Abstract
In many animals the rate of protein synthesis is higher in slow-twitch, oxidative than fast-twitch, glycolytic muscles. To discover if muscles in the human body also show such differences, we measured [13C]leucine incorporation into proteins of anatomically distinct muscles of markedly different fibre-type composition (vastus lateralis, triceps, soleus) after an overnight fast and during infusion of a mixed amino acid solution (75 mg amino acids kg−1 h−1) in nine healthy, young men. Type-1 fibres contributed 83 ± 4% (mean ±s.e.m.) of total fibres in soleus, 59 ± 3% in vastus lateralis and 22 ± 2% in triceps. The basal myofibrillar and sarcoplasmic protein fractional synthetic rates (FSR, % h−1) were 0.034 ± 0.001 and 0.064 ± 0.001 (soleus), 0.031 ± 0.001 and 0.060 ± 0.001 (vastus), and 0.027 ± 0.001 and 0.055 ± 0.001 (triceps). During amino acid infusion, myofibrillar protein FSR increased to 3-fold, and sarcoplasmic to 2-fold basal values (P < 0.001). The differences between muscles, although significant statistically (triceps versus soleus and vastus lateralis, P < 0.05), were within ∼15%, biologically probably insignificant. The rates of collagen synthesis were not affected by amino acid infusion and varied by < 5% between muscles and experimental conditions.
The main features of the regulation of muscle mass (i.e. maintenance, growth and loss) are generally well understood. Muscle mass is maintained by a balance between muscle protein accretion as a result of anabolic stimuli (e.g. meals, exercise) and protein loss as a result of catabolic stimuli (e.g. fasting, immobilization) (Rennie et al. 2004). It seems likely that most of the anabolic effect of food on muscle is due to the stimulatory action of increased plasma amino acid availability on muscle protein synthesis (Bennet et al. 1989; Bohe et al. 2001, 2003; Nygren & Nair, 2003). Amino acids are also required to achieve a positive protein net balance in response to contractile activity, which in itself promotes muscle protein synthesis, although in the absence of extra amino acids not enough to overcome the simultaneous stimulatory effect on muscle protein breakdown (Biolo et al. 1997; Phillips et al. 1997; Rennie, 2001).
One major short-coming in our understanding of human muscle protein metabolism is due to the fact that most of our current information has been obtained from measuring the rate of muscle protein synthesis in the m. quadriceps femoris, usually because of convenience and the relative difficulty of sampling other muscles. Furthermore, it is commonly assumed (with little evidence of validity) that the metabolic behaviour of skeletal muscle is the same throughout the human body, and therefore it has become acceptable to extrapolate the results obtained in the quadriceps to total skeletal muscle and to whole-body protein metabolism. In animals, however, muscle protein synthesis rates differ markedly between muscles, probably due to differences in muscle fibre-type composition. Slow-twitch, oxidative muscles, with predominately Type-1 fibres, have much higher (as much as > 2-fold) basal protein turnover rates than fast-twitch, glycolytic muscles, with predominantly Type-2 fibres (Laurent et al. 1978; Bates & Millward, 1983; Kelly et al. 1984; Garlick et al. 1989; Lang et al. 2003), and basal rates of protein synthesis in nine limb muscles of rats are correlated with the content of slow-twitch, oxidative fibres (Garlick et al. 1989). Also, in rodents at least, protein synthesis in slow- and fast-twitch muscles exhibit different sensitivity to anabolic (e.g. exercise, muscle loading/hypergravity, feeding) (Bates & Millward, 1983; Fluckey et al. 1996b; Kita et al. 1998) and catabolic (e.g. starvation, ageing, ethanol administration, burn injury, tumour necrosis factor (TNF) administration, blocking of insulin-like growth factor 1 (IGF-1)) (Bates & Millward, 1983; Preedy & Peters, 1988; Fang et al. 1995; Fluckey et al. 1996a; Lang et al. 2003) stimuli. Recently, while the present work was under review, Carroll et al. (2004) published a report concerning mixed muscle protein synthesis rates in human vastus lateralis and soleus, studied in the basal, postabsorptive state and during amino acid infusion. The authors concluded that there were no differences in the rates of mixed muscle protein synthesis but it is difficult to accept that conclusion as definitive on the basis of the data presented because the variances in the measured rates of protein synthesis in the two muscles were sufficiently large to obscure possible differences of up to 50% of the mean values reported.
Attempts to discover the influence of fibre-type composition on the rate of muscle protein synthesis in human muscle have been made by evaluating the relationship between fibre-type composition of the quadriceps and the basal rate of quadriceps protein synthesis, with negative results, i.e. no association was found; but this work cannot be thought to be definitive because of the narrow and skewed range of variation in fibre-type composition (i.e. 22–52% Type-1 fibres) of the muscle samples studied (Nair et al. 1988). Muscle protein turnover in human muscles other than the quadriceps and with distinctly different fibre-type composition (i.e. tibialis anterior, soleus, biceps brachii, deltoid) has been measured in very few studies, with varying experimental conditions (Bennet et al. 1989; Chesley et al. 1992; MacDougall et al. 1995; Tipton et al. 1996; Gamrin et al. 1998; Fowles et al. 2000) but no studies have compared basal rates of protein turnover and/or the response of protein turnover to various anabolic/catabolic stimuli in different muscles simultaneously. Hence, it is not known if the observations made in animals are mirrored in human beings.
