Abstract
Whole-cell patch recordings were made from parasympathetic preganglionic neurones (P-PGNs) and unidentified intermediolateral (IML) neurones in thick slices of the lower lumbar and sacral spinal cord of 14- to 21-day-old rats. The P-PGNs and IML neurones examined were similar in terms of soma sizes, input resistance and capacitance, and displayed a sag conductance as well as rebound firing. In the absence of drugs, the neurones responded with either tonic or adapting firing to depolarizing current steps. However, in the presence of the group I metabotropic glutamate receptor agonist (RS)-3,5-dihydroxyphenylglycine (DHPG), almost half of the neurones displayed accelerating firing rates during the constant current injection, followed by a sustained after-discharge. In the presence of TTX, plateau potentials were observed. The firing changes and plateaux were blocked by nifedipine, an L-type Ca2+ channel blocker, and (S)-(−)-Bay K8644 was able to produce these firing changes and plateaux in the absence of DHPG, demonstrating the involvement of an L-type Ca2+ conductance. Ca2+-activated nonspecific cationic conductances also appear to contribute to the firing changes. A few neurones displayed membrane oscillations and burst firing in the presence of DHPG. The results suggest that the firing characteristics of both P-PGNs and other neurones likely to be involved in caudal spinal reflex control are not static but, rather, quite dynamic and under metabotropic glutamate receptor modulatory control. Such changes in firing patterns may be involved in normal pelvic parasympathetic reflex function during micturition, defaecation and sexual reflexes, and may contribute to the abnormal output patterns seen with loss of descending brainstem input and visceral or perineal sensory disturbances.
Micturition, defecation and penile erectile reflexes are regulated by parasympathetic preganglionic neurones (P-PGNs) located in the intermediolateral region of the caudal lumbar and rostral sacral spinal segments in the rat. While P-PGNs have been examined using a variety of anatomical approaches in both adult and neonatal animals (for review see de Groat et al. 1996), there is only limited information regarding their synaptic inputs, intrinsic electrical membrane properties and firing characteristics. In vitro studies using neonatal spinal cord preparations have provided most of the information we have about the electrophysiological properties of lumbosacral P-PGNs (Araki, 1994; Araki & de Groat, 1996; Miura et al. 2000, 2001, 2002). In addition to the parasympathetic preganglionic neurones, the intermediolateral area of the caudal lumbosacral spinal grey matter contains a variety of neurones thought to be interposed in segmental, ascending and descending pathways controlling the parasympathetic preganglionic neurones (Nadelhaft et al. 1992; Marson, 1997). Several of these neurone populations have been identified as segmental interneurones that mediate direct excitation and inhibition of P-PGNs (Araki & de Groat, 1996). While there is limited basic information about the membrane electrical features of these presumed interneurones in the neonatal rat, as with the P-PGNs, the presence of active intrinsic membrane properties capable of modulating neurone firing has not been examined in any detail. Other spinal cord neurone populations, including motoneurones, deep dorsal horn neurones and sympathetic preganglionic neurones, possess endogenous properties such as plateau potentials and membrane oscillations that are thought to play an important role in determining neurone output (Hounsgaard et al. 1988; Morisset & Nagy, 1999; Derjean et al. 2003a). The control of the expression of such membrane properties appears to be by various neuromodulators. In the deep dorsal horn, plateau potentials and membrane oscillations are highly dependent on metabotropic glutamatergic receptor activation (Morisset & Nagy, 1996; Derjean et al. 2003a). In ventral horn motoneurones, plateau properties are facilitated by serotonin and noradrenalin (Hounsgaard et al. 1988; Perrier et al. 2002), and metabotropic glutamatergic receptor activation (Svirskis & Hounsgaard, 1998). In thoracolumbar sympathetic preganglionic neurones, membrane oscillations and burst firing occur in the presence of serotonin (Pickering et al. 1994) and metabotropic glutamate receptor activation (Spanswick et al. 1995). It is believed that in each of these neurone populations, plasticity in the firing properties plays a significant role in producing functional changes in sensory, motor and autonomic pathways.
In the present study, the intrinsic electrophysiological properties of P-PGNs, and neurones within 250 μm of the P-PGNs, were examined using whole-cell patch-clamp recordings in thick spinal cord slices from 14- to 21-day-old rats. Our results show that both P-PGNs and unidentified neurones in the intermediolateral grey matter express plateau properties and, less frequently, membrane potential oscillations that appear to be dependent on the activation of group I metabotropic glutamate receptors (mGluR I). In the absence of TTX, the evoked plateaux are characterized by an accelerating firing rate during a step depolarizing current injection, and a sustained after-discharge or membrane depolarization following the termination of the current injection. The plateaux are nifedipine sensitive, and are enhanced by the CaV1.2/1.3 (L-type) channel agonist (S)-(−)-Bay K8644. In the presence of flufenamic acid, a blocker of Ca2+-activated nonspecific cationic channels, there is a reduction in the evoked firing and sustained after-discharge/depolarization, suggesting a contribution of a Ca2+-activated nonspecific cationic current (ICAN) to the firing pattern. Some of these results have been presented in a preliminary form (Derjean et al. 2003b).
Methods
All procedures were carried out in compliance with the guidelines stipulated by the Canadian Council for Animal Care, and the University of Manitoba. Sprague-Dawley rats of both sexes, 14–21 days old, were deeply anaesthetized with isoflurane 5%, and then decapitated. The spinal cord was exposed with a laminectomy, and the lumbosacral spinal cord was removed and further dissected in a cold oxygenated artificial cerebrospinal fluid (ACSF) containing (mm): 101 NaCl, 3.8 KCl, 1 CaCl2, 1.2 KH2PO4, 18 MgCl2, 10 N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid (Hepes) and 25 glucose (pH = 7.4). Transverse slices (400 μm) of the L6–S1 segments were cut using a vibratome (Pelco 101, series 1000). The slices were incubated for 1–2 h in a warm (29°C) Krebs solution (mm: 130.5 NaCl, 2.4 KCl, 2.4 CaCl2, 19.5 NaHCO3, 1.3 MgSO4, 1.2 KH2PO4, 1.25 Hepes and 10 glucose, pH = 7.4, saturated with 95% O2 and 5% CO2).
