Abstract
5-Hydroxytryptamine (5-HT; serotonin)-containing neurones contribute to reflex activation of parasympathetic outflow in a number of species, but the 5-HT receptors mediating these effects have yet to be fully determined. The present experiments demonstrate that central 5-HT7 receptors are involved in the vagal bradycardia evoked during the cardiopulmonary reflex, baroreflexes and the chemoreflex, as well as other autonomic changes caused by these reflexes. The experiments examined the effects of the selective 5-HT7 receptor antagonists SB-269970 and SB-656104 on these reflexes. For the cardiopulmonary reflex, when compared to time-matched vehicle control experiments, intracisternal application of SB-269970 (30–300 μg kg−1, i.c.) dose-dependently attenuated the evoked bradycardia. At the highest dose, SB-269970 also attenuated the reflex hypotension and sympathoinhibition. The structurally different 5-HT7 receptor antagonist SB-656104 (100 μg kg−1, i.c.) similarly attenuated the reflex bradycardia and hypotension. SB-269970 (100 μg kg−1, i.c.) also attenuated the bradycardias evoked by electrical stimulation of aortic nerve afferents and the baroreflex evoked by the pressor response to phenylephrine (3–25 μg kg−1, i.v.). The gain of the baroreflex was also significantly attenuated (0.15 ± 0.06 versus 0.34 ± 0.06 ms mmHg−1). Finally, SB-269970 (100 μg kg−1, i.c.) significantly attenuated both the bradycardia and sympathoexcitation evoked by the chemoreflex. These data indicate that central 5-HT7 receptors play an important facilitatory role in the reflex activation of vagal outflow to the heart.
Central 5-hydroxytryptamine (5-HT)-containing neurones make an important contribution to the reflex activation of parasympathetic outflow in a number of species (Ramage, 2001; Jordan, 2005). Blockade of 5-HT1A receptors within the CNS attenuates reflex parasympathetic outflow to the heart in rats (Bogle et al. 1990), rabbits (Futuro-Neto et al. 1993; Dando et al. 1998; Skinner et al. 2002) and cats (Wang & Ramage, 2001), to the airways in cats (Bootle et al. 1996) and guinea pigs (Bootle et al. 1998), and to the bladder in rats (Conley et al. 2001). Recently, blockade of supraspinal but not spinal 5-HT7 receptors has been shown to attenuate the micturition reflex (i.e. parasympathetic outflow to the bladder) in rats (Read et al. 2003). This is in contrast to blockade of sacral 5-HT1A receptors where blockade of both supraspinal (Secker et al. 2002) and spinal (Read et al. 2003) receptors attenuated the reflex. These reports suggest that both 5-HT1A and 5-HT7 receptor activation is necessary for successful reflex function, posing the question of whether brainstem 5-HT7 receptors contribute also to other parasympathetic reflexes. Interestingly, mRNA for 5-HT7 receptors has been localized in the nucleus tractus solitarii (NTS) (Gustafson et al. 1996), the site of termination of vagal afferents (Jordan & Spyer, 1986), and an area densely innervated by 5-HT immunoreactive fibres (Steinbusch, 1981).
The aim of this study was to investigate the role of brainstem 5-HT7 receptors in the control of reflex activation of vagal outflow to the heart in anaesthetized rats, comparing cardiopulmonary C-fibre, baroreceptor, and chemoreceptor reflexes using the selective 5-HT7 receptor antagonists SB-269970 (Hagan et al. 2000; Lovell et al. 2000) and SB-656104; the latter is structurally distinct although from the same chemical series as SB-269970 (Thomas et al. 2003) and has been previously shown to be effective in blocking micturition reflexes in rats (Read et al. 2003). Preliminary accounts of the results have been published (Kellett et al. 2003, 2004b).
Methods
The experiments were carried out under the Animals (Scientific Procedures) Act 1986. After completion of experiments, animals were humanely killed by an overdose of pentobarbitone sodium (i.v.)
General preparation
Experiments were performed on 45 adult male Sprague-Dawley rats (300–400 g body weight) anaesthetized with isoflurane (5% in oxygen), and maintained with α-chloralose (initially 80 mg kg−1, i.v., supplemented with 10–15 mg kg−1, i.v., when necessary). Core body temperature was maintained at 37–38°C by a homeothermic blanket system (Harvard Apparatus, South Natick, MA, USA). The left femoral vein and artery were cannulated (Portex nonsterile tubing, o.d. 0.96 mm, i.d. 0.58 mm) for administration of drugs and fluids, and for measurement of arterial pressure and collection of arterial blood samples, respectively. The trachea was cannulated low in the neck for mechanical ventilation (Harvard Apparatus model 683). Arterial and tracheal pressures were measured with pressure transducers (model P23XL, Statham, Hato Rey, Puerto Rico). Mechanical ventilation with oxygen-enriched room air was commenced once the animal was in the stereotaxic frame. The urinary bladder was also cannulated to prevent any reflex effects of bladder distension. In all experiments, a cannula was inserted via the right external jugular vein so that the tip lay within or close to the right atrium. This cannula was prefilled with 50 μg ml−1 phenylbiguanide (PBG). In some experiments, a cannula was also inserted into the right femoral vein, and prefilled with either 0.5 mg ml−1 sodium cyanide (NaCN) or 0.1 mg ml−1 phenylephrine.
The animals were then placed in a stereotaxic head frame and the atlanto–occipital membrane was exposed by retraction of the nuchal muscles. A 23 gauge needle was inserted into the cisterna magna as a guide cannula. The right phrenic nerve was exposed by reflecting the right scapula laterally. The nerve was dissected and desheathed, crushed distally to block afferent traffic, and placed on a bipolar silver hook electrode. The nerve and electrode were covered in polyvinylsiloxane dental impression material (Super Dent, Carlisle Laboratories, NY, USA). This prevented the nerve drying out or excess fluid short-circuiting the electrode. Whole-nerve activity was amplified (Digitimer NL 104; gain 10 000–20 000) and filtered (Digitimer NL 125; 500–1000 Hz). The signal was also passed through a solid-state electronic integrator (Royal Free Medical Electronics), which quantified activity in 5 s bins above background noise as a measure of both frequency and size of burst firing. For illustrations, raw signal was digitally rectified and smoothed (100 ms time constant). In some experiments the right aortic depressor nerve was dissected from the cervical vagus and sympathetic trunk, placed on a bipolar silver hook electrode, and also covered with polyvinylsiloxane. The electrode was connected to a constant current stimulator (Digitimer DS2) triggered by a digital programmer (Digitimer D4030). The left renal nerve was exposed by a retroperitoneal approach, placed on a bipolar platinum hook electrode, and also covered with polyvinylsiloxane. Whole-nerve activity was amplified (Digitimer NL 104; gain 20 000) and filtered (Digitimer NL 125; 100–500 Hz). The electrocardiogram (ECG) was recorded from lead II via needles inserted under the skin. The signal was amplified (Digitimer NL 104; gain 20 000) and filtered (Digitimer NL115; 10–100 Hz). The R-wave was discriminated using a Spike Trigger (Neurolog, NL201).
Once surgery was completed, animals were neuromuscularly blocked with α-bungarotoxin (75 μg per animal, i.v.). During neuromuscular blockade, depth of anaesthesia was assessed by continuous monitoring of the stability of blood pressure, heart rate and phrenic nerve activity, and the absence of cardiovascular response to noxious stimuli. An intravenous infusion was commenced (6 ml kg−1 h−1; Gilson Minipuls 2) consisting of 50% Gelofusine plasma substitute, and 50% distilled water containing 100 mm sodium bicarbonate and 10 mm glucose. This helped to maintain blood volume and prevent metabolic acidosis. At regular intervals, arterial blood samples were collected in heparinized capillary tubes, and analysed using a pH/blood gas analyser (Ciba Corning 238). Arterial blood gases were maintained at PO2 90–120 mmHg, PCO2 40–50 mmHg and pH 7.3–7.4 by adjusting the rate and/or stroke volume of the ventilator. A PCO2 range of slightly higher than physiological norms was chosen to ensure that phrenic nerve discharge was entrained to the ventilator cycle. Also, to ensure a bradycardia rather than tachycardia in response to NaCN injection, PO2 was maintained at 85–100 mmHg in experiments involving the chemoreflex. The appropriate stimulation parameters for the reflex to be tested were then determined. The preparation was then left to stabilize for at least 30 min before a particular experimental protocol was carried out. At the end of the experiment, the position of the intracisternal (i.c.) cannula was verified by injecting 10 μl pontamine sky-blue dye through the cannula. The heaviest staining was seen on the dorsal surface of the medulla just caudal to the obex, but considerable staining was also seen around the ventral surface of the medulla and pons. Light staining continued rostrally along the ventral surface as far as the optic chiasm, and caudally to approximately C2. No staining was seen in cerebellar or cortical areas, or in more caudal parts of the spinal cord.
