Abstract
The potential roles of insulin and dietary amino acids in the regulation of skeletal muscle protein synthesis were examined in adult and old rats. Animals were fed over 1 h with either a 25% or a 0% amino acid/protein meal. In each nutritional condition, postprandial insulin secretion was either maintained or blocked with diazoxide injections. Protein synthesis in gastrocnemius and soleus muscles was assessed in vivo using the flooding dose method. Insulin suppression decreased protein synthesis in both muscles irrespective of the nutritional condition and age of the rats. Moreover, reduced insulinaemia was associated with 4E-BP1 dephosphorylation, enhanced assembly of the 4E-BP1−eIF4E inactive complex and hypophosphorylation of eIF4E, p70S6k and protein kinase B, key intermediates in the regulation of translation initiation and protein synthesis. Old rats did not differ from adult rats. The lack of amino acids in the meal of insulin-suppressed rats did not result in any additional decrease in protein synthesis. In the presence of insulin secretion, dietary amino acid suppression significantly decreased gastrocnemius protein synthesis in adult but not in old rats. Amino acid suppression was associated with reduced phosphorylation of 4E-BP1 and p70S6k in adults. Along with protein synthesis, only the inhibition of p70S6k phosphorylation was abolished in old rats. We concluded that insulin is required for the regulation of muscle protein synthesis irrespective of age and that the effect of dietary amino acids is blunted in old rats.
Many studies have shown that amino acids and insulin play a major role in promoting postprandial protein anabolism (Volpi et al. 1996; Giordano et al. 2000; Balage et al. 2001). However, their respective and relative contributions remain unclear. Although in vitro studies show that insulin and amino acids can independently regulate skeletal muscle protein synthesis and degradation (Fulks et al. 1975; Li & Jefferson, 1978; Stirewalt & Low, 1983), the assessment of their respective roles in vivo is complicated by the concomitant changes in their concentration that usually accompany experimental changes in plasma insulin and amino acid concentrations. Removal of amino acids from the diet blunted the stimulation of protein synthesis in rodents (Yoshizawa et al. 1995) or humans (Volpi et al. 1996), suggesting that amino acids are essential in postprandial stimulation of protein synthesis. Provision of anti-insulin antibodies before re-feeding prevents (Preedy & Garlick, 1986; Yoshizawa et al. 1995) or attenuates (Millward et al. 1983; Svanberg et al. 1996) the stimulation of skeletal muscle protein synthesis in response to food intake in rodents, suggesting that insulin also plays a role in the stimulation of protein synthesis after a meal. Others have suggested that insulin would have a permissive effect on synthesis (Kimball et al. 2002a; O'Connor et al. 2003). In fact we have recently demonstrated that insulin and amino acids are both required to stimulate skeletal muscle protein synthesis in response to feeding in growing rats (Sinaud et al. 1999; Balage et al. 2001).
The anabolic actions of insulin and amino acids on muscle protein metabolism have been shown to occur through a stimulation of translation initiation which, in part, involves the phosphatidylinositol 3-kinase (PI3-kinase)/mammalian target of rapamycin (mTOR) pathway (Kelly & Jefferson, 1985; Yoshizawa et al. 1995). Within this pathway, protein kinase B (PKB) (Kimball et al. 1996), eukaryotic initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1), and ribosomal protein S6 kinase (p70S6k) are key intermediates involved in the regulation of translation initiation and protein synthesis (Ueki et al. 1998). Activation of PKB, a threonine/serine kinase downstream of PI3-kinase, promotes the phosphorylation and activation of mTOR (Kimball et al. 1996; Coffer et al. 1998), which, in turn, phosphorylates 4E-BP1 and p70S6k. Dephosphorylated 4E-BP1 represses mRNA translation initiation by binding to eIF4E. Phosphorylation of 4E-BP1 frees eIF4E, which can then associate with eIF4G to form the preinitiation complex and initiate protein synthesis. Phosphorylation of p70S6k increases the phosphorylation of ribosomal protein S6 and facilitates the synthesis of some ribosomal proteins, initiation factors and elongation factors that play important roles in protein synthesis (Ueki et al. 1998).
The study was carried out in order to determine whether or not the regulation of muscle protein synthesis by insulin and amino acids is altered in older animals. A previous study (Kimball et al. 2002b) has reported a developmental decline in signal transduction pathways regulating protein synthesis in muscle. A significant increase in muscle protein synthesis under physiological hyperinsulinaemia has been reported in young growing animals after a short period of fasting, but the effect was blunted in adult and old animals (Baillie & Garlick, 1992; McNulty et al. 1993; Mosoni et al. 1993). A significant effect of amino acids is obtained in older rodents, but only by using very high- or long-term hyperaminoacidaemia (McNulty et al. 1993; Mosoni et al. 1993; Tauveron et al. 1994). In elderly humans, amino acids also stimulate protein synthesis (Volpi et al. 1999). However, the muscle of aged rats is less sensitive to the effect of amino acids but is still able to respond if the increase in aminoacidaemia is high enough (Dardevet et al. 2000, 2002).
The aim of the present experiment, therefore, was to assess the role(s) played by insulin and/or amino acids on muscle protein synthesis in adult and old rats. This study was conducted similarly to studies in young rats (Balage et al. 2001). A postprandial state was obtained after feeding either a 25% or a 0% amino acid/protein meal. In each condition, plasma insulin was either allowed to increase or was blocked with diazoxide injections.
Methods
Animals and experimental design
The present study was performed in accordance with current legislation on animal experimentation in France. Thirty-two adult male Wistar rats (8 months old) and 26 old male Wistar rats (20 months old) were housed individually under controlled environmental conditions (temperature 22°C; 12 h dark period starting at 10.00 h) and were fed between 10.00 and 16.30 h ad libitum with a semi-liquid 18.5% casein diet (Table 1); water was provided freely. Rats were acclimated to their surroundings for 1 month, which is the time required for adaptation to the individual housing and to the diet. At the beginning of acclimation, body weights were significantly higher in old than in adult rats (700 ± 20 versus 502 ± 10 g, respectively; P < 0.05). During the adaptation period, body weights of adult animals significantly increased whereas body weights of old rats significantly decreased (+16.4 ± 3.8 versus−40.4 ± 6.6 g, respectively; P < 0.05). However, at the end of the adaptation period mean body weights of old rats were still significantly higher than the body weight of adults (659 ± 17 versus 519 ± 9 g, respectively; P < 0.05). During the adaptation period the mean daily food consumption was similar between the groups (20.4 ± 0.4 and 20.9 ± 0.4 g of dry matter, in adult and old rats, respectively). On the day of the experiment, after a 17.5 h fasting period, adult and old rats were fed either a 25% amino acid (AA)/protein meal (n = 16 for adult and n = 13 for old rats) or a 0% AA/protein meal (n = 16 for adult and n = 13 for old rats) over 1 h (i.e. from 10.30 to 11.30 h). Food intake was not significantly different among the old rats over this 1 h feeding period (10.24 ± 0.97 and 9.84 ± 0.78 g of dry matter for the 25% AA/protein meal and the 0% AA/protein meal, respectively). Adults rats exhibited the same food intake as old rats except after being fed the 25% AA/protein meal when it was significantly decreased (7.20 ± 0.67 and 9.52 ± 0.68 g of dry matter, respectively, for corresponding values).
Table 1.
Composition of the diet and experimental meals
| Casein dietb | 25% AA/protein meal | 0% AA/protein meal | |
|---|---|---|---|
| Casein | 18.5 | 5 | — |
| l-Amino acid mixturea | — | 20 | — |
| Peanut oil | 2.4 | 2.4 | 4 |
| Colza oil | 3 | 3 | 5 |
| Sunflower oil | 0.6 | 0.6 | 1 |
| Saccharose | — | 20 | 20 |
| Wheat starch | 64.5 | 44 | 61 |
| Agar-agar | 3 | 5 | 9 |
| Supplements | |||
| Multivitamin mixture | 1 | — | — |
| Multimineral-oligoelement mixture | 7 | — | — |
| Energy (MJ (100 g dry matter)−1) | 1.7 | 1.8 | 1.8 |
Composition of the diets is expressed in terms of dry matter as a percentage of the total mixture.
Composition of the l-amino acid mixture was (in g (100 g mixture)−1): aspartic acid 6.84, asparagine 4.58, threonine 5.29, serine 4.90, glutamic acid 10.97, glutamine 5.87, proline 4.13, glycine 2.00, alanine 4.65, valine 4.84, cystine 2.90, methionine 2.00, isoleucine 5.03, leucine 12.32, tyrosine 3.48, phenylalanine 3.61, lysine 9.74, histidine 1.87, arginine 2.84, and tryptophan 2.13.
Casein diet was from Unité de Préparation d'Aliments Expérimentaux, INRA Jouy-en-Josas, France. They were prepared according to the Nutrient Requirements of Laboratory Animals (4th Edition, 1995, National Academy, Washington DC). These two experimental diets were isoenergetic.
