Abstract
Skeletal muscle has been recognized as an endocrine organ, and muscle cell cultures express several cytokines with potential hormonal effects. Interleukin-8 (IL-8), a chemokine, which induces angiogenesis, is expressed in working muscles; however, the cell source of origin has not been identified. We aimed to elucidate if IL-8 protein is: (1) expressed in contracting muscle fibres and (2) whether there is a release of IL-8 from exercising muscle. Seventeen healthy male volunteers were included in two independent protocols: 3 h of ergometer bicycle exercise at 60% of ![]() (n = 6) or rest (n = 5), and 3 h of two-legged knee-extensor exercise at 60% of maximal workload (n = 6). Repetitive muscle biopsy samples were obtained from the vastus lateralis in all experiments. A marked increase in IL-8 mRNA was found in muscle biopsy samples obtained after exercise. A marked IL-8 protein expression was demonstrated within the cytoplasm of muscle fibres in biopsy samples obtained in the recovery phase following 3 h of bicycle exercise, and the peak occurred 3–6 h postexercise. A small transient net release of IL-8 from working muscle was found at 1.5 h of knee-extensor exercise. However, the small release of IL-8 from muscle did not result in an increase in the systemic plasma concentration of IL-8, suggesting that muscle-derived IL-8 may play a local role, e.g. in angiogenesis.
(n = 6) or rest (n = 5), and 3 h of two-legged knee-extensor exercise at 60% of maximal workload (n = 6). Repetitive muscle biopsy samples were obtained from the vastus lateralis in all experiments. A marked increase in IL-8 mRNA was found in muscle biopsy samples obtained after exercise. A marked IL-8 protein expression was demonstrated within the cytoplasm of muscle fibres in biopsy samples obtained in the recovery phase following 3 h of bicycle exercise, and the peak occurred 3–6 h postexercise. A small transient net release of IL-8 from working muscle was found at 1.5 h of knee-extensor exercise. However, the small release of IL-8 from muscle did not result in an increase in the systemic plasma concentration of IL-8, suggesting that muscle-derived IL-8 may play a local role, e.g. in angiogenesis.
The immunological and endocrine function of skeletal muscle is an area of increasing interest. Skeletal muscle cell cultures express several cytokines, such as interleukin (IL)-6, IL-8, interferon (IFN)-γ and tumour necrosis factor (TNF)-α (Saghizadeh et al. 1996; De Rossi et al. 2000; Alvarez et al. 2002). IL-8 is produced by monocytes and macrophages, as well as by most tissues (Baggiolini et al. 1994). IL-8 is induced by a number of stimuli, including pro-inflammatory cytokines (Hoffmann et al. 2002), and acts as a chemokine on neutrophils. IL-8 belongs to the CXC family of chemokines. The CXC nomenclature relates to the presence of two conserved cysteine residues, separated by one amino acid, at the amino terminus. IL-8 belongs to a subdivision of the CXC chemokines, and this subgroup has an amino acid sequence Glu-Leu-Arg (ELR) preceding the first conserved cysteine amino acid residue in the primary structure of these proteins (Baggiolini, 2001). CXC chemokines with the ELR motif are potent angiogenic factors in vivo (Koch et al. 1992; Norrby, 1996; Bek et al. 2002). IL-8 acts as an angiogenic factor in human microvascular endothelial cells (Heidemann et al. 2003). Keane et al. (1997) have found an association between IL-8 and angiogenesis in idiopathic pulmonary fibrosis patients. In addition, IL-8 is associated with insulin resistance and obesity (Bruun et al. 2002, 2003).
The plasma concentration of IL-8 increases in response to exhaustive exercise comprised of both eccentric and concentric components, such as running (Ostrowski et al. 2001; Nieman et al. 2001, 2002, 2003; Suzuki et al. 2003). It is unclear whether concentric exercise alone, which is not associated with an inflammatory response, results in an increased IL-8 plasma concentration (Mucci et al. 2000; Henson et al. 2000; Chan et al. 2004). Incremental bicycle ergometer exercise to exhaustion resulted in a small increase in plasma IL-8 concentration (Mucci et al. 2000), whereas plasma IL-8 concentration remained unaltered during rowing or bicycle exercise for 1 h or 2 h, respectively (Henson et al. 2000; Chan et al. 2004). Therefore, this indicates that concentric exercise alone is not related to a marked increase in IL-8 plasma concentration.
