Abstract
The key concept of proteomics (looking at many proteins at once) opens new avenues in the search for clinically useful biomarkers of disease, treatment response and ageing. As the number of proteins that can be detected in plasma or serum (the primary clinical diagnostic samples) increases towards 1000, a paradoxical decline has occurred in the number of new protein markers approved for diagnostic use in clinical laboratories. This review explores the limitations of current proteomics protein discovery platforms, and proposes an alternative approach, applicable to a range of biological/physiological problems, in which quantitative mass spectrometric methods developed for analytical chemistry are employed to measure limited sets of candidate markers in large sets of clinical samples. A set of 177 candidate biomarker proteins with reported associations to cardiovascular disease and stroke are presented as a starting point for such a ‘directed proteomics’ approach.
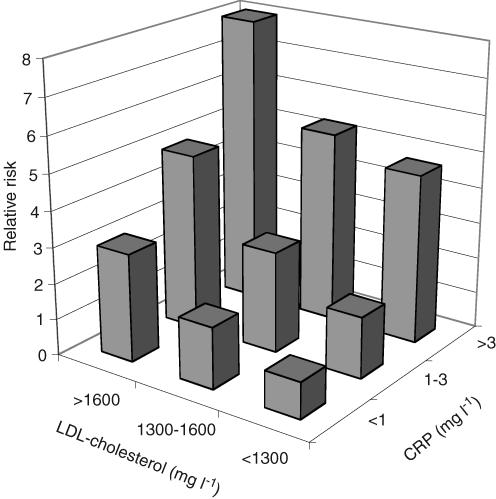
Proteomics has been defined from the biochemist's viewpoint (in a remark by Kenneth Mann) as the study of more than one protein at a time, a perspective that recognizes the importance of complex relationships between the functional parts of living systems while resisting the temptation to insist on a genome style complete (and perhaps unattainable) description at the protein level. While the technologies of proteomics have made rapid strides in recent years, providing tools that have been applied to many disease processes, there is a conspicuous lack of important disease markers discovered through proteomics and now established in the clinic. In fact if the rate of new plasma diagnostic protein markers is examined over the last decade, it has actually declined from one to two per year to near zero today (Anderson & Anderson, 2002). The reasons behind this paradox deserve study because the potential importance of accessible protein biomarkers of both normal and abnormal physiology is so great, particularly if we can believe the attractive but unproven hypothesis that all abnormal physiological states leave some specific fingerprint in the composition of circulating proteins. Evidence for this hypothesis, most recently in the field of cancer detection (Petricoin et al. 2002), has been accumulating for many years in the related fields of metabolite analysis (Jellum et al. 1981) and clinical chemistry (Robertson et al. 1980). These studies add support to the general statistical argument that a panel of independent disease-related proteins considered in the aggregate should be less prone to the influence of genetic and environmental ‘noise’ than is the level of a single marker protein. The heterogeneity of disease processes, and the genetic differences between individuals in the human population, both tend to obscure what might otherwise be clear disease associations. However, if there are multiple markers affected by the disease which are not strongly correlated with one another, then a composite index combining these markers may provide a much more robust indication of disease. In measuring the acute phase response, for example, a composite index summarizing a panel of weak acute phase reactants (Doherty et al. 1998) can provide a more robust indicator of inflammation than a single marker (e.g. C-reactive protein (CRP) or serum amyloid A). Similarly the relative risk of coronary heart disease is better predicted (Rifai & Ridker, 2003) by CRP and low-density lipoprotein (LDL)-cholesterol together than by either alone (Fig. 1, replotted from published data: Rifai & Ridker, 2003). More sophisticated multiplex panels have emerged from work with microarrays. One such example is the Netherlands breast cancer study (van't Veeret al. 2002), which sought to distinguish between patients with the same stage of disease but different response to treatment and overall outcome. The success of this initial study motivated a more extensive independent follow-up study involving 295 patients (van de Vijver et al. 2002) which led to a nationwide clinical trial in the Netherlands in which gene expression profiles for 70 classifier genes are being collected on all breast cancer patients and used as an adjunct to classical clinical staging. The belief that this phenomenon will be general for both proteins and mRNA, and that combinations of markers can be found that will identify and stage a wide range of diseases with useful specificity and sensitivity, is among the most important hypotheses of current biomedical research.
Figure 1.
Data replotted from Rifai & Ridker, 2003 showing the improved discrimination of relative cardiovascular disease risk when two different markers (in this case LDL-cholesterol and C-reactive protein) are considered jointly.
The difficulty of finding and using new biomarkers in the blood, even given the impressive advances in proteomics technologies, becomes clear when we compare the characteristics of the plasma proteome with the capabilities of current proteome analysis strategies and technology platforms. An exploration of this juxtaposition, set out in the following sections, provides the basis for an alternative candidate-based (targeted) approach proposed in the remainder of the paper.
Challenges of the plasma proteome
Plasma, which (together with its close cognate serum) is the primary biochemically useful clinical specimen, comprises the largest and deepest version of the human proteome. This makes it the most difficult sample to work with in proteomics, despite the relatively good behaviour (i.e. solubility) of its protein components. The daunting size of the plasma proteome is a reflection of the sheer number of different proteins to be detected. A rough calculation of this number can be made as follows. (1) Assume that 10% of the ∼30 000 genes encode secreted proteins, that each of these is made in an average of three splice forms, that two cleaved versions of each exist, and that there are an average of five post-translational modifications for each protein (a low estimate given the extreme carbohydrate microheterogeneity of most major plasma proteins). Since all these events can occur independently of the others, we obtain 3000 × 3 × 2 × 5 = 90 000 different secreted molecules. (2) Assume that all the non-secreted human proteins and their various modified forms are released into plasma at some low level as a result of cell turnover in the tissues. Using levels of modification similar to the secreted proteins, we obtain a further 810 000 protein species present at low levels. (3) Finally, assume that there are ∼10 000 000 distinct clonal immunoglobulin sequences present in plasma reflecting the immune history of the individual. The sum of these admittedly rough estimates is > 106 different molecules representing products of all ∼30 000 genes: in other words, plasma is the largest version of the human proteome in one sample. Proteomics technologies can typically resolve ∼100 different species per dimension of separation, indicating that 3 or more perfectly independent separative dimensions would be required, or more probably 4–5 dimensions of realistically implementable technology.
The enormous ‘depth’ of the plasma proteome is a reflection of the dynamic range (difference between the highest and lowest concentration) over which proteins must be detected. Approximately half of the total protein mass in plasma is accounted for by one protein (albumin, present at ∼55 000 000 000 pg ml−1), while roughly 10 proteins together make up 90% of the total. At the other end of the concentration histogram are the cytokines, such as interleukin-6 (IL-6), which is normally present at 1–5 pg ml−1. The difference in concentration between albumin and IL-6 is thus ∼1010. This range, of course, covers the proteins we know and consider useful as markers today, and ignores potentially valuable markers to be found in the future at even lower concentrations. The fact that we know anything about the concentrations of these proteins, and hence have been able to use them as biomarkers, is due to the power of specific protein tests, typically immunoassays of one protein at a time, and not to proteomics as currently defined, where currently technology is limited to a dynamic range of 103–104 (see Fig. 2).
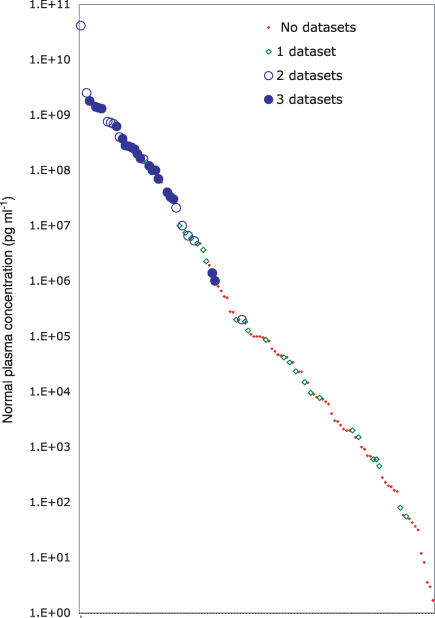
Figure 2.
A plot in which normal plasma concentrations for 115 proteins from Table 1 (distributed along the X-axis but unlabelled because of legibility limitations) are plotted on a log scale (pg ml−1 along the Y-axis). The proteins are sorted by abundance to reveal the smooth distribution across > 10 logs of concentration. Each protein is represented by a symbol that indicates in how many of three proteomics datasets (see text) it was detected.
Proteomic strategies for the discovery and validation of biomarkers in plasma
Given the analytical challenges inherent in the plasma proteome, what practical strategies exist for finding and confirming protein biomarkers? The problem can be approached from two opposite directions: (1) complete analysis (to see all differences) and (2) targeted analysis (to measure one or more hypothesis-generated candidates). The advantages of complete analysis, if it is possible, are substantial. Complete analysis would allow the direct selection of optimal biomarker proteins at the outset, thus skipping over what is currently a very long and laborious iterative process. Not surprisingly, progress towards complete analysis has been the focus of most proteomics research for the past decade. The number of proteins detectable in plasma has risen from 40 in 1975 (Anderson & Anderson, 1977) to 300–1000 reported in various recent studies (Adkins et al. 2002; Pieper et al. 2003a; Tirumalai et al. 2003). The latter datasets have been combined (Anderson et al. 2004b) to generate a non-redundant set of 1173 proteins, which revealed surprisingly small commonality between the results of these three different proteomics platforms (respectively multidimensional chromatography of proteins followed by 2-D electrophoresis and mass spectrometry (MS) identification of resolved proteins; tryptic digestion and multidimensional chromatography of peptides followed by MS identification; and tryptic digestion and multidimensional chromatography of peptides from low-molecular weight plasma components followed by MS identification). When these datasets are searched for a group of candidate disease markers (the cardiovascular candidates described below) for which plasma concentration normal values exist, the result illustrates the limited sensitivity of the platforms as a means of complete plasma proteome analysis (Fig. 2). Most proteins in the top 3 logs of the concentration distribution are detected by two or three of the three platforms, a fair proportion of the proteins in the middle two logs are seen by at least one of the platforms, and very few of the proteins in the bottom 5 logs are detected by any of the three. Thus it appears that current proteomics technology is unlikely to be able to provide a complete analysis of the most relevant diagnostic samples (e.g. serum and plasma). An additional important feature of this plot is that the candidate proteins show a smooth distribution between 10 and 109 pg ml−1, demonstrating that presumed disease relationships appear to occur independently of a protein's plasma concentration. In particular there does not seem to be a bias towards either very low abundance proteins (e.g. cytokines) or high abundance molecules. Since plasma concentration was not a criterion in the selection of these proteins (just a relationship with cardiovascular disease or stroke), this observation is probably meaningful.
Targeted analysis, which emerged as a means of searching for disease marker associations in the 1950s (in the form of enzyme assays), has a longer history than proteomics (which emerged in the form of 2-D electrophoresis in the mid-1970s), and has produced most of the protein markers now in diagnostic use. Typically a researcher interested in a specific protein develops hypotheses regarding a specific disease, and arranges to apply a lab bench assay to sets of samples from patients and controls. The specific assays involved are usually immunoassays, which, because of the great specificity of antibodies, are often able to detect proteins in plasma at much lower concentrations than current proteomics platforms. While this approach adheres to the conventions of hypothesis-driven research (and is thus fundable through grants), it has a substantial weakness in the poor probability of success when one marker is tested in one disease at a time: there are, as indicated, at least tens of thousands of candidate protein forms, and at least hundreds of disease entities. Even if it were the case that there is one protein capable of serving as a robust marker of each disease state, this method will take a long time to find them, and unfortunately it will take as much effort to find the last such marker as it took to find the first. More discouraging yet is the fact that any disease state in which several markers need to be considered together to produce an accurate result (i.e. a multiplex panel) would represent an enormous combinatorial discovery problem: since the experimental assays are typically developed in separate laboratories, bringing them together for application as a prototype panel is an organizational challenge, compounded by the increased sample requirement of multiple separate assays.
Thus both the complete and targeted analytical approaches have important limitations (sensitivity and mono-analyte focus, respectively) that diminish the output of novel disease marker proteins. This situation has led in recent years to consideration of hybrid approaches, in which a set of preselected proteins could be measured at high sensitivity. By focusing on a limited number of candidate biomarker proteins, assay technologies providing higher sensitivity and dynamic range than current proteomics could be used. By looking at multiple proteins, instead of one, the odds of finding useful disease associations, and effective panels, would be increased. The odds can be further improved through intelligent selection of candidate markers: here there is an opportunity to make use of information from many sources in addition to proteomics: expression microarray data suggesting tissue-specific or disease-altered synthesis of specific proteins, relationships of proteins to disease pathways, and classical biochemistry. Such a hybrid approach, combining the multiprotein view of proteomics and the advantages of targeted specific assays can be termed targeted proteomics.
Technology platforms for targeted proteomics of candidate markers
The central technical issue in targeted proteomics is how best to measure a limited set of proteins in complex samples such as plasma. Two broad strategies are developing: miniaturized, mulitplexed immunoassays and quantitative mass spectrometry. The former approach, which includes antibody arrays in both planar and particle suspension formats (recently reviewed by Joos (Joos et al. 2002) and a review in this series), has the advantage that immunoassays are well-understood, sensitive and specific. Antibody arrays are limited, however, by the availability of suitable antibodies, and this has proved to be a critical bottleneck for the development of immunoassays for new marker content. While a single research immunoassay costs less to assemble than the $2–4 million required for a commercial diagnostic test, each additional new marker assay costs the same again as the first, typically requiring development of two different high-affinity antibodies. It thus appears that substantial time will be required to generate large sets of new immunoassays to candidate markers, and that an alternative approach based on quantitative mass spectrometry may serve to evaluate candidate biomarkers prior to investment in immunoassays. Here I focus on the emerging MS methodologies for specific protein quantification.
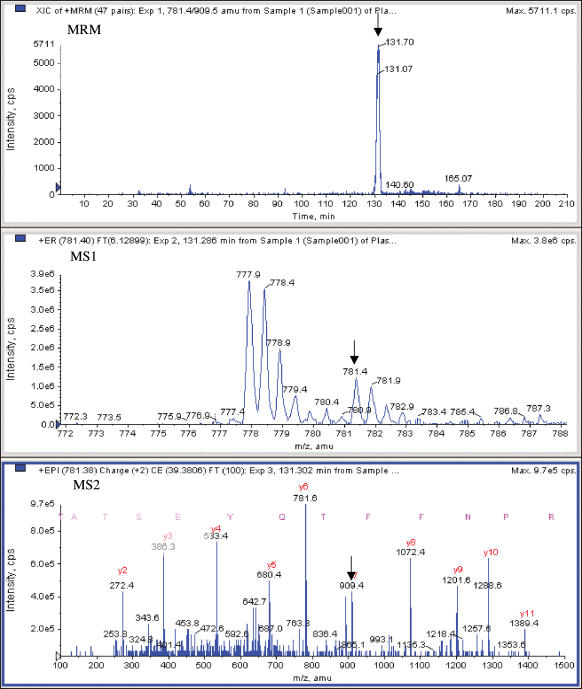
Mass spectrometry is widely used for the quantitative measurement of specific small molecules (e.g. drugs (Streit et al. 2002, 2004), drug metabolites (Kostiainen et al. 2003), hormones (Tai et al. 2004), and pesticides (Sannino et al. 2004)), with excellent precision (Tai et al. 2004) and very high throughput (Bakhtiar et al. 2002; Deng et al. 2002). In these methods, a sample is typically subjected to some form of high-throughput prefractionation (e.g. solid phase extraction; SPE) followed by a rapid reversed-phase chromatography separation, and the resulting output stream is introduced through an ionizing spray interface into a triple-quadrupole MS (TQMS). Within the MS, the first mass analyser (MS1) is set to pass the parent molecule (the ‘analyte’), rejecting components of other mass-to-charge ratios (m/z). The analyte is then fragmented in a collision chamber and passed to a second mass analyser (MS2) set to pass a known specific fragment. This two-stage selection of parent and fragment ions (selected reaction monitoring: SRM) affords great specificity, with the result that the detected signal usually traces a peak in the chromatogram at the expected retention time corresponding to the selected analyte. Integrating this peak gives a measure of the quantity of the analyte. Figure 3 presents an example of this approach in which a specific tryptic peptide of the coagulation protease prothrombin is measured in a tryptic digest of plasma. This measurement, based on precise molecular characteristics of the peptide, is in fact more specific for prothrombin than a typical immunoassay (in which lack of perfect antibody specificity is usually overlooked). An internal standard is often spiked into the sample to provide a reference signal to which the analyte is compared for absolute quantification. Lower limits of quantification (LLOQ) of 5–25 ng ml−1 (∼20 nm) can be obtained for drug metabolites (Zhang et al. 2000a), and < 10 ng ml−1 for pesticides in vegetable samples (Sannino, 2004). In serum and plasma, methods based on two-stage mass spectrometry (MS/MS) quantify the drugs mycophenolic acid (Streit et al. 2004) (0.5 ng ml−1) and sirolimus (Streit et al. 2002) (0.25 ng ml−1), as well as hormones and metabolites such as thyroid hormone T3 (Tai et al. 2004) (a reference method with coefficient of variation (CV) < 3%), homocysteine (Magera et al. 1999; Arndt et al. 2004; Stabler & Allen, 2004), S-adenosylmethionine and S-adenosylhomocysteine (Struys et al. 2000) (LLOQs of 3 and 1 ng ml−1, respectively, with CV < 8%).
Figure 3.
An example showing MS/MS detection of a prothrombin peptide (TATSEYQTFFNPR) in a tryptic digest of unfractionated plasma, using the SRM transition 781.4/909.7 (parent/fragment masses). Prothrombin is present in normal plasma at 100 mg ml−1, and the peptide is detected at a signal-to-noise ratio (S/N, smoothed peak height/3 s background) of 85. In the figure, the arrow in panel MS1 shows the peak in the peptide MS spectrum selected as the parent, the arrow in panel MS2 shows the fragment chosen from the MS/MS spectrum (the y7 ion), and panel MRM shows the ion current detected at this parent/fragment SRM transition (with unit mass windows) over the entire course of a 3 h LC run. The MS/MS spectrum in MS2 unambiguously identifies the prothrombin peptide by sequence, providing absolute specificity better than immunoassay.
The SPE–LC–MS/MS approach (where LC is liquid chromatography) has also been successfully applied to peptides, which typically have higher masses than the small molecules discussed above. Peptides yield specific fragments suitable for MS/MS measurement, and suitable internal standard peptides can be prepared by chemical synthesis. Small amounts (picomoles) of neuropeptides (enkephalins (Desiderio & Kai, 1983), endorphins (Dass et al. 1989), substance P (Lisek et al. 1989)) were detected by MS/MS and measured against stable isotope-labelled standards in the 1980s. More recently this approach has been used in standardized assays for larger peptides in serum such as 3 kDa thymosin a1 (LLOQ 0.5 ng ml−1 (Tuthill et al. 2000) CV < 10%) and for small proteins like the 10 kDa recombinant protein rK5 (LOQ 100 ng ml−1 in monkey serum (Ji et al. 2003) and later 10 ng ml−1 in human serum (Ji et al. 2004), CV of 3%). The structural specificity of MS/MS allows better analyte discrimination than immunoassays: particular forms of insulin and its fragments can be selectively detected (Kippen et al. 1997), and in fact MS/MS is now used as a reference against which to standardize different immunoassays for C-peptide (Fierens et al. 2003).
However, the method as described above is not generally useful for proteins larger than about 10 kDa, whose higher mass is not as well resolved by current MS or LC systems as peptides, which do not fragment efficiently into a few discrete pieces, and for which labelled internal standards are significantly more expensive. MS analysis of whole proteins from plasma is typically restricted to non-quantitative applications in which an available high affinity antibody is used to capture the protein, which is then eluted and analysed by MS (Kiernan et al. 2003; Nepomuceno et al. 2004), or digested to peptides that can be subjected to MS/MS for structural analysis (Labugger et al. 2003; Nedelkov et al. 2004). Such methods are useful for detecting protein sequence variants and post-translational modifications (PTMs), and can be quantitative in the rare cases where a purified cross-reacting homologue from another species is available to serve as an internal standard (e.g. the assay of 7.6 kDa IGF1 (Nelson et al. 2004) at ∼100 pg ml−1).