Using an Olympus microscope (BX51WI) equipped with IR-150 camera and ×40 lens, visual whole-cell patch-clamp recordings were made. The tissue was placed in a recording chamber continuously superfused with the Krebs solution (1 ml min−1, 29°C). The patch pipettes (8–10 MΩ) were filled with a solution containing (mm): 120 potassium gluconate, 20 KCl, 0.1 CaCl2, 1.3 MgCl2, 1 EGTA, 10 Hepes, 0.1 GTP, 0.2 cAMP, 0.1 leupeptin, 3 Na2ATP and 77 d-mannitol, pH = 7.3. Added to the solution was 0.05% biocytin for later visualization of the cell morphology. Recordings were made with a Multiclamp 700A amplifier, digitized (25 or 50 kHz) with a Digidata 1322A, and stored using pCLAMP 8 software (Axon Instruments) for later analysis. The junction potential in each recording was corrected in the final value of the membrane potential. All the drugs were stored as frozen stock solutions and bath-applied. Ionotropic synaptic inputs were blocked by a mixture of bicuculline (20 μm), strychnine (50 μm) (±)-2-amino-5-phosphonopentanoic acid (AP5; 50 μm), and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 20 μm). When needed, the following drugs were added: nifedipine (10 μm), (RS)-3,5-dihydroxyphenylglycine (DHPG; 25 μm), (S)-4-carboxyphenylglycine (4-CPG; 500 μm), (S)-(−)-Bay K8644 (BayK8644; 1 μm), flufenamic acid (FFA; 500 μm), tetrodotoxin (TTX; 1 μm). Nifedipine, BayK8644 and FFA were freshly dissolved in dimethylsulfoxide (DMSO) for each experiment, and nifedipine was protected from light. DMSO had no effects per se at the concentration (0.5%) used. All chemicals were from Sigma, except the CNQX, DHPG and 4-CPG (Tocris Cookson, Inc., USA). In all experiments the membrane input resistance (Rin) was monitored during each pharmacological condition. Membrane capacitance (Cm) was measured off-line with Clampfit 8 software (Axon Instruments) for each experimental condition. Results from neurones with more than a 25% variation in Cm during the recordings were excluded. The acceleration index for firing was calculated as the instantaneous firing frequency of the last two spikes, divided by the instantaneous firing frequency of the first two spikes during the current injection step.
Slices were postfixed in 4% paraformaldehyde solution for 12 h. The biocytin-filled neurones were visualized with successive incubations with streptavidin (Vectastain ABC kit, Vector Laboratories, Canada), diamino-benzidine (DAB) and hydrogen peroxide solutions. Individually labelled neurones were identified as P-PGN based on their location in the intermediolateral grey matter and visual confirmation of an axon exiting the ventral horn or rootlet. In the absence of an observable axon, neurones were classified simply as intermediolateral (IML) neurones, a category which could include P-PGNs, interneurones or tract cells. Data were analysed using two-population t tests (paired), Mann–Whitney tests or Fisher exact tests (SigmaStat, SPSS, IL, USA). Significance was accepted when P < 0.05. Results are presented as means ± standard error of the mean (s.e.m.).
Results
Passive membrane properties and action potentials
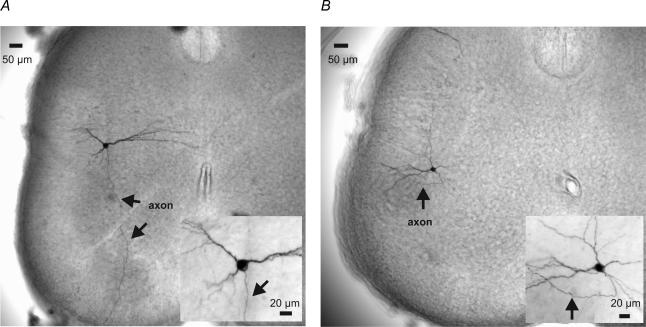
The cell shown in Fig. 1A is typical of the neurones identified as P-PGNs: the soma was within the IML region, and an identified axon (indicated by the arrows) could be traced to the ventral horn. The mean P-PGN soma diameter in the current sample was 24 ± 1.5 μm (n = 29). One neurone was labelled in a given slice, and there was no evidence of anatomical coupling of neurones as might be revealed by the transfer of biocytin between cells. Five consecutive hyperpolarizing current pulses (−50 pA, 100 ms) were averaged to determine the membrane resistance, and the exponential curve fit of the mean responses was used to determine the capacitance. The 29 identified P-PGNs recorded had a mean input resistance of 343 ± 37 MΩ, and capacitance of 86 ± 16 pF (see summary in Fig. 2). The mean amplitude of the current-evoked action potential was 66 ± 3 mV. The half-duration of the action potential, measured as the time between the rising and falling phase of the spike at the point equal to half the spike maximum amplitude, was 1.49 ± 0.2 ms. This method of determining the half-duration was selected to allow comparisons between cells in which spiking occurred under quite variable conditions (i.e. different firing frequencies and membrane potentials). Each of the measures for a given parameter was arranged from the minimum to maximum values, and plotted, resulting in a single linear distribution of values (linear regression, r > 0.85), suggesting that the sample was single population.
Figure 1. Morphology of the neurones sampled.
A, a biocytin-filled parasympathetic preganglionic neurone (P-PGN) identified by the location of its soma and the axon (arrow) projecting through the ventral horn. Note that the intracellular filling of one cell did not reveal labelling of surrounding neurones, suggesting that there was no dye coupling between cells. B, this unidentified intermediolateral (IML) neurone, near the P-PGNs, was characterized by a lateral projecting axon (indicated by the arrow). Higher magnifications of the neurone soma and proximal processes can be seen in the inset pictures.
Figure 2. Passive and active properties: P-PGN and IML neurones.
A, example of a current-clamp recording in a neurone expressing a hyperpolarization-induced sag rectification. Hyperpolarizing pulses between −80 and −65 pA evoked the sag conductance, and a postinhibitory rebound with firing. Ba, a postinhibitory rebound in the absence of a sag rectification was observed in a different neurone. Bb, the same neurone as shown in Ba showed different responses to 50, 75 and 100 pA depolarizing currents. At the holding potential of −80 mV, with 50 pA depolarizing current, there was only a passive depolarization (spontaneous postsynaptic potentials are evident); with 75 pA, a single action potential is evoked; with 100 pA, an initial burst of two action potentials was followed by continued firing throughout the current injection. Ca, a 50 pA depolarizing current pulse evoked from the resting membrane potential of −50 mV resulted in a short burst of action potentials at the start of the depolarization, with no subsequent firing after about 400 ms. Cb, when the neurone was hyperpolarized from its resting potential prior to the depolarizing current step, a progressive depolarization of the membrane occurred with a single action potential appearing 750 ms after the start of the depolarization. The table on the right provides a summary of the values and comparisons of the various passive membrane properties, and the incidence of active properties, observed for the sampled P-PGN and IML neurones. Rin, input resistance; Cm, membrane capacitance; IH, sag current. Results are expressed as means ± s.e.m.