In the 25% AA/protein meal, amino acids originated from both casein (5%) and a specific amino acid mixture (20%) (Table 1). A combination of protein and free amino acids was used to obtain a rapid postprandial increase in plasma amino acids. In the 0% AA/protein meal, wheat starch was used as a substitute for protein and amino acids. Peanut, colza and sunflower oils were also increased in the 0% diet; agar-agar (indigestible) was included in the 0% meal to obtain the same consistency between the two types of meals. After feeding, one-half of each fed group received two intraperitoneal injections of diazoxide in order to suppress postprandial insulin secretion, one at the end of the meal (15 mg (100 g body weight)−1 in 0.05 mol l−1 NaOH) (Hyperstat diazoxide, Schering-plough; France) and another 90 min later (6 mg (100 g body weight)−1). Animals were transiently anaesthetized by inhalation with Forène (isoflurane, Abbott Laboratories Ltd, UK) during the injections. At the same time, the other half of the animals received vehicle under the same conditions.
The four groups used at each age were as follows. (1) Control group (C): rats fed with the 25% AA/protein meal, injected with vehicle. (2) Insulin-deprived group (INS−): rats fed with the 25% AA/protein meal and injected with diazoxide. (3) AA/protein-deprived group (AA–): rats fed with the 0% AA/protein meal and injected with vehicle. (4) Insulin- and AA/protein-deprived group (INS–/AA–): rats fed with the 0% AA/protein meal and injected with diazoxide.
Measurements of in vivo muscle protein synthesis
Protein synthesis rates were assessed in gastrocnemius and soleus muscles 120 min after the end of feeding using the flooding dose method (Davis et al. 1999). Each rat was injected intravenously with valine (150 μmol (100 g body weight)−1) to flood the precursor pools with l-[13C]valine (99% enriched 13C, Euriso-Top Gif-sur-Yvette, France). 13C-Valine was diluted as 450 μmol in 1 ml of physiological serum. The incorporation time averaged 39.9 ± 0.39 min. Note that the valine flood (around 0.10 g rat−1) was almost equal to valine coming from the meal (around 0.13 g rat−1).
Rats were anaesthetized with pentobarbital sodium (18 mg (100 g body weight)−1; Sanofi Santé Animal, France) just before killing by exsanguination; blood was rapidly collected and centrifuged at 3000 g for 4 min. Plasma was collected and kept frozen at −20°C until assayed for insulin, glucose and amino acids. Gastrocnemius and soleus muscles were excised, weighed, frozen in liquid nitrogen and stored at −80°C until protein synthesis was measured and amino acids assayed. Part of the gastrocnemius was immediately homogenized for Western blot analysis (see below).
Free and bound valine enrichments were determined as follows. Muscle free amino acids (in gastrocnemius and soleus) were extracted using 0.6 mol l−1 trichloroacetic acid (TCA) as previously described (Savary et al. 1998). Free valine enrichment determination was then performed by gas chromatography/mass spectrometry, with a HP 5972 organic mass spectrometer quadrupole coupled to a HP 5890 GC (Hewlett-Packard, Les Ulis, France). Valine was measured as the tertiary butyl-dimethylsilyl derivative under electron impact ionization. The ionic mass to charge ratios (m/z) of 288 and 289 were monitored by selective ion recording to determine the 13C-valine enrichment.
Muscle protein precipitates were hydrolysed in 6 mol l−1 HCl during 48 h. Aliquots were used to measure tissue protein and RNA content as previously described (Mosoni et al. 1993). Another aliquot was used to measure the enrichment of protein-bound valine using the N-acetyl-propyl-amino acid derivative and gas chromatography–combustion–isotope ratio mass spectrometry (Micromass Isochrom II, Fisons Instruments, Middlewich, UK).
Calculations
In vivo protein fractional synthesis rates (FSR, as percentage per day) were calculated according to the method described by Garlick et al. 1983):
where t is the incorporation time, expressed in days, EP and EA are the enrichment of protein-bound and tissue-free valine at the time of killing, respectively, and EN is the natural enrichment of protein-bound valine which was measured in each muscle studied. EP, EN and EA were expressed in APE (atom% excess). Absolute synthesis rates (ASR, in mg day−1) were calculated by multiplying FSR by the total tissue protein content. Protein synthetic capacity (in mg RNA (g protein)−1) was estimated as the ratio of RNA to protein because most of the RNA in tissues is ribosomal. An estimate of translational efficiency was obtained from the amount of protein synthesized per milligram of RNA per day (mg protein (mg RNA)−1 day−1).
Plasma insulin, glucose and amino acid measurements
Plasma insulin concentrations were analysed using a commercial radioimmunoassay kit (Bio-Rad, France). Plasma glucose was determined enzymatically using a glucose oxidase kit (Boehringer Mannheim, D-68298 Mannheim, Germany). Plasma free amino acids were determined by ion-exchange chromatography after protein precipitation: 500 μl of plasma were added to 125 μl of a sulfosalicylic acid solution (1 mol l−1 in ethanol with 0.5 mol l−1 thiodiglycol) previously evaporated to dryness. Samples were incubated on ice for 1 h and then centrifuged at 3500 g at 4°C for 1 h. An aliquot (250 μl) of the supernatant was combined with 125 μl of 0.1 mol l−1 lithium acetate buffer, pH 2.2.
Skeletal muscle free amino acids were extracted from 0.5 g of gastrocnemius which were previously powdered in liquid nitrogen. The powder was homogenized in 8 volumes of ice-cold 10% TCA containing 2.5% thiodiglycol. The acid-soluble fraction containing free amino acids was separated from the protein precipitate by centrifugation. The procedure was repeated once more. Then, the pooled supernatants were passed through columns of cation-exchange resin (AG 50 W-X8, 100–200 mesh, Bio-Rad, Richmond, CA, USA). Purified amino acids eluted from the column by 4 mol l−1 NH4OH, were dried and reconstituted in 1 ml of 0.1 mol l−1 lithium acetate buffer, pH 2.2. Amino acid concentrations were determined on extracts from both plasma and muscle by means of an amino acid analyser (HPLC system; Bio-Tek Instruments).
Western blot analysis
Muscle samples were prepared for western blot analysis as described previously (Sinaud et al. 1999; Anthony et al. 2000b). One portion of gastrocnemius muscle was homogenized in 7 volumes of buffer consisting of (in mmol l−1): 20 Hepes (pH 7.4), 100 potassium chloride, 0.2 EDTA, 2 EGTA, 1 dithiothreitol, 50 sodium fluoride (NaF), 50 glycerophosphate, 0.1 phenylmethylsulphonyl fluoride, 1 benzamidine, and 0.5 sodium vanadate (Na3VO4), and 0.001 microcystin LR, using a Polytron homogenizer. The homogenate was centrifuged at 10 000 g at 4°C for 10 min and the supernatant stored at −80°C until analysis.
Measurements of eukaryotic initiation factors were performed as previously described (Anthony et al. 2000a). The phosphorylated and unphosphorylated forms of eIF4E were separated by isoelectric focusing on a slab gel and quantified by protein immunoblot analysis. The proportion of eIF4E present in the phosphorylated state was measured by densitometric scanning of the films and expressed as a percentage of the total eIF4E content (i.e. phosphorylated + unphosphorylated). 4E-BP1 was immunoprecipitated from aliquots of 10 000 g supernatants using an anti-4E-BP1 monoclonal antibody and was subjected to protein immunoblot.
eIF4E and 4E-BP1–eIF4E complexes were immunoprecipitated from aliquots of 10 000 g supernatants by means of an anti-eIF4E monoclonal antibody. Samples were subjected to immunoblot analysis using a polyclonal antibody to 4E-BP1 to determine the association of 4E-BP1 with eIF4E.
The phosphorylation state of the 70 kDa protein p70S6k was analysed on 10 000 g supernatants by protein immunoblot analysis using a rabbit polyclonal p70S6k antibody (Santa Cruz Biotechnology, Santa Cruz A) (Gautsch et al. 1998; Anthony et al. 2000b).
Phosphorylation of p70S6k on Thr389 was determined in 10 000 g supernatants by protein immunoblot analysis (Kimball et al. 1997). Membranes were incubated with a rabbit polyclonal antibody, which specifically recognizes phosphorylation of p70S6k on Thr389 (New England Biolabs).
Phosphorylation of PKB on Ser473 (Santa Cruz Biotechnology) was determined by protein immunoblot analysis with antibodies that specifically recognize phosphorylation on Ser473.
Statistical analysis
Values are given as means ±s.e.m. The effects of age, type of meal and diazoxide and their interactions were analysed using a three-way ANOVA. In Tables 2 and 3, statistical evaluation of the different data was performed by three-way ANOVA to analyse the effects of age, type of meal (25 or 0% AA/protein), insulin status (diazoxide treatment effect) and their interaction (i.e. age × insulin status). When a significant overall effect was detected, differences among individual means were assessed with Student's t tests. Moreover, a significant interaction between age, meal or insulin status could be revealed. Differences among the means were considered significant when P≤ 0.05. StatView program (version 4.5; Abacus Concepts, Berkley, CA, USA) was used for the statistical analyses.
Table 2.
