Abstract
The ability of postnatal testosterone propionate (TP) to masculinize both behaviour and gonadal cyclicity in the female rat is well documented. We have investigated whether postnatal androgen also has an organizational effect on another sexually dimorphic neuroendocrine system – the hypothalamo-pituitary-adrenal (HPA) axis. Female rats were exposed to a single injection of testosterone propionate (TP) or oil within 24 h of birth. As adults, rats were either ovariectomized and given 17β-oestradiol replacement (OVXE2) or sham ovariectomized with cholesterol implants (SHOVX). An automated sampling system collected blood from unanaesthetized adult female rats every 10 min over a 24-h period, during a mild psychological stress (noise) and following an immunological lipopolysaccharide stress (LPS). Neonatal TP-treated SHOVX rats had a significant reduction in the number, height, frequency and amplitude of corticosterone pulses over the basal 24-h period, compared to both the neonatal oil-treated and TP-treated OVXE2 animals. The corticosterone response to both noise and LPS was also significantly decreased for the TP-treated SHOVX females. Three hours post-LPS administration, TP females had significantly lower values of paraventricular nucleus (PVN) corticotrophin releasing hormone (CRH), arginine vasopressin (AVP) and anterior pituitary proopiomelanocortin (POMC) mRNAs and greater PVN glucocorticoid receptor (GR) mRNA expression compared to the oil-treated controls. E2 replacement in adult TP rats normalized all the mRNA levels, except for PVN GR mRNA which did fall towards the levels of the oil-control animals. A single injection of TP within 24 h of birth disrupts the development of the characteristic female pattern of corticosterone secretion and the normal female HPA response to stress, resulting in a pattern similar to that seen in males. These effects can be reversed by E2 treatment in the adult TP female rat.
The ability of postnatal environmental changes to alter the subsequent activity of the hypothalamo-pituitary-adrenal (HPA) axis in the adult rat (Levine, 1957; Meaney et al. 1989; Gotz et al. 1993; Shanks et al. 2000; Kamphuis et al. 2002) clearly shows that this axis is very plastic and can be programmed by both physical and behavioural stimuli. Sexual differentiation is completed during the early postnatal period (Maclusky & Naftolin, 1981) and is another example of a neuroendocrine system that has a critical time window during which gonadal hormones programme the brain of the rat to exhibit characteristically male or female behaviour. The classic paradigm is the exposure of the neonatal female rat to exogenous androgens resulting in masculinization of the reproductive axis (Barraclough & Gorski, 1961). Despite the well known sexual diergism of HPA function in the adult rat, the possible organizational effects of neonatal androgens on this axis are unknown.
Early studies on the organizational effects of postnatal androgens used the transplantation of testes into female rats (Pfeiffer, 1936). These studies were simplified by the demonstration that exposure of neonatal female rats to testosterone resulted in the occurrence of characteristically masculine behavioural traits in female rats (Selye, 1940). This had led to the frequent use of neonatal testosterone propionate (TP) to study the influence of androgen activity on the development of the masculinized female rat (Barraclough & Gorski, 1961; Gorski, 1971). TP is only effective when administered during the first 10 days post-parturition, implying the existence of a specific androgen-sensitive period in the female pup for the development of organizational effects (Barraclough, 1961). The resultant androgenized female has an increased body weight (Swanson & Van Der Werff Ten Bosch, 1963; Tartellin et al. 1975), polyfollicular non-ovulatory ovaries devoid of corpora lutea (Gerall & Kenny, 1970; McDonald & Doughty, 1972), enhanced male sexual behaviour (Baum, 1979) and a failure to display the lordosis response when sexually active (Harris & Levine, 1965). It has been proposed that the morphological changes in the ovaries of masculinized rats result from disrupted gonadotrophin regulation by the CNS (Pfeiffer, 1936; Barraclough, 1962). Indeed the ability of postnatal androgen administration to alter the characteristically cyclic nature of gonadotrophin secretion in female rats into a more tonic release reminiscent of the male rat (Gorski & Barraclough, 1963; Harris & Levine, 1965), provides evidence for an organizational role of androgens at the hypothalamic level. Studies on the organizational effect of neonatal androgen on hypothalamic mechanisms have focused on the changes in gonadal secretion and sexual behaviour (Barraclough, 1961; Gorski & Barraclough, 1963; Clemens et al. 1969) and not addressed another sexually differentiated system controlled by the hypothalamus – the HPA axis.
The basal levels of plasma corticosterone secretion and the stress-induced responses of the HPA axis are much greater in adult female than adult male rats (Kitay, 1961; Critchlow et al. 1963; Le Mevel et al. 1979; Patchev et al. 1995; Seale et al. 2004). An activational effect of oestrogen clearly contributes to this difference (Viau & Meaney, 1991; Burgess & Handa, 1992). However, given the importance of early gonadal steroid exposure in disrupting feminine behavioural gonadotrophin and anatomical development, the possibility arises that neonatal androgen exposure might also affect the development of the HPA axis and regulation of corticosterone secretion. As early as 1986, Hary et al. investigated ACTH responses of newborn rats to ether, following castration and testosterone administration, but other HPA axis parameters were not measured.
We have now directly addressed the issue whether neonatal androgenization with TP can programme changes in both basal and stress-induced HPA axis activity of the adult masculinized female, and if so to what degree this might be mediated by changes in circulating 17β-oestradiol (E2) concentrations in the adult.
Methods
Animals and postnatal treatment
Sprague-Dawley rats were time-mated to produce litters over four consecutive weeks. Within 24 h of birth, half of the female pups from each litter were injected subcutaneously with 80 μg of TP in 20 μl of olive oil. This method is effective in inducing masculinization in female pups (Tsai et al. 1995) The remaining half of the female pups from each litter were given a subcutaneous injection of 20 μl of olive oil. All female pups were randomly assigned to one of the treatment groups. Female pups were left with their littermates for 10 weeks to ensure they reached a weight within the range of 225–250 g. Rats were maintained under a 14 h light.10 h dark schedule with ad libitum access to food and water.