Our aim was to investigate whether the basal rates of muscle protein synthesis and the stimulation of it by increased amino acid availability are different in different muscles in the human body and to what extent any differences are due to variations in fibre-type composition of the muscles. Thus, we measured in healthy, young men, rates of protein synthesis in three muscles with markedly different fibre-type composition (quadriceps, soleus and triceps) both in the postabsorptive state, after an overnight fast, and during infusion of a mixed amino acid solution. Since in the steady-state (i.e. maintenance of muscle protein mass) the rates of muscle protein synthesis should be equal and opposite to those of protein breakdown, any fibre-type specific difference would be expected to be of the same order for each process.
Methods
Subjects
Nine healthy, recreationally active but untrained men (age: 26 ± 1 years; body weight: 76 ± 2 kg; BMI: 23 ± 1 kg m−2; means ±s.e.m.) participated in this project. All subjects were considered to be in good health after completing a comprehensive medical evaluation, which included a history and physical examination and standard blood tests. All subjects were instructed to adhere to their regular diet and to refrain from vigorous exercise 3 days before the study. The study was conducted according to the Declaration of Helsinki; written informed consent was obtained from all subjects before their participation in the study, which was approved by the local (Copenhagen and Fredriksberg Communities, Denmark) ethics committee.
Experimental protocol
At 07.00 h, subjects arrived at the laboratory in the Copenhagen Muscle Research Center after an overnight fast. At ∼07.30 h, a cannula was inserted into an antecubital vein for the infusion of the stable isotope labelled tracer and mixed amino acid solution as described below; another one was inserted into a vein of the contralateral forearm for blood sampling. At ∼08.00 h, a baseline blood sample and a muscle biopsy from the vastus lateralis portion of the m. quadriceps femoris were obtained (see ‘Sample collection and storage’) to determine the background enrichment of plasma and muscle. All of our subjects were ‘tracer virgins’ (i.e. had not received a tracer infusion before participation in our study) which obviated the need for initial biopsies in all three muscles, since they have had the same basal leucine enrichment in protein. Following the first biopsy, a primed constant infusion of [1,2-13C]leucine (priming dose: 7.8 μmol (kg body wt)−1, infusion rate: 0.13 μmol (kg body wt)−1 min−1) was started and maintained until completion of the study some 6 h later. After 180 min from the start of the tracer infusion, additional muscle biopsies were obtained from the mm. quadriceps femoris (vastus lateralis portion), soleus and triceps bracchii to determine basal rates of muscle protein synthesis (as incorporation of [1,2-13C]leucine into protein; see ‘Calculations’), and muscle fibre-type composition. After completion of the biopsy procedures, a primed constant infusion of a mixed balanced amino acid solution (Vamin 18EF, Kabi Fresenius, Uppsala, Sweden) was infused (priming dose: 25 mg (kg body wt)−1, infusion rate: 75 mg (kg body wt)−1 h−1) until completion of the study. At the onset of the mixed amino acid infusion, the infusion rate of [1,2-13C]leucine was increased to 0.18 μmol (kg body wt)−1 min−1 to match the increased availability of leucine, thus maintaining a tracer steady state. Muscle biopsies from each of the three muscles (vastus, soleus and triceps) were obtained again 180 min after the start of the mixed amino acid infusion to determine the amino acid stimulated rates of muscle protein synthesis, and muscle fibre-type composition. Biopsy samples from the three different muscles were obtained in randomized order, via new incisions, and the exact time of biopsy was noted for the calculation of muscle protein synthetic rates. Blood samples were obtained before and every 30 min throughout the tracer infusion to determine plasma leucine and α-ketoisocaproic acid (KIC; an index of intramuscular free leucine enrichment (Matthews et al. 1982; Chinkes et al. 1996)) enrichment. All infusions were stopped and cannulae were removed after the last biopsy procedure was completed.
Sample collection and storage
Muscle tissue (∼100 mg) was obtained under local anaesthesia (lidocaine, 2%) by using the Bergström needle technique. An aliquot of the tissue was immediately frozen in liquid nitrogen for subsequent determination of muscle free and protein-bound leucine enrichment (i.e. protein synthesis rate), and the remaining sample was embedded in Tissue Tek (Sakura Finetek, Zoeterwoude, the Netherlands) and frozen in isopentane cooled in liquid nitrogen for subsequent fibre-type analysis. The deep frozen samples were stored at −70°C until final analyses. Blood samples (∼5 ml) were collected in pre-chilled tubes containing EDTA, plasma was separated immediately and stored at −70°C until final analyses.
Sample preparation and analyses
All sample analyses are routinely performed in our laboratories and have previously been described in detail. To determine muscle fibre-type (1, 2a and 2x) composition, serial sections (10 μm) of each muscle sample, embedded in Tissue Tek, were cut in a cryostat at −20°C, and myofibrillar ATPase histochemistry was performed at pH 9.40 after pre-incubation at pH 4.37, 4.60 and 10.30 by using a computer image analysis system (TEMA, Scan Beam ApS, Hadsund, Denmark) (Andersen & Aagaard, 2000). The relative proportion of the various fibre-types was determined based on an average of 116 ± 5 representative cells in each sample. The proportional fibre-type composition between the two biopsies (end of basal period and end of amino acid infusion) in each muscle was generally in good agreement; therefore, we used the average value of the two biopsies for presentation of our results.