Thus in order to effectively leverage the successful methods of LC–MS/MS quantification to proteins in a sample such as plasma, one must ‘disassemble’ each protein quantitatively into its constituent peptides by complete chemical or enzymatic cleavage. Within this digest one can select a monitor peptide to serve as a quantitative surrogate for the protein, and achieve accurate quantification by spiking with a stable isotope-labelled version of the same peptide as internal standard (Stemmann et al. 2001). Such ‘postdigest’ assays have been generated for some higher-abundance plasma proteins such as ApoA-I lipoprotein (Barr et al. 1996) (CV < 4%) and Hb A1C (Jeppsson et al. 2002) (an International Federation of Clinical Chemistry reference method in which a glycated peptide is measured with interlaboratory CVs of 1.4–2.3%). Attempts to assay the 26 kDa cancer marker prostate-specific antigen (PSA) (Barnidge et al. 2004) using a standard LC–MS/MS system yielded a detection limit of 4.5 mg ml−1 (0.17 mg ml−1 of the monitored peptide, a level ∼1000 times higher than the clinically relevant level), while measurement of CRP (after a molecular weight enrichment by SDS gel) yielded quantitative measurements at < 1 mg ml−1 (Kuhn et al. 2004).
While individual analytes within each class of molecule vary, the published data lead us to conclude that serum concentrations in the order of 1 ng ml−1 for drugs, 1–10 ng ml−1 for plasma peptides, and ∼100 ng ml−1 for peptides in a complex plasma digest can be measured by existing LC–MS/MS-based assay methods. On average, proteins in plasma are ∼34 times as large as the roughly 10 amino acid-long monitor peptides chosen to represent them, and thus the protein detection limit (measuring a peptide in a digest) would be expected to be roughly 3 mg ml−1.
Two additional elements are required to enable quantitative MS/MS for targeted proteomics: the capability to assay many proteins at a time and a means to extend sensitivity downwards to the level of low abundance biomarkers such as cytokines (∼10 pg ml−1).
Multi-analyte methods are implemented in TQMS by rapidly switching between pairs of MS/MS parameters during the LC run. Published methods have measured up to 29 pesticides in one run (Barr et al. 2002) and prototype studies of up to 200 multiple-reaction monitoring (MRM) analytes performed. Sensitivity of MS assays can be increased by additional stages of fractionation prior to LC–MS/MS. Two such methods of particular promise involve the subtraction of specific high-abundance plasma proteins (e.g. albumin, transferrin, Igs, haptoglobin, etc.) using specific antibody columns (Pieper et al. 2003b), and the specific enrichment of selected monitor peptides through binding and release from antipeptide antibody columns (Anderson et al. 2004a). The former method provides a 10-fold improvement in sensitivity (by subtracting 90% of the mass of protein in plasma), while the latter method yields an additional 100-fold average improvement using relatively crude rabbit polyclonal antibodies. These extensions provide a reasonable basis for the expectation that panels of 20–50 protein analytes taken from the top 6 or 7 (of 10) orders of magnitude plasma concentration should be accessible for routine MS/MS measurement.
Candidate markers of cardiovascular disease
Given a technology platform for measuring a limited number of identified proteins, intelligent candidate selection is a high priority. As an example of a set of candidates to start with, I present here a table of proteins reported to have some connection with cardiovascular disease (here considered in a broad sense, and including heart disease, stroke, vascular disease, hyper- and hypo-coagulation) from literature and other sources (Table 1).
Table 1.
A table of 177 candidate markers of cardiovascular disease (CVD) and stroke, assembled through literature search
| Name | Accession | Normal concentration (pg ml−1) | Source for concentration | Reason | Coagulation | Lipoprotein | Acute Phase | |
|---|---|---|---|---|---|---|---|---|
| 1 | activin A | P08476 | 6.0E +02 | (Eldar-Geva et al. 2001) | Released by heparin from vascular endothelium (Phillips et al. 2000) | |||
| 2 | adiponectin (ADPN) | Q15848 | 4.8E +06 | (Mallamaci et al. 2002) | Higher levels in essential hypertensives (Mallamaci et al. 2002) | |||
| 3 | albumin | P02768 | 4.1E +10 | (Specialty Laboratories, 2001) | Negative acute phase reactant, lower levels associated with increased risk of cardiovascular mortality (Shaper et al. 2004) | + | ||
| 4 | aldolase C | P09972 | 4.0E +03 | (Asaka et al. 1990) | A more specific and sensitive marker of cerebrovascular diseases than aldolase A (Asaka et al. 1990) | |||
| 5 | alpha 2 antiplasmin (alpha 2 AP) | P08697 | 7.0E +07 | Progen test insert | An important regulator of the fibrinolytic system | + | ||
| 6 | alpha 2 macroglobulin (alpha 2 m) | P01023 | 1.8E +09 | (Specialty Laboratories, 2001) | Major plasma protease inhibitor | |||
| 7 | alpha(1)- antichymotrypsin (ACT) | P01011 | 4.2E +07 | (Putnam, 1975) | Major plasma protease inhibitor | + | ||
| 8 | alpha1 acid-glycoprotein (AAG) | P02763 | 6.9E +08 | (Specialty Laboratories, 2001) | Acute phase reactant | + | ||
| 9 | alpha1-antitrypsin (AAT) | P01009 | 1.4E +09 | (Specialty Laboratories, 2001) | Major plasma protease inhibitor | |||
| 10 | angiotensin- converting enzyme (ACE) | P12821 | Lower in stroke patients than controls (Catto et al. 1996) | |||||
| 11 | angiotensinogen | P01019 | 1.5E +06 | (Bloem et al. 1995) | Precursor of major blood pressure control peptide | |||
| 12 | antithrombin III (AT III) | P01008 | 2.0E +08 | (Kalafatis et al. 1997) | Major inhibitor of thrombin | + | ||
| 13 | apolipoprotein A-I | P02647 | 1.4E +09 | (Glowinska et al. 2003) | Low level associated with mortality and myocardial infarction five years after CABG(Skinner et al. 1999) | + | + | |
| 14 | apolipoprotein A-II | P02652 | 2.4E +08 | (Luo & Liu, 1994) | Lipoprotein | + | ||
| 15 | apolipoprotein A-IV | P06727 | 1.6E +08 | (Kondo et al. 1989) | A relatively independent risk factor for CHD (Warner et al. 2001) | + | ||
| 16 | apolipoprotein B | P04114 | 7.3E +08 | (Glowinska et al. 2003) | Major component of LDL | + | ||
| 17 | apolipoprotein C-I | P02654 | 6.1E +07 | (Riesen & Sturzenegger, 1986) | Lipoprotein | + | ||
| 18 | apolipoprotein C-II | P02655 | 3.3E +07 | (Bury et al. 1986) | Lipoprotein | + | ||
| 19 | apolipoprotein CIII | P02656 | 1.2E +08 | (Onat et al. 2003) | Marker of CHD independent of cholesterol (Onat et al. 2003) | + | ||
| 20 | apolipoprotein D | P05090 | Lipoprotein | + | ||||
| 21 | apolipoprotein E | P02649 | 4.0E +07 | Presence of epsilon4 allele a strong independent predictor of adverse events (Brscic et al. 2000) | + | |||
| 22 | apolipoprotein L1 | O14791 | Lipoprotein | + | ||||
| 23 | aspartate aminotransferase, mitochondrial (m-type) | P00505 | Giagnostic for early detection of myocardial infarction (Yoneda et al. 1992) | |||||
| 24 | basic fibroblast gth factor (bFGF) | P09038 | 6.0E +03 | (Song et al. 2002) | sICAM-1level increases in acute cerebral infarction (Song et al. 2002) | |||
| 25 | beta(2)-glycoprotein I, nicked | P02749 | May control extrinsic fibrinolysis via a negative feedback pathway loop (Yasuda et al. 2004) | + | ||||
| 26 | B-type neurotrophic gth factor (BNGF) | P01138 | 7.0E +02 | (Reynolds et al. 2003) | Candidate stroke marker (Reynolds et al. 2003) | |||
| 27 | cathepsin B | P07858 | 2.1E +03 | (Kos et al. 1998) | Potential biomarker for vulnerable plaques (Chen et al. 2002) | |||
| 28 | CD105 (endoglin) | P17813 | 3.4E +04 | (Takahashi et al. 2001) | Potential myocardial infarction and stroke marker (Li et al. 1998) | |||
| 29 | CD40 ligand, soluble (sCD40L)(= CD154) | P29965 | 2.9E +03 | (Schonbeck et al. 2001) | Patients with unstable angina have elevated plasma levels of soluble CD40L (Schonbeck et al. 2001) | |||
| 30 | ceruloplasmin | P00450 | 2.8E +08 | (Kim et al. 2002) | Ceruloplasmin reported to be an independent risk factor for cardiovascular disease (Kim et al. 2002) | + | ||
| 31 | chitotriosidase | Q13231 | Significantly increased in individuals suffering from atherosclerosis disease (Artieda et al. 2003) | |||||
| 32 | cholesteryl ester transfer protein (CETP) | P11597 | 1.9E +06 | (Sasai et al. 1998) | Alleles affect CVD (Blankenberg et al. 2003) | + | ||
| 33 | chromogranin A | P10645 | 1.1E +05 | (Ceconi et al. 2002) | Increased in chronic heart failure (Ceconi et al. 2002) | |||
| 34 | clusterin | P10909 | 3.7E +08 | (Hogasen et al. 1993) | Induced in media and neointima after vascular injury (Miyata et al. 2001) | + | ||
| 35 | coagulation Factor IX | P00740 | 5.1E +06 | (Kalafatis et al. 1997) | Coagulation | + | ||
| 36 | coagulation Factor V | P12259 | 6.6E +06 | (Kalafatis et al. 1997) | Most common genetic CVD risk factor to date is a single point mutation (FV Leiden) (Dahlback, 2003) | + | ||
| 37 | coagulation Factor VII | P08709 | 5.0E +05 | (Kalafatis et al. 1997) | Coagulation | + | ||
| 38 | coagulation Factor VII-activating protease | Q14520 | 7.5E +06 | (Romisch et al. 1999) | Coagulation | + | ||
| 39 | coagulation Factor VIII | P00451 | 2.0E +05 | (Kalafatis et al. 1997) | Coagulation | + | ||
| 40 | coagulation Factor X | P00742 | 1.0E +07 | (Kalafatis et al. 1997) | Target for novel antithrombotic agents | + | ||
| 41 | coagulation Factor XI | P03951 | 4.8E +06 | (Kalafatis et al. 1997) | Coagulation | + | ||
| 42 | coagulation Factor XII | P00748 | 3.0E +07 | (Kalafatis et al. 1997) | Coagulation | + | ||
| 43 | coagulation Factor XIIa | P00748 | 2.0E +03 | (McLaren et al. 2002) | Levels of 2 ng ml−1 or more have an increased risk of CHD (McLaren et al. 2002) | + | ||
| 44 | coagulation Factor XIII | P00488, P05160 | 1.0E +07 | (Katona et al. 2000) | Coagulation | + | ||
| 45 | collagen I degradation byproduct (ICTP) | 0 | Altered in hypertrophic cardiomyopathy (Lombardi et al. 2003) | |||||
| 46 | collagen I synthesis byproduct (PICP) | 0 | Altered in hypertrophic cardiomyopathy (Lombardi et al. 2003) | |||||
| 47 | collagen I synthesis byproduct (PINP) | 0 | Altered in hypertrophic cardiomyopathy (Lombardi et al. 2003) | |||||
| 48 | collagen I synthesis byproduct (PIP) | 0 | 1.0E +05 | (Lopez et al. 2001) | May be useful to assess the cardioreparative properties of antihypertensive treatment in hypertensives (Lopez et al. 2001) | |||
| 49 | collagen III propeptide (PIIIP) | 0 | (Nomura et al. 2003) | |||||
| 50 | collagen III synthesis byproduct (PIIINP) | 0 | 5.0E +03 | (Poulsen et al. 2000) | Correlates with infarct size in MI (Poulsen et al. 2000) | |||
| 51 | complement C1 inactivator | P05155 | 3.0E +08 | (Oshitani et al. 1988) | Can preserve ischaemic myocardium from reperfusion injury (Buerke et al. 1995) | |||
| 52 | complement C3 | P01024 | 1.3E +09 | (Specialty Laboratories, 2001) | C3 is more strongly associated with previous myocardial infarction than other risk factors (Muscari et al. 2000) | + | ||
| 53 | complement C4 | P01028 | 2.7E +08 | (Specialty Laboratories, 2001) | Associated with previous myocardial infarction (Muscari et al. 1995) | + | ||
| 54 | C-reactive protein (CRP) | P02741 | 2.3E +06 | (Menon et al. 2003) | CRP levels strongly predicts cardiovascular death (Park et al. 2002) | + | ||
| 55 | creatine kinase-MB | P12277, P06732 | Specific biochemical marker of myocardial injury (Ay et al. 2002) | |||||
| 56 | endothelial cell protein C receptor (EPCR) | Q9UNN8 | 1.0E +05 | (Kurosawa et al. 1997) | Protein C activation is augmented by EPCR (Esmon, 2003) | |||
| 57 | endothelial leucocyte adhesion molecule 1 (ELAM-1) | P16581 | 9.2E +02 | (Carson et al. 1993) | Stroke caused an initial transient increase of sELAM-1 (Fassbender et al. 1995) | |||
| 58 | endothelin-1 (ET-1) | P05305 | 3.6E +00 | (Tsutamoto et al. 1995) | ET-1 levels are elevated in acute MI (Monge, 1998) | |||
| 59 | endothelin-1, Big | P05305 | 1.2E +01 | (Erbas et al. 2000) | Elevated Big endothelin-1 is a strong predictor of atrial fibrillation (Masson et al. 2000) | |||
| 60 | enolase, beta, skeletal muscle | P13929 | Concentrations significantly increased in acute MI (Nomura et al. 1987) | |||||
| 61 | enolase, gamma, neurone-specific | P09104 | 9.6E +03 | (Oh et al. 2002) | May be a useful marker for severity in acute ischaemic stroke (Oh et al. 2002) | |||
| 62 | erythropoietin (EPO) | P01588 | 2.6E +02 | (Masaki et al. 1992) | Protects neurones from hypoxic/ischaemic injury (Ehrenreich et al. 2002) | |||
| 63 | E-selectin, soluble | P16581 | 1.5E +04 | (Galvani et al. 2000) | sE-selectin significantly elevated in the acute stage of ischaemic stroke (Frijns et al. 1997) | |||
| 64 | Fas, soluble (APO-1/CD95) | P25445 | 2.0E +03 | (Ohtsuka et al. 1999) | Increased plasma sFas levels are predictive of future CVD (Troyanov et al. 2003) | |||
| 65 | fatty acid-binding protein, heart-type (H-FABP) | P05413 | 2.0E +03 | (Glatz et al. 1998) | Performs as well as myoglobin as a marker of cardiac reperfusion (de Groot et al. 2001) | |||
| 66 | ferritin | P02792 + P02794 | 8.2E +04 | (Zuyderhoudt et al. 1978) | Possible relationship with carotid atherosclerosis potentiated by LDL cholesterol (Wolff et al. 2004) | + | ||
| 67 | fibrinogen | P02671 + P02675 + P02679 | 2.5E +09 | (Glowinska et al. 2003) | Strongly related to cardiovascular risk (Koenig, 2003) | + | + | |
| 68 | fibrinopeptide A | P02671 | 9.0E +02 | (Cronlund et al. 1976) | iIncreased in patients with ACS and is associated with adverse outcome (Ottani & Galvani, 2001) | |||
| 69 | fibrinopeptide B beta 1–42 | P02675 | May be predictive of recurrent ischaemia (Scharfstein et al. 1996) | |||||
| 70 | fibrinopeptide B beta 15–42 | P02675 | Candidate haemostasis marker (Fareed et al. 1998) | |||||
| 71 | fibronectin | P02751 | 1.4E +06 | (Castellanos et al. 2004) | Cellular fibronectin may be a marker protein for endothelial cell activation (Kanters et al. 2001) | |||
| 72 | follistatin | P19883 | 6.0E +02 | (Eldar-Geva et al. 2001) | Released by heparin from vascular endothelium (Phillips et al. 2000) | |||
| 73 | gamma- glutamyltransferase (GGT) | P19440 | Marker of liver dysfunction, alcohol intake and stroke (Whitfield, 2001) | |||||
| 74 | glial fibrillary acidic protein (GFAP) | P14136 | 4.5E +02 | (van Geel et al. 2002) | Marker of brain damage (Herrmann et al. 2000) | |||
| 75 | glycogen phosphorylase BB, cardiac | P11216 | 3.0E +03 | (Hofmann et al. 1989) | Classical cardiac marker | |||
| 76 | GMP-140 (soluble P-selectin) | P16109 | 2.0E +05 | (Facer & Theodoridou, 1994) | Elevated in elderly hypertensives (Li et al. 2001b) | |||
| 77 | gp130, soluble (sgp130) | P40189 | 2.7E +05 | (Li et al. 2001a) | Correlated with variables reflecting deranged haemodynamic status (Aukrust et al. 1999) | |||
| 78 | GPIIb/IIIa, soluble | P08514 | Implicated in the pathogenesis of acute coronary syndromes (Wagner et al. 1998) | |||||
| 79 | gth hormone (GH) | P01241 | 2.0E +02 | (Krassas et al. 2003) | Associated with an increased incidence of cardiovascular disease (Vahl et al. 1999) | |||
| 80 | haptoglobin | P00737 | 6.2E +08 | (Specialty Laboratories, 2001) | Subjects with Hp 2–2 had significantly higher serum total and free cholesterol concentration (Braeckman et al. 1999) | |||
| 81 | haemopexin | P02790 | 7.6E +08 | (Jakob, 2002) | Acute phase protein | |||
| 82 | heparin cofactor II (HCII) | P05546 | Protein inhibitor of coalgulation (Mann et al. 2003) | + | ||||
| 83 | hepatocyte gth factor (HGF) | P14210 | 2.0E +02 | (Matsumori et al. 2000) | Reflects the clinical course in patients with acute MI (Sato et al. 1997) | |||
| 84 | hexosaminidase A | P06865 | Subjects in the 95–100%ile showed significantly increased frequency of myocardial infarction of their fathers and of stroke in their mothers (Hultberg et al. 1994) | |||||
| 85 | hydroxybutyrate dehydrogenase (HBDH) | Q02338 | 1.3E +05 | (Akenzua et al. 1992) | Mitochondrial enzyme useful for estimation of infarct size in MI (van der Laarse et al. 1984). | |||
| 86 | immunoglobulin G | 0 | 9.8E +09 | (Specialty Laboratories, 2001) | Acute phase protein | |||
| 87 | insulin | P01308 | 2.0E +03 | (Green et al. 1976) | Serum insulin quantitatively associated with cardiovascular risk factors (Chen et al. 1999) | |||
| 88 | insulin C-peptide | P01308 | 1.7E +03 | (Donatelli et al. 1991) | C-peptide quantitatively associated with cardiovascular risk factors (Chen et al. 1999) | |||
| 89 | insulin precursor (proinsulin) | P01308 | 4.3E +01 | (Burtis & Ashwood, 1999) | Increased concentrations predict death and morbidity caused by CHD over a period of 27 years, independent of other major cardiovascular risk factors (Zethelius et al. 2002) | |||
| 90 | insulin-like gth factor binding protein-1 (IGFBP-1) | P08833 | 6.0E +04 | (Wacharasindhu et al. 2002) | Correlated negatively with several established cardiovascular factors (Heald et al. 2001) | |||
| 91 | insulin-like gth factor-1 (IGF-1) | P01343 | 1.9E +05 | (Oh et al. 2004) | May be a risk factor for certain cardiac disorders (Ren et al. 1999) | |||
| 92 | intercellular adhesion molecule 1, soluble (sICAM-1) | P05362 | 5.3E +05 | (Song et al. 2003) | sICAM-1 related to the estimated risk of coronary heart disease (Witte et al. 2003) | |||
| 93 | interleukin-1 beta (IL-1 beta) | P01584 | 1.2E +00 | (Lu et al. 2004) | Higher in MI group or UA (Wang et al. 2004) | |||
| 94 | interleukin-1 receptor antagonist (IL-1Ra) | P18510 | Plasma levels appear to be a valuable independent predictive factor of major adverse cardiac events in unselected patients undergoing PCI (Patti et al. 2002) | |||||
| 95 | interleukin-1 receptor family member, ST2 | Q01638 | Increased in the serum 1 day after myocardial infarction (Weinberg et al. 2002) | |||||
