Abstract
Mitogen-activated protein kinases (MAPKs) have been implicated in the signal transduction of the endothelial response to growth factors and inflammatory stimuli. The objective of this study was to test the hypothesis that the p42/44 MAPK pathway plays a common role in mediating the microvascular hyperpermeability response to vascular endothelial growth factor (VEGF) and histamine. The apparent permeability coefficient of albumin was measured in isolated and perfused coronary venules. Application of VEGF induced a rapid increase in venular permeability, and the effect was blocked by PD98059 and UO126, selective inhibitors of the mitogen-activated protein kinase kinase MEK1/2, in a dose-dependent pattern. The same MEK1/2 inhibitors dose-dependently attenuated the increase in venular permeability caused by histamine. In addition, the increases in venular permeability caused by agents that are known to activate the nitric oxide pathway, including the calcium ionophore ionomycin, the nitric oxide donor S-nitroso-N-acetylpenicillamine, and the protein kinase G activator 8-bromo-cGMP, were significantly attenuated in venules pretreated with the MEK1/2 inhibitors. Furthermore, transfection of venules with active MEK1 increased baseline permeability. In contrast, transfection of active ERK1, a downstream target of MEK1/2, did not significantly alter the basal permeability of venules. Moreover, inhibition of ERK1/2 with a specific inhibiting peptide did not prevent the hyperpermeability response to VEGF or histamine. The results suggest that activation of MEK1/2 may play a central role in the signal transduction of microvascular hyperpermeability in response to growth factors and inflammatory mediators.
The mitogen-activated protein kinase (MAPK) family consists of a network of signalling cascades that mediate diverse cellular responses to growth factors, physical and chemical stress, and inflammatory cytokines (Pearson et al. 2001; Kyriakis & Avruch, 2001). In mammalian cells, extracellular signal-related kinases (p42/44 MAPK or ERK1/2), c-jun N-terminal kinases (JNK), and p38 MAPK are terminal kinases of three different MAPK cascades that have been well characterized (Chang & Karin, 2001). It is known that the p42/44 MAP kinase kinase MEK1/2 and its downstream target ERK1/2 can be activated by mitogens and morphogens, thereby controlling cell proliferation and differentiation, respectively (Pearson et al. 2001). Within this context, the importance of ERK1/2 in the signal transduction of angiogenesis induced by vascular endothelial growth factor (VEGF) has been established (Berra et al. 2000; Zachary & Gliki, 2001). Recent evidence indicates that certain inflammatory mediators, including histamine, thrombin, and intracellular calcium-elevating agents, are able to activate the MEK–ERK cascade in cultured endothelial cells (Wheeler-Jones & Pearson, 1995; Fleming et al. 1995; Katoch & Moreland, 1995; Gorenne et al. 1998; Koch et al. 2000; Robinson & Dickenson, 2001; Bates et al. 2001). While the precise molecular mechanisms underlying these reactions remain elusive, the fact that hyperpermeability is a common form of the venular response to both VEGF and histamine raises the possibility that the activation of MAPK cascades is involved in the regulation of endothelial barrier function in postcapillary venules. Another important consideration is the demonstration in cultured endothelial monolayers that ERK1/2 activation may serve as a mechanism to increase barrier permeability (Verin et al. 2000; Breslin et al. 2003). The involvement of ERK1/2 and p38 MAPK in the regulation of basal and VEGF-stimulated permeability in endothelial monolayers has also been documented (Lal et al. 2001; Varma et al. 2002).
The endothelial lining of microvessels provides a semi-permeable barrier to the transvascular flux of plasma fluid and proteins. The permeability of the microvascular endothelium can be increased by an array of mediators, including VEGF and histamine, resulting in microvascular leakage and tissue oedema (Lum & Malik, 1994; Ferrara & Davis-Smyth, 1997; Dvorak et al. 1999; Yuan, 2000). This process has been implicated in angiogenesis, ischaemic heart disease, inflammation, trauma, sepsis, and many other pathological conditions. Tremendous effort has been devoted to identifying key signalling molecules that are responsible for the hyperpermeability reaction. Our previous investigations (Yuan et al. 1993b; Wu et al. 1996, 1999) suggest that VEGF- and histamine-induced microvascular hyperpermeability are both mediated by a signalling cascade triggered by receptor binding and transduced by a serial activation of intracellular enzymes, including phospholipase C (PLC), endothelial nitric oxide synthase (eNOS), soluble guanylate cyclase (sGC), and protein kinase G (PKG). Subsequently, the VEGF-activated NO–PKG pathway was linked to ERK1/2-mediated proliferation of cultured endothelial cells via phosphorylation and activation of the upstream p42/44 MAPK cascade component RAF by PKG (Hood & Granger, 1998). However, whether the same mechanism is also involved in regulating endothelial barrier function in postcapillary venules from porcine heart has yet to be determined. Therefore, the purpose of this study was to examine the potential contribution of the p42/44 MAPK cascade to microvascular hyperpermeability in response to VEGF and histamine. The results suggest that MEK1/2 activation serves as a common signal downstream of the NO–PKG cascade in mediating coronary venular hyperpermeability elicited by VEGF and histamine.
Methods
Materials
An albumin-physiological salt solution (APSS) was used as a bathing solution while the microvessels were being dissected. It had the following composition (mm): NaCl 145.0, KCl 4.7, CaCl2 2.0, MgSO4 1.17, NaH2PO4 1.2, glucose 5.0, pyruvate 2.0, EDTA 0.02, and 3-N-morpholinopropanesulphonic acid buffer 3.0. After adding 1% bovine serum albumin, the solution was buffered to a pH of 7.40 at 4°C and then filtered through a Millex-PF 0.22 μm filter unit (Millipore, Bedford, MA, USA). The APSS used to perfuse the vessels during permeability measurements had the same composition as mentioned above, but was buffered to a pH of 7.40 at 37°C. The chemicals used to make the perfusate, including fluorescein isothiocyanate (FITC)-albumin, were purchased from Sigma (St Louis, MO, USA). Bovine serum albumin was obtained from United States Biochemical (Cleveland, OH, USA). Cell culture supplies including the culture media Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum were from Gibco (Gaithersburg, MD, USA). VEGF was from R & D Systems (Minneapolis, MN, USA) and histamine was from Sigma. The calcium ionophore ionomycin, the nitric oxide donor S-nitroso-N-acetylpenicillamine (SNAP), the protein kinase G activator 8-bromo-cGMP, and the selective MEK1/2 inhibitors PD98059 and UO126 were purchased from Calbiochem (San Diego, CA, USA). The cell-permeable ERK1/2-inhibiting peptide stearyl-MPKKPTPIQLNP was ordered from SynPep (Dublin, CA, USA). Human recombinant active MEK1 and ERK1 were acquired from Upstate (Lake Placid, NY, USA) and Calbiochem, respectively.