In the example of an unidentified IML neurone shown in Fig. 1B, one can see one of the many possible dendritic patterns and axon trajectories encountered. In this case the axon projected laterally to the lateral funiculus. In contrast to the variability in neurone dendritic and axon patterns, the somas of these non-PGN neurones were similar in shape and size, with a mean long soma axis diameter of 22 ± 1.2 μm. A comparison of the 63 nonclassified IML neurones with the P-PGN population sampled (refer to Fig. 2) did not reveal a statistical difference in the mean input resistance (414 ± 37 MΩ, P = 0.394) or membrane capacitance (69 ± 4 pF, P = 0.344). The similarity of membrane capacitances was confirmed by the histological observation that the soma size of the IML neurones (22 ± 1.2 μm) sampled was not statistically different (P = 0.201) from those of the P-PGNs. The means for the action potential amplitude (65 ± 2 mV) and half-duration (1.2 ± 0.08 ms) were also similar (P = 0.913 and 0.910, respectively) between the two populations (summarized in Fig. 2). As with the P-PGNs, the measured values were arranged from minimum to maximum, and plotted, resulting in a linear distribution (linear regression, r > 0.94) without evidence of subpopulations of neurones based on any measure.
Active membrane properties
In addition to the passive membrane properties, we determined if these cells displayed a sag (IH) current sensitive to caesium (McCormick & Pape, 1990; Maccaferri et al. 1993), and also used current injection protocols that could reveal delayed firing (suggestive of slow inactivating K+ conductances; Storm, 1988; Wu & Barish, 1992) and postinhibitory rebound firing in the absence of sag rectifications (Llinas et al. 1972; Bossu & Feltz, 1986).
In contrast to the presence of a 4-aminopyridine sensitive transient K+ conductance in 100% of neonatal rat spinal parasympathetic preganglionic neurones sampled by Miura et al. (2000), only 24% (n = 7/29) of recorded P-PGNs and 16% (n = 10/63) of the unidentified IML neurones in the present study displayed delayed firing in response to depolarizing current injection (Fig. 2C). A depolarizing sag (Fig. 2A) was observed in 62% (n = 18/29) of the P-PGNs, and 65% (n = 41/63) of the IML neurones. In 3/3 cells tested, the sag conductance was blocked by caesium (data not shown), thus confirming it as an IH. In 45% (n = 13/29) of the sample, a postinhibitory rebound burst of action potentials or membrane depolarization was observed in the absence of a sag conductance (Fig. 2B). These neurones expressed bursts of action potentials followed by a spike train when stimulated (initial bursting category in Figs 2B and 3). In almost half of the cells (n = 29/63), there was no statistical difference between the sampled identified P-PGN and surrounding unidentified neurones with regard to the proportion of cells in each group that expressed the IH (Fisher exact test, P = 0.818), delayed firing (P = 0.391), or postinhibitory rebound firing in the absence of a sag rectification (P = 1.0).
Figure 3. Example of the plasticity of firing patterns in a given neurone.
A, a 50 pA depolarizing current injection from this IML neurone's resting membrane potential evoked a few action potentials. B, when the injected current was increased to 75 pA, an initial burst of firing and adapting sustained firing was evoked (acceleration index = 0.21). C, 100 pA of current injection evoked higher-frequency firing, but with the high frequency of firing in the initial burst, the acceleration index (0.2) remained the same. D, smaller current intensity (25 pA), at more depolarized holding membrane potential, did not evoke the initial burst of action potentials; the cell displayed a more tonic mode of firing (acceleration index = 0.67).
Firing patterns
We used 1 and 2 s depolarizing current steps of varying magnitudes to characterize neurone firing properties before and after the addition of agents to the bath. As illustrated in Fig. 3, in the absence of any drugs or synaptic blockers, long current injections evoked one of two types of firing when the cell was at its resting membrane potential (usually between −60 and −45 mV). Forty-one per cent of the P-PGNs and 46% of unclassified IML neurones fired spike trains throughout the current injection (see examples in Figs 4Aa and Ba, and 7Ba), with no adaptation in the instantaneous firing frequency. The remaining neurones showed varying degrees of firing adaptation (for examples see Figs 3, 7Aa and 8A). In six neurones recorded in the absence of TTX, in which there was no deterioration in recording conditions detected (i.e. similar or unchanged capacitance measurements), no significant change in the firing acceleration index was detected between control conditions (index 0.75 ± 0.06) and in the presence of the fast excitatory and inhibitory synaptic blockers (0.76 ± 0.04; P = 0.885). The proportion of cells displaying firing adaptation was similar for the P-PGNs and unclassified IML neurones (P = 0.657, Fisher exact test). Although one of two general patterns of firing could be observed at the resting membrane potential, the firing pattern in a given cell could be modified by changing the holding membrane potential and the amount of current injection used (Figs 2Bb and 3). However, in the absence of any agents added to the bath, none of the sampled neurones showed an acceleration in firing during the depolarizing current injection, or a sustained after-discharge following termination of the current injection, regardless of the membrane holding potential.
Figure 4. Plateau potentials observed in the presence of the group I metabotropic glutamate receptor (mGluR I) agonist (RS)-3,5-dihydroxyphenylglycine (DHPG).
Aa, this P-PGN neurone displayed a tonic firing pattern in response to current injection prior to the addition of the DHPG. Ab, following addition of DHPG, the neurone responded to 25 pA depolarizing current injection with a progressive depolarization, and an accelerating firing rate during the current injection, which was followed by an after-discharge. Ac, the evoked after-discharge could be terminated with a brief 60 pA hyperpolarizing current pulse. Ad, the instantaneous firing frequencies increased from ∼30 Hz in control conditions, to 60 Hz in the presence of DHPG. Ba, the tonic 16 Hz firing of this unidentified IML neurone was replaced with accelerating firing to 40 Hz during the 40 pA depolarizing current injection in the presence of DHPG (Bb). A sustained 20 Hz after-discharge was also evident. Bc, although DHPG was present, the smaller 25 pA current injection brought the cell near the threshold for the plateau, and this was manifested by a delayed firing that, once begun, accelerated with continued current injection. This was followed by a 400 ms membrane depolarization after termination of the current injection. The holding membrane potential was −40 mV throughout the recordings.