The contracting skeletal muscle responds to a 3 h treadmill run by increasing IL-8 mRNA several fold, concomitant with increased plasma levels of IL-8 (Nieman et al. 2003). Similarly, skeletal muscle IL-8 mRNA increased in response to 1 h of bicycle exercise without affecting IL-8 plasma concentration (Chan et al. 2004). A muscle biopsy contains several cell types in addition to myocytes. Indeed, IL-8 can be expressed by human endothelial cells (vascular tissue) in vitro (Zhao et al. 2003), and could thus serve as the cellular source of IL-8 in muscle biopsy samples. Whether myocytes are the cellular source of IL-8 in muscle biopsy samples has not been investigated. Furthermore, it is not known if the IL-8 protein is expressed in or released from skeletal muscle.
We hypothesized that: (1) concentric exercise would induce expression of IL-8 mRNA and protein in skeletal muscle cells and (2) concentrically contracting skeletal muscle would release IL-8.
Methods
Study 1. The effect of bicycle exercise on muscle IL-8 mRNA and protein expression
Bicycle ergometer exercise was chosen as the mode of exercise in this study since this type of exercise is mainly concentric and induces minimal muscle damage and subsequent inflammation. Furthermore, it has been used in several of the aforementioned studies investigating IL-8 plasma and gene-expression changes as a response to exercise, and would thus allow for a comparison of results. Eleven healthy men with a median age of 26 years (range 21–28 years), a median height of 186 cm (range 164–197 cm), and a median weight of 77.5 kg (range 65–96 kg), participated in the study. One week before the first experimental day, subjects performed a maximal oxygen uptake test ![]() on an ergometer bicycle. Measurement of
on an ergometer bicycle. Measurement of ![]() was performed on an electrically braked cadence independent cycle ergometer (Monark 839E, Monark Ltd, Varberg, Sweden). Subjects cycled for 3 min at 100 W as a warm-up, followed by cycling at progressively higher work rates (increasing 50 W every 3 min for 9 min), and then increasing the workload by 25 W every minute until subjects were unable to maintain a cadence of 60 r.p.m. Expired oxygen and carbon dioxide concentrations were recorded online. Median
was performed on an electrically braked cadence independent cycle ergometer (Monark 839E, Monark Ltd, Varberg, Sweden). Subjects cycled for 3 min at 100 W as a warm-up, followed by cycling at progressively higher work rates (increasing 50 W every 3 min for 9 min), and then increasing the workload by 25 W every minute until subjects were unable to maintain a cadence of 60 r.p.m. Expired oxygen and carbon dioxide concentrations were recorded online. Median ![]() was 50.4 ml kg −1 min−1 (range 45.9–58.4). On the experimental day, subjects arrived at 08.00 h, after an overnight fast, and were randomised to either exercise or rest. A venous catheter was placed in an antecubital vein. There was no difference between the two groups with regard to age, weight, height or
was 50.4 ml kg −1 min−1 (range 45.9–58.4). On the experimental day, subjects arrived at 08.00 h, after an overnight fast, and were randomised to either exercise or rest. A venous catheter was placed in an antecubital vein. There was no difference between the two groups with regard to age, weight, height or ![]() . Subjects performed 3 h of cycling at 60%
. Subjects performed 3 h of cycling at 60% ![]() , followed by 6 h of recovery. The following day they reported to the laboratory after an overnight fast. Muscle biopsy samples were obtained from the vastus lateralis prior to the exercise (0), immediately after exercise (3 h), and at 4.5, 6, 9 and 24 h, using the percutaneous needle biopsy technique with suction. Control subjects rested in the laboratory for 9 h, reported to the laboratory the day after, and had biopsy samples taken at the same time points.
, followed by 6 h of recovery. The following day they reported to the laboratory after an overnight fast. Muscle biopsy samples were obtained from the vastus lateralis prior to the exercise (0), immediately after exercise (3 h), and at 4.5, 6, 9 and 24 h, using the percutaneous needle biopsy technique with suction. Control subjects rested in the laboratory for 9 h, reported to the laboratory the day after, and had biopsy samples taken at the same time points.