Protocols
At the start of each protocol, anaesthesia was supplemented by giving 15 mg kg−1α-chloralose i.v. to ensure a stable level of anaesthesia during the protocol. In addition, animals were pretreated with the selective β1-adrenoceptor antagonist atenolol (1 mg kg−1, i.v.) to block sympatho–adrenal drive to the heart, so that changes in heart rate could be assumed to reflect changes in cardiac vagal outflow (Bogle et al. 1990). Atenolol was chosen as it penetrates the central nervous system poorly (Street et al. 1979) and has negligible affinity for 5-HT receptors (Middlemiss et al. 1977). A 10 min stabilization period was allowed to elapse before control reflexes were elicited. After three stable controls, the test solution for i.c. injection was administered using a 25 μl glass syringe (Hamilton, Reno, NV, USA) attached via a short length of polythene tubing to a 27 gauge steel cannula. This was inserted into the cisterna magna via the guide needle. The test solutions (10 μl) were injected over 20 s, and the injector left in place for 5 min to ensure diffusion. At the end of some experiments, atenolol was administered again and was not found to cause any overt effect on baseline heart rate, indicating there was still full β-adrenoceptor blockade.
Protocol 1: activation of cardiopulmonary afferents and the baroreflex
In this protocol the effects of the selective 5-HT7 receptor antagonists SB-269970 and SB-656104, and the 5-HT1A receptor antagonist WAY-100635, on the cardiopulmonary reflex were investigated, whilst for the baroreflex, only the effects of SB-269970 were investigated. Ten minutes after the administration of α-chloralose and atenolol, PBG (3–15 μg kg−1) was injected intra-atrially, the dose having been adjusted to evoke submaximal bradycardia (40–100 beats min−1). PBG was then given intra-atrially every 10 min until between three and four stable control bradycardias (less than 10% variability) had been obtained. Five minutes after the final control, 10 μl of the test solution was administered (i.c.). PBG challenges were then given at 5, 15 and 25 min after i.c. injection (and in some experiments at 35 and 45 min to ensure recovery of control values). In experiments using phenylephrine (3–25 μg kg−1, i.v.), the same protocol was used, but phenylephrine given instead of PBG. The dose had been adjusted to evoke submaximal bradycardia (30–100 beats min−1). In three baroreflex control experiments (in the phenylephrine saline control group), following the last dose of phenylephrine, rats were left for 30 min, then 15 mg kg−1 chloralose and 1 mg kg−1 atenolol were given i.v. and protocol 1 was repeated, giving three control PBG reflex stimuli, SB-269970 (100 μg kg−1, i.v.), then a PBG reflex at 5 min only.
Protocol 2: activation of the aortic depressor nerve and the chemoreflex
In this protocol only the 5-HT7 receptor antagonist SB-269970 was studied. Two alternating stimuli were given, always separated by 5 min. Ten minutes after administering α-chloralose and atenolol, the aortic depressor nerve was stimulated with 5 s trains of 40 Hz pulses (0.1 ms pulse duration, 0.1–1.0 mA). The current had been adjusted to evoke a submaximal bradycardia (40–100 beats min−1). Five minutes later, the chemoreflex was elicited by i.v. injection of 75–150 μg kg−1 NaCN (Franchini & Kreiger, 1993), the dose having been adjusted to evoke submaximal bradycardia (30–100 beats min−1). Alternating challenges were delivered every 5 min until between three and four stable control bradycardias had been obtained for each reflex. As soon as variables had returned to baseline after the final control NaCN challenge (<2 min), SB-269970 (100 μg kg−1) was administered i.c. Aortic depressor nerve stimuli were given 5, 15 and 25 min, and NaCN injections were given at 10, 20 and 30 min after administration of the SB-269970. In some experiments, at the end of the protocol, the effects of atropine methonitrate (1 mg kg−1, i.v.) on the NaCN-evoked changes in heart rate were noted to control for possible direct cardiac effects of NaCN.
Analysis of data
Arterial blood pressure, heart rate (from ECG), raw renal nerve activity, and raw and integrated phrenic nerve activity were recorded onto a computer hard disk using a CED 1401+ interface and Spike2 data collection software (Cambridge Electronic Design). For nerve activity, the noise levels were verified at the end of the experiment by slowly giving pentobarbitone sodium (60 mg per animal, i.v.) to abolish activity. The background noise was then determined and subtracted from the measured values. All baseline variables were calculated from the 30 s period prior to a reflex stimulation. Control baselines were averaged from the three control reflexes, and postdrug or vehicle baselines were measured from the 30 s periods preceding each subsequent reflex. Baseline mean arterial pressure (MAP; calculated as: diastolic pressure + (pulse pressure/3)) was measured over the 30 s before a reflex using best-fit horizontal cursors. Then MAP at the peak response following a reflex was measured, and absolute changes in MAP (peak response MAP − baseline MAP) were calculated for each reflex. Baseline heart rate (HR) was measured over the 30 s before a reflex using best-fit horizontal cursors, and the HR at the peak response was also measured. HR values were then converted to R-R interval (60 × 1000/HR; ms), and the absolute change in R-R interval (peak R-R interval − baseline R-R interval) was calculated. Raw renal nerve activity was rectified and smoothed (1 s time constant) using Spike2 functions. The resulting integrated renal nerve activity (IRNA) baseline was measured by averaging the 30 s prior to a reflex using Spike2 cursor functions. The peak change after a reflex was measured using a horizontal cursor, and calculated as the absolute fall for the cardiopulmonary reflex, and as the absolute rise within 10 s of NaCN injection for the chemoreflex. Baseline integrated phrenic nerve activity (IPNA) was calculated by measuring the mean height of the six 5 s bins prior to a reflex. For the chemoreflex, peak change was measured as the largest 5 s bin in the 10 s following the NaCN injection. For the cardiopulmonary reflex, phrenic changes were too inconsistent to be quantified. Since the absolute values of both phrenic and renal integrated nerve activity vary substantially between animals, the baselines prior to each stimulation were normalized to 100%, and changes were expressed as percentage change. For aortic depressor nerve stimulations, nerve activities were not quantified due to interference of stimulus artefacts, although a sympathoinhibition was detectable from the audio output. For phenylephrine-evoked baroreflexes, variables were measured as for the cardiopulmonary reflex, but RNA was quantified by comparing the mean of the 60 s period before and after phenylephrine injection, and converting changes to percentage of control. Reflex gain was quantified as previously described (Su et al. 1992). Using a Spike2 script, each arterial pressure pulse during a phenylephrine-evoked pressure ramp was converted to MAP and plotted against the R-R interval at the corresponding time point up to the maximum increase in R-R interval. A regression line was plotted through these points (Sigma Plot), to measure slope (milliseconds per millimetre of mercury) and correlation coefficient (r). In order to achieve the maximum correlation between the points (r > 0.8), the MAP values were staggered one beat at a time with respect to their corresponding R-R intervals. Typically, MAPs would be staggered by up to 10 beats to achieve the maximum correlation coefficient. The slope of this regression line is the baroreflex gain – a measure of reflex sensitivity.
Raw data were collated and expressed as means ±s.e.m. Statistical analysis was performed using a two-way analysis of variance (ANOVA) to compare each drug-treated experimental group with its time-matched vehicle control group. A priori analysis was performed using the least significant difference (LSD) test, to calculate significant differences between means of drug- and saline-treated groups at specific time points. In addition, vehicle-treated groups were analysed using one-way ANOVA followed by Tukey's a posteriori analysis to compare the averaged control baselines and control reflexes with the baselines and reflexes at various times after vehicle. For all statistical analysis, differences between groups were considered significant when P < 0.05.
Drugs and solutions
Drugs and chemicals were obtained from the following sources. The selective 5-HT7 receptor antagonists SB-269970 ((R)-1-[3-hydroxyphenyl)sulphonyl]-2-[2-(4-methyl-1-piperidinyl)ethyl]pyrrolidine) and SB- 656104 (6-((R)-2-{2-[4-(4-chloro-phenoxy)-piperidin-1-yl]-ethyl}-pyrrolidine-1-sulphonyl)-1H-indole hydrochloride) were synthesized at GlaxoSmithKline (GSK), Harlow, UK. The selective 5-HT1A receptor antagonist WAY-100635 (N-[2-[4-2-methoxyphenyl-1-piperazinyl]ethyl]-N-(2-pyridinyl)cyclohexanecarbox-amide maleate), atenolol, atropine methylnitrate, α-bungarotoxin, 1-phenylbiguanide, sodium tetraborate (Borax), polyethylene glycol and dimethyl sulfoxide were from Sigma Aldrich Chemicals, Poole, UK. α–Chloralose was from Vickers Laboratories Ltd, Pudsey, UK. Sodium chloride, d-glucose, sodium cyanide and sodium bicarbonate were from BDH, Poole, UK. Isoflurane (Aerrane) was from Baxter Healthcare Ltd, Thetford, UK. Pentobarbitone sodium (Sagatal) was from Rhône-Mérieux Ltd, Harlow, UK. Gelofusine was from Braun Medical Ltd, Sheffield, UK. Heparin was from CP Pharmaceuticals, Wrexham, UK. All chemicals were dissolved in 0.9% saline, with the following exceptions: SB-656104 was dissolved in 27.5% polyethylene glycol 400, 27.5% dimethyl sulfoxide and 45% saline; α–chloralose was dissolved in 0.9% saline containing 2.5% (w/v) borax, to give a 2.5% (w/v) solution.