| 96 | interleukin-10 (IL-10) | P22301 | Increased serum levels detected in stroke patients (Dziedzic et al. 2002) | |||||
| 97 | interleukin-18 (IL-18) | Q14116 | 5.9E +01 | (Blankenberg et al. 2002) | Significantly increased in unstable angina and MI (Mallat et al. 2002) | |||
| 98 | interleukin-2 (IL-2) | P60568 | 5.1E +01 | (Mizia-Stec et al. 2003) | Significantly higher in patients with MI (Mizia-Stec et al. 2003) | |||
| 99 | interleukin-6 (IL-6) | P05231 | Increased serum level was a significant predictor of death or new heart failure episodes (Orus et al. 2000) | |||||
| 100 | interleukin-6 receptor, soluble (sIL-6R) | P08887 | 1.0E +05 | (Disthabanchong et al. 2002) | Increased in MI and UA (Bossowska et al. 2003) | |||
| 101 | interleukin-8 (IL-8) | P10145 | 1.7E +00 | (Zhang et al. 2003) | Level higher in UA (Romuk et al. 2002) | |||
| 102 | leptin | P41159 | Patients with advanced CHF show elevated serum levels (Schulze et al. 2003) | |||||
| 103 | leptin receptor, soluble | P48357 | 2.3E +04 | (Schulze et al. 2003) | Patients with advanced CHF show elevated serum levels (Schulze et al. 2003) | |||
| 104 | lipoprotein lipase (LPL) | P06858 | 2.8E +05 | (Dugi et al. 2002) | Significant association between the LPL protein mass and NYHA class (Kastelein et al. 2000) | + | ||
| 105 | lipoprotein receptor-related protein 1, soluble (sLRP1) (alpha-2- macroglobulin receptor) | Q07954 | 6.0E +06 | (Quinn et al. 1997) | May antagonize the clearance of ligands by cell bound LRP perturbing lipid metabolism (Quinn et al. 1997) | + | ||
| 106 | lipoprotein(a) (Lp(a)) | P08519 | 1.4E +08 | (Glowinska et al. 2003) | An index of atherosclerosis risk (Malaguarnera et al. 1996) | + | ||
| 107 | lipoprotein- associated phospholipase A2 (Lp-PLA2) | P04054 | 1.5E +03 | (Kugiyama et al. 1999) | Potential biomarker of coronary heart disease, plays a proinflammatory role in the progression of atherosclerosis (Dada et al. 2002) | + | ||
| 108 | l-selectin, soluble (sL-selectin) (CD62L) | P14151 | 6.7E +05 | (Atalar et al. 2002) | CD62L expression increased during cardiopulmonary bypass (Hambsch et al. 2002) | |||
| 109 | macrophage colony-stimulating factor (MCSF) | P09603 | 6.8E +02 | (Saitoh et al. 2000) | Mean concentration in patients with coronary events was significantly higher than controls (Saitoh et al. 2000) | |||
| 110 | matrix metalloproteinase-1 (MMP-1) | P03956 | Patients with atrial fibrillation (AF) had lower levels of MMP-1 (Marin et al. 2003) | |||||
| 111 | matrix metalloproteinase-2 (MMP-2) | P08253 | 8.1E +05 | (Noji et al. 2004) | Higher in hypertrophic cardiomyopathy than controls (Lombardi et al. 2003). | |||
| 112 | matrix metalloproteinase-3 (MMP-3) | P08254 | 8.0E +03 | (Sangiorgi et al. 2001) | Levels are strongly associated with carotid lesions (Beaudeux et al. 2003) | |||
| 113 | matrix metalloproteinase-9 (MMP-9) | P14780 | 9.0E +03 | (Sangiorgi et al. 2001) | Predicts haemorrhagic transformation in acute ischaemic stroke (Castellanos et al. 2003) | |||
| 114 | monocyte chemoattractant protein-1 (MCP-1) | P13500 | 1.6E +02 | (de Lemos et al. 2003) | Appears to play a crucial role at multiple stages of atherosclerosis (de Lemos et al. 2003) | |||
| 115 | myelin basic protein (MBP) | P02686 | 2.5E +03 | Marker of cerebral damage (Zhou et al. 1992) | ||||
| 116 | myeloperoxidase (MPO) | P05164 | Predicts increased risk for subsequent cardiovascular events (Baldus et al. 2003) | |||||
| 117 | myoglobin, cardiac (Mb) | P02144 | 4.2E +04 | (Burtis & Ashwood, 1999) | Cardiac muscle damage marker | |||
| 118 | myosin heavy chain, cardiac | P13533, P12883 | Cardiac muscle damage marker | |||||
| 119 | myosin light chain I, cardiac | P08590 | 1.0E +03 | (Uji et al. 1991) | Cardiac muscle damage marker | |||
| 120 | myosin light chain II, cardiac | P10916 | 2.0E +03 | (Hirayama et al. 1990) | Cardiac muscle damage marker | |||
| 121 | natriuretic peptide, atrial, C-terminal (C-ANP) | P01160 | Diagnostic utility in detecting left ventricular dysfunction (Lee et al. 2002) | |||||
| 122 | natriuretic peptide, atrial (ANP) | P01160 | 5.6E +01 | (Goto et al. 2002) | Diagnostic utility in detecting left ventricular dysfunction (Lee et al. 2002) | |||
| 123 | natriuretic peptide, atrial, N-terminal (N-ANP) | P01160 | Diagnostic utility in detecting left ventricular dysfunction (Lee et al. 2002) | |||||
| 124 | natriuretic peptide, atrial, propeptide (31–67) | P01160 | Increased moderately with primary pulmonary hypertension (Goetze et al. 2004) | |||||
| 125 | natriuretic peptide, brain (BNP) | P16860 | 1.9E +02 | (Goto et al. 2002) | Diagnostic utility in detecting left ventricular dysfunction (Lee et al. 2002) | |||
| 126 | natriuretic peptide, brain, N-terminal (NT-BNP) | P16860 | Diagnostic utility in detecting left ventricular dysfunction | |||||
| 127 | natriuretic peptide, brain, pro-form (proBNP) | P16860 | 40-fold increase in primary pulmonary hypertension (Goetze et al. 2004) | |||||
| 128 | neurone-specific enolase (NSE) | P09104 | 8.0E +01 | (Oh et al. 2002) | Significantly elevated in patients with acute cerebral infarction (Oh et al. 2002) | |||
| 129 | neutral endopeptidase 24.11 (NEP) | P08473 | 2.5E +02 | (Zhang et al. 1994) | A target for ACE-inhibitor-like drugs | |||
| 130 | neutrophil gelatinase-associated lipocalin (NGAL) | P80188 | 8.7E +04 | (Elneihoum et al. 1997) | Levels higher in stroke (Falke et al. 2000) | |||
| 131 | neutrophil protease-4 (NP4) | P24158 | 2.3E +04 | (Elneihoum et al. 1997) | Levels higher in stroke (Elneihoum et al. 1996) | |||
| 132 | osteoprotegerin (OPG) | O00300 | 2.3E +02 | (Bner et al. 2001) | Serum levels associated with cardiovascular mortality, may be a marker for vascular calcification (Bner et al. 2001) | |||
| 133 | paraoxonase (PON1, 2, 3) | (P27169, Q15165, Q15166) | 5.9E +07 | (Kujiraoka et al. 2000) | Plasma levels influence the risk of developing cardiovascular disease (Getz & Reardon, 2004). | + | ||
| 134 | phosphoglycerate mutase (PGM) B-type | P18669 | Novel marker for diagnosis of cerebral stroke and its severity (Hayashi & Matuo, 2001) | |||||
| 135 | plasminogen | P00747 | 1.0E +08 | (Marchal et al. 1996) | Major enzyme of thrombolysis | + | ||
| 136 | plasminogen activator inhibitor (PAI)-1-antigen | P05121 | 4.2E +04 | (Glowinska et al. 2003) | High plasma levels reported in coronary artery disease and stroke (Diamantopoulos et al. 2003) | + | + | |
| 137 | platelet endothelial cell adhesion molecule-1, soluble (sPECAM-1) | P16284 | 6.6E +03 | (Zeisler et al. 2001) | Stroke patients displayed statistically significant higher levels of sPECAM-1 in sera (Zaremba & Losy, 2002) | |||
| 138 | platelet factor 4 | P02776 | 7.7E +03 | (Cella et al. 1983) | Elevated in brain lacunar infarctions with long-lasting signs (Oishi et al. 1999) | |||
| 139 | platelet-activating factor (PAF) acetylhydrolase | Q13093 | Deficiency associated with stroke, myocardial infarction, brain haemorrhage, and non-familial cardiomyopathy (Tjoelker & Stafforini, 2000) | |||||
| 140 | platelet-derived gth factor (PDGF) | P04085 + P01127 | 1.7E +02 | (Cimminiello et al. 1994) | iIncreased levels in chronic arterial obstructive disease (Cimminiello et al. 1994) | |||
| 141 | pregnancy-associated plasma protein A (PAPP-A) | Q13219 | Elevated in acute coronary syndromes (Bayes-Genis et al. 2001) | |||||
| 142 | prorenin | P00797 | 3.7E +01 | (Sealey, 1991) | Involved in blood pressure regulation | |||
| 143 | protein C | P04070 | 3.7E +06 | (Yan & Dhainaut, 2001) | Major regulator of haemostasis (Yan & Dhainaut, 2001) | + | ||
| 144 | protein C inhibitor (PCI) | P05154 | 5.3E +06 | (Laurell et al. 1992) | Inhibitor of key component of natural anticoagulant pathway | + | ||
| 145 | protein C, activated (APC) | P04070 | 2.0E +03 | (Yan & Dhainaut, 2001) | Key component of natural anticoagulant pathway | + | ||
| 146 | protein S | P07225 | 2.1E +07 | (Kalafatis et al. 1997) | Deficiency of protein S constitutes a major risk factor of venous thrombosis (Dahlback, 2004) | + | ||
| 147 | protein Z | P22891 | In the context of juvenile stroke, high plasma levels may represent a prothrombotic condition (Lichy et al. 2004) | + | ||||
| 148 | prothrombin | P00734 | 1.0E +08 | (Kalafatis et al. 1997) | Coagulation | + | ||
| 149 | prothrombin fragment 1.2 | P00734 | 1.2E +03 | (McKenzie et al. 1999) | Stroke patients had higher values than controls (Soncini et al. 2000) | + | ||
| 150 | P-selectin glycoprotein ligand-1 (PSGL-1) | Q14242 | Serum levels decreased during CV surgery (Osmancik et al. 2002) | |||||
| 151 | P-selectin, soluble (GMP-140) | P16109 | 4.7E +04 | (Carter et al. 2003) | Significantly elevated in the acute stage of ischaemic stroke (Frijns et al. 1997) | |||
| 152 | resistin | Q9HD89 | 1.5E +04 | (Fujinami et al. 2004) | Concentrations of adipocytokines such as resistin and adiponectin determine inflammation status of vasculature, and in turn the progress of Atherosclerosis (Kawanami et al. 2004) | |||
| 153 | S-100beta | P04271 | A promising early biochemical marker for cerebral injury following cardiac surgery (Farsak et al. 2003) | |||||
| 154 | serum amyloid A protein (SAA) | P02735 | Classical inflammation marker (with CRP) | + | ||||
| 155 | serum placenta gth factor | P49763 | Associated with the occurrence of subsequent preeclampsia (Su et al. 2001) | |||||
| 156 | sex hormone-binding globulin (SHBG) | P04278 | A biological marker for insulin resistance, which is linked to cardiovascular risk in African-American women (Sherif et al. 1998) | |||||
| 157 | smooth muscle myosin heavy chain | P35749 | Intracoronary level may be a biochemical marker for the prediction of restenosis (Tsuchio et al. 2000) | |||||
| 158 | tau protein | P10636 | Correlated with brain infarct volume and disability after 3 months (Bitsch et al. 2002) | |||||
| 159 | thrombin activatable fibrinolysis inhibitor (TAFI) | Q9P2Y6 | 3.5E +06 | (Wada et al. 2002) | Indirectly affects clot stability (Mann et al. 2003) | + | ||
| 160 | thrombomodulin, soluble (sTM) | P07204 | 4.5E +04 | (Blann et al. 1997) | Strong, graded, inverse association with incident coronary heart disease (Salomaa et al. 1999) | + | ||
| 161 | thrombospondin-1 | P07996 | 2.0E +05 | (Hayden et al. 2000) | Might function as an alternative substrate for thrombus formation (Jurk et al. 2003) | + | ||
| 162 | tissue factor (TF) | P13726 | 2.8E +02 | (Zemanova et al. 2003) | Good predictor of cardiac allograft vasculopathy (CAV) (Yen et al. 2002) | + | ||
| 163 | tissue factor pathway inhibitor (TFPI) | P10646 | 2.3E +04 | (Nomura et al. 2003) | Significantly higher in acute MI (He et al. 2002) | + | ||
| 164 | tissue inhibitor of metalloproteinases-1 (TIMP-1) | P01033 | 9.5E +04 | (Noji et al. 2001) | Significantly higher in HCM patients than in control subjects (Noji et al. 2004) | |||
| 165 | tissue inhibitor of metalloproteinases-2 (TIMP-2) | P16035 | 3.4E +04 | (Noji et al. 2004) | Significantly higher in patients with HCM accompanied by systolic dysfunction (Noji et al. 2004) | |||
| 166 | tissue plasminogen activator (t-PA) | P00750 | 7.3E +03 | (Glowinska et al. 2003) | Predicted coronary events during a very long-term follow-up (Niessner et al. 2003) | + | ||
| 167 | transforming gth factor-beta (TGF-beta) | P01137 | 4.5E +03 | (Shariat et al. 2001) | Concentrations decreased in patients with coronary artery disease (CAD) (Tashiro et al. 2002) | |||
| 168 | tropomyosin 1 alpha chain | P09493 | 2.0E +03 | (Cummins et al. 1981) | Elevated ∼50-fold in MI (Cummins et al. 1981) | |||
| 169 | troponin I, cardiac | P19429 | 1.0E +03 | (Kini et al. 2004) | A clinical marker of cardiac muscle damage | |||
| 170 | troponin T, cardiac | P45379 | 3.0E +00 | (Xue et al. 2003) | A clinical marker of cardiac muscle damage | |||
| 171 | tumour necrosis factor receptor I, soluble (sTNF-RI) | P19438 | 8.9E +02 | (Weiss et al. 1996) | Significant independent predictor of cardiovascular mortality (Falke et al. 2000) | |||
| 172 | tumour necrosis factor receptor II, soluble (sTNF-RII) | P20333 | 1.7E +03 | (Weiss et al. 1996) | Increased in patients with CHF (Nowak et al. 2002) | |||
| 173 | tumour necrosis factor-alpha (TNF-alpha) | P01375 | 8.3E +00 | (Mizia-Stec et al. 2003) | Levels were elevated in all CAD groups (Mizia-Stec et al. 2003) | |||
| 174 | vascular endothelial gth factor (VEGF) | P15692 | 3.2E +01 | (Lavie et al. 2002) | Levels increased in patients with peripheral artery disease (PAD) (Makin et al. 2003) | |||
| 175 | vitronectin | P04004 | 2.6E +08 | (Hogasen et al. 1993) | A cofactor for rapid inhibition of activated protein C by plasminogen activator inhibitor-1 (Gechtman & Shaltiel, 1997) | |||
| 176 | von Willebrand Factor (vWF) | P04275 | Elevated plasma concentrations are increasingly recognized as a cardiovascular risk factor (Vischer et al. 1997) | + | ||||
| 177 | von Willebrand Factor, propeptide (vWf:AgII) | P04275 | 7.0E +05 | (Vischer et al. 1997) | Could provide a sensitive plasma marker of acute endothelial secretion (Vischer et al. 1997) | + |
The common name, Swissprot sequence accession number, normal plasma concentration (and source of concentration measurement), justification for inclusion, and membership in one of three general CVD-related groups (coagulation pathway, lipid transport, and acute phase reactants) are tabulated. Concentrations are mean values where given, or a geometric average of high and low normal values where a range was given. Blanks occur where the search has not yet found reliable published values. Some entries have multiple accessions (multiple subunits separated by +, or lack of sufficient information to select among homologues separated by commas), and in some cases multiple candidates share a single accession (when different processed forms of one protein are considered separately).
Cardiovascular disease (CVD) is the leading cause of death in the United States (∼40% of all deaths), and a major economic burden ($227 billion in direct medical costs this year) (2003). In 2001, there were more than 4 million visits to emergency departments with a primary diagnosis of CVD, and more than 6 million inpatient cardiovascular operations and procedures were performed (American Heart Association, 2003).
Cardiovascular disease includes a range of phenomena differing markedly in timescale, physical size, and relative effects of genes and environment. It includes slow processes such as atherosclerosis, which can evolve over decades, and very rapid events such as myocardial infarction, which can be lethal in a matter of minutes. It involves subtle changes at the molecular level, as coagulation enzymes are activated at the site of a ruptured arterial plaque, and large-scale physical consequences, when a blood clot physically plugs a major coronary artery. Genetic factors (e.g. familial hypercholesterolaemia or levels of lipoprotein (a) (Lp(a)) are strongly involved, as are environmental and lifestyle factors, the most obvious of which are lipid intake and smoking. Largely on account of this breadth of causes and effects, and the diversity of treatment strategies that this makes possible, major progress has been made in the development of life-saving interventions. Damaged hearts can be repaired physically, by coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI), or enzymatically, by administering recombinant human tissue plasminogen activator (tPA) to digest a clot; elevated blood pressure can be controlled by several different classes of drugs, and coagulation can be enhanced (in treatment of haemophilia by replacement of missing clotting factor proteins) or diminished (with aspirin, heparins, and platelet GP IIb/IIIA receptor antagonists).
A major challenge in medicine is thus deciding when, and upon whom, these effective interventions should be carried out. A patient presenting with chest pain may have an acute myocardial infarction (MI) requiring immediate PCI or tPA treatment, stable angina requiring nitroglycerine, oesophageal spasm with no cardiovascular consequences, etc. Given the urgency of this issue, the cardiology community has promulgated detailed guidelines concerning triage of chest-pain patients (Ryan et al. 1996; Braunwald et al. 2000). Perhaps most importantly, there is a window of opportunity, while conditions such as atherosclerosis and hypertension gradually worsen, in which the ability to anticipate an imminent acute event (e.g. MI or stroke) can have immense benefit. Where causal molecules or telltale molecular fingerprints can be identified, objective and reproducible laboratory tests can be created, helping to implement best medical practices at institutions large and small. Such tests are typically inexpensive in relation to drug treatment or surgical intervention, providing a major health economic benefit. And they can be fast, providing critical results in < 15 min when implemented in automated instruments near the patient.
History of protein markers in CVD
Cardiovascular disease is the most likely area in the spectrum of human disease to yield protein markers in plasma. Most pathologies of the cardiovascular system involve plasma proteins directly (e.g. the coagulation cascade with its positive and negative modulators (> 29 proteins), or proteins of lipid transport involved in atherosclerosis (> 16 proteins)), or proteins that interact with vessel walls, platelets, or both. In addition to these, numerous inflammatory modulators transported in the blood have direct and indirect relationships to cardiovascular disease, while release of proteins from the heart itself provides evidence of cardiac damage.