Plasma and skeletal muscle essential free amino acid concentrations
| Adult | Old | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25% AA/protein | 0% AA/protein | 25% AA/protein | 0% AA/protein | Analysis of variance, significants effects | |||||||||||
| C | INS– | AA– | INS–/AA– | C | INS– | AA– | INS–/AA– | RSE | A | M | I | AM | AI | MI | |
| Plasma | |||||||||||||||
| Arginine | 70 | 78 | 71 | 59 | 63 | 62 | 67 | 70 | 27 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Histidine | 72 | 76 | 64 | 73 | 75 | 71 | 65 | 73 | 11 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Isoleucine | 135 | 146 | 53a | 99a, b | 117 | 127 | 59a | 91a, b | 25 | n.s. | < 0.0001 | < 0.001 | n.s. | n.s. | 0.03 |
| Leucine | 305 | 284 | 81a | 166a | 256 | 249 | 84a | 149a | 59 | n.s. | < 0.0001 | 0.05 | n.s. | n.s. | 0.005 |
| Lysine | 585 | 629 | 364a | 422a | 493 | 563 | 381a | 366a | 113 | n.s. | < 0.0001 | n.s. | n.s. | n.s. | n.s. |
| Methionine | 26 | 27 | 19 | 21 | 22 | 24 | 19 | 16a | 7 | n.s. | < 0.01 | n.s. | n.s. | n.s. | n.s. |
| Phenylalanine | 62 | 83b | 49 | 71b | 66 | 81b | 57 | 75b | 14 | n.s. | < 0.01 | < 0.0001 | n.s. | n.s. | n.s. |
| Threonine | 363 | 313 | 223a | 266 | 362 | 315 | 238a | 239a | 61 | n.s. | < 0.0001 | n.s. | n.s. | n.s. | < 0.03 |
| Tryptophan | 72 | 80 | 85 | 60 | 62 | 84 | 60 | 50 | 28 | n.s. | n.s. | n.s. | n.s. | n.s. | < 0.03 |
| Tyrosine | 62 | 98b | 39a | 77a, b | 82 | 116b | 48a | 73a | 22 | n.s. | < 0.0001 | < 0.0001 | n.s. | n.s. | n.s. |
| Valine | 1363 | 1412 | 1364 | 1364 | 1498 | 1667 | 1296 | 1692 | 385 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Total | 3116 | 3226 | 2412a | 2680a | 3097 | 3347 | 2374a | 2892a | 453 | n.s. | < 0.0001 | n.s. | n.s. | n.s. | n.s. |
| Gastrocnemius | |||||||||||||||
| Arginine | 221 | 247 | 215 | 232 | 292 | 269 | 470a, c | 336c | 78 | < 0.0001 | < 0.01 | n.s. | < 0.01 | < 0.05 | n.s. |
| Histidine | 196 | 216 | 201 | 215 | 201 | 208 | 229a | 232 | 25 | n.s. | < 0.03 | n.s. | n.s. | n.s. | n.s. |
| Isoleucine | 89 | 107b | 43a | 82a, b | 103 | 97 | 54a | 82b | 18 | n.s. | < 0.0001 | < 0.0001 | n.s. | n.s. | < 0.01 |
| Leucine | 208 | 207 | 68a | 135a | 236 | 188 | 79a | 131 | 46 | n.s. | < 0.0001 | n.s. | n.s. | n.s. | < 0.001 |
| Lysine | 1305 | 1316 | 874 | 975 | 1505 | 1383 | 1846c | 1265 | 378 | < 0.001 | n.s. | n.s. | < 0.02 | < 0.04 | n.s. |
| Methionine | 48 | 50 | 40 | 43 | 51 | 47 | 44 | 42 | 9 | n.s. | < 0.01 | n.s. | n.s. | n.s. | n.s. |
| Phenylalanine | 48 | 74b | 46 | 62b | 64c | 82b | 50a | 67a, b | 13 | < 0.02 | < 0.002 | < 0.0001 | n.s. | n.s. | n.s. |
| Threonine | 572 | 515 | 489a | 500 | 584 | 573 | 523 | 462a | 74 | n.s. | < 0.001 | n.s. | n.s. | n.s. | n.s. |
| Tryptophan | 377 | 387 | 540a | 497a | 370 | 373 | 555a | 480 | 109 | n.s. | < 0.0001 | n.s. | n.s. | n.s. | n.s. |
| Tyrosine | 78 | 121b | 51a | 99b | 124c | 149c | 61a | 95a, b | 27 | < 0.005 | < 0.0001 | < 0.0001 | < 0.02 | n.s. | n.s. |
| Valine | 1129 | 1236 | 1250 | 1185 | 1349 | 1377 | 1161 | 1475 | 367 | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Total | 4271 | 4477 | 3818 | 4025 | 4877 | 4745 | 5072c | 4666 | 605 | < 0.001 | n.s. | n.s. | n.s. | n.s. | n.s. |
Plasma and gastrocnemius essential free amino acid concentrations (nmol g−1 of plasma or muscle) were measured in adult and old rats in the four groups: C, fed a 25% AA/protein meal in the presence of insulin secretion; INS−, fed a 25% AA/protein meal in the absence of insulin secretion; AA–, fed a 0% AA/protein meal in the presence of insulin secretion; INS–/AA–, fed a 0% AA/protein meal in the absence of insulin secretion. Values are means and residual s.e.m. (RSE) for 7−8 determinations per group. Three-way analyses of variance were performed to discriminate among effect of age (A), type of meal (M), insulin (I), and their interaction (AM, AI, MI) on amino acid concentrations; n.s., not significant.
P < 0.05 AA–versus C and INS–/AA–versus INS−.
P < 0.05 INS−versus C and INS–/AA–versus AA–.
P < 0.05 old versus adult rats in the corresponding groups. Valine: Valine levels are not in the range of the other amino acids due to the flooding dose of 13C-valine.
Table 3.
Muscle protein metabolism
| Adult | Old | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25% AA/protein | 0% AA/protein | 25% AA/protein | 0% AA/protein | Analysis of variance, significant effects | ||||||||
| C | INS– | AA– | INS–/AA– | C | INS– | AA– | INS–/AA– | RSE | A | M | I | |
| Gastrocnemius | ||||||||||||
| Protein concentration (mg (g tissue)−1) | 199 | 205 | 200 | 203 | 194 | 192c | 196 | 194c | 8 | < 0.002 | n.s. | n.s. |
| RNA concentration (mg (g tissue)−1) | 0.99 | 0.97 | 0.97 | 0.99 | 1.05 | 1.05c | 1.04c | 1.04 | 0.06 | < 0.001 | n.s. | n.s. |
| Capacity for protein synthesis (mg RNA (g protein)−1) | 4.97 | 4.74 | 4.83 | 4.89 | 5.44c | 5.46c | 5.32c | 5.39c | 0.34 | < 0.0001 | n.s. | n.s. |
| Ribosomal efficiency (mg protein synthesized (mg RNA)−1 day−1) | 7.99 | 4.16b | 6.91 | 4.33b | 6.69c | 3.78b | 6.86 | 3.80b | 0.92 | < 0.03 | n.s. | < 0.0001 |
| Absolute synthesis rates (mg protein synthesized day−1) | 21.19 | 10.61b | 18.3 | 11.41b | 18.25 | 10.75b | 18.08 | 10.25b | 3.05 | n.s. | n.s. | < 0.0001 |
| Soleus | ||||||||||||
| Protein concentration (mg (g tissue)−1) | 185 | 185 | 182 | 184 | 179 | 178 | 177 | 174c | 8 | < 0.002 | n.s. | n.s. |
| RNA concentration (mg (g tissue)−1) | 1.61 | 1.66 | 1.62 | 1.57 | 1.53 | 1.43c | 1.49c | 1.57 | 0.10 | < 0.001 | n.s. | n.s. |
| Capacity for protein synthesis (mg RNA (g protein)−1) | 8.69 | 8.99 | 8.94 | 8.53 | 8.60 | 8.11 | 8.43 | 8.99 | 0.61 | n.s. | n.s. | n.s. |
| Ribosomal efficiency (mg protein synthesized (mg RNA)−1 day−1) | 7.70 | 5.71b | 7.28 | 5.54b | 7.17 | 5.67b | 7.48 | 5.54b | 0.68 | n.s. | n.s. | < 0.0001 |
| Absolute synthesis rates (mg protein synthesized day−1) | 4.63 | 3.46b | 4.74 | 3.39b | 4.75 | 3.62b | 4.94 | 3.60b | 0.59 | n.s. | n.s. | < 0.0001 |
Characteristics of gastrocnemius and soleus protein metabolism were measured in adult and old rats in the four groups: C, INS−, AA–, INS–/AA–. Protein synthesis was measured using the l-[13C]valine flooding dose. Ten rats were used to measure basal protein-bound valine enrichment. Values are means and residual s.e.m. (RSE), n = 6–8. Three-way analyses of variance were performed to discriminate among effects of age (A), type of meal (M), insulin (I), on gastrocnemius and soleus protein metabolism. n.s., not significant. No interaction between the effect of age, insulin secretion state and amino acid/protein meal was significant.
P < 0.05 AA–versus C and INS–/AA–versus INS−.
P < 0.05 INS−versus C and INS–/AA–versus AA–.
