Abstract
Fatigue is increased during hyperthermia, and torque declines more rapidly in sustained maximal voluntary contractions (MVCs). This can be caused by a greater decline in voluntary activation of muscle (i.e. ‘central fatigue’). The present study aimed to localize the site of failure of voluntary drive during hyperthermia. Seven subjects made brief (2–3 s) and sustained (2 min) MVCs of elbow flexor muscles in two experiments. Core temperature was normal (∼37°C) in the first experiment, and elevated (∼38.5°C) by passive heating in the second. During some MVCs, transcranial magnetic stimulation of the motor cortex (TMS) was delivered, and the evoked torque (superimposed twitch) and EMG responses were measured. During hyperthermia, voluntary torque was reduced by ∼2.4% during brief MVCs (P = 0.03), and decreased further (∼12%) during sustained MVCs (P = 0.01). The superimposed twitch amplitude in the sustained MVC was ∼50% larger (P = 0.01). Thus, the ability to drive the muscle maximally in a sustained fashion was decreased, and some motor cortical output, which could have increased torque, remained untapped by voluntary drive. The additional central fatigue was not associated with altered motor cortical ‘excitability’, as EMG responses produced by TMS were similar at the two temperatures. However, the peak relaxation rate of muscle increased by ∼20% (P = 0.005) during hyperthermia. Hence, faster motor unit firing rates would be required to produce fusion of force. The increased central fatigue during hyperthermia may represent a failure of descending voluntary drive to compensate for changed muscle properties, despite the availability of additional cortical output.
Increased muscle temperature changes muscle contractile properties, and an increased core temperature (Tc; hyperthermia) can impair voluntary muscle performance. Exercise in a hot environment markedly reduces endurance (e.g. Saltin et al. 1972; MacDougall et al. 1974; Galloway & Maughan, 1997; Parkin et al. 1999), and stresses the thermoregulatory and cardiovascular systems because of the need to pump blood to exercising muscles and to dissipate heat (for review see Rowell, 1974). Exercise in the heat also increases muscle glycogenolysis (e.g. Edwards et al. 1972; Febbraio et al. 1994) and lactate accumulation (e.g. Edwards et al. 1972; Young et al. 1985; Febbraio et al. 1994; Parkin et al. 1999). However, the impaired voluntary endurance is more strongly associated with an elevated Tc than changes in the cardiovascular system or muscle metabolism (Gonzalez-Alonso et al. 1999). During prolonged exercise in the heat, exhaustion in humans appears to coincide with attainment of a particular Tc which is independent of the initial Tc or the rate of heat storage (e.g. MacDougall et al. 1974; Nielsen et al. 1993; Gonzalez-Alonso et al. 1999).
Hyperthermia may impair endurance through increased muscle fatigue (Parkin et al. 1999; Nybo & Nielsen, 2001a; Morrison et al. 2004). Muscle fatigue is an exercise-induced reduction in the ability to produce muscle force or power (e.g. Gandevia et al. 1995), and is largely caused by processes within the muscle, such as disturbances in excitation–contraction coupling and accumulation of metabolites (i.e. ‘peripheral fatigue’; for reviews see Fitts, 1994; Allen & Westerblad, 2001). However, some muscle fatigue arises from a progressive exercise-induced reduction in the voluntary activation of muscle, termed ‘central fatigue’ (e.g. Bigland-Ritchie et al. 1978; for review see Gandevia, 2001). In hyperthermia, changes in the periphery, such as an increased speed of muscle contraction and relaxation (e.g. Davies et al. 1982; Wiles & Edwards, 1982; Ranatunga et al. 1987; De Ruiter & De Haan, 2000), may directly affect voluntary activation because temperature changes the motor-unit firing frequency required for tetanic fusion.
Brück & Olschewski (1987) suggested that central fatigue may be greater during hyperthermia, and this was later tested by Nybo & Nielsen (2001a). In their study, subjects cycled to exhaustion in a hot environment to elevate Tc to ∼40°C, and then performed maximal voluntary contractions (MVCs) of the knee extensors. After cycling with hyperthermia, central fatigue was greater during a sustained MVC, but not during brief MVCs, when compared to performance after cycling at a similar intensity with a Tc of 38°C (Nybo & Nielsen, 2001a). However, testing voluntary activation when exercise induces hyperthermia is complicated because not only has temperature increased, but there have been different metabolic, thermoregulatory and cardiovascular requirements. These differences are exacerbated when the tested muscles have been used in the exercise, but they are reduced when hyperthermia is induced by passive heating rather than exercise.
During passive elevation of Tc to ∼39.5°C, voluntary activation of the knee extensor muscles was lower during MVCs lasting 10 s (Morrison et al. 2004). This decline reversed with passive cooling back to a normal Tc (Morrison et al. 2004). Together, the studies using active and passive heating suggest that hyperthermia impairs voluntary activation, and that the impairment increases with the duration of the contraction. The effect occurs at a site at or above the level of the motoneurones, because voluntary activation was estimated by interpolation of a single stimulus to the motor nerve during an isometric voluntary contraction (‘twitch interpolation’; Merton, 1954). The extra force evoked by the superimposed stimulus during a maximal effort indicates that either the stimulated axons were not all recruited voluntarily, or they were discharging at subtetanic rates (e.g. Merton, 1954; Belanger & McComas, 1981; Herbert & Gandevia, 1999; Babault et al. 2001).
The aim of the present study was to further understand the mechanisms and site for the failure of voluntary drive during passive hyperthermia. To do this, we estimated voluntary activation with transcranial magnetic stimulation of the motor cortex (TMS) under control conditions and during hyperthermia for both brief and sustained MVCs. If extra force is evoked by stimulation of the motor cortex during maximal effort it suggests that failure of voluntary drive must occur at or above the level of cortical output (Gandevia et al. 1996; Taylor et al. 2000; Todd et al. 2003). We also investigated whether changes in voluntary drive were accompanied by changes in motor cortical ‘excitability’ as assessed by the EMG responses to motor cortex stimulation and the length of the subsequent EMG silence (‘silent period’). Because a rise in muscle temperature increases contractile speed, we assessed the contractile properties of muscle both with motor nerve stimulation and during the silent period following motor cortex stimulation. We hypothesized that hyperthermia may change the force–frequency relationship for the muscle, and thus alter voluntary activation and the development of central fatigue.