Animal preparation
Yorkshire pigs weighing 9–13 kg were anaesthetized with sodium pentobarbital (30 mg kg−1, i.v.) and heparinized (250 units kg−1, i.v.). Following tracheotomy and intubation, animals were ventilated with room air. A left thoracotomy was performed and the heart was electrically fibrillated, excised, and placed in 4°C physiological saline. The pigs were killed by the surgical removal of the heart. The animals were housed and handled in accordance with the protocols approved by the Institutional Animal Care and Use Committee of Texas A & M University, and Scott and White Hospital.
Isolation and perfusion of coronary venules
The coronary sinus was cannulated, and India ink–gelatin–physiological salt solution was infused to clearly define venular microvessels. This solution was prepared by adding 0.2 ml of India ink (Koh-I-Noor, Bloomsbury, NJ, USA) and 0.35 g of porcine skin gelatin to 10 ml of warm physiological salt solution and filtered through P8 filter paper (Fisher Scientific, Pittsburgh, PA, USA). Information regarding the validation and limitation of the ink-perfusion procedure has been provided in our previous publication (Yuan et al. 1993a). The method for isolating and cannulating coronary venules has also been described in detail in that study. Briefly, a suitable venule (length 0.8–1.2 mm, diameter 20–60 μm) was dissected from the surrounding myocardium in a dissecting chamber containing APSS at 4°C with the aid of a Zeiss stereo dissecting microscope. The vessel was transferred to a cannulating chamber which was mounted on a Zeiss axiovert microscope. The isolated vessel was cannulated with an inflow and outflow micropipette on each end and secured with 11-0 suture (Alcon, Fort Worth, TX, USA). A third smaller pipette was inserted into the inflow pipette. The vessel was perfused with either APSS through the outer inflow pipette or APSS containing FITC-albumin through the inner inflow pipette. Each cannulating micropipette was connected to a reservoir and the vessel was perfused at a relatively constant intraluminal pressure and flow rate. The bath solution in the chamber was maintained at 37°C and pH 7.4 throughout the experiments. The image of the vessel was projected onto a Hamamatsu charged coupled device-intensified camera and was displayed on a high resolution monochromatic video monitor and recorded onto a VHS video recorder. The diameter of the vessel was measured on-line with a video caliper (Cardiovascular Research Institute, College Station, TX, USA).
Measurement of venular permeability
The permeability of the vessel was measured with a fluorescence ratioing technique (Huxley et al. 1987; Yuan et al. 1993a). Using an optical window of a video photometer positioned over the venules and adjacent space on the monitor, the fluorescent intensity from the window was measured and digitized on-line. In each measurement, the isolated venule was first perfused with APSS through the outer inflow pipette to establish a baseline intensity. The venular lumen was then rapidly filled with APSS containing FITC-albumin by switching the perfusion to the inner inflow pipette. This produced an initial step increase, followed by a gradual increase, in fluorescence intensity. There was a step decrease of intensity when the fluorescently labelled molecules were washed out of the vessel lumen by switching the perfusion back to the outer inflow pipette. The apparent solute permeability coefficient of albumin (Pa) was calculated using the equation Pa = (1/ΔIf)(dIf/dt)o(r/2), where ΔIf is the initial step increase in fluorescence intensity, (dIf/dt)o is the initial rate of gradual increase in intensity as the fluorescently labelled solutes diffuse out of the vessel into the extravascular space, and r is the venular radius.
In each experiment, the cannulated venule was perfused at a constant perfusion pressure of 20 cmH2O. The preparation was equilibrated for 45–60 min after cannulation and the measurements were conducted at 36–37°C and a pH of 7.35–7.45. A limited number (< 3) of interventions were applied to each vessel. Between interventions the preparation was washed three times and allowed to equilibrate for 10–15 min. In some vessels, the permeability was monitored over 6 h to ensure that the barrier property of the venules was not significantly altered with time.
VEGF (10−10m) or histamine (10−5m) was topically added into the suffusion bath and the permeability of the vessels was measured before and after exposure to the mediators up to 15 min. To study the role of MEK in hyperpermeability responses, venules were preincubated for 30 min with selective MEK inhibitors PD98059 (10−7–10−5m) or UO126 (10−7–10−4m) (Davies et al. 2000). The Pa values were then determined before and after adding VEGF or histamine for 15 min in the presence of the inhibitor. To further verify that the p42/44 MAPK signal is located downstream of the NO pathway, the same experiment was repeated in separate vessels that were treated with the MEK inhibitors before stimulation by the NO cascade activators ionomycin (10−5m), SNAP (10−5m), and 8-bromo-cGMP (10−5m).
In order to specify the effect of the MEK downstream target, ERK1/2, we measured the permeability response to VEGF and histamine in venules that were treated with a specific ERK1/2-inhibiting peptide (50 μm) (Kelemen et al. 2002). Furthermore, we tested the direct effects of MEK and ERK on venular permeability by transfecting active MEK1 or active ERK1 into venular endothelium. The MEK1 protein is a recombinant full-length human MEK1 expressed in E. coli that is activated by in vitro phosporylation with active RAF1 (Alessi et al. 1995). The ERK1 protein is a recombinant human MAPK expressed in E.coli activated by phosphorylation with a constitutively active MEK1 bound to glutathione agarose beads (Charest et al. 1993). The technique of transfecting proteins into intact microvascular endothelium has been described and evaluated in detail in our previous publications (Tinsley et al. 1998, 2001). Venules were perfused for 1 h with a transfection mixture containing the polyamine reagent TransIT (Pan Vera) at 10 μl ml−1 and active MEK1 (0.1 U ml−1) or ERK1 (5 μg ml−1). After transfection, the vessels were washed with the regular perfusate and then subjected to chemical stimulation as described above.
Data analysis
In the intact vessel studies, Pa was measured two to three times in each venule at each experimental intervention and the values were averaged. For each experimental condition, the values of Pa from different vessels were averaged, normalized to the basal values obtained before drug treatments, and reported as a percentage of the basal value as mean ±s.e.m. For all experiments n is given as the number of vessels studied, with each vessel representing a separate animal. Analysis of variance was used to evaluate the significance of intergroup differences. A value of P < 0.05 was considered significant for the comparisons.
Results
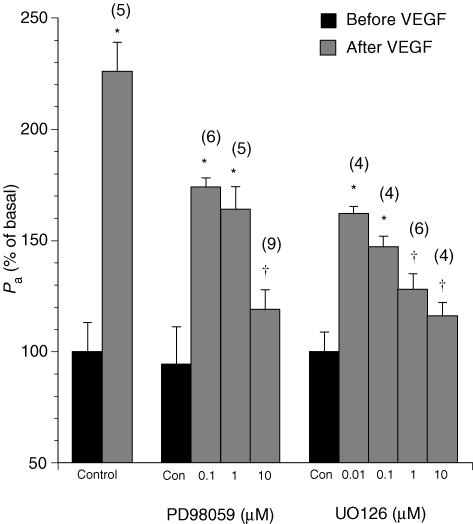
VEGF induced a significant increase in venular permeability (Fig. 1). In agreement with our previous observations (Wu et al. 1996, 1999), the hyperpermeability response was rapid, peaking at 3–5 min and recovering within 15 min. At 10−10m VEGF, Pa rose to a peak value of 232 ± 17% of the basal permeability (Pa from (2.45 ± 0.20) × 10−6 cm s−1 to (5.69 ± 0.62) × 10−6 cm s−1, n = 8). The MEK inhibitors did not affect the basal barrier property of the venules, but blocked VEGF-induced hyperpermeability in a dose-dependent fashion. Neither the time course to peak response nor the duration of recovery was significantly altered by the inhibitors. As shown in Fig. 1, the peak Pa values after treatment with VEGF (10−10m) were reduced to 189 ± 12% of basal in the presence of PD98059 at 10−7m. The peak Pa value was further reduced to 164 ± 26% at 10−6m and 120 ± 9% at 10−5m, respectively. Similarly, UO126-treated venules decreased VEGF-stimulated Pa to 162 ± 5% at 10−7m, 148 ± 6% at 10−6m, 125 ± 3% at 10−5m, and 120 ± 15% at 10−4m, respectively. The results indicated that the activation of MEK was involved in the mechanism of VEGF-induced hyperpermeability.