Results
Effects of SB-269970, SB-656104 and WAY-100635 on baseline variables
The resting baseline values of the cardiovascular, sympathetic and respiratory variables (based on all the cardiopulmonary reflex experiments) before, and 5 and 15 min following each i.c. drug treatment are shown in Table 1. The i.c. administration of saline (10 μl, n = 5, pH 5.8) had no significant effect on MAP, renal nerve activity (RNA) or phrenic nerve activity (PNA), but it produced a statistically significant, but small, fall in R-R interval (equivalent to an increase in heart rate of 13 beats min−1) after 15 min when compared with baseline control. SB-269970 i.c. at all doses had no significant effect on MAP, R-R interval or RNA when compared with saline. However, baseline PNA was significantly attenuated at the higher doses of SB-269970 when compared with saline. SB-656104, (100 μg kg−1, i.c., n = 5) when compared to its vehicle (10 μl, n = 5; see Methods), had no significant effect on any of the variables measured. However, the vehicle did cause a small but significant increase in MAP after 5 min when compared to baseline control values. The 5-HT1A receptor antagonist WAY-100635 (100 μg kg−1, i.c., n = 5) had no significant effect on any baseline variable.
Table 1.
Baseline values of mean arterial pressure (MAP), R-R interval, integrated renal (IRNA) and integrated phrenic nerve activity (IPNA) for all cardiopulmonary reflex experimental groups (protocol 1) in anaesthetized, neuromuscular-blocked and atenolol-pretreated rats
| MAP (mmHg) | R-R interval (ms) | IRNA (% of control) | IPNA (% of control) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Experimental group | n | Control | 5 min | 15 min | Control | 5 min | 15 min | 5 min | 15 min | 5 min | 15 min |
| Saline | |||||||||||
| 10 μl | 5 | 112 ± 3 | 116 ± 5 | 118 ± 5 | 180 ± 7 | 175 ± 7 | 173 ± 7† | 96 ± 15 | 99 ± 18 | 116 ± 13 | 121 ± 10 |
| SB-269970 | |||||||||||
| 30 μg kg−1 | 5 | 113 ± 10 | 110 ± 11 | 114 ± 10 | 177 ± 4 | 175 ± 5 | 173 ± 5 | 99 ± 2 | 103 ± 4 | 131 ± 18 | 123 ± 21 |
| 100 μg kg−1 | 5 | 120 ± 6 | 119 ± 10 | 126 ± 6 | 182 ± 3 | 179 ± 3 | 179 ± 3 | 102 ± 4 | 103 ± 5 | 143 ± 18 | 88 ± 18* |
| 300 μg kg−1 | 5 | 111 ± 9 | 116 ± 14 | 131 ± 16 | 187 ± 5 | 183 ± 3 | 175 ± 2† | 104 ± 4 | 115 ± 7 | 56 ± 9* | 24 ± 12** |
| SB-656104 | |||||||||||
| Vehicle 10 μl | 5 | 107 ± 2 | 117 ± 1† | 118 ± 2† | 190 ± 4 | 186 ± 5 | 184 ± 5 | 100 ± 10 | 129 ± 29 | 88 ± 10 | 114 ± 23 |
| 100 μg kg−1 | 5 | 105 ± 6 | 115 ± 9 | 128 ± 8 | 187 ± 4 | 185 ± 4 | 180 ± 4 | 145 ± 40 | 139 ± 49 | 77 ± 8 | 144 ± 38 |
| WAY-100635 | |||||||||||
| 100 μg kg−1 | 5 | 115 ± 3 | 118 ± 4 | 118 ± 3 | 170 ± 2 | 170 ± 2 | 165 ± 2 | 95 ± 4 | 107 ± 5 | 93 ± 26 | 87 ± 29 |
P < 0.05
P < 0.01 compared to vehicle;
P < 0.05 compared to baseline control.
Effects of SB-269970, SB-656104 and WAY-100635 on cardiopulmonary afferent-evoked changes
Right atrial bolus administration of PBG (1–5 μg, 20–100 μl, n = 35) evoked an increase in R-R interval of 49 ± 3 ms from a baseline of 181 ± 2 ms, a fall in MAP of 34 ± 2 mmHg from a baseline of 110 ± 3 mmHg, and a fall in renal nerve activity of 46 ± 5%. Additionally either apnoea or tachypnoea was observed; these changes have not been quantified. Reflex changes usually occurred within 2 s of PBG administration, and returned to baseline within 15–30 s (Fig. 1). Right atrial bolus administration of the same volume of saline (20–100 μl, n = 5) had no significant effects. Saline (i.c.) had no significant effect on the PBG-evoked fall in MAP or the increase in R-R interval. The reflex sympathoinhibition, however, was significantly potentiated at all time points after saline compared to control baseline values (Fig. 2).
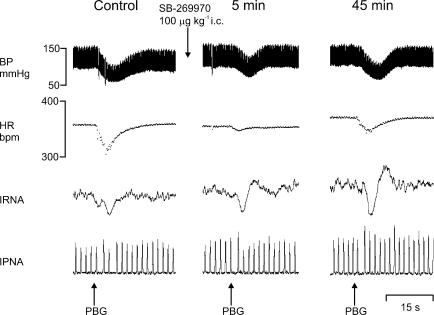
Figure 1. Effect of SB-269970 on cardiopulmonary reflexes.
Experimental traces from an anaesthetized, neuromuscular-blocked and atenolol-pretreated rat illustrating the effects on blood pressure (BP), heart rate (HR), integrated renal nerve activity (IRNA), and integrated phrenic nerve activity (IPNA) evoked by stimulation of cardiopulmonary afferents with intra-atrial phenylbiguanide (PBG; 2 μg) before, and 5 min and 45 min following SB-269970 (100 μg kg−1, intracisternal, i.c.).
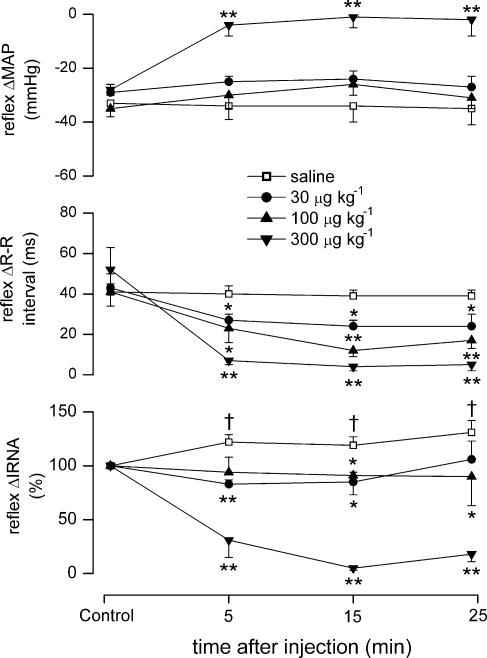
Figure 2. Effect of differing doses of SB-269970 on cardiopulmonary reflex responses.
Graphs showing the effects (mean ±s.e.m) of saline (10 μl, i.c., n = 5), and SB-269970 (30, 100 and 300 μg kg−1, i.c.; n = 5) on changes (Δ) in mean arterial pressure (MAP), R-R interval and IRNA evoked by cardiopulmonary reflex stimulation with phenylbiguanide in anaesthetized, neuromuscular-blocked and atenolol-pretreated rats. *P < 0.05,**P < 0.01 (compared to vehicle); †P < 0.05 (compared to control).
SB-269970 significantly attenuated the response to stimulating cardiopulmonary afferents (Figs 1 and 2). Within the first 5 min, it dose-dependently attenuated the reflex increase in R-R interval, with the greatest change after 15 min. In some experiments this effect was followed, and recovery occurred after 35 min (data not illustrated). SB-269970 had no significant effect on the reflex-evoked fall in MAP, except at the highest dose of 300 μg kg−1 (34 ± 5 versus 6 ± 2 mmHg at 5 min). The reflex renal sympathoinhibition was also strongly attenuated by this high dose. The two lower doses significantly prevented the potentation of the reflex-evoked increase in RNA that was observed with vehicle (Fig. 2). In a separate set of experiments (see protocol 1), intravenous administration of SB-269970 (100 μg kg−1, n = 3) had no effects on either baseline, or cardiopulmonary reflex-evoked changes in MAP, R-R interval or RNA (data not illustrated).
SB-656104 (100 μg kg−1, i.c., n = 5) also produced a significant and long-lasting attenuation of the reflex-evoked increase in R-R interval (55 ± 8 versus 9 ± 5 ms) and of the fall in MAP (35 ± 12 versus 15 ± 5 mmHg; Fig. 3). The reflex sympathoinhibition was unchanged. The vehicle for SB-656104 (10 μl, i.c., n = 5) had no significant effect on any component of the reflex.