Study 2. The effect of knee-extensor exercise on IL-8 release from working muscles
Two-legged knee extensor exercise was chosen as the mode of exercise in this study. This mode of exercise is mainly concentric, and isolates the quadriceps muscle, and allows for catheterization of the femoral artery and vein. Therefore, it is an advantageous method for studying arterial–venous difference across the quadriceps muscle.
Six healthy physically active male subjects ranging in age from 22 to 33 years (median 26 years), with a median height of 187 cm (range 175–193 cm) and a median weight of 78.1 kg (range 70–93 kg), participated in this study. The subjects completed a trial of two-legged knee-extensor exercise on a modified Krogh cycle ergometer (Andersen & Saltin, 1985).
One week before the trial, a two-legged knee-extensor exercise test was performed to determine the maximal workload. Resistance was increased every 2 min until a kicking frequency of 60 extensions per minute could no longer be maintained. The highest workload the subject could maintain for 2 min was set as the maximum workload. After an overnight fast, the subjects reported to the laboratory at 07.30 h on the day of the trial, voided, changed into appropriate exercise attire and rested in a supine position. After resting for 10 min, a catheter was inserted in the femoral artery and the femoral vein under local anaesthesia (20 mg ml−1), as previously described (Andersen & Saltin, 1985). At rest, blood was sampled from the femoral artery and the femoral vein simultaneously, and femoral arterial blood flow was measured using an ultrasound Doppler. In brief, an annular phased array transducer probe (11.5 mm diameter) was placed just below the inguinal ligament on the common femoral artery. The probe, operating at an imaging frequency of 7.5 MHz and at variable Doppler frequencies of 4.0–6.0 MHz, was used to measure the diameter of the femoral artery at rest, and the blood flow at rest and during exercise, as described and validated (Radegran, 1997). Immediately following, a muscle biopsy sample was taken from the vastus lateralis. The subjects then performed two-legged knee-extensor exercise for 3 h at ∼60% of their maximum workload. Blood flow was measured using the ultrasound Doppler method, and blood was sampled from the femoral vein and femoral artery, simultaneously, after 30 min, 1.5 and 3 h of exercise, and at 2 h of recovery. Muscle biopsy samples were obtained from the vastus lateralis at 30 min, 1.5 and 3 h of exercise, and at 2 h of recovery (5 h).
Ethics
The subjects were given both oral and written information about the experimental procedures before giving their written informed consent. All studies were approved by the Copenhagen and Frederiksberg Ethics Committee, Denmark, and performed according to the Declaration of Helsinki.
Blood analysis
In both studies, blood samples were collected at the same time points as muscle biopsy samples. Plasma was immediately separated from blood cells by centrifugation, and stored at −80°C until analysed. Quantification of plasma IL-8 was performed using an Enzyme-linked immunosorbent assay (ELISA) for IL-8 (Sanquin, Amsterdam, The Netherlands). The range and sensitivity of the assay are 1–240 pg ml−1 and 1 pg ml−1, respectively.
Calculations of IL-8 release
The femoral venous–arterial (fv–a) IL-8 difference was calculated by subtracting the femoral arterial concentration of IL-8 from the femoral venous concentration. The fv–a was then multiplied with the blood flow of the leg (Fick principle) to get the IL-8 balance (net release/uptake) across the leg.
Muscle biopsy samples
Biopsy samples were immediately placed on an ice-cold glass plate, cleaned of connective tissue and blood, and frozen in liquid nitrogen (20–40 mg) for further analysis.
RNA isolation
Total RNA was isolated from ∼20–25 mg of tissue by a modified guanidinium thiocyanate (GT)–phenol–chloroform extraction method, which was adapted from Chomczynski & Sacchi (1987), as previously described (Pilegaard et al. 2000).