P < 0.05versus adult rats in the corresponding groups.
Results
Plasma insulin, glucose and amino acids
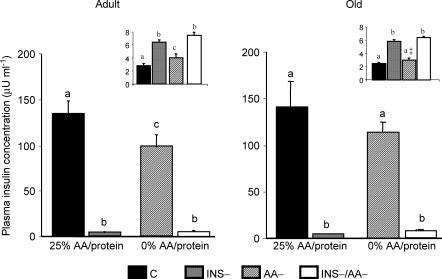
Plasma insulin concentrations (Fig. 1) were similar in adult and old control groups (C groups). In adult rats it was significantly lower after feeding the 0% AA/protein meal (AA– group) than the 25% AA/protein meal (97.5 ± 13.6 versus 133.5 ± 14.9 μU ml−1, respectively, P < 0.05). The same trend was observed in old rats, but the difference was not statistically significant (113.5 ± 11.9 versus 140.7 ± 27.7 μU ml−1, respectively). As previously observed, diazoxide injections dramatically reduced plasma insulin to the same levels in every circumstance.
Figure 1. Plasma insulin and glucose concentrations in adult and old rats.
Plasma insulin and glucose concentrations were measured in adult and old rats in the four groups: C, fed a 25% AA/protein meal in the presence of insulin secretion; INS−, fed a 25% AA/protein meal in the absence of insulin secretion; AA–, fed a 0% AA/protein meal in the presence of insulin secretion; INS–/AA–, fed a 0% AA/protein meal in the absence of insulin secretion. Results are means ±s.e.m. for 7–8 determinations per group. Plasma insulin is indicated by the columns and the plasma insulin level in diazoxide-treated rats is on average 5.2 ± 0.5 μU ml−1. Plasma glucose concentrations (in g l−1) are indicated in the insets. Three-way analyses of variance were performed to discriminate among effect of age, type of meal, insulin, and their interaction on insulin and glucose concentrations. Values not sharing the same letter differ significantly (P < 0.05). ‡P < 0.05versus adult rats in the corresponding groups.
Plasma glucose concentration (Fig. 1, insets) was also similar in adult and old control groups. The effect of treatments on plasma glucose concentrations was consistant with the observed changes in plasma insulin. As expected, plasma glucose concentration was dramatically increased in rats injected with diazoxide (P < 0.05). There was a higher value after feeding the 0% AA/protein meal than the 25% AA/protein meal in adult rats (P < 0.05) but not in old rats.
Plasma and muscle amino acid concentrations are presented in Table 2. Note that plasma and muscle valine concentrations were elevated in each group due to the flooding dose method used to measure muscle protein synthesis.
Age effect
Plasma essential free amino acid concentrations were similar in adult and old rats irrespective of nutritional status or plasma insulin concentration.
In gastrocnemius muscle, free arginine and tyrosine levels were higher in old rats when compared to adult rats after feeding on both 0% and 25% AA/protein meals. This was also the case for lysine and phenylalanine, but only in the presence of insulin secretion. All the other muscle free amino acids did not differ in adult and old rats. The sum of essential free amino acids was higher in old rats than adults especially after feeding the 0% AA/protein meal in the presence of insulin secretion.
Meal composition effect
Compared to the 25% AA/protein meal, feeding the 0% AA/protein meal decreased total essential plasma free amino acids, especially isoleucine, leucine, lysine, threonine and tyrosine, independently of age or plasma insulin concentration (all P < 0.05 except for threonine in adults in the absence of insulin secretion). Methionine only decreased in old rats in the absence of insulin secretion.
Although not always significantly at P < 0.05, free isoleucine, leucine, threonine and tyrosine also decreased in gastrocnemius muscle whereas lysine did not change in 0% compared to 25% AA/protein-fed rats. The 0% AA/protein meal resulted in an additional decrease in free phenylalanine in muscle from old rats independent of insulin concentration (P < 0.05). Conversely, it further increased free muscle arginine, histidine and tryptophan in old rats when insulin was present (P < 0.05). Only an increase in free muscle tryptophan was recorded in adult rats (P < 0.05) independent of insulin concentration.
Diazoxide injection effect
Insulin suppression by diazoxide resulted in an increase in free phenylalanine and tyrosine in both plasma and skeletal muscle, independent of age (all P < 0.05 except with tyrosine for old rats in plasma after feeding the 0% AA/protein meal and in muscle after feeding the 25% AA/protein meal). Diazoxide injection also resulted in a significant increase in free isoleucine in both plasma and muscle after feeding the 0% AA/protein meal whatever the age. An additional increase in free isoleucine was recorded in muscle from adults after feeding the 25% AA/protein meal.
With regard to non-essential free amino acids (not shown), there was a general decrease in their plasma levels after feeding the 0% AA/protein meal that was independent of age (P < 0.05). No other change was observed.
Muscle protein metabolism
See Table 3 and Fig. 2. Gastrocnemius weights were similar in adult and old rats (2.66 ± 0.04 and 2.60 ± 0.03 g, respectively) whereas soleus weights were higher in old than in adult rats (0.433 ± 0.008 and 0.385 ± 0.010 g, respectively). However, relative to body weight, both gastrocnemius and soleus weights significantly decreased with age (0.401 ± 0.009% in old versus 0.503 ± 0.013% in adult rats for gastrocnemius and 0.067 ± 0.002% in old versus 0.074 ± 0.001% in adult rats for soleus, all P < 0.05).
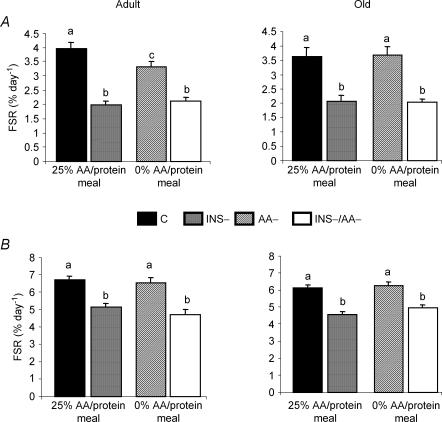
Figure 2. Fractional protein synthesis rates (FSRs).
FSRs were measured in gastrocnemius (A) and soleus (B) muscles from adult and old rats in the four groups: C, INS−, AA–, INS–/AA–. Values are means ±s.e.m. and are expressed as percentage protein synthetized per day; n = 6–9 per group. Values not sharing the same letter differ significantly (P < 0.05) by ANOVA.
Protein concentration was significantly lower in old compared to adult rats (194 ± 1 versus 202 ± 1 mg (g tissue)−1 and 177 ± 1 versus 184 ± 1 mg (g tissue)−1 in gastrocnemius and soleus, respectively, P < 0.05). RNA concentration was higher in gastrocnemius from old than from adult rats (1.05 ± 0.01 versus 0.98 ± 0.01 mg (g tissue)−1, P < 0.05) whereas it was lower in soleus (1.51 ± 0.03 versus 1.62 ± 0.02 mg (g tissue)−1, P < 0.05). In gastrocnemius the capacity for protein synthesis was higher in old than in adult rats (5.40 ± 0.03 versus 4.86 ± 0.05 mg RNA (g protein)−1), whereas in soleus it was constant whatever the age (Table 3). Ribosomal efficiency (mg protein synthesized (mg RNA)−1 day−1) was lower in old than in adult rats in gastrocnemius but not in soleus. This parameter was significantly reduced by diazoxide treatment in every case (4.02 ± 0.14 versus 7.11 ± 0.30 mg protein (mg RNA)−1 day−1 in gastrocnemius in diazoxide-treated and untreated rats, respectively; 5.62 ± 0.04 versus 7.40 ± 0.12 mg protein (mg RNA)−1 day−1 in soleus in diazoxide-treated and untreated rats, respectively).
In both gastrocnemius and soleus, FSR of the C groups did not differ significantly between adult and old rats (Fig. 2). Insulin suppression in the 25% AA/protein-fed rats (INS− groups) dramatically depressed FSR in gastrocnemius whatever the age (−50 and −43%, respectively, in adult and old rats, P < 0.0001). The same significant effect was observed in soleus, although the magnitude of the change was less (−23 and −26% for corresponding values, P < 0.0001). Feeding the 0% AA/protein meal compared to the 25% AA/protein meal (AA– group versus C group) had no effect on FSR in soleus whatever the age of rats. This was also the case in gastrocnemius from old rats. Conversely, we observed a moderate, but significant, decrease of the FSR in gastrocnemius from adult animals fed the 0% AA/protein meal (3.33 ± 0.17 versus 3.97 ± 0.21% day−1 in AA– and C groups, respectively, P < 0.05). In both gastrocnemius and soleus, the FSR exhibited a constant value in the absence of insulin, independent of meal and age of the animals. As expected, both insulin and amino acid suppression (INS–/AA– groups) highly impaired FSR in both gastrocnemius and soleus, whatever the age of the animals, compared to C or AA– groups. However, FSR in INS–/AA– groups was not lower compared with INS– group.