Methods
Seven subjects (37 ± 11 years, five females, two males) each took part in two experiments performed on separate days. For measures of muscle performance, subjects sat with the dominant arm held firmly at the wrist in an isometric myograph which measured elbow flexion torque (Fig. 1A). Subjects were positioned with the dominant shoulder and elbow flexed at 90° with the forearm vertical and fully supinated. All experimental procedures were approved by the institutional ethics committee, and conducted according to the Declaration of Helsinki. Written informed consent was obtained.
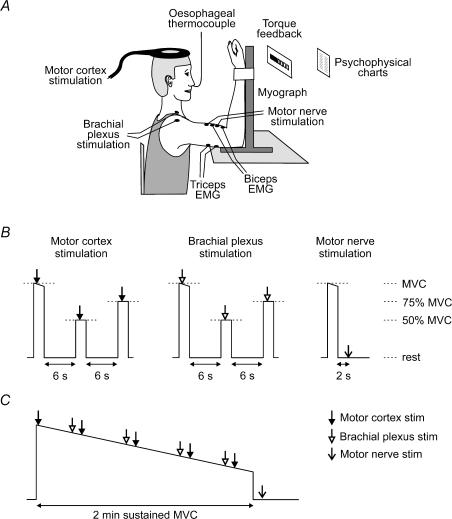
Figure 1. Experimental arrangement.
A, experimental apparatus. B and C, experimental protocol. Subjects performed sets of brief contractions (B) followed by a sustained maximal voluntary contraction (MVC; C). Each contraction set comprised a single MVC, or a MVC followed by two submaximal voluntary contractions. Brachial plexus stimulation or transcranial magnetic stimulation of the motor cortex (TMS) was delivered during sets containing three contractions. Motor nerve stimulation was delivered at rest after single MVCs. Arrows indicate the timing of stimuli.
Force and EMG recordings
Isometric elbow flexion torque was measured using a linear strain gauge (Xtran, Melbourne, Australia: linear to 2 kN). Electromyographic activity (EMG) was recorded with surface electrodes (Ag–AgCl, 10 mm diameter) over the biceps brachii and triceps brachii muscles. Care was taken to standardize electrode locations. Surface EMG signals were amplified (×100–300), filtered (16–1000 Hz), and sampled (2000 Hz) for later analysis using a data acquisition system (CED 1401 interface with Signal and Spike2 software, Cambridge Electronic Design, Cambridge, UK).
Stimulation
Three forms of stimulation were used: stimulation of the brachial plexus, stimulation of the biceps and brachialis motor nerve (termed ‘motor nerve stimulation’), and TMS.
Stimulation of the brachial plexus
Single electrical stimuli of 100 μs duration were delivered to the brachial plexus via a cathode in the supraclavicular fossa and an anode on the acromion (constant current, DS7, Digitimer Ltd, Welwyn Garden City, Hertfordshire, UK, modified to deliver up to 1 A). During the experiment setup, the stimulation intensity (90–240 mA) was set at 50% above the level required to produce a maximal compound muscle action potential (Mmax) in the biceps and triceps muscles at rest. The average amplitude of the resting Mmax was 17.7 ± 8.1 mV (mean ± s.d.) for biceps, and 7.4 ± 3.4 mV for triceps. In the experimental protocol, stimulation of the brachial plexus was only delivered during voluntary contractions.
Stimulation of the biceps brachii/brachialis motor nerve
Single electrical stimuli of 100 μs duration were delivered (constant current, DS7, Digitimer) via a surface cathode located approximately midway between the anterior edge of the deltoid and the elbow crease with the elbow flexed at 90°, and a surface anode positioned over the bicipital tendon (2–3 cm proximal to the elbow). During the experiment set-up, the stimulation intensity (132–418 mA) was set 10% above the level required to produce a resting twitch of maximal amplitude. In the experimental protocol, twitches were evoked at rest when the muscle was potentiated by a prior MVC.
TMS
A circular coil (13.5 cm outside diameter) positioned over the vertex elicited motor evoked potentials (MEPs) recorded from the biceps and triceps muscles (Magstim 200, Magstim Co., Dyfed, UK). The direction of current flow in the coil preferentially activated the motor cortex in the hemisphere which innervated the dominant arm. The stimulator output (50–80% of maximum) was set to produce a large MEP in the agonist biceps muscle (minimum amplitude: 50–60% of Mmax) during brief elbow flexion MVCs, and only a small MEP in the antagonist triceps muscle (amplitude <10% of Mmax). TMS was delivered during voluntary contractions only, and the stimulus intensity was similar for the two experiments.
Thermometry
Tc was measured with a thermocouple inserted transnasally into the oesophagus to a depth approximating the position of the right atrium (type T, Physitemp Instruments, Inc., Clifton, NJ, USA). Depth was estimated with the formula: depth (cm) = 0.479 × sitting height (cm) − 4.44 cm (Mekjavic & Rempel, 1990). Tc was recorded continuously at 10 Hz (Thermalert Model TH-8, Physitemp Instruments, Inc.), except when subjects were immersed in a water bath. During this time, Tc was recorded every 2 min. Air and water temperature were also measured.
Heart rate and blood pressure
Heart rate and blood pressure were continuously measured from the resting, nondominant hand at the digital or radial artery (Finapres Medical Systems, Amsterdam, The Netherlands; CBM-7000, Colin Medical Instruments Corp., San Antonio, TX, USA). In addition, heart rate and blood pressure were measured automatically from the brachial artery at set intervals.
Psychophysical measures
Whole-body thermal sensation and thermal discomfort were measured at rest. The thermal sensation scale ranged from 1 (unbearably cold) to 13 (unbearably hot), with 7 being neutral, while the thermal discomfort scale ranged between 1 (comfortable) and 5 (extremely uncomfortable) (Gagge et al. 1967). Ratings were obtained before and after the sets of brief contractions, during immersion in the bath, and during recovery.