Reverse transcription and PCR
Reverse transcription (RT) of total RNA samples was performed using the Superscript II RNase H-system (Gibco-BRL), as previously described (Pilegaard et al. 2000; Hildebrandt & Neufer, 2000). RT products of total RNA samples were diluted to a total of 110–220 μl, depending on the relative differences in total RNA yield among samples. Skeletal muscle IL-8 and GAPDH mRNA content were determined by PCR using predeveloped assay reagents (Applied Biosystems). GAPDH was used as an endogenous control to normalize the mRNA content of the target genes in each sample. The mRNA content of the selected genes was determined by fluorescence-based real-time PCR technology (7900 ABI PRISM Sequence Detection System, Applied Biosystems). Seven microlitres of the diluted RT product (template) were mixed with 2× TaqMan Universal Master Mix, forward primer, reverse primer, probe and nuclease-free water, to a final volume of 36 μl. The Master Mix (Applied Biosystems) contained AmpliTaq Gold DNA polymerase, AmpErase UNG, dNTPs with dUTP, ROX as passive reference, and optimized buffer components. The final mix was added to the PCR plate and each sample in a volume of 10 (l, and was analysed in triplicate. The PCR cycle profile used was: 50°C for 2 min +95°C for 10 min + ((95°C for 15 s + 60°C for 1 min) × 50 cycles). The triplicates had a threshold cycle coefficient of variation of no more than 2%. Data were analysed by using a comparative critical threshold (Ct) method, where the amount of target normalized to the amount of endogenous control and relative to the (pre) control sample is given by 2−ΔΔCt (Applied Biosystems). For each subject, all samples were run together, allowing relative comparison of the samples from a given subject. To determine the specificity of the IL-8 predeveloped assay reagent, the PCR-product was separated by gel (2.5% agarose) electrophoresis, stained with ethidium bromide, and visualized by UV exposure using a CCD integrating camera (Gel Doc, Bio-Rad, Hercules, CA, USA). The agarose gel electrophoresis of the PCR product resulted in a single clearly defined band between 100 and 125 base pairs.
Immunohistochemistry
Muscle tissue was cut into 6 μm consecutive sections on a cryostat, and the sections were immediately collected on glass slides to be used for general histology and immunohistochemistry.
For epitope retrieval, sections were preincubated overnight in tris-EGTA (TEG) buffer (1.211 g Tris, 0.95 g EGTA, 1 l distilled H2O) at 60°C, and afterwards in 0.5% H2O2 in Tris-buffered saline (TBS)/Nonidet (TBS: 0.05 m Tris, pH 7.4, 0.15 m NaCl; with 0.01% Nonidet P-40) (Sigma-Aldrich) for 15 min at room temperature to quench endogenous peroxidase. Afterwards, sections were incubated in 10% goat serum (In Vitro, Denmark) in TBS/Nonidet for 30 min at room temperature in order to block nonspecific binding.
Sections were stained for myofibrillar ATPase following preincubation at pH 4.6 in order to identify muscle fibre types. Myofibrillar ATPase staining was performed according to standard procedures (Brooke & Kaiser, 1970). Other sections were incubated overnight at 4°C with the following primary antibody: mouse antihuman IL-8 diluted 1:50 (Biosource, Germany).
These primary antibodies were detected using biotinylated goat antimouse IgG diluted 1:200 (Sigma-Aldrich), followed by the streptavidin–biotin–peroxidase complex (StreptABComplex/HRP, DakoCytomation, Denmark), prepared at manufacturer's recommended dilutions, for 30 min at room temperature. Afterwards, sections were incubated with biotinylated tyramide and streptavidin–peroxidase complex (NEN, Life Science Products, USA), which were prepared following manufacturer's recommendations. The immunoreaction was visualized using 0.015% H2O2 in 3,3-diaminobenzidine-tetrahydrochloride (DAB)/TBS for 10 min at room temperature. The sections stained using immunohistochemistry were always processed simultaneously, and under the same laboratory conditions.
In order to evaluate the extent of nonspecific binding in the immunohistochemical experiments, control sections were incubated in the absence of primary or secondary antibody, or in the blocking serum. Results were considered only if these controls were negative.