Surgery
At 9–10 weeks of age (275–300 g), all rats were anaesthetized using hypnorm (0.32 mg kg−1 fentanyl citrate and 10 mg kg−1 fluanisone, Janssen Pharmaceuticals, UK) and diazepam (2.6 mg kg−1, Phoenix Pharmaceuticals, UK). Half of the masculinized (TP) rats were bilaterally ovariectomized and oestradiol-replaced (TP-OVXE2), receiving a subcutaneous silastic implant (length, 0.5 cm; i.d., 1.57 mm; o.d., 3.17 mm; Legan et al. 1976) containing crystalline 17β-oestradiol (Sigma, UK). Half of the oil-treated females were also ovariectomized and implanted with an oestradiol pellet (oil-OVXE2). The remaining animals from the maculinized (TP-SHOVX) and oil-treated (Oil-SHOVX) groups were sham ovariectomized and given subcutaneous cholesterol implants (length, 0.5 cm; i.d., 1.57 mm; o.d., 3.17 mm). Sham ovariectomy entailed the same surgical procedures except for the removal of the gonads.
To allow for connection to an automated sampling system, a silastic-tipped polythene cannula (i.d.: 0.58 mm, Portex, Hythe, UK) filled with heparinized saline (10 U ml−1 heparin, CP pharmaceuticals Ltd) was inserted into the right jugular vein of each rat. The cannula was exteriorized at the crown of the rat's head and fed through a spring attached to a 360° mechanical swivel allowing the rat free movement within its cage. Rats were connected to an automated blood sampling system which collected blood samples at preset time points, avoiding the confounding variables characteristic of manual sampling techniques (detailed in Windle et al. 1998a). All animal procedures were carried out in accordance with the Animal (Scientific procedures) Act 1986.
Sampling procedure
Sampling began at 07.00 h, day 5 post-surgery. For each rat, blood samples were collected every 10 min over a period of 24 h under basal environmental conditions. At 07.00 h, day 6 post-surgery, rats were exposed to a 10 min white noise stress (110 db) after which blood samples were taken every 10 min for a further 2 h (07.00 h–09.00 h). At 09.00 h day 6 post-surgery, each rat was administered 100 μl of LPS (i.v., 250 μg ml−1, Sigma, UK). Blood samples were collected every 15 min for 3 h post-LPS administration (09.00 h–12.00 h). At 12.00 h, rats were overdosed with Euthatal (i.v.) and decapitated. Trunk blood, brains and pituitaries were collected. Previous work has reported LPS-induced increases in corticotrophin releasing hormone (CRH) mRNA using this serotype (E. coli; 055:B5) 3 h post-LPS administration (Conde et al. 1999). Up to 10 animals were prepared for each group. However, problems with the fraction collector or the occasional blocked line reduced this to seven to eight rats per group, although the remaining animals could still be used for assessing mRNA levels at the end of the sampling procedure.
Corticosterone radioimmunoassay
A total of 169 samples were collected for each rat over the complete sampling period (07.00 h, day 5 post-surgery to 12.00 h, day 6 post-surgery). Each blood sample from the automated sampling system consisted of 37.7 μl of blood diluted 1.5 in heparinized saline; 20 μl of each blood sample was further diluted into 80 μl of a citrate buffer (pH 3.0). Samples were processed in duplicate and incubated overnight at 4°C with 50 μl of 125I corticosterone tracer (ICN Flow) and 50 μl of rabbit antirat corticosterone primary antibody (kindly donated by G. Makara, Hungary). On day 2, a charcoal/dextran solution was added to all samples, which were then centrifuged (15 min, 4000 rpm, 4°C), aspirated and loaded onto a gamma counter. Intra- and interassay coefficients of variation of the corticosterone assays were 12.4% and 16%, respectively.
ACTH radioimmunoassay
Plasma collected 3 h post-LPS administration was analysed for adrenocorticotrophic hormone (ACTH) using a rabbit antirat ACTH primary antibody (kindly donated by G. Makara, Hungary) and 125I ACTH (Amersham, UK). A polyethylene glycol solution, sheep antirabbit secondary antibody (1.50 in SAB buffer with 0.4% normal rabbit serum) and centrifugation were used to separate the bound from the unbound hormone fraction into a pellet. Pellets were then counted on a gamma counter.
CBG assay
A pre-LPS corticosteroid-binding globulin (CBG) level was determined by pooling plasma samples from 08.00 h to 08.50 h, day 6 pre-LPS administration. Plasma collected 3 h post-LPS administration were used to provide a post-LPS level. Plasma samples were stripped of endogenous corticosterone, eluted and incubated overnight in a total binding or non-specific binding [3H]corticosterone solution (Windle et al. 1998a). On day 2, incubates were processed in triplicate through LH20 columns, eluted and counted on a beta scintillation counter as previously described (Windle et al. 1998a). The Bradford method was used to determine protein content (Bradford, 1976). Resultant values are reported as picomoles of [3H] corticosterone bound per mg protein.
Oestradiol radioimmunoassay
Plasma collected upon termination of the experiment was assayed for E2 levels via a double antibody 125I oestradiol kit (Diagnostic Products Corporation, USA). 200 μl of plasma was incubated with 100 μl of oestradiol antiserum for 2 h at room temperature; 100 μl of 125I oestradiol was added to all tubes and left to incubate for 1 h at room temperature. All samples were incubated for 10 min at room temperature with 1 ml of cold precipitating solution, followed by centrifugation for 15 min at 3000 g. Samples were decanted and counted on a gamma counter. The limit of detection of the assay was 8 pg ml−1.
Testosterone radioimmunoassay
Plasma collected 3 h post-LPS administration from each male rat was assayed for testosterone levels using a coat-a-count 125I testosterone Kit (Diagnostic Products Corporation, USA); 50 μl of plasma was incubated at 37°C for 3 h in testosterone antibody-coated tubes with 1 ml of 125I testosterone. Samples were decanted before being counted on a gamma counter. The limit of detection of the assay was 0.09 ng ml−1.