Figure 7. Contribution of L-type Ca2+ channels to firing changes and plateau potential expression.
A, the adapting firing pattern of this unidentified IML neurone in (Aa) was converted to a pattern characterized by an increased firing frequency during the current injection and an after-discharge in the presence of DHPG (Ab). In Ac, the addition of 10 μm nifedipine (n = 7) blocked the DHPG-induced after-discharge, and the spiking showed adaptation during the current injection. Ba, activation of L-type Ca2+ channels with (S)-(−)-Bay K8644 (s-Bay K8644) (n = 8) converted the tonic firing in this IML neurone (Ba) into a pattern characterized by accelerating firing during the current injection followed by an after-discharge (Bb). Bc, the abolition of the after-discharge and the presence of adaptation during the slower firing during the current injection as a consequence of the addition of nifedipine (n = 3/3). Ad and Bd, plots of the instantaneous firing frequency of the raw data presented.
Figure 8. Contribution of a Ca2+-activated nonspecific cationic current (ICAN) to plateau potential expression.
A, the slowly adapting firing during current injection recorded in this IML neurone became three times faster in the presence of DHPG, and an after-discharge/depolarization was observed (B). C, in the presence of 0.5 mm flufenamic acid (FFA), a specific ICAN antagonist, the DHPG-induced firing changes were partially inhibited (n = 4). The firing frequency of the neurone during current injection was strongly reduced (C and D), and the after-discharge (C) was eliminated leaving only a brief depolarization. Recordings were all made at −61 mV.
Effects of group I mGluR activation
In the presence of fast synaptic blockers (strychnine, bicuculline, CNQX and AP5), the effect of mGluR I activation on the firing properties of P-PGNs and neurones within the IML region was investigated. In nine neurones, in which the recording conditions and cell health remained stable throughout the duration of the recording, the addition of the fast excitatory and inhibitory synaptic blockers alone resulted in a modest increase (273.7 ± 65.53 to 293.6 ± 62.8 mΩ; P = 0.124, near the limit of significance) in the input resistance. However, none of the 14 neurones systematically examined before and after the addition of synaptic blockers displayed a significant change in evoked firing acceleration during the current injection, or an after-discharge. The effects of DHPG, as well as all the drugs used, were seen within 10 min of adding them to the perfusion solution. Given the flow rate and the distance travelled from the reservoir to the slice recording chamber, there was a 5 min delay until the agent reached the slice. Within 5 min of exposure to DHPG, effects were noted. The DHPG actions remained as long as DHPG was present in the perfusion solution; no evidence of habituation was observed.
In the presence of 25 μm DHPG, a group I mGluR specific agonist, 46% of the 93 recorded neurones examined, including P-PGNs and the unidentified IML neurones, displayed a high-frequency accelerating discharge during the constant depolarizing current injection, followed by a sustained after-discharge. Examples of the effects of DHPG can be seen in Fig. 4Ab, Ac and Bb. The instantaneous firing frequency routinely increased from a control maximum rate of ∼20 Hz to 40–60 Hz in the presence of DHPG. In this case, the acceleration index increased from 0.75 ± 0.04 to 1.85 ± 0.21 (P < 0.001). Perfusion of the broad-spectrum metabotropic receptor agonist 1S,3R-1-aminocyclopentane-1,3-dicarboxylic acid (1S,3R-ACPD) resulted in similar firing pattern changes (n = 4).
As shown in Fig. 4Ab and Bb, in the presence of DHPG, this P-PGN responded to a constant-intensity depolarizing current injection with an acceleration in firing during the current step, and an after-discharge/depolarization typically lasting 1–10 s after the current injection. Figure 4Ac shows how the sustained after-discharge could be abruptly terminated by injection of a brief hyperpolarizing pulse. In Fig. 4Bb and Bc, the need for sufficient depolarizing current injection to evoke the plateau potential is illustrated. In Fig. 4Bc, it is evident that with less current (25 versus 40 pA) the neurone is near the threshold for the sustaining plateau conductance, and begins firing only near the end of the current injection, and once recruited, the firing rate accelerates until the pulse is terminated. Presumably the activation of the current(s) required for plateau was not fully developed.
The expression of the after-discharge appeared to be dependent on the duration of the current injection. While shorter duration current steps (i.e. 1 s) could reveal an increase in firing rate during the current injection, after-discharges were not always observed. However, when the duration of current injection was increased to 2 s, both the acceleration and after-discharge were present (data not shown). This voltage- and time-dependence of the membrane response is typical of endogenous plateau potentials (Morisset & Nagy, 1996, 1999).
The plateau properties induced by mGluR I were blocked by bath application of the mGluR-I-specific antagonist 4-CPG (500 m). In Fig. 5A, the effects of DHPG and 4-CPG on a neurone include both a slowing of the firing rate (Fig. 5Ac) and the abolition of the after-discharge in the presence of the antagonist. In this particular example, no fast synaptic blockers were present in the bath and hence the background synaptic activity on the recording traces. In each of the four cells tested with 4-CPG, the accelerating firing pattern and after-discharge were eliminated. Figure 5B shows one of two cells in which the DHPG-evoked plateau potential recorded in the presence of TTX was blocked with 4-CPG. Depolarization of the membrane did not reveal a masked plateau in the presence of 4-CPG.
Figure 5. DHPG-induced plateaux are reversed by the mGluR I antagonist (S)-4-carboxyphenylglycine (4-CPG).
Aa, in the presence of DHPG, this unidentified IML neurone displayed a sustained depolarization, with three superimposed spikes, following the termination of the injection pulse. Ab, in the presence of DHPG and 4-CPG, the after-discharge and depolarization was abolished. Ac, the tonic firing during the current injection was slower and more variable compared to that with DHPG alone (also, compare Ab with Aa). The synaptic noise seen on the recordings are present because no fast synaptic blockers had been administered prior to the DHPG and 4-CPG. B, a plateau-expressing P-PGN recorded in presence of TTX showed progressive depolarization during the 25 pA current injection, followed by a sustained after-depolarization (TTX + DHPG trace). When 4-CPG (500 μm) was applied through the bath during recording from this P-PGN neurone, the 35 pA stimulation necessary to compensate the resistance shift was not sufficient to produce progressive depolarization or a sustained after-depolarization (n = 2).