Consistent with this expectation, a number of very successful protein diagnostics have emerged in cardiovascular medicine. The most definitive of these is cardiac troponin I (TnI, or the alternative TnT, both muscle contractile proteins) as a primary indicator of myocardial infarction (Jaffe, 2001), often in combination with the cardiac isozyme of creatine kinase (CK-MB) and myoglobin. In this case, the diagnosis of MI typically includes a finding of elevated cardiac marker (e.g. TnI > 1 ng ml−1), leading to initiation of reperfusion treatment based on the knowledge that the marker signals destruction of cardiac muscle tissue surrounding an infarct. Brain-type natriuretic peptide (Maeda et al. 1998) (BNP or NTproBNP), a molecule produced in and released by the left ventricle, has recently been adopted as an effective test for congestive heart failure. Because of the clinical importance of these tests, they are performed in very large numbers: ∼85 million troponin assays and ∼10 million BNP assays are performed each year. Similarly the levels of inflammation markers like C-reactive protein (Ridker et al. 1998) (CRP), lipoprotein(a) (Agewall & Fagerberg, 2002), fibrinogen (Kannel et al. 1992), and the apportionment of cholesterol between high- and low-density lipoproteins (Luria et al. 1991) (usually distinguished in assays by their protein components) all serve as valuable measures of cardiovascular risk.
In fact, many proteins in plasma show changes associated with cardiovascular disease states. Thus the strategy of seeking single-protein tests (each with a defined reference interval, or normal range, outside of which a patient value is clearly diseased) has been vigorously pursued. Unfortunately, in most cases these changes are not sufficiently specific to provide a test of useful predictive value: the change may be real but too small in relation to genetic and environmental ‘noise’, or it occurs with other diseases as well. Where useful biomarkers have emerged, the discovery and development of each test was the result of efforts over a number of years. The appearance of cardiac troponin in plasma in MI was reported in 1987 (Cummins et al. 1987), the test was introduced commercially in 1995, and it emerged as the core parameter for MI diagnosis in 2000 (Alpert et al. 2000; Braunwald et al. 2000). BNP, probably the most rapidly adopted new diagnostic test in CVD, was shown to be diagnostic for congestive heart failure (CHF) in 1996 (Yamamoto et al. 1996) and introduced as a commercial test in 2002. However, most markers have been under investigation for many years: myoglobin since 1977 (Rosano et al. 1977), cardiac fatty acid-binding protein (FABP) since 1992 (Kleine et al. 1992) and cardiac myosin light chain 1 since 1994 (Uchino et al. 1994). On average, there appears to be a delay of approximately 10 years between discovery of a CVD marker and its commercial implementation in a form that can benefit clinical medicine (assuming it is specific and sensitive). Reducing this time lag while maintaining the rigor of clinical validation is a high priority.
Collection of candidate CVD markers
Table 1 presents a set of proteins that are confirmed or potential plasma markers of some aspect of cardiovascular disease (in the heart, vessels or brain). To my knowledge, no comparable list of proteins associated with a specific disease area has been assembled and published. Results from several sources were pooled to generate this list. A large set (> 2000) of papers was selected through keyword searches on cardiovascular disease and stroke, and these were classified and clustered using the RefViz program where titles and abstracts were scanned for protein names. A table of these proteins was constructed in an Excel spreadsheet, to which was added additional ‘pathway’-derived potential markers derived from a literature survey of the protein components of coagulation and thrombolysis pathways, as well as acute phase reactants and known inflammatory markers. The resulting list comprised 177 protein targets, some of which were composed of multiple subunits, and some of which were different fragments of a single protein. Where possible, the normal plasma concentration was extracted from the literature references, or, in the case of existing clinical markers, from the normal range values used in test interpretation. These values are of critical importance in developing strategies for measurement: the 50 most abundant candidates are likely to be measurable by MS/MS (as in Fig. 3) without additional enrichment steps, while the others may require more elaborate sample preparation or fractionation prior to quantification.
While almost all of these candidates have been evaluated in some form of CVD or stroke, none has been surveyed across all forms of these diseases, and very few have been investigated jointly in the same sample sets. Thus these candidates include many proteins that have disease relationships that are significant (though not definitive enough to provide a specific single protein test): precisely the kinds of candidates from which multiplex panels of great specificity might be drawn.
Table 2 presents 28 additional known or candidate biomarkers of CVD that are not individual proteins. These include specific protein complexes, protein modifications, antibodies against specific proteins and smaller molecules (typically metabolites). While these markers are not directly accessible to the MS-based approach outlined here, they can be measured by immunoassay or by alternative MS-based methodologies.
Table 2.
Other candidate CVD markers
| fibrinogen d-dimer | (Ince et al. 1999) | |
| plasmin-alpha(2)-antiplasmin complex (PAP) | (Sakkinen et al. 1999) | |
| thrombin-antithrombin III complex (TAT) | (Brodin et al. 2004) | |
| tissue factor pathway inhibitor-factor Xa (TFPI-Xa) complex | (Ohkura et al. 1999) | |
| tissue plasminogen activator (tPA)-plasminogen activator | ||
| inhibitor-1 (PAI-1) complex (tPA/PAI-1 complex) | (Johansson et al. 2000) | |
| Protein modification | haemoglobin, glycated (HbA1c) | (Schillinger et al. 2003) |
| lipoprotein(a), glycated | (Zhang et al. 2000b) | |
| Antibodies to: | angiotensin II receptor (AT1) | (Fu et al. 2000) |
| beta 2-glycoprotein I (beta2-GPI) | (Ebeling et al. 2003) | |
| cardiac actin | (Dangas et al. 2000) | |
| cardiac myosin | (Ebeling et al. 2003) | |
| cardiolipin (aCL) | (Dangas et al. 2000) | |
| chlamydial LPS | (Lowe, 2001) | |
| heat shock protein 65 | (Birnie et al. 1998) | |
| oxidized LDL | (Ogawa et al. 2001) | |
| phospholipid [lupus anticoagulant (LA)] | (Guerin et al. 1998) | |
| prothrombin | (Guerin et al. 1998) | |
| Smaller molecules | asymmetric dimethylarginine (ADMA) | (Tarnow et al. 2004) |
| dehydroepiandrosterone sulphate (DHEAS) | (Jansson et al. 1998) | |
| folate | (Riddell et al. 2000) | |
| homocysteine (HCY) | (Abbate et al. 2003) | |
| kallidin (a tissue kinin) | (Wagner et al. 2002) | |
| malonyldialdehyde (MDA) | (Belboul et al. 2001) | |
| marinobufagenin (MBG) | (Fridman et al. 2002) | |
| melatonin | (Grote, 2004) | |
| N-acetyl-aspartate | (Stevens et al. 1999) | |
| oxidized phosphatidylcholine (OxPC, formed in OxLDL) | (Itabe, 2002) | |
| uric acid | (Leyva et al. 1998) |
Twenty-eight candidate markers of other types relevant to cardiovascular disease and stroke. These occur in four categories: protein complexes (where the amount of protein in heteromultimer complexes provides separate information from the concentrations of individual components); protein modifications (where the amount of specifically modified protein is relevant); antibodies (where the corresponding antigen is specified); and smaller molecules (which are not proteins, but rather metabolites). The first three categories are ultimately accessible to modified proteomics approaches. A citation is provided for each, illustrative of the connection to cardiovascular disease or stroke.
Discussion
This paper makes an argument for a candidate-based approach to protein biomarker development, supplementing the methods of classical proteomics that seek a complete analysis of a target proteome. Specific features of the plasma proteome, including its complexity and dynamic range, make it resistant to complete analysis in the near future. A targeted proteomics approach, aimed at selected candidates, can provide greater sensitivity and thus greater coverage of markers across the 10 orders of magnitude spanning known markers.
The fact that a non-exhaustive search for candidates related to CVD and stroke produced 177 different proteins (and protein forms) is revealing. A great deal of exploratory work has already been done, providing a targeted approach with an excellent starting point. The fact that most of these proteins have not yet become stand-alone clinical markers does not prevent them from providing incremental statistical improvement to multiprotein panels yielding improved specificity.
Two other factors also motivate a targeted approach. In the limiting case, the number of human genes is relatively small (∼25 000), and it might be reasonable to design specific MS-based assays (and ultimately antibodies for immunoassays) for all of these. Quantifying a major form of each human protein as a candidate disease marker is an attractive goal, though obviously far less comprehensive than the complete analysis goal (all forms of all proteins) implicit in the aims commonly expressed in proteomics.
A second and more practical factor favouring targeted assays is quantification itself. Most of the methods currently employed in proteomics can detect many proteins, but generally with poor quantitative accuracy. In particular when aiming for greatest sensitivity, proteome surveys of plasma detect quite variable subsets of proteins, even in repeat runs on the same sample. This makes it very difficult to assemble a coherent analytical dataset, since proteins are typically detected in one run but not the next: the dataset is filled with holes. This is acceptable when one is looking for hints as to the involvement of individual proteins in specific processes, but it is a major disadvantage when trying to develop a statistical case associating a protein with a disease in the human population. In this case accurate determinations of a protein in each sample are needed, as one obtains from specific assays.
By fusing the approaches taken by proteomics, analytical chemistry and clinical chemistry, hybrid methods should emerge capable of rapidly expanding the range of biomarkers for the study of disease, ageing and physiology.
Acknowledgments
I wish to thank my collaborator Dr Christie Hunter of Applied Biosystems (Foster City, CA, USA) for generating Fig. 3.
References
- Abbate R, Sofi F, Brogi D, Marcucci R. Emerging risk factors for ischemic stroke. Neurol Sci. 2003;24(Suppl. 1):S11–S12. doi: 10.1007/s100720300027. [DOI] [PubMed] [Google Scholar]
- Adkins JN, Varnum SM, Auberry KJ, Moore RJ, Angell NH, Smith RD, Springer DL, Pounds JG. Toward a human blood serum proteome: analysis by multidimensional separation coupled with mass spectrometry. Mol Cell Proteomics. 2002;1:947–955. doi: 10.1074/mcp.m200066-mcp200. [DOI] [PubMed] [Google Scholar]
- Agewall S, Fagerberg B. Lipoprotein(a) was an independent predictor for major coronary events in treated hypertensive men. Clin Cardiol. 2002;25:287–290. doi: 10.1002/clc.4960250609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Heart Association. Heart Disease and Stroke Statistics – 2004 Update. Dallas, TX, USA: American Heart Association; 2003. [Google Scholar]
- Akenzua GI, Ihongbe JC, Asemota HN. Alpha-hydroxybutyrate dehydrogenase and the diagnosis of painful crisis in sickle cell anaemia. Afr J Med Med Sci. 1992;21:13–17. [PubMed] [Google Scholar]
- Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined – a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–969. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- Anderson L, Anderson NG. High resolution two-dimensional electrophoresis of human plasma proteins. Proc Natl Acad Sci U S A. 1977;74:5421–5425. doi: 10.1073/pnas.74.12.5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics. 2002;1:845–867. doi: 10.1074/mcp.r200007-mcp200. [DOI] [PubMed] [Google Scholar]
- Anderson NL, Anderson NG, Haines LR, Hardie DB, Olafson RW, Pearson TW. Mass spectrometric quantitation of peptides and proteins using Stable Isotope Standards and Capture by Anti-Peptide Antibodies (SISCAPA) J Proteome Res. 2004a;3:235–244. doi: 10.1021/pr034086h. [DOI] [PubMed] [Google Scholar]
- Anderson NL, Polanski M, Pieper R, Gatlin T, Tirumalai RS, Conrads TP, Veenstra TD, Adkins JN, Pounds JG, Fagan R, Lobley A. The human plasma proteome: A non-redundant list developed by combination of four separate sources. Mol Cell Proteomics. 2004b;3:311–326. doi: 10.1074/mcp.M300127-MCP200. [DOI] [PubMed] [Google Scholar]
- Arndt T, Guessregen B, Hohl A, Heicke B. Total plasma homocysteine measured by liquid chromatography-tandem mass spectrometry with use of 96-well plates. Clin Chem. 2004;50:755–757. doi: 10.1373/clinchem.2003.029686. [DOI] [PubMed] [Google Scholar]
- Artieda M, Cenarro A, Ganan A, Jerico I, Gonzalvo C, Casado JM, Vitoria I, Puzo J, Pocovi M, Civeira F. Serum chitotriosidase activity is increased in subjects with atherosclerosis disease. Arterioscler Thromb Vasc Biol. 2003;23:1645–1652. doi: 10.1161/01.ATV.0000089329.09061.07. [DOI] [PubMed] [Google Scholar]
- Asaka M, Kimura T, Nishikawa S, Saitoh M, Miyazaki T, Takatori T, Alpert E. Serum aldolase isozyme levels in patients with cerebrovascular diseases. Am J Med Sci. 1990;300:291–295. doi: 10.1097/00000441-199011000-00004. [DOI] [PubMed] [Google Scholar]
- Atalar E, Ozmen F, Haznedaroglu I, Acil T, Ozer N, Ovunc K, Aksoyek S, Kes S. Effects of short-term atorvastatin treatment on global fibrinolytic capacity, and sL-selectin and sFas levels in hyperlipidemic patients with coronary artery disease. Int J Cardiol. 2002;84:227–231. doi: 10.1016/s0167-5273(02)00148-1. [DOI] [PubMed] [Google Scholar]
- Aukrust P, Ueland T, Lien E, Bendtzen K, Muller F, Andreassen AK, Nordoy I, Aass H, Espevik T, Simonsen S, Froland SS, Gullestad L. Cytokine network in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1999;83:376–382. doi: 10.1016/s0002-9149(98)00872-8. [DOI] [PubMed] [Google Scholar]
- Ay H, Arsava EM, Saribas O. Creatine kinase-MB elevation after stroke is not cardiac in origin: comparison with troponin T levels. Stroke. 2002;33:286–289. doi: 10.1161/hs0102.101544. [DOI] [PubMed] [Google Scholar]
- Bakhtiar R, Lohne J, Ramos L, Khemani L, Hayes M, Tse F. High-throughput quantification of the anti-leukemia drug STI571 (Gleevec) and its main metabolite (CGP 74588) in human plasma using liquid chromatography-tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2002;768:325–340. doi: 10.1016/s1570-0232(01)00611-0. [DOI] [PubMed] [Google Scholar]
- Baldus S, Heeschen C, Meinertz T, Zeiher AM, Eiserich JP, Munzel T, Simoons ML, Hamm CW. Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003;108:1440–1445. doi: 10.1161/01.CIR.0000090690.67322.51. [DOI] [PubMed] [Google Scholar]
- Barnidge DR, Goodmanson MK, Klee GG, Muddiman DC. Absolute quantification of the model biomarker prostate-specific antigen in serum by LC-Ms/MS using protein cleavage and isotope dilution mass spectrometry. J Proteome Res. 2004;3:644–652. doi: 10.1021/pr049963d. [DOI] [PubMed] [Google Scholar]
- Barr DB, Barr JR, Maggio VL, Whitehead RD, Jr, Sadowski MA, Whyatt RM, Needham LL. A multi-analyte method for the quantification of contemporary pesticides in human serum and plasma using high-resolution mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2002;778:99–111. doi: 10.1016/s0378-4347(01)00444-3. [DOI] [PubMed] [Google Scholar]
- Barr JR, Maggio VL, Patterson DG, Jr, Cooper GR, Henderson LO, Turner WE, Smith SJ, Hannon WH, Needham LL, Sampson EJ. Isotope dilution – mass spectrometric quantification of specific proteins: model application with apolipoprotein A-I. Clin Chem. 1996;42:1676–1682. [PubMed] [Google Scholar]
- Bayes-Genis A, Conover CA, Overgaard MT, Bailey KR, Christiansen M, Holmes DR, Jr, Virmani R, Oxvig C, Schwartz RS. Pregnancy-associated plasma protein A as a marker of acute coronary syndromes. N Engl J Med. 2001;345:1022–1029. doi: 10.1056/NEJMoa003147. [DOI] [PubMed] [Google Scholar]
- Beaudeux JL, Giral P, Bruckert E, Bernard M, Foglietti MJ, Chapman MJ. Serum matrix metalloproteinase-3 and tissue inhibitor of metalloproteinases-1 as potential markers of carotid atherosclerosis in infraclinical hyperlipidemia. Atherosclerosis. 2003;169:139–146. doi: 10.1016/s0021-9150(03)00149-7. [DOI] [PubMed] [Google Scholar]
- Belboul A, Roberts D, Borjesson R, Johnsson J. Oxygen free radical generation in healthy blood donors and cardiac patients: the protective effect of allopurinol. Perfusion. 2001;16:59–65. doi: 10.1177/026765910101600109. [DOI] [PubMed] [Google Scholar]
- Birnie DH, Holme ER, McKay IC, Hood S, McColl KE, Hillis WS. Association between antibodies to heat shock protein 65 and coronary atherosclerosis. Possible mechanism of action of Helicobacter pylori and other bacterial infections in increasing cardiovascular risk. Eur Heart J. 1998;19:387–394. doi: 10.1053/euhj.1997.0618. [DOI] [PubMed] [Google Scholar]
