gestation was reported by our laboratory to significantly reduce plasma volume expansion and promote intra-uterine growth restriction (IUGR) while further activating the RAAS (Roy-Clavel et al. 1999). Comparable alterations in plasma volume are observed in women with idiopathic IUGR (Salas et al. 1993).
Several factors contribute to the haemodynamic changes observed during pregnancy. One of the most important is certainly the RAAS (Barron, 1987) through the vascular and adrenal effects of angiotensin II (ANGII) and the antinatriuretic action of aldosterone. The RAAS normally plays an important role in maintaining vascular tone as well as fluid and electrolyte balance. The electrolyte-regulating steroid, aldosterone, released from adrenal cortical cells stimulated by ANGII, promotes Na+ resorption at the expense of K+ and Mg2+ excretion in kidney and colon as well as sweat and salivary glands (Weber, 2003). The biological activity of renin–angiotensin is mediated primarily by the octapeptide ANGII through its binding to and activation of specific transmembrane G protein-coupled ANGII receptors, type I (AT1) and type II (AT2).
The AT1 receptor subtype, was cloned in 1991 (Sasaki et al. 1991; Murphy et al. 1991) and shown to mediate most of the classical effects of ANGII such as vasoconstriction as well as electrolyte and water homeostasis. Consistent with this, AT1, although ubiquitous in the adult, is most abundantly expressed in blood vessels (where it induces vasoconstriction), adrenal cortex (where it promotes aldosterone release), kidney (where it controls water and Na+ retention), liver (where it acts to control glycogen metabolism) and brain (where it promotes vasopressin release, thirst and salt appetite and controls blood pressure regulation and sympathetic output).
In contrast, while AT2 is expressed ubiquitously in fetal tissues, it is maintained at high levels in adulthood in vascular endothelium, adrenal gland, uterus, ovary and certain brain areas (Timmermans et al. 1993). Expression patterns for this receptor in vivo as well as findings from a number of in vitro studies and transgenic/knockout animal models strongly suggest important roles for AT2 in development and repair as well as to counterbalance or oppose the effects of AT1 (Dzau et al. 1994). In this regard, AT2 receptor activation has been shown to promote neuronal cell morphological differentiation (Gendron et al. 1999), induce apoptosis (Gallinat et al. 1999) and activate kinin and nitric oxide systems so to promote vasorelaxation (Tsutsumi et al. 1999). Finally, although expression of both ANGII receptor types are subject to modulation in a number of pathophysiological states, there is ample evidence, particularly for AT2, that these receptors may undergo reversible physiological changes throughout life to facilitate adaptation and modulation of the RAAS to distinct physiological events. One such change has been demonstrated in the human myometrium during pregnancy (de Gasparo et al. 1994).
Despite marked activation of the RAAS during pregnancy, sensitivity to ANGII has been shown to be somewhat altered. A diminished vasoconstrictive response to ANGII has been reported in normotensive pregnant women when compared to non-pregnant women (Gant et al. 1994). This decreased sensitivity has also been demonstrated in the rat in isolated vessels such as the aorta (Hart, 1982) and mesenteric arteries (Massicotte et al. 1987). In agreement with these findings, ANGII receptor density has been documented to decrease in mesenteric arteries and renal glomeruli of pregnant rabbits (Brown & Venuto, 1986). In contrast, Bird et al. (1997) reported an increase in AT1 expression in the uterine artery endothelium, which remained unaltered in arterial smooth muscle in pregnant sheep. On the other hand, the number and affinity of ANGII receptors have been shown not to change during gestation in the mesenteric vascular bed (Parent et al. 1991), isolated hepatocytes (Massicotte et al. 1990) and adrenal cortex of rats (Forcier et al. 1995) and rabbits (Brown & Venuto, 1986).
Alterations in Na+ intake or plasma ANGII levels have been repeatedly shown to affect the regulation of ANGII receptors. Indeed, Na+ restriction has been documented to promote increases in AT1 receptor levels in the adrenal cortex in rats (LeHoux et al. 1997). Low dietary Na+ has been reported to variably affect AT1 receptor levels in the kidney (Jo et al. 1996; Schmid et al. 1997). In cultured rat endothelial cells, ANGII has been reported to down-regulate AT2 mRNA expression via AT1 binding (De Paolis et al. 1999). Co-treatment of cells transfected with the AT2 gene promoter with an AT1 antagonist and ANGII was shown to increase AT2 mRNA expression (De Paolis et al. 1999). In nephrectomized rats on Na+-restricted diets, the administration of DuP753, a specific AT1 antagonist, was found to promote notable decreases in AT1 mRNA and concomitant increases in AT2 mRNA in the adrenal cortex (Gigante et al. 1997) suggesting once again the existence of an important functional ‘cross-talk’ between ANGII receptor types, one that may be particularly sensitive to Na+.
In view of the above, we propose that some of the important haemodynamic and vascular adaptive mechanisms that come into play during normal pregnancy to ensure maternal well-being and fetal growth implicate changes in the expression of ANGII receptor subtypes in certain target tissues. Furthermore, Na+ restriction during the last week of gestation in the rat may reduce plasma volume expansion and promote IUGR despite increased activation of the RAAS by differentially altering levels of AT1 and AT2 in these specific target tissues. Similar alterations in these receptors may be found in women with idiopathic IUGR. Therefore, the aim of the present investigation was to study, in the face of an important RAAS activation, the effects of Na+ restriction during pregnancy on ANGII receptor regulation in selected tissues thought to be involved in the maintenance of vascular tone, haemodynamic homeostasis and fetal growth. Changes in ANGII receptor expression will be measured and compared to the state of activation of the RAAS, important renal clearance indices, uterine artery measurements and fetal growth.
Methods
Animals, experimental design, diet, housing and physiological measurements
Experimental procedures were reviewed and approved by the local Animal Care Committee, accredited by the Canadian Council on Animal Care. A total of 96 female Sprague-Dawley rats (Charles River Canada, St-Constant, Québec, Canada) were used. Fifty-six pregnant near-term rats (15 days gestation, weighing 225–250 g) obtained as detailed previously (Roy-Clavel et al. 1999), and 40 age-matched non-pregnant females (chosen randomly throughout their oestrous cycle) were randomly assigned to two groups that were placed on normal or Na+-restricted diets for 7 days. This 7-day experimental period corresponded to the last week of gestation for the pregnant animals.
The four groups of animals were housed under controlled illumination on a 12 h light–dark cycle under ambient temperature and fed and watered ad libitum. The normal diet consisted of 0.2% sodium (Basal Diet 5755; PMI Feed Inc., Ren's Feed and Supplies, Oakville, Ontario, Canada) and tap water. The diet restricted in Na+ contained 0.03% sodium (Low-sodium Diet 5881; PMI Feed Inc.) and demineralized water.
Animals were housed in metabolic cages for the last three experimental days so to measure 24 h water intake and urine output. At the end of the treatment period (day 7), all animals were killed by decapitation (at 9.00–9.30 h), body weight was recorded, blood was collected for hormonal and electrolyte analyses, and tissues (hypothalamus, pituitary, adrenal cortex, kidney, uterine arteries and placenta) were removed as previously described (Beausejour et al. 2003) for receptor measurements. Weights of fetoplacental units were recorded after the animals were killed, but before further tissue dissection. The number of animals used for measuring the numerous parameters varied according to specific procedural and technical requirements.
Haematology, clinical biochemistry and urine analysis
The following haematological and clinical indices were measured as previously described (Beauséjour et al. 2003). For haematocrit measures, blood drawn into microhaematocrit capillary tubes (Fisherbrand, Fisher) was centrifuged at 800 g for 3 min (Triac Centrifuge, Becton-Dickinson, Franklin Lakes, NJ, USA) and the result was read manually. For all other analyses, blood was drawn, at the time the animal was killed, into heparinized vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ, USA) and centrifuged at 800 g for 20 min at 4°C for collection of plasma. Aliquots of plasma and 24-h urine samples collected during the last three experimental days from animals housed in metabolic cages were used for the following determinations: Na+ and K+ concentrations using specific electrodes, urea levels using conductibility electrodes, creatinine concentration by the standard enzymatic colorimetric test, and osmolality using a vapour osmometer.