Oligonucleotide in situ hybridization
Paraventricular nucleus (PVN) and pituitary sections (12 μm) were cut. PVN sections were taken through the PVN where the magnocellular and parvocellular regions were well defined to incorporate the dorsomedial parvocellular cells (approximate bregma −1.88). Inspection of 1% toluidine blue-stained ‘test’ brain slices by a microscope (Nikon FX35A, Japan) was used to determine the required region in accordance with a rat brain atlas (Paxinos & Watson, 1998). Pituitary sections contained the posterior, anterior and intermediate lobe regions of the pituitary. All sections were mounted onto gelatin-coated slides and stored at −80°C. Slides were fixed in 4% formaldehyde (5 min) and taken through a prehybridization washing procedure as previously described (Harbuz & Lightman, 1989). Slides were incubated overnight at 37°C in hybridization buffer (Young et al. 1986a) containing 1 m dithiothreitol (DTT) and the required 35S-dATP oligonucleotide probe. Approximately 100 000–200 000 c.p.m. were applied to each slide in 45 μl of hybridization buffer. The specificity of the oligonucleotide probes has been previously determined for arginine vasopressin (AVP) (Young et al. 1986a), CRH (Young et al. 1986b) and proopiomelanocortin (POMC) (Takahashi et al. 1983). The specific activities of the probes were 2.21 × 1018 d.p.m. mol−1 (CRH), 1.92 × 1018 d.p.m. mol−1 (AVP) and 1.97 × 1018 d.p.m. mol−1 (POMC). All sham and experimental sections were hybridized in the same reaction. On day 2, slides were taken through four changes of 1 × saline sodium citrate (SSC) and washed in 1 × SSC at 37°C (4 × 15 min), 1 × SSC at room temperature (2 × 30 min), dipped in distilled water and dried. Slides were exposed (AVP: 3 days, POMC: 5 days and CRH: 14 days) to Hyperfilm MP (Amersham, Bucks., UK). The amount of bound probe to mRNA was analysed in comparison to 14C-labelled standards using image analysis software (Image 1.6.2, W. Rasband, NIH, Bethesda, MD, USA). For each rat, analysis of CRF and AVP mRNA in the parvocellular PVN was undertaken in four PVN sections and a mean value was produced for each brain according to previously published methods (Harbuz & Lightman, 1989). Parvocellular message was analysed by using a threshold tool on the image analysis software to threshold out the magnocellular region (Kinoshita et al. 2000). The image analysis software was used to highlight and measure POMC mRNA in the anterior region only of each pituitary section. For every rat, six pituitary sections were analysed and a mean value was calculated for each pituitary.
Riboprobe in situ hybridization
Antisense 35S-labelled glucocorticoid receptor (GR) and mineralocorticoid (MR) riboprobes (kindly donated by J. Seckl, University of Edingburgh) were used to detect GR mRNA expression in PVN and hippocampal sections (12 μm) and MR mRNA in hippocampal sections only. GR and MR were reverse transcribed as previously described (Seckl et al. 1991). Sections were fixed in 4% paraformaldehyde, processed through prehybridization washes and incubated overnight at 55°C in hybridization buffer containing the required 35S radiolabelled riboprobe (previously described in Seckl et al. 1991). Approximately 1000 000 c.p.m. were applied to each slide in 45 μl of hybridization buffer. The specific activities of the probes were 1.85 × 1019 d.p.m. mol−1 (GR), 1.61 × 1019 d.p.m. mol−1 (MR). On the second day sections were taken through three changes of 1 × SSC and washed in 50% formamide in 1 × SSC three times for 15 min (55°C). Sections were briefly dipped in 1 × SSC (37°C) and incubated in RNase A (25 mg ml−1, 37°C) for 30 min. Slides were dipped in 1 × SSC (37°C), washed in 50% formamide in 1 × SSC (3 × 15 min, 55°C), 1 × SSC (2 × 5 min, room temperature), dipped in distilled water and dried. Sections were exposed to Hyperfilm for two weeks and analysed using image analysis software as described above. Sense 35S-labelled GR and MR riboprobes were included as negative controls and displayed no radioactive signal on the treated hippocampal sections.
Statistical analysis
Data are presented as mean plasma corticosterone blood levels within each group (TP-SHOVX, TP-OVXE2, oil-SHOVX and oil-OVXE2 females) over the 24-h stress-free period. A pulsar analysis program (previously reported by Merriam & Wachter, 1982; Windle et al. 1998b) analysed pulse number, frequency, amplitude and height. ANOVA and post hoc Tukey tests were used to detect any differences in these parameters between the groups.
For statistical analysis of the post-noise stress response, the mean corticosterone level at a single time point (20 min post noise onset) was averaged for each group. The 2 min time point chosen was based on previous findings of maximal corticosterone secretion 20 min post-noise onset (Windle et al. 1998b). A baseline control value was obtained using the mean of values from 06.00 h to 06.50 h according to the recommendation of Festing et al. 2002). ANOVA and post hoc Tukey tests compared the stress response between each group, and the baseline and stress response within each group. To clarify any statistical differences in corticosterone secretion occurring at this single time point between the treatment groups, an area under the curve measurement from the time of noise onset (07.00 h, day 6 post-surgery) to the time at which values had returned to baseline (07.50 h, day 6) was calculated for each rat. ANOVA compared the areas under the curve measurements between the four groups.
A similar statistical procedure analysed corticosterone levels post-LPS administration. In this situation, the mean of values from 08.00 h to 08.50 h was used to obtain a baseline control value. Mean corticosterone levels 90 min following LPS administration were taken as an indication of LPS-induced stress response. This time point was based on previous findings demonstrating maximal corticosterone secretion between one and two hours post-LPS administration (Harbuz et al. 1999). For further statistical analysis of the LPS stress response, an area under the curve, measured using the trapezoid rule, from LPS administration (09.00 h) to experimental termination (12.00 h, day 6) was calculated for each rat. An ANOVA was used to compare area under the curve measurements between the four groups.
ANOVA and post hoc Tukey tests were used to compare ACTH levels between the four groups. An ANOVA was also used to compare pre and post-LPS CBG levels within and between the treatment groups in each study.
For mRNA analysis four PVN or hippocampal or six pituitary sections were analysed for each rat. A mean value of the sections per rat was then calculated so that each rat contributed a single mean value which was used to calculate a group mean. Statistical analysis was performed on the data set prior to calculation of percentage change values used for comparative purposes across the different mRNAs measured.
Results
E2 levels were not significantly different for oil-SHOVX (28.66 ± 7.8 pg ml−1), oil-OVXE2 (30.02 ± 4.3 pg ml−1) and TP-OVXE2-replaced females (27.82 ± 2.4 pg ml−1). The levels of E2 for TP-SHOVX however, were below the limit of detection of the assay. The weights of ovaries removed from ovariectomized rats at the time of surgery and removed from sham-operated rats upon termination of the experiment were pooled to produce a mean ovarian weight for oil-treated and a mean weight for TP-treated rats. TP treated females demonstrated significantly (P < 0.001) smaller ovaries (mean: 50 mg ± 4 mg, n = 20) compared to oil-treated females (mean: 90 mg ± 5 mg, n = 20) females. Testosterone levels of each rat were below the limit of detection of the assay.