To determine plasma and tissue free leucine and plasma KIC enrichment (tracer-to-tracee ratio, TTR), proteins were precipitated and the supernatant, containing free amino and imino acids, was collected to prepare the t-BDMS (leucine) and ortho-phenylenediamine-tertiary-butyldimethylsilyl (OPDA-t-BDMS) (KIC) derivative for analysis by gas-chromatography/mass-spectrometry (GC/MS; MD800, Fisons Plc, Ipswich, UK) by using electron-ionization and selective ion monitoring (Schwenk et al. 1984; Babraj et al. 2002).
To prepare myofibrillar, sarcoplasmic and collagen fractions, frozen muscle (20–30 mg) was ground in liquid nitrogen to a fine powder, hand homogenized in a buffer containing 0.15 m NaCl–0.1% Triton X-100–0.02 m Tris-HCl (pH 7.4), and then centrifuged at 1600 g, 4°C for 20 min to pellet the myofibrils and collagen; the supernatant containing sarcoplasmic proteins was cleared of all membrane fractions by precipitating the sarcoplasmic proteins with ethanol (Bohe et al. 2001). The pellet (containing myofibrils and collagen) was washed with 0.7 m KCl to solubilize myofibrillar proteins, centrifuged as above, and the collagen pellet was washed with acetic acid and acetic acid–pepsin leaving an insoluble mature collagen pellet (Bohe et al. 2001; Miller et al. in press); myofibrillar proteins were precipitated from the KCl supernatant with ethanol (Bohe et al. 2001). All protein extracts (myofibrillar, sarcoplasmic, collagen) were hydrolysed in 0.05 m HCl: Dowex 50W-X8-200 (Sigma Ltd, Poole, UK), and the liberated amino acids purified on cation-exchange columns (Dowex 50W-X8-200) (Balagopal et al. 1997; Bohe et al. 2001). The amino acids were then converted to their N-acetyl-n-propyl ester (NAP)-derivative and the leucine TTR determined by gas-chromatography-combustion-isotope ratio mass spectrometry (GC-C-IRMS, Delta-plus XL, Thermofinnigan, Hemel Hempstead, UK) (Rennie et al. 1996; Meier-Augenstein, 1999). Due to technical difficulties during sample preparations we were unable to obtain data for myofibrillar protein synthesis in the vastus and soleus for one subject and collagen synthesis in the soleus for another subject.
Calculations
The fractional synthesis rate (FSR) of myofibrillar and sarcoplasmic proteins and muscle collagen was calculated based on the incorporation rate of [1,2-13C]leucine into muscle proteins using a standard precursor-product model as follows:
where ΔEp is the change in enrichment (TTR) of protein-bound leucine in two subsequent biopsies, Eic is the mean enrichment over time of the precursor for protein synthesis (i.e. leucyl-t-RNA) and t is the time between biopsies. Venous KIC was chosen to represent the immediate precursor for muscle non-collagen protein synthesis (i.e. leucyl-t-RNA) (Matthews et al. 1982; Watt et al. 1991; Chinkes et al. 1996). Because fibroblasts, like myocytes, contain a branched-chain amino acid transaminase (Wendel & Langenbeck, 1984), we also used the plasma KIC TTR as precursor for muscle (probably perimysial) collagen synthesis. We have recently validated this approach (Miller et al. in press). All values for FSR are expressed as percentage per hour.
Statistical analysis
Data from these studies were analysed by repeated measures analysis of variance (ANOVA); significant F ratios were followed by Tukey's post hoc comparisons procedure. The Pearson Product Correlation was determined to assess the relationship between relative muscle fibre-type composition (e.g. % Type-1 fibres) and muscle protein synthesis rate. A P value of ≤ 0.05 was considered statistically significant.
Results
Fibre-type composition
Evaluation of fibre-type composition based on either total fibre number or fibre area gave similar results. Type-1 fibres were most and Type-2 fibres least abundant in soleus and vice versa, Type-2 fibres were most and Type-1 fibres least abundant in triceps; vastus consisted of approximately equal numbers of Type-1 and Type-2 fibres (Table 1).
Table 1.
Fibre-type profile in soleus, vastus and triceps biopsies
| Type-1 | Type-2a | Type-2x | ||||
|---|---|---|---|---|---|---|
| Number | Area | Number | Area | Number | Area | |
| Soleus | 83 ± 4 (65–98) | 82 ± 4 (65–96) | 14 ± 3 (2–31) | 15 ± 3 (4–27) | 3 ± 1 (0–11) | 3 ± 1 (0–15) |
| Vastus | 59 ± 3 (42–75) | 57 ± 3 (34–71) | 26 ± 3 (13–41) | 28 ± 3 (14–53) | 15 ± 3 (2–29) | 15 ± 3 (2–35) |
| Triceps | 22 ± 2 (14–33) | 17 ± 1 (9–23) | 39 ± 5 (24–77) | 41 ± 6 (25–82) | 39 ± 5 (9–57) | 42 ± 5 (9–61) |
Values (% of total fibre number and total fibre area, respectively) are means ±s.e.m. with range in parentheses; they represent the average value of two biopsy samples (at the end of basal conditions and the end of the amino acid infusion) for each muscle and subject.