- Bitsch A, Horn C, Kemmling Y, Seipelt M, Hellenbrand U, Stiefel M, Ciesielczyk B, Cepek L, Bahn E, Ratzka P, Prange H, Otto M. Serum tau protein level as a marker of axonal damage in acute ischemic stroke. Eur Neurol. 2002;47:45–51. doi: 10.1159/000047946. [DOI] [PubMed] [Google Scholar]
- Blankenberg S, Rupprecht HJ, Bickel C, Jiang XC, Poirier O, Lackner KJ, Meyer J, Cambien F, Tiret L. Common genetic variation of the cholesteryl ester transfer protein gene strongly predicts future cardiovascular death in patients with coronary artery disease. J Am Coll Cardiol. 2003;41:1983–1989. doi: 10.1016/s0735-1097(03)00408-x. [DOI] [PubMed] [Google Scholar]
- Blankenberg S, Tiret L, Bickel C, Peetz D, Cambien F, Meyer J, Rupprecht HJ. Interleukin-18 is a strong predictor of cardiovascular death in stable and unstable angina. Circulation. 2002;106:24–30. doi: 10.1161/01.cir.0000020546.30940.92. [DOI] [PubMed] [Google Scholar]
- Blann AD, Amiral J, McCollum CN. Prognostic value of increased soluble thrombomodulin and increased soluble E-selectin in ischaemic heart disease. Eur J Haematol. 1997;59:115–120. doi: 10.1111/j.1600-0609.1997.tb00735.x. [DOI] [PubMed] [Google Scholar]
- Bloem LJ, Manatunga AK, Tewksbury DA, Pratt JH. The serum angiotensinogen concentration and variants of the angiotensinogen gene in white and black children. J Clin Invest. 1995;95:948–953. doi: 10.1172/JCI117803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossowska A, Kiersnowska-Rogowska B, Bossowski A, Galar B, Sowinski P. Cytokines in patients with ischaemic heart disease or myocardial infarction. Kardiol Pol. 2003;59:105–114. [PubMed] [Google Scholar]
- Braeckman L, De Bacquer D, Delanghe J, Claeys L, De Backer G. Associations between haptoglobin polymorphism, lipids, lipoproteins and inflammatory variables. Atherosclerosis. 1999;143:383–388. doi: 10.1016/s0021-9150(98)00330-x. [DOI] [PubMed] [Google Scholar]
- Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, Jones RH, Kereiakes D, Kupersmith J, Levin TN, Pepine CJ, Schaeffer JW, Smith EE, 3rd, Steward DE, Theroux P, Gibbons RJ, Alpert JS, Eagle KA, Faxon DP, Fuster V, Gardner TJ, Gregoratos G, Russell RO, Smith SC., Jr ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee on the management of patients with unstable angina) Circulation. 2000;102:1193–1209. doi: 10.1161/01.cir.102.10.1193. [DOI] [PubMed] [Google Scholar]
- Brodin E, Borvik T, Sandset PM, Bonaa KH, Nordoy A, Hansen JB. Coagulation activation in young survivors of myocardial infarction (MI) – a population-based case-control study. Thromb Haemost. 2004;92:178–184. doi: 10.1160/TH03-11-0674. [DOI] [PubMed] [Google Scholar]
- Browner WS, Lui LY, Cummings SR. Associations of serum osteoprotegerin levels with diabetes, stroke, bone density, fractures, and mortality in elderly women. J Clin Endocrinol Metab. 2001;86:631–637. doi: 10.1210/jcem.86.2.7192. [DOI] [PubMed] [Google Scholar]
- Brscic E, Bergerone S, Gagnor A, Colajanni E, Matullo G, Scaglione L, Cassader M, Gaschino G, Di Leo M, Brusca A, Pagano GF, Piazza A, Trevi GP. Acute myocardial infarction in young adults: prognostic role of angiotensin-converting enzyme, angiotensin II type I receptor, apolipoprotein E, endothelial constitutive nitric oxide synthase, and glycoprotein IIIa genetic polymorphisms at medium-term follow-up. Am Heart J. 2000;139:979–984. doi: 10.1067/mhj.2000.106165. [DOI] [PubMed] [Google Scholar]
- Buerke M, Murohara T, Lefer AM. Cardioprotective effects of a C1 esterase inhibitor in myocardial ischemia and reperfusion. Circulation. 1995;91:393–402. doi: 10.1161/01.cir.91.2.393. [DOI] [PubMed] [Google Scholar]
- Burtis CA, Ashwood ER. Tietz Textbook of Clinical Chemistry. Philadelphia: W. B. Saunders Company; 1999. [Google Scholar]
- Bury J, Michiels G, Rosseneu M. Human apolipoprotein C-II quantitation by sandwich enzyme-linked immunosorbent assay. J Clin Chem Clin Biochem. 1986;24:457–463. doi: 10.1515/cclm.1986.24.7.457. [DOI] [PubMed] [Google Scholar]
- Carson CW, Beall LD, Hunder GG, Johnson CM, Newman W. Serum ELAM-1 is increased in vasculitis, scleroderma, and systemic lupus erythematosus. J Rheumatol. 1993;20:809–814. [PubMed] [Google Scholar]
- Carter AM, Anagnostopoulou K, Mansfield MW, Grant PJ. Soluble P-selectin levels, P-selectin polymorphisms and cardiovascular disease. J Thromb Haemost. 2003;1:1718–1723. doi: 10.1046/j.1538-7836.2003.00312.x. [DOI] [PubMed] [Google Scholar]
- Castellanos M, Leira R, Serena J, Blanco M, Pedraza S, Castillo J, Davalos A. Plasma cellular-fibronectin concentration predicts hemorrhagic transformation after thrombolytic therapy in acute ischemic stroke. Stroke. 2004;35:1671–1676. doi: 10.1161/01.STR.0000131656.47979.39. [DOI] [PubMed] [Google Scholar]
- Castellanos M, Leira R, Serena J, Pumar JM, Lizasoain I, Castillo J, Davalos A. Plasma metalloproteinase-9 concentration predicts hemorrhagic transformation in acute ischemic stroke. Stroke. 2003;34:40–46. [PubMed] [Google Scholar]
- Catto A, Carter AM, Barrett JH, Stickland M, Bamford J, Davies JA, Grant PJ. Angiotensin-converting enzyme insertion/deletion polymorphism and cerebrovascular disease. Stroke. 1996;27:435–440. [PubMed] [Google Scholar]
- Ceconi C, Ferrari R, Bachetti T, Opasich C, Volterrani M, Colombo B, Parrinello G, Corti A. Chromogranin A in heart failure; a novel neurohumoral factor and a predictor for mortality. Eur Heart J. 2002;23:967–974. doi: 10.1053/euhj.2001.2977. [DOI] [PubMed] [Google Scholar]
- Cella G, Colby SI, Taylor AD, McCracken L, Parisi AF, Sasahara AA. Platelet factor 4 (PF4) and heparin-released platelet factor 4 (HR-PF4) in patients with cardiovascular disorders. Thromb Res. 1983;29:499–509. doi: 10.1016/0049-3848(83)90345-6. [DOI] [PubMed] [Google Scholar]
- Chen CH, Tsai ST, Chou P. Correlation of fasting serum C-peptide and insulin with markers of metabolic syndrome-X in a homogenous Chinese population with normal glucose tolerance. Int J Cardiol. 1999;68:179–186. doi: 10.1016/s0167-5273(98)00366-0. [DOI] [PubMed] [Google Scholar]
- Chen J, Tung CH, Mahmood U, Ntziachristos V, Gyurko R, Fishman MC, Huang PL, Weissleder R. In vivo imaging of proteolytic activity in atherosclerosis. Circulation. 2002;105:2766–2771. doi: 10.1161/01.cir.0000017860.20619.23. [DOI] [PubMed] [Google Scholar]
- Cimminiello C, Arpaia G, Aloisio M, Uberti T, Rossi F, Pozzi F, Bonfardeci G. Platelet-derived growth factor (PDGF) in patients with different degrees of chronic arterial obstructive disease. Angiology. 1994;45:289–293. doi: 10.1177/000331979404500405. [DOI] [PubMed] [Google Scholar]
- Cronlund M, Hardin J, Burton J, Lee L, Haber E, Bloch KJ. Fibrinopeptide A in plasma of normal subjects and patients with disseminated intravascular coagulation and systemic lupus erythematosus. J Clin Invest. 1976;58:142–151. doi: 10.1172/JCI108443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins B, Auckland ML, Cummins P. Cardiac-specific troponin-I radioimmunoassay in the diagnosis of acute myocardial infarction. Am Heart J. 1987;113:1333–1344. doi: 10.1016/0002-8703(87)90645-4. [DOI] [PubMed] [Google Scholar]
- Cummins P, McGurk B, Littler WA. Radioimmunoassay of human cardiac tropomyosin in acute myocardial infarction. Clin Sci (Lond) 1981;60:251–259. doi: 10.1042/cs0600251. [DOI] [PubMed] [Google Scholar]
- Dada N, Kim NW, Wolfert RL. Lp-PLA2: an emerging biomarker of coronary heart disease. Expert Rev Mol Diagn. 2002;2:17–22. doi: 10.1586/14737159.2.1.17. [DOI] [PubMed] [Google Scholar]
- Dahlback B. The discovery of activated protein C resistance. J Thromb Haemost. 2003;1:3–9. doi: 10.1046/j.1538-7836.2003.00016.x. [DOI] [PubMed] [Google Scholar]
- Dahlback B. Progress in the understanding of the protein C anticoagulant pathway. Int J Hematol. 2004;79:109–116. doi: 10.1532/ijh97.03149. [DOI] [PubMed] [Google Scholar]
- Dangas G, Konstadoulakis MM, Epstein SE, Stefanadis CI, Kymionis GD, Toutouza MG, Liakos C, Sadaniantz A, Cohen AM, Chesebro JH, Toutouzas PK. Prevalence of autoantibodies against contractile proteins in coronary artery disease and their clinical implications. Am J Cardiol. 2000;85:870–872. doi: 10.1016/s0002-9149(99)00883-8. [DOI] [PubMed] [Google Scholar]
- Dass C, Fridland GH, Tinsley PW, Killmar JT, Desiderio DM. Characterization of beta-endorphin in human pituitary by fast atom bombardment mass spectrometry of trypsin-generated fragments. Int J Pept Protein Res. 1989;34:81–87. doi: 10.1111/j.1399-3011.1989.tb01494.x. [DOI] [PubMed] [Google Scholar]
- de Groot MJ, Muijtjens AM, Simoons ML, Hermens WT, Glatz JF. Assessment of coronary reperfusion in patients with myocardial infarction using fatty acid binding protein concentrations in plasma. Heart. 2001;85:278–285. doi: 10.1136/heart.85.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lemos JA, Morrow DA, Sabatine MS, Murphy SA, Gibson CM, Antman EM, McCabe CH, Cannon CP, Braunwald E. Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation. 2003;107:690–695. doi: 10.1161/01.cir.0000049742.68848.99. [DOI] [PubMed] [Google Scholar]
- Deng Y, Wu JT, Lloyd TL, Chi CL, Olah TV, Unger SE. High-speed gradient parallel liquid chromatography/tandem mass spectrometry with fully automated sample preparation for bioanalysis: 30 seconds per sample from plasma. Rapid Commun Mass Spectrom. 2002;16:1116–1123. doi: 10.1002/rcm.688. [DOI] [PubMed] [Google Scholar]
- Desiderio DM, Kai M. Preparation of stable isotope-incorporated peptide internal standards for field desorption mass spectrometry quantification of peptides in biologic tissue. Biomed Mass Spectrom. 1983;10:471–479. doi: 10.1002/bms.1200100806. [DOI] [PubMed] [Google Scholar]
- Diamantopoulos EJ, Andreadis EA, Vassilopoulos CV, Theodorides TG, Giannakopoulos NS, Chatzis NA, Christopoulou-Kokkinou VD. Increased plasma plasminogen activator inhibitor-1 levels: a possible marker of hypertensive target organ damage. Clin Exp Hypertens. 2003;25:1–9. doi: 10.1081/ceh-120017736. [DOI] [PubMed] [Google Scholar]
- Disthabanchong S, Gonzalez EA, Martin KJ. Soluble IL-6 receptor levels in patients on chronic hemodialysis. Clin Nephrol. 2002;58:289–295. doi: 10.5414/cnp58289. [DOI] [PubMed] [Google Scholar]
- Doherty NS, Littman BH, Reilly K, Swindell AC, Buss JM, Anderson NL. Analysis of changes in acute-phase plasma proteins in an acute inflammatory response and in rheumatoid arthritis using two-dimensional gel electrophoresis. Electrophoresis. 1998;19:355–363. doi: 10.1002/elps.1150190234. [DOI] [PubMed] [Google Scholar]
- Donatelli M, Scarpinato A, Bucalo ML, Russo V, Iraci T, Vassallo G. Stepwise increase in plasma insulin and C-peptide concentrations in obese, in obese hypertensive, and in obese hypertensive diabetic subjects. Diabetes Res. 1991;17:125–129. [PubMed] [Google Scholar]
- Dugi KA, Schmidt N, Brandauer K, Ramacher D, Fiehn W, Kreuzer J. Activity and concentration of lipoprotein lipase in post-heparin plasma and the extent of coronary artery disease. Atherosclerosis. 2002;163:127–134. doi: 10.1016/s0021-9150(01)00752-3. [DOI] [PubMed] [Google Scholar]
- Dziedzic T, Bartus S, Klimkowicz A, Motyl M, Slowik A, Szczudlik A. Intracerebral hemorrhage triggers interleukin-6 and interleukin-10 release in blood. Stroke. 2002;33:2334–2335. doi: 10.1161/01.str.0000027211.73567.fa. [DOI] [PubMed] [Google Scholar]
- Ebeling F, Petaja J, Alanko S, Hirvasniemi A, Holm T, Lahde M, Nuutila A, Pesonen H, Vahtera E, Rasi V. Infant stroke and beta-2-glycoprotein 1 antibodies: six cases. Eur J Pediatr. 2003;162:678–681. doi: 10.1007/s00431-003-1285-9. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, Rustenbeck HH, Breiter N, Jacob S, Knerlich F, Bohn M, Poser W, Ruther E, Kochen M, Gefeller O, Gleiter C, Wessel TC, De Ryck M, Itri L, Prange H, Cerami A, Brines M, Siren AL. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- Eldar-Geva T, Spitz IM, Groome NP, Margalioth EJ, Homburg R. Follistatin and activin A serum concentrations in obese and non-obese patients with polycystic ovary syndrome. Hum Reprod. 2001;16:2552–2556. doi: 10.1093/humrep/16.12.2552. [DOI] [PubMed] [Google Scholar]
- Elneihoum AM, Falke P, Axelsson L, Lundberg E, Lindgarde F, Ohlsson K. Leukocyte activation detected by increased plasma levels of inflammatory mediators in patients with ischemic cerebrovascular diseases. Stroke. 1996;27:1734–1738. doi: 10.1161/01.str.27.10.1734. [DOI] [PubMed] [Google Scholar]
- Elneihoum AM, Falke P, Hedblad B, Lindgarde F, Ohlsson K. Leukocyte activation in atherosclerosis: correlation with risk factors. Atherosclerosis. 1997;131:79–84. doi: 10.1016/s0021-9150(96)06077-7. [DOI] [PubMed] [Google Scholar]
- Erbas T, Erbas B, Kabakci G, Aksoyek S, Koray Z, Gedik O. Plasma big-endothelin levels, cardiac autonomic neuropathy, and cardiac functions in patients with insulin-dependent diabetes mellitus. Clin Cardiol. 2000;23:259–263. doi: 10.1002/clc.4960230407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon CT. Coagulation and inflammation. J Endotoxin Res. 2003;9:192–198. doi: 10.1179/096805103125001603. [DOI] [PubMed] [Google Scholar]
- Facer CA, Theodoridou A. Elevated plasma levels of P-selectin (GMP-140/CD62P) in patients with Plasmodium falciparum malaria. Microbiol Immunol. 1994;38:727–731. doi: 10.1111/j.1348-0421.1994.tb01848.x. [DOI] [PubMed] [Google Scholar]
- Falke P, Elneihoum AM, Ohlsson K. Leukocyte activation: relation to cardiovascular mortality after cerebrovascular ischemia. Cerebrovasc Dis. 2000;10:97–101. doi: 10.1159/000016037. [DOI] [PubMed] [Google Scholar]
- Fareed J, Hoppensteadt DA, Leya F, Iqbal O, Wolf H, Bick R. Useful laboratory tests for studying thrombogenesis in acute cardiac syndromes. Clin Chem. 1998;44:1845–1853. [PubMed] [Google Scholar]
- Farsak B, Gunaydin S, Yorgancioglu C, Zorlutuna Y. Elevated levels of s-100beta correlate with neurocognitive outcome after cardiac surgery. J Cardiovasc Surg (Torino) 2003;44:31–35. [PubMed] [Google Scholar]
- Fassbender K, Mossner R, Motsch L, Kischka U, Grau A, Hennerici M. Circulating selectin- and immunoglobulin-type adhesion molecules in acute ischemic stroke. Stroke. 1995;26:1361–1364. doi: 10.1161/01.str.26.8.1361. [DOI] [PubMed] [Google Scholar]
- Fierens C, Stockl D, Baetens D, De Leenheer AP, Thienpont LM. Standardization of C-peptide measurements in urine by method comparison with isotope-dilution mass spectrometry. Clin Chem. 2003;49:992–994. doi: 10.1373/49.6.992. [DOI] [PubMed] [Google Scholar]
- Fridman AI, Matveev SA, Agalakova NI, Fedorova OV, Lakatta EG, Bagrov AY. Marinobufagenin, an endogenous ligand of alpha-1 sodium pump, is a marker of congestive heart failure severity. J Hypertens. 2002;20:1189–1194. doi: 10.1097/00004872-200206000-00032. [DOI] [PubMed] [Google Scholar]
- Frijns CJ, Kappelle LJ, van Gijn J, Nieuwenhuis HK, Sixma JJ, Fijnheer R. Soluble adhesion molecules reflect endothelial cell activation in ischemic stroke and in carotid atherosclerosis. Stroke. 1997;28:2214–2218. doi: 10.1161/01.str.28.11.2214. [DOI] [PubMed] [Google Scholar]
- Fu ML, Herlitz H, Schulze W, Wallukat G, Micke P, Eftekhari P, Sjogren KG, Hjalmarson A, Muller-Esterl W, Hoebeke J. Autoantibodies against the angiotensin receptor (AT1) in patients with hypertension. J Hypertens. 2000;18:945–953. doi: 10.1097/00004872-200018070-00017. [DOI] [PubMed] [Google Scholar]
- Fujinami A, Obayashi H, Ohta K, Ichimura T, Nishimura M, Matsui H, Kawahara Y, Yamazaki M, Ogata M, Hasegawa G, Nakamura N, Yoshikawa T, Nakano K, Ohta M. Enzyme-linked immunosorbent assay for circulating human resistin: resistin concentrations in normal subjects and patients with type 2 diabetes. Clin Chim Acta. 2004;339:57–63. doi: 10.1016/j.cccn.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Galvani M, Ferrini D, Ottani F, Nanni C, Ramberti A, Amboni P, Iamele L, Vernocchi A, Nicolini FA. Soluble E-selectin is not a marker of unstable coronary plaque in serum of patients with ischemic heart disease. J Thromb Thrombolysis. 2000;9:53–60. doi: 10.1023/a:1018656530541. [DOI] [PubMed] [Google Scholar]
- Gechtman Z, Shaltiel S. Phosphorylation of vitronectin on Ser362 by protein kinase C attenuates its cleavage by plasmin. Eur J Biochem. 1997;243:493–501. doi: 10.1111/j.1432-1033.1997.0493a.x. [DOI] [PubMed] [Google Scholar]
- Getz GS, Reardon CA. Paraoxonase, a cardioprotective enzyme: continuing issues. Curr Opin Lipidol. 2004;15:261–267. doi: 10.1097/00041433-200406000-00005. [DOI] [PubMed] [Google Scholar]
- Glatz JF, van der Vusse GJ, Simoons ML, Kragten JA, van Dieijen-Visser MP, Hermens WT. Fatty acid-binding protein and the early detection of acute myocardial infarction. Clin Chim Acta. 1998;272:87–92. doi: 10.1016/s0009-8981(97)00255-6. [DOI] [PubMed] [Google Scholar]
- Glowinska B, Urban M, Koput A, Galar M. [Selected new atherosclerosis risk factors and markers of fibrinolysis in children and adolescents with obesity, hypertension and diabetes] Przegl Lek. 2003;60:12–17. [PubMed] [Google Scholar]
- Goetze JP, Videbaek R, Boesgaard S, Aldershvile J, Rehfeld JF, Carlsen J. Pro-brain natriuretic peptide as marker of cardiovascular or pulmonary causes of dyspnea in patients with terminal parenchymal lung disease. J Heart Lung Transplant. 2004;23:80–87. doi: 10.1016/s1053-2498(03)00060-3. [DOI] [PubMed] [Google Scholar]