Figure 1. Changes in the apparent permeability coefficient of albumin upon treatment with vascular endothelial growth factor (VEGF; 10−10m) in venules pretreated with the MEK1/2 inhibitors PD98059 or UO126.
Controls show the effects of vehicle (0.1% DMSO) alone. Numbers in parentheses represent the numbers of vessels studied. * Significant difference (P < 0.05)versus the basal Pa value in untreated venules. † Significant difference (P < 0.05)versus the Pa value after VEGF without PD98059 or UO126 treatment.
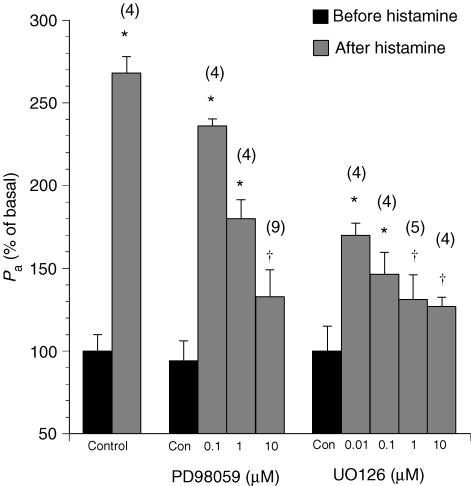
Treatment of the venules with histamine rapidly increased venular permeability by 268 ± 34% over the basal value (Pa = (2.55 ± 0.11) × 10−6 cm s−1 before histamine, and Pa = (6.85 ± 0.96) × 10−6 cm s−1 after histamine, n = 4). Similar to the VEGF response, the histamine response peaked at 3–5 min and dissipated after 15–20 min. The hyperpermeability was greatly attenuated during inhibition of MEK without a significant change in the duration or time of peak response (Fig. 2). Specifically, PD98059 reduced the peak Pa upon histamine to 237 ± 11% over the basal value at 10−7m, 180 ± 17% at 10−6m and 133 ± 15% at 10−5m, respectively. In the group of vessels pretreated with UO126, the permeability responses to histamine were attenuated to 171 ± 13% at 10−7m, 146 ± 15% at 10−6m, 131 ± 12% at 10−5m and 128 ± 7% at 10−4m, respectively.
Figure 2. Changes in the apparent permeability coefficient of albumin (Pa) upon treatment with histamine (10−5m) in venules pretreated with the MEK1/2 inhibitors PD98059 or UO126.
Controls show the effects of vehicle (0.1% DMSO) alone. Numbers in parentheses represent the numbers of vessels studied. * Significant difference (P < 0.05)versus the basal Pa value in untreated venules. † Significant difference (P < 0.05)versus the Pa value after histamine without PD98059 or UO126 treatment.
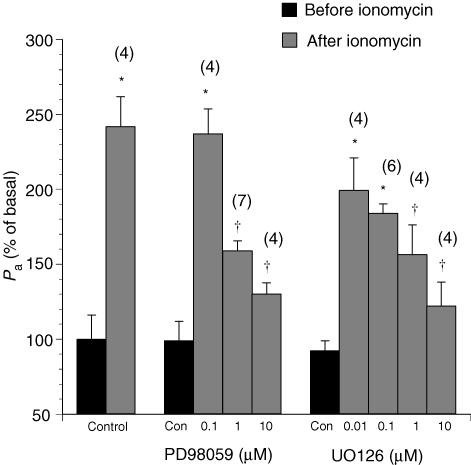
Previous studies indicated that the p42/44 MAPK cascade is downstream of the NO–PKG pathway (Hood & Granger, 1998), which has been shown to mediate VEGF- and histamine-elicited hyperpermeability responses in venules. In control venules, the calcium ionophore ionomycin, the exogenous NO donor SNAP, and the PKG activator 8-bromo-cGMP produced a hyperpermeability effect with a time course and peak response comparable to those of histamine. The MEK inhibitors PD98059 and UO126 displayed a dose-related inhibition of the peak response to all of these agonists without affecting the time course of hyperpermeability (Figs 3, 4, 5). In Fig. 3, ionomycin (10−5m) increased Pa to 234 ± 33% from its basal value in the absence of the inhibitors (Pa was from (2.38 ± 0.31) × 10−6 cm s−1 to (5.27 ± 0.38) × 10−6 cm s−1, n = 4). In the PD98059- treated group, the increases were reduced to 184 ± 32% at 10−7m, 177 ± 13% at 10−6m and 128 ± 18% at 10−5m, respectively. Similarly, ionomycin-induced increases in Pa were attenuated, in the presence of UO126, to 199 ± 22% at 10−7m, 185 ± 6% at 10−6m, 156 ± 20% at 10−5m and 122 ± 17% at 10−4m, respectively.
Figure 3. Changes in the apparent permeability coefficient of albumin upon treatment with the calcium ionophore ionomycin (10−5m) in venules pretreated with the MEK1/2 inhibitors PD98059 or UO126.
Controls show the effects of vehicle (0.1% DMSO) alone. Numbers in parentheses represent the numbers of vessels studied. * Significant difference (P < 0.05)versus the basal Pa value in untreated vessels. † Significant difference (P < 0.05)versus the Pa value after ionomycin without PD98059 or UO126 treatment.
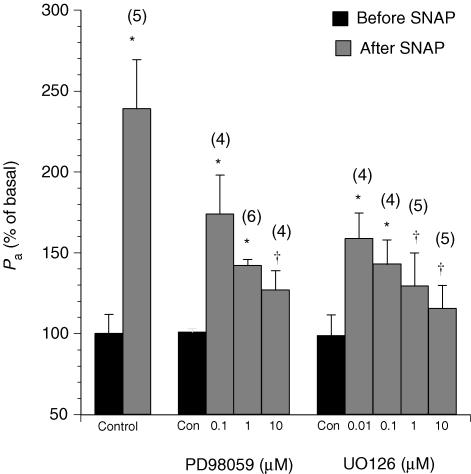
Figure 4. Changes in the apparent permeability coefficient of albumin upon treatment with the exogenous NO donor S-nitroso-N-acetylpenicillamine (SNAP, 10−5m) in venules pretreated with the MEK1/2 inhibitors PD98059 or UO126.