Plasma KIC labelling
In the 3 h study period, both of the postabsorptive state and amino acid infusion, plasma KIC labelling was at steady state. The plasma KIC labelling values were (mean ±s.e.m.) 6.14 ± 0.23 and 5.78 ± 0.19% at 30 and 180 min after the start of the tracer infusion, respectively; similarly, during the amino acid infusion, when the tracer leucine infusion rate was increased appropriately to match the increased appearance of unlabelled leucine in plasma, the KIC labelling was not different at 30 min (6.77 ± 0.17%) and 180 min (6.85 ± 0.22%) after the start of the amino acid infusion.
Myofibrillar protein synthesis
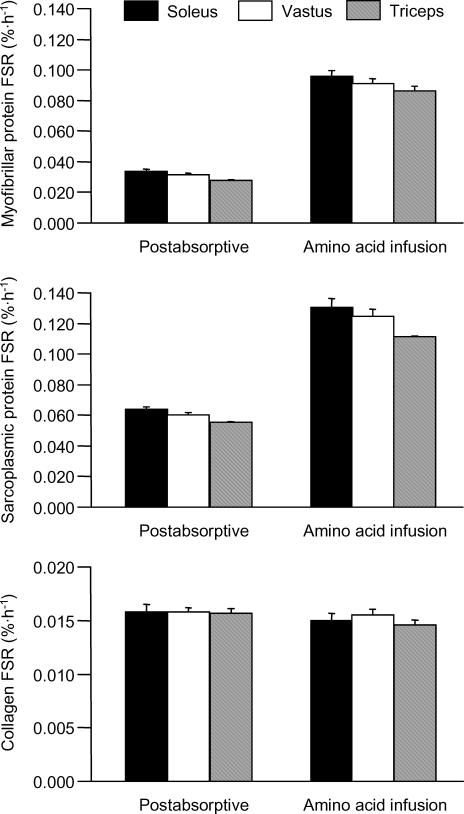
The basal rate of myofibrillar protein synthesis (FSR, % h−1) was 0.034 ± 0.001 in the soleus, 0.031 ± 0.001 in the vastus, and 0.027 ± 0.001 in the triceps (Fig. 1). Myofibrillar FSR increased in all muscles during the mixed amino acid infusion to approximately 3-times basal values (Figs 1 and 2). ANOVA revealed a statistically significant main effect of feeding on the FSR (P < 0.001) and a statistically significant difference between the FSR in soleus and triceps (P < 0.05) and vastus and triceps (P < 0.05) but no significant muscle–feeding interaction.
Figure 1. Synthesis rates of myofibrillar (top) and sarcoplasmic (middle) proteins and muscle collagen (bottom) in soleus, vastus and triceps during basal, postabsorptive conditions and mixed amino acid infusion.
Values are means ±s.e.m. ANOVA revealed a statistically significant main effect of feeding on myofibrillar and sarcoplasmic protein FSR (P < 0.001) and a statistically significant difference between the FSR (for both myofibrillar and sarcoplasmic proteins) in soleus and triceps (P < 0.05) and vastus and triceps (P < 0.05) but no significant muscle–feeding interaction.
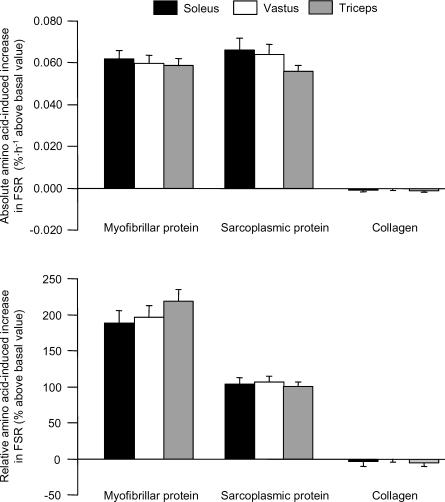
Figure 2. Absolute (top) and relative (bottom) increase above basal, postabsorptive values in the fractional synthesis rate (FSR) of myofibrillar and sarcoplasmic proteins and muscle collagen in soleus, vastus and triceps in response to mixed amino acid infusion.
See text for details. Values are means ±s.e.m.
Sarcoplasmic protein synthesis
The basal rate of sarcoplasmic protein synthesis (FSR, % h−1) was 0.064 ± 0.001 in the soleus, 0.060 ± 0.001 in the vastus, and 0.055 ± 0.001 in the triceps (Fig. 1). The mixed amino acid infusion resulted in a doubling of the FSR in all three muscles (Figs 1 and 2). ANOVA revealed a statistically significant main effect of feeding on the FSR (P < 0.001) and a statistically significant difference between the FSR in soleus and triceps (P < 0.05) and vastus and triceps (P < 0.05) but no significant muscle–feeding interaction.
Collagen synthesis
The rates of collagen synthesis were not different between muscles and experimental conditions (grand mean values for collagen FSR in % h−1: 0.0154 ± 0.0005 in the soleus, 0.0157 ± 0.0003 in the vastus, and 0.0151 ± 0.0004 in the triceps; Figs 1 and 2).