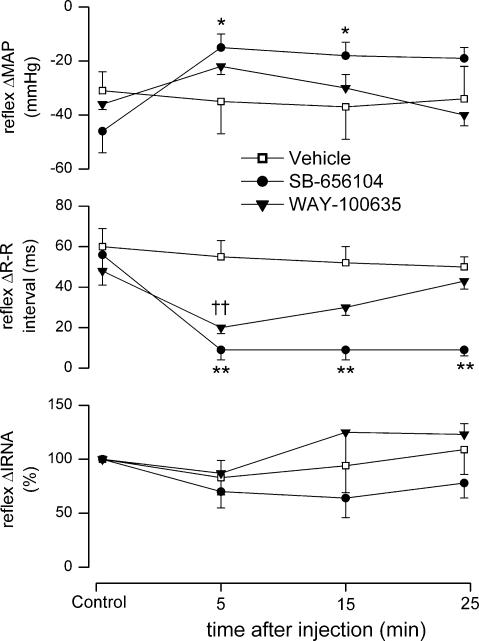
Figure 3. Effect of SB-6856104 and WAY-100635 on cardiopulmonary reflex responses.
Graphs showing the effects (mean ±s.e.m) of vehicle for SB-656104 (10 μl, i.c., n = 5), SB-656104 (100 μg kg−1, i.c., n = 5) and WAY-100635 (100 μg kg−1, i.c., n = 5) on changes in MAP, R-R interval and IRNA evoked by cardiopulmonary reflex stimulation with phenylbiguanide in anaesthetized, neuromuscular-blocked and atenolol-pretreated rats. For clarity, the vehicle for WAY-100635 (saline; see Fig. 2) has been omitted. *P < 0.05,**P < 0.01 (compared to vehicle); ††P < 0.01 (compared to saline vehicle).
WAY-100635 (100 μg kg−1, i.c., n = 5) also significantly attenuated the reflex increase in R-R interval (40 ± 4 versus 20 ± 3 ms; Fig. 3) when compared with saline, but it was without significant effects on the reflex-evoked falls in MAP or RNA.
Effect of SB-269970 on responses evoked by electrical stimulation of the aortic depressor nerve
Electrical stimulation of the right aortic depressor nerve (n = 10) evoked an increase in R-R interval of 52 ± 7 ms from a baseline of 175 ± 3 ms, and a fall in MAP of 28 ± 3 mmHg from a baseline of 106 ± 5 mmHg. These reflex responses began within 1 s of electrical stimulation, peaked within 5 s, and usually returned to baseline within 20 s (Fig. 4).
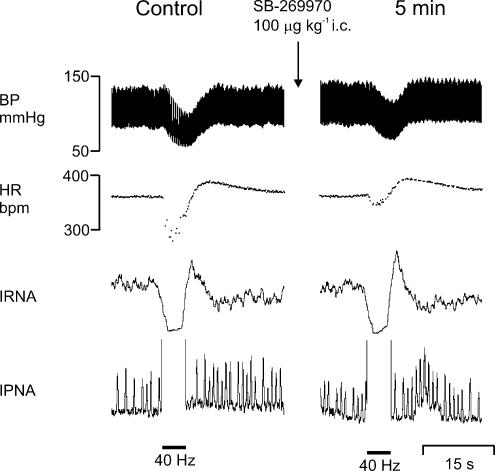
Figure 4. Effect of SB-269970 on responses evoked by aortic depressor nerve stimulation.
Experimental traces from an anaesthetized, neuromuscular-blocked and atenolol-pretreated rat illustrating the effects on BP, HR, IRNA and IPNA evoked by electrical stimulation (40 Hz) of the right aortic depressor nerve before and 5 min following SB-269970 (100 μg kg−1, i.c.).
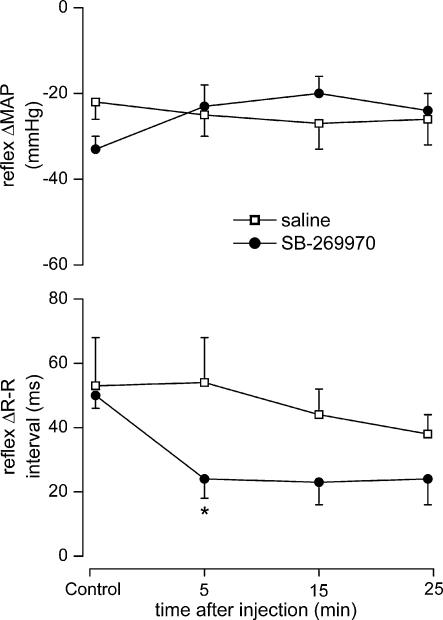
SB-269970 (100 μg kg−1, n = 5) significantly attenuated the reflex increase in R-R interval 5 min after administration (54 ± 14 versus 24 ± 6 ms), but had no significant effect on the reflex-evoked fall in MAP when compared with saline (Figs 4 and 5). Administration of saline (10 μl, i.c., pH 5.8, n = 5) had no significant effect on the reflex MAP or R-R interval changes (Fig. 5).
Figure 5. Effect of SB-269970 on responses evoked by aortic depressor nerve stimulation.
Graphs showing the effects (mean ±s.e.m) of saline (10 μl, i.c., n = 5), and SB-269970 (100 μg kg−1, i.c., n = 5), on changes in MAP and R-R interval evoked by electrical stimulation of the right aortic depressor nerve in anaesthetized, neuromuscular blocked and atenolol pretreated rats. *P < 0.05 (compared to saline).
Effect of SB-269970 on the baroreflex-evoked changes caused by I.V. phenylephrine
Bolus administration of phenylephrine (3–25 μg kg−1, i.v., n = 10) evoked an increase in MAP of 77 ± 2 mmHg from a baseline of 98 ± 5 mmHg, a reflex increase in R-R interval of 22 ± 3 ms from a baseline of 184 ± 4 ms, and renal sympathoinhibition of 44 ± 6%. Traces from one of these experiments are shown in Fig. 6. Baroreflex gain was calculated to be 0.24 ± 0.03 ms mmHg−1. These reflex responses began within 4 s of phenylephrine injection, peaked within 10 s, and usually returned to baseline within 120 s. Administration of saline (10 μl, i.c., pH 5.8, n = 5) had no significant effect on phenylephrine-evoked increases in MAP, R-R interval or renal sympathoinhibition, although the gain was significantly potentiated 5 min after saline (from 0.25 ± 0.04 to 0.34 ± 0.06 ms mmHg−1).
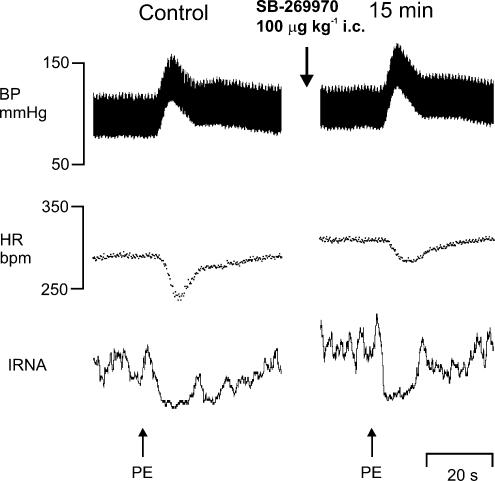
Figure 6. Effect of SB-269970 on responses evoked by intravenous phenylephrine.
Experimental traces from an anaesthetized, neuromuscular-blocked and atenolol-pretreated rat illustrating changes in BP, HR and IRNA (neurogram is rectified and smoothed, time constant 1 s) evoked by phenylephrine (PE; 3 μg, i.v.) before (control) and 15 min following SB-269970 (100 μg kg−1, i.c.).
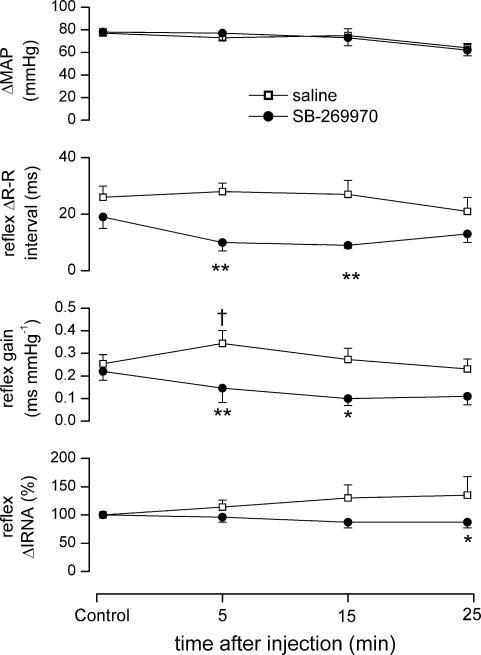
SB-269970 (100 μg kg−1, i.c., n = 5), when compared with saline, significantly attenuated the reflex-evoked increase in R-R interval (28 ± 8 versus 10 ± 3 ms) and gain (0.34 ± 0.06 versus 0.15 ± 0.06 ms mmHg−1), but had no significant effect on the reflex-evoked renal sympathoinhibition (Fig. 7).