Controls show the effects of vehicle (0.1% DMSO) alone. Numbers in parentheses represent the numbers of vessels studied. * Significant difference (P < 0.05)versus the basal Pa value in untreated venules. † Significant difference (P < 0.05)versus the Pa value after SNAP without PD98059 or UO126 treatment.
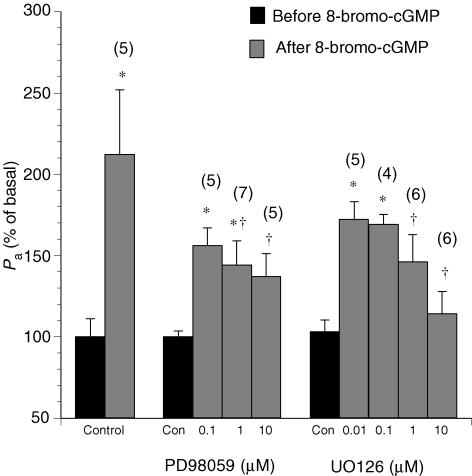
Figure 5. Changes in the apparent permeability coefficient of albumin upon treatment with the protein kinase G (PKG) activator 8-bromo-cGMP (10−5m) in venules pretreated with the MEK1/2 inhibitors PD98059 or UO126.
Controls show the effects of vehicle (0.1% DMSO) alone. Numbers in parentheses represent the numbers of vessels studied. * Significant difference (P < 0.05)versus the basal Pa value in untreated venules. † Significant difference (P < 0.05)versus the Pa value after cGMP without PD98059 or UO126 treatment.
The NO donor SNAP increased the basal permeability to 240 ± 31% (Fig. 4). The effect of SNAP on Pa was significantly attenuated to 127 ± 12% in the presence of PD98059 (10−5m) and to 140 ± 21% in the presence of UO126 (10−5m). In addition, the increase in Pa induced by 8-bromo-cGMP was attenuated during MEK inhibition (Fig. 5). In control venules, 8-bromo-cGMP elevated Pa to 231 ± 39% of the basal value. In contrast, the Pa value in response to 8-bromo-cGMP was 137 ± 15% in PD98059 (10−5m)-treated venules. In UO126 (10−5m)-treated venules, Pa was 147 ± 17% of basal after 8-bromo-cGMP.
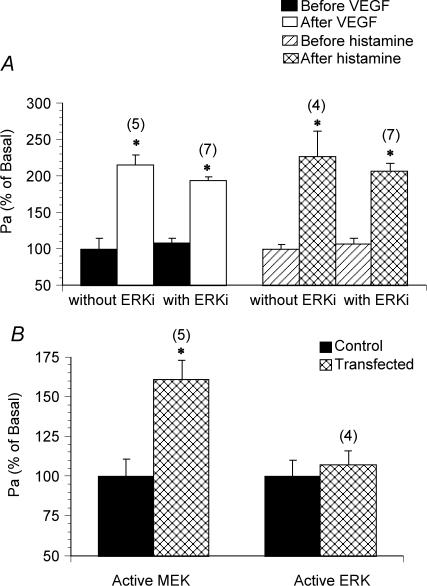
Pretreatment of venules with an ERK1/2-specific inhibiting peptide (50 μm ERKi) did not alter the basal permeability, nor did it prevent VEGF- and histamine-induced hyperpermeability (Fig. 6A). However, transfection of venules with active MEK1 (0.1 U ml−1, which equals an enzymatic activity of 0.3 nmol phosphate (mg myelin basic protein)−1 min−1) significantly increased Pa by 161 ± 22% (Fig. 6B). In contrast, transfection with active ERK1 (5 μg ml−1, an enzymatic activity of 0.5 nmol phosphate (mg myelin basic protein)−1 min−1) did not elevate the permeability (Fig. 6B). The transfection procedure per se did not alter the endothelial barrier function as indicated by an unchanged basal permeability.
Figure 6. Changes of venular permeability after inhibition of ERK1/2 and transfections of active MEK and ERK.
Changes in the apparent permeability coefficient of albumin upon treatment with VEGF (10−10m) and histamine (10−5m) in venules pretreated with an ERK1/2-inhibiting peptide (ERKi, 50 μm) (A). B, effects of transfection of active MEK1 (0.1 U ml−1) and active ERK1 (5 μg ml−1) on venular permeability. Controls show the effects of vehicle (the transfection reagent TransIT) alone. Numbers in parentheses represent the numbers of vessels studied. * Significant difference (P < 0.05)versus the basal Pa value in untreated venules.
Discussion
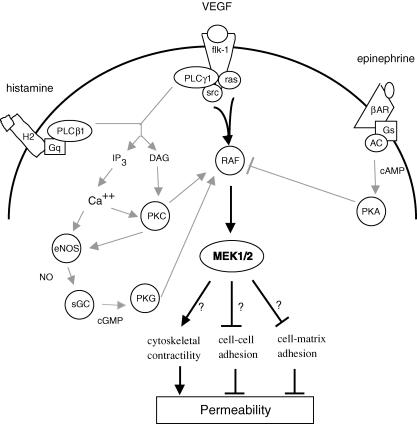
Previous studies on isolated, perfused postcapillary venules from the porcine heart support the convergence of histamine (Yuan et al. 1993b; Huang & Yuan, 1997) and VEGF (Wu et al. 1996, 1999) signalling on the PLC–eNOS–sGC–PKG cascade. As illustrated in Fig. 7, histamine activates the β1 isoform of PLC through the association of the ligated H2 receptor with the G protein Gq. VEGF binding to the flk-1/KDR receptor tyrosine kinase results in phosphorylation and activation of PLCγ1. Both PLC isoforms catalyse the formation of inositol 1,4,5-trisphosphate (IP3) and diacylgyclerol (DAG) from phosphatidylinositol 4,5-bisphosphate molecules imbedded in the inner leaflet of the plasma membrane. DAG activates protein kinase C, and IP3 stimulates the release of calcium from internal stores. The subsequent rise in cytosolic calcium activates eNOS, and facilitates the stimulation of PKC by DAG. Activated PKC phosphorylates eNOS and further stimulates NO production by eNOS. In turn, NO turns on soluble guanylate cyclase, thereby increasing the production of the second messenger cyclic GMP. The cyclic nucleotide binds to protein kinase G, resulting in stimulation of catalytic activity. The operation of this common signalling cascade in porcine coronary venules has been established through the use of inhibitors of PLC, eNOS, sGC and the binding and catalytic domains of PKG. Moreover, exposure of the venules to ionomycin, NO donors and cell-permeable cGMP analogues leads to hyperpermeability responses that are blocked by inhibitors that bind to catalytic sites on PKG. Finally, hyperpermeability due to increased shear rate may also converge on this signalling cascade since inhibition of NO production blocks the response in coronary venules (Yuan et al. 1992).
Figure 7. Hypothetical model of permeability signalling in postcapillary venules of porcine heart.