About 10% (8/93) of neurones spontaneously displayed slow membrane potential oscillations and burst firing in the presence of DHPG. These oscillations varied in amplitude, shape and frequency (0.02–0.4 Hz). One such example is illustrated in Fig. 6 in which the membrane potential of this P-PGN oscillated from −80 to −10 mV, with very high-frequency firing (up to 90 Hz) during the depolarized phase of each oscillation. The oscillations were likely to be due to intrinsic properties of neurones, as shown by the voltage sensitivity of the frequency of oscillations. At more hyperpolarized holding potentials, the duration of the bursts decreased, and the frequency of the oscillations increased (Fig. 6B compared with 6A). The relatively infrequent occurrence of these oscillatory responses made a full assessment of their characteristics beyond the scope of this investigation.
Figure 6. Endogenous membrane oscillations in the presence of the mGluR I agonist DHPG.
A, spontaneous rhythmic membrane oscillations occurred in this P-PGN neurone in the presence of DHPG and the fast synaptic blockers. B, at more negative holding potentials, the membrane potential fluctuations were larger in amplitude, shorter in duration and higher in frequency.
Ionic mechanisms sustaining plateau potentials within the IML region
Plateau potentials described in the central nervous system are known to be sustained by activation of persistent Na+ channels (Li & Bennett, 2003) or L-type Ca2+-channel-dependent mechanisms (Morisset & Nagy, 1999; Svirskis & Hounsgaard, 1998). The plateau-potential-like features described in this study were insensitive to TTX blockade (n = 8; Fig. 5B). We investigated the contribution of L-type Ca2+ channels to the firing acceleration and after-discharge using L-type Ca2+ channel modulators. As illustrated in Fig. 7Aa–Ad, bath application of the dihydropyridine L-type antagonist nifedipine (10 m; Fox et al. 1987) blocked the acceleration of firing during the current injection and after-discharge induced by DHPG. Similar results were obtained in 7/7 neurones tested. In addition, as shown in Fig. 7Bb–Bd, plateau potentials could be evoked using the L-type Ca2+ channel activator BayK8644. Figure 7Bc illustrates the ability of nifedipine to block the BayK8644-evoked plateau (n = 6), similar to the block observed when the firing acceleration and after-discharge were evoked with DHPG (Fig. 7Ac). Once again, depolarizing the holding potential failed to re-establish the firing acceleration or after-discharge.
We also explored the potential contribution of ICAN conductance on firing changes using the ICAN blocker flufenamic acid (FFA, 0.5 mm; Fig. 8). ICAN is known to be one of depolarizing components of plateau potentials expressed in rat dorsal horn neurones (Morisset & Nagy, 1999). In 4/4 neurones recorded within the sacral IML region, perfusion of FFA strongly reduced the sustained after-discharge and depolarization induced in the presence of mGluR I agonists. In Fig. 8C, with the addition of FFA, the firing rate of the neurone during the current injection (at the same holding potential) was much slower when only DHPG was added to the bath (Fig. 8B), but remained faster than firing in control conditions (Fig. 8Cversus 8A). In the presence of this concentration of FFA, there remained a small membrane depolarization lasting less than 500 ms following the current injection, which was not unexpected since the L-channel contribution was not specifically blocked.
Discussion
The passive membrane properties and firing characteristics of the neurones sampled in this study, P-PGN and surrounding unidentified neurones, were similar and distributed linearly, suggesting a electrophysiologically homogeneous group of neurones. These neurones expressed various characteristics including an IH, initial bursting and delayed firing. The two conductances associated with postinhibitory rebound, the IH (>60%) and initial burst in the absence of a sag conductance (∼45%), were most frequently observed. There is no reason to believe that the apparent homogeneity of the sample reported here is due to a bias associated with a subset of particular neurones, since prelabelled neurones were not visualized prior to patching. Neurones were selected based only on their apparent health (soma integrity) and location in the region of the lateral intermediate grey matter.
In the absence of neuroactive substances in the perfusion solution, at their resting membrane potential, P-PGN and nearby unidentified neurones displayed one of two firing patterns in response to depolarizing current injection. One group of cells displayed firing adaptation during the evoked train of action potentials, while the other fired tonically without adaptation. There was a tendency (P = 0.022) for those cells that displayed firing adaptation at their resting membrane potential to display an initial burst of firing (postinhibitory rebound) following the membrane hyperpolarization, suggesting that these initial bursts and postinhibitory rebound in the absence of a sag rectification could correspond to a low-threshold Ca2+ conductance (IT; Llinas et al. 1972; Bossu & Feltz, 1986). Whatever the firing pattern of a given cell at its resting membrane potential, there was the possibility of changing that pattern by modifying the holding potential or the intensity of the injected current. This flexibility in firing pattern, observed in both P-PGNs and unidentified IML neurones, suggested that the output of these cells is quite dynamic.
The present results and conclusions regarding firing of P-PGN neurones differ from those previously reported by Miura et al. (2000). We did not find neurones that fired one or two action potentials in response to current steps in our preparation, unless there was a significant decrease in the cell's membrane capacitance (indicating cell damage or deterioration) during the recording period. In rare cases (3/93 recorded neurones within IML area) were only a few action potentials evoked during step current injection, and this was attributed to the presence of a slowly inactivating K+ conductance or strong firing frequency adaptation. However, in these three neurones, this particular firing pattern was observed only at a certain range of holding membrane potentials (−90 to −60 mV), presumably reflecting the voltage-dependent variability in the expression of the involved conductances. In the current study, a relatively low proportion (24%) of neurones expressed the slow-inactivating transient voltage-dependent K+ current, while in the younger rats, all P-PGNs sampled were sensitive to 4-AP, an agent commonly used to block the hyperpolarization-activated transient potassium current (ID) (Wu & Barish, 1992). Administration of 4-AP unmasked tonic firing in phasic neurones, and amplified firing frequency in tonic neurones in 5- to 11-day-old rats (Miura et al. 2000). A probable decrease of ID or IA (similar to ID but with different kinetics and less sensitivity to 4-aminopyridine) expression or effectiveness (Beck et al. 1992; Gao & Ziskind-Conhaim, 1998) after the second week postnatal period could explain the presence of the more tonic firing pattern in our preparation.
There may be several explanations for the differences between the present results and those previously reported by Miura et al. (2000). First, the age of the animals in the present study was between day 14 and day 21, at least 1 week older than the pups used by Miura and coworkers. de Groat and coworkers (Thor et al. 1986; Kruse & de Groat, 1990; de Groat, 2002) have described significant changes in the spinal networks controlling lower urinary tract function during the first weeks postnatal. Thus, the phasic firing mode described by Miura et al. (2000) in the very young pups could be a transitory characteristic of developing spinal cord neurones at a time when all membrane conductances are not fully functional or distributed (Gao & Ziskind-Conhaim, 1998). Second, our intracellular recording solution included classic ‘support’ elements, such as GTP and cAMP, which may be necessary for the maintenance of particular conductances during the whole-cell recordings. Others (Wilson et al. 2002; Schneider, 2003) who have used intracellular solutions similar to ours have reported cell mean input resistance values comparable to those of the present study.