Signal transduction pathway
4E-BP1 phosphorylation
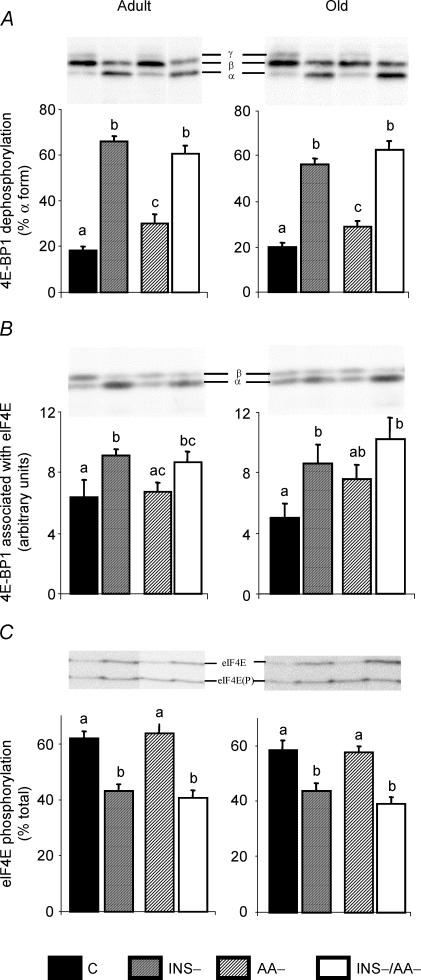
Figure 3A depicts the proportion of 4E-BP1 present in the α form (i.e. the least phosphorylated form of 4E-BP1) in each experimental group. Old rats did not differ from adult rats. Control groups fed the 25% AA/protein meal in the presence of insulin showed the lowest amount of hypophosphorylated 4E-BP1. Feeding the 0% AA/protein meal compared to the 25% AA/protein meal (AA–versus C groups) resulted in a moderate, but significant, increase in the amount of hypophosphorylated 4E-BP1. Insulin suppression caused a large increase in the proportion of 4E-BP1 in the hypophosphorylated form. Note that this change in 4E-BP1 phosphorylation was independent of the amino acid content of the meal.
Figure 3.
4E-BP1 dephosphorylation (A), 4E-BP1 associated with eIF4E (B) and eIF4E phosphorylation (C) in gastrocnemius from adult and old rats in the four groups: C, INS−, AA–, INS–/AA–. Values are expressed as means ±s.e.m.; n = 7–9 in each group. At each age, values not sharing the same letter differ significantly, P < 0.05.
eIF4E–4E-BP1 association
The association of 4E-BP1 with eIF4E (Fig. 3B) increased to the same levels in the four cases of insulin deficiency compared to the C groups, which showed the lowest amount of eIF4E associated with 4E-BP1. Feeding the 0% AA/protein meal in the presence of insulin did not significantly modify the association of 4E-BP1 and eIF4E compared to C group.
eIF4E phosphorylation
The phosphorylation state of eIF4E (Fig. 3C) was also assessed in the present study. We found that insulin deprivation reduced the phosphorylation state of eIF4E (P < 0.05) whereas feeding an amino acid-deficient diet had no effect when compared to C groups.
Phosphorylation of p70S6k
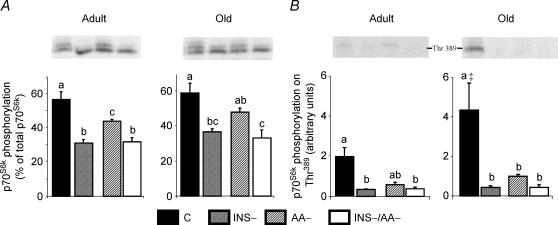
Hyperphosphorylation of p70S6k was greatest in control rats fed the 25% AA/protein meal (Fig. 4A). Insulin deprivation markedly decreased p70S6k phosphorylation to the same values, independent of the amino acid content of the meal. Feeding the 0% AA/protein meal in the presence of insulin also decreased p70S6k phosphorylation when compared to the control 25% AA/protein meal (P < 0.05 only in adults). Only control groups fed the 25% AA/protein meal, especially old rats, exhibited a high amount of p70S6k phosphorylated on Thr389 (Fig. 4B). This phosphorylation was very low in all the other groups.
Figure 4.
p70S6k phosphorylation (A) and p70S6k phosphorylation on Thr389 (B) in gastrocnemius from adult and old rats in the four groups: C, INS−, AA–, INS–/AA–. Values are expressed as means ±s.e.m.; n = 7–9 in each group. At each age, values not sharing the same letter differ significantly, P < 0.05. ‡P < 0.05versus adult in the corresponding group.
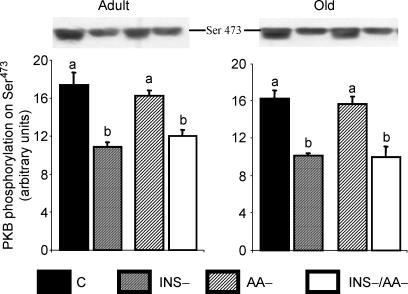
Protein kinase B phosphorylation on Ser473
The total PKB content was similar in all groups (not shown). Insulin deprivation significantly decreased the phosphorylation of PKB on Ser473 (Fig. 5), independent of the amino acid content of the meals.
Figure 5.
PKB phosphorylation on Ser473 in gastrocnemius from adult and old rats in the four groups: C, INS−, AA–, INS–/AA–. Values are expressed as means ±s.e.m.; n = 7–9 in each group. At each age, values not sharing the same letter differ significantly, P < 0.05.
Discussion
In the present study, the contribution of dietary amino acids and insulin in the regulation of skeletal muscle protein synthesis was assessed in adult and old rats using a model of postprandial insulin suppression that we previously developed in young rats (Sinaud et al. 1999; Balage et al. 2001). Our experimental design allowed us to analyse the contribution of hyperinsulinaemia with low amino acids (AA– groups), hyperaminoacidaemia alone (INS– groups), and the combination of insulin and amino acids in the regulation of muscle protein metabolism (C groups). Using a similar experimental design, we previously demonstrated that insulin and amino acids are both required to stimulate skeletal muscle protein synthesis in young growing rats (Sinaud et al. 1999; Balage et al. 2001). In the present study using older rats, we show that insulin is also required for muscle protein synthesis whatever the age, but the response to dietary amino acids is apparently blunted in old rats.
Effect of insulin and amino acids on muscle protein synthesis
Diazoxide injection dramatically decreased the absolute and fractional protein synthesis rates in skeletal muscles of adult and old rats receiving the AA/protein-rich diet (INS–versus C groups). The difference between the C and INS– groups reflected changes in plasma insulin concentrations. They were not related to different food intakes, because animals from INS− and C groups consumed similar quantities of the diet over the 1 h feeding period. Moreover, differences could not be due to a decrease in plasma and muscle free amino acid concentrations. Indeed most amino acids were unchanged under these conditions. Also, concentrations of some of the amino acids were increased rather than decreased (tyrosine and phenylalanine in every case, isoleucine in muscle of adults). These increases correlated with reduced amino acid utilization for protein synthesis. The increases could also be due to enhanced proteolysis. Indeed, we previously demonstrated that diazoxide injection to young rats significantly stimulated protein degradation in epitrochlearis muscle incubated in vitro (Sinaud et al. 1999; Balage et al. 2001). The lack of a general accumulation of free amino acids suggests that amino acid catabolism might also be stimulated. Alternatively, as the animals were in a postprandial state, a decreased amino acid absorption and transport from the gut could not be ruled out.
Both plasma insulin and skeletal muscle protein synthesis rates in old rats increased to the same levels in response to feeding the AA/protein-free meal as with feeding the AA/protein-rich meal (AA–versus C groups). This result suggests that the increase in plasma insulin per se is required for the increase in skeletal muscle protein synthesis in old animals. In contrast, plasma insulin and gastrocnemius protein synthesis in adults were lower after feeding the AA/protein-free meal compared with feeding the AA/protein-rich meal. As food intake was lower in the C group compared to the AA– group, the difference between the two groups might be underestimated because insulin and skeletal muscle protein synthesis both have been shown to increase as a function of food intake (Reeds & Fuller, 1983; Grizard et al. 1995). Thus this altered response of gastrocnemius protein synthesis in adults fed the AA/protein-free meal could be related to this lower plasma insulin. However, based on the dose−response curves for muscle protein synthesis as a function of plasma insulin (Garlick & Grant, 1988; Millward et al. 1988; Davis et al. 1998; Yoshizawa et al. 1998; Fedele et al. 2000; Balage et al. 2001; O'Connor et al. 2003), the insulin levels in adult rats fed the AA/protein-free meal seemed to be high enough to elicit a maximum response of protein synthesis and that any additional increase in plasma insulin would not further increase protein synthesis. Instead, it may be inferred that a portion of postprandial muscle protein synthesis was not driven by insulin in adult rats but by amino acids as previously observed in young rats (Balage et al. 2001). The effect of amino acids on gastrocnemius protein synthesis in the adult was modest (i.e. 19% increase) and well below that of insulin. Our results agree with those of Mosoni et al. (1993), but not those of Garlick & Grant (1988) or McNulty et al. (1993) who recorded no change in protein synthesis with amino acid feeding using the flooding dose method in adult rats. As an amino acid itself might artificially elevate tissue protein synthesis (Smith et al. 1992, 1998; McNulty et al. 1993; Smith & Rennie, 1996,), it may be speculated that in the present experiment, all groups were under amino acid action. The higher gastrocnemius protein synthesis in the C group than in the AA– group could be therefore a reflection of the highest hyperaminoacidaemia in the C group (3116 versus 2412 nmol g−1 in the C and AA– groups, respectively, P < 0.05). Alternatively the potential effect of the flooding dose of amino acid might be poor when using valine because it has been shown that a bolus administration of valine in rats did not stimulate muscle protein synthesis or initiation translation factors (Anthony et al. 2000b). In that case the difference between the C and AA– groups would be due to a true effect of dietary amino acids. Accordingly a number of other studies have shown that feeding amino acids stimulates muscle protein synthesis markedly (Biolo et al. 1997; Tipton et al. 1999; Volpi et al. 1999, 2000; Rasmussen et al. 2000, 2002; Borsheim et al. 2002; Miller et al. 2003; Paddon-Jones et al. 2004). All these studies used a prime constant infusion and tissue enrichments of a tracer amino acid which presumably could not stimulate protein synthesis as a bolus does; the three-pool model of amino acid kinetics (Biolo et al. 1992) shows that stimulation of the net tissue uptake is generally a reflection of an increase in protein synthesis. Bohe et al. (2003) provided evidence that even small doses of amino acids stimulated muscle protein synthesis when using the constant infusion measurement, suggesting that the phenomena was very sensitive.