In the centre, the MAPK cascade integrates positive (lines with filled arrow) and negative (lines with perpendicular at tip) influences at the level of RAF. Phosphorylation of RAF by src, PKC or PKG increases its kinase activity and leads to activation of MEK1/2. MEK1/2 may affect endothelial barrier function by altering the cytoskeletal arrangement or cell–cell and cell–matrix adhesions. Phosphorylation of RAF by PKA inhibits the activity of the MAPK cascade and results in reduced permeability. Not shown are phosphodiesterases that catalyse cGMP and cAMP degradation, and phosphatases that dephosphorylate RAF, MEK, ERK and other phosphoproteins. βAR, β-adrenergic receptor; Gs, stimulatory.
The present study represents the first attempt to map the hyperpermeability signalling pathway in coronary venules beyond the PLC–eNOS–sGC–PKG cascade. Because previous work from this laboratory demonstrated that PKG phosphorylates and activates RAF1 in cultured endothelial cells (Hood & Granger, 1998), we hypothesize that the hyperpermeability signals are transmitted from PKG and other kinases to the RAF–MEK cascade. Figure 7 shows our hypothetical model of permeability signalling in postcapillary venules. Multiple pathways are proposed, based on our studies with intact isolated venules (Yuan et al. 1993b; Wu et al. 1996, 1999; Huang & Yuan, 1997; Yuan, 2000) and results from other laboratories using intravital microscopy (Bates & Harper, 2003; Aramoto et al. 2004), as well as in vitro experiments with cultured endothelial cell monolayers (Lum et al. 1999; Lal et al. 2001; Varma et al. 2002). Additional support for this hypothesis in the literature includes: (1) histamine (Huang & Yuan, 1997; Robinson & Dickenson, 2001) and VEGF (Hood & Granger, 1998; He et al. 1999; Takahashi et al. 1999; Lal et al. 2001; Varma et al. 2002) activate PKG, PKC and/or Src, three known stimulators of RAF1 kinase activity and the p42/44 and p38 MAPK cascades in cultured endothelial cells; (2) other hyperpermeability stimuli such as shear stress (Jo et al. 1997; Traub et al. 1997), hyperosmolarity (Duzgun et al. 2000), and hydrogen peroxide (Kevil et al. 2000) elicit transient phosphorylation of ERK; (3) MEK and ERK phosphorylation transients mirror coronary venular hyperpermeability responses, peaking at 3–5 min and dissipating within 15–30 min (Parenti et al. 1998; Takahashi et al. 1999; Breslin et al. 2003); and (4) activation of PKA, a known inhibitor of RAF1 kinase activity, by cAMP in cultured endothelial cells leads to a reduction in both p42/44 MAPK cascade activity and monolayer permeability (Lum et al. 1999; Liu et al. 2001).
At the micromolar concentrations used in this study, PD98059 and UO126 exert potent inhibitory effects on MEK1 and MEK2 by preventing association with their activating partner RAF1; however, they do not affect any other protein kinase (Davies et al. 2000). Furthermore, at the concentrations used in this study, neither of these inhibitors act on MEKs associated with JNK or p38 kinase, the two other major MAP kinases. The use of two different types of inhibitor for MEK1/2 minimized the risk of studying non-specific effects. The current study demonstrates the importance of MEK1/2 activation as a common signal in the transduction of the venular hyperpermeability response to VEGF and histamine. Moreover, hyperpermeability responses elicited by ionomycin, SNAP and 8-bromo-cGMP, all putative activators of PKG, are also blocked by UO126 and PD98059. Furthermore, the upstream p42/44 MAPK component RAF1 is a major participant in the transmission of the hyperpermeability signal elicited by VEGF, histamine, NO and cyclic GMP. This view is supported by the ability of PD98059 and UO126, inhibitors of RAF1 binding to MEK1/2, to reduce the permeability responses to these mediators.
In addition to the pharmacological approach, we examined the effect of ERK1/2 as a potential downstream hyperpermeability target of MEK1/2 by treating venules with a specific ERK1/2-inhibiting peptide. The cell-permeable inhibitory peptide, stearyl-MPKKKPTPIQLNP, is derived from the amino terminus sequence of MEK1 and prevents ERK1 and ERK2 activation by interfering with MEK binding to ERK at the docking site. This inhibition of ERK activity is concentration dependent with an IC50 of 2.5 μmin vitro and 13 μmin vivo (Kelemen et al. 2002). The peptide fails to inhibit JNK and p38 protein kinases, even at concentrations of 100 μmin vitro (Kelemen et al. 2002). At a concentration equal to 4 times the in vivo IC50, the specific ERK1/2-inhibiting peptide failed to alter baseline permeability and to diminish the hyperpermeability elicited by VEGF. This finding suggests that the hyperpermeability signal is not transmitted to the terminal component of the p42/44 MAPK cascade.
Although others have shown reduced hyperpermeability reactions in endothelial monolayers chronically transfected with ERK antisense oligonucleotides (Verin et al. 2000; Breslin et al. 2003), acute insertion of active ERK protein into coronary venules did not elevate baseline albumin transport above control. In contrast, baseline albumin transport was augmented after introducting active MEK1 into the endothelial cells of isolated postcapillary venules. Taken together, these findings using active forms of MEK1 and ERK1 support the notion that the hyperpermeability signal associated with histamine and VEGF travels through RAF1 and MEK1/2 but does not extend to ERK1/2. In other words, the hyperpermeability signal under these specific circumstances exits the p42/44 MAP kinase cascade at MEK1/2 in coronary venules. By contrast the mitogenic signal has been shown by many others to continue through ERK1/2.
A search of potential MEK targets using the consensus phosphorylation motif TXY produces important proteins associated with endothelial permeability including paxillin, focal adhesion kinase (FAK), β4 integrin, ZO1, VE-cadherin, catenin, glycogen synthase kinase-3β, F-actin, endothelial actin-binding protein, vimentin and caveolin; all have been linked to the mechanisms underlying paracellular or transcellular permeability. Within this context, our recent study (Wu et al. 2003) showed that VEGF stimulated FAK phosphorylation and focal adhesion redistribution with a time course similar to the increase in venular permeability; the biochemical morphological and physiological responses were all attenuated by a specific FAK inhibitor. Thus, FAK activation may serve as an important signalling event downstream of MEK1/2 in VEGF regulation of endothelial barrier function. The precise molecular interaction between MEK and FAK remains to be elucidated. We do not rule out the possibility that FAK participates in VEGF signalling at an initial stage upstream from MEK1/2 activation.