Protocol
The first experiment assessed voluntary activation of elbow flexor muscles in a thermoneutral environment (control condition). Subjects performed nine sets of brief (1–2 s) contractions, performed in a sequence and separated by at least 1 min to avoid fatigue. Three contraction sets were performed for each type of stimulation (Fig. 1B). For brachial plexus and motor cortex stimulation, the contraction sets involved a MVC followed by a 50% MVC and a 75% MVC. The target contractions were calculated from the preceding MVC, and were displayed on a visual feedback device. Within a set, the contractions were separated by 6 s of rest, and a stimulus was delivered during each contraction. For motor nerve stimulation, one MVC was performed followed by a stimulus delivered at rest, 2 s after completion of the MVC. After completing the brief contractions, subjects then performed a 2 min sustained MVC during which a series of stimuli were delivered. Motor cortex stimuli were delivered at 2, 25, 55, 85 and 110 s, and brachial plexus stimuli were delivered at 20, 50, 80 and 105 s. A motor nerve stimulus was also delivered at rest, 5 s after completion of the sustained MVC. To monitor recovery, subjects then commenced a series of brief contraction sets, similar to those performed prior to the sustained MVC. The rest interval between the sets of brief contractions varied between 15 and 30 s.
In the second experiment, voluntary activation of elbow flexor muscles was assessed during hyperthermia elicited by passive heating. The experiment began as in the first one with nine sets of brief contractions in a thermoneutral environment. Subjects then changed into swimwear and entered a bath containing water of 39.5 ± 1.4°C to passively induce hyperthermia (Modena, 1800, Decina Bathroomware, Carole Park, Australia). The water temperature was gradually increased to 40.9 ± 0.6°C by recirculating water through a pump with a heating element in series (XS300HD, Davey Products, Scoresby, Australia). Subjects were submerged up to the neck, and wore a warm hat to minimize heat loss from the head. Subjects remained in the bath until Tc increased to 39.5°C, which took on average 19 ± 7 min. Subjects consumed warm water ad libitum. Subjects then stepped out of the bath, quickly dried, entered a prewarmed laboratory, and put on warm clothing. Maximality of the brachial plexus and motor nerve stimulation was checked at rest. Subjects then performed six sets of brief contractions, followed by a 2 min sustained MVC with superimposed stimuli. Finally, subjects performed a series of brief contraction sets to monitor recovery. The protocol for the sustained MVC and recovery period was similar to that in the control experiment.
Data analysis
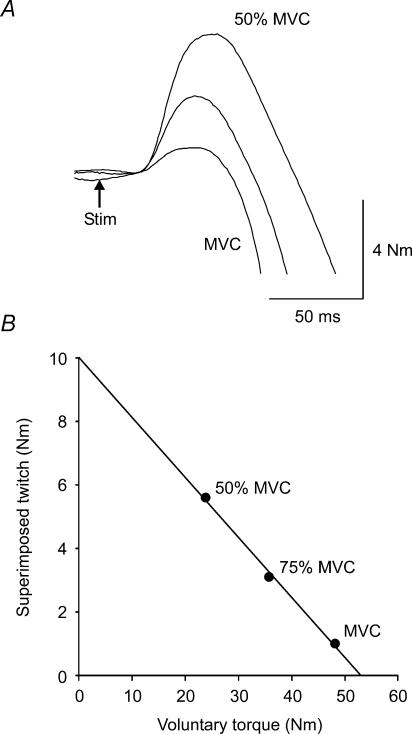
Voluntary activation was quantified by measurement of the torque responses to motor cortex stimulation. Any increment in elbow flexion torque evoked during a contraction (superimposed twitch; Fig. 2A) was expressed as a fraction of the estimated amplitude of the response evoked by the same stimulus at rest (Todd et al. 2003, 2004). The amplitude of the resting twitch was estimated rather than measured directly because motor cortical and spinal cord excitability increase with activity (e.g. Hess et al. 1987; Ugawa et al. 1995; Di Lazzaro et al. 1998; for review see Rothwell et al. 1991). For each subject, estimation involved linear regression between voluntary torque and the amplitude of the superimposed twitch evoked by motor cortex stimulation. One regression was performed per set of brief contractions (a MVC followed by a 50% MVC and a 75% MVC). The y-intercept was taken as the amplitude of the resting twitch evoked by motor cortex stimulation (Fig. 2B, Todd et al. 2003, 2004). Thus, each set of brief contractions, with superimposed motor cortex stimuli, yielded one ‘estimated resting twitch’. The level of drive was quantified as a percentage using the equation: Voluntary activation (%) = (1–superimposed twitch/estimated resting twitch) × 100.
Figure 2. Single-subject data for torque increments produced by motor cortex stimulation.
A, raw torque traces of the superimposed twitch during a 50% MVC, 75% MVC, and a MVC. Arrow indicates the timing of stimulus. B, the relationship between the superimposed twitch and voluntary torque during one set of brief contractions.
During contractions in which motor cortex or brachial plexus stimulation was delivered, the areas of MEPs and Mmax were measured between set cursors for the biceps and triceps muscles. To account for activity-dependent changes in the muscle-fibre action potentials, the area of each MEP was normalized to the area of Mmax elicited during nearby contractions of the same strength (e.g. Gandevia et al. 1999; Taylor et al. 1999). For biceps, the voluntary root mean square (r.m.s.) EMG amplitude was expressed relative to the r.m.s. EMG amplitude of Mmax to account for any temperature-related changes in the muscle-fibre action potentials. The contractile properties of muscle were assessed by measurement of the resting twitch evoked by motor nerve stimulation, before and after exercise. The relaxation rate of muscle was also calculated during each MVC by measurement of the steepest rate of decline of torque in the silent period immediately following motor cortex stimulation (see Fig. 3). The peak rate of decline was then normalized to the total torque (voluntary plus evoked) prior to the silent period. This value reflects the relative relaxation rate for all elbow flexor muscle fibres active in the ongoing contraction or activated in the MEP. Voluntary torque was measured by calculation of the mean torque over a 100 ms period immediately prior to the stimulus. For each subject, voluntary torque was normalized to the largest MVC recorded on the day during the sets of brief contractions performed in the thermoneutral environment.