- Goto T, Takase H, Toriyama T, Sugiura T, Kurita Y, Tsuru N, Masuda H, Hayashi K, Ueda R, Dohi Y. Increased circulating levels of natriuretic peptides predict future cardiac event in patients with chronic hemodialysis. Nephron. 2002;92:610–615. doi: 10.1159/000064100. [DOI] [PubMed] [Google Scholar]
- Green G, Dyce D, Gimovsky A, Lo DH. Automated approach to radioimmunoassays of somatotropin (human growth hormone) and insulin. Clin Chem. 1976;22:1510–1515. [PubMed] [Google Scholar]
- Grote L. [Influence of circadian rhythms on cardiovascular function] Internist (Berl) 2004;45:994–1005. doi: 10.1007/s00108-004-1258-8. [DOI] [PubMed] [Google Scholar]
- Guerin J, Smith O, White B, Sweetman G, Feighery C, Jackson J. Antibodies to prothrombin in antiphospholipid syndrome and inflammatory disorders. Br J Haematol. 1998;102:896–902. doi: 10.1046/j.1365-2141.1998.00876.x. [DOI] [PubMed] [Google Scholar]
- Hambsch J, Osmancik P, Bocsi J, Schneider P, Tarnok A. Neutrophil adhesion molecule expression and serum concentration of soluble adhesion molecules during and after pediatric cardiovascular surgery with or without cardiopulmonary bypass. Anesthesiology. 2002;96:1078–1085. doi: 10.1097/00000542-200205000-00009. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Matuo Y. A new stroke marker as detected by serum phosphoglycerate mutase B-type isozyme. Biochem Biophys Res Commun. 2001;287:843–845. doi: 10.1006/bbrc.2001.5666. [DOI] [PubMed] [Google Scholar]
- Hayden K, Tetlow L, Byrne G, Bundred N. Radioimmunoassay for the measurement of thrombospondin in plasma and breast cyst fluid: validation and clinical application. Ann Clin Biochem. 2000;37:319–325. doi: 10.1258/0004563001899212. [DOI] [PubMed] [Google Scholar]
- He M, Wen Z, He X, Xiong S, Liu F, Xu J, Li J, Xie Q, Jian Z, Chen F, Xiao B, Pu X, He S. Observation on tissue factor pathway and some other coagulation parameters during the onset of acute cerebrocardiac thrombotic diseases. Thromb Res. 2002;107:223–228. doi: 10.1016/s0049-3848(02)00331-6. [DOI] [PubMed] [Google Scholar]
- Heald AH, Cruickshank JK, Riste LK, Cade JE, Anderson S, Greenhalgh A, Sampayo J, Taylor W, Fraser W, White A, Gibson JM. Close relation of fasting insulin-like growth factor binding protein-1 (IGFBP-1) with glucose tolerance and cardiovascular risk in two populations. Diabetologia. 2001;44:333–339. doi: 10.1007/s001250051623. [DOI] [PubMed] [Google Scholar]
- Herrmann M, Vos P, Wunderlich MT, de Bruijn CH, Lamers KJ. Release of glial tissue-specific proteins after acute stroke: a comparative analysis of serum concentrations of protein S-100B and glial fibrillary acidic protein. Stroke. 2000;31:2670–2677. doi: 10.1161/01.str.31.11.2670. [DOI] [PubMed] [Google Scholar]
- Hirayama A, Arita M, Takagaki Y, Tsuji A, Kodama K, Inoue M. Clinical assessment of specific enzyme immunoassay for the human cardiac myosin light chain II (MLC II) with use of monoclonal antibodies. Clin Biochem. 1990;23:515–522. doi: 10.1016/0009-9120(90)80042-h. [DOI] [PubMed] [Google Scholar]
- Hofmann U, Rabitzsch G, Loster K, Handschack W, Noll F, Krause EG. Immunenzymometric assay for the heart specific glycogen phosphorylase BB in human serum using monoclonal antibodies. Biomed Biochim Acta. 1989;48:S132–S136. [PubMed] [Google Scholar]
- Hogasen K, Mollnes TE, Tschopp J, Harboe M. Quantitation of vitronectin and clusterin. Pitfalls and solutions in enzyme immunoassays for adhesive proteins. J Immunol Meth. 1993;160:107–115. doi: 10.1016/0022-1759(93)90014-x. [DOI] [PubMed] [Google Scholar]
- Hultberg B, Isaksson A, Nilsson JA, Lindgarde F. Serum beta-hexosaminidase isoenzymes are related to risk factors for atherosclerosis in a large population of postmenopausal women. Clin Chim Acta. 1994;227:59–68. doi: 10.1016/0009-8981(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Ince B, Bayram C, Harmanci H, Ulutin T. Hemostatic markers in ischemic stroke of undetermined etiology. Thromb Res. 1999;96:169–174. doi: 10.1016/s0049-3848(99)00097-3. [DOI] [PubMed] [Google Scholar]
- Itabe H. [Atherosclerosis and sensitive determination of oxidized LDL using monoclonal antibody] Yakugaku Zasshi. 2002;122:745–753. doi: 10.1248/yakushi.122.745. [DOI] [PubMed] [Google Scholar]
- Jaffe AS. New standard for the diagnosis of acute myocardial infarction. Cardiol Rev. 2001;9:318–322. doi: 10.1097/00045415-200111000-00006. [DOI] [PubMed] [Google Scholar]
- Jakob M. Normal Values Pocket. Ashland, OH, USA: Börm Bruckmeier Publishing; 2002. [Google Scholar]
- Jansson JH, Nilsson TK, Johnson O. von Willebrand factor, tissue plasminogen activator, and dehydroepiandrosterone sulphate predict cardiovascular death in a 10 year follow up of survivors of acute myocardial infarction. Heart. 1998;80:334–337. doi: 10.1136/hrt.80.4.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellum E, Bjornson I, Nesbakken R, Johansson E, Wold S. Classification of human cancer cells by means of capillary gas chromatography and pattern recognition analysis. J Chromatogr. 1981;217:231–237. doi: 10.1016/s0021-9673(00)88077-2. [DOI] [PubMed] [Google Scholar]
- Jeppsson JO, Kobold U, Barr J, Finke A, Hoelzel W, Hoshino T, Miedema K, Mosca A, Mauri P, Paroni R, Thienpont L, Umemoto M, Weykamp C. Approved IFCC reference method for the measurement of HbA1c in human blood. Clin Chem Lab Med. 2002;40:78–89. doi: 10.1515/CCLM.2002.016. [DOI] [PubMed] [Google Scholar]
- Ji QC, Rodila R, Gage EM, El-Shourbagy TA. A strategy of plasma protein quantitation by selective reaction monitoring of an intact protein. Anal Chem. 2003;75:7008–7014. doi: 10.1021/ac034930n. [DOI] [PubMed] [Google Scholar]
- Ji QC, Rodila R, Gage EM, El-Shourbagy TA. Mass spectrometric approaches for protein quantitation in drug development. Am Pharma Rev. 2004 May/June, 2004. [Google Scholar]
- Johansson L, Jansson JH, Boman K, Nilsson TK, Stegmayr B, Hallmans G. Tissue plasminogen activator, plasminogen activator inhibitor-1, and tissue plasminogen activator/plasminogen activator inhibitor-1 complex as risk factors for the development of a first stroke. Stroke. 2000;31:26–32. doi: 10.1161/01.str.31.1.26. [DOI] [PubMed] [Google Scholar]
- Joos TO, Stoll D, Templin MF. Miniaturised multiplexed immunoassays. Curr Opin Chem Biol. 2002;6:76–80. doi: 10.1016/s1367-5931(01)00289-7. [DOI] [PubMed] [Google Scholar]
- Jurk K, Clemetson KJ, de Groot PG, Brodde MF, Steiner M, Savion N, Varon D, Sixma JJ, Van Aken H, Kehrel BE. Thrombospondin-1 mediates platelet adhesion at high shear via glycoprotein Ib (GPIb): an alternative/backup mechanism to von Willebrand factor. Faseb J. 2003;17:1490–1492. doi: 10.1096/fj.02-0830fje. [DOI] [PubMed] [Google Scholar]
- Kalafatis M, Egan JO, Van't Veer C, Cawthern KM, Mann KG. The regulation of clotting factors. Crit Rev Eukaryot Gene Expr. 1997;7:241–280. doi: 10.1615/critreveukargeneexpr.v7.i3.40. [DOI] [PubMed] [Google Scholar]
- Kannel WB, D'Agostino RB, Belanger AJ. Update on fibrinogen as a cardiovascular risk factor. Ann Epidemiol. 1992;2:457–466. doi: 10.1016/1047-2797(92)90095-8. [DOI] [PubMed] [Google Scholar]
- Kanters SD, Banga JD, Algra A, Frijns RC, Beutler JJ, Fijnheer R. Plasma levels of cellular fibronectin in diabetes. Diabetes Care. 2001;24:323–327. doi: 10.2337/diacare.24.2.323. [DOI] [PubMed] [Google Scholar]
- Kastelein JJ, Jukema JW, Zwinderman AH, Clee S, van Boven AJ, Jansen H, Rabelink TJ, Peters RJ, Lie KI, Liu G, Bruschke AV, Hayden MR. Lipoprotein lipase activity is associated with severity of angina pectoris. REGRESS Study Group. Circulation. 2000;102:1629–1633. doi: 10.1161/01.cir.102.14.1629. [DOI] [PubMed] [Google Scholar]
- Katona E, Haramura G, Karpati L, Fachet J, Muszbek L. A simple, quick one-step ELISA assay for the determination of complex plasma factor XIII (A2B2) Thromb Haemost. 2000;83:268–273. [PubMed] [Google Scholar]
- Kawanami D, Maemura K, Takeda N, Harada T, Nojiri T, Imai Y, Manabe I, Utsunomiya K, Nagai R. Direct reciprocal effects of resistin and adiponectin on vascular endothelial cells: a new insight into adipocytokine–endothelial cell interactions. Biochem Biophys Res Commun. 2004;314:415–419. doi: 10.1016/j.bbrc.2003.12.104. [DOI] [PubMed] [Google Scholar]
- Kiernan UA, Tubbs KA, Nedelkov D, Niederkofler EE, McConnell E, Nelson RW. Comparative urine protein phenotyping using mass spectrometric immunoassay. J Proteome Res. 2003;2:191–197. doi: 10.1021/pr025574c. [DOI] [PubMed] [Google Scholar]
- Kim CH, Park JY, Kim JY, Choi CS, Kim YI, Chung YE, Lee MS, Hong SK, Lee KU. Elevated serum ceruloplasmin levels in subjects with metabolic syndrome: a population-based study. Metabolism. 2002;51:838–842. doi: 10.1053/meta.2002.33348. [DOI] [PubMed] [Google Scholar]
- Kini AS, Lee P, Marmur JD, Agarwal A, Duffy ME, Kim MC, Sharma SK. Correlation of postpercutaneous coronary intervention creatine kinase-MB and troponin I elevation in predicting mid-term mortality. Am J Cardiol. 2004;93:18–23. doi: 10.1016/j.amjcard.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Kippen AD, Cerini F, Vadas L, Stocklin R, Vu L, Offord RE, Rose K. Development of an isotope dilution assay for precise determination of insulin, C-peptide, and proinsulin levels in non-diabetic and type II diabetic individuals with comparison to immunoassay. J Biol Chem. 1997;272:12513–12522. doi: 10.1074/jbc.272.19.12513. [DOI] [PubMed] [Google Scholar]
- Kleine AH, Glatz JF, Van Nieuwenhoven FA, Van der Vusse GJ. Release of heart fatty acid-binding protein into plasma after acute myocardial infarction in man. Mol Cell Biochem. 1992;116:155–162. doi: 10.1007/BF01270583. [DOI] [PubMed] [Google Scholar]
- Koenig W. Fibrin(ogen) in cardiovascular disease: an update. Thromb Haemost. 2003;89:601–609. [PubMed] [Google Scholar]
- Kondo K, Allan C, Fidge N. Quantitation of apolipoprotein A-IV in human plasma using a competitive enzyme-linked immunosorbent assay. J Lipid Res. 1989;30:939–944. [PubMed] [Google Scholar]
- Kos J, Nielsen HJ, Krasovec M, Christensen IJ, Cimerman N, Stephens RW, Brunner N. Prognostic values of cathepsin B and carcinoembryonic antigen in sera of patients with colorectal cancer. Clin Cancer Res. 1998;4:1511–1516. [PubMed] [Google Scholar]
- Kostiainen R, Kotiaho T, Kuuranne T, Auriola S. Liquid chromatography/atmospheric pressure ionization-mass spectrometry in drug metabolism studies. J Mass Spectrom. 2003;38:357–372. doi: 10.1002/jms.481. [DOI] [PubMed] [Google Scholar]
- Krassas GE, Papadopoulou P, Koliakos G, Konstantinidis T, Kalothetou K. Growth hormone, insulin growth factor-1, and igf binding protein-3 axis relationship with bone mineral density among healthy men. Arch Androl. 2003;49:191–199. doi: 10.1080/01485010390196724. [DOI] [PubMed] [Google Scholar]
- Kugiyama K, Ota Y, Takazoe K, Moriyama Y, Kawano H, Miyao Y, Sakamoto T, Soejima H, Ogawa H, Doi H, Sugiyama S, Yasue H. Circulating levels of secretory type II phospholipase A(2) predict coronary events in patients with coronary artery disease. Circulation. 1999;100:1280–1284. doi: 10.1161/01.cir.100.12.1280. [DOI] [PubMed] [Google Scholar]
- Kuhn E, Wu J, Karl J, Liao H, Zolg W, Guild B. Quantification of C-reactive protein in the serum of patients with rheumatoid arthritis using multiple reaction monitoring mass spectrometry and 13C-labeled peptide standards. Proteomics. 2004;4:1175–1186. doi: 10.1002/pmic.200300670. [DOI] [PubMed] [Google Scholar]
- Kujiraoka T, Oka T, Ishihara M, Egashira T, Fujioka T, Saito E, Saito S, Miller NE, Hattori H. A sandwich enzyme-linked immunosorbent assay for human serum paraoxonase concentration. J Lipid Res. 2000;41:1358–1363. [PubMed] [Google Scholar]
- Kurosawa S, Stearns-Kurosawa DJ, Hidari N, Esmon CT. Identification of functional endothelial protein C receptor in human plasma. J Clin Invest. 1997;100:411–418. doi: 10.1172/JCI119548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labugger R, Simpson JA, Quick M, Brown HA, Collier CE, Neverova I, Van Eyk JE. Strategy for analysis of cardiac troponins in biological samples with a combination of affinity chromatography and mass spectrometry. Clin Chem. 2003;49:873–879. doi: 10.1373/49.6.873. [DOI] [PubMed] [Google Scholar]
- Laurell M, Christensson A, Abrahamsson PA, Stenflo J, Lilja H. Protein C inhibitor in human body fluids. Seminal plasma is rich in inhibitor antigen deriving from cells throughout the male reproductive system. J Clin Invest. 1992;89:1094–1101. doi: 10.1172/JCI115689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie L, Kraiczi H, Hefetz A, Ghandour H, Perelman A, Hedner J, Lavie P. Plasma vascular endothelial growth factor in sleep apnea syndrome: effects of nasal continuous positive air pressure treatment. Am J Respir Crit Care Med. 2002;165:1624–1628. doi: 10.1164/rccm.20110-040OC. [DOI] [PubMed] [Google Scholar]
- Lee SC, Stevens TL, Sandberg SM, Heublein DM, Nelson SM, Jougasaki M, Redfield MM, Burnett JC., Jr The potential of brain natriuretic peptide as a biomarker for New York Heart Association class during the outpatient treatment of heart failure. J Card Fail. 2002;8:149–154. doi: 10.1054/jcaf.2002.125368. [DOI] [PubMed] [Google Scholar]
- Leyva F, Anker SD, Godsland IF, Teixeira M, Hellewell PG, Kox WJ, Poole-Wilson PA, Coats AJ. Uric acid in chronic heart failure: a marker of chronic inflammation. Eur Heart J. 1998;19:1814–1822. doi: 10.1053/euhj.1998.1188. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang Y, Qi L. [Determination of serum soluble interleukin-6 receptor and soluble gp130 levels in patient with pregnancy induced hypertension and its significance] Zhonghua Fu Chan Ke Za Zhi. 2001a;36:18–19. [PubMed] [Google Scholar]
- Li CG, Wilson PB, Bernabeu C, Raab U, Wang JM, Kumar S. Immunodetection and characterisation of soluble CD105-TGFbeta complexes. J Immunol Meth. 1998;218:85–93. doi: 10.1016/s0022-1759(98)00118-5. [DOI] [PubMed] [Google Scholar]
- Li YN, Yuan H, Zhang MX. [Study on endothelial dysfunction and platelet activation in elderly hypertensive patients complicated with cerebral infarction] Hunan Yi Ke Da Xue Xue Bao. 2001b;26:143–145. [PubMed] [Google Scholar]
- Lichy C, Kropp S, Dong-Si T, Genius J, Dolan T, Hampe T, Stoll F, Reuner K, Grond-Ginsbach C, Grau A. A common polymorphism of the protein Z gene is associated with protein Z plasma levels and with risk of cerebral ischemia in the young. Stroke. 2004;35:40–45. doi: 10.1161/01.STR.0000106909.75418.E4. [DOI] [PubMed] [Google Scholar]
- Lisek CA, Bailey JE, Benson LM, Yaksh TL, Jardine I. Quantitation of endogenous substance P by on-line microcolumn liquid chromatography/continuous-flow fast-atom bombardment mass spectrometry. Rapid Commun Mass Spectrom. 1989;3:43–46. doi: 10.1002/rcm.1290030211. [DOI] [PubMed] [Google Scholar]
- Lombardi R, Betocchi S, Losi MA, Tocchetti CG, Aversa M, Miranda M, D'Alessandro G, Cacace A, Ciampi Q, Chiariello M. Myocardial collagen turnover in hypertrophic cardiomyopathy. Circulation. 2003;108:1455–1460. doi: 10.1161/01.CIR.0000090687.97972.10. [DOI] [PubMed] [Google Scholar]
- Lopez B, Querejeta R, Varo N, Gonzalez A, Larman M, Martinez Ubago JL, Diez J. Usefulness of serum carboxy-terminal propeptide of procollagen type I in assessment of the cardioreparative ability of antihypertensive treatment in hypertensive patients. Circulation. 2001;104:286–291. doi: 10.1161/01.cir.104.3.286. [DOI] [PubMed] [Google Scholar]
- Lowe GD. The relationship between infection, inflammation, and cardiovascular disease: an overview. Ann Periodontol. 2001;6:1–8. doi: 10.1902/annals.2001.6.1.1. [DOI] [PubMed] [Google Scholar]
- Lu KC, Wang JY, Lin SH, Chu P, Lin YF. Role of circulating cytokines and chemokines in exertional heatstroke. Crit Care Med. 2004;32:399–403. doi: 10.1097/01.CCM.0000108884.74110.D9. [DOI] [PubMed] [Google Scholar]
- Luo J, Liu B. [ELISA for measurement of human serum apolipoprotein A II] Hua Xi Yi Ke Da Xue Xue Bao. 1994;25:229–232. [PubMed] [Google Scholar]
- Luria MH, Erel J, Sapoznikov D, Gotsman MS. Cardiovascular risk factor clustering and ratio of total cholesterol to high-density lipoprotein cholesterol in angiographically documented coronary artery disease. Am J Cardiol. 1991;67:31–36. doi: 10.1016/0002-9149(91)90094-2. [DOI] [PubMed] [Google Scholar]
- McKenzie ME, Pothula A, Gurbel PA, Fuzaylov SY, O'Connor CM, Gattis WA, Serebruany VL. Failure of thrombin generation markers to triage patients presenting with chest pain. Cardiology. 1999;92:53–58. doi: 10.1159/000006946. [DOI] [PubMed] [Google Scholar]
- McLaren M, Alkaabi J, Connacher M, Belch JJ, Valenete E. Activated factor XII in rheumatoid arthritis. Rheumatol Int. 2002;22:182–184. doi: 10.1007/s00296-002-0219-6. [DOI] [PubMed] [Google Scholar]
- Maeda K, Tsutamoto T, Wada A, Hisanaga T, Kinoshita M. Plasma brain natriuretic peptide as a biochemical marker of high left ventricular end-diastolic pressure in patients with symptomatic left ventricular dysfunction. Am Heart J. 1998;135:825–832. doi: 10.1016/s0002-8703(98)70041-9. [DOI] [PubMed] [Google Scholar]
- Magera MJ, Lacey JM, Casetta B, Rinaldo P. Method for the determination of total homocysteine in plasma and urine by stable isotope dilution and electrospray tandem mass spectrometry. Clin Chem. 1999;45:1517–1522. [PubMed] [Google Scholar]
- Makin AJ, Chung NA, Silverman SH, Lip GY. Vascular endothelial growth factor and tissue factor in patients with established peripheral artery disease: a link between angiogenesis and thrombogenesis? Clin Sci (Lond) 2003;104:397–404. doi: 10.1042/CS20020182. [DOI] [PubMed] [Google Scholar]