Relationship between the rate of protein synthesis and muscle fibre-type composition
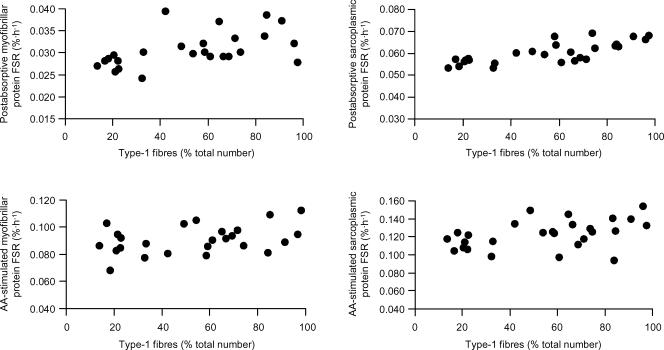
No statistically significant relationship between muscle fibre-type composition and the rate of protein synthesis was revealed when values for the rates of myofibrillar and sarcoplasmic protein synthesis from all three muscles (soleus, vastus and triceps) were combined (Fig. 3).
Figure 3. Relationship between the proportional contribution of Type-1 fibres to total fibre number and the fractional synthesis rate (FSR) of myofibrillar (left) and sarcoplasmic (right) proteins (in soleus, vastus and triceps) during basal, postabsorptive conditions (top) and mixed amino acid infusion (bottom).
See text for details. Similar results were obtained using data based on per cent of total fibre area instead of total fibre number on the x-axes (data not shown). AA: amino acid
Discussion
We set out to discover first, whether or not a relationship between muscle fibre-type composition and the rate of muscle protein synthesis exists during basal, postabsorptive conditions; and second, to what extent fibre-type composition influences the response of muscle protein synthesis to increased amino acid availability. We found small (< 15%), but nonetheless statistically significant, differences in the mean rates of myofibrillar and sarcoplasmic protein synthesis between muscles, in the basal postabsorptive state and during extra amino acid availability, with the rates being highest in the slow-twitch soleus and lowest in the fast-twitch triceps. However, we could not find any absolute or relative difference in the responses to increased amino acid availability. Furthermore, we could not identify muscle fibre-type composition per se as a major determinant of the rate of human muscle protein synthesis when evaluating the composite of the data from all three muscles, despite having data covering a broad range of fibre-type composition (14–98% of total fibre number Type-1 and, respectively, 2–86% Type-2 fibres). Anatomical location and/or muscle function (e.g. flexor, extensor, weight bearing) therefore appear to have a stronger, though in our opinion still negligible, effect on the rates of myofibrillar and sarcoplasmic protein synthesis. The rates of collagen synthesis were not different between muscles and were not affected by the availability of extra amino acids. These results suggest strongly that muscle fibroblasts do not share the nutrient-sensing pathways present in myocytes.
Qualitatively, our results are comparable with the observations made in other species, predominantly rodents (Laurent et al. 1978; Bates & Millward, 1983; Kelly et al. 1984; Garlick et al. 1989; Lang et al. 2003); but the extent to which protein synthesis rates varied between different human muscles was much less in our study than that in studies of animals. It has been speculated that fibre-type composition is responsible for the differences between muscles. However, close inspection of previous results suggests that although the proportional amount of Type-1 fibres in nine different muscles in female rats was a significant predictor of the rate of muscle protein synthesis, it could only explain part of the observed variation; and, the inclusion of the relative contribution of different Type-2 fibres (2a, 2x) in the model did not further improve the fit (Garlick et al. 1989), corroborating our view that, muscle fibre-type composition, in animal as well as human muscles, is not an overwhelmingly important factor setting the basal rate of muscle protein synthesis. We are not certain what does cause the rates of protein turnover to vary between muscles but speculate that this phenomenon may be related to intrinsic factors, such as the capacity for protein synthesis (e.g. amount of RNA) and the myofibre nuclei content (Millward et al. 1973; Laurent et al. 1978; Lang et al. 1999) as well as muscle action and location.
Although we carried out our studies with our subjects at rest, we cannot rule out a possible residual effect of contractile activity (which probably varies greatly between the soleus, vastus and triceps in the course of the day), to have contributed to the observed differences in their rates of muscle protein synthesis. Muscle protein synthesis increases within a few hours after a bout of strenuous exercise and remains elevated for at least 24 h (MacDougall et al. 1995; Phillips et al. 1997; Miller et al. in press), probably returning to normal after 72 h (Miller et al. in press). It is therefore possible that the higher rates of synthesis in soleus and vastus compared with triceps, are in part due to their weight-bearing function and regular involvement in movement. However, if this were the case, we would have expected a relative greater increase in the rate of synthesis in response to the extra amino acids (Biolo et al. 1997) in the soleus and vastus than the triceps, which we did not see. Furthermore, it might be expected, a priori, by comparison with other metabolic characteristics of muscle, that accustomed activity would cause minor perturbations, as indeed appears to be the case for muscle protein synthesis (Phillips et al. 1999). So, it is unlikely that differences in muscle use contributed significantly to the differences in protein synthesis rates, especially considering that our subjects refrained from vigorous exercise for 3 days before the study. In any case, our observations were made under circumstances that reflect normal daily conditions and are, to that extent, likely to be representative.