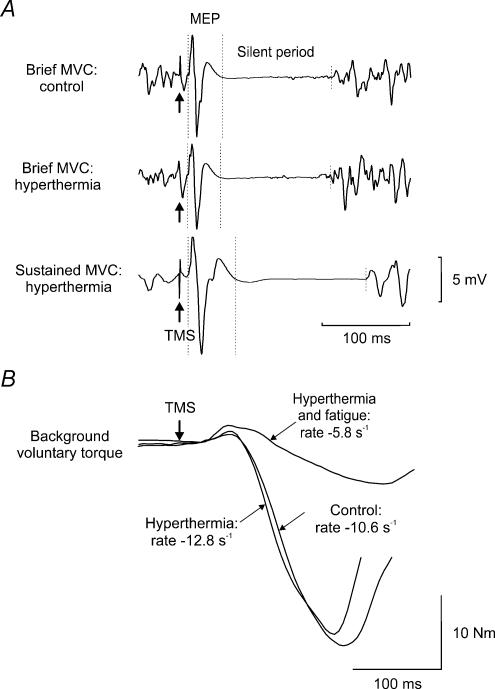
Figure 3. Raw traces of biceps EMG and elbow flexion torque for one subject during the second experiment.
A, biceps EMG. B, elbow flexion torque. The data were recorded during MVCs performed with a normal core temperature (control) or with hyperthermia. Motor cortex stimuli (arrows labelled TMS) were delivered during MVCs. B, arrows indicate the peak relaxation rate of muscle, calculated by measurement of the steepest rate of decline of torque in the silent period (shown in A) immediately following TMS. The rate of decline was normalized to the total torque prior to the silent period (unit, s−1). Background voluntary torque is superimposed for comparison between traces. MEP, motor evoked potential.
In the text, group data are presented as the mean ± standard deviation (s.d.), whereas in figures, mean ± standard error of the mean (s.e.m.) values are shown. Student's paired t tests were performed to compare data collected during the sets of brief contractions performed in the thermoneutral environment in the two experiments. Student's paired t tests also compared data collected during the sets of brief contractions performed in the thermoneutral environment with those during hyperthermia in the second experiment. Two-way repeated measures analysis of variance (ANOVA) was used for comparison between the brief control contractions and various time points during the sustained MVC, and for comparison between the brief control contractions and the recovery contractions. Two-way repeated measures ANOVA was also used to determine the onset of muscle fatigue during the sustained MVCs. Non-parametric data were transformed to ranks, and a two-way repeated measures ANOVA on ranks was performed. Post-hoc discrimination between means was made with the Student–Newman–Keuls procedure (SigmaStat 2.03, SPSS, Inc.). Statistical significance was set at the 5% level.
Results
Torque and EMG responses to stimulation of the motor pathway were measured during brief elbow flexion contractions of varying strengths and during a sustained maximal effort. Subjects performed the contractions in a thermoneutral environment and during passively induced hyperthermia.
Brief MVCs
Immersion in the hot bath induced hyperthermia and elevated ratings of thermal sensation and thermal discomfort (Table 1). Heart rate and systolic blood pressure increased with hyperthermia, but diastolic blood pressure decreased (P < 0.05). Mean arterial pressure was similar at the two temperatures. Table 2 shows that hyperthermia also affected the properties of muscle fibres. The half-relaxation time of the resting twitch evoked by motor nerve stimulation diminished (P = 0.048). Furthermore, the normalized peak relaxation rate of the elbow flexors in a cortically induced silent period (see Methods) was faster during hyperthermia (−11.0 ± 5.5 s−1) than at the normal Tc (−9.0 ± 5.0 s−1, P = 0.002; see also Fig. 3B). The size of the twitch torque was not affected by hyperthermia. In biceps, the area of the resting Mmax decreased with hyperthermia (P = 0.005). Although the sampling frequency limited the precision of measurement, the latency of biceps Mmax at rest was ∼0.4 ms shorter during hyperthermia than at the normal Tc (P = 0.01).
Table 1.
The average (± S.D.) core temperature, psychophysical ratings, and brachial artery (a.) blood pressure and heart rate, prior to performing sets of brief contractions in the second experiment
| Variable | Control | Hyperthermia |
|---|---|---|
| Core temperature (°C) | 37.2 ± 0.3 | 38.5 ± 0.4* |
| Thermal sensation | 7.6 ± 0.6 (neutral to slightly warm) | 10.3 ± 1.0* (hot to very hot) |
| Thermal discomfort | 1.2 ± 0.3 (comfortable) | 2.9 ± 1.1* (uncomfortable) |
| Brachial a. SBP (mmHg) | 112 ± 8 | 118 ± 12* |
| Brachial a. DBP (mmHg) | 70 ± 11 | 63 ± 9* |
| Brachial a. MAP (mmHg) | 84 ± 9 | 81 ± 8 |
| Brachial a. HR (beats min−1) | 74 ± 17 | 112 ± 21* |
Data were collected with a normal core temperature (control) and during hyperthermia.
Significant difference between the control and hyperthermic conditions (P < 0.05).
SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; HR, heart rate.
Table 2.
Characteristics of the potentiated resting twitch evoked by motor nerve stimulation, the estimated resting twitch evoked by motor cortex stimulation (TMS), and the resting maximal compound muscle action potential (Mmax) evoked by brachial plexus stimulation with a normal core temperature (control) and during hyperthermia
| Response | Control | Hyperthermia |
|---|---|---|
| Potentiated motor nerve resting twitch | ||
| Amplitude (Nm) | 5.3 ± 1.4 | 4.7 ± 1.0 |
| Time-to-peak (ms) | 57 ± 11 | 50 ± 13 |
| Half-relaxation time (ms) | 86 ± 23 | 74 ± 27* |
| Estimated TMS resting twitch | ||
| Amplitude (Nm) | 11.1 ± 2.1 | 13.0 ± 3.2 |
| Biceps Mmax at rest | ||
| Area (mV s−1) | 0.15 ± 0.06 | 0.13 ± 0.05* |
| Amplitude (mV) | 17.7 ± 7.6 | 16.2 ± 6.4 |
Significant difference between control and hyperthermic conditions (P < 0.05).
With hyperthermia, voluntary torque during brief MVCs was slightly but significantly less than at the normal Tc (93.4 ± 4.8 versus 95.7 ± 3.9% MVC, P = 0.03). However, hyperthermia did not affect the average size of the superimposed twitch evoked by motor cortex stimulation (normal Tc: 1.1 ± 0.7%; hyperthermia: 1.4 ± 1.2% MVC) or the calculated voluntary activation (normal Tc: 94.9 ± 2.4%; hyperthermia: 94.0 ± 4.4%). During brief MVCs, the area of the MEP in biceps and triceps (relative to Mmax), and the duration of the silent period evoked by cortical stimulation, were unaffected by hyperthermia (see Fig. 3A). The level of voluntary biceps EMG in brief maximal efforts was the same under the two conditions. Overall, during hyperthermia there was a small reduction in torque in the brief MVCs, and an increase in the relaxation rate of muscle.