Miura et al. (2000) hypothesized the existence of two populations of parasympathetic preganglionic neurones in the neonatal rat based on differences in firing patterns and responses to 4-AP. Phasic neurones were hypothesized to be bladder-related, and tonic firing neurones to be related to the colon. In the sample of P-PGN neurones reported in the present study, no such distinction was evident, and the overwhelming feature was the dynamic nature of any given P-PGN's firing response. Furthermore, we found that the membrane characteristics and firing properties of P-PGNs were not different from those of the surrounding neurones. This suggests that neurones within the IML region of lumbosacral spinal cord, although potentially quite different in terms of anatomy and connection (interneurones, and ascending and propriospinal neurones), are relatively electrically homogeneous. It would appear the control of these electrical properties may be the key to their function in normal reflex function, and may be underlying some of the pathological states that may modify pelvic reflex function.
The plasticity in firing output of the neurones sampled in the lower lumbar and rostral sacral region of the young adult rat, including parasympathetic preganglionic neurones, was enhanced in the presence of mGluR I agonists. The basic firing pattern in response to current injections, be it adapting or tonic, could be profoundly modified in the presence of mGluR I agonists. In about half the cells, an acceleration in neurone firing during constant current injection, as well as after-discharges, were observed, as previously described in the spinal cord literature (Russo & Hounsgaard, 1996; Lee & Heckman, 1998). These firing features are commonly thought to be supported by plateau potentials. This is the first description of neurotransmitter-induced firing plasticity of P-PGN and surrounding neurones in the IML grey matter of the lower lumbar and sacral spinal cord. Contrary to turtle's spinal dorsal horn neurones (Russo et al. 1998), fast synaptic blockers, such as strychnine and bicuculline, alone could not induce plateau potentials in the absence of mGluR I agonists. This suggests that the inward currents sustaining plateaux were not masked by synaptic glycinergic or GABAergic inhibition.
Our results indicate that two ionic conductances contributing to the observed plateau potentials, an L-type Ca2+ conductance and an ICAN. The implication of the L-type Ca2+ channels is not surprising since the L-type Ca2+ current has been shown to be the principal component of plateau potentials in a variety of spinal cord neurones, including motoneurones (Svirskis & Hounsgaard, 1998; Perrier et al. 2002; Li & Bennett, 2003) and deep dorsal horn neurones (Russo & Hounsgaard, 1996; Morisset & Nagy, 1999). Noteworthy, is the fact that like several other neurone types in the rat (Morisset & Nagy, 1999; Egorov et al. 2002), an ICAN appears to contribute to the firing changes observed in the cells sampled in the IML region. In turtle motoneurones, although ICAN are expressed, they are not involved in plateau potential expression (Perrier & Hounsgaard, 1999), and the principal charge carriers contributing to the prolonged firing are likely to be other conductances or ionic movements that are activated subsequent to L-type Ca2+ channel opening. Intracellular ryanodine-sensitive Ca2+ stores have been recently implicated (Mejia-Gervacio et al. 2004). At this point, the participation of internal Ca2+ stores and other ionic mechanisms, such as K+ channel inhibition (Spanswick et al. 1995; Derjean et al. 2003a), cannot be excluded for neurones expressing plateaux in the lower lumbar and sacral intermediate rat spinal cord. Nor can we dismiss the possibility of species-related differences in this phenomenon.
The acceleration in the firing rate during the current step, which may or may not be followed with an after-discharge, is one way that the message from the firing neurone may be amplified (Derjean et al. 2003a). Given the same input, the output may be enhanced in the presence of such facilitation. The release of both classical neurotransmitters and neuropeptides by the same neurone, depending upon the firing frequencies of the neurone, has been reported (for review see Bartfai et al. 1988), and Sasek et al. (1984) have described the presence of a variety of peptides in fibres and cells bodies within the rat sacral parasympathetic nuclei. We hypothesize that one potential role for the plateaux may be to alter the compliment of transmitters released within the spinal parasympathetic nuclei and/or by the parasympathetic preganglionic neurones depending upon the state of the circuitry and reflexes demands.
The sustained after-discharge is another manifestation of the amplification of output in the sacral system. For those cells expressing an after-discharge, the need for constant pelvic sensory input to depolarize the spinal neurones and maintain their firing is alleviated if brief synaptic excitation could be translated into sustained firing. Interestingly, in very young neonatal animals, the absence of plateau potential expression in bladder-related P-PGNs may be hypothesized as one factor contributing to the need for continuous afferent (perineal) stimulation to drive to bladder P-PGNs and achieve complete bladder emptying (Thor et al. 1986; Kruse & de Groat, 1990). Perhaps with maturation of the conductances underlying the plateaux, briefer bursts of bladder afferent or descending input is sufficient to ensure plateau expression and adequate P-PGN output. In the present study, the duration of the after-discharges observed in the presence of fast synaptic blockers was usually less than 10 s. After-discharges have also been described in neonatal rat P-PGNs in the presence of prostaglandin E2 (Miura et al. 2002). The potential for other modulators to regulate conductances that enhance the duration of the after-discharges remains to be investigated (see Derjean et al. 2003b). At this point, however, we have no reason to believe that a categorization into partial and fully expressed plateau potentials (see discussion by Lee & Heckman, 1998) is necessary in caudal spinal cord autonomic-related neurones. To our knowledge, there has been no testing or documentation of the expression of plateau-like firing in the parasympathetic preganglionic neurones during normal or pathological micturition, defaecation or erection reflexes. Unfortunately, however, most in vivo studies of parasympathetic preganglionic firing patterns in the cat model were undertaken using anaesthetics, or recording and stimulation conditions that are not conducive for revealing the presence of plateau potentials (de Groat & Ryall, 1968a). Furthermore, the proposed recurrent inhibitory pathways described by de Groat & Ryall (1968a) may function to limit parasympathetic output, at least in the cat (de Groat & Ryall, 1968b), and limit the expression of plateaux.