Figure 7. Effect of SB-269970 on baroreflex responses and bareoreflex gain.
Graphs showing the effects (mean ±s.e.m) of saline (10 μl, i.c., n = 5), and SB-269970 (100 μg kg−1, i.c., n = 5), on changes in MAP, R-R interval, reflex gain and IRNA evoked by intravenous phenylephrine in anaesthetized, neuromuscular-blocked and atenolol-pretreated rats. *P < 0.05, **P < 0.01 (compared to saline); †P < 0.05 (compared to control).
Effects of SB-269970 on chemoreflex-evoked changes
Bolus injection of NaCN (75–150 μg kg−1, i.v., n = 10) evoked an increase in R-R interval of 26 ± 3 ms from a baseline of 174 ± 3 ms, a fall in MAP of 30 ± 2 mmHg from a baseline of 110 ± 3 mmHg, and increases in RNA and PNA of 79 ± 18 and 232 ± 63%, respectively. These reflex responses began within 4 s of NaCN administration, peaked within 10 s (except RNA and PNA, which sometimes continued to rise for another 10 s), and usually returned to baseline within 30–60 s (except PNA which took up to 2 min to recover; Fig. 8). Saline (0.1 ml, i.v., n = 5) had no significant effect on any of the measured variables. To control for possible direct cardiac effects of NaCN, atropine methonitrate (1 mg kg−1, i.v., n = 3) was administered at the end of a protocol, and it abolished the increase in R-R interval evoked by NaCN.
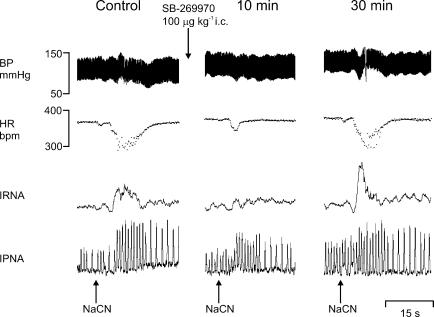
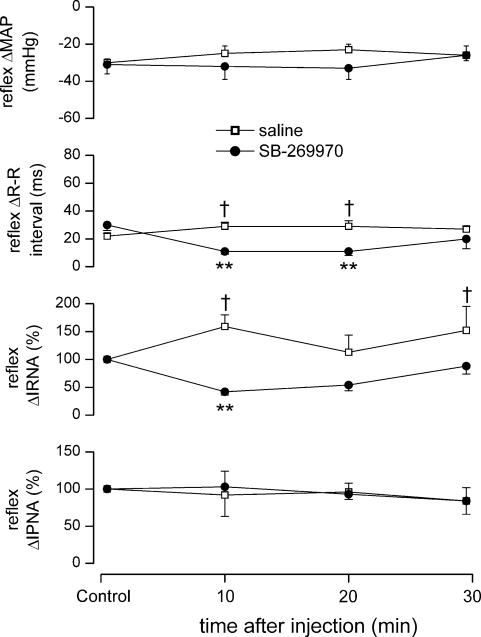
Figure 8. Effect of SB-269970 on chemororeflexes.
Experimental traces from an anaesthetized, neuromuscular-blocked and atenolol-pretreated rat illustrating the effects on BP, HR, IRNA and IPNA evoked by stimulation of chemoreceptor afferents with sodium cyanide (NaCN; 50 μg i.v.) before, and 10 and 30 min following SB-269970 (100 μg kg−1, i.c.).
SB-269970 (100 μg kg−1, i.c., n = 5) caused a significant attenuation of the NaCN-evoked increase in R-R interval (29 ± 3 versus 11 ± 2 ms) and RNA (159 ± 21 versus 42 ± 6% of control), with recovery after 30 min when compared with i.c. saline (Figs 8 and 9). SB-269970 had no significant effect on either the NaCN-induced fall in MAP or the increase in PNA. Saline (10 μl, i.c., pH 5.8, n = 5) had no significant effect on the NaCN-evoked changes in MAP or PNA. Interestingly, compared to control, there were significant increases in the NaCN-evoked increase in R-R interval and renal sympathoexcitation (Fig. 9).
Figure 9. Effect of SB-269970 on chemoreflex responses.
Graphs showing the effects (mean ±s.e.m) of saline (10 μl, i.c.; n = 5) and SB-269970 (100 μg kg−1, i.c., n = 5) on changes in MAP, R-R interval, IRNA and IPNA evoked by chemoreflex stimulation with NaCN (75–150 μg kg−1, i.c.) in anaesthetized, neuromuscular-blocked and atenolol-pretreated rats. **P < 0.01 (compared to saline); †P < 0.05 (compared to control).
Discussion
These experiments demonstrate, for the first time, that central 5-HT7 receptors are involved in the reflex activation of parasympathetic outflow to the heart in anaesthetized rats. Central application of the selective 5-HT7 receptor antagonist SB-269970 (Hagan et al. 2000; Lovell et al. 2000) dose-dependently attenuated the vagally mediated cardiac slowing evoked during reflexes activated by stimulating cardiopulmonary afferent fibres, arterial baroreceptor and chemoreceptor afferents. This effect is most likely due to blockade of 5-HT7 receptors and is not compound specific, as SB-656104, another selective 5-HT7 receptor antagonist (Thomas et al. 2003), also strongly attenuated the HR component of the cardiopulmonary reflex. SB-656104, although from the same chemical series, is sufficiently structurally distinct and has a different selectivity profile (see Table 2) to rule out this latter possibility. Furthermore, the selectivity of SB-269970 is nearly 10 000-fold higher (Table 2) for 5-HT7 receptors over 5-HT1A receptors, the other identified 5-HT receptor involved in the reflex control of parasympathetic outflow to the heart. These effects are most likely to be due to actions on 5-HT7 receptors located centrally within the brainstem, since the same dose of SB-269970 given intravenously was without effect. However, as with any pharmacological study, it is always possible that another, currently unidentified receptor, for which these compounds have affinity could be responsible for these actions. The present data also confirm that central 5-HT1A receptors are also involved in initiating the cardiopulmonary-reflex-evoked vagal bradycardia in the rat, as the selective 5-HT1A receptor antagonist WAY-100635 (Fletcher et al. 1996) given i.c. also attenuated this bradycardia. The present data, however, indicate that 5-HT7 receptors play a more ubiquitous role in the control of cardiac vagal outflow than 5-HT1A receptors, since blockade of 5-HT1A receptors in the rabbit brainstem attenuated cardiopulmonary and baroreflex bradycardias, but did not interfere with carotid chemoreceptor-evoked bradycardias (Skinner et al. 2002). A dual involvement of these two receptors has also been previously demonstrated for the micturition reflex (Read et al. 2003), and in thermoregulation (Hedlund et al. 2004). This is particularly intriguing as these two receptors have opposite effects on adenylyl cyclase; 5-HT7 receptors being positively coupled while 5-HT1A receptors are negatively coupled (Alexander et al. 2004). Interestingly, there is evidence to suggest that 5-HT1A receptor activation can augment 5-HT7 receptor-mediated stimulation of adenylyl cyclase, at least in guinea-pig hippocampal membranes (Thomas et al. 1999). Again, these data demonstrate the importance of central 5-HT containing neurones in the control of cardiac vagal outflow. Further, as with 5-HT1A receptors, it would appear that, at least in the anaesthetized rat, 5-HT7 receptors are not tonically active but are recruited during cardiorespiratory reflexes as neither selective antagonist significantly changed baseline values of HR or blood pressure. In similar experiments performed in awake rats, SB-269970 also attenuated cardiopulmonary reflex bradycardia and hypotension without significantly changing baseline blood pressure or HR (Kellett et al. 2004a).
Table 2.
Receptor binding profiles for SB-269970 and SB-656104
| SB-656104 | SB-269970 | |
|---|---|---|
| Receptor | pKi | pKi |
| 5-HT1A | <5.0 | 6.25 |
| 5-HT1B | 6.0 | 6.20 |
| 5-HT1D | 5.8 | 7.60 |
| 5-ht1E | <5.2 | <5.30 |
| 5-HT1F | <5.5 | <5.70 |
| 5-HT2A | <5.5 | <7.20 |
| 5-HT2B | 5.0 | 7.04 |
| 5-HT2C | <5.0 | 6.57 |
| 5-HT4 | 5.9 | 5.72 |
| 5-ht5A | 7.2 | 6.74 |
| 5-HT6 | 5.2 | 6.07 |
| 5-HT7 | 8.9 | 8.70 |
| α1B | <5.0 | 6.67 |
| D2 | 6.5 | 7.01 |
| D3 | 5.6 | 6.49 |
The receptor binding profiles were obtained from radioligand binding assays using human cloned receptors taken for SB-269970 from Lovell et al. (2000) and for SB-656104 from Thomas et al. (2003).