Plasma renin activity, aldosterone and corticosterone analyses
Plasma renin activity (PRA) was determined indirectly as already described (Roy-Clavel et al. 1999). Briefly, angiotensin I, generated per 2-h incubation period, was quantified by radioimmunoassay using rabbit anti-human ANG I (RAS 7001) that cross-reacts with rat, human, fowl and bullfrog ANG I (Peninsula, Inc., Belmont, CA, USA). Aldosterone extracted from plasma by solid phase using C18 September – Pak cartridges (Millipore; Waters, Montréal, Québec, Canada) was measured by radioimmunoassay (Roy-Clavel et al. 1999). Cortisterone plasma levels, determined to assess possible stress inflicted by animal manipulation, were determined using a commercially available radioimmunoassay kit (Medicorp, Montréal, Québec, Canada). Fetal corticosterone plasma determinations were performed similarly on pooled blood samples from each litter. Number of Na+-restricted pregnant rats was smaller not because of death or abortion but because samples were lost in storage.
RNA isolation
Total cellular RNA from excised tissues was extracted using TRIzol Reagent according to the procedure detailed by the manufacturer (Invitrogen Canada Inc., Burlington, Ontario, Canada). Final RNA pellets were dissolved in appropriate volumes of 100% deionized formamide, and stored at −20°C until further use. Concentration of RNA was determined from absorbance measurements at 260 nm, and sample quality verified by ethidium bromide fluorescence.
RNase protection assay
Preparation of probes
A 182-base DraI fragment excised from pcDNAI plasmid was used for the preparation of a high-activity RNA antisense probe for the AT1 receptor (Murphy et al. 1991). A 215-base HindIII-XbaI fragment excised from pcDNAI/Amp plasmid was cloned into the multiple cloning sites of the Bluescript vector, linearized with PvuII and used for the preparation of a high-activity RNA antisense probe for the AT2 receptor (Hunyady et al. 1994). A 334-base fragment of β-actin gene (Ambion RNA Company, Austin, TX, USA) served as an internal control. The antisense probes were prepared by transcription in vitro by the Ambion Maxiscript Technique (Ambion RNA Company) in the presence of T7 polymerase for AT2, SP6 polymerase for AT1 and β-actin, and [α-32P]UTP (Mandel, Boston, MA, USA). The radioactive probes were separated from free [α-32P]UTP by washing with 75% ethanol−25% sodium acetate.
RNase protection
Total RNA (25 μg) was incubated at 65°C for 10 min with the antisense probes (100 000 counts min−1 for AT1 and AT2, 15 000 counts min−1 for β-actin) in 5 X Pipes buffer (200 mm 1,4-piperazine diethanesulphonic acid, pH 6.4, 2 m NaCl, 5 mm EDTA) and 80% deionized formamide. After denaturation of RNA at 85°C for 5 min, hybridization was performed overnight at 47°C. Non-hybridized RNA was digested using RNase T1/A (RNase cocktail from Ambion RNA Company) in RNase digestion buffer containing 1 m Tris-HCl (pH 7.5), 0.5 m EDTA and 0.3 m NaCl for 1 h at 30°C. After treatment with 20% sodium dodecyl sulphate (SDS) and 10 mg ml−1 proteinase K for 15 min at 37°C, the hybridized RNA was purified by phenol–chloroform extraction, precipitated in 100% ethanol and resuspended in 10 μl of electrophoresis buffer containing 80% deionized formamide, 1 mm EDTA, 0.1% bromophenol blue, 0.1% xylene cyanol and 0.1% SDS. The RNA was denatured at 85°C for 5 min and then separated by electrophoresis on 5% acrylamide−7 m urea gels at 40 W. After separation, the gel was fixed for 15 min in 10% methanol−5% acetic acid and dried for 1 h. The dried gel was exposed to X-ray film (Kodak X-Omat AR5) using intensifying screens at −80°C for 1–7 days. Relative intensities of AT1, AT2 and β-actin bands were determined by gel analysis with Scion Image computer software (Scion Corporation, National Institutes of Health, Frederick, MD, USA). The steady-state mRNA levels for AT1 and AT2 were expressed in arbitrary units, and standardized by comparison with hybridization results obtained from the housekeeping β-actin gene.
Preparation of tissue membranes
To prepare each tissue-specific membrane the following amount of tissue was needed: adrenal cortices from four rats, pituitaries from two rats, 120 mg of the kidney cortex and placenta pooled from each experimental pregnant group. Tissues were minced with scissors and then homogenized in 0.25 m sucrose by 2 × 10 s pulses with a Polytron at setting 8 (Brinkman, Rexdale, Ontario, Canada). The homogenates were centrifuged at 1500 g for 10 min at 4°C and supernatants thus collected centrifuged at 50 000 g for 30 min at 4°C. The resulting particulate fractions were resuspended in buffer (25 mm Tris-HCl, 120 mm NaCl, 5 mm MgCl2, 0.0002% NaN3, 5 mm EDTA, 1 μm leupeptide, 1 μm aprotinin, 1 μm pepstatin, and 100 μm phenylmethylsulfonyl fluoride, pH 7.4) and membrane protein concentrations measured by the Bradford colorimetric method. Membranes were then diluted to a final protein concentration of 1 μg μl−1 and stored at 80°C until further use.
Photoaffinity labelling procedures
Iodination of peptides
Purified 1Sar-5Val-8Bpa-ANGII was radiolabelled using Na125I by the iodogen method as described by Fraker & Speck (1978). Radiolabelled peptides were purified to homogeneity by high performance liquid chromatography (reverse phase C-18), and specific radioactivity measured using a gamma counter (Packard Cobra II Model 5003, Canberra Packard Canada, Mississauga, Ontario, Canada). Specific activity for 125I-ANGII-Bpa was calculated at 2000 Ci mmol−1.
Photoaffinity labelling
Briefly, tissue membranes (100 μg of protein) were incubated with 2 μCi (or 5 nm) of 125I-labelled 1Sar-5Val-8Bpa-ANGII in 500 μl of binding buffer (25 mm Tris-HCl 100 mm NaCl, 5 mm MgCl2, 1 mm EDTA, 0.1% bovine serum albumin (BSA), 2 mg ml−1 bacitracin and 50 μg ml−1 soybean trypsin inhibitor, pH 7.4) for 45 min in the dark at room temperature. To selectively label AT1 or AT2 receptors, 1 μm PD123319 (AT2 antagonist) or L-158 809 (AT1 antagonist) was added to the incubation mixture, respectively. Non-specific binding was determined by incubating in the presence of 10−5m ANGII. Following incubation, the membranes were washed by centrifugation (13 000 g for 10 min) with binding buffer prepared without BSA and then irradiated under UV light (365 nm) (Long Wave B-100SP, UVP Inc., Upland, CA, USA) for 45 min at 4°C. Non-covalently bound ligand was removed by successive washes with citrate buffer (pH 5.5). The washed pellets were then incubated for 60 min at 37°C in denaturing buffer (60 mm Tris-HCl, 10% (v/v) glycerol, 2% (w/v) SDS, 0.3% bromophenol blue and 125 mm dithiothreitol, pH 6.8) and denatured photolabelled protein complexes (40 μl) separated electrophoretically on polyacrylamide gels (9% running and 5% stacking) at 25 mA (stacking) and 50 mA (running) for approximately 5 h. For comparative analyses, samples from each experimental animal group were routinely separated on the same gel. Gels were dried and then exposed to Kodak X-Omat AR film for 1–3 days at −80°C. Relative intensities of the AT1 and AT2 bands for all samples from all four groups of animals were determined by gel analysis using Scion Image Computer Software (Scion Corporation, National Institutes of Health) and expressed in arbitrary units relative to values from non-pregnant rats fed a normal diet which were set constant at 1 arbitrary unit.
Mechanical uterine vessel measurement
Vessel mechanical measurements were performed as previously described (St-Louis et al. 1997, 2001). An arcuate artery, at the midpoint of the uterine arcade, was cleaned of adhering tissues. A section (1.5 mm) was isolated and two tungsten wires (20 μm) were inserted into the lumen to secure the vessel to supports of the myograph, one attached to a force transducer and the other to a micrometer device. Force was recorded through data acquisition package (Workbench, Kent Scientific, Litchfield, CT, USA). The artery was bathed in 7 ml oxygenated (95% O2−5% CO2) Krebs bicarbonate solution (KBS). The arterial segment was stretched in steps of ∼30 μm in length (internal circumference) until ∼1.5 mN mm−1 was reached (1 mN = 102 mg) and held at each length for 2–3 min while wall tension at peak response to stretch was recorded. The passive length–tension relationship generated was fitted to an exponential equation that intercepted the line of Laplace (pressure, 50 mmHg) at the diameter used as passive tension.