In order to exclude false-positive staining due to endogenous biotin, we pretreated sections sequentially with 0.01–0.1% avidin (Sigma-Aldrich) followed by 0.001–0.01% biotin (Sigma-Aldrich), each step for 20 min, before the immunohistochemistry was performed. Comparing immunohistochemistry ±biotin blocking showed that in the tissue used muscular endogenous biotin is unlikely to induce false-positive immunostainings by binding to the streptavidin used. In order to control the specificity of the primary IL–8 antibody, we preabsorbed the primary antibody with its corresponding antigenic protein. For this purpose, we used human IL-8 protein (Biosource, Germany).
Results were considered only if this preabsorption of primary antibodies resulted in negative immunostaining. For the simultaneous examination and recording of the staining, a Zeiss Axioplan2 light microscope was used.
Statistics
Content of mRNA was normalized to GAPDH mRNA levels, and results were expressed relative to the corresponding resting (pre) sample result, which was set to 1.0. All data was tested for normality. The distribution of mRNA fold-change data was normally distributed after log transformation. All other data were normally distributed. The mRNA fold-change values are presented as geometric mean ± geometric s.e.m. All other values are presented as means ± s.e.m. One-way analysis of variance (ANOVA) for repeated measures (RM) was used to analyse the main effect of time on all variables. A two-way RM ANOVA was used to evaluate the effect of exercise and time in study 1. Student–Newman–Keuls post hoc test was used to locate specific differences across time. Differences were considered significant at P < 0.05. Data were analysed using Sigma Stat 3.0 (SPSS, Inc., Chicago, USA).
Results
Study 1. The effect of bicycle exercise on muscle IL-8 mRNA and protein expression
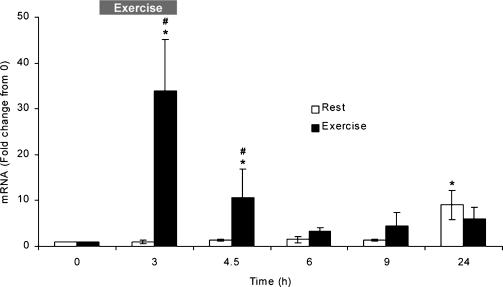
Skeletal muscle IL-8 mRNA increased in response to exercise when compared to pre-exercise samples, and when compared to resting values (Fig. 1). A significant increase in IL-8 mRNA was seen at two time points in the exercise group, 3 h and 4.5 h, which were also significantly different from the rest group (P < 0.05). A significant increase in IL-8 mRNA compared to the pre sample was seen at 24 h in the rest group (Fig. 1). This change was not significantly different from the exercise group (Fig. 1).
Figure 1. Muscle IL-8 mRNA in rest and exercise groups.
The exercise group: pre (0 h), immediately after 3 h of bicycle exercise (3 h), and 1.5 (4.5 h), 3 (6 h), 6 (9 h) and 21 h (24 h) postexercise. The rest group: pre (0 h), after 3, 4.5, 6 and 9 h of rest, and 24 h after the presample. Values are means ± s.e.m. (n = 6 subjects). *Significant (P < 0.05) difference from pre sample (0 h), and #significant (P < 0.05) difference from rest group.
The IL-8 protein was not expressed in muscle tissue before exercise (n = 12, Fig. 2A), and repetitive muscle biopsy samples at rest did not show induction of IL-8 expression (n = 6, data not shown). By the end of the exercise period (3 h), the immunohistochemistry for IL-8 was still not increased in the muscle tissue (Fig. 2B). At time points 4.5 (Fig. 2C), 6 (Fig. 2E), 9 (Fig. 2F, H and I) and 24 h (Fig. 2G), there was a strong immunoreaction for IL-8 within muscle fibres, the peak being at 6–9 h (Fig. 2E, F, H and I). At time point 4.5 h, a parallel section was stained for myofibrillar ATPase to identify muscle fibre types (Fig. 2D). As shown, IL-8 expression was increased similarly in type 1 and 2 muscle fibres at time point 4.5 h (Fig. 2C and D). Moreover, this IL-8 fibre-type expression pattern was observed at all the time points studied (not shown).
Figure 2. IL-8 immunohistochemical expression in skeletal muscle tissue before and after 3 h of bicycle exercise.