Site(s) of action
The precise location of these 5-HT7 receptors was not determined in the present experiments. The sensory afferents stimulated in this study all terminate within the NTS, where visceral afferent information is processed and integrated (see Jordan & Spyer, 1986). From there, activity passes (via multisynaptic pathways) to the dorsal vagal nuclei and nucleus ambiguus (the location of parasympathetic cardiac preganglionic neurones) (Taylor et al. 1999) and the rostral ventrolateral medulla (RVLM), the location of premotor sympathetic neurones (Guyenet, 1990). All these sites are probably within diffusion distance of the site of ligand application so any one (or more) of these sites may be involved in the modulations described in the present paper, since all receive substantial innervation by 5-HT-containing nerve fibres (Steinbusch, 1981). However, to date, only the NTS has been reported to contain 5-HT7 receptor mRNA (Gustafson et al. 1996), and in this respect the NTS is known to contain many other 5-HT receptor subtypes (see Jordan, 2005). For instance, activation of 5-HT3 receptors facilitates transmission in the NTS, and blockade of these receptors can attenuate cardiopulmonary afferent excitation of these neurones (Jeggo et al. 2000b, 2001). Activation of different 5-HT2 receptor subtypes can have opposing effects on NTS neurones, and this depends on the type of NTS neurones (Sévoz-Couche et al. 2000). Regarding 5-HT1 receptors, 5-HT1B and 5-HT1D receptors also have opposing actions on NTS neurones (Jeggo et al. 2000a), while activating 5-HT1A receptors, using the archetypal agonist 8-OH-DPAT, has variable effects (Wang et al. 1997). Since antagonism of these responses was not attempted, and it is now known that 8-OH-DPAT has affinity also for 5-HT7 receptors (Lovenberg et al. 1993), the excitatory effects may be due to activation of this receptor. In this respect, application of SB269970 to the dorsal surface of the medulla reduced vagal-evoked discharge of NTS neurones (Kellett et al. 2004c). If 5-HT7 receptors are involved in facilitating vagal afferent input to cardiac vagal preganglionic neurones at the level of the NTS, then the question arises of how these receptors interact with 5-HT3 receptors, which also facilitate vagal afferent input to NTS neurones presumably by facilitating glutamate release (Jeggo et al. 2000b, 2001). It is now well documented that glutamate receptors are important in synaptic transmission at both the first and at subsequent synapses within the NTS (Zhang & Mifflin, 1998; Jones et al. 2002). In addition, vagal afferents are known to contain both glutamate (Saha et al. 1995) and 5-HT (Sykes et al. 1994), and at least some of the 5-HT3 receptors are located on these vagal afferent terminals (Pratt & Bowery, 1989). However, the cellular location of the 5-HT7 receptors remains to be determined.
At the level of vagal preganglionic neurones, 5-HT had an excitatory action when applied at low ionophoretic currents, but a predominantly depressant action when applied at higher currents to rat dorsal vagal neurones (Wang et al. 1995). In addition, 8-OH-DPAT also had both excitatory and inhibitory action on rat dorsal vagal neurones (Wang et al. 1995) and cat cardiac vagal preganglionic neurones in the nucleus ambiguus (Wang & Ramage, 2001). Further, when a 5-HT1A receptor antagonist was given locally, it was found that it could attenuate cardiopulmonary excitation of these cardiac vagal preganglionic neurones, suggesting that the 5-HT1A-receptor-mediated action could be at this level of the brain (Wang & Ramage, 2001). However, only the excitatory effects of 5-HT and 8-OH-DPAT were attenuated by selective 5-HT1A receptor antagonists (Wang et al. 1995; Wang & Ramage, 2001), suggesting that the inhibitions were mediated by some other 5-HT receptor. There was no evidence to suggest that 8-OH-DPAT was causing excitation through 5-HT7 receptors.
Sympathetic outflow, arterial blood pressure and respiration
The data presented suggest that the NTS may be the primary site at which 5-HT7 receptors are recruited in these reflex pathways. If so, and as the NTS is the site at which afferent input is integrated (see Jordan & Spyer, 1986), then it may be expected that blockade of these receptors in the NTS would also interfere with other components of these reflexes. Thus, if 5-HT7 receptors were acting in the reflex pathway antecedent to the particular NTS neurones involved in processing the sympathetic components of these reflexes, then both reflex-evoked sympathoinhibition and excitation should be attenuated by the antagonist. Indeed, the renal sympathoexcitation evoked by the chemoreflex was attenuated but the baroreflex sympathoinhibition was unaffected. The data on the sympathoinhibitory response of SB-269970 on the cardiopulmonary reflex is not clear-cut in that the two lower doses only inhibited the tendency of reflex-evoked renal sympathoinhibition to get larger with time, this was not dose-dependent and was not inhibited by the other 5-HT7 receptor antagonist SB-656104. The observed inhibition of the cardiopulmonary sympathoinhibitory response by the highest dose of SB-269970 could be due to SB-269970 interfering with another 5-HT receptor. One possibility is the 5HT1A receptor, since central activation of these receptors is well known to evoke sympathoinhibition, although the primary site for this is the RVLM (Ramage, 2001). However, this is unlikely since the 5-HT1A receptor antagonist WAY-100635 did not alter the cardiopulmonary-evoked renal sympathoinhibition. Intriguingly, SB-269970 does have an affinity for one of the least-understood 5-HT receptors, the 5-ht5A receptor, having a pKi of 7.2 compared with 8.9 on the 5-HT7 receptor (Table 2), and this receptor is negatively coupled to adenylyl cyclase (Thomas et al. 2004). In addition, 5-ht5A receptor immunoreactivity has been visualized in the NTS and brainstem raphe nuclei, but not as yet within the RVLM (Oliver et al. 2000). Thus, this receptor could be involved in the control of sympathetic outflow mediated by the cardiopulmonary reflex.
The data for chemoreflex-evoked sympathoexcitation are clearer, and would suggest that 5-HT7 receptors are involved, and this is consistent with 5-HT7 receptors increasing adenylyl cyclase activity. Again, whether this is occurring at the level of the NTS, the RVLM and/or some other site(s) remains to be determined. During the chemoreflex evoked in the present study, renal sympathoexcitation was observed without an associated rise in blood pressure, suggesting that there was no global effect on sympathetic outflow to the cardiovascular system. The failure to observe an increase in blood pressure, although renal sympathetic nerve activity increased, may be due to anaesthesia blunting the overall sympathoexcitatory response. Additionally, the fall in cardiac output associated with the bradycardia may prevent an increase in blood pressure being observed. Indeed, in their original study Franchini & Kreiger (1993) also observed a decrease in blood pressure in anaesthetized rats, but a rise in pressure in awake rats, when using this method to activate the chemoreceptor reflex.
Effects of 5-HT7 receptors in other systems
In a number of systems, activation of 5-HT7 receptors has been shown to increase neuronal excitability. In both hippocampal CA3 (Gill et al. 2002) and CA1 (Tokarski et al. 2003) pyramidal cells, and in trigeminal jaw-closing motoneurones (Inoue et al. 2002), they reduce the amplitude of the slow afterhyperpolarization, which results in an increase in bursting activity. In addition, 5-HT7 receptors enhance excitability in thalamic (Chapin & Andrade, 2001), CA1 (Bickmeyer et al. 2002) and dorsal root ganglion (DRG) (Cardenas et al. 1999) neurones by enhancing hyperpolarization-activated current (IH).
Although this is the first report of an action on cardiac parasympathetic outflow, 5-HT7 receptors have been implicated in other reflex responses. Supraspinal 5-HT7 receptors appear to be required for normal functioning of the micturition reflex (Read et al. 2003), and, at the hypothalamic level, the hypothermia induced by 5-CT or 8-OH-DPAT application is reduced by blockade of 5-HT7 receptors (Hagan et al. 2000; Guscott et al. 2003) and absent in 5-HT7-receptor-knockout mice (Guscott et al. 2003; Hedlund et al. 2004). In addition, these receptors have been shown to phase shift the circadian rhythm (Sprouse et al. 2004), and to modulate the onset and duration of REM sleep (Thomas et al. 2003). At the spinal level, 5-HT7 receptors localized in DRG cells (Cardenas et al. 1999; Meuser et al. 2002) and laminae I and II (Meuser et al. 2002) have been implicated in nociceptor activation of lumbar primary afferents by 5-HT (Meuser et al. 2002) and in enhancing reflex activation gastrocnemius muscle by sural nerve afferents (Ogilvie et al. 1999), but they do not seem to be involved in micturition reflexes (Read et al. 2003).
Conclusion
In the present experiments in rats, all the reflex activations of cardiac vagal preganglionic neurones studied involved the activation of 5-HT7 receptors. This is in contrast with previous studies which showed that 5-HT1A receptors were only involved in cardiopulmonary reflex responses in rats and cats, and additionally, in aortic depressor nerve reflex responses in rabbits. This difference in the function between these receptors is intriguing, and may indicate differential localization of the different receptor subtypes in the different reflex pathways. In conclusion, the present data demonstrate that in addition to 5-HT1A, 5-HT2 and 5-HT3 receptors (see Ramage, 2001; Jordan, 2005), another 5-HT receptor plays an important role central autonomic regulation, and adds further evidence for the critical importance of 5-HT-containing neurones in the control of vagal outflow to the heart.