Statistical analysis
Data were compared by two-way analysis of variance. Unpaired Student's t test was performed group by group if interaction between gestation and diet was significant. Otherwise, post hoc Newman–Keuls test was used. Minimum level of significance of P < 0.05 was used. The results were expressed as means ± s.e.m. with numbers in parentheses corresponding to numbers of animals studied. Where the data were not normally distributed (PRA, aldosterone and corticosterone), they were analysed using the Kruskal–Wallis test. In this case, data are presented as their median values and interquartile ranges.
Drugs and chemicals
All salts used in the experiments were of analytical grade and obtained from Fisher Scientific (Montreal, Quebec, Canada) or Sigma Chemical (St Louis, MO, USA).
Results
Physiological changes induced by Na+ restriction and pregnancy
Sodium restriction promotes significant decreases in mean body weights in both non-pregnant (277 ± 4 g (14) on normal versus 254 ± 5 g (13) on low-Na+ diet, P < 0.001) and pregnant rats (370 ± 7 g (13) on normal versus 325 ± 6 g (12), on low-Na+ diet, P < 0.001). In addition, the mean fetoplacental unit weight is significantly (P < 0.01) lower in pregnant rats on Na+-restricted diet (85 ± 2 g (12)) when compared to those from rats on normal diet (101 ± 4 g (13)). Furthermore, Na+ restriction appears to induce premature delivery as seven of the 28 rats on the low-Na+ diet delivered the morning of gestational day 22. In contrast, none of the 28 pregnant rats on the normal diet delivered before they were killed. Nevertheless, Na+ restriction does not affect litter size (14.5 ± 0.6 pups (13) for normally fed animals versus 15.0 ± 0.6 pups (12), for rats on low-Na+ diet).
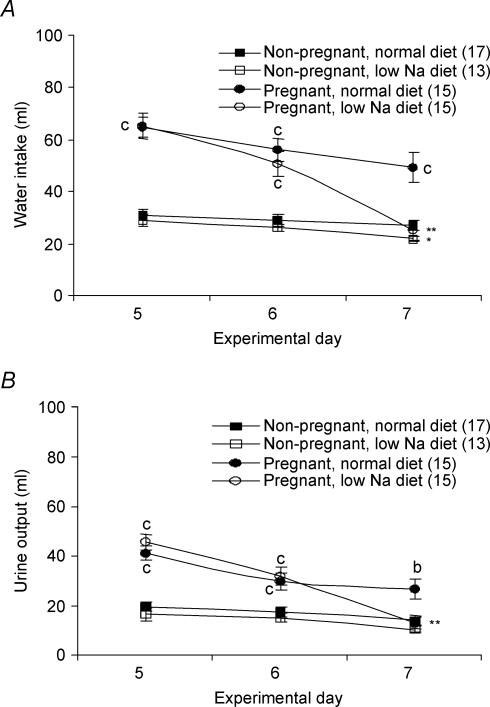
As shown in Fig. 1, pregnancy alone is associated with an increase in both water intake (Fig. 1A) and urine output (Fig. 1B). These increases are significant and independent of diet. During the last experimental day, water intake significantly decreased in both pregnant (P < 0.01; Fig. 1A) and non-pregnant (P < 0.05; Fig. 1A) animals on the low-Na+ diet while urinary output only decreased significantly (P < 0.01; Fig. 1B) in the pregnant rats on the low-Na+ diet.
Figure 1. Effects of low-Na+ diet and pregnancy on water homeostasis: water intake (A) and urine output (B).
Experimental days 5 to 7 correspond to days 19 to 21 of gestation for the pregnant animals. Results are expressed as means ± s.e.m. with numbers in parentheses representing the number of rats. Values significantly different from those obtained for animals (same group) fed a normal diet indicated by *P < 0.05, **P < 0.01, ***P < 0.001. Values significantly different from those obtained from non-pregnant animals (same diet) indicated by aP < 0.05, bP < 0.01, cP < 0.001.
Effects of Na+ restriction and pregnancy on plasma hormone levels
As shown in Table 1, Na+ restriction promotes increases in PRA and plasma aldosterone in non-pregnant and pregnant rats. Pregnancy increases both PRA and aldosterone levels in rats fed normal or low-Na+ diets (see Table 1). In contrast, as shown in Table 1, plasma corticosterone levels are not affected by diet or gestation. However, fetal corticosterone levels are significantly higher (P < 0.05) in fetuses from rats on the low-Na+ diet (1.07 ± 0.07 nmol ml−1, n = 3 litters) when compared to fetuses from rats on the normal diet (0.82 ± 0.07 nmol ml−1, n = 3 litters).
Table 1.
Effects of low-Na+ diet and pregnancy on plasma hormone levels
| Non-pregnant rats | Pregnant rats | |||
|---|---|---|---|---|
| Normal | Low-Na+ | Normal | Low-Na+ | |
| Plasma renin activity | 2.1 | 2.8 | 5.0* | 9.8 |
| ((pmol angiotensin I) ml−1 h−1) | [1.5–2.7](12) | [1.1–5.3](12) | [4.2–8.3](12) | [5.0–26.5](8) |
| Aldosterone (pmol ml−1) | 2.2 | 4.8 | 6.8a | 24.0*b |
| [1.7–2.4](14) | [3.6–6.5](13) | [4.7–8.5](12) | [18.1–28.2](10) | |
| Corticosterone (nmol ml−1) | 1.3 | 1.5 | 1.2 | 1.1 |
| [0.8–1.8](13) | [1.0–1.6](12) | [0.9–1.4](13) | [0.9–1.3](11) | |
Results are expressed as medians and interquartile ranges. Numbers in parentheses represent the number of rats. Significantly different from animals (same group) fed a normal diet indicated by
P < 0.05. Significantly different from non-pregnant animals (same diet) indicated by
P < 0.05,
P < 0.01.
Effects of Na+ restriction and pregnancy on haematocrit, electrolyte and solute measurememts
As shown in Table 2, Na+ restriction promotes a significant (P < 0.05) increase in haematocrit in the non-pregnant rat group without affecting plasma osmolality (Posm) or solute concentrations. Pregnancy alone is found to significantly decrease plasma Na+, urea and osmolality as well as haematocrit levels while significantly increasing plasma creatinine values in rats fed a normal diet (Table 2). Sodium restriction during pregnancy significantly decreases plasma Na+ concentrations and osmolality further while promoting significant increases in both creatinine and haematocrit values (see Table 2). These findings clearly show that although Na+ restriction elicits a contraction of extracellular fluid volume in both non-pregnant and pregnant rats, volume contraction is greatest in the pregnant rat group.
Table 2.
Impact of low-Na+ diet and pregnancy on plasma osmolality, solute concentrations and haematocrit
| Non-pregnant rats | Pregnant rats | |||
|---|---|---|---|---|
| Normal | Low-Na+ | Normal | Low-Na+ | |
| Osmolality (mosmol (kg H2O)−1) | 295 ± 2 (13) | 292 ± 3 (13) | 279 ± 3c (14) | 259 ± 5***c (12) |
| Sodium (mmol l−1) | 139 ± 1 (13) | 140 ± 1 (12) | 132 ± 1c (11) | 118 ± 2***c (12) |
| Potassium (mmol l−1) | 6.4 ± 0.2 (10) | 6.2 ± 0.2 (11) | 5.7 ± 0.7 (8) | 6.6 ± 0.8 (9) |
| Urea (mmol l−1) | 5.1 ± 0.2 (13) | 4.9 ± 0.2 (12) | 4.0 ± 0.5a (14) | 9.2 ± 0.6***c (11) |
| Creatinine (μmol l−1) | 35 ± 1 (14) | 37 ± 2 (12) | 46 ± 3a (13) | 55 ± 2*a (11) |
| Haematocrit (%) | 42 ± 1 (14) | 46 ± 1* (11) | 33 ± 1a (13) | 39 ± 1*a (12) |
Results are expressed as means ± s.e.m. Numbers in parentheses represent the number of rats. Significantly different from animals (same group) fed a normal diet indicated by
P < 0.05,
P < 0.001. Significantly different from non-pregnant animals (same diet) indicated by
P < 0.05,
P < 0.001.
The effects of both diet and gestation on urinary parameters measured on the last experimental day corresponding to gestational day 22 for the pregnant rat groups are presented in Table 3. Restricting dietary Na+ significantly (P < 0.001; Table 3) reduces Na+ excretion in non-pregnant rats. Pregnancy significantly (P < 0.01; Table 3) increases urinary volume while significantly decreasing Na+ (P < 0.001; Table 3), potassium (P < 0.001; Table 3) and urea (P < 0.01; Table 3) excretion in the normally fed animals. However, Na+ restriction significantly depresses urinary volume (P < 0.01; Table 3), Na+ (P < 0.05; Table 3) and creatinine excretion (P < 0.001; Table 3) and significantly increases urinary excretion in pregnant rats (P < 0.01; Table 3).