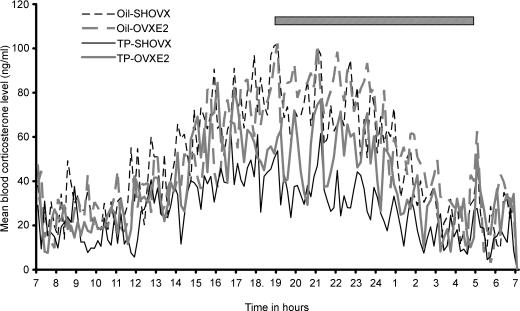
Blood samples collected over 24 h at 10 min intervals were assayed in duplicate for corticosterone from seven oil-SHOVX, seven oil-OVXE2 females, eight TP-SHOVX and eight TP-OVXE2 females. The mean corticosterone level for each 10 min time point was calculated for each group across the 24 h to produce a mean group corticosterone profile (Fig. 1). In both the individual profiles and the resultant mean group profiles, TP-SHOVX rats had lower corticosterone secretion over 24 h than any of the other treatment groups. Corticosterone release of the TP-OVXE2 females was also slightly lower than that found for the oil-treated control groups. Pulsar analysis of the individual corticosterone profiles demonstrated a significantly lower number (P < 0.001), frequency (P < 0.001), amplitude (P < 0.001) and height (P < 0.001) of corticosterone pulses for the TP-SHOVX rats compared to both oil-treated groups. E2 replacement in TP-OVX females resulted in a significantly increased number (P < 0.001), frequency (P < 0.001), amplitude (P < 0.05) and height (P < 0.05) of corticosterone pulses compared to TP-SHOVX females (Table 1).
Figure 1. Mean blood corticosterone levels for oil-SHOVX, oil-OVXE2, TP-SHOVX and TP-OVXE2 rats over a 24-h period (n = 7–8).
Dark phase (19.00 h to 05.00 h) is represented by hatched bar.
Table 1.
Pulsar parameter measurements
| Mean number of pulses over 24 h | Mean height of pulses | Mean frequency of pulses per hour | Mean amplitude of pulses | |
|---|---|---|---|---|
| Oil-SHOVX | 24 ± 1 | 89 ± 2 | 1.06 ± 0.04 | 66 ± 2 |
| Oil-OVXE2 | 22 ± 1.5 | 87 ± 4 | 0.95 ± 0.06 | 61 ± 4 |
| TP-SHOVX | 14 ± 0.8*†† | 67 ± 6*† | 0.59 ± 0.03*†† | 44 ± 3*† |
| TP- OVXE2 | 22 ± 1.4 | 80 ± 4 | 0.94 ± 0.05 | 58 ± 2 |
Values represent pulsar parameter measurements for oil-SHOVX, oil-OVXE2, TP-SHOVX and TP-OVXE2 rats (Mean ±s.e.m., n = 7–8). TP-SHOVX rats demonstrated significantly lower levels of all the parameters measured compared to the remaining treatment groups. No significant differences were found between oil-SHOVX, oil-OVXE2 and TP-OVXE2 rats.
P < 0.001 compared to oil-SHOVX and oil-OVXE2 females,
P < 0.05
P < 0.001 compared to TP-OVXE2 female rats.
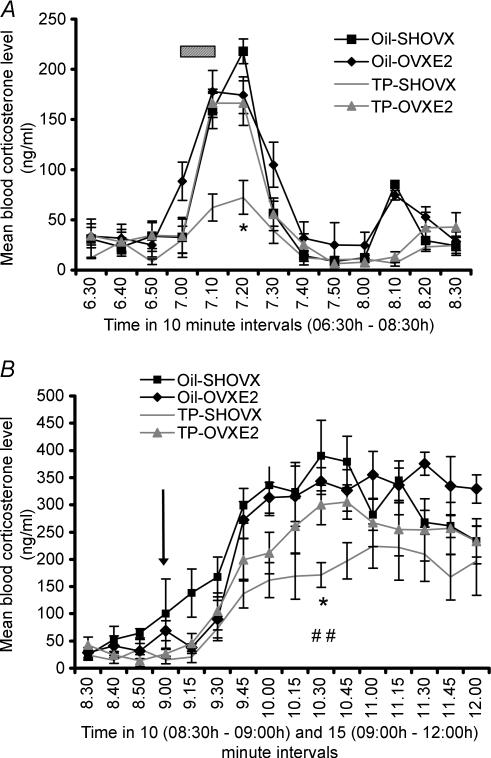
Following 24 h basal blood sampling, rats were exposed to a 10-minute noise stress (07.00 h). Blood samples were collected for 2 h post-noise onset and mean values for each rat group are shown in Fig. 2A. All rats demonstrated a significant increase in corticosterone release in response to the noise stress (P < 0.001). TP-SHOVX rats demonstrated significantly decreased corticosterone secretion compared to TP-OVXE2 animals and both oil-treated groups at the 20 min post-noise timepoint chosen for comparison purposes (P < 0.001). The area under the curve from 07.00 h to 07.50 h post-noise stress was significantly lower (P < 0.01) for the TP-SHOVX rats (mean = 1218 ± 198) compared to the TP-OVXE2 (mean = 4957 ± 281), oil-SHOVX (mean = 5134 ± 264) and oil-OVXE2 (mean = 5216 ± 256) rats. No significant differences in area under the curve measurements were found between the oil-treated control groups and TP-OVXE2 rats.
Figure 2. Corticosterone stress response.
A, values represent blood corticosterone levels of oil-SHOVX, oil-OVXE2, TP-SHOVX and TP-OVXE2 rats 30 min pre- and 90 min post-noise stress administration at 07.00 h (Mean ±s.e.m., n = 7–8). Hatched bar represents noise stress (07.00 h to 07.10 h). *P < 0.001 compared to oil-SHOVX, oil-OVXE2 and TP-OVXE2 rats. Statistical comparison was made between the groups at the 20 min timepoint according to the recommendations of Festing et al. (2002). B, values represent blood corticosterone levels of oil-SHOVX, oil-OVXE2, TP-SHOVX and TP-OVXE2 rats 30 min pre- and 180 min post-LPS administration at 09.00 h (Mean ±s.e.m., n = 7–8). Arrow represents time of LPS injection. *P < 0.05 compared to TP-OVXE2 rats; ##P < 0.01 compared to oil-SHOVX and oil-OVXE2 rats. Statistical comparison was made between the groups 90 min post-LPS administration.