Acknowledgments
This work was supported by a British Heart Foundation studentship to D.O.K. SB-269970 and SB-656104 were generous gifts from GSK, Harlow, UK.
References
- Alexander SPH, Mathie A, Peters JA. 5-Hydroxytryptamine receptors. Br J Pharmacol. 2004;141:S36–S37. doi: 10.1038/sj.bjp.0705672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickmeyer U, Heine M, Manzke T, Richter DW. Differential modulation of Ih by 5-HT receptors in mouse CA1 hippocampal neurons. Eur J Neurosci. 2002;16:209–218. doi: 10.1046/j.1460-9568.2002.02072.x. [DOI] [PubMed] [Google Scholar]
- Bogle RG, Pires JG, Ramage AG. Evidence that central 5-HT1A receptors play a role in the von Bezold–Jarisch reflex in the rat. Br J Pharmacol. 1990;100:757–760. doi: 10.1111/j.1476-5381.1990.tb14088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootle DJ, Adcock JJ, Ramage AG. Involvement of central 5-HT1A receptors in the reflex activation of pulmonary vagal motoneurones by inhaled capsaicin in anaesthetized cats. Br J Pharmacol. 1996;117:724–728. doi: 10.1111/j.1476-5381.1996.tb15250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootle DJ, Adcock JJ, Ramage AG. The role of central 5-HT receptors in the bronchoconstriction evoked by inhaled capsaicin in anaesthetised guinea-pigs. Neuropharmacology. 1998;37:243–250. doi: 10.1016/s0028-3908(98)00019-7. [DOI] [PubMed] [Google Scholar]
- Cardenas CG, Mar LP, Vysokanov AV, Arnold PB, Cardenas LM, Surmeier DJ, Scroggs RS. Serotonergic modulation of hyperpolarization-activated current in acutely isolated rat dorsal root ganglion neurons. J Physiol. 1999;518:507–523. doi: 10.1111/j.1469-7793.1999.0507p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin EM, Andrade R. A 5-HT7 receptor-mediated depolarization in the anterodorsal thalamus. II. Involvement of the hyperpolarization-activated current I(h) J Pharmacol Exp Ther. 2001;297:403–409. [PubMed] [Google Scholar]
- Conley RK, Williams TJ, Ford AP, Ramage AG. The role of α1 adrenoceptors and 5-HT1A receptors in the control of the micturition reflex in male anaesthetized rats. Br J Pharmacol. 2001;133:61–72. doi: 10.1038/sj.bjp.0704043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dando SB, Skinner MR, Jordan D, Ramage AG. Modulation of the vagal bradycardia evoked by stimulation of upper airway receptors by central 5-HT1 receptors in anaesthetized rabbits. Br J Pharmacol. 1998;125:409–417. doi: 10.1038/sj.bjp.0702085. 10.1038/sj.bjp.0702085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher A, Forster EA, Bill DJ, Brown G, Cliffe IA, Hartley JE, et al. Electrophysiological, biochemical, neurohormonal and behavioural studies with WAY-100635, a potent, selective and silent 5-HT1A receptor antagonist. Behav Brain Res. 1996;73:337–353. doi: 10.1016/0166-4328(96)00118-0. 10.1016/0166-4328(96)00118-0. [DOI] [PubMed] [Google Scholar]
- Franchini KG, Kreiger EM. Cardiovascular responses of conscious rats to carotid body chemoreceptor stimulation by intravenous KCN. J Auton Nerv Syst. 1993;42:63–70. doi: 10.1016/0165-1838(93)90342-r. 10.1016/0165-1838(93)90342-R. [DOI] [PubMed] [Google Scholar]
- Futuro-Neto HA, Pires JG, Gilbey MP, Ramage AG. Evidence for the ability of central 5-HT1A receptors to modulate the vagal bradycardia induced by stimulating the upper airways of anesthetized rabbits with smoke. Brain Res. 1993;629:349–354. doi: 10.1016/0006-8993(93)91345-s. 10.1016/0006-8993(93)91345-S. [DOI] [PubMed] [Google Scholar]
- Gill CH, Soffin EM, Hagan JJ, Davies CH. 5-HT7 receptors modulate synchronized network activity in rat hippocampus. Neuropharmacology. 2002;42:82–92. doi: 10.1016/s0028-3908(01)00149-6. 10.1016/S0028-3908(01)00149-6. [DOI] [PubMed] [Google Scholar]
- Guscott MR, Egan E, Cook GP, Stanton JA, Beer MS, Rosahl TW, et al. The hypothermic effect of 5-CT in mice is mediated through the 5-HT7 receptor. Neuropharmacology. 2003;44:1031–1037. doi: 10.1016/s0028-3908(03)00117-5. 10.1016/S0028-3908(03)00117-5. [DOI] [PubMed] [Google Scholar]
- Gustafson EL, Durkin MM, Bard JA, Zgombick J, Branchek TA. A receptor autoradiographic and in situ hybridization analysis of the distribution of the 5ht7 receptor in rat brain. Br J Pharmacol. 1996;117:657–666. doi: 10.1111/j.1476-5381.1996.tb15241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG. Role of the ventral medulla oblongata in blood pressure regulation. In: Loewy AD, Spyer KM, editors. Central Regulation of Autonomic Function. New York: Oxford University Press; 1990. pp. 145–167. [Google Scholar]
- Hagan JJ, Price GW, Jeffrey P, Deeks NJ, Stean T, Piper D, et al. Characterization of SB-269970-A, a selective 5-HT7 receptor antagonist. Br J Pharmacol. 2000;130:539–548. doi: 10.1038/sj.bjp.0703357. 10.1038/sj.bjp.0703357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund PB, Kelly L, Mazur C, Lovenberg T, Sutcliffe JG, Bonaventure P. 8-OH-DPAT acts on both 5-HT1A and 5-HT7 receptors to induce hypothermia in rodents. Eur J Pharmacol. 2004;487:125–132. doi: 10.1016/j.ejphar.2004.01.031. 10.1016/j.ejphar.2004.01.031. [DOI] [PubMed] [Google Scholar]
- Inoue T, Itoh S, Wakisaka S, Ogawa S, Saito M, Morimoto T. Involvement of 5-HT7 receptors in serotonergic effects on spike afterpotentials in presumed jaw-closing motoneurons of rats. Brain Res. 2002;954:202–211. doi: 10.1016/s0006-8993(02)03286-9. 10.1016/S0006-8993(02)03286-9. [DOI] [PubMed] [Google Scholar]
- Jeggo RD, Wang Y, Jordan D, Ramage AG. The effects of 5-HT1B/1D/1F receptor ligands on the activity of nucleus tractus solitarius (NTS) neurones in anaesthetized rats: an in vivo ionophoretic study. Br J Pharmacol. 2000a;129:63P. 10.1038/sj.bjp.0703007. [Google Scholar]
- Jeggo RD, Wang Y, Jordan D, Ramage AG. The role of 5-HT3 receptors in the cardiopulmonary afferent-evoked response of NTS neurones in the anaesthetized rat. J Physiol. 2000b;523.P:258–259. [Google Scholar]
- Jeggo RD, Wang Y, Jordan D, Ramage AG. Evidence to suggest that presynaptic 5-HT3 receptors are involved in the excitation of NTS neurones in anaesthetised rats. J Physiol. 2001;533.P:92–93. [Google Scholar]
- Jones GA, Llewellyn-Smith IJ, Jordan D. Physiological, pharmacological, and immunohistochemical characterisation of juxtacellularly labelled neurones in rat nucleus tractus solitarius. Auton Neurosci. 2002;98:12–16. doi: 10.1016/s1566-0702(02)00022-x. 10.1016/S1566-0702(02)00022-X. [DOI] [PubMed] [Google Scholar]
- Jordan D. Vagal control of the heart: Central serotonergic (5-HT) mechanisms. Exp Physiol. 2005 doi: 10.1113/expphysiol.2004.029058. 10.1113/expphysiol.2004.02905810.1113/expphysiol.2004.029058. [DOI] [PubMed] [Google Scholar]
- Jordan D, Spyer KM. Brainstem integration of cardiovascular and pulmonary afferent activity. Prog Brain Res. 1986;67:295–314. doi: 10.1016/s0079-6123(08)62769-7. [DOI] [PubMed] [Google Scholar]
- Kellett DO, Damasco EL, Bonagamba LGH, Machado BH, Jordan D, Ramage AG. Involvement of central 5-HT7 receptors in the autonomic responses to cardiopulmonary reflex activation in awake and anaesthetised rat. Brazilian Biol Soc (FESBE) Meeting. 2004a (in press) [Google Scholar]
- Kellett DO, Ramage AG, Jordan D. Role of central 5-HT7 as well as 5-HT1A receptors in cardiopulmonary reflex control in anaesthetised rats. J Physiol. 2003;551.P:C55. [Google Scholar]
- Kellett DO, Ramage AG, Jordan D. Evidence that vagal bradycardias evoked by baroreceptor and chemoreceptor afferents involve the activation of central 5-HT7 receptors. J Physiol. 2004b;555.P:C25. [Google Scholar]