The permeability of microvascular endothelium is maintained by an equilibrium between the contractile force generated by the endothelial cytoskeleton and adhesive forces produced at endothelial cell–cell junctions and cell–matrix focal contacts (Lum & Malik, 1994; Garcia & Schaphorst, 1995; Moy et al. 2000). Over the past few years, multiple signalling cascades have been proposed to be involved in the regulation of these structural elements. For example, the cAMP–PKA cascade has been shown to maintain barrier function through an adhesion-dependent mechanism (Lampugnani et al. 1990). Activation of myosin light chain kinase or the Rho GTPases is known to cause endothelial cell contraction (Yuan, 2000; van Nieuw Amerongen et al. 2000). The PKC pathway has been linked to stress fibre formation and junction dissociation (Siflinger-Birnboim & Johnson, 2003). The results from this study, along with our previous findings, lead to the delineation of the PLC–eNOS–sGC–PKG–RAF–MEK cascade. While the current study demonstrates a common role for MEK1/2 in the hyperpermeability response to two different types of stimuli, cross-talk between MEK1/2 and other pathways could occur at different levels and redundancy may exist. We hypothesize that MEK1/2 activation is required for conveying the hyperpermeability signal to final adhesion, cytoskeletal or membrane effectors in coronary venules (Fig. 7). Further studies are necessary not only to establish the importance of the MEK pathway relative to others, but also to identify the routes of transendothelial movement of fluid and proteins modulated by this signalling cascade. Within this context, the effects of VEGF on hydraulic conductivity, reflection coefficient, and diffusive permeability have all been investigated in intact vessels (Bates & Harper, 2003). However, the findings in this study using measurements of apparent permeability coefficient for albumin offer limited information on the relative roles of different modes of transport activated by the p42/44 MAPK cascade.
The involvement of MEK1/2 as potential common mediators for venular permeability signals has important implications. First, the findings imply a direct linkage between mitogenic and hyperpermeability signals in vascular endothelium. We have previously shown that NO and cyclic GMP are mitogenic in cultured venular and venous endothelial cells (Ziche et al. 1993a; Morbidelli et al. 1996; Hood et al. 1998), as well as acting as hyperpermeability factors in isolated coronary venules (Yuan et al. 1993b). Moreover, agents normally considered as purely hyperpermeability factors, such as histamine, bradykinin and substance P, are capable of stimulating endothelial cell proliferation in culture through NO-dependent mechanisms (Marks et al. 1986; Ziche et al. 1993b, 1994; Parenti et al. 2001). Second, our results suggest that any growth factor capable of turning on MEK1/2 is a potential mediator of venular hyperpermeability. In this regard, only VEGF has received significant attention as a hyperpermeability factor. Previous studies have failed to demonstrate acute opening of the endothelial barrier after exposure to FGF, PDGF and other tyrosine receptor kinases capable of activating the p42/44 MAPK cascade (Murohara et al. 1998; Rissanen et al. 2003). However, recent experiments from our laboratory (authors' unpublished data) suggest that both FGF and PDGF are capable of increasing the permeability of isolated coronary venules from the porcine heart. Thus, a consistent linkage between mitogenic and permeability signalling through the p42/44 MAPK pathway amongst endothelial growth factors has not yet been conclusively established. Finally, our findings hint at the potential for new mechanisms of signalling hyperpermeability, namely, through modulation of the phosphatases that terminate the actions of RAF and MEK1/2 through dephosphorylation (Haneda et al. 1999). For example, signalling pathways that lead to inhibition of these phosphatases could lead to hyperpermeability responses of greater magnitude and greater duration. By contrast, activation of the phosphatases could prevent the subsequent actions of inflammatory mediators that normally signal hyperpermeability reactions via the p42/44 MAPK pathway.
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute Grants HL-58062, HL-21498, HL61507 and HL70752.
References
- Alessi DR, Cohen P, Ashworth A, Cowley S, Leevers SJ, Marshall CJ. Assays and expression of mitogen-activated protein kinase, MAP kinase kinase, and Raf. Meth Enzymol. 1995;255:279–290. doi: 10.1016/s0076-6879(95)55031-3. [DOI] [PubMed] [Google Scholar]
- Aramoto H, Breslin JW, Pappas PJ, Hobson RW, II, Duran WN. Vascular endothelial growth factor stimulates differential signaling pathways in in vivo microcirculation. Am J Physiol Heart Circ Physiol. 2004;287:H1590–H1598. doi: 10.1152/ajpheart.00767.2003. 10.1152/ajpheart.00767.2003. [DOI] [PubMed] [Google Scholar]
- Bates DO, Harper SJ. Regulation of vascular permeability by vascular endothelial growth factors. Vascular Pharmacol. 2003;39:225–237. doi: 10.1016/s1537-1891(03)00011-9. [DOI] [PubMed] [Google Scholar]
- Bates DO, Heald RI, Curry FE, Williams B. Vascular endothelial growth factor increases Rana vascular permeability and compliance by different signalling pathways. J Physiol. 2001;533:263–272. doi: 10.1111/j.1469-7793.2001.0263b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berra E, Milanini J, Richard DE, Le Gall M, Vinals F, Gothie E, Roux D, Pages G, Pouyssegur J. Signaling angiogenesis via p42/p44 MAP kinase and hypoxia. Biochem Pharmacol. 2000;60:1171–1178. doi: 10.1016/s0006-2952(00)00423-8. [DOI] [PubMed] [Google Scholar]
- Breslin JW, Pappas PJ, Cerveira JJ, Hobson RW, Duran WN. VEGF increases endothelial permeability by separate signaling pathways involving ERK-1/2 and nitric oxide. Am J Physiol Heart Circ Physiol. 2003;284:H92–H100. doi: 10.1152/ajpheart.00330.2002. [DOI] [PubMed] [Google Scholar]