LPS was administered at 09.00 h, after which blood samples were collected every 15 min for 3 h, analysed for corticosterone secretion and mean values obtained for each group (Fig. 2B). LPS resulted in a significant increase in the corticosterone release of each group (P < 0.01). TP-SHOVX rats had significantly lower corticosterone levels at the chosen comparative timepoint (90 min post-LPS) compared to oil-SHOVX (P < 0.01), oil-OVXE2 (P < 0.01) and TP-OVXE2 rats (P < 0.05). A significantly lower (P < 0.01) area under the curve measurement post-LPS administration was found for the TP-SHOVX rats (mean = 24104 ± 2119) compared to the TP-OVXE2 (mean = 45097 ± 3083), oil-SHOVX (mean = 45541 ± 2878) and oil-OVXE2 (mean = 48348 ± 3332) rats. No significant differences were found between the oil-treated control groups and TP-OVXE2 animals.
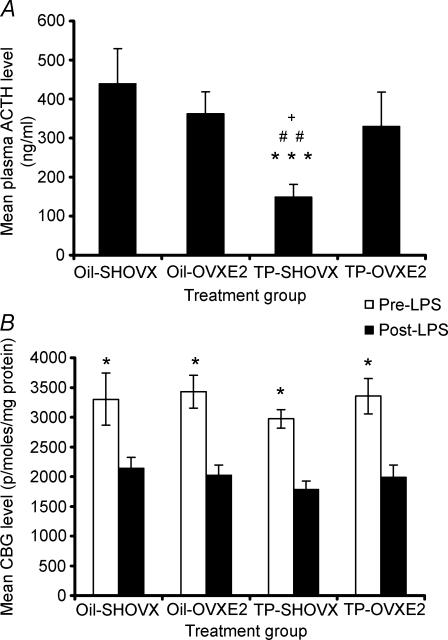
Plasma collected at the end of the sampling period was assayed for ACTH levels (Fig. 3A). Significantly lower levels of ACTH were found for TP-SHOVX compared to the oil-SHOVX (P < 0.001), oil-OVXE2 (P < 0.01) and TP-OVXE2 rats (P < 0.05). Post-LPS CBG levels were significantly reduced in comparison to pre-LPS CBG levels for each treatment group (P < 0.001). No significant differences in CBG levels were found between the four groups at analogous timepoints (Fig. 3B).
Figure 3. LPS-induced ACTH and CBG levels.
A, plasma ACTH levels (Mean ±s.e.m., n = 7–8) for oil-SHOVX, oil- OVXE2, TP-SHOVX and TP-OVXE2 rats 3 h hours post-LPS administration. ***P < 0.001 compared to oil-SHOVX; ##P < 0.01 compared to oil-OVXE2; +P < 0.05 compared to TP-OVXE2 rats. B, plasma CBG (pmoles (mg protein)−1) levels (Mean ±s.e.m., n = 7–8) for oil-SHOVX, oil-OVXE2, TP-SHOVX and TP-OVXE2 rats pre- and post-LPS administration. *P < 0.001 compared to post-LPS condition.
The mean percentage change in PVN CRH, AVP and GR, anterior pituitary POMC and hippocampal GR and MR mRNA was calculated for each group in comparison to a control group (designated as oil-SHOVX) 3 h post-LPS administration (Table 2). Significantly decreased levels of PVN CRH mRNA were found for TP-SHOVX compared to TP-OVXE2 (P < 0.05), oil-OVXE2 (P < 0.01) and oil-SHOVX rats (P < 0.001). PVN parvocellular AVP mRNA levels were significantly lower in TP-SHOVX animals in comparison to both oil-treated controls and TP-OVXE2 animals (P < 0.05). GR PVN was significantly elevated in TP-SHOVX compared to oil-SHOVX (P < 0.01) and oil-OVXE2 females (P < 0.05). POMC mRNA in the anterior pituitary was significantly decreased in TP-SHOVX compared to both oil-treated controls (P < 0.001) and TP- OVXE2 rats (P < 0.05) (Table 2).
Table 2.
AVP, CRH and GR mRNA in the PVN and POMC mRNA levels in the anterior pituitary for oil-SHOVX, oil-OVXE2, TP-SHOVX and TP-OVXE2 female rats (Mean ±s.e.m, n = 7–8)
| Treatment group | AVP mRNA (%±s.e.m. of Oil-SHOVX controls) | CRH mRNA (%±s.e.m. of Oil-SHOVX controls) | GR mRNA (%±s.e.m. of Oil-SHOVX controls) | POMC mRNA (%±s.e.m. of Oil-SHOVX controls) |
|---|---|---|---|---|
| Oil-SHOVX | 100 (9) | 100 (12) | 100 (9) | 100 (13) |
| Oil-OVXE2 | 101.2 (11) | 97.2 (13) | 96.3 (7) | 114 (15) |
| TP-SHOVX | 65.4 (8)*†‡ | 51.5 (10)***††‡ | 150.9 (8)**† | 47.3 (10)***†††‡ |
| TP-OVXE2 | 96.3 (11) | 90 (14) | 114.4 (8) | 89.5 (14) |
TP-SHOVX rats demonstrated significantly lower levels of AVP, CRH and POMC mRNA compared to the remaining groups and significantly greater levels of PVN GR mRNA compared to oil-treated controls.
P < 0.05;
P < 0.01.
P < 0.001 compared to oil-SHOVX;
P < 0.05;
P < 0.01;
P < 0.001 compared to oil-OVXE2;
P < 0.05 compared to TP-OVXE2 rats.
GR and MR mRNA in the CA1, CA2, CA3 and dentate gyrus regions of the hippocampus were not significantly different (data not shown) between the four groups post-LPS administration.
Discussion
Exposure of the female neonate to a single injection of TP within 24 h of birth resulted in a significantly reduced height of corticosterone pulses over a 24-h period in adult animals compared to the levels from oil-treated females and TP-OVXE2 rats. The decrease in pulse height was accompanied by a significantly lower number, amplitude and frequency of corticosterone pulses throughout the 24-h period. This reduced corticosterone profile for the neonatal TP-SHOVX rats resembles that previously reported for intact adult male rats (Seale et al. 2004). In addition to this male-like corticosterone profile, responsiveness to both a psychological stress (noise) and immunological stress (LPS) was reduced. PVN AVP and CRH and adenohypophysial POMC mRNA measured at the termination of blood sampling were significantly reduced in the TP-SHOVX rats compared to the oil-treated and TP-OVXE2-replaced animals. In parallel with this, GR mRNA in the PVN was significantly elevated for the TP-SHOVX rats compared to the oil-treated female groups. The stimulatory influence of E2 replacement on the activity of the HPA axis in the adult TP-treated rats suggests that this major neonatal organizational influence on the HPA hyporeactivity is secondary to the reduced levels of circulating E2 found in these masculinized animals.