A, before the exercise began, IL-8 expression was almost absent in the muscle tissue. B, By 3 h, when the exercise had just ended, the muscle tissue showed a comparable IL-8 expression relative to the level seen before exercise. C, by 4.5 h after exercise, the IL-8 expression was significantly increased in the skeletal muscle tissue, which showed a high level of IL-8 both in general in the cytoplasm and related to the cell membranes, as well as in vessels in the muscle. D, ATPase-stained section. This is the neighbouring section to that seen in C. By comparing C and D, it is seen that both muscle fibre types express IL-8 after exercise. E and F, IL-8 expression was still very high, and peaked at 6 (E) and 9 h (F) following the bicycle exercise. G, after 24 h, the levels of IL-8 protein had decreased again, and the staining appeared homogeneous and mildly increased in the fibres. H and I, higher magnification of skeletal muscle tissue at 9 h following exercise. As shown, IL-8 protein is mainly expressed in the cytoplasm, and it is also expressed in the membranes, including the nuclei. We also detected intermittently vascular IL-8 expression in the vessel endothelium of the muscle tissue. Scale bars: A–G, 50 μm; H, 29 μm; I, 14 μm.
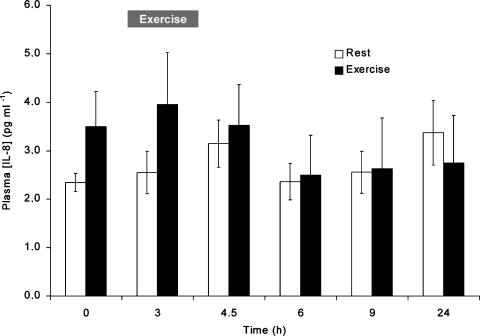
The plasma concentrations in the rest group and the exercise group did not change significantly over time, and the IL-8 plasma concentrations in the two groups were not significantly different from each other (Fig. 3).
Figure 3. IL-8 venous plasma concentrations in rest and exercise groups.
The exercise group: pre (0 h), immediately after 3 h of bicycle exercise (3 h), and 1.5 (4.5 h), 3 (6 h), 6 (9 h) and 21 h (24 h) postexercise. The rest group: pre (0 h), after 3, 4.5, 6, and 9 h of rest, and 24 h after the presample. Values are means ± s.e.m. (n = 6 subjects).
Study 2. The effect of knee-extensor exercise on IL-8 release from working muscles
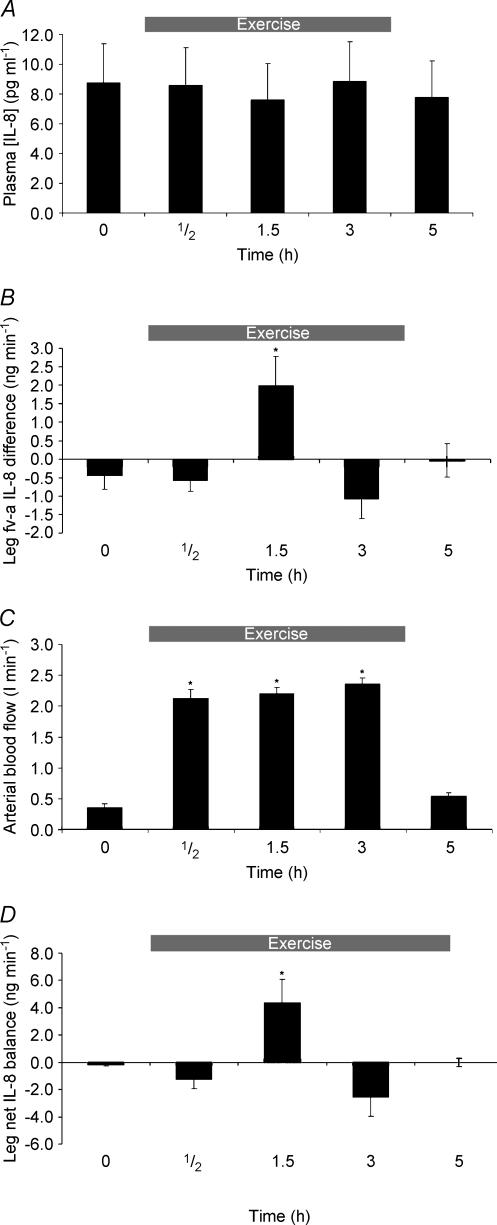
IL-8 mRNA content in skeletal muscle increased in response to knee-extensor exercise (Fig. 4). At 1.5, 3 and 5 h, IL-8 mRNA was significantly increased when compared with pre-exercise (P < 0.05). The level of IL-8 mRNA peaked after 3 h of exercise, reaching a 225-fold increase (Fig. 4). As depicted in Fig. 5A, mean arterial plasma IL-8 concentration averaged 8.7 ± 2.6 pg ml−1 at rest, and was not altered during or after exercise. However, the leg fv–a IL-8 difference was affected by exercise
Figure 4. Muscle IL-8 mRNA before (0), during (0.5 and 1.5 h) and after 3 h of two-legged knee-extensor exercise, and 2 h after the end of exercise (5 h).