The effect of amino acids on protein synthesis was not visible in old rats. Differences between ages could not be explained by plasma or muscle free valine levels which were similar in adult and old rats. It is possible that the old rats were more sensitive to valine, thus resulting in the lack of an additional effect of the AA/protein meal above the level obtained with the amino acid-free meal. In contrast assuming that valine would not be able to stimulate muscle protein synthesis, we may hypothesize that dietary amino acids should have a significant effect. Indeed muscle protein synthesis normally responded to hyperaminoacidaemia in old subjects when measured either by the flooding dose method in rats (Mosoni et al. 1993) or the constant infusion in human (Volpi et al. 1998, 1999; Paddon-Jones et al. 2004). To explain this discrepancy we may first consider that the amount of dietary amino acids was not big enough in our experiment. Accordingly, it appears that in order for amino acids to stimulate maximally protein anabolism in older people they need to rapidly flood the system (with either proteins that are rapidly digested and absorbed, Dangin et al. 2002, or protein pulse feeding, Arnal et al. 1999) whereas this is not the case in the younger individual. Similarly, the ability of leucine to stimulate muscle protein synthesis was altered in old rats (Dardevet et al. 2000) and the stimulation can be restored by a leucine-supplemented meal (Dardevet et al. 2002; Rieu et al. 2003). Secondly our finding was observed in the presence of other nutrients but not with amino acids alone as in previous studies (Mosoni et al. 1993; Volpi et al. 1998, 1999; Paddon-Jones, 2004). Volpi hypothesized that an insulin-resistant state in old subjects might alter the action of the endogenous insulin response due to glucose and other nutrients (Volpi et al. 2000). This hypothesis seems unlikely based on the present results which failed to demonstrate any insulin resistance in old rats. Surprisingly, postprandial protein synthesis after feeding the control meal was not higher in adult than old rats in gastrocnemius. This may arise from the lower food intake in the adult rats. In contrast to gatrocnemius the soleus muscle did not respond to amino acids whatever the age of the rats. This can also be explained by the bias following the use of the flooding dose (see above). In fact soleus muscle has been studied extensively as a highly oxidative muscle, and has been shown to have higher protein synthesis rates, but to be less responsive than more glycolytic muscles, e.g. gastrocnemius.
The alteration in the effect of dietary amino acids on gastrocnemius protein synthesis in old rats could be due to a reduced availability of amino acids to the peripheral tissues. Indeed, there is strong evidence that the action of insulin in increasing skeletal muscle blood flow is affected by age and obesity (Steinberg & Baron, 2002). It appears also that the hepatic extraction of amino acids differs between old and adult, potentially resulting in different amounts of amino acids being delivered to the muscle. Although, blood flow and splanchnic extraction of amino acids were not measured in the present experiment, muscle free amino acid levels were not lower (even occasionally higher) in old than adult rats, suggesting that their delivery to the muscle was maintained in old rats. In keeping with this statement it has been demonstrated that the oral administration of mixed amino acids in young and older volunteers results in the same improvement in the delivery of phenylalanine to the leg whatever the age despite a higher phenylalanine first pass splanchnic extraction in the elderly (Volpi et al. 1999).
Our experiment failed to demonstrate any significant effect of amino acids in diazoxide-treated rats whatever the age (INS–versus INS–/AA–) as we had previously failed to show in the younger rat (Balage et al. 2001). This occurred at very low insulin concentrations. Our results did not fit with the data from Garlick & Grant (1988) or O'Connor et al. (2003) which demonstated an improvement of muscle protein synthesis due to amino acids within the low physiological range of plasma insulin. In fact the presence of insulin appears to be permissive rather than regulatory. The amount of insulin required for the effect of amino acid is low, and a concentration of the hormone that approximates to that observed in fasting animals is sufficient for maximal stimulation (Kimball et al. 2002a). Plasma insulin in the diazoxide-treated rats might be not sufficient to allow an amino acid action.
Effect of insulin and amino acids on translation initiation
To examine the potential mechanisms leading to the decreased muscle protein synthesis in insulin- and/or amino acid-deprived rats, we investigated the components of the insulin and/or amino acid signal transduction pathways in the gastrocnemius.
Many studies report that regulation of muscle protein synthesis by insulin and amino acids involves modulation of the initiation phase of mRNA translation (Kelly & Jefferson, 1985; Preedy & Garlick, 1986; Yoshizawa et al. 1995). The phosphorylation status of 4E-BP1 is a primary step involved in the regulation of translation initiation by modulating the assembly of the inactive 4E-BP1−eIF4E complex. Because dephosphorylation of 4E-BP1 is generally proportional to the amount of 4E-BP1 bound to eIF4E, we examined the amount of 4E-BP1 associated with eIF4E (Fig. 3B). Moreover, in vitro, studies have demonstrated that increases in the rate of translation are proportional to the increase in eIF4E phosphorylation (Karim et al. 2001). Our results from gastrocnemius muscle are consistent with these mechanisms. The presence of insulin and amino acids yielded the lowest levels of 4E-BP1 dephosphorylation, 4E-BP1 associated with eIF4E, but the highest eIF4E phosphorylation (C groups versus INS–/AA– groups) (Fig. 3). Moreover these changes correlated with alterations in muscle protein synthesis, especially as far as insulin action was concerned. In contrast a stimulatory effect of amino acids on 4E-BP1 dephosphorylation was recorded at both ages and not only in adults as was the case for protein synthesis. The effect of amino acids was not observed for eIF4E phosphorylation or 4E-BP1 associated with eIF4E whatever the age, suggesting that the difference in the effect of amino acids between adult and old rats does not originate from these sites of regulation.
Another signal transduction pathway parallel to the eIF4E–4E-BP1 phosphorylation pathway involves activation of the p70 S6 kinase (p70S6k). In vitro, phosphorylation of threonine389 (Thr389), in conjunction with phosphorylation of Thr229, results in maximal p70S6k activity. Thus, in the present study we also investigated the phosphorylation of p70S6k on a specific amino acid residue, i.e. Thr389 (Fig. 4B). The p70S6k branch of the signalling pathway has been shown to be important for protein synthesis regulation by both insulin and amino acids (Kimball et al. 1998a, b; Yoshizawa et al. 1999; Anthony et al. 2000b). Because p70S6k phosphorylation (% of total p70S6k) and muscle protein synthesis exhibited parallel differences between the four groups, our results strongly suggest that p70S6k plays a major role in the regulation of protein synthesis by insulin and amino acids in adult and old rats. In other words, the blocking of the effect of amino acids in old rats may arise from the p70S6k. Consistent with this we have shown previously that there is a similar alteration in the sensitivity of muscle protein synthesis and sensitivity of p70S6k activation to leucine (Dardevet et al. 2000). Unfortunately, the phosphorylation of p70S6k was higher in old than adult rats, suggesting a more complicated mechanism than the direct action of leucine. The protein kinase B (PKB) is upstream to p70S6k in the insulin regulation of protein synthesis but does not seem to be involved in the signal transduction of amino acids (Peyrollier et al. 2000; Liu et al. 2002). So, in order to assess whether early insulin signalling events were modified in the different groups, we examined the phosphorylation of PKB on serine473 (Ser473) (Fig. 5), a residue whose phosphorylation is associated with increased activation of the protein. Accordingly the PKB phosphorylation on Ser473 was correlated with the portion of protein synthesis which was driven by insulin alone. Again there was no evidence of insulin resistance in old rats.