Organizational effects of perinatal gonadal steroid activity on behavioural development were originally described in 1959 by the demonstration of masculine behaviour in the female offspring of dams exposed to testosterone during pregnancy (Phoenix et al. 1959). It has subsequently been demonstrated that these masculinized rats have polyfollicular non-ovulatory ovaries devoid of corpora lutea and are permanently sterile (Gerall & Kenny, 1970; McDonald & Doughty, 1972). Behaviourally the androgenized female demonstrates enhanced male sexual behaviour (Baum, 1979). Within the brain itself there is a morphological sexual dimorphism that is especially pronounced in the medial preoptic nucleus (Gorski et al. 1977).
Since the HPA axis is highly susceptible to neonatal programming (Levine et al. 1967; Plotsky & Meaney, 1993; Viau et al. 1993; Shanks et al. 2000) and is sexually dimorphic (Critchlow et al. 1963; Le Mevel et al. 1979; Patchev et al. 1995; Seale et al. 2004), it could also be sensitive to neonatal testosterone exposure. Any studies of this phenomenon must however, control for the E2 status of the masculinized female rat. In the present study, TP-treated rats had significantly smaller ovaries whose function was clearly compromised as evidenced by the undetectable plasma levels of E2. These data support the anatomical changes previously reported in masculinized rats (Gerall & Kenny, 1970; Tartellin et al. 1975).
TP-SHOVX females display a lower corticosterone profile over a 24-h period compared to the TP-OVXE2 and oil-treated rats with levels and pulse parameters similar to those we have previously reported for the intact males (Seale et al. 2004). This reduced corticosterone release in the TP-SHOVX rats was also found in response to both an acute noise stress and an LPS immune-mediated challenge. The changes in corticosterone we describe cannot be accounted for by alterations in plasma levels of basal CBG as these were similar in all four experimental groups, which also all showed a similar response to LPS exposure. The administration of E2 to TP-treated adult females reversed the effects of neonatal TP. The ability of E2 to elevate CBG levels in male adults implicates a stimulatory influence of this hormone on CBG levels (McCormick et al. 2002). In contrast, ovariectomy does not significantly alter CBG levels in adult female rats, whereas castration results in an elevated CBG response in adult males indicating a more prevalent role for testosterone compared to E2 in determining CBG levels (Gala & Westphal, 1965). In light of this latter study, the presence of non-detectable E2 levels in TP-sham females would have little effect on CBG activity in these rats. The demonstration of significantly decreased post-LPS compared to pre-LPS CBG levels within each group confirms previous studies demonstrating an inhibitory influence of immune activation on CBG levels at this timepoint (Neufeld et al. 1994; Shanks et al. 1994; Tannenbaum et al. 1997).
The reduced ACTH and corticosterone responses to LPS in the TP-SHOVX group was also accompanied by a decreased expression of AVP and CRH mRNA in the PVN, POMC mRNA in the anterior pituitary and significantly higher GR mRNA in the PVN. This suggests that a primary site of action of the neonatal TP might be the enhancement of negative feedback by an increase of GR mRNA in the PVN. This would result in consequent inhibition of HPA axis activity at a hypothalamic level. The administration of E2 to adult TP-OVX rats resulted in normalization of PVN AVP and CRH and anterior pituitary POMC mRNA expression similar to that of the oil-treated controls suggesting an important role for hypo-oestrogenization in the HPA inhibition of these masculinized animals. This is reminiscent of the finding that E2 replacement in masculinized animals can re-instate lordosis behaviour (Clemens et al. 1969; Sodersten, 1976). E2 replacement in TP-OVX adults also decreased GR PVN mRNA towards the levels found for oil-treated females, although this decrease did not reach statistical significance.
In addition to the possibility of a primary action at the level of PVN GR mRNA, the presence of androgen, oestrogen and corticosteroid receptors in the hippocampus (Simerley et al. 1990) suggests that this could also be an important regulatory locus. The present study, however, failed to demonstrate any difference in GR or MR mRNA in the CA1, CA2, CA3 and dentate gyrus hippocampal regions, providing no evidence for an organizational influence of early androgen exposure in this area. The inability to find any differences in corticosteroid receptor mRNA levels between castrated and intact male (Handa et al. 1994; Seale et al. 2004), and ovariectomized and intact female (Karandrea et al. 2002; Seale et al. 2004) adult rats further supports an absence of gonadal steroid influence on hippocampal receptor activity despite major changes in the functioning of the HPA axis.
In summary, our data demonstrate the ability of a single neonatal injection of TP to result in a masculinization of the HPA axis with a secretion pattern characteristic of that previously reported for intact male rats (Seale et al. 2004). This reduction in HPA axis activity can be reversed by the administration of physiological levels of E2, suggesting that as adults these females are still receptive to the excitatory influence of E2 on HPA axis activity and corticosterone secretion.