Values are means ± s.e.m. (n = 6). *Significant (P < 0.05) difference from before (0 h).
Figure 5.
A, IL-8 arterial plasma concentration before (0 h), during (0.5 and 1.5 h) and after 3 h of two-legged knee-extensor exercise, and 2 h (5 h) after the end of exercise. Values are means ± s.e.m. (n = 6). B, leg plasma femoral venous–arterial (fv–a) IL-8 difference before (0 h), during (0.5 and 1.5 h) and after 3 h of two-legged knee-extensor exercise, and 2 h (5 h) post exercise. Values are means ± s.e.m. (n = 6). *Significant difference (P < 0.05) from before (0 h). C, leg blood flow before (0 h), during (0.5 and 1.5 h) and after 3 h of two-legged knee-extensor exercise, and 2 h (5 h) after the end of exercise. Values are means ± s.e.m. (n = 6). *Significant difference (P < 0.05) from before (0 h). D, leg net IL-8 balance (ng min−1) values before (0 h), during (0.5 and 1.5 h) and after 3 h of two-legged knee-extensor exercise, and 2 h postexercise (5 h). Values are means ± s.e.m. (n = 6). *Significant difference (P < 0.05) from before (0 h).
(Fig. 5B). After 1.5 h of exercise, the venous concentration of IL-8 was slightly increased, resulting in an fv–a for IL-8 of 1.95 ± 0.8 pg ml−1, which was significantly different from all other time points. None of the other time points were significantly different from the pre (0 h) value or from each other (Fig. 5B). Prior to exercise, mean arterial blood flow measured 351.5 ± 59.7 ml min−1. Exercise increased blood flow significantly (P < 0.05) at all time points during the exercise period as depicted in Fig. 5C. Two hours post-exercise, mean arterial blood flow was reduced to 550.5 ± 59.3 ml kg−1, which was not significantly different from blood flow prior to exercise (P = 0.091). The IL-8 balance over the working limb was calculated as previously described. Thus, following 1.5 h of exercise, a significant IL-8 net release of 5.1 ± 1.7 ng min−1 was observed across the thigh (Fig. 5D). The difference was significantly different from all other time points. No other observation deviated significantly from the pre sample.
Discussion
This is the first study to demonstrate that exercise induces expression of the IL-8 protein in the cytoplasm of skeletal muscle fibres. The finding of a marked increase of IL-8 mRNA in muscle biopsy samples during and following exercise, and IL-8 protein expression within skeletal muscle fibres in the recovery from exercise, strongly indicates that exercise per se stimulates muscle cells to produce IL-8. This finding is in accordance with the finding that muscle cells in vitro have the capacity to express IL-8 both at the mRNA and protein levels (De Rossi et al. 2000).