In conclusion, the results of the present study analyse the potential respective roles of insulin and dietary amino acids in the regulation of skeletal muscle protein synthesis during ageing in rats. Insulin appears to be similarly required in the regulation of muscle protein synthesis in adult and old rats. The stimulatory effect of insulin was associated with an increase in 4E-BP1 and eIF4E phosphorylation states leading to a dissociation of the inactive 4E-BP1–eIF4E complex. Insulin also activates other signal transduction pathways parallel and upstream to 4E-BP1–eIF4E through phosphorylation of p70S6k and PKB, respectively. Dietary amino acids induced a significant improvement of protein synthesis in adult rats along with an increase in the phosphorylation states of 4E-BP1 and p70S6k. The effect of amino acids is completely abolished in old rats and correlated with an impairment of p70S6k phosphorylation. However, our model of insulin suppression used to investigate the effect of insulin was too drastic to analyse the permissive role of physiological low insulin levels in amino acid action.
Acknowledgments
We thanks S. Rannels for outstanding technical expertise, G. Bayle for amino acids analysis, J. Prugnaud and C. Giraudet for mass spectrometry assays and H. Lafarge for management of the bibliography. We also thank C. Lafarge for care of the animals. This project was supported by a grant from the French Ministry of Research and Technology and Dr Jefferson's Grant DK-15658 (National Institutes of Health).
References
- Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr. 2000a;130:139–145. doi: 10.1093/jn/130.2.139. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin sensitive pathway. J Nutr. 2000b;130:2413–2419. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- Arnal MA, Mosoni L, Boirie Y, Houlier ML, Morin L, Verdier E, et al. Pulse protein feeding improves protein retention in elderly women. Am J Clin Nutr. 1999;69:1202–1208. doi: 10.1093/ajcn/69.6.1202. [DOI] [PubMed] [Google Scholar]
- Baillie AGS, Garlick PJ. Attenuated responses of muscle protein synthesis to fasting and insulin in adult female rats. Am J Physiol. 1992;262:E1–E5. doi: 10.1152/ajpendo.1992.262.1.E1. [DOI] [PubMed] [Google Scholar]
- Balage M, Sinaud S, Prod'homme M, Dardevet D, Vary TC, Kimball SR, et al. Amino acids and insulin are both required to regulate assembly of the eIF4E.eIF4G complex in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2001;281:E565–E574. doi: 10.1152/ajpendo.2001.281.3.E565. [DOI] [PubMed] [Google Scholar]
- Biolo G, Chinkes D, Zhang XJ, Wolfe RR. A new model to determine in vivo the relationship between amino acid transmembrane transport and protein kinetics in muscle. J Parenter Enteral Nutr. 1992;16:305–315. doi: 10.1177/0148607192016004305. [DOI] [PubMed] [Google Scholar]
- Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol. 1997;273:E122–E129. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- Bohe J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose–response study. J Physiol. 2003;552:315–324. doi: 10.1113/jphysiol.2003.050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsheim E, Tipton KD, Wolf SE, Wolfe RR. Essential amino acids and muscle protein recovery from resistance exercise. Am J Physiol Endocrinol Metab. 2002;283:E648–E657. doi: 10.1152/ajpendo.00466.2001. [DOI] [PubMed] [Google Scholar]
- Coffer PJ, Jin J, Woodgett JR. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangin M, Boirie Y, Guillet C, Beaufrère B. Influence of the protein digestion rate on protein turnover in young and elderly subjects. J Nutr. 2002;132:3228S–3233S. doi: 10.1093/jn/131.10.3228S. [DOI] [PubMed] [Google Scholar]
- Dardevet D, Sornet C, Balage M, Grizard J. Stimulation of in vitro rat muscle protein synthesis by leucine decreases with age. J Nutr. 2000;130:2630–2635. doi: 10.1093/jn/130.11.2630. [DOI] [PubMed] [Google Scholar]
- Dardevet D, Sornet C, Bayle G, Prugnaud J, Pouyet C, Grizard J. Postprandial stimulation of muscle protein synthesis in old rats can be restored by a leucine-supplemented meal. J Nutr. 2002;132:95–100. doi: 10.1093/jn/132.1.95. [DOI] [PubMed] [Google Scholar]
- Davis TA, Burrin DG, Fiorotto ML, Reeds PJ, Jahoor F. Roles of insulin and amino acids in the regulation of protein synthesis in the neonate. J Nutr. 1998;128:347S–350S. doi: 10.1093/jn/128.2.347S. [DOI] [PubMed] [Google Scholar]
- Davis TA, Fiorotto ML, Nguyen HV, Burrin DG. Aminoacyl-tRNA and tissue free amino acid pools are equilibrated after a flooding dose of phenylalanine. Am J Physiol. 1999;277:E103–E109. doi: 10.1152/ajpendo.1999.277.1.E103. [DOI] [PubMed] [Google Scholar]
- Fedele MJ, Hernandez JM, Lang CH, Vary TC, Kimball SR, Jefferson LS, et al. Severe diabetes prohibits elevations in muscle protein synthesis after acute resistance exercise in rats. J Appl Physiol. 2000;88:102–108. doi: 10.1152/jappl.2000.88.1.102. [DOI] [PubMed] [Google Scholar]
- Fulks R, Li JB, Goldberg AL. Effects of insulin, glucose and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975;250:290–298. [PubMed] [Google Scholar]
- Garlick PJ, Fern M, Preedy VR. The effect of insulin infusion and food intake on muscle protein synthesis in postabsorptive rats. Biochem J. 1983;210:669–676. doi: 10.1042/bj2100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick PJ, Grant I. Amino acid infusion increases the sensitivity of muscle protein synthesis in vivo to insulin. Effect of branched-chain amino acids. Biochem J. 1988;254:579–584. doi: 10.1042/bj2540579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautsch TA, Anthony JC, Kimball SR, Paul GL, Layman DK, Jefferson LS. Availability of eIF4E regulates skeletal muscle protein synthesis during recovery from exercise. Am J Physiol. 1998;274:C406–C414. doi: 10.1152/ajpcell.1998.274.2.C406. [DOI] [PubMed] [Google Scholar]
- Giordano M, Castellino P, Ohno A, Defronzo RA. Differential effects of amino acid and ketoacid on protein metabolism in humans. Nutrition. 2000;16:15–21. doi: 10.1016/s0899-9007(99)00211-7. 10.1016/S0899-9007(99)00211-7. [DOI] [PubMed] [Google Scholar]
- Grizard J, Dardevet D, Papet I, Mosoni L, Patureau Mirand P, Attaix D, et al. Nutrient regulation of skeletal muscle protein metabolism in animals. The involvement of hormones and substrates. Nutr Res Rev. 1995;8:67–91. doi: 10.1079/NRR19950007. [DOI] [PubMed] [Google Scholar]
- Karim MM, Hughes JMX, Warwicker J, Scheper GC, Proud CG, McCarthy JEG. A quantitative molecular model for modulation of mammalian translation by the eIF4E-binding protein 1. J Biol Chem. 2001;276:20750–20757. doi: 10.1074/jbc.M011068200. 10.1074/jbc.M011068200. [DOI] [PubMed] [Google Scholar]
- Kelly FJ, Jefferson LS. Control of peptide-chain initiation in rat skeletal muscle. Development of methods for preparation of native ribosomal subunits and analysis of the effect of insulin on formation of 40 S initiation complexes. J Biol Chem. 1985;260:6677–6683. [PubMed] [Google Scholar]
- Kimball SR, Farrell PA, Jefferson LS. Exercise effects on muscle insulin signalling and action – Invited review: Role of insulin in translational control of protein synthesis in skeletal muscle by amino acids or exercise. J Appl Physiol. 2002a;93:1168–1180. doi: 10.1152/japplphysiol.00221.2002. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Farell PA, Nguyen HV, Jefferson LS, Davis TA. Developmental decline in components of signal transduction pathways regulating protein synthesis in pig muscle. Am J Physiol Endocrinol Metab. 2002b;282:E585–E592. doi: 10.1152/ajpendo.00269.2001. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Horetsky RL, Jefferson LS. Implication of eIF2B rather than eIF4E in the regulation of global protein synthesis by amino acids in L6 myoblasts. J Biol Chem. 1998a;273:30945–30953. doi: 10.1074/jbc.273.47.30945. 10.1074/jbc.273.47.30945. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Horetsky RL, Jefferson LS. Signal transduction pathways involved in the regulation of protein synthesis by insulin in L6 myoblasts. Am J Physiol. 1998b;274:C221–C228. doi: 10.1152/ajpcell.1998.274.1.C221. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Jefferson LS, Fadden P, Haystead TAJ, Lawrence JC. Insulin and diabetes cause reciprocal changes in the association of eIF-4E and PHAS-I in rat skeletal muscle. Am J Physiol. 1996;270:C705–C709. doi: 10.1152/ajpcell.1996.270.2.C705. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Jurasinski CV, Lawrence JC, Jefferson LS. Insulin stimulates protein synthesis in skeletal muscle by enhancing the association of eIF-4E and eIF-4G. Am J Physiol. 1997;272:C754–C759. doi: 10.1152/ajpcell.1997.272.2.C754. [DOI] [PubMed] [Google Scholar]