Less frequently observed in the presence of mGluR I agonists were membrane oscillations and rhythmic bursting. The capacity for mammal sympathetic neurones to oscillate has been described (Spanswick & Logan, 1990), and these oscillations are facilitated by metabotropic glutamate receptor activation (Spanswick et al. 1995) and serotonin (Pickering et al. 1994). Endogenous oscillations described in the current study displayed a relatively slow rhythm, which may be explained by the use of fast synaptic blockers that could remove synaptic drives contributing to the oscillations. Unlike the sympathetic preganglionic neurones reported by Spanswick and coworkers, spontaneously bursting neurones in the absence of mGluR I agonists were not observed in P-PGNs or surrounding neurones in our preparation and, thus, the role of such oscillations in these neurones under physiological conditions remains to be determined.
The present observations introduce a novel dimension to our understanding parasympathetic preganglionic neurone control and function. These neurones, as well as the presumed interneurones and tract neurones in their vicinity, may not respond in a singular manner to excitatory inputs but, rather, are subject to modulation, adaptation, and short-term plasticity due to postsynaptic mGluR I actions. This plasticity could explain the adaptability of spinal autonomic components, and may contribute to changes in lower urinary tract, bowel and sexual function following disruption in normal sensory and descending inputs to these neurones.
Acknowledgments
The authors thank Shannon Deschamps and Leila Monteils for their excellent technical assistance. D.D. was funded by the NIH (R01-NS40846), and the project costs were funded through a grant from the Canadian Institutes for Health Research (MGP-37757) to S.J.S.
References
- Araki I. Inhibitory postsynaptic currents and the effects of GABA on visually identified sacral parasympathetic preganglionic neurons in neonatal rats. J Neurophysiol. 1994;72(6):2903–2910. doi: 10.1152/jn.1994.72.6.2903. [DOI] [PubMed] [Google Scholar]
- Araki I, de Groat WC. Unitary excitatory synaptic currents in preganglionic neurons mediated by two distinct groups of interneurons in neonatal rat sacral parasympathetic nucleus. J Neurophysiol. 1996;76:215–226. doi: 10.1152/jn.1996.76.1.215. [DOI] [PubMed] [Google Scholar]
- Bartfai T, Iverfeldt K, Fisone G, Serfozo P. Regulation of the release of coexisting neurotransmitters. Annu Rev Pharmacol Toxicol. 1988;28:285–310. doi: 10.1146/annurev.pa.28.040188.001441. [DOI] [PubMed] [Google Scholar]
- Beck H, Ficker E, Heinemann U. Properties of two voltage-activated potassium currents in acutely isolated juvenile rat dentate gyrus granule cells. J Neurophysiol. 1992;68:2086–2099. doi: 10.1152/jn.1992.68.6.2086. [DOI] [PubMed] [Google Scholar]
- Bossu JL, Feltz A. Inactivation of the low-threshold transient calcium current in rat sensory neurones: evidence for a dual process. J Physiol. 1986;376:341–357. doi: 10.1113/jphysiol.1986.sp016157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC. Plasticity of bladder reflex pathways during postnatal development. Physiol Behav. 2002;77:689–692. doi: 10.1016/s0031-9384(02)00919-8. 10.1016/S0031-9384(02)00919-8. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Ryall RW. The identification and characterization of sacral parasympathetic preganglionic neurones. J Physiol. 1968a;196:563–577. doi: 10.1113/jphysiol.1968.sp008523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Ryall RW. Recurrent inhibition in sacral parasympathetic pathways to the bladder. J Physiol. 1968b;196:579–591. doi: 10.1113/jphysiol.1968.sp008524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Vizzard MA, Araki I, Roppolo J. Spinal interneurons and preganglionic neurons in sacral autonomic reflex pathways. Prog Brain Res. 1996;107:97–111. doi: 10.1016/s0079-6123(08)61860-9. [DOI] [PubMed] [Google Scholar]
- Derjean D, Bertrand S, Le Masson G, Landry M, Morisset V, Nagy F. Dynamic balance of metabotropic inputs causes dorsal horn neurons to switch functional states. Nat Neurosci. 2003a;6:274–281. doi: 10.1038/nn1016. 10.1038/nn1016. [DOI] [PubMed] [Google Scholar]
- Derjean D, Bertrand S, Nagy F, Shefchyk SJ. Rat sacral parasympathetic preganglionic neurons express plateau potentials sustained by L-type calcium channels. Abstr Soc Neurosci. 2003b:377.3. [Google Scholar]
- Egorov AV, Hamam BN, Fransen E, Hasselmo ME, Alonso AA. Graded persistent activity in entorhinal cortex neurons. Nature. 2002;420:173–178. doi: 10.1038/nature01171. 10.1038/nature01171. [DOI] [PubMed] [Google Scholar]
- Fox AP, Nowycky MC, Tsien RW. Kinetic and pharmacological properties distinguishing three types of calcium currents in chick sensory neurones. J Physiol. 1987;394:149–172. doi: 10.1113/jphysiol.1987.sp016864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao BX, Ziskind-Conhaim L. Development of ionic currents underlying changes in action potential waveforms in rat spinal motoneurons. J Neurophysiol. 1998;80:3047–3061. doi: 10.1152/jn.1998.80.6.3047. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol. 1988;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse MN, de Groat WC. Micturition reflexes in decerebrate and spinalized neonatal rats. Am J Physiol Regul Integr Comp Physiol. 1990;258:R1508–1511. doi: 10.1152/ajpregu.1990.258.6.R1508. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. J Neurophysiol. 1998;80:583–593. doi: 10.1152/jn.1998.80.2.583. [DOI] [PubMed] [Google Scholar]
- Li Y, Bennett DJ. Persistent sodium and calcium currents cause plateau potentials in motoneurons of chronic spinal rats. J Neurophysiol. 2003;90:857–869. doi: 10.1152/jn.00236.2003. [DOI] [PubMed] [Google Scholar]
- Llinas R, Blinks JR, Nicholson C. Calcium transient in presynaptic terminal of squid giant synapse: detection with aequorin. Science. 1972;176:1127–1129. doi: 10.1126/science.176.4039.1127. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, Mangoni M, Lazzari A, DiFrancesco D. Properties of the hyperpolarization-activated current in rat hippocampal CA1 pyramidal cells. J Neurophysiol. 1993;69:2129–2136. doi: 10.1152/jn.1993.69.6.2129. [DOI] [PubMed] [Google Scholar]