Table 3.
Effects of low-Na+ diet and pregnancy on urinary parameters measured on the last experimental day
| Non-pregnant rats | Pregnant rats | |||
|---|---|---|---|---|
| Normal | Low-Na+ | Normal | Low-Na+ | |
| Urine volume (ml day−1) | 14.1 ± 1.7 (17) | 10.4 ± 1.3 (14) | 26.7 ± 4.1b (16) | 13.0 ± 2.2** (15) |
| Osmolality (mosmol (kg H2O)−1) | 1393 ± 121 (16) | 1347 ± 104 (14) | 476 ± 60c (15) | 806 ± 121*b (16) |
| UVosmol (μmol day−1) | 17.3 ± 1.6 (16) | 12.5 ± 1.0* (14) | 10.0 ± 0.9a (15) | 8.1 ± 0.6a (16) |
| UVSodium (μmol day−1) | 0.89 ± 0.12 (13) | 0.09 ± 0.01*** (12) | 0.29 ± 0.06c (13) | 0.12 ± 0.02* (13) |
| UVPotassium (μmol day−1) | 1.24 ± 0.15 (13) | 1.59 ± 0.17 (12) | 0.82 ± 0.11c (14) | 0.81 ± 0.07c (13) |
| UVUrea (μmol day−1) | 8.88 ± 1.32 (12) | 6.78 ± 0.61 (12) | 5.24 ± 1.18b (14) | 3.81 ± 0.29b (13) |
| UVCreatinine (μmol day−1) | 0.075 ± 0.007 (13) | 0.065 ± 0.003 (12) | 0.079 ± 0.003 (14) | 0.051 ± 0.003***b (13) |
Results are expressed as means ± s.e.m. with numbers in parentheses representing the number of rats. UV, urinary excretion. Significantly different from animals (same group) fed a normal diet indicated by
P < 0.05,
P < 0.01,
P < 0.001. Significantly different from non-pregnant animals (same diet) indicated by
P < 0.05,
P < 0.01,
P < 0.001.
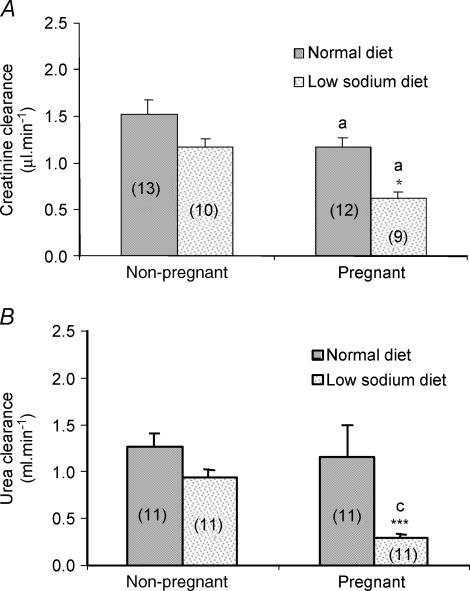
Creatinine clearance is not affected by restricting Na+ intake in non-pregnant rats (Fig. 2A). In contrast, while pregnancy significantly decreases creatinine clearance from rats fed either diet, decreases were most marked (see Fig. 2A) in rats on the low Na+ diet. Urea clearance is not affected by diet in non-pregnant rats nor is it altered by pregnancy alone. However, Na+ restriction significantly (P < 0.001; Fig. 2B) decreases urea clearance from pregnant animals.
Figure 2. Effects of low-Na+ diet and pregnancy on clearance of creatinine (A) and urea (B).
Results are expressed as means ± s.e.m. with numbers in parentheses representing the number of rats. Values significantly different from those obtained for animals (same group) fed a normal diet indicated by *P < 0.05, ***P < 0.001. Values significantly different from those obtained for non-pregnant animals (same diet) indicated by aP < 0.05, cP < 0.001.
Restricting Na+ differentially affects uterine artery measurements
Diameter of the uterine arcuate arteries was measured to get an index of blood flow to the fetoplacental unit. In non-pregnant rats, Na+ restriction is found to produce a non-significant increase in mean uterine artery diameter from 121 ± 7 μm (n = 11) in normally fed animals to 140 ± 10 μm (n = 11). Pregnancy significantly (P < 0.001) increases mean artery diameter in normally fed animals to 269 ± 11 μm (n = 10). However, Na+ restriction significantly (P < 0.01) reduces mean artery diameter measured in the pregnant animals to 198 ± 15 μm (n = 10).
Pregnancy and Na+ restriction affect AT1 and AT2 protein and mRNA expression
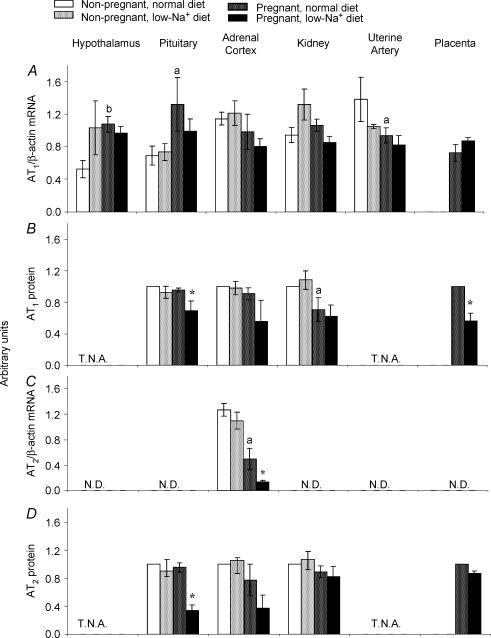
The effects of pregnancy and restricting dietary Na+ on AT1 and AT2 mRNA and protein levels in several tissues that are implicated in volume regulation (hypothalamus, pituitary, adrenal cortex and kidney) and fetal growth (uterine artery and placenta) are depicted in Fig. 3. Sensitivity of the RNase protection assay limited detection of AT2 mRNA to the adrenal cortex. In addition, receptor proteins could not be measured in hypothalamus and uterine arteries due to limited availability of these tissues.
Figure 3. Effects of low-Na+ diet on AT1 and AT2 receptor mRNA and protein expression in tissues from non-pregnant and pregnant rats.
Data for mRNA are expressed as AT1 or AT2/β-actin ratio (arbitrary units) and results shown (A and C) represent means ± s.e.m. from five separate experiments. Membrane proteins were prepared and photoaffinity labelling performed as described in Methods. Data for receptor protein were compared to those from normal non-pregnant controls and results shown (B and D) represent means ± s.e.m. from three separate experiments. Significantly different from values obtained for non-pregnant rats fed a normal diet indicated by aP < 0.05, bP < 0.01. Significantly different from values obtained for pregnant animals on a normal diet indicated by *P < 0.05. T.N.A., tissues not available. N.D., not detected.
Hypothalamus
In non-pregnant rats, Na+ restriction does not alter the AT1 receptor steady-state mRNA levels in the hypothalamus (Fig. 3A). However, pregnancy does promote a significant (P < 0.01; Fig. 3A) increase in AT1 mRNA levels in the hypothalamus that is not affected further by the low Na+ diet.
Pituitary
Both ANGII receptor subtypes are present in the pituitary (Fig. 3A, B and D). In non-pregnant rats, restricting dietary Na+ does not alter expression of AT1 (mRNA and protein) or AT2 (protein). Furthermore, pregnancy does significantly (P < 0.05; Fig. 3A) increase AT1 mRNA levels in the pituitary without affecting receptor protein (Fig. 3B). Finally, AT1 and AT2 protein levels in pituitaries of pregnant rats on Na+-restricted diets are both significantly (P < 0.05; Fig. 3B and D) lower than those from normally fed pregnant animals.
Adrenal cortex
As shown in Fig. 3, AT1 receptor steady-state mRNA and protein levels in the adrenal cortex are not affected by diet, gestation or the combination of Na+ restriction and pregnancy. Although Na+ restriction does not alter AT2 mRNA or protein in the adrenal cortex of non-pregnant rats, pregnancy significantly (P < 0.05; Fig. 3C) decreases AT2 steady-state mRNA levels in normally fed animals. Furthermore, Na+ restriction significantly (P < 0.05; Fig. 3D) decreases AT2 mRNA levels in the adrenal cortex of pregnant rats further.