Sustained MVCs
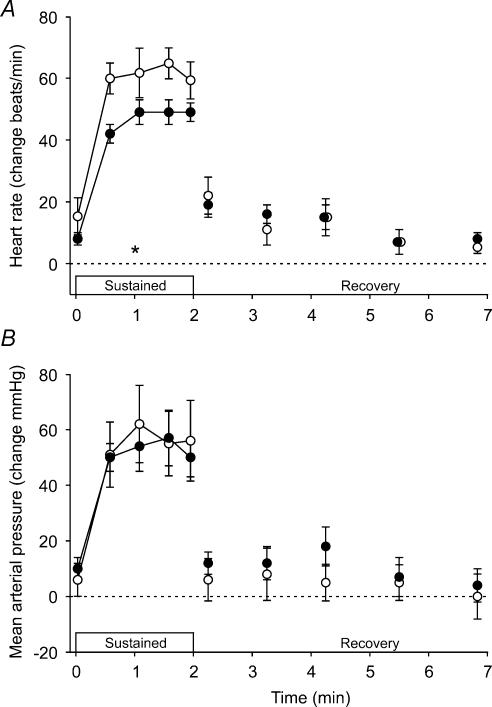
During each sustained MVC, heart rate (F5,28 = 66.4) and mean arterial pressure (F5,25 = 45.0) increased (P < 0.001; Fig. 4), but Tc remained unchanged. Figure 4 shows that the increase in mean arterial pressure was similar in the two conditions, but the increase in heart rate was less during hyperthermia (F1,28 = 60.6, P < 0.001).
Figure 4. Average cardiovascular responses during sustained MVCs and brief recovery MVCs.
A, heart rate. B, mean arterial pressure. Contractions were performed with a normal core temperature (control; ○) and during hyperthermia (•). Data are expressed relative to values obtained during the brief MVCs performed in the two conditions (dashed line). *Significant difference between the control and hyperthermic conditions during the sustained MVC (P < 0.05).
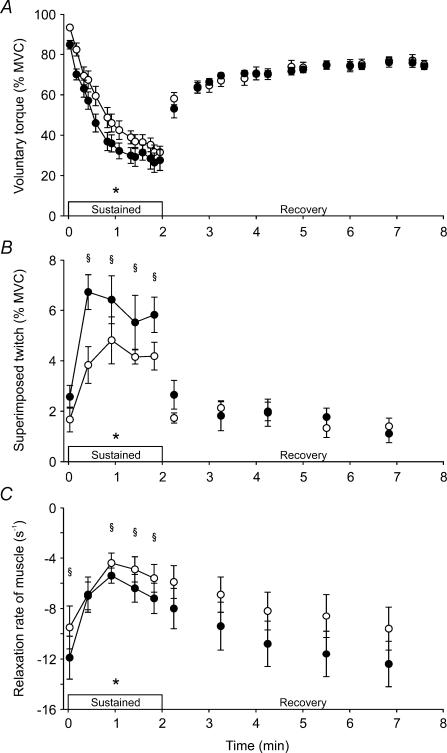
Muscle fatigue was greater during the 2 min sustained MVC performed with hyperthermia (Fig. 5A). The area under the torque trace was ∼12% lower during hyperthermia (P = 0.012). Muscle fatigue also developed more quickly during hyperthermia than in the control condition. Statistically, the torque decline was evident at 6 s with hyperthermia, and at 8 s in the control condition (F9,45 = 2.58, P = 0.017). Again the level of voluntary biceps EMG was similar in the two conditions during the sustained effort. As fatigue developed during each sustained MVC, the peak relaxation rate of elbow flexor muscles declined (F5,28 = 8.3; P < 0.001), but the relaxation rate remained higher with hyperthermia (average: −7.5 ± 2.8 s−1) than in the control condition (average: −6.1 ± 2.8 s−1) for four of the five time points (F5,28 = 2.77, P = 0.037; see Methods; Fig. 5C).
Figure 5. Average torque responses during sustained MVCs and brief recovery MVCs.
A, voluntary torque. B, superimposed twitch evoked by motor cortex stimulation. C, Peak relaxation rate of elbow flexor muscles. Peak relaxation rate was measured during the silent period following motor cortex stimulation. Contractions were performed with a normal core temperature (control; ○) and during hyperthermia (•). Data in A and B are relative to the largest voluntary torque recorded during brief control MVCs. *Significant difference between the control and hyperthermic conditions during the sustained MVC (P < 0.05). §Significant difference between conditions for individual time points.
During the sustained MVCs, muscle fatigue was partly caused by a progressive reduction in voluntary activation. Hence, the amplitude of the superimposed twitch evoked by motor cortex stimulation increased during the sustained maximal efforts (F5,28 = 18.6, P < 0.001; Fig. 5B). The amplitude of the initial superimposed twitch, evoked 2 s into the sustained MVC, was similar to that evoked during the brief MVCs, but thereafter its amplitude increased (P < 0.001). The increase in amplitude was ∼50% greater during hyperthermia than in the control condition (F1,28 = 13.6, P = 0.01). Across the sustained contraction, the average amplitudes of the superimposed twitch evoked by the motor cortex stimulus were 3.6 ± 0.8 and 5.4 ± 1.4% MVC in the control condition and during hyperthermia, respectively.
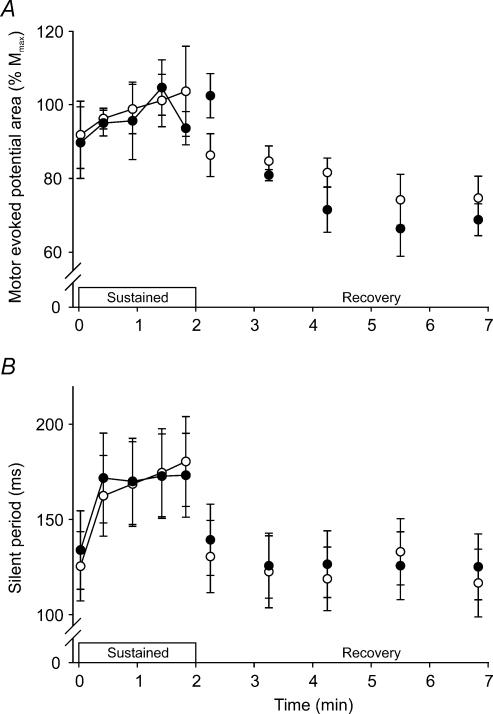
The area of the MEP in biceps was larger during the sustained MVCs than in brief MVCs (F5,29 = 7.3, P < 0.001; Fig. 6A), and its size was similar at the two temperatures. The duration of the silent period in biceps lengthened during the sustained MVC (F5,29 = 24.1, P < 0.001) by a similar amount in the two conditions (∼40–55 ms; Fig. 6B). In the antagonist triceps muscle, the area of the MEP was similar during the brief (9 ± 3% Mmax) and sustained MVCs (13 ± 5% Mmax) in the control condition, and it did not change with hyperthermia.