- Kellett DO, Ramage AG, Jordan D. Excitation of rat nucleus tractus solitarius (NTS) neurones by vagal afferents involves central 5-HT7 and AMPA receptors. J Physiol. 2004c (in press) (Cork meeting) [Google Scholar]
- Lovell PJ, Bromidge SM, Dabbs S, Duckworth DM, Forbes IT, Jennings AJ, et al. A novel, potent, and selective 5-HT7 antagonist: (R)-3-(2-(2-(4-methylpiperidin-1-yl) ethyl) pyrrolidine-1-sulfonyl) phenol (SB-269970) J Med Chem. 2000;43:342–345. doi: 10.1021/jm991151j. 10.1021/jm991151j. [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Baron BM, de Lecea L, Miller JD, Prosser RA, Rea MA, et al. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron. 1993;11:449–458. doi: 10.1016/0896-6273(93)90149-l. 10.1016/0896-6273(93)90149-L. [DOI] [PubMed] [Google Scholar]
- Meuser T, Pietruck C, Gabriel A, Xie GX, Lim KJ, Pierce PP. 5-HT7 receptors are involved in mediating 5-HT-induced activation of rat primary afferent neurons. Life Sci. 2002;71:2279–2289. doi: 10.1016/s0024-3205(02)02011-8. 10.1016/S0024-3205(02)02011-8. [DOI] [PubMed] [Google Scholar]
- Middlemiss DN, Blakeborough L, Leather SR. Direct evidence for an interaction of β-adrenergic blockers with the 5-HT receptor. Nature. 1977;267:289–290. doi: 10.1038/267289a0. [DOI] [PubMed] [Google Scholar]
- Ogilvie J, Wigglesworth M, Appleby L, Kingston TO, Clarke RW. On the role of 5-HT1B/1D receptors in modulating transmission in a spinal reflex pathway in the decerebrated rabbit. Br J Pharmacol. 1999;128:781–787. doi: 10.1038/sj.bjp.0702832. 10.1038/sj.bjp.0702832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver KR, Kinsey AM, Wainwright A, Sirinathsinghji DJ. Localization of 5-ht5A receptor-like immunoreactivity in the rat brain. Brain Res. 2000;867:131–142. doi: 10.1016/s0006-8993(00)02273-3. 10.1016/S0006-8993(00)02273-3. [DOI] [PubMed] [Google Scholar]
- Pratt GD, Bowery NG. The 5-HT3 receptor ligand, [3H]BRL 43694, binds to presynaptic sites in the nucleus tractus solitarius of the rat. Neuropharmacology. 1989;28:1367–1376. doi: 10.1016/0028-3908(89)90012-9. 10.1016/0028-3908(89)90012-9. [DOI] [PubMed] [Google Scholar]
- Ramage AG. Central cardiovascular regulation and 5-hydroxytryptamine receptors. Brain Res Bull. 2001;56:425–439. doi: 10.1016/s0361-9230(01)00612-8. 10.1016/S0361-9230(01)00612-8. [DOI] [PubMed] [Google Scholar]
- Read KE, Sanger GJ, Ramage AG. Evidence for the involvement of central 5-HT7 receptors in the micturition reflex in anaesthetized female rats. Br J Pharmacol. 2003;140:53–60. doi: 10.1038/sj.bjp.0705399. 10.1038/sj.bjp.0705399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Batten TF, McWilliam PN. Glutamate immunoreactivity in identified vagal afferent terminals of the cat: a study combining horseradish peroxidase tracing and postembedding electron microscopic immunogold staining. Exp Physiol. 1995;80:193–202. doi: 10.1113/expphysiol.1995.sp003839. [DOI] [PubMed] [Google Scholar]
- Secker AG, Naylor AM, Ramage AG. A role for supraspinal 5-HT1A receptors in the control of micturition in urethane anaesthetized rats. Pharmacologist. 2002;44:A186. [Google Scholar]
- Sévoz-Couche C, Spyer KM, Jordan D. In vivo modulation of vagal-identified dorsal medullary neurones by activation of different 5-hydroxytryptamine2 receptors in rats. Br J Pharmacol. 2000;131:1445–1453. doi: 10.1038/sj.bjp.0703722. 10.1038/sj.bjp.0703722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MR, Ramage AG, Jordan D. Modulation of reflexly evoked vagal bradycardias by central 5-HT1A receptors in anaesthetized rabbits. Br J Pharmacol. 2002;137:861–873. doi: 10.1038/sj.bjp.0704941. 10.1038/sj.bjp.0704941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprouse J, Reynolds L, Li X, Braselton J, Schmidt A. 8-OH-DPAT as a 5-HT7 agonist: phase shifts of the circadian biological clock through increases in cAMP production. Neuropharmacology. 2004;46:52–62. doi: 10.1016/j.neuropharm.2003.08.007. 10.1016/j.neuropharm.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Steinbusch HW. Distribution of serotonin immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Street JA, Hemsworth BA, Roach AG, Day MD. Tissue levels of several radiolabelled β-adrenoceptor antagonists after intravenous administration in rats. Arch Int Pharmacodyn. 1979;237:180–190. [PubMed] [Google Scholar]
- Su DF, Cerutti C, Barres C, Julien C, Vincent M, Paultre C, Sassard J. Arterial baroreflex control of heart period is not related to blood pressure variability in conscious hypertensive and normotensive rats. Clin Exp Pharmacol Physiol. 1992;19:767–776. doi: 10.1111/j.1440-1681.1992.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Sykes RM, Spyer KM, Izzo PN. Central distribution of substance P, calcitonin gene-related peptide and 5-hydroxytryptamine in vagal sensory afferents in the rat dorsal medulla. Neuroscience. 1994;59:195–210. doi: 10.1016/0306-4522(94)90110-4. 10.1016/0306-4522(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Taylor EW, Jordan D, Coote JH. Central control of the cardiovascular and respiratory systems and their interactions in vertebrates. Physiol Rev. 1999;79:855–916. doi: 10.1152/physrev.1999.79.3.855. [DOI] [PubMed] [Google Scholar]
- Thomas DR, Larminie CG, Lyons HR, Fosberry A, Hill MJ, Hayes PD. Cloning and pharmacological characterisation of the guinea pig 5-ht5A receptor. Eur J Pharmacol. 2004;494:91–99. doi: 10.1016/j.ejphar.2004.04.027. 10.1016/j.ejphar.2004.04.027. [DOI] [PubMed] [Google Scholar]
- Thomas DR, Melotto S, Massagrande M, Gribble AD, Jeffrey P, Stevens AJ, et al. SB-656104-A, a novel selective 5-HT7 receptor antagonist, modulates REM sleep in rats. Br J Pharmacol. 2003;139:705–714. doi: 10.1038/sj.bjp.0705290. 10.1038/sj.bjp.0705290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DR, Middlemiss DN, Taylor SG, Nelson P, Brown AM. 5-CT stimulation of adenylyl cyclase activity in guinea-pig hippocampus: evidence for involvement of 5-HT7 and 5-HT1A receptors. Br J Pharmacol. 1999;128:158–164. doi: 10.1038/sj.bjp.0702759. 10.1038/sj.bjp.0702759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokarski K, Zahorodna A, Bobula B, Hess G. 5-HT7 receptors increase the excitability of rat hippocampal CA1 pyramidal neurons. Brain Res. 2003;993:230–234. doi: 10.1016/j.brainres.2003.09.015. 10.1016/j.brainres.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jones JF, Ramage AG, Jordan D. Effects of 5-HT and 5-HT1A receptor agonists and antagonists on dorsal vagal preganglionic neurones in anaesthetized rats: an ionophoretic study. Br J Pharmacol. 1995;116:2291–2297. doi: 10.1111/j.1476-5381.1995.tb15067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ramage AG. The role of central 5-HT1A receptors in the control of B-fibre cardiac and bronchoconstrictor vagal preganglionic neurones in anaesthetized cats. J Physiol. 2001;536:753–767. doi: 10.1111/j.1469-7793.2001.00753.x. 10.1111/j.1469-7793.2001.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ramage AG, Jordan D. In vivo effects of 5-hydroxytryptamine receptor activation on rat nucleus tractus solitarius neurones excited by vagal C-fibre afferents. Neuropharmacology. 1997;36:489–498. doi: 10.1016/s0028-3908(97)00063-4. 10.1016/S0028-3908(97)00063-4. [DOI] [PubMed] [Google Scholar]
- Zhang J, Mifflin SW. Differential roles for NMDA and non-NMDA receptor subtypes in baroreceptor afferent integration in the nucleus of the solitary tract of the rat. J Physiol. 1998;511:733–745. doi: 10.1111/j.1469-7793.1998.733bg.x. 10.1111/j.1469-7793.1998.733bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]