Kidney
As shown in Fig. 3, neither Na+ restriction nor pregnancy affect AT1 mRNA and AT2 protein levels in the rat kidney. In contrast, pregnancy promotes a significant (P < 0.05; Fig. 3B) decrease in AT1 protein in the kidney that is not affected further by restricting dietary Na+.
Uterine arteries
While pregnancy significantly (P < 0.05; Fig. 3A) decreases AT1 mRNA levels in uterine arteries, restricting dietary Na+ is without effect. For the reasons detailed earlier, neither mRNA for AT1 and AT2 nor protein values for AT2 in uterine arteries could be obtained.
Placenta
Finally, while restricting dietary Na+ does not affect AT1 mRNA or AT2 protein levels in the placenta, it significantly (P < 0.05; Fig. 3B) decreases AT1 protein values.
Discussion
In the present study we show that maternal adaptive haemodynamic changes that take place during normal pregnancy in the rat, are noted concurrently with alterations in the expression of ANGII receptors in some tissues involved in volume regulation and fetal development. However, restricting dietary Na+ during the last week of gestation disrupts some of these adaptive vascular changes, impedes fetal growth, and induces modifications in the expression of the ANGII receptors that are in some instances greater, and in others, distinct, from those noted during normal pregnancy. Finally, although extracellular volume contraction is also seen in non-pregnant rats on a low-Na+ diet, plasma electrolyte levels, kidney clearance indices and ANGII receptor expression in all the tissues examined in these animals are unaltered.
Changes in ANGII receptor expression in the hypothalamus
In specific areas of the hypothalamus (thirst centre), ANGII has been shown to stimulate water intake via AT1 (Beresford & Fitzsimons, 1992) and AT2 receptor activation (Hogarty et al. 1992; Li et al. 2003). In our present study, we found that AT1 expression in the rat hypothalamus increased significantly during pregnancy. This higher expression may account for the increase in water intake we note in our animals during the last few days of pregnancy. Similar increases in water intake and decreases in urine output have been reported during the last week of gestation in the rat as well (Atherton et al. 1982). However, we find that although water intake declines significantly during these last days of gestation in the pregnant rats on the low-Na+ diet, high levels of AT1 are maintained in the hypothalamus and the RAAS is significantly activated in these animals. In this instance, Na+ restriction may in itself stimulate these changes in hypothalamic receptor levels. In support of this, Jo et al. (1996) have shown that mRNA for both AT1a and AT1b, the two isoforms of the rat AT1 receptor, increased in the non-pregnant rat hypothalamus following Na+ restriction. In our study, Na+ restriction alone does not promote increases in AT1 receptor levels in the non-pregnant rat hypothalamus. A likely explanation may be that if Na+ restriction had been prolonged, as in the study by Jo et al. (1996), to 20 instead of 7 days, statistical significance could have been reached. Alternatively, gestation and Na+ restriction may differentially modulate AT2 receptor levels in the hypothalamus leading to increases and decreases in water intake, respectively. These changes may be more important to fluid intake than those noted in AT1. In this regard, the importance of the AT2 receptor in the thirst response in rodents is clearly illustrated by Hein et al. (1995) who showed that water intake was significantly reduced in AT2 receptor gene knock-out mice. Nevertheless, an assay with greater sensitivity than the RNase protection assay that was used in our study is needed to test the importance of this receptor subtype in the rat hypothalamus.
ANGII receptor expression in the pituitary
In response to ANGII, the posterior pituitary releases vasopressin (AVP) which in turn enhances water and salt reabsorption in the renal tubules (Bonjour & Malvin, 1970). However, the role of each ANGII receptor subtype in the control of AVP release from the rat pituitary is somewhat controversial. In this regard, Qadri et al. (1993) reported that ANGII-induced AVP release in the rat is attenuated by the central administration of losartan (an AT1 antagonist) and unaffected by centrally administered PD123177 (an AT2 antagonist), while blockade of either AT1 or AT2 was shown to suppress ANGII-induced increases in plasma AVP (Hogarty et al. 1992).
During normal pregnancy in the rat, circulating AVP is found in sufficiently high enough levels to promote water retention (Lindheimer et al. 1987) and thus contribute to maternal extracellular fluid expansion. In the present study, we found that expression of AT1 mRNA increased in the rat pituitary during pregnancy while AT1 and AT2 protein levels were unaltered. This increase in receptor expression in the rat during pregnancy is supported by the findings of Seltzer et al. (1992) who showed that oestrogen levels influenced ANGII receptor expression in the anterior pituitary of female rats. However, in pregnant rats on the low-Na+ diet, AT1 and AT2 protein levels decreased by 25% and 64%, respectively. Should both ANGII receptor subtypes be implicated in promoting AVP release (see Hogarty et al. 1992), this decrease in receptor expression may underlie decreased AVP release and thus, decreased water retention (which we noted in the pregnant animals on the low-Na+ diet).
Changes in ANGII receptor expression in the adrenal cortex
Aldosterone release from the adrenal cortex is stimulated by ANGII via AT1 activation to promote Na+ resorption and subsequently, water retention (Chung et al. 1999). Here we found that Na+ restriction does not affect transcription or translation of either of the ANGII receptor subtypes in the adrenal cortex of the non-pregnant rats. Furthermore, in agreement with the earlier study of Forcier et al. (1995) we found that pregnancy did not affect AT1 expression in the adrenal cortex of the rat. However, both AT2 mRNA and protein levels declined in the adrenal cortex of our pregnant animals, most probably in response to the high plasma levels of ANGII. In this regard, it has been shown that ANGII can, via AT1 binding, decrease AT2 mRNA stability (Ouali et al. 1997). Finally, it is possible that the ratio of AT1 to AT2 is most important to the final adrenal response to ANGII. Therefore, when levels of AT2 decrease during pregnancy, the AT1: AT2 ratio increases and stimulation of aldosterone release is favoured. This would explain the hyperaldosteronism we observed in the pregnant rats, irrespective of diet.
Renal function, osmoregulation and ANGII receptor expression
During pregnancy, the kidney has been shown to play an important role in resetting plasma tonicity in the rat to a new narrow range (Lindheimer et al. 1987). The decrease we observed in Posm in the normally fed pregnant rats is consistent with such a re-adjustment. Furthermore, plasma volume expansion found during pregnancy is associated with a rise in the glomerular filtration rate in the rat (Baylis, 1987), which in turn increases Na+ filtration and reabsorption rates. Increases in Na+ reabsorption are observed during gestation in both proximal tubules and distal nephrons of the rat kidney (Atherton et al. 1988). In view of the above, the decrease we found in plasma urea concentration in the pregnant rats on the normal diet may be due to both extracellular fluid expansion and the decrease in Posm. Furthermore, the decrease in Na+ excretion we, and others (Atherton et al. 1982) observed in the pregnant rat, as well as the dilution of urine (indicated by a decrease in urinary osmolality) we noted in our pregnant animals may serve to maintain homeostasis during pregnancy.
In the kidney, water and salt reabsorption are stimulated in part by ANGII primarily via AT1 activation (Chung et al. 1999). The effect of Na+ restriction on renal ANGII receptors is indeed controversial. For example, low Na+ intake has been shown to decrease expression of AT1a and AT1b mRNA in the renal afferent artery (Ruan et al. 1997). On the other hand, total renal expression of AT1 is documented to increase in rats on a Na+-restricted diet (Jo et al. 1996). In our present study, we found that Na+ restriction did not affect AT1 mRNA or AT1 and AT2 protein levels in the non-pregnant rats. However, and quite surprisingly, we found that AT1 protein decreased rather than increased in the rat kidney during pregnancy, suggesting that the renal involvement in fluid retention during normal pregnancy may implicate physiological mediators other than ANGII such as AVP and aldosterone.
In pregnant rats on the low-Na+ diet we found that osmoregulation was somewhat perturbed while urine volume and Na+ excretion were reduced. Furthermore, although we expected Na+ reabsorption to be greater in pregnant than non-pregnant rats on the low-Na+diet, in order to conserve the small amount of Na+ available, Na+ excretion was similar in both groups of animals. Therefore, the plasma constriction we observed in the pregnant rats on the low-Na+ diet is apparently secondary to deficient renal Na+ reabsorption. The kidney in this instance has probably reached its maximum capacity for Na+ reabsorption.