- Li JB, Jefferson LS. Influence of amino acid availability on protein turnover in perfused skeletal muscle. Biochim Biophys Acta. 1978;544:351–359. doi: 10.1016/0304-4165(78)90103-4. [DOI] [PubMed] [Google Scholar]
- Liu Z, Jahn LA, Wei L, Long W, Barrett EJ. Amino acids stimulate translation initiation and protein synthesis through an Akt-independent pathway in human skeletal muscle. J Clin Endocrinol Metab. 2002;87:5553–5558. doi: 10.1210/jc.2002-020424. 10.1210/jc.2002-020424. [DOI] [PubMed] [Google Scholar]
- McNulty PH, Young LH, Barrett EJ. Response of rat heart and skeletal muscle protein in vivo to insulin and amino acid infusion. Am J Physiol. 1993;264:E958–E965. doi: 10.1152/ajpendo.1993.264.6.E958. [DOI] [PubMed] [Google Scholar]
- Miller SL, Tipton KD, Chinkes DL, Wolf SE, Wolfe RR. Independent and combined effects of amino acids and glucose after resistance exercise. Med Sci Sport Exer. 2003;35:449–455. doi: 10.1249/01.MSS.0000053910.63105.45. [DOI] [PubMed] [Google Scholar]
- Millward DJ, Brown JG, Van Beuren J. Influence of plasma concentration of T3 on the acute increase in insulin and muscle protein synthesis in the refed fasted rat. J Endocrinol. 1988;118:417–422. doi: 10.1677/joe.0.1180417. [DOI] [PubMed] [Google Scholar]
- Millward DJ, Odedra B, Bates PC. The role of insulin, corticosterone and other factors in the acute recovery of muscle protein synthesis on refeeding food-deprived rats. Biochem J. 1983;216:583–587. doi: 10.1042/bj2160583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosoni L, Houlier ML, Patureau Mirand P, Bayle G, Grizard J. Effect of amino acids alone or with insulin on muscle and liver protein synthesis in adult and old rats. Am J Physiol. 1993;264:E614–E620. doi: 10.1152/ajpendo.1993.264.4.E614. [DOI] [PubMed] [Google Scholar]
- O'Connor PMJ, Bush JA, Suryawan A, Nguyen HV, Davis TA. Insulin and amino acids independently stimulate skeletal muscle protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab. 2003;284:E110–E119. doi: 10.1152/ajpendo.00326.2002. [DOI] [PubMed] [Google Scholar]
- Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, et al. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol. 2004;286:E321–E328. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- Peyrollier K, Hajduch E, Blair AS, Hyde R, Hundal HS. L-Leucine availability regulates phosphatidylinositol 3-kinase, p70, S6 kinase and glycogen synthase kinase-3 activity in L6 muscle cells: evidence for the involvement of the mammalian target of rapamycin (mTOR) pathway in the L-leucine-induced up-regulation of the system A amino acid transport. Biochem J. 2000;350:361–368. 10.1042/0264-6021:3500361. [PMC free article] [PubMed] [Google Scholar]
- Preedy VR, Garlick PJ. The response of muscle protein synthesis to nutrient intake in postabsorptive rats: the role of insulin and amino acids. Biosci Rep. 1986;6:177–183. doi: 10.1007/BF01115004. [DOI] [PubMed] [Google Scholar]
- Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol. 2000;88:386–392. doi: 10.1152/jappl.2000.88.2.386. [DOI] [PubMed] [Google Scholar]
- Rasmussen BB, Wolfe RR, Volpi E. Oral and intravenously administered amino acids produce similar effects on muscle protein synthesis in the elderly. J Nutr Health Aging. 2002;6:358–362. [PMC free article] [PubMed] [Google Scholar]
- Reeds PJ, Fuller MF. Nutrient intake and protein turnover. Proc Nutr Soc. 1983;42:463–471. doi: 10.1079/pns19830053. [DOI] [PubMed] [Google Scholar]
- Rieu I, Sornet C, Bayle G, Prugnaud J, Pouyet C, Balage M, et al. Leucine-supplemented meal feeding for ten days beneficially affects postprandial muscle protein synthesis in old rats. J Nutr. 2003;133:1198–1205. doi: 10.1093/jn/133.4.1198. [DOI] [PubMed] [Google Scholar]
- Savary I, Debras E, Dardevet D, Sornet C, Capitan P, Prugnaud J, et al. Effect of glucocorticoid excess on skeletal muscle and heart protein synthesis in adult and old rats. Br J Nutr. 1998;79:297–304. doi: 10.1079/bjn19980047. [DOI] [PubMed] [Google Scholar]
- Sinaud S, Balage M, Bayle G, Dardevet D, Vary TC, Kimball SR, et al. Diazoxide-induced insulin deficiency greatly reduced muscle protein synthesis in rats: involvement of eIF4E. Am J Physiol. 1999;276:E50–E61. doi: 10.1152/ajpendo.1999.276.1.E50. [DOI] [PubMed] [Google Scholar]
- Smith K, Barua JM, Watt PW, Scrimgeour CM, Rennie MJ. Flooding with L-1-13C-leucine stimulates human muscle protein incorporation of continuously infused 1-13C-valine. Am J Physiol. 1992;262:E372–E376. doi: 10.1152/ajpendo.1992.262.3.E372. [DOI] [PubMed] [Google Scholar]
- Smith K, Rennie MJ. The measurement of tissue protein turnover. Bailliere's Clin Endocrinol Metab. 1996;10:469–495. doi: 10.1016/s0950-351x(96)80651-3. [DOI] [PubMed] [Google Scholar]
- Smith K, Reynolds N, Downie S, Patel A, Rennie MJ. Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am J Physiol. 1998;275:E73–E78. doi: 10.1152/ajpendo.1998.275.1.E73. [DOI] [PubMed] [Google Scholar]
- Steinberg HO, Baron AD. Vascular function, insulin resistance and fatty acids. Diabetologia. 2002;45:623–634. doi: 10.1007/s00125-002-0800-2. 10.1007/s00125-002-0800-2. [DOI] [PubMed] [Google Scholar]
- Stirewalt WS, Low RB. Effects of insulin in vitro on protein turnover in rat epitrochlearis muscle. Biochem J. 1983;210:323–330. doi: 10.1042/bj2100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svanberg E, Zachrisson H, Ohlsson C, Iresjo BM, Lundholm KG. Role of insulin and IGF-I in activation of muscle protein synthesis after oral feeding. Am J Physiol. 1996;270:E614–E620. doi: 10.1152/ajpendo.1996.270.4.E614. [DOI] [PubMed] [Google Scholar]
- Tauveron I, Larbaud D, Champredon C, Debras E, Tesseraud S, Bayle G, et al. Effect of hyperinsulinemia and hyperaminoacidemia on muscle and liver protein synthesis in lactating goats. Am J Physiol. 1994;267:E877–E885. doi: 10.1152/ajpendo.1994.267.6.E877. [DOI] [PubMed] [Google Scholar]
- Tipton KD, Ferrando AA, Phillips SM, Doyle DJ, Wolfe RR. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol. 1999;276:E628–E634. doi: 10.1152/ajpendo.1999.276.4.E628. [DOI] [PubMed] [Google Scholar]
- Ueki K, Yamamoto-Honda R, Kaburagi Y, Yamauchi T, Tobe K, Burgering BMT, et al. Potential role of protein kinase B in insulin-induced glucose transport, glycogen synthesis, and protein synthesis. J Biol Chem. 1998;273:5315–5322. doi: 10.1074/jbc.273.9.5315. 10.1074/jbc.273.9.5315. [DOI] [PubMed] [Google Scholar]
- Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest. 1998;101:2000–2007. doi: 10.1172/JCI939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi E, Lucidi P, Cruciani G, Monacchia F, Reboldi G, Brunetti P, et al. Contribution of amino acids and insulin to protein anabolism during meal absorption. Diabetes. 1996;45:1245–1252. doi: 10.2337/diab.45.9.1245. [DOI] [PubMed] [Google Scholar]
- Volpi E, Mittendorfer BB, Rasmussen RR, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85:4481–4490. doi: 10.1210/jcem.85.12.7021. 10.1210/jc.85.12.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol. 1999;277:E513–E520. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- Yoshizawa F, Endo M, Ide H, Yagasaki K, Funabiki R. Translational regulation of protein synthesis in the liver and skeletal muscle of mice in response to refeeding. J Nutr Biochem. 1995;6:130–136. 10.1016/0955-2863(95)00018-U. [Google Scholar]
- Yoshizawa F, Kido T, Nagasawa T. Stimulative effect of dietary protein on the phosphorylation of p70, S6 kinase in the skeletal muscle and liver of food-deprived rats. Biosci Biotechnol Biochem. 1999;63:1803–1805. doi: 10.1271/bbb.63.1803. [DOI] [PubMed] [Google Scholar]
- Yoshizawa F, Kimball SR, Vary TC, Jefferson LS. Effect of dietary protein on translation initiation in rat skeletal muscle and liver. Am J Physiol. 1998;275:E814–E820. doi: 10.1152/ajpendo.1998.275.5.E814. [DOI] [PubMed] [Google Scholar]