Figure 6. Average biceps EMG responses to motor cortex stimulation during sustained MVCs and brief recovery MVCs.
A, area of the motor evoked potential (expressed relative to the maximal compound muscle action potential, Mmax). B, duration of the silent period. Contractions were performed with a normal core temperature (○) and during hyperthermia (•).
Following the sustained MVC, the time course of the recovery of voluntary torque (Fig. 5A), responses evoked by motor cortex stimulation (Figs 5B and C, and 6), cardiovascular measures (Fig. 4), and the amplitude of the potentiated resting twitch evoked by motor nerve stimulation, were similar in the two conditions.
Discussion
This study assessed voluntary activation with motor cortex stimulation in brief and sustained MVCs of elbow flexor muscles during passive hyperthermia. Two aspects of the results will be discussed in detail. First, voluntary torque was less during brief MVCs performed with hyperthermia, and second, central fatigue was greater during the sustained MVC performed with hyperthermia. We have shown for the first time that additional failure of voluntary drive during hyperthermia occurs at or above the level of motor cortical output, and it is not associated with any changes in motor cortical excitability or intracortical inhibition as assessed by single-pulse TMS. A second novel observation is that some of the central fatigue may develop because the contraction speed of muscle increases during hyperthermia.
Brief MVCs
Temperature-induced changes within the muscle probably contributed to the small decline (by ∼2%) in voluntary torque. The resting twitch evoked by motor nerve stimulation relaxed faster and the peak relaxation rate of elbow flexor muscles measured in the silent period after motor cortex stimulation increased. This change means that higher rates of motoneurone discharge would be required to generate maximal force. Previous studies in which muscles were cooled showed that temperature-induced slowing of contraction time does not alter motor unit firing rates (Marsden et al. 1983; Bigland-Ritchie et al. 1992). Hence, if firing rates are also unchanged during hyperthermia, voluntary activation would be impaired because they would not be quite sufficient to achieve the same level of fusion from the faster muscle. This concept is supported by modelling. Changes in relaxation rate of the size measured here would produce a reduction of up to 2% in maximal voluntary activation based on a realistic model of twitch interpolation (see Herbert & Gandevia, 1999). However, voluntary activation measured with motor cortex stimulation during brief MVCs was not changed by hyperthermia. Thus, it is possible that in brief MVCs, the motor unit discharge rates can be increased somewhat by volition sufficient to maintain the control level of muscle voluntary activation, but any such increase may not be sustainable in contractions lasting more than 2–3 s (see Sustained MVCs).
The presence of suboptimal motoneurone firing rates during MVCs performed with hyperthermia is suggested by the data of Morrison et al. (2004). They reported that passive hyperthermia (Tc 38.5°C) reduced voluntary torque by 13% and voluntary activation by 11% during 10 s MVCs of the knee extensor muscles. This reduction in voluntary torque was greater than in the present study during the first 10 s of the sustained MVC of the elbow flexors (∼7% reduction). Apart from the muscle groups tested, a difference between the two studies was the provision of feedback of performance. We provided subjects with verbal encouragement and visual feedback of voluntary torque during all MVCs to ensure maximal effort (Gandevia, 2001), whereas Morrison et al. (2004) did not. This lack of feedback could also have contributed to the poor average voluntary activation (83%) during the 10 s MVCs performed in a thermoneutral environment (Morrison et al. 2004). In contrast, in other studies in which hyperthermia was actively generated by exercising in a hot environment, voluntary torque and activation during brief MVCs were unaffected by hyperthermia (Nybo & Nielsen, 2001a; Saboisky et al. 2003).
Sustained MVCs
Muscle fatigue was greater during a sustained MVC of the elbow flexor muscles during passive hyperthermia than when Tc was normal. Some of this extra fatigue was caused by additional central fatigue. The use of motor cortex stimulation has enabled us to show that some additional failure of voluntary drive occurs at or above the level of motor cortical output. The larger superimposed twitch evoked by motor cortex stimulation during hyperthermia is produced through synaptic activation of motoneurones, and thus, any fall in motoneurone activity is not due to an inability of motor cortical neurones to produce additional motoneuronal output. Furthermore, the additional failure of voluntary drive is not due to any obvious differences in motor cortical ‘excitability’ because changes in the MEP and the duration of the silent period were similar at the two temperatures.
We argue that temperature-induced changes in the contractile properties of muscle probably contributed to the additional central fatigue during sustained MVCs performed with hyperthermia. The peak relaxation rate of muscle remained ∼20% faster throughout the contraction at an elevated temperature, and so higher motor unit firing rates would be needed to fuse force. Even if motor unit firing rates can be transiently increased in brief MVCs during hyperthermia (see above), the significantly larger superimposed twitches evoked by motor cortex stimulation later in the sustained MVC indicate that any higher discharge rates cannot be maintained during a 2 min maximal voluntary effort. It is as if the motor cortical output can no longer keep up with or compensate for the increased contractile speed.
Changes in the contractile properties of muscle may not be the only factor contributing to the additional central fatigue during hyperthermia. Many studies have noted the effects of hyperthermia on brain function. For example, prolonged exercise in the heat can lead to a reduced blood velocity in the middle cerebral artery (Nybo & Nielsen, 2001b), and altered electroencephalographic activity (Nielsen et al. 2001) and brain metabolism (Gonzalez-Alonso et al. 2004). At higher temperatures, the motor pathways may even be affected by altered output from the thermoregulatory centres in the hypothalamus to other structures, including the cerebral cortex (Saper, 2004).