- Malaguarnera M, Giugno I, Trovato BA, Panebianco MP, Restuccia N, Ruello P. Lipoprotein(a) in cirrhosis. A new index of liver functions? Curr Med Res Opin. 1996;13:479–485. doi: 10.1185/03007999609115228. [DOI] [PubMed] [Google Scholar]
- Mallamaci F, Zoccali C, Cuzzola F, Tripepi G, Cutrupi S, Parlongo S, Tanaka S, Ouchi N, Kihara S, Funahashi T, Matsuzawa Y. Adiponectin in essential hypertension. J Nephrol. 2002;15:507–511. [PubMed] [Google Scholar]
- Mallat Z, Henry P, Fressonnet R, Alouani S, Scoazec A, Beaufils P, Chvatchko Y, Tedgui A. Increased plasma concentrations of interleukin-18 in acute coronary syndromes. Heart. 2002;88:467–469. doi: 10.1136/heart.88.5.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann KG, Butenas S, Brummel K. The dynamics of thrombin formation. Arterioscler Thromb Vasc Biol. 2003;23:17–25. doi: 10.1161/01.atv.0000046238.23903.fc. [DOI] [PubMed] [Google Scholar]
- Marchal E, Montagne P, Cuilliere ML, Bene MC, Faure G. Microparticle-enhanced nephelometric immunoassay of human plasminogen. J Clin Lab Anal. 1996;10:85–90. doi: 10.1002/(SICI)1098-2825(1996)10:2<85::AID-JCLA5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Marin F, Roldan V, Climent V, Garcia A, Marco P, Lip GY. Is thrombogenesis in atrial fibrillation related to matrix metalloproteinase-1 and its inhibitor, TIMP-1? Stroke. 2003;34:1181–1186. doi: 10.1161/01.STR.0000065431.76788.D9. [DOI] [PubMed] [Google Scholar]
- Masaki Y, Oka N, Furuya H, Ohguni S, Takagi C, Sato T, Notsu K, Kato Y. [Clinical use of serum erythropoietin determination by the recombigen EPO RIA kit] Kaku Igaku. 1992;29:701–707. [PubMed] [Google Scholar]
- Masson S, Gorini M, Salio M, Lucci D, Latini R, Maggioni AP. Clinical correlates of elevated plasma natriuretic peptides and Big endothelin-1 in a population of ambulatory patients with heart failure. A substudy of the Italian Network on Congestive Heart Failure (IN-CHF) registry. IN-CHF Investigators. Ital Heart J. 2000;1:282–288. [PubMed] [Google Scholar]
- Matsumori A, Miyazaki S, Takano H, Ono K, Okada M, Miyamoto T, Nonogi H, Daikoku S, Mitsudo K, Matsunaga Y, Ohnishi T, Daikuhara Y, Sasayama S. Circulating hepatocyte growth factor as a marker of thrombus formation in unstable angina pectoris. Jpn Circ J. 2000;64:805–807. doi: 10.1253/jcj.64.805. [DOI] [PubMed] [Google Scholar]
- Menon V, Wang X, Greene T, Beck GJ, Kusek JW, Marcovina SM, Levey AS, Sarnak MJ. Relationship between C-reactive protein, albumin, and cardiovascular disease in patients with chronic kidney disease. Am J Kidney Dis. 2003;42:44–52. doi: 10.1016/s0272-6386(03)00407-4. [DOI] [PubMed] [Google Scholar]
- Miyata M, Biro S, Kaieda H, Eto H, Orihara K, Kihara T, Obata H, Matsushita N, Matsuyama T, Tei C. Apolipoprotein J/clusterin is induced in vascular smooth muscle cells after vascular injury. Circulation. 2001;104:1407–1412. doi: 10.1161/hc3701.095583. [DOI] [PubMed] [Google Scholar]
- Mizia-Stec K, Gasior Z, Zahorska-Markiewicz B, Janowska J, Szulc A, Jastrzebska-Maj E, Kobielusz-Gembala I. Serum tumour necrosis factor-alpha, interleukin-2 and interleukin-10 activation in stable angina and acute coronary syndromes. Coron Artery Dis. 2003;14:431–438. doi: 10.1097/00019501-200309000-00003. [DOI] [PubMed] [Google Scholar]
- Monge JC. Neurohormonal markers of clinical outcome in cardiovascular disease: is endothelin the best one? J Cardiovasc Pharmacol. 1998;32(Suppl. 2):S36–S42. doi: 10.1097/00005344-199800004-00006. [DOI] [PubMed] [Google Scholar]
- Muscari A, Bozzoli C, Massarelli G, Puddu GM, Palareti G, Legnani C, D'Atena T, Mazzuca A, Miniero R, Toscano V, et al. Complement components and fibrinogen: correlations and association with previous myocardial infarction. Cardiology. 1995;86:232–237. doi: 10.1159/000176879. [DOI] [PubMed] [Google Scholar]
- Muscari A, Massarelli G, Bastagli L, Poggiopollini G, Tomassetti V, Drago G, Martignani C, Pacilli P, Boni P, Puddu P. Relationship of serum C3 to fasting insulin, risk factors and previous ischaemic events in middle-aged men. Eur Heart J. 2000;21:1081–1090. doi: 10.1053/euhj.1999.2013. [DOI] [PubMed] [Google Scholar]
- Nedelkov D, Tubbs KA, Niederkofler EE, Kiernan UA, Nelson RW. High-throughput comprehensive analysis of human plasma proteins: a step toward population proteomics. Anal Chem. 2004;76:1733–1737. doi: 10.1021/ac035105+. [DOI] [PubMed] [Google Scholar]
- Nelson RW, Nedelkov D, Tubbs KA, Kiernan UA. Quantitative mass spectrometric immunoassay of insulin like growth factor 1. J Proteome Res. 2004;3:851–855. doi: 10.1021/pr0499388. [DOI] [PubMed] [Google Scholar]
- Nepomuceno AI, Mason CJ, Muddiman DC, Bergen HR, 3rd, Zeldenrust SR. Detection of genetic variants of transthyretin by liquid chromatography-dual electrospray ionization fourier-transform ion-cyclotron-resonance mass spectrometry. Clin Chem. 2004;50:1535–1543. doi: 10.1373/clinchem.2004.033274. [DOI] [PubMed] [Google Scholar]
- Niessner A, Graf S, Nikfardjam M, Speidl WS, Huber-Beckmann R, Zorn G, Wojta J, Huber K. Circulating t-PA antigen predicts major adverse coronary events in patients with stable coronary artery disease – a 13-year follow-up. Thromb Haemost. 2003;90:344–350. doi: 10.1160/TH02-10-0185. [DOI] [PubMed] [Google Scholar]
- Noji Y, Kajinami K, Kawashiri MA, Todo Y, Horita T, Nohara A, Higashikata T, Inazu A, Koizumi J, Takegoshi T, Mabuchi H. Circulating matrix metalloproteinases and their inhibitors in premature coronary atherosclerosis. Clin Chem Lab Med. 2001;39:380–384. doi: 10.1515/CCLM.2001.060. [DOI] [PubMed] [Google Scholar]
- Noji Y, Shimizu M, Ino H, Higashikata T, Yamaguchi M, Nohara A, Horita T, Shimizu K, Ito Y, Matsuda T, Namura M, Mabuchi H. Increased circulating matrix metalloproteinase-2 in patients with hypertrophic cardiomyopathy with systolic dysfunction. Circ J. 2004;68:355–360. doi: 10.1253/circj.68.355. [DOI] [PubMed] [Google Scholar]
- Nomura F, Ihara A, Yoshitatsu M, Tamura K, Katayama A, Ihara K. Relationship between coagulation cascade, cytokine, adhesion molecule and aortic aneurysm. Eur J Cardiothorac Surg. 2003;23:1034. doi: 10.1016/s1010-7940(03)00156-8. discussion 1038–1038. [DOI] [PubMed] [Google Scholar]
- Nomura M, Kato K, Nagasaka A, Shiga Y, Miyagi Y, Fukui R, Nakano H, Abo Y, Okajima S, Nakai A, et al. Serum beta-enolase in acute myocardial infarction. Br Heart J. 1987;58:29–33. doi: 10.1136/hrt.58.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak J, Rozentryt P, Szewczyk M, Gierlotka M, Duszanska A, Szygula B, Wojnicz R, Hawranek M, Polonski L, Zembala M. [Tumor necrosis factor receptors sTNF-RI and sTNF-RII in advanced chronic heart failure] Pol Arch Med Wewn. 2002;107:223–229. [PubMed] [Google Scholar]
- Ogawa H, Soejima H, Takazoe K, Miyamoto S, Kajiwara I, Shimomura H, Sakamoto T, Yoshimura M, Kugiyama K, Kimura M, Yasue H. Increased autoantibodies against oxidized low-density lipoprotein in coronary circulation in patients with coronary spastic angina. Angiology. 2001;52:167–174. doi: 10.1177/000331970105200302. [DOI] [PubMed] [Google Scholar]
- Oh SH, Lee JG, Na SJ, Park JH, Kim WJ. The effect of initial serum neuron-specific enolase level on clinical outcome in acute carotid artery territory infarction. Yonsei Med J. 2002;43:357–362. doi: 10.3349/ymj.2002.43.3.357. [DOI] [PubMed] [Google Scholar]
- Oh JC, Wu W, Tortolero-Luna G, Broaddus R, Gershenson DM, Burke TW, Schmandt R, Lu KH. Increased plasma levels of insulin-like growth factor 2 and insulin-like growth factor binding protein 3 are associated with endometrial cancer risk. Cancer Epidemiol Biomarkers Prev. 2004;13:748–752. [PubMed] [Google Scholar]
- Ohkura N, Soe G, Kohno I, Kumeda K, Wada H, Kamikubo Y, Shiku H, Kato H. Monoclonal antibody specific for tissue factor pathway inhibitor-factor Xa complex: its characterization and application to plasmas from patients with disseminated intravascular coagulation and pre-disseminated intravascular coagulation. Blood Coagul Fibrinolysis. 1999;10:309–319. [PubMed] [Google Scholar]
- Ohtsuka T, Hamada M, Sasaki O, Suzuki M, Hara Y, Shigematsu Y, Ohtani T, Honda T, Hiwada K. Clinical implications of circulating soluble Fas and Fas ligand in patients with acute myocardial infarction. Coron Artery Dis. 1999;10:221–225. doi: 10.1097/00019501-199906000-00003. [DOI] [PubMed] [Google Scholar]
- Oishi M, Mochizuki Y, Shikata E. Single lacunar brain infarction with transient signs versus those with long-lasting signs. Int Angiol. 1999;18:206–209. [PubMed] [Google Scholar]
- Onat A, Hergenc G, Sansoy V, Fobker M, Ceyhan K, Toprak S, Assmann G. Apolipoprotein C-III, a strong discriminant of coronary risk in men and a determinant of the metabolic syndrome in both genders. Atherosclerosis. 2003;168:81–89. doi: 10.1016/s0021-9150(03)00025-x. [DOI] [PubMed] [Google Scholar]
- Orus J, Roig E, Perez-Villa F, Pare C, Azqueta M, Filella X, Heras M, Sanz G. Prognostic value of serum cytokines in patients with congestive heart failure. J Heart Lung Transplant. 2000;19:419–425. doi: 10.1016/s1053-2498(00)00083-8. [DOI] [PubMed] [Google Scholar]
- Oshitani N, Kitano A, Nakamura S, Obata A, Hashimura H, Hiki M, Matsumoto T, Okawa K, Kobayashi K. Dissociation between the functional activity and immunoreactive concentration of C1 esterase inhibitor in active and quiescent Crohn's disease. Scand J Gastroenterol. 1988;23:973–976. doi: 10.3109/00365528809090156. [DOI] [PubMed] [Google Scholar]
- Osmancik P, Hambsch J, Schneider P, Bellinghausen W, Tarnok A. Soluble endothelial adhesion molecules during paediatric cardiovascular surgery with or without cardiopulmonary bypass. Cardiol Young. 2002;12:130–137. doi: 10.1017/s1047951102000288. [DOI] [PubMed] [Google Scholar]
- Ottani F, Galvani M. Prognostic role of hemostatic markers in acute coronary syndromes patients. Clin Chim Acta. 2001;311:33–39. doi: 10.1016/s0009-8981(01)00555-1. [DOI] [PubMed] [Google Scholar]
- Park CW, Shin YS, Kim CM, Lee SY, Yu SE, Kim SY, Choi EJ, Chang YS, Bang BK. Increased C-reactive protein following hemodialysis predicts cardiac hypertrophy in chronic hemodialysis patients. Am J Kidney Dis. 2002;40:1230–1239. doi: 10.1053/ajkd.2002.36891. [DOI] [PubMed] [Google Scholar]
- Patti G, Di Sciascio G, D'Ambrosio A, Dicuonzo G, Abbate A, Dobrina A. Prognostic value of interleukin-1 receptor antagonist in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2002;89:372–376. doi: 10.1016/s0002-9149(01)02254-8. [DOI] [PubMed] [Google Scholar]
- Petricoin EF, Ardekani AM, Hitt BA, Levine PJ, Fusaro VA, Steinberg SM, Mills GB, Simone C, Fishman DA, Kohn EC, Liotta LA. Use of proteomic patterns in serum to identify ovarian cancer. Lancet. 2002;359:572–577. doi: 10.1016/S0140-6736(02)07746-2. [DOI] [PubMed] [Google Scholar]
- Phillips DJ, Jones KL, McGaw DJ, Groome NP, Smolich JJ, Parsson H, de Kretser DM. Release of activin and follistatin during cardiovascular procedures is largely due to heparin administration. J Clin Endocrinol Metab. 2000;85:2411–2415. doi: 10.1210/jcem.85.7.6666. [DOI] [PubMed] [Google Scholar]
- Pieper R, Gatlin CL, Makusky AJ, Russo PS, Schatz CR, Miller SS, Su Q, McGrath AM, Estock MA, Parmar PP, Zhao M, Huang ST, Zhou J, Wang F, Esquer-Blasco R, Anderson NL, Taylor J, Steiner S. The human serum proteome: Display of nearly 3700 chromatographically separated protein spots on two-dimensional electrophoresis gels and identification of 325 distinct proteins. Proteomics. 2003a;3:1345–1364. doi: 10.1002/pmic.200300449. [DOI] [PubMed] [Google Scholar]
- Pieper R, Su Q, Gatlin CL, Huang ST, Anderson NL, Steiner S. Multi-component immunoaffinity subtraction chromatography: An innovative step towards a comprehensive survey of the human plasma proteome. Proteomics. 2003b;3:422–432. doi: 10.1002/pmic.200390057. [DOI] [PubMed] [Google Scholar]
- Poulsen SH, Host NB, Jensen SE, Egstrup K. Relationship between serum amino-terminal propeptide of type III procollagen and changes of left ventricular function after acute myocardial infarction. Circulation. 2000;101:1527–1532. doi: 10.1161/01.cir.101.13.1527. [DOI] [PubMed] [Google Scholar]
- Putnam FW. The Plasma Proteins Structure, Function, and Genetic Control. New York: Academic Press; 1975. [Google Scholar]
- Quinn KA, Grimsley PG, Dai YP, Tapner M, Chesterman CN, Owensby DA. Soluble low density lipoprotein receptor-related protein (LRP) circulates in human plasma. J Biol Chem. 1997;272:23946–23951. doi: 10.1074/jbc.272.38.23946. [DOI] [PubMed] [Google Scholar]
- Ren J, Samson WK, Sowers JR. Insulin-like growth factor I as a cardiac hormone: physiological and pathophysiological implications in heart disease. J Mol Cell Cardiol. 1999;31:2049–2061. doi: 10.1006/jmcc.1999.1036. [DOI] [PubMed] [Google Scholar]
- Reynolds MA, Kirchick HJ, Dahlen JR, Anderberg JM, McPherson PH, Nakamura KK, Laskowitz DT, Valkirs GE, Buechler KF. Early biomarkers of stroke. Clin Chem. 2003;49:1733–1739. doi: 10.1373/49.10.1733. [DOI] [PubMed] [Google Scholar]
- Riddell LJ, Chisholm A, Williams S, Mann JI. Dietary strategies for lowering homocysteine concentrations. Am J Clin Nutr. 2000;71:1448–1454. doi: 10.1093/ajcn/71.6.1448. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation. 1998;97:425–428. doi: 10.1161/01.cir.97.5.425. [DOI] [PubMed] [Google Scholar]
- Riesen WF, Sturzenegger E. Enzyme-linked immunosorbent assay for apolipoprotein C-I. J Clin Chem Clin Biochem. 1986;24:723–727. doi: 10.1515/cclm.1986.24.10.723. [DOI] [PubMed] [Google Scholar]
- Rifai N, Ridker PM. Population distributions of C-reactive protein in apparently healthy men and women in the United States: implication for clinical interpretation. Clin Chem. 2003;49:666–669. doi: 10.1373/49.4.666. [DOI] [PubMed] [Google Scholar]
- Robertson EA, Van Steirteghem AC, Byrkit JE, Young DS. Biochemical individuality and the recognition of personal profiles with a computer. Clin Chem. 1980;26:30–36. [PubMed] [Google Scholar]
- Romisch J, Vermohlen S, Feussner A, Stohr H. The FVII activating protease cleaves single-chain plasminogen activators. Haemostasis. 1999;29:292–299. doi: 10.1159/000022515. [DOI] [PubMed] [Google Scholar]
- Romuk E, Skrzep-Poloczek B, Wojciechowska C, Tomasik A, Birkner E, Wodniecki J, Gabrylewicz B, Ochala A, Tendera M. Selectin-P and interleukin-8 plasma levels in coronary heart disease patients. Eur J Clin Invest. 2002;32:657–661. doi: 10.1046/j.1365-2362.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- Rosano TG, Sanders LA, Johnson ES, Kenny MA, Clayson KJ, Strandjord PE. Myoglobin concentrations and muscle-enzyme activities in serum after myocardial infarction and cardiac arrhythmia. Clin Chem. 1977;23:868–870. [PubMed] [Google Scholar]
- Ryan TJ, Anderson JL, Antman EM, Braniff BA, Brooks NH, Califf RM, Hillis LD, Hiratzka LF, Rapaport E, Riegel BJ, Russell RO, Smith EE, Jr, Weaver WD. ACC/AHA guidelines for the management of patients with acute myocardial infarction. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Acute Myocardial Infarction) J Am Coll Cardiol. 1996;28:1328–1428. doi: 10.1016/s0735-1097(96)00392-0. [DOI] [PubMed] [Google Scholar]
- Saitoh T, Kishida H, Tsukada Y, Fukuma Y, Sano J, Yasutake M, Fukuma N, Kusama Y, Hayakawa H. Clinical significance of increased plasma concentration of macrophage colony-stimulating factor in patients with angina pectoris. J Am Coll Cardiol. 2000;35:655–665. doi: 10.1016/s0735-1097(99)00583-5. [DOI] [PubMed] [Google Scholar]
- Sakkinen PA, Cushman M, Psaty BM, Rodriguez B, Boineau R, Kuller LH, Tracy RP. Relationship of plasmin generation to cardiovascular disease risk factors in elderly men and women. Arterioscler Thromb Vasc Biol. 1999;19:499–504. doi: 10.1161/01.atv.19.3.499. [DOI] [PubMed] [Google Scholar]
- Salomaa V, Matei C, Aleksic N, Sansores-Garcia L, Folsom AR, Juneja H, Chambless LE, Wu KK. Soluble thrombomodulin as a predictor of incident coronary heart disease and symptomless carotid artery atherosclerosis in the Atherosclerosis Risk in Communities (ARIC) Study: a case-cohort study. Lancet. 1999;353:1729–1734. doi: 10.1016/s0140-6736(98)09057-6. [DOI] [PubMed] [Google Scholar]
- Sangiorgi G, D'Averio R, Mauriello A, Bondio M, Pontillo M, Castelvecchio S, Trimarchi S, Tolva V, Nano G, Rampoldi V, Spagnoli LG, Inglese L. Plasma levels of metalloproteinases-3 and -9 as markers of successful abdominal aortic aneurysm exclusion after endovascular graft treatment. Circulation. 2001;104:I288–I295. doi: 10.1161/hc37t1.094596. [DOI] [PubMed] [Google Scholar]
- Sannino A. Evaluation of a method based on liquid chromatography/electrospray tandem mass spectrometry for analyzing eight triazolic and pyrimidine fungicides in extracts of processed fruits and vegetables. J AOAC Int. 2004;87:991–996. [PubMed] [Google Scholar]
- Sannino A, Bolzoni L, Bandini M. Application of liquid chromatography with electrospray tandem mass spectrometry to the determination of a new generation of pesticides in processed fruits and vegetables. J Chromatogr A. 2004;1036:161–169. doi: 10.1016/j.chroma.2004.02.078. [DOI] [PubMed] [Google Scholar]
- Sasai K, Okumura-Noji K, Hibino T, Ikeuchi R, Sakuma N, Fujinami T, Yokoyama S. Human cholesteryl ester transfer protein measured by enzyme-linked immunosorbent assay with two monoclonal antibodies against rabbit cholesteryl ester transfer protein: plasma cholesteryl ester transfer protein and lipoproteins among Japanese hypercholesterolemic patients. Clin Chem. 1998;44:1466–1473. [PubMed] [Google Scholar]