Failure to reproduce the findings from studies in animals (with regard to the magnitude of differences) is not surprising, however. First, despite evidence of a wide variance in the proportional contribution of Type-1 and Type-2 fibres to total fibre content in vastus lateralis (∼30–70%, see Table 1), the most frequently studied human muscle, the published basal rates for muscle protein synthesis in human vastus do not show a similar variability. Second, the published basal rates of muscle protein synthesis in other human muscles (Bennet et al. 1989; Chesley et al. 1992; MacDougall et al. 1995; Tipton et al. 1996; Gamrin et al. 1998; Fowles et al. 2000) are fairly similar despite their markedly different fibre-type composition, although the use of different analysis techniques weakens this comparison, especially if the differences between muscles are (as we have shown) of a smaller magnitude than those in animals. This is why we designed our studies to achieve greatest possible power to detect differences: (1) we chose [13C]leucine as tracer in combination with GC-C-IRMS analysis because this gives a much higher precision and reproducibility for measurement of muscle protein synthesis rates (personal unpublished observation and Nair et al. 1988) compared with other tracers and analysis techniques (e.g. Ferrando et al. 1997; Biolo et al. 1999; Volpi et al. 2001) resulting in the lowest hitherto published population standard deviation for our study end-point (muscle protein synthesis); (2) we have shown that muscle protein synthesis responds rapidly (within 30 min) to increased availability of amino acids but then returns to basal values (after ∼3 h) despite continued amino acid availability (Bohe et al. 2001); thus, assessing the response of muscle protein synthesis to amino acid infusion during 3 h should have given us a near-maximal response in the three muscles; (3) we elected to analyse the major muscle protein fractions separately because myofibrillar proteins are more responsive to feeding/extra amino acids than sarcoplasmic proteins (personal observation and Bates & Millward, 1983), and we would have expected that differences in the responsiveness between muscles, if they existed, would be most apparent in the myofibrillar protein fraction. We achieved our aim by being able to demonstrate statistically significant differences but which were of such small magnitude that they are unlikely to be biologically relevant. In other words, we did not observe differences of the magnitude such as occur with a physiological intervention, e.g. feeding or exercise (which cause a 2- to 3-fold increase above basal sarcoplasmic and myofibrillar protein synthetic rates (Rennie et al. 2004)) between human muscles of different fibre-type composition either in the basal state (even though the measured rates were statistically significantly different) or during amino acid infusion.
In summary, our findings indicate that observations regarding muscle protein turnover made in many animals (especially lower vertebrates) are not fully mirrored by human muscle. Since the differences in the rates of muscle protein synthesis in the three muscles we studied that were markedly different with regard to fibre-type composition, anatomical location, locomotor function and extent of weight bearing was, in our opinion, negligible we suggest that it is reasonable to extrapolate the findings made in one skeletal muscle in the postabsorptive or amino acid-fed states to all skeletal muscles in the human body. Furthermore, animals with large variations in muscle protein turnover between muscles and in the responsiveness of different muscles to anabolic or catabolic perturbations are likely to be relatively poor models for investigation of conditions likely to affect human muscle protein turnover.
Acknowledgments
The authors wish to thank Bente Klarlund Pedersen for medical supervision of the project, Gitte Wilkens and Inge Luise Madsen for technical assistance, and the study subjects for their participation. This study was supported by US National Institutes of Health grants HD 01459 (B.M.), AR 49869 (B.M. and M.J.R.), DK 56341 (Clinical Nutrition Research Unit at Washington University), and grants from the Novo Nordisk Foundation (B.M.), the Danish Natural Sciences Foundation (B.M.), the Danish National Research Foundation (Copenhagen Muscle Research Center grant 504-14), The Wellcome Trust (M.J.R.), UK MRC (M.J.R.), UK BBSRC (M.J.R.), Diabetes UK (M.J.R.), Chief Scientist's Office of the Scottish Executive (M.J.R.), and the University of Dundee (M.J.R.).
References
- Andersen JL, Aagaard P. Myosin heavy chain IIX overshoot in human skeletal muscle. Muscle Nerve. 2000;23:1095–1104. doi: 10.1002/1097-4598(200007)23:7<1095::aid-mus13>3.0.co;2-o. 10.1002/1097-4598(200007)23:7<1095::AID-MUS13>3.3.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Babraj J, Cuthbertson DJ, Rickhuss P, Meier-Augenstein W, Smith K, Bohe J, Wolfe RR, Gibson JN, Adams C, Rennie MJ. Sequential extracts of human bone show differing collagen synthetic rates. Biochem Soc Trans. 2002;30:61–65. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Balagopal P, Rooyackers OE, Adey DB, Ades PA, Nair KS. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am J Physiol. 1997;273:E790–800. doi: 10.1152/ajpendo.1997.273.4.E790. [DOI] [PubMed] [Google Scholar]