References
- Barraclough CA. Production of anovulatory, sterile rats by single injections of testosterone propionate. Endocrinology. 1961;68:62–67. doi: 10.1210/endo-68-1-62. [DOI] [PubMed] [Google Scholar]
- Barraclough CA. Studies on mating behaviour in the androgen-steralized female rat in relation to the hypothalamic regulation of sexual behaviour. Endocrinology. 1962;25:175–182. doi: 10.1677/joe.0.0250175. [DOI] [PubMed] [Google Scholar]
- Barraclough CA, Gorski RA. Evidence that the hypothalamus is responsible for androgen-induced sterility in the female rat. Endocrinology. 1961;68:68–79. doi: 10.1210/endo-68-1-68. [DOI] [PubMed] [Google Scholar]
- Baum MJ. Differentation of coital behaviour in mammals: a comparative analysis. Neurosci Biobehav Rev. 1979;3:265–284. doi: 10.1016/0149-7634(79)90013-7. [DOI] [PubMed] [Google Scholar]
- Bradford MA. A rapid and sensitive method for quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burgess LH, Handa RJ. Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion and glucocoticoid receptor-mediated functions in female rats. Endocrinology. 1992;131:1261–1269. doi: 10.1210/endo.131.3.1324155. [DOI] [PubMed] [Google Scholar]
- Clemens L, Hiroi GM, Gorski RA. Induction and facilitation of female mating behaviour in rats treated neonatally with low doses of testosterone propionate. Endocrinology. 1969;84:1430–1438. doi: 10.1210/endo-84-6-1430. [DOI] [PubMed] [Google Scholar]
- Conde GL, Renshaw D, Zubelewicz B, Lightman SL, Harbuz MS. Central LPS-induced c-fos expression in the PVN and the A1/A2 brainstem noradrenergic cell groups is altered by adrenalectomy. Neuroendocrinology. 1999;70:175–185. doi: 10.1159/000054474. [DOI] [PubMed] [Google Scholar]
- Critchlow V, Liebelt A, Barsela M, Mountcastle W, Lipscomb HS. Sex differences in resting pituitary-adrenal function in the rat. Am J Physiol. 1963;205:807–815. doi: 10.1152/ajplegacy.1963.205.5.807. [DOI] [PubMed] [Google Scholar]
- Festing MFW, Overend P, Gaines Das R, Borja MC, Berdoy M. The Design of Animal Experiments. London: Royal Society of Medicine Press Limited; 2002. [Google Scholar]
- Gala RR, Westphal U. Corticosteroid-binding globulin in the rat: studies on the sex difference. Endocrinology. 1965;77:841–851. doi: 10.1210/endo-77-5-841. [DOI] [PubMed] [Google Scholar]
- Gerall AA, Kenny AM. Neonatally androgenised females' responsiveness to estrogen and progesterone. Endocrinology. 1970;87:560–566. doi: 10.1210/endo-87-3-560. [DOI] [PubMed] [Google Scholar]
- Gorski RA. Frontiers in Neuroendocrinology. New York: Oxford University Press; 1971. [Google Scholar]
- Gorski RA, Barraclough CA. Effects of low dosages of androgen on the differentation of hypothalamic regulatory control of ovulation in the rat. Endocrinology. 1963;73:210–216. doi: 10.1210/endo-73-2-210. [DOI] [PubMed] [Google Scholar]
- Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1977;148:333–346. doi: 10.1016/0006-8993(78)90723-0. 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- Gotz F, Dorner G, Malz U, Rohde W, Stahl F, Poppe I, Schulze M, Plagemann A. Short – and long-term effects of perinatal interleukin-1β-application in rats. Neuroendocrinology. 1993;58:344–351. doi: 10.1159/000126560. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Harbuz MS, Lightman SL. Responses of hypothalamic and pituitary mRNA to physical and psychological stress in the rat. J Endocrinol. 1989;122:705–711. doi: 10.1677/joe.0.1220705. [DOI] [PubMed] [Google Scholar]
- Harbuz MS, Windle RJ, Jessop DS, Renshaw D, Ingram CD, Lightman SL. Differential effects of psychological and immunological challenge on the hypothalamo-pituitary-adrenal axis function in adjuvant-induced arthritis. Ann N Y Acad Sci. 1999;876:43–52. doi: 10.1111/j.1749-6632.1999.tb07621.x. [DOI] [PubMed] [Google Scholar]
- Harris GW, Levine S. Sexual differentiation of the brain and its experimental control. J Physiol. 1965;181:379–400. doi: 10.1113/jphysiol.1965.sp007768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hary L, Dupouy JP, Gregoire I. Effects of castration and testosterone on the pituitary and adrenal responses of the newborn rat to ether inhalation. Neuroendocrinology. 1986;42:137–142. doi: 10.1159/000124264. [DOI] [PubMed] [Google Scholar]
- Kamphuis PJG, Bakker JM, Broekhoven MH, Kunne C, Croiset G, Lentjes EG, Tilders FJH, van Bel F, Wiegert VM. Enhanced glucocorticoid feedback inhibition of hypothalamo-pituitary-adrenal responses to stress in adult rats neonatally treated with dexamethasone. Neuroendocrinology. 2002;76:158–169. doi: 10.1159/000064526. 10.1159/000064526. [DOI] [PubMed] [Google Scholar]
- Karandrea D, Kittas C, Kitraki E. Forced swimming differentially affects male and female brain corticosteroid receptors. Neuroendocrinology. 2002;75:217–226. doi: 10.1159/000054713. 10.1159/000054713. [DOI] [PubMed] [Google Scholar]
- Kinoshita H, Jessop DS, Finn DP, Harbuz MS. Cyanamide-induced activation of the hypothalamo-pituitary-adrenal axis. J Neuroendocinol. 2000;12:255–262. doi: 10.1046/j.1365-2826.2000.00449.x. 10.1046/j.1365-2826.2000.00449.x. [DOI] [PubMed] [Google Scholar]
- Kitay JI. Sex differences in adrenal cortical secretion in the rat. Endocrinology. 1961;68:818–824. doi: 10.1210/endo-68-5-818. [DOI] [PubMed] [Google Scholar]
- Le Mevel JC, Abitbol S, Beraud G, Maniey J. Temporal changes in plasma adrenocorticotropin concentration after repeated neurotropic stress in male and female rats. Endocrinology. 1979;105:812–817. doi: 10.1210/endo-105-3-812. [DOI] [PubMed] [Google Scholar]
- Legan SJ, Coon GA, Karsch FJ. Role of estrogen as initiator of daily surges in the ovariectomised rats. Endocrinology. 1976;96:50–56. doi: 10.1210/endo-96-1-50. [DOI] [PubMed] [Google Scholar]
- Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126:406–406. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- Levine S, Haltmeyer GC, Karas GG, Denenberg VH. Physiological and behavioral effects of infantile stimulation. Physiol Behav. 1967;2:55–63. 10.1016/0031-9384(67)90011-X. [Google Scholar]
- Maclusky NJ, Naftolin F. Sexual differentation of the central nervous system. Science. 1981;211:1294–1303. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Linkroum W, Sallinen BJ, Miller NW. Peripheral and central sex steroids have differential effects on the HPA axis of male and female rats. Stress. 2002;5:235–247. doi: 10.1080/1025389021000061165. [DOI] [PubMed] [Google Scholar]