The magnitude of the additional central fatigue related to hyperthermia was smaller than that reported by Nybo & Nielsen (2001a). The disparity may result from a difference between hyperthermia elicited by active and passive heating, the muscle groups tested, the method used to measure voluntary activation, or the absence of visual feedback of voluntary force in the study conducted by Nybo & Nielsen (2001a). In their study, voluntary activation was measured with the use of motor nerve stimulation. This differs from that measured with motor cortex stimulation. A superimposed twitch evoked by motor nerve stimulation implies that failure of voluntary drive must have occurred at a site at or above the point of stimulation, the motor axons. However, one cannot determine whether the failure is due to inadequate input to the motoneurone pool, or an inability of motoneurones to respond to adequate input. In contrast, a superimposed twitch evoked by motor cortex stimulation implies that voluntary output from the motor cortex was not sufficient to drive the motoneurone pool optimally. Furthermore, the finding of large MEPs evoked in agonist muscles relative to Mmax, suggests that motoneurones were able to respond to descending synaptic input.
This study has used novel techniques to examine limits to voluntary force production imposed at the motor cortex and at the muscle in hyperthermia. We have confirmed that central fatigue is greater during sustained MVCs performed with hyperthermia than at a normal body temperature. In addition, by using motor cortex stimulation, we have shown that the additional central fatigue is partly caused by a failure of voluntary drive at or above the level of motor cortical output. Based on measurement of changes in muscle contractile properties during the exercise, we argue that some of the failure of voluntary drive to produce maximal force can be accounted for by temperature-related changes in the contractile properties of muscle.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia (3206). We are grateful to Decina Bathroomware Pty Ltd and Davey Products Pty Ltd for their donation of equipment. We thank Peter Martin for comments on the manuscript.
References
- Allen DG, Westerblad H. Role of phosphate and calcium stores in muscle fatigue. J Physiol. 2001;536:657–665. doi: 10.1111/j.1469-7793.2001.t01-1-00657.x. 10.1111/j.1469-7793.2001.t01-1-00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babault N, Pousson M, Ballay Y, Van Hoecke J. Activation of human quadriceps femoris during isometric, concentric, and eccentric contractions. J Appl Physiol. 2001;91:2628–2634. doi: 10.1152/jappl.2001.91.6.2628. [DOI] [PubMed] [Google Scholar]
- Belanger AY, McComas AJ. Extent of motor unit activation during effort. J Appl Physiol. 1981;51:1131–1135. doi: 10.1152/jappl.1981.51.5.1131. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Jones DA, Hosking GP, Edwards RHT. Central and peripheral fatigue in sustained maximum voluntary contractions of human quadriceps muscle. Clin Sci Mol Med. 1978;54:609–614. doi: 10.1042/cs0540609. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Thomas CK, Rice CL, Howarth JV, Woods JJ. Muscle temperature, contractile speed, and motoneuron firing rates during human voluntary contractions. J Appl Physiol. 1992;73:2457–2461. doi: 10.1152/jappl.1992.73.6.2457. [DOI] [PubMed] [Google Scholar]
- Brück K, Olschewski H. Body temperature related factors diminishing the drive to exercise. Can J Physiol Pharmacol. 1987;65:1274–1280. doi: 10.1139/y87-203. [DOI] [PubMed] [Google Scholar]
- Davies CT, Mecrow IK, White MJ. Contractile properties of the human triceps surae with some observations on the effects of temperature and exercise. Eur J Appl Physiol Occup Physiol. 1982;49:255–269. doi: 10.1007/BF02334074. [DOI] [PubMed] [Google Scholar]
- De Ruiter CJ, De Haan A. Temperature effect on the force/velocity relationship of the fresh and fatigued human adductor pollicus muscle. Pflügers Arch. 2000;440:163–170. doi: 10.1007/s004240000284. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Effects of voluntary contraction on descending volleys evoked by transcranial stimulation in conscious humans. J Physiol. 1998;508:625–633. doi: 10.1111/j.1469-7793.1998.625bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RH, Harris RC, Hultman E, Kaijser L, Koh D, Nordesjo LO. Effect of temperature on muscle energy metabolism and endurance during successive isometric contractions, sustained to fatigue, of the quadriceps muscle in man. J Physiol. 1972;220:335–352. doi: 10.1113/jphysiol.1972.sp009710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio MA, Snow RJ, Hargreaves M, Stathis CG, Martin IK, Carey MF. Muscle metabolism during exercise and heat stress in trained men: effect of acclimation. J Appl Physiol. 1994;76:589–597. doi: 10.1152/jappl.1994.76.2.589. [DOI] [PubMed] [Google Scholar]
- Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- Gagge AP, Stolwijk JAJ, Hardy JD. Comfort and thermal sensations and associated physiological responses at various ambient temperatures. Environ Res. 1967;1:1–20. doi: 10.1016/0013-9351(67)90002-3. [DOI] [PubMed] [Google Scholar]
- Galloway SD, Maughan RJ. Effects of ambient temperature on the capacity to perform prolonged cycle exercise in man. Med Sci Sports Exerc. 1997;29:1240–1249. doi: 10.1097/00005768-199709000-00018. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Allen GM, Butler JE, Taylor JL. Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. J Physiol. 1996;490:529–536. doi: 10.1113/jphysiol.1996.sp021164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, Allen GM, McKenzie DK. Central fatigue. Critical issues, quantification and practical implications. Adv Exp Med Biol. 1995;384:281–294. [PubMed] [Google Scholar]