- Bates PC, Millward DJ. Myofibrillar protein turnover. Synthesis rates of myofibrillar and sarcoplasmic protein fractions in different muscles and the changes observed during postnatal development and in response to feeding and starvation. Biochem J. 1983;214:587–592. doi: 10.1042/bj2140587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennet WM, Connacher AA, Scrimgeour CM, Smith K, Rennie MJ. Increase in anterior tibialis muscle protein synthesis in healthy man during mixed amino acid infusion: studies of incorporation of [1-13C]leucine. Clin Sci. 1989;76:447–454. doi: 10.1042/cs0760447. [DOI] [PubMed] [Google Scholar]
- Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol. 1997;273:E122–129. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- Biolo G, Williams BD, Fleming RY, Wolfe RR. Insulin action on muscle protein kinetics and amino acid transport during recovery after resistance exercise. Diabetes. 1999;48:949–957. doi: 10.2337/diabetes.48.5.949. [DOI] [PubMed] [Google Scholar]
- Bohe J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol. 2001;532:575–579. doi: 10.1111/j.1469-7793.2001.0575f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohe J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose–response study. J Physiol. 2003;552:315–324. doi: 10.1113/jphysiol.2003.050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll CC, Fluckey JD, Williams RH, Sullivan DH, Trappe TA. Human soleus and vastus lateralis muscle protein metabolism with an amino acid infusion. Am J Physiol Endocrinol Metab. 2004 doi: 10.1152/ajpendo.00393.2004. (in press) 10.1152/ajpendo.00393.2004. [DOI] [PubMed] [Google Scholar]
- Chesley A, MacDougall JD, Tarnopolsky MA, Atkinson SA, Smith K. Changes in human muscle protein synthesis after resistance exercise. J Appl Physiol. 1992;73:1383–1388. doi: 10.1152/jappl.1992.73.4.1383. [DOI] [PubMed] [Google Scholar]
- Chinkes D, Klein S, Zhang XJ, Wolfe RR. Infusion of labeled KIC is more accurate than labeled leucine to determine human muscle protein synthesis. Am J Physiol. 1996;270:E67–71. doi: 10.1152/ajpendo.1996.270.1.E67. [DOI] [PubMed] [Google Scholar]
- Fang CH, James HJ, Ogle C, Fischer JE, Hasselgren PO. Influence of burn injury on protein metabolism in different types of skeletal muscle and the role of glucocorticoids. J Am Coll Surg. 1995;180:33–42. [PubMed] [Google Scholar]
- Ferrando AA, Tipton KD, Bamman MM, Wolfe RR. Resistance exercise maintains skeletal muscle protein synthesis during bed rest. J Appl Physiol. 1997;82:807–810. doi: 10.1152/jappl.1997.82.3.807. [DOI] [PubMed] [Google Scholar]
- Fluckey JD, Vary TC, Jefferson LS, Evans WJ, Farrell PA. Insulin stimulation of protein synthesis in rat skeletal muscle following resistance exercise is maintained with advancing age. J Gerontol a Biol Sci Med Sci. 1996a;51:B323–330. doi: 10.1093/gerona/51a.5.b323. [DOI] [PubMed] [Google Scholar]
- Fluckey JD, Vary TC, Jefferson LS, Farrell PA. Augmented insulin action on rates of protein synthesis after resistance exercise in rats. Am J Physiol. 1996b;270:E313–319. doi: 10.1152/ajpendo.1996.270.2.E313. [DOI] [PubMed] [Google Scholar]
- Fowles JR, MacDougall JD, Tarnopolsky MA, Sale DG, Roy BD, Yarasheski KE. The effects of acute passive stretch on muscle protein synthesis in humans. Can J Appl Physiol. 2000;25:165–180. doi: 10.1139/h00-012. [DOI] [PubMed] [Google Scholar]
- Gamrin L, Berg HE, Essen P, Tesch PA, Hultman E, Garlick PJ, McNurlan MA, Wernerman J. The effect of unloading on protein synthesis in human skeletal muscle. Acta Physiol Scand. 1998;163:369–377. doi: 10.1046/j.1365-201X.1998.t01-1-00391.x. [DOI] [PubMed] [Google Scholar]
- Garlick PJ, Maltin CA, Baillie AG, Delday MI, Grubb DA. Fiber-type composition of nine rat muscles. II. Relationship to protein turnover. Am J Physiol. 1989;257:E828–832. doi: 10.1152/ajpendo.1989.257.6.E828. [DOI] [PubMed] [Google Scholar]
- Kelly FJ, Lewis SE, Anderson P, Goldspink DF. Pre- and postnatal growth and protein turnover in four muscles of the rat. Muscle Nerve. 1984;7:235–242. doi: 10.1002/mus.880070309. 10.1002/mus.880070309. [DOI] [PubMed] [Google Scholar]
- Kita F, Koike K, Metori K, Takahashi S. Protein synthesis in fast and slow muscles of developing cockerels loaded with 2G for 3 weeks. Aviat Space Environ Med. 1998;69:145–148. [PubMed] [Google Scholar]
- Lang CH, Vary TC, Frost RA. Acute in vivo elevation of insulin-like growth factor (IGF) binding protein-1 decreases plasma free IGF-I and muscle protein synthesis. Endocrinology. 2003;144:3922–3933. doi: 10.1210/en.2002-0192. 10.1210/en.2002-0192. [DOI] [PubMed] [Google Scholar]