Uraemia and decreased urea clearance were observed in the pregnant rats on the low-Na+ diet. Plasma urea may increase in these rats as a result of decreased urinary flow that serves to counterbalance plasma volume constriction. We also found that expression (mRNA and protein) of the two ANGII receptor subtypes were not different in the kidneys from these rats compared to their pregnant controls. The high levels of plasma creatinine and low creatinine clearance rates in the pregnant rats on the low-Na+ diet strongly suggest that the glomerular filtration rate declined in these animals as a result of plasma volume constriction. Taken together, the physiological changes we observed in the pregnant rats on the Na+-restricted diet are consistent with a diagnosis of functional renal insufficiency. Functional renal insufficiency in humans is characterized clinically by urea/creatinine plasma ratio values > 100, Uosm > 500 mosmol (kg water)−1 and urinary sodium < 20 mmol l−1 (Faber et al. 1993). Similar urinary and plasma values were seen in these rats.
The IUGR response: changes in uterine diameter and ANGII receptor expression in the placenta
The mean uterine arcuate artery diameter increased significantly in the normally fed pregnant rats. This is consistent with one aspect of remodelling of the uterine vasculature that normally takes place during pregnancy (Osol & Cipolla, 1993). This increase in artery diameter would allow for the higher uteroplacental blood flow (Ahokas et al. 1983) that is needed for adequate fetal growth. In support of this, the decrease we noted in mean artery diameter in the pregnant rats on the Na+-restricted diet may contribute to the diminished mean fetoplacental unit weight.
To the best of our knowledge, there are no published data on ANGII receptor expression in the rat uterine artery, most probaby due to the low availability of tissue in this animal. In our present study we showed that AT1 mRNA levels do decline during gestation in the rat uterine artery. This decrease is similar in pregnant animals on the Na+-restricted diet. The significance of these changes in AT1 receptor expression to those in vessel diameter is unclear. Furthermore, although functional studies have shown the presence of the AT2 receptor subtype in rat uterine arteries (St-Louis et al. 2001) and this receptor subtype may play a role in cell proliferation (Rizkalla et al. 2003) and in turn influence vessel diameter, AT2 could not, unfortunately, be detected in the rat uterine artery with our current techniques.
In the placenta, the predominant ANGII receptor subtype is AT1 (Li et al. 1998). Although ANGII is not a potent vasoconstrictor in the human placenta (McCarthy et al. 1994) it may, via AT1 receptor activation, play an important role in controlling fetoplacental circulation and thus, fetal growth. In this regard, it has been recently demonstrated that ANGII can, via AT1 receptor activation, increase expression of vascular epidermal growth factor (VEGF) (Rizkalla et al. 2003; Zhang et al. 2004), a growth factor that plays an important ro3le in the remodelling of the uteroplacental and fetoplacental vasculature (Ni et al. 1997). Of particular relevance to our study, increases in VEGF mRNA in the uterine tissues and placenta are most evident in late pregnancy (Ni et al. 1997), corresponding to the last gestational week in the rat and the time for maximum fetal growth. In addition, ANGII has been shown, via AT1 activation, to stimulate human placental lactogen release (Kalenga et al. 1994) which can act directly, or indirectly, via insulin growth factor-1, to promote fetal growth. Therefore, in view of the above, Na+ restriction during this crucial period of gestation in the rat may jeopardize fetal growth (as reflected by the decrease in mean fetoplacental weight) by decreasing levels of AT1 in the placenta. Indeed levels of this ANGII receptor are significantly decreased in the rats on the low-Na+ diet. This decrease in placental AT1 protein noted in these animals on the low-Na+ diet may translate into diminished synthesis of VEGF and placental lactogen that could, in turn, impede vascular growth, fetoplacental circulation and fetal growth. In support of this, as well as the relevance of this animal model for idiopathic IUGR in humans, Li et al. (1998) found that AT1 mRNA and protein decreased in syncytiotrophoblasts and cytotrophoblasts of the placenta in human IUGR.
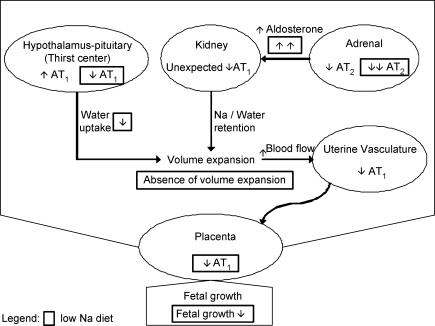
In summary, we report here that dietary Na+ restriction has a greater impact on water/electrolyte homeostasis in the rat during pregnancy. Furthermore, Na+ restriction during the last week of gestation significantly jeopardizes fetal growth and modifies the expression of ANGII receptors in a number of tissues involved in maintaining haemodynamics and sustaining fetal growth. We propose that some of these changes in ANGII receptor expression correlate well with the adaptive vascular mechanisms that come into play during normal gestation while others (noted in our pregnant animals where Na+ intake is reduced) underlie a maladaptive process that leads to IUGR. In Fig. 4 we summarize some of our findings that show that the increase in AT1 expression in the hypothalamic–pituitary axis in the normally fed pregnant rats may serve to increase water consumption. At the level of the kidney, the unexpected decrease in AT1 we see during pregnancy may simply reflect a down-regulatory response. Other renal mechanisms implicating AVP and aldosterone may account for the increase in water retention at the level of the kidney during normal gestation in the rat. In the adrenal cortex, the decrease in AT2 levels would favour ANGII binding to the AT1 receptor subtype ultimately leading to an increase in aldosterone secretion which would act on the kidney to increase Na+ and water retention. These three aforementioned phenomena favour the volume expansion that occurs during normal pregnancy. The increase in mean uterine artery diameter during normal gestation helps maintain normal fetal growth. On the other hand, when dietary Na+ is restricted during pregnancy, the decrease in AT1 levels in the hypothalamic–pituitary axis may serve to suppress thirst. At the level of the adrenal cortex, Na+ restriction results in a greater decrease in AT2 expression that correlates well with the increase in plasma aldosterone in these animals. However, this increase does not serve to compensate for the decrease in water intake and thus, volume expansion is dramatically reduced. The decreased mean artery diameter we measured in the pregnant rats on the low-Na+ diet, combined with the diminished levels of AT1 in the placenta, serve to inhibit fetal growth by restricting uteroplacental circulation and decreasing production of fetal growth factors, respectively. How these findings correlate with those taking place in any clinical conditions of human pregnancy is speculative, considering the present state of characterization of our animal models. However, the model allows the investigation of pregnancy-associated mechanisms, which cannot be studied in human.
Figure 4. Schematic illustration of interactions between angiotensin receptors, maternal homeostasis and fetal growth.
The modifications occurring in pregnant rats are compared to non-pregnant animals. The changes during low-Na+ diet are illustrated by dotted boxes only if they differ from the pregnant animals on normal diet. See text for detailed discussion.
Acknowledgments
We are grateful to Drs Patrick Vinay, Pierre Moreau, André Tremblay and Rhoda Kenigsberg as well as the reviewers of this manuscript for their suggestions and valuable comments that helped improve the quality of this manuscript. We would also like to thank Mr Patrick Lapointe and Mr Serge Picard for technical assistance, Dr Kathy Griendling (Emory University, Atlanta, GA, USA) for the AT1 probe, and Dr Gaétan Guillemette (Université de Sherbrooke, Quebec, Canada) for the AT2 probe as well as the probe used for the photoaffinity procedure. This study was supported by grants from the Heart and Stroke Foundation of Quebec and the Canadian Institutes of Health Research (CIHR) (Grant MOP-37902). Personal support was provided by Fondation de l'Hôpital Sainte-Justine to S.B. (studentship) and Fonds de la Recherche en Santé du Québec (FRSQ) to M.B. (Chercheur-Bousier, Senior).