- Sato T, Yoshinouchi T, Sakamoto T, Fujieda H, Murao S, Sato H, Kobayashi H, Ohe T. Hepatocyte growth factor (HGF): a new biochemical marker for acute myocardial infarction. Heart Vessels. 1997;12:241–246. doi: 10.1007/BF02766790. [DOI] [PubMed] [Google Scholar]
- Scharfstein JS, Abendschein DR, Eisenberg PR, George D, Cannon CP, Becker RC, Sobel B, Cupples LA, Braunwald E, Loscalzo J. Usefulness of fibrinogenolytic and procoagulant markers during thrombolytic therapy in predicting clinical outcomes in acute myocardial infarction. TIMI-5 Investigators. Thrombolysis in Myocardial Infarction. Am J Cardiol. 1996;78:503–510. doi: 10.1016/s0002-9149(96)00353-0. [DOI] [PubMed] [Google Scholar]
- Schillinger M, Exner M, Amighi J, Mlekusch W, Sabeti S, Rumpold H, Wagner O, Minar E. Joint effects of C-reactive protein and glycated hemoglobin in predicting future cardiovascular events of patients with advanced atherosclerosis. Circulation. 2003;108:2323–2328. doi: 10.1161/01.CIR.0000095267.24234.00. [DOI] [PubMed] [Google Scholar]
- Schonbeck U, Varo N, Libby P, Buring J, Ridker PM. Soluble CD40L and cardiovascular risk in women. Circulation. 2001;104:2266–2268. doi: 10.1161/hc4401.099447. [DOI] [PubMed] [Google Scholar]
- Schulze PC, Kratzsch J, Linke A, Schoene N, Adams V, Gielen S, Erbs S, Moebius-Winkler S, Schuler G. Elevated serum levels of leptin and soluble leptin receptor in patients with advanced chronic heart failure. Eur J Heart Fail. 2003;5:33–40. doi: 10.1016/s1388-9842(02)00177-0. [DOI] [PubMed] [Google Scholar]
- Sealey JE. Plasma renin activity and plasma prorenin assays. Clin Chem. 1991;37:1811–1819. [PubMed] [Google Scholar]
- Shaper AG, Wannamethee SG, Whincup PH. Serum albumin and risk of stroke, coronary heart disease, and mortality: the role of cigarette smoking. J Clin Epidemiol. 2004;57:195–202. doi: 10.1016/j.jclinepi.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Shariat SF, Shalev M, Menesses-Diaz A, Kim IY, Kattan MW, Wheeler TM, Slawin KM. Preoperative plasma levels of transforming growth factor beta(1) (TGF-beta(1) strongly predict progression in patients undergoing radical prostatectomy. J Clin Oncol. 2001;19:2856–2864. doi: 10.1200/JCO.2001.19.11.2856. [DOI] [PubMed] [Google Scholar]
- Sherif K, Kushner H, Falkner BE. Sex hormone-binding globulin and insulin resistance in African-American women. Metabolism. 1998;47:70–74. doi: 10.1016/s0026-0495(98)90195-0. [DOI] [PubMed] [Google Scholar]
- Skinner JS, Farrer M, Albers CJ, Neil HA, Adams PC. High apolipoprotein AI concentrations are associated with lower mortality and myocardial infarction five years after coronary artery bypass graft surgery. Heart. 1999;81:488–494. doi: 10.1136/hrt.81.5.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soncini M, Gasparini P, Lorena M, Motta A, Cimminiello C. Prognostic significance of markers of thrombin generation in the acute and chronic phases of non cardioembolic ischemic stroke. Minerva Cardioangiol. 2000;48:349–356. [PubMed] [Google Scholar]
- Song SJ, Wen SQ, Huang JZ. [Serum levels of soluble intercellular adhesion molecule-1 in patients with cerebral infarct] ZheJiang Da Xue Xue Bao Yi Xue Ban. 2003;32:56–58. doi: 10.3785/j.issn.1008-9292.2003.01.014. [DOI] [PubMed] [Google Scholar]
- Song S, Zheng X, Wen S, Huang J, Ding D. [Change of serum soluble intercellular adhesion molecule and basic fibroblast growth factor in patients with acute cerebral infarction and its clinical significance] Zhonghua Yi Xue Za Zhi. 2002;82:1447–1449. [PubMed] [Google Scholar]
- Specialty Laboratories. Santa Monica, CA, USA: 2001. Directory of Services, and Use and Interpretation of Tests. [Google Scholar]
- Stabler SP, Allen RH. Quantification of serum and urinary S-adenosylmethionine and S-adenosylhomocysteine by stable-isotope-dilution liquid chromatography-mass spectrometry. Clin Chem. 2004;50:365–372. doi: 10.1373/clinchem.2003.026252. [DOI] [PubMed] [Google Scholar]
- Stemmann O, Zou H, Gerber SA, Gygi SP, Kirschner MW. Dual inhibition of sister chromatid separation at metaphase. Cell. 2001;107:715–726. doi: 10.1016/s0092-8674(01)00603-1. [DOI] [PubMed] [Google Scholar]
- Stevens H, Jakobs C, de Jager AE, Cunningham RT, Korf J. Neurone-specific enolase and N-acetyl-aspartate as potential peripheral markers of ischaemic stroke. Eur J Clin Invest. 1999;29:6–11. doi: 10.1046/j.1365-2362.1999.00408.x. [DOI] [PubMed] [Google Scholar]
- Streit F, Armstrong VW, Oellerich M. Rapid liquid chromatography-tandem mass spectrometry routine method for simultaneous determination of sirolimus, everolimus, tacrolimus, and cyclosporin A in whole blood. Clin Chem. 2002;48:955–958. [PubMed] [Google Scholar]
- Streit F, Shipkova M, Armstrong VW, Oellerich M. Validation of a rapid and sensitive liquid chromatography-tandem mass spectrometry method for free and total mycophenolic acid. Clin Chem. 2004;50:152–159. doi: 10.1373/clinchem.2003.024323. [DOI] [PubMed] [Google Scholar]
- Struys EA, Jansen EE, de Meer K, Jakobs C. Determination of S-adenosylmethionine and S-adenosylhomocysteine in plasma and cerebrospinal fluid by stable-isotope dilution tandem mass spectrometry. Clin Chem. 2000;46:1650–1656. [PubMed] [Google Scholar]
- Su YN, Lee CN, Cheng WF, Shau WY, Chow SN, Hsieh FJ. Decreased maternal serum placenta growth factor in early second trimester and preeclampsia. Obstet Gynecol. 2001;97:898–904. doi: 10.1016/s0029-7844(01)01341-2. [DOI] [PubMed] [Google Scholar]
- Tai SS, Bunk DM, White ET, Welch MJ. Development and evaluation of a reference measurement procedure for the determination of total 3,3′,5-triiodothyronine in human serum using isotope-dilution liquid chromatography-tandem mass spectrometry. Anal Chem. 2004;76:5092–5096. doi: 10.1021/ac049516h. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kawanishi-Tabata R, Haba A, Tabata M, Haruta Y, Tsai H, Seon BK. Association of serum endoglin with metastasis in patients with colorectal, breast, and other solid tumors, and suppressive effect of chemotherapy on the serum endoglin. Clin Cancer Res. 2001;7:524–532. [PubMed] [Google Scholar]
- Tarnow L, Hovind P, Teerlink T, Stehouwer CD, Parving HH. Elevated plasma asymmetric dimethylarginine as a marker of cardiovascular morbidity in early diabetic nephropathy in type 1 diabetes. Diabetes Care. 2004;27:765–769. doi: 10.2337/diacare.27.3.765. [DOI] [PubMed] [Google Scholar]
- Tashiro H, Shimokawa H, Sadamatu K, Yamamoto K. Prognostic significance of plasma concentrations of transforming growth factor-beta in patients with coronary artery disease. Coron Artery Dis. 2002;13:139–143. doi: 10.1097/00019501-200205000-00001. [DOI] [PubMed] [Google Scholar]
- Tirumalai RS, Chan KC, Prieto DA, Issaq HJ, Conrads TP, Veenstra TD. Characterization of the low molecular weight human serum proteome. Mol Cell Proteomics. 2003;2:1096–1103. doi: 10.1074/mcp.M300031-MCP200. [DOI] [PubMed] [Google Scholar]
- Tjoelker LW, Stafforini DM. Platelet-activating factor acetylhydrolases in health and disease. Biochim Biophys Acta. 2000;1488:102–123. doi: 10.1016/s1388-1981(00)00114-1. [DOI] [PubMed] [Google Scholar]
- Troyanov S, Hebert MJ, Masse M, Vigneault N, Sirois I, Madore F. Soluble Fas: a novel predictor of atherosclerosis in dialysis patients. Am J Kidney Dis. 2003;41:1043–1051. doi: 10.1016/s0272-6386(03)00202-6. [DOI] [PubMed] [Google Scholar]
- Tsuchio Y, Naito S, Nogami A, Hoshizaki H, Oshima S, Taniguchi K, Katoh H, Suzuki T, Kurabayashi M, Hasegawa A, Nagai R. Intracoronary serum smooth muscle myosin heavy chain levels following PTCA may predict restenosis. Jpn Heart J. 2000;41:131–140. doi: 10.1536/jhj.41.131. [DOI] [PubMed] [Google Scholar]
- Tsutamoto T, Hisanaga T, Fukai D, Wada A, Maeda Y, Maeda K, Kinoshita M. Prognostic value of plasma soluble intercellular adhesion molecule-1 and endothelin-1 concentration in patients with chronic congestive heart failure. Am J Cardiol. 1995;76:803–808. doi: 10.1016/s0002-9149(99)80231-8. [DOI] [PubMed] [Google Scholar]
- Tuthill CW, Rudolph A, Li Y, Tan B, Fitzgerald TJ, Beck SR, Li YX. Quantitative analysis of thymosin alpha1 in human serum by LC-MS/MS. AAPS PharmSciTech. 2000;1:E11. doi: 10.1208/pt010211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino T, Belboul A, El-Gatit A, Roberts D, Berglin E, William-Olsson G. Assessment of myocardial damage by circulating cardiac myosin light chain I after heart transplantation. J Heart Lung Transplant. 1994;13:418–423. [PubMed] [Google Scholar]
- Uji Y, Sugiuchi H, Okabe H. Measurement of human ventricular myosin light chain-1 by monoclonal solid-phase enzyme immunoassay in patients with acute myocardial infarction. J Clin Lab Anal. 1991;5:242–246. doi: 10.1002/jcla.1860050404. [DOI] [PubMed] [Google Scholar]
- Vahl N, Klausen I, Christiansen JS, Jorgensen JO. Growth hormone (GH) status is an independent determinant of serum levels of cholesterol and triglycerides in healthy adults. Clin Endocrinol (Oxf) 1999;51:309–316. doi: 10.1046/j.1365-2265.1999.00772.x. [DOI] [PubMed] [Google Scholar]
- van't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- van de Vijver MJ, He YD, van't Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, Witteveen A, Glas A, Delahaye L, van der Velde T, Bartelink H, Rodenhuis S, Rutgers ET, Friend SH, Bernards R. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347:1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- van der Laarse A, Hermens WT, Hollaar L, Jol M, Willems GM, Lemmers HE, Liem AH, Souverijn JH, Oudhof JH, de Hooge J, et al. Assessment of myocardial damage in patients with acute myocardial infarction by serial measurement of serum alpha-hydroxybutyrate dehydrogenase levels. Am Heart J. 1984;107:248–260. doi: 10.1016/0002-8703(84)90372-7. [DOI] [PubMed] [Google Scholar]
- van Geel WJ, de Reus HP, Nijzing H, Verbeek MM, Vos PE, Lamers KJ. Measurement of glial fibrillary acidic protein in blood: an analytical method. Clin Chim Acta. 2002;326:151–154. doi: 10.1016/s0009-8981(02)00330-3. [DOI] [PubMed] [Google Scholar]
- Vischer UM, Ingerslev J, Wollheim CB, Mestries JC, Tsakiris DA, Haefeli WE, Kruithof EK. Acute von Willebrand factor secretion from the endothelium in vivo: assessment through plasma propeptide (vWf: AgII) levels. Thromb Haemost. 1997;77:387–393. [PubMed] [Google Scholar]
- Wada H, Nobori T, Watanabe R, Shiku H, Sakuragawa N. plasma levels of plasminogen activator inhibitor-1 (PAI-1) and thrombin activatable fibrinolysis inhibitor (TAFI) in patients with disseminated intravascular coagulation (DIC) Turk J Haematol. 2002;19:235–237. [PubMed] [Google Scholar]
- Wagner KR, Giles WH, Johnson CJ, Ou CY, Bray PF, Goldschmidt-Clermont PJ, Croft JB, Brown VK, Stern BJ, Feeser BR, Buchholz DW, Earley CJ, Macko RF, McCarter RJ, Sloan MA, Stolley PD, Wityk RJ, Wozniak MA, Price TR, Kittner SJ. Platelet glycoprotein receptor IIIa polymorphism P1A2 and ischemic stroke risk: the Stroke Prevention in Young Women Study. Stroke. 1998;29:581–585. doi: 10.1161/01.str.29.3.581. [DOI] [PubMed] [Google Scholar]
- Wagner S, Kalb P, Lukosava M, Hilgenfeldt U, Schwaninger M. Activation of the tissue kallikrein-kinin system in stroke. J Neurol Sci. 2002;202:75–76. doi: 10.1016/s0022-510x(02)00208-3. [DOI] [PubMed] [Google Scholar]
- Wang YN, Che SM, Ma AQ. Clinical significance of serum cytokines IL-1beta, sIL-2R, IL-6, TNF-alpha, and IFN-v in acute coronary syndrome. Chin Med Sci J. 2004;19:120–124. [PubMed] [Google Scholar]
- Warner MM, Guo J, Zhao Y. The relationship between plasma apolipoprotein A-IV levels and coronary heart disease. Chin Med J (Engl) 2001;114:275–279. [PubMed] [Google Scholar]
- Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, Rouleau JL, Lee RT. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;106:2961–2966. doi: 10.1161/01.CIR.0000038705.69871.D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M, Martignoni M, Petropoulou T, Solder B, Belohradsky BH. Increased serum levels of soluble tumor necrosis factor receptors (sTNF-Rs) in children and adolescents with vertically and horizontally transmitted HIV infection. Infection. 1996;24:301–308. doi: 10.1007/BF01743365. [DOI] [PubMed] [Google Scholar]
- Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38:263–355. doi: 10.1080/20014091084227. [DOI] [PubMed] [Google Scholar]
- Witte DR, Broekmans WM, Kardinaal AF, Klopping-Ketelaars IA, van Poppel G, Bots ML, Kluft C, Princen JM. Soluble intercellular adhesion molecule 1 and flow-mediated dilatation are related to the estimated risk of coronary heart disease independently from each other. Atherosclerosis. 2003;170:147–153. doi: 10.1016/s0021-9150(03)00253-3. [DOI] [PubMed] [Google Scholar]
- Wolff B, Volzke H, Ludemann J, Robinson D, Vogelgesang D, Staudt A, Kessler C, Dahm JB, John U, Felix SB. Association between high serum ferritin levels and carotid atherosclerosis in the study of health in Pomerania (SHIP) Stroke. 2004;35:453–457. doi: 10.1161/01.STR.0000114875.31599.1C. [DOI] [PubMed] [Google Scholar]
- Xue C, Yu H, Li R, Wo J, Cui J, Cheng H, Wang H, Guan Q, Suo X, Jia R. Clinical significance of serum cardiac troponin T in patients with congestive heart failure. Chin Med J (Engl) 2003;116:469–471. [PubMed] [Google Scholar]
- Yamamoto K, Burnett JC, Jr, Jougasaki M, Nishimura RA, Bailey KR, Saito Y, Nakao K, Redfield MM. Superiority of brain natriuretic peptide as a hormonal marker of ventricular systolic and diastolic dysfunction and ventricular hypertrophy. Hypertension. 1996;28:988–994. doi: 10.1161/01.hyp.28.6.988. [DOI] [PubMed] [Google Scholar]
- Yan SB, Dhainaut JF. Activated protein C versus protein C in severe sepsis. Crit Care Med. 2001;29:S69–S74. doi: 10.1097/00003246-200107001-00024. [DOI] [PubMed] [Google Scholar]
- Yasuda S, Atsumi T, Ieko M, Matsuura E, Kobayashi K, Inagaki J, Kato H, Tanaka H, Yamakado M, Akino M, Saitou H, Amasaki Y, Jodo S, Amengual O, Koike T. Nicked beta2-glycoprotein I: a marker of cerebral infarct and a novel role in the negative feedback pathway of extrinsic fibrinolysis. Blood. 2004;103:3766–3772. doi: 10.1182/blood-2003-08-2712. [DOI] [PubMed] [Google Scholar]
- Yen MH, Pilkington G, Starling RC, Ratliff NB, McCarthy PM, Young JB, Chisolm GM, Penn MS. Increased tissue factor expression predicts development of cardiac allograft vasculopathy. Circulation. 2002;106:1379–1383. doi: 10.1161/01.cir.0000028588.73765.b4. [DOI] [PubMed] [Google Scholar]
- Yoneda K, Katayama Y, Koike T, Tanimizu I. A homogeneous assay system of aspartate aminotransferase iso-enzymes using proteases and application for clinical evaluation of myocardial infarction. J Clin Lab Anal. 1992;6:362–367. doi: 10.1002/jcla.1860060605. [DOI] [PubMed] [Google Scholar]
- Zaremba J, Losy J. sPECAM-1 in serum and CSF of acute ischaemic stroke patients. Acta Neurol Scand. 2002;106:292–298. doi: 10.1034/j.1600-0404.2002.01339.x. [DOI] [PubMed] [Google Scholar]
- Zeisler H, Livingston JC, Schatten C, Tempfer C, Knofler M, Husslein P. Serum levels of adhesion molecules in women with pregnancy-induced hypertension. Wien Klin Wochenschr. 2001;113:588–592. [PubMed] [Google Scholar]
- Zemanova P, Opatrny K, Jr, Opatrna S, Vit L, Sefrna F, Racek J. Tissue factor, its pathway inhibitor, and metabolic disturbances in long-term peritoneal dialysis. Kidney Blood Press Res. 2003;26:368–375. doi: 10.1159/000073944. [DOI] [PubMed] [Google Scholar]
- Zethelius B, Byberg L, Hales CN, Lithell H, Berne C. Proinsulin is an independent predictor of coronary heart disease: Report from a 27-year follow-up study. Circulation. 2002;105:2153–2158. doi: 10.1161/01.cir.0000015855.04844.e7. [DOI] [PubMed] [Google Scholar]
- Zhang N, Fountain ST, Bi H, Rossi DT. Quantification and rapid metabolite identification in drug discovery using API time-of-flight LC/MS. Anal Chem. 2000a;72:800–806. doi: 10.1021/ac9911701. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gu Y, Li H, Lucas MJ, Wang Y. Increased endothelial monolayer permeability is induced by serum from women with preeclampsia but not by serum from women with normal pregnancy or that are not pregnant. Hypertens Pregnancy. 2003;22:99–108. doi: 10.1081/PRG-120017008. [DOI] [PubMed] [Google Scholar]
- Zhang M, Niehus J, Schnellbacher T, Muller S, Graf K, Schultz KD, Baumgarten CR, Lucas C, Kunkel G. ELISA for the neuropeptide degrading endopeptidase 3.4.24.11 in human serum and leukocytes. Peptides. 1994;15:843–848. doi: 10.1016/0196-9781(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ren S, Shen GX. Glycation amplifies lipoprotein(a)-induced alterations in the generation of fibrinolytic regulators from human vascular endothelial cells. Atherosclerosis. 2000b;150:299–308. doi: 10.1016/s0021-9150(99)00381-0. [DOI] [PubMed] [Google Scholar]
- Zhou L, Hu C, Yuan G, Xu W, Chen J, Lai L. [Radioimmunoassay of serum and CSF myelin basic protein and its application to patients with acute cerebrovascular accident] Hua Xi Yi Ke Da Xue Xue Bao. 1992;23:362–366. [PubMed] [Google Scholar]
- Zuyderhoudt FM, Boers W, Linthorst C, Jorning GG, Hengeveld P. An enzyme-linked immunoassay for ferritin in human serum and rat plasma and the influence of the iron in serum ferritin on serum iron measurement, during acute hepatitis. Clin Chim Acta. 1978;88:37–44. doi: 10.1016/0009-8981(78)90146-8. [DOI] [PubMed] [Google Scholar]