- Lang CH, Wu D, Frost RA, Jefferson LS, Kimball SR, Vary TC. Inhibition of muscle protein synthesis by alcohol is associated with modulation of eIF2B and eIF4E. Am J Physiol. 1999;277:E268–276. doi: 10.1152/ajpendo.1999.277.2.E268. [DOI] [PubMed] [Google Scholar]
- Laurent GJ, Sparrow MP, Bates PC, Millward DJ. Turnover of muscle protein in the fowl (Gallus domesticus). Rates of protein synthesis in fast and slow skeletal, cardiac and smooth muscle of the adult fowl. Biochem J. 1978;176:393–401. doi: 10.1042/bj1760393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall JD, Gibala MJ, Tarnopolsky MA, MacDonald JR, Interisano SA, Yarasheski KE. The time course for elevated muscle protein synthesis following heavy resistance exercise. Can J Appl Physiol. 1995;20:480–486. doi: 10.1139/h95-038. [DOI] [PubMed] [Google Scholar]
- Matthews DE, Schwarz HP, Yang RD, Motil KJ, Young VR, Bier DM. Relationship of plasma leucine and alpha-ketoisocaproate during a L-[1-13C]leucine infusion in man: a method for measuring human intracellular leucine tracer enrichment. Metabolism. 1982;31:1105–1112. doi: 10.1016/0026-0495(82)90160-3. 10.1016/0026-0495(82)90160-3. [DOI] [PubMed] [Google Scholar]
- Meier-Augenstein W. Use of gas chromatography- combustion-isotope ratio mass spectrometry in nutrition and metabolic research. Curr Opin Clin Nutr Metab Care. 1999;2:465–470. doi: 10.1097/00075197-199911000-00005. 10.1097/00075197-199911000-00005. [DOI] [PubMed] [Google Scholar]
- Miller BF, Olesen JL, Hansen M, Døssing S, Crameri R, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, Smith K, Rennie MJ. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. Proceedings of the Nat Acad Sci. in press doi: 10.1113/jphysiol.2005.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward DJ, Garlick PJ, James WP, Nnanyelugo DO, Ryatt JS. Relationship between protein synthesis and RNA content in skeletal muscle. Nature. 1973;241:204–205. doi: 10.1038/241204a0. [DOI] [PubMed] [Google Scholar]
- Nair KS, Halliday D, Griggs RC. Leucine incorporation into mixed skeletal muscle protein in humans. Am J Physiol. 1988;254:E208–213. doi: 10.1152/ajpendo.1988.254.2.E208. [DOI] [PubMed] [Google Scholar]
- Nygren J, Nair KS. Differential regulation of protein dynamics in splanchnic and skeletal muscle beds by insulin and amino acids in healthy human subjects. Diabetes. 2003;52:1377–1385. doi: 10.2337/diabetes.52.6.1377. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol. 1997;273:E99–107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Tipton KD, Ferrando AA, Wolfe RR. Resistance training reduces the acute exercise-induced increase in muscle protein turnover. Am J Physiol. 1999;276:E118–124. doi: 10.1152/ajpendo.1999.276.1.E118. [DOI] [PubMed] [Google Scholar]
- Preedy VR, Peters TJ. Acute effects of ethanol on protein synthesis in different muscles and muscle protein fractions of the rat. Clin Sci. 1988;74:461–466. doi: 10.1042/cs0740461. [DOI] [PubMed] [Google Scholar]
- Rennie MJ. Control of muscle protein synthesis as a result of contractile activity and amino acid availability: implications for protein requirements. Int J Sport Nutr Exerc Metab. 2001;11(l):S170–176. doi: 10.1123/ijsnem.11.s1.s170. [DOI] [PubMed] [Google Scholar]
- Rennie MJ, Meier-Augenstein W, Watt PW, Patel A, Begley IS, Scrimgeour CM. Use of continuous-flow combustion MS in studies of human metabolism. Biochem Soc Trans. 1996;24:927–932. doi: 10.1042/bst0240927. [DOI] [PubMed] [Google Scholar]
- Rennie MJ, Wackerhage H, Spangenburg EE, Booth FW. Control of the size of the human muscle mass. Annu Rev Physiol. 2004;66:799–828. doi: 10.1146/annurev.physiol.66.052102.134444. 10.1146/annurev.physiol.66.052102.134444. [DOI] [PubMed] [Google Scholar]
- Schwenk WF, Berg PJ, Beaufrere B, Miles JM, Haymond\ MW. Use of t-butyldimethylsilylation in the gas chromatographic/mass spectrometric analysis of physiologic compounds found in plasma using electron-impact ionization. Anal Biochem. 1984;141:101–109. doi: 10.1016/0003-2697(84)90431-7. 10.1016/0003-2697(84)90431-7. [DOI] [PubMed] [Google Scholar]
- Tipton KD, Ferrando AA, Williams BD, Wolfe RR. Muscle protein metabolism in female swimmers after a combination of resistance and endurance exercise. J Appl Physiol. 1996;81:2034–2038. doi: 10.1152/jappl.1996.81.5.2034. [DOI] [PubMed] [Google Scholar]
- Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. Jama. 2001;286:1206–1212. doi: 10.1001/jama.286.10.1206. 10.1001/jama.286.10.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt PW, Lindsay Y, Scrimgeour CM, Chien PA, Gibson JN, Taylor DJ, Rennie MJ. Isolation of aminoacyl-tRNA and its labeling with stable-isotope tracers: Use in studies of human tissue protein synthesis. Proc Natl Acad Sci U S A. 1991;88:5892–5896. doi: 10.1073/pnas.88.13.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendel U, Langenbeck U. Intracellular levels and metabolism of leucine and alpha-ketoisocaproate in normal and maple syrup urine disease fibroblasts. Biochem Med. 1984;31:294–302. doi: 10.1016/0006-2944(84)90085-1. 10.1016/0006-2944(84)90085-1. [DOI] [PubMed] [Google Scholar]