- Chang L, Karin M. Mammalian MAP kinase signaling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Charest DL, Mordret G, Harder KW, Jirik F, Pelech SL. Molecular cloning, expression, and characterization of the human mitogen activated protein kinase p44 erk 1. Mol Cell Biol. 1993;13:4679–4690. doi: 10.1128/mcb.13.8.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duzgun SA, Rasque H, Kito H, Azuma N, Li W, Basson MD, Gahtan V, Dudrick SJ, Sumpio BE. Mitogen-activated protein phosphorylation in endothelial cells exposed to hyperosmolar conditions. J Cell Biochem. 2000;76:567–571. doi: 10.1002/(sici)1097-4644(20000315)76:4<567::aid-jcb5>3.0.co;2-w. 10.1002/(SICI)1097-4644(20000315)76:4<567::AID-JCB5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Dvorak HF, Nagy JA, Feng D, Brown LF. Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr Top Microbiol Immunol. 1999;237:97–132. doi: 10.1007/978-3-642-59953-8_6. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. 10.1210/er.18.1.4. [DOI] [PubMed] [Google Scholar]
- Fleming I, Fisslthaler B, Busse R. Calcium signaling in endothelial cells involves activation of tyrosine kinases and leads to activation of mitogen-activated protein kinases. Circ Res. 1995;76:522–529. doi: 10.1161/01.res.76.4.522. [DOI] [PubMed] [Google Scholar]
- Garcia JG, Schaphorst KL. Regulation of endothelial cell gap formation and paracellular permeability. J Investig Med. 1995;43:117–126. [PubMed] [Google Scholar]
- Gorenne I, Su X, Moreland RS. Inhibition of p42 and p44 MAP kinase does not alter smooth muscle contraction in swine carotid artery. Am J Physiol. 1998;275:H131–H138. doi: 10.1152/ajpheart.1998.275.1.H131. [DOI] [PubMed] [Google Scholar]
- Haneda M, Sugimoto T, Kikkawa R. Mitogen-activated protein kinase phosphatase: a negative regulator of the mitogen-activated protein kinase cascade. Eur J Pharmacol. 1999;365:1–7. doi: 10.1016/s0014-2999(98)00857-7. 10.1016/S0014-2999(98)00857-7. [DOI] [PubMed] [Google Scholar]
- He H, Venema VJ, Gu X, Venema RC, Marrero MB, Caldwell RB. Vascular endothelial growth factor signals endothelial cell production of nitric oxide and prostacyclin through flk-1/KDR activation of c-Src. J Biol Chem. 1999;274:25130–25135. doi: 10.1074/jbc.274.35.25130. 10.1074/jbc.274.35.25130. [DOI] [PubMed] [Google Scholar]
- Hood J, Granger HJ. Protein kinase G mediates vascular endothelial growth factor-induced Raf-1 activation and proliferation in human endothelial cells. J Biol Chem. 1998;273:23504–23508. doi: 10.1074/jbc.273.36.23504. 10.1074/jbc.273.36.23504. [DOI] [PubMed] [Google Scholar]
- Hood JD, Meininger CJ, Ziche M, Granger HJ. VEGF upregulates ecNOS message, protein, and NO production in human endothelial cells. Am J Physiol. 1998;274:H1054–H1058. doi: 10.1152/ajpheart.1998.274.3.H1054. [DOI] [PubMed] [Google Scholar]
- Huang Q, Yuan SY. Interaction of PKC and NOS in signal transduction of microvascular hyperpermeability. Am J Physiol. 1997;273:H2442–H2451. doi: 10.1152/ajpheart.1997.273.5.H2442. [DOI] [PubMed] [Google Scholar]
- Huxley VH, Curry FE, Adamson RH. Quantitative fluorescence microscopy on single capillaries: alpha-lactalbumin transport. Am J Physiol. 1987;252:H188–H197. doi: 10.1152/ajpheart.1987.252.1.H188. [DOI] [PubMed] [Google Scholar]
- Jo H, Sipos K, Go YM, Law R, Rong J, McDonald JM. Differential effect of shear stress on extracellular signal-regulated kinase and N-terminal Jun kinase in endothelial cells. Gi2- and Gbeta/gamma-dependent signaling pathways. J Biol Chem. 1997;272:1395–1401. doi: 10.1074/jbc.272.2.1395. 10.1074/jbc.272.2.1395. [DOI] [PubMed] [Google Scholar]
- Katoch SS, Moreland RS. Agonist and membrane depolarization induced activation of MAP kinase in the swine carotid artery. Am J Physiol. 1995;269:H222–H229. doi: 10.1152/ajpheart.1995.269.1.H222. [DOI] [PubMed] [Google Scholar]
- Kelemen BR, Hsiao K, Goueli SA. Selective in vivo inhibition of mitogen-activated protein kinase activation using cell-permeable peptides. J Biol Chem. 2002;10:8741–8748. doi: 10.1074/jbc.M108459200. 10.1074/jbc.M108459200. [DOI] [PubMed] [Google Scholar]
- Kevil CG, Oshima T, Alexander B, Coe LL, Alexander JS. H2O2-mediated permeability: role of MAPK and occludin. Am J Physiol. 2000;279:C21–C30. doi: 10.1152/ajpcell.2000.279.1.C21. [DOI] [PubMed] [Google Scholar]
- Koch A, Nasuhara Y, Barnes PJ, Lindsay MA, Giembycz MA. Extracellular signal-regulated kinase 1/2 control Ca2+-independent force development in histamine-stimulated bovine tracheal smooth muscle. Br J Pharmacol. 2000;131:981–989. doi: 10.1038/sj.bjp.0703663. 10.1038/sj.bjp.0703663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- Lal BK, Varma S, Pappas PJ, Hobson RW, Duran WN. VEGF increases permeability of the endothelial cell monolayer by activation of PKB/akt, endothelial nitric oxide synthase, and MAP kinase pathways. Microvasc Res. 2001;62:252–261. doi: 10.1006/mvre.2001.2338. 10.1006/mvre.2001.2338. [DOI] [PubMed] [Google Scholar]
- Lampugnani MG, Giorgi M, Baboli M, Dejana E, Marchisio PC. Endothelial cell motility, integrin receptor clustering, and microfilament organization are inhibited by agents that increase intracellular cAMP. Lab Invest. 1990;63:521–531. [PubMed] [Google Scholar]
- Liu F, Verin AD, Borbiev T, Garcia JG. Role of cAMP-dependent protein kinase A activity in endothelial cell cytoskeleton rearrangement. Am J Physiol. 2001;280:L1309–L1317. doi: 10.1152/ajplung.2001.280.6.L1309. [DOI] [PubMed] [Google Scholar]
- Lum H, Jaffe HA, Schulz IT, Masood A, RayChaudhury A, Green RD. Expression of PKA inhibitor (PKI) gene abolishes cAMP-mediated protection to endothelial barrier dysfunction. Am J Physiol. 1999;277:C580–C588. doi: 10.1152/ajpcell.1999.277.3.C580. [DOI] [PubMed] [Google Scholar]
- Lum H, Malik AB. Regulation of vascular endothelial barrier function. Am J Physiol. 1994;267:L223–L241. doi: 10.1152/ajplung.1994.267.3.L223. [DOI] [PubMed] [Google Scholar]
- Marks RM, Roche WR, Czerniecki M, Penny R, Nelson DS. Mast cell granules cause proliferation of human microvascular endothelial cells. Lab Invest. 1986;55:289–294. [PubMed] [Google Scholar]
- Morbidelli L, Chang CH, Douglas JG, Granger HJ, Ledda F, Ziche M. Nitric oxide mediates mitogenic effect of VEGF on coronary venular endothelium. Am J Physiol. 1996;270:H411–H415. doi: 10.1152/ajpheart.1996.270.1.H411. [DOI] [PubMed] [Google Scholar]
- Moy AB, Winter M, Kamath A, Blackwell K, Reyes G, Giaever I, Keese C, Shasby DM. Histamine alters endothelial barrier function at cell-cell and cell-matrix sites. Am J Physiol Lung Cell Mol Physiol. 2000;278:L888–L898. doi: 10.1152/ajplung.2000.278.5.L888. [DOI] [PubMed] [Google Scholar]
- Murohara T, Horowitz JR, Silver M, Tsurumi Y, Chen D, Sullivan A, Isner JM. Vascular endothelial growth factor/vascular permeability factor enhances vascular permeability via nitric oxide and prostacyclin. Circulation. 1998;97:99–107. doi: 10.1161/01.cir.97.1.99. [DOI] [PubMed] [Google Scholar]