- Gandevia SC, Petersen N, Butler JE, Taylor JL. Impaired response of human motoneurones to corticospinal stimulation after voluntary exercise. J Physiol. 1999;521:749–759. doi: 10.1111/j.1469-7793.1999.00749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Dalsgaard MK, Osada T, Volianitis S, Dawson EA, Yoshiga CC, Secher NH. Brain and central haemodynamics and oxygenation during maximal exercise in humans. J Physiol. 2004;557:331–342. doi: 10.1113/jphysiol.2004.060574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Teller C, Andersen SL, Jensen FB, Hyldig T, Nielsen B. Influence of body temperature on the development of fatigue during prolonged exercise in the heat. J Appl Physiol. 1999;86:1032–1039. doi: 10.1152/jappl.1999.86.3.1032. [DOI] [PubMed] [Google Scholar]
- Herbert RD, Gandevia SC. Twitch interpolation in human muscles: mechanisms and implications for measurement of voluntary activation. J Neurophysiol. 1999;82:2271–2283. doi: 10.1152/jn.1999.82.5.2271. [DOI] [PubMed] [Google Scholar]
- Hess CW, Mills KR, Murray NMF. Responses in small hand muscles from magnetic stimulation of the human brain. J Physiol. 1987;388:397–419. doi: 10.1113/jphysiol.1987.sp016621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall JD, Reddan WG, Layton CR, Dempsey JA. Effects of metabolic hyperthermia on performance during heavy prolonged exercise. J Appl Physiol. 1974;36:538–544. doi: 10.1152/jappl.1974.36.5.538. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Meadows JC, Merton PA. ‘Muscular wisdom’ that minimizes fatigue during prolonged effort in man: Peak rates of motoneurone discharge and slowing of discharge during fatigue. In: Desmedt JE, editor. Advances in Neurology, Vol. 39. Motor Control Mechanisms in Health and Disease. New York, USA: Raven Press; 1983. pp. 169–211. [PubMed] [Google Scholar]
- Mekjavic IB, Rempel ME. Determination of esophageal probe insertion length based on standing and sitting height. J Appl Physiol. 1990;69:376–379. doi: 10.1152/jappl.1990.69.1.376. [DOI] [PubMed] [Google Scholar]
- Merton PA. Voluntary strength and fatigue. J Physiol. 1954;123:553–564. doi: 10.1113/jphysiol.1954.sp005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S, Sleivert GG, Cheung SS. Passive hyperthermia reduces voluntary activation and isometric force production. Eur J Appl Physiol. 2004;91:729–736. doi: 10.1007/s00421-004-1063-z. [DOI] [PubMed] [Google Scholar]
- Nielsen B, Hales JR, Strange S, Christensen NJ, Warberg J, Saltin B. Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. J Physiol. 1993;460:467–485. doi: 10.1113/jphysiol.1993.sp019482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen B, Hyldig T, Bidstrup F, Gonzalez-Alonso J, Christoffersen GR. Brain activity and fatigue during prolonged exercise in the heat. Pflügers Arch. 2001;442:41–48. doi: 10.1007/s004240100515. [DOI] [PubMed] [Google Scholar]
- Nybo L, Nielsen B. Hyperthermia and central fatigue during prolonged exercise in humans. J Appl Physiol. 2001a;91:1055–1060. doi: 10.1152/jappl.2001.91.3.1055. [DOI] [PubMed] [Google Scholar]
- Nybo L, Nielsen B. Middle cerebral artery blood velocity is reduced with hyperthermia during prolonged exercise in humans. J Physiol. 2001b;534:279–286. doi: 10.1111/j.1469-7793.2001.t01-1-00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin JM, Carey MF, Zhao S, Febbraio MA. Effect of ambient temperature on human skeletal muscle metabolism during fatiguing submaximal exercise. J Appl Physiol. 1999;86:902–908. doi: 10.1152/jappl.1999.86.3.902. [DOI] [PubMed] [Google Scholar]
- Ranatunga KW, Sharpe B, Turnbull B. Contractions of a human skeletal muscle at different temperatures. J Physiol. 1987;390:383–395. doi: 10.1113/jphysiol.1987.sp016707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC, Thompson PD, Day BL, Boyd S, Marsden CD. Stimulation of the human motor cortex through the scalp. Exp Physiol. 1991;76:159–200. doi: 10.1113/expphysiol.1991.sp003485. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev. 1974;54:75–159. doi: 10.1152/physrev.1974.54.1.75. [DOI] [PubMed] [Google Scholar]
- Saboisky J, Marino FE, Kay D, Cannon J. Exercise heat stress does not reduce central activation to non-exercised human skeletal muscle. Exp Physiol. 2003;88:783–790. doi: 10.1113/eph8802611. [DOI] [PubMed] [Google Scholar]
- Saltin B, Gagge AP, Bergh U, Stolwijk JA. Body temperatures and sweating during exhaustive exercise. J Appl Physiol. 1972;32:635–643. doi: 10.1152/jappl.1972.32.5.635. [DOI] [PubMed] [Google Scholar]
- Saper CB. Hypothalamus. In: Paxinos G, Mai JK, editors. The Human Nervous System. London: Elsevier. Academic Press; 2004. pp. 513–550. [Google Scholar]
- Taylor JL, Allen GM, Butler JE, Gandevia SC. Supraspinal fatigue during intermittent maximal voluntary contractions of the human elbow flexors. J Appl Physiol. 2000;89:305–313. doi: 10.1152/jappl.2000.89.1.305. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Butler JE, Gandevia SC. Altered responses of human elbow flexors to peripheral-nerve and cortical stimulation during a sustained maximal voluntary contraction. Exp Brain Res. 1999;127:108–115. doi: 10.1007/s002210050779. [DOI] [PubMed] [Google Scholar]
- Todd G, Taylor JL, Gandevia SC. Measurement of voluntary activation of fresh and fatigued human muscles using transcranial magnetic stimulation. J Physiol. 2003;551:661–671. doi: 10.1113/jphysiol.2003.044099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd G, Taylor JL, Gandevia SC. Reproducible measurement of voluntary activation of human elbow flexors with motor cortical stimulation. J Appl Physiol. 2004;97:236–242. doi: 10.1152/japplphysiol.01336.2003. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Terao Y, Hanajima R, Sakai K, Kanazawa I. Facilitatory effect of tonic voluntary contraction on responses to motor cortex stimulation. Electroencephalogr Clin Neurophysiol. 1995;97:451–454. doi: 10.1016/0924-980x(95)00214-6. [DOI] [PubMed] [Google Scholar]
- Wiles CM, Edwards RH. Metabolic heat production in isometric ischaemic contractions of human adductor pollicis. Clin Physiol. 1982;2:499–512. doi: 10.1111/j.1475-097x.1982.tb00056.x. [DOI] [PubMed] [Google Scholar]
- Young AJ, Sawka MN, Levine L, Cadarette BS, Pandolf KB. Skeletal muscle metabolism during exercise is influenced by heat acclimation. J Appl Physiol. 1985;59:1929–1935. doi: 10.1152/jappl.1985.59.6.1929. [DOI] [PubMed] [Google Scholar]