- Marson P. Identification of central nervous system neurons that innervate the bladder body, bladder base, or external urethral sphincter of female rats: a transneuronal tacing study using pseudorabies virus. J Comp Neurol. 1997;389:584–602. 10.1002/(SICI)1096-9861(19971229)389:4<584::AID-CNE4>3.3.CO;2-A. [PubMed] [Google Scholar]
- McCormick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejia-Gervacio S, Hounsgaard J, Diaz-Munoz M. Roles of ryanodine and inositol triphosphate receptors in regulation of plateau potentials in turtle spinal motoneurons. Neuroscience. 2004;123:123–130. doi: 10.1016/j.neuroscience.2003.08.049. 10.1016/j.neuroscience.2003.08.049. [DOI] [PubMed] [Google Scholar]
- Miura A, Kawatani M, Araki I, de Groat WC. Electrophysiological properties of lumbosacral preganglionic neurons in the neonatal rat spinal cord. Brain Res. 2000;872:54–63. doi: 10.1016/s0006-8993(00)02448-3. 10.1016/S0006-8993(00)02448-3. [DOI] [PubMed] [Google Scholar]
- Miura A, Kawatani M, de Groat WC. Excitatory synaptic currents in lumbosacral parasympathetic preganglionic neurons elicited from the lateral funiculus. J Neurophysiol. 2001;86:1587–1593. doi: 10.1152/jn.2001.86.4.1587. [DOI] [PubMed] [Google Scholar]
- Miura A, Kawatani M, Maruyama T, de Groat WC. Effect of prostaglandins on parasympathetic neurons in the rat lumbosacral spinal cord. Neuroreport. 2002;113:557–562. doi: 10.1097/00001756-200208270-00014. [DOI] [PubMed] [Google Scholar]
- Morisset V, Nagy F. Modulation of regenerative membrane properties by stimulation of metabotropic glutamate receptors in rat deep dorsal horn neurons. J Neurophysiol. 1996;76:2794–2798. doi: 10.1152/jn.1996.76.4.2794. [DOI] [PubMed] [Google Scholar]
- Morisset V, Nagy F. Ionic basis for plateau potentials in deep dorsal horn neurons of the rat spinal cord. J Neurosci. 1999;19:7309–7316. doi: 10.1523/JNEUROSCI.19-17-07309.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadelhaft I, Vera PL, Card JP, Miselis RR. Central nervous system neurons labeled following the injection of pseudorabies virus into the rat urinary bladder. Neurosci Lett. 1992;143:271–274. doi: 10.1016/0304-3940(92)90281-b. 10.1016/0304-3940(92)90281-B. [DOI] [PubMed] [Google Scholar]
- Perrier JF, Alaburda A, Hounsgaard J. Spinal plasticity mediated by postsynaptic L-type Ca2+ channels. Brain Res Brain Res Rev. 2002;40:223–229. doi: 10.1016/s0165-0173(02)00204-7. 10.1016/S0165-0173(02)00204-7. [DOI] [PubMed] [Google Scholar]
- Perrier JF, Hounsgaard J. Ca2+-activated nonselective cationic current (I (CAN) in turtle motoneurons. J Neurophysiol. 1999;82:730–735. doi: 10.1152/jn.1999.82.2.730. [DOI] [PubMed] [Google Scholar]
- Pickering AE, Spanswick D, Logan SD. 5-Hydoxytryptamine evokes depolarizations and membrane potential oscillations in rat sympathetic preganglionic neurones. J Physiol. 1994;480:109–121. doi: 10.1113/jphysiol.1994.sp020345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo RE, Hounsgaard J. Plateau-generating neurones in the dorsal horn in an in vitro preparation of the turtle spinal cord. J Physiol. 1996;493:39–54. doi: 10.1113/jphysiol.1996.sp021363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo RE, Nagy F, Hounsgaard J. Inhibitory control of plateau properties in dorsal horn neurones in the turtle spinal cord in vitro. J Physiol. 1998;506:795–808. doi: 10.1111/j.1469-7793.1998.795bv.x. 10.1111/j.1469-7793.1998.795bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasek CA, Seybold VS, Elde RP. The immunohistochemical localization of nine peptides in the sacral parasympathetic nucleus and the dorsal gray commissure in rat spinal cord. Neuroscience. 1984;12:855–873. doi: 10.1016/0306-4522(84)90175-1. 10.1016/0306-4522(84)90175-1. [DOI] [PubMed] [Google Scholar]
- Schneider SP. Spike frequency adaptation and signaling properties of identified neurons in rodent deep spinal dorsal horn. J Neurophysiol. 2003;90:245–258. doi: 10.1152/jn.01012.2002. [DOI] [PubMed] [Google Scholar]
- Spanswick D, Logan SD. Sympathetic preganglionic neurones in neonatal rat spinal cord in vitro: electrophysiological characteristics and the effects of selective excitatory amino acid receptor agonists. Brain Res. 1990;525:181–188. doi: 10.1016/0006-8993(90)90862-6. 10.1016/0006-8993(90)90862-6. [DOI] [PubMed] [Google Scholar]
- Spanswick D, Pickering AE, Gibson IC, Logan SD. Excitation of sympathetic preganglionic neurons via metabotropic excitatory amino acid receptors. Neuroscience. 1995;68:1247–1261. doi: 10.1016/0306-4522(95)00216-6. 10.1016/0306-4522(95)00216-6. [DOI] [PubMed] [Google Scholar]
- Storm JF. Temporal integration by a slowly inactivating K+ current in hippocampal neurons. Nature. 1988;336:379–381. doi: 10.1038/336379a0. 10.1038/336379a0. [DOI] [PubMed] [Google Scholar]
- Svirskis G, Hounsgaard J. Transmitter regulation of plateau properties in turtle motoneurons. J Neurophysiol. 1998;79:45–50. doi: 10.1152/jn.1998.79.1.45. [DOI] [PubMed] [Google Scholar]
- Thor KM, Kawatani M, de Groat WC. Plasticity in the reflex pathways to the lower urinary tract of the cat during postnatal development and following spinal cord injury. In: Goldberger MR, Gorio A, Murray M, editors. Development and Plasticity of the Mammalian Spinal Cord. III. Padua, Italy: Liviana Press; 1986. [Google Scholar]
- Wilson JM, Codene E, Renaud LP, Spanswick D. Active and passive membrane properties of rat sympathetic preganglionic neurones innervating the adrenal medulla. J Physiol. 2002;545:945–960. doi: 10.1113/jphysiol.2002.023390. 10.1113/jphysiol.2002.023390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu RL, Barish ME. Two pharmacologically and kinetically distinct transient potassium currents in cultured embryonic mouse hippocampal neurons. J Neurosci. 1992;12:2235–2246. doi: 10.1523/JNEUROSCI.12-06-02235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]