- Parenti A, Morbidelli L, Cui X, Douglas JG, Hoods JD, Granger HJ, Ledda F, Ziche M. Nitric oxide is an upstream signal of vascular endothelial growth factor-induced extracellular signal-regulated kinase1/2 activation in postcapillary endothelium. J Biol Chem. 1998;273:4220–4226. doi: 10.1074/jbc.273.7.4220. 10.1074/jbc.273.7.4220. [DOI] [PubMed] [Google Scholar]
- Parenti A, Morbidelli L, Ledda F, Granger HJ, Ziche M. The bradykinin/B1 receptor promotes angiogenesis by up-regulation of endogenous FGF-2 in endothelium via the nitric oxide synthase pathway. FASEB J. 2001;15:1487–1489. [PubMed] [Google Scholar]
- Pearson G, Robinson F, Gibson TB, Xu B, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological function. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. 10.1210/er.22.2.153. [DOI] [PubMed] [Google Scholar]
- Rissanen TT, Markkanen JE, Arve K, Rutanen J, Kettunen MI, Vajanto I, Jauhiainen S, Cashion L, Gruchala M, Narvanen O, Taipale P, Kauppinen RA, Rubanyi GM, Yla-Herttuala S. Fibroblast growth factor 4 induces vascular permeability, angiogenesis and arteriogenesis in a rabbit hindlimb ischemia model. FASEB J. 2003;17:100–102. doi: 10.1096/fj.02-0377fje. [DOI] [PubMed] [Google Scholar]
- Robinson AJ, Dickenson JM. Activation of the p38 and p42/p44 mitogen-activated protein kinase families by the histamine H1 receptor in DDT(1)MF-2 cells. Br J Pharmacol. 2001;133:1378–1386. doi: 10.1038/sj.bjp.0704200. 10.1038/sj.bjp.0704200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siflinger-Birnboim A, Johnson A. Protein kinase C modulates pulmonary endothelial permeability: a paradigm for acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2003;284:L435–L451. doi: 10.1152/ajplung.00106.2002. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Ueno H, Shibuya M. VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene. 1999;18:2221–2230. doi: 10.1038/sj.onc.1202527. 10.1038/sj.onc.1202527. [DOI] [PubMed] [Google Scholar]
- Tinsley JH, Hawker J, Yuan Y. Efficient protein transfection of cultured coronary venular endothelial cells. Am J Physiol. 1998;275:H1873–H1878. doi: 10.1152/ajpheart.1998.275.5.H1873. [DOI] [PubMed] [Google Scholar]
- Tinsley JH, Zawieja DC, Ustinova EE, Wu MH, Xu W, Yuan SY. Protein transfection of intact microvessels specifically modulates vasoreactivity and permeability. J Vas Res. 2001;38:444–452. doi: 10.1159/000051077. 10.1159/000051077. [DOI] [PubMed] [Google Scholar]
- Traub O, Monia BP, Dean NM, Berk BC. PKC-epsilon is required for mechano-sensitive activation of ERK1/2 in endothelial cells. J Biol Chem. 1997;272:31251–31257. doi: 10.1074/jbc.272.50.31251. 10.1074/jbc.272.50.31251. [DOI] [PubMed] [Google Scholar]
- van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ Res. 2000;87:335–340. doi: 10.1161/01.res.87.4.335. [DOI] [PubMed] [Google Scholar]
- Varma S, Breslin JW, Lal BK, Pappas PJ, Hobson RW, Duran WN. p42/44MAPK regulates baseline permeability and cGMP-induced hyperpermeability in endothelial cells. Microvasc Res. 2002;63:172–178. doi: 10.1006/mvre.2001.2381. 10.1006/mvre.2001.2381. [DOI] [PubMed] [Google Scholar]
- Verin AD, Liu F, Bogatcheva N, Borbiev T, Hershenson MB, Wang P, Garcia JG. Role of ras-dependent ERK activation in phorbol ester-induced endothelial cell barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2000;279:L360–L370. doi: 10.1152/ajplung.2000.279.2.L360. [DOI] [PubMed] [Google Scholar]
- Wheeler-Jones CP, Pearson JD. Thrombin and histamine phosphorylated the 42kDa mitogen-activated protein kinase in HUVEC. Biochem Soc Trans. 1995;23:203S. doi: 10.1042/bst023203s. [DOI] [PubMed] [Google Scholar]
- Wu MH, Guo M, Yuan SY, Granger HJ. Focal adhesion kinase mediates porcine venular hyperpermeability elicited by vascular endothelial growth factor. J Physiol. 2003;552:691–699. doi: 10.1113/jphysiol.2003.048405. 10.1113/jphysiol.2003.048405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HM, Huang Q, Yuan Y, Granger HJ. VEGF induces NO-dependent hyperpermeability in coronary venules. Am J Physiol. 1996;271:H2735–H2739. doi: 10.1152/ajpheart.1996.271.6.H2735. [DOI] [PubMed] [Google Scholar]
- Wu HM, Yuan Y, Zawieja DC, Tinsley J, Granger HJ. Role of phospholipase C, protein kinase C, and calcium in VEGF-induced venular hyperpermeability. Am J Physiol. 1999;276:H535–H542. doi: 10.1152/ajpheart.1999.276.2.H535. [DOI] [PubMed] [Google Scholar]
- Yuan SY. Signal transduction pathways in enhanced microvascular permeability. Microcirculation. 2000;7:395–403. 10.1038/sj.mn.7300123. [PubMed] [Google Scholar]
- Yuan Y, Chilian WM, Granger HJ, Zawieja DC. Permeability to albumin in isolated coronary venules. Am J Physiol. 1993a;265:H543–H552. doi: 10.1152/ajpheart.1993.265.2.H543. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Granger HJ, Zawieja DC, Chilian WM. Flow modulates venular permeability by a nitric oxide-related mechanism. Am J Physiol. 1992;263:H641–H646. doi: 10.1152/ajpheart.1992.263.2.H641. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Granger HJ, Zawieja DC, DeFily DV, Chilian WM. Histamine increases venular permeability via a phospholipase C-NO synthase-guanylate cyclase cascade. Am J Physiol. 1993b;264:H1734–H1739. doi: 10.1152/ajpheart.1993.264.5.H1734. [DOI] [PubMed] [Google Scholar]
- Zachary I, Gliki G. Signal transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res. 2001;49:568–581. doi: 10.1016/s0008-6363(00)00268-6. 10.1016/S0008-6363(00)00268-6. [DOI] [PubMed] [Google Scholar]
- Ziche M, Morbidelli L, Masini E, Amerini S, Granger HJ, Maggi CA, Geppetti P, Ledda F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. 1994;94:2036–2044. doi: 10.1172/JCI117557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziche M, Morbidelli L, Masini E, Granger HJ, Geppetti P, Ledda F. Nitric oxide promotes DNA synthesis and cyclic GMP formation in endothelial cells from postcapillary venules. Biochem Biophys Res Commun. 1993a;192:1198–1203. doi: 10.1006/bbrc.1993.1543. 10.1006/bbrc.1993.1543. [DOI] [PubMed] [Google Scholar]
- Ziche M, Morbidelli L, Parenti A, Amerini S, Granger HJ, Maggi CA. Substance P increases cyclic GMP levels on coronary postcapillary venular endothelial cells. Life Sci. 1993b;53:PL229–PL234. doi: 10.1016/0024-3205(93)90556-i. 10.1016/0024-3205(93)90556-I. [DOI] [PubMed] [Google Scholar]