References
- Ahokas RA, Anderson GD, Lipshitz J. Effect of dietary restriction, during the last week only or throughout gestation, on cardiac output and uteroplacental blood flow in pregnant rats. J Nutr. 1983;113:1766–1776. doi: 10.1093/jn/113.9.1766. [DOI] [PubMed] [Google Scholar]
- Atherton JC, Bielinska A, Davison JM, Haddon I, Kay C, Samuels R. Sodium and water reabsorption in the proximal and distal nephron in conscious pregnant rats and third trimester women. J Physiol. 1988;396:457–470. doi: 10.1113/jphysiol.1988.sp016972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton JC, Dark JM, Garland HO, Morgan MR, Pidgeon J, Soni S. Changes in water and electrolyte balance, plasma volume and composition during pregnancy in the rat. J Physiol. 1982;330:81–93. doi: 10.1113/jphysiol.1982.sp014330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron WM. Volume homeostasis during pregnancy in the rat. Am J Kidney Dis. 1987;9:296–302. doi: 10.1016/s0272-6386(87)80125-7. [DOI] [PubMed] [Google Scholar]
- Baylis C. The determinants of renal hemodynamics in pregnancy. Am J Kidney Dis. 1987;9:260–264. doi: 10.1016/s0272-6386(87)80119-1. [DOI] [PubMed] [Google Scholar]
- Beaudoin AR. Embryology and teratology. In: Baker HJ, Lindsey JR, Weisbroth SH, editors. The Laboratory Rat. New York: Academic Press; 1980. pp. 75–101. [Google Scholar]
- Beausejour A, Auger K, St Louis J, Brochu M. High-sodium intake prevents pregnancy-induced decrease of blood pressure in the rat. Am J Physiol Heart Circ Physiol. 2003;285:H375–H383. doi: 10.1152/ajpheart.01132.2002. [DOI] [PubMed] [Google Scholar]
- Beresford MJ, Fitzsimons JT. Intracerebroventricular angiotensin II-induced thirst and sodium appetite in rat are blocked by the AT1 receptor antagonist, Losartan (DuP 753), but not by the AT2 antagonist, CGP 42112B. Exp Physiol. 1992;77:761–764. doi: 10.1113/expphysiol.1992.sp003643. [DOI] [PubMed] [Google Scholar]
- Bird IM, Zheng J, Cale JM, Magness RR. Pregnancy induces an increase in angiotensin II type-1 receptor expression in uterine but not systemic artery endothelium. Endocrinology. 1997;138:490–498. doi: 10.1210/endo.138.1.4879. [DOI] [PubMed] [Google Scholar]
- Bonjour JP, Malvin RL. Stimulation of ADH release by the renin-angiotensin system. Am J Physiol. 1970;218:1555–1559. doi: 10.1152/ajplegacy.1970.218.6.1555. [DOI] [PubMed] [Google Scholar]
- Brown GP, Venuto RC. Angiotensin II receptor alterations during pregnancy in rabbits. Am J Physiol. 1986;251:E58–E64. doi: 10.1152/ajpendo.1986.251.1.E58. [DOI] [PubMed] [Google Scholar]
- Chung O, Csikos T, Unger T. Angiotensin II receptor pharmacology and AT1-receptor blockers. J Hum Hypertens. 1999;13(Suppl. 1):S11–S20. doi: 10.1038/sj.jhh.1000744. [DOI] [PubMed] [Google Scholar]
- De Paolis P, Porcellini A, Gigante B, Giliberti R, Lombardi A, Savoia C, Rubattu S, Volpe M. Modulation of the AT2 subtype receptor gene activation and expression by the AT1 receptor in endothelial cells. J Hypertens. 1999;17:1873–1877. doi: 10.1097/00004872-199917121-00015. 10.1097/00004872-199917121-00015. [DOI] [PubMed] [Google Scholar]
- Dowell RT, Kauer CD. Maternal hemodynamics and uteroplacental blood flow throughout gestation in conscious rats. Methods Find Exp Clin Pharmacol. 1997;19:613–625. [PubMed] [Google Scholar]
- Dzau VJ, Mukoyama M, Pratt RE. Molecular biology of angiotensin receptors: target for drug research ? J Hypertens Suppl. 1994;12:S1–S5. [PubMed] [Google Scholar]
- Faber MD, Kupin WL, Gopal KG, Narins RG. The differential diagnosis of acute renal failure. In: Lazarus JM, Brenner BM, editors. Acute Renal Failure. New York: Churchill Livingstone; 1993. pp. 133–192. [Google Scholar]
- Forcier I, St-Louis J, Brochu M. Angiotensin II receptor subtypes in the adrenals of pregnant rats. Mol Cell Endocrinol. 1995;114:177–186. doi: 10.1016/0303-7207(95)96798-m. 10.1016/0303-7207(95)96798-M. [DOI] [PubMed] [Google Scholar]
- Fraker PJ, Speck JC., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide,1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978;80:849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gallinat S, Busche S, Schutze S, Kronke M, Unger T. AT2 receptor stimulation induces generation of ceramides in PC12W cells. FEBS Lett. 1999;443:75–79. doi: 10.1016/s0014-5793(98)01675-5. 10.1016/S0014-5793(98)01675-5. [DOI] [PubMed] [Google Scholar]
- Gant NF, Whalley PJ, Everett RB, Worley R, MacDonald PC. Control of vascular reactivity in pregnancy. Am J Kidney Dis. 1994;9:303–307. doi: 10.1016/s0272-6386(87)80126-9. [DOI] [PubMed] [Google Scholar]
- de Gasparo M, Whitebread S, Kalenga MK, De Hertogh R, Crevoisier P, Thomas K. Down regulation of the angiotensin II receptor subtype AT2 in human myometrium during pregnancy. Regul Pept. 1994;53:39–45. doi: 10.1016/0167-0115(94)90157-0. 10.1016/0167-0115(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Gendron L, Laflamme L, Rivard N, Asselin C, Payet MD, Gallo-Payet N. Signals from the AT2 (angiotensin type 2) receptor of angiotensin II inhibit p21ras and activate MAPK (mitogen-activated protein kinase) to induce morphological neuronal differentiation in NG108-15 cells. Mol Endocrinol. 1999;13:1615–1626. doi: 10.1210/mend.13.9.0344. 10.1210/me.13.9.1615. [DOI] [PubMed] [Google Scholar]
- Gigante B, Rubattu S, Russo R, Porcellini A, Enea I, De Paolis P, Savoia C, Natale A, Piras O, Volpe M. Opposite feedback control of renin and aldosterone biosynthesis in the adrenal cortex by angiotensin II AT1-subtype receptors. Hypertension. 1997;30:563–568. doi: 10.1161/01.hyp.30.3.563. [DOI] [PubMed] [Google Scholar]
- Hart JL. Barium responsiveness of the rat aorta and femoral artery during pregnancy. Life Sci. 1982;30:163–169. doi: 10.1016/0024-3205(82)90648-8. 10.1016/0024-3205(82)90648-8. [DOI] [PubMed] [Google Scholar]
- Hein L, Barsh GS, Pratt RE, Dzau VJ, Kobilka BK. Behavioural and cardiovascular effects of disrupting the angiotensin II type-2 receptor in mice. Nature. 1995;377:744–747. doi: 10.1038/377744a0. 10.1038/377744a0. [DOI] [PubMed] [Google Scholar]
- Hogarty DC, Speakman EA, Puig V, Phillips MI. The role of angiotensin, AT1 and AT2 receptors in the pressor, drinking and vasopressin responses to central angiotensin. Brain Res. 1992;586:289–294. doi: 10.1016/0006-8993(92)91638-u. 10.1016/0006-8993(92)91638-U. [DOI] [PubMed] [Google Scholar]
- Hunyady L, Bor M, Balla T, Catt KJ. Identification of a cytoplasmic Ser-Thr-Leu motif that determines agonist-induced internalization of the AT1 angiotensin receptor. J Biol Chem. 1994;269:31378–31382. [PubMed] [Google Scholar]
- Jo H, Yang EK, Lee WJ, Park KY, Kim HJ, Park JS. Gene expression of central and peripheral renin-angiotensin system components upon dietary sodium intake in rats. Regul Pept. 1996;67:115–121. doi: 10.1016/s0167-0115(96)00119-x. 10.1016/S0167-0115(96)00119-X. [DOI] [PubMed] [Google Scholar]
- Kalenga MK, de Gasparo M, Thomas K, De Hertogh R. Angiotensin II induces human placental lactogen and pregnancy-specific beta 1-glycoprotein secretion via an angiotensin AT1 receptor. Eur J Pharmacol. 1994;268:231–236. doi: 10.1016/0922-4106(94)90193-7. 10.1016/0922-4106(94)90193-7. [DOI] [PubMed] [Google Scholar]