- McDonald PG, Doughty C. Inhibition of androgen-sterilization in the female rat by administration of an antioestrogen. J Endocrinol. 1972;55:455–456. doi: 10.1677/joe.0.0550455. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitkin DH, Viau V, Sharma S, Sarrieau A. Neonatal handling alters adrenocortical negative feedback sensitivity and hippocampal type II glucocorticoid receptor binding in the rat. Neuroendocrinology. 1989;50:597–604. doi: 10.1159/000125287. [DOI] [PubMed] [Google Scholar]
- Merriam GR, Wachter KW. Algorhythms for the study of episodic hormone secretion. Am J Physiol. 1982;243:E310–E318. doi: 10.1152/ajpendo.1982.243.4.E310. [DOI] [PubMed] [Google Scholar]
- Neufeld JH, Breen L, Hauger R. Extreme posture elevate corticosterone in a forced ambulation model of chronic stress in rats. Pharm Biochem Behav. 1994;47:223–240. doi: 10.1016/0091-3057(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Hayashi S, Orikasa C, Almeida OFX. Implications of estrogen dependent brain organisation for gender differences in hypothalamo-pituitary-adrenal regulation. FASEB J. 1995;9:419–423. doi: 10.1096/fasebj.9.5.7896013. [DOI] [PubMed] [Google Scholar]
- Pfeiffer CA. Sexual differences of hypotheses and their determination by gonads. Am J Anat. 1936;58:195–225. [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organising action of prenatally administered testosterone propionate on the tissues mediating mating behaviour in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early postnatal experience alters hypothalamic corticotropin-releasing hormone (CRH) mRNA, median eminence CRH content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. 10.1016/0169-328X(93)90189-V. [DOI] [PubMed] [Google Scholar]
- Seale JV, Wood SA, Atkinson HC, Bate E, Lightman SL, Ingram CD, Jessop DS, Harbuz MS. Gonadectomy reverses the sexually diergic patterns of circadian and stress-induced hypothalamo-pituitary-adrenal axis activity in male and female rats. J Neuroendocrinol. 2004;16:516–524. doi: 10.1111/j.1365-2826.2004.01195.x. 10.1111/j.1365-2826.2004.01195.x. [DOI] [PubMed] [Google Scholar]
- Seckl JR, Dickson KL, Yates C, Fink G. Distribution of glucocorticoid and mineralcorticoid receptor messenger RNA expression on human postmortem hippocampus. Brain Res. 1991;562:332–337. doi: 10.1016/0006-8993(91)91612-5. 10.1016/0006-8993(91)91612-5. [DOI] [PubMed] [Google Scholar]
- Selye H. Production of persistent changes in the genital organs of immature female rats treated with testosterone. Endocrinology. 1940;27:657–660. [Google Scholar]
- Shanks N, McCormick CM, Meaney MJ. Hypothalamic-pituitary-adrenal activation following endotoxin administration in the developing rat: a CRH-mediated effect. J Neuroendocrinol. 1994;6:375–383. doi: 10.1111/j.1365-2826.1994.tb00596.x. [DOI] [PubMed] [Google Scholar]
- Shanks N, Windle RJ, Harbuz MS, Jessop DJ, Ingram CD, Lightman SL. Early-life exposure to endotoxin alters hypothalamic-pituitary-adrenal function and predisposition to inflammation. Proc Natl Acad Sci U S A. 2000;97:5645–5650. doi: 10.1073/pnas.090571897. 10.1073/pnas.090571897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerley R, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA containing cells in the rat brain: an in situ hybridisation study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Sodersten P. Lordosis behaviour in male, female and androgenised female rats. J Endocrinol. 1976;70:409–420. doi: 10.1677/joe.0.0700409. [DOI] [PubMed] [Google Scholar]
- Swanson HE, Van Der Werff Ten Bosch JJ. Sex differences in growth of rats and their modification by a single injection of testosterone propionate shortly after birth. J Endocrinol. 1963;26:197–207. doi: 10.1677/joe.0.0260197. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Hakamata Y, Wantabe Y, Kikuno R, Miyata T, Numa S. POMC probe. Nucleic Acids Res. 1983;11:6647–6858. doi: 10.1093/nar/11.19.6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannenbaum B, Rowe W, Sharma S, Diorio J, Steverman A, Walker M, Meaney MJ. Dynamic variations in plasma corticosteroid-binding globulin and basal activity following acute stress in adult rats. J Neuroendocrinol. 1997;9:163–168. doi: 10.1046/j.1365-2826.1997.t01-1-00550.x. 10.1046/j.1365-2826.1997.t01-1-00550.x. [DOI] [PubMed] [Google Scholar]
- Tartellin MF, Shryne JE, Gorski RA. Patterns of body weight change in rats following neonatal hormone manipulation: a critical period for androgen-induced growth increases. Acta Endocrinol. 1975;79:177–191. doi: 10.1530/acta.0.0790177. [DOI] [PubMed] [Google Scholar]
- Tsai Y, Chen TJ, Pi W, Tai M, Huang R, Chiueh C, Peng M. Effects of fetal brain grafting on adult behavioural masculinisation and defeminisation in neonatally androgenised female rats. Neurosci Lett. 1995;190:97–100. doi: 10.1016/0304-3940(95)11510-4. 10.1016/0304-3940(95)11510-4. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney J. Basal and stress hypothalamic-adrenal activity in cycling and ovariectomised-steroid treated rats. Endocrinology. 1991;129:2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- Viau V, Sharma S, Plotsky PM, Meaney MJ. Increased plasma ACTH responses to stress in nonhandled compared with handled rats require levels of corticosterone and are associated with increased levels of ACTH secretagogues in the median eminence. J Neurosci. 1993;13:1097–1105. doi: 10.1523/JNEUROSCI.13-03-01097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle RJ, Wood SA, Lightman SL, Ingram CD. The pulsatile characteristics of the hypothalamo-pituitary-adrenal activity in female Lewis and Fischer 344 rats and its relationship to differential stress responses. Endocrinology. 1998b;139:4044–4052. doi: 10.1210/endo.139.10.6238. 10.1210/en.139.10.4044. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Wood SA, Shanks N, Lightman SL, Ingram CD. Ultradian rhythm of basal corticosterone release in the female rat: dynamic interaction with the response to acute stress. Endocrinology. 1998a;139:443–450. doi: 10.1210/endo.139.2.5721. 10.1210/en.139.2.443. [DOI] [PubMed] [Google Scholar]
- Young WS, III, Mezey E, Siegel RE. Vasopressin and oxytocin mRNAs in adrenalectomised and Brattleboro rats: Analysis by quantitative in situ hybridisation histochemistry. Mol Brain Res. 1986a;1:231–241. doi: 10.1016/0169-328x(86)90029-x. 10.1016/0169-328X(86)90029-X. [DOI] [PubMed] [Google Scholar]
- Young WS, III, Mezey E, Siegel RE. Quantitative in situ hybridisation histochemistry reveals increased levels of corticotrophin-releasing factor mRNA after adrenalectomy in rats. Neurosci Lett. 1986b;70:198–203. doi: 10.1016/0304-3940(86)90463-5. 10.1016/0304-3940(86)90463-5. [DOI] [PubMed] [Google Scholar]