- LeHoux JG, Bird IM, Briere N, Martel D, Ducharme L. Influence of dietary sodium restriction on angiotensin II receptors in rat adrenals. Endocrinology. 1997;138:5238–5247. doi: 10.1210/endo.138.12.5612. 10.1210/en.138.12.5238. [DOI] [PubMed] [Google Scholar]
- Li Z, Iwai M, Wu L, Shiuchi T, Jinno T, Cui TX, Horiuchi M. Role of AT2 receptor in the brain in regulation of blood pressure and water intake. Am J Physiol Heart Circ Physiol. 2003;284:H116–H121. doi: 10.1152/ajpheart.00515.2002. [DOI] [PubMed] [Google Scholar]
- Li X, Shams M, Zhu J, Khalig A, Wilkes M, Whittle M, Barnes N, Ahmed A. Cellular localization of AT (1) receptor mRNA and protein in normal placenta and its reduced expression in intrauterine growth restriction. Angiotensin II stimulates the release of vasorelaxants. J Clin Invest. 1998;101:442–454. doi: 10.1172/JCI119881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindheimer MD, Barron WM, Durr J, Davison JM. Water homeostasis and vasopressine release during rodent and human gestation. Am J Kidney Dis. 1987;9:270–275. doi: 10.1016/s0272-6386(87)80121-x. [DOI] [PubMed] [Google Scholar]
- McCarthy AL, Woolfson RG, Evans BJ, Davies DR, Raju SK, Poston L. Functional characteristics of small placental arteries. Am J Obstet Gynecol. 1994;170:945–951. doi: 10.1016/s0002-9378(94)70311-6. [DOI] [PubMed] [Google Scholar]
- Massicotte G, Coderre L, Chiasson JL, Thibault G, Schiffrin EL, St-Louis J. Regulation of ANG II and AVP receptors in isolated hepatocytes of pregnant rats. Am J Physiol. 1990;258:E597–E605. doi: 10.1152/ajpendo.1990.258.4.E597. [DOI] [PubMed] [Google Scholar]
- Massicotte G, St Louis J, Parent A, Schiffrin EL. Decreased in vitro responses to vasoconstrictors during gestation in normotensive and spontaneously hypertensive rats. Can J Physiol Pharmacol. 1987;65:2466–2471. doi: 10.1139/y87-391. [DOI] [PubMed] [Google Scholar]
- Murphy TJ, Alexander RW, Griendling KK, Runge MS, Bernstein KE. Isolation of a cDNA encoding the vascular type-1 angiotensin II receptor. Nature. 1991;351:233–236. doi: 10.1038/351233a0. 10.1038/351233a0. [DOI] [PubMed] [Google Scholar]
- Ni Y, May V, Braas K, Osol G. Pregnancy augments uteroplacental vascular endothelial growth factor gene expression and vasodilator effects. Am J Physiol. 1997;273:H938–H944. doi: 10.1152/ajpheart.1997.273.2.H938. [DOI] [PubMed] [Google Scholar]
- Osol G, Cipolla M. Pregnancy-induced changes in the three-dimensional mechanical properties of pressurized rat uteroplacental (radial) arteries. Am J Obstet Gynecol. 1993;168:268–274. doi: 10.1016/s0002-9378(12)90924-2. [DOI] [PubMed] [Google Scholar]
- Ouali R, Berthelon M-C, Bégeot M, Saez JM. Angiotensin II receptor subtypes AT1 and AT2 are down-regulated by angiotensin II through AT1 receptor by different mechanisms. Endocrinology. 1997;138:725–733. doi: 10.1210/endo.138.2.4952. 10.1210/en.138.2.725. [DOI] [PubMed] [Google Scholar]
- Parent A, Schiffrin E, St-Louis J. Receptors for Arg8−vasopressin, angiotensin II, and atrial natriuretic peptide in the mesenteric vasculature of pregnant rats. Can J Physiol Pharmacol. 1991;69:137–144. doi: 10.1139/y91-020. [DOI] [PubMed] [Google Scholar]
- Qadri F, Culman J, Veltmar A, Maas K, Rascher W, Unger T. Angiotensin II-induced vasopressin release is mediated through alpha-1 adrenoceptors and angiotensin II AT1 receptors in the supraoptic nucleus. J Pharmacol Exp Ther. 1993;267:567–574. [PubMed] [Google Scholar]
- Rizkalla B, Forbes JM, Cooper ME, Cao Z. Increased renal vascular endothelial growth factor and angiopoietins by angiotensin II infusion is mediated by both AT1 and AT2 receptors. J Am Soc Nephrol. 2003;14:3061–3071. doi: 10.1097/01.asn.0000099374.58607.c9. 10.1097/01.ASN.0000099374.58607.C9. [DOI] [PubMed] [Google Scholar]
- Roy-Clavel E, Picard S, St-Louis J, Brochu M. Induction of intrauterine growth restriction with low-sodium diet fed to pregnant rats. Am J Obstet Gynecol. 1999;180:608–613. doi: 10.1016/s0002-9378(99)70262-0. [DOI] [PubMed] [Google Scholar]
- Ruan X, Wagner C, Chatziantoniou C, Kurtz A, Arendshorst WJ. Regulation of angiotensin II receptor AT1 subtypes in renal afferent arterioles during chronic changes in sodium diet. J Clin Invest. 1997;99:1072–1081. doi: 10.1172/JCI119235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas SP, Rossa P, Espinoza R, Robert JA, Valdes G, Donoso E. Maternal plasma volume expansion and hormonal changes in women with idiopathic fetal growth retardation. Obstet Gynecol. 1993;81:1029–1033. [PubMed] [Google Scholar]
- Sasaki K, Yamano Y, Bardhan S, Iwai N, Murray J, Hasegawa M, Matsuda Y, Inagami T. Cloning and expression of a complementary DNA encoding a bovine adrenal angiotensin II type -1 receptor. Nature. 1991;351:230–233. doi: 10.1038/351230a0. 10.1038/351230a0. [DOI] [PubMed] [Google Scholar]
- Schmid C, Castrop H, Reitbauer J, Della Bruna R, Kurtz A. Dietary salt intake modulates angiotensin II type 1 receptor gene expression. Hypertension. 1997;29:923–929. doi: 10.1161/01.hyp.29.4.923. [DOI] [PubMed] [Google Scholar]
- Seltzer A, Pinto JE, Viglione PN, Correa FM, Libertun C, Tsutsumi K, Steele MK, Saavedra JM. Estrogens regulate angiotensin-converting enzyme and angiotensin receptors in female rat anterior pituitary. Neuroendocrinology. 1992;55:460–467. doi: 10.1159/000126157. [DOI] [PubMed] [Google Scholar]
- St-Louis J, Massicotte G. Chronic decrease of blood pressure by rat relaxin in sponstaneously hypertensive rats. Life Sci. 1985;37:1351–1357. doi: 10.1016/0024-3205(85)90251-6. 10.1016/0024-3205(85)90251-6. [DOI] [PubMed] [Google Scholar]
- St-Louis J, Paré H, Sicotte B, Brochu M. Increased reactivity of uterine artery of the rat throughout gestation and post-partum. Am J Physiol. 1997;273:H1148–H1153. doi: 10.1152/ajpheart.1997.273.3.H1148. [DOI] [PubMed] [Google Scholar]
- St-Louis J, Sicotte B, Bedard S, Brochu M. Blockade of angiotensin receptor subtypes in arcuate uterine artery of pregnant and postpartum rats. Hypertension. 2001;38:1017–1023. doi: 10.1161/hy1101.095008. [DOI] [PubMed] [Google Scholar]
- Timmermans PB, Wong PC, Chiu AT, Herblin WF, Benfield P, Carini DJ, Lee RJ, Wexler RR, Saye JA, Smith RD. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993;45:205–251. [PubMed] [Google Scholar]
- Tsutsumi Y, Matsubara H, Masaki H, Kurihara H, Murasawa S, Takai S, et al. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J Clin Invest. 1999;104:925–935. doi: 10.1172/JCI7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkeste CM, Slangen BF, Dubelaar ML, van Kreel BK, Peeters LL. Mechanism of volume adaptation in the awake early pregnant rat. Am J Physiol. 1998;274:H1662–H1666. doi: 10.1152/ajpheart.1998.274.5.H1662. [DOI] [PubMed] [Google Scholar]
- Weber KT. Aldosteronism revisted: perspectives on less well-recognized actions of aldosterone. J Lab Clin Med. 2003;142:71–82. doi: 10.1016/S0022-2143(03)00062-3. 10.1016/S0022-2143(03)00062-3. [DOI] [PubMed] [Google Scholar]
- Zhang X, Lassila M, Cooper ME, Cao Z. Retinal expression of vascular endothelial growth factor is mediated by angiotensin type 1 and type 2 receptors. Hypertension. 2004;43:276–281. doi: 10.1161/01.HYP.0000113628.94574.0f. 10.1161/01.HYP.0000113628.94574.0f. [DOI] [PubMed] [Google Scholar]