The finding of increased IL-8 mRNA and expression of IL-8 protein in skeletal muscle biopsy samples obtained during and after exercise adds to the findings of the pioneering work by Nieman and coworkers. Those authors reported increased IL-8 mRNA in skeletal muscle biopsy samples after 3 h of exhaustive treadmill running (Nieman et al. 2003). Treadmill running involves a substantial eccentric component leading to skeletal muscle damage and an associated inflammatory response (Brenner et al. 1999). It is possible that invading monocytes and macrophages represent the cell sources of IL-8 mRNA in the study by Nieman et al. (2003). However, our finding of a marked increase in IL-8 mRNA and IL-8 protein within muscle fibres in two independent concentric exercise models adds strong support to previous indications that IL-8 is produced by the muscle (Figs 1, 2 and 4). In support of our finding, an increase in IL-8 mRNA following concentric exercise has recently been found by others (Chan et al. 2004). Concentric exercise is not associated with muscle damage or inflammation (Sorichter et al. 1997; Brenner et al. 1999), which rules out an inflammatory response being the cause of the IL-8 expression in exercising skeletal muscle in this study. Several tissues exhibit stimulus-dependent expression of IL-8. The promoter region of the IL-8 gene contains a nuclear factor (NF)-κB binding site, and binding of NF-κB is sufficient for gene transcription in all cells studied (Hoffmann et al. 2002). Yu and coworkers have shown intracellular Ca2+-dependent activation of IL-8 gene expression in human colonic epithelial cells (Yu et al. 2001), and adenosine has also been shown to mediate IL-8 gene expression in mast cells (Feoktistov et al. 1999). Adenosine increases in the interstitial space as a response to exercise (Hellsten et al. 1998), and the concentration of calcium (Ca2+) rises temporarily in association with skeletal muscle contraction. Adenosine and Ca2+ could thus represent possible pathways of IL-8 gene transcription activation in skeletal muscle as a response to exercise. However, no studies attempting to elucidate the induction pathway of IL-8 gene expression have been performed in skeletal muscle cells, which leaves the transcriptional activation mechanism unknown
Muscle biopsy samples obtained by the percutaneus needle biopsy method contain a relatively small portion of endothelial cells in the form of capillaries (Qu et al. 1997). Human endothelial cells express IL-8 when stimulated with IL-1 or TNF-αin vitro (Zhao et al. 2003). It is unlikely that capillary endothelial cells are the source of IL-8 mRNA, as IL-1 and TNF-α plasma concentrations are not elevated in response to concentric exercise (Pedersen et al. 1998). There was a slight increase in IL-8 mRNA in the control group at 24 h, which was not significantly different from the exercise group (Fig. 1). Multiple biopsy samples can induce an inflammatory response in the muscle (Malm et al. 2000), which might explain the slight increase in mRNA. Alternatively, it could reflect light exercise activity before the subjects returned to the laboratory the following morning.
There is a clear staining for IL-8 in the cytoplasm of the muscle cell, which indicates that myocytes are the true source of IL-8. A clear expression of the protein is seen at 4.5 h, with peaks at 6 and 9 h (Fig. 2A–C and E–G). A marked increase in the concentration of systemic IL-8 has been a consistent finding in studies with an eccentric exercise component (Nieman et al. 2001, 2002, 2003; Suzuki et al. 2003), whereas the findings are less clear in concentric exercise. Mucci et al. (2000) found a minimal increase (2 pg ml−1) in plasma IL-8 during intense ergometer bicycling for 10–15 min, whereas neither Henson and coworkers nor Chan et al. found a change in plasma IL-8 after 2 h of rowing or 1 h of bicycle exercise (Henson et al. 2000; Chan et al. 2004), respectively. Possibly, the systemic increase in IL-8 as seen during exercise with an eccentric component is mainly due to an inflammatory response. In accordance, we observed no increase in the systemic IL-8 plasma concentration during or after exercise in study 1 or 2. A small and transient fv–a difference, and thus a net release of IL-8 (Fig. 5C and D), which did not result in an increase in the systemic IL-8 plasma concentration, was found at 1.5 h of exercise in study 2 (Fig. 5A). However, we found no release of IL-8 after 3 h (Fig. 5D). The finding that a high local IL-8 expression takes place in working muscle with only a small and transient release could indicate that muscle-derived IL-8 exerts its effect in an endocrine or paracrine fashion. Moreover, it is possible that an exercise factor inhibits the release of IL-8. Dexamethasone has been shown to inhibit IL-8 release in cultured epithelial cells (Chang et al. 2001). Plasma concentration of cortisol increases during prolonged exercise (Kjaer et al. 1988; Viru et al. 1992). It may be speculated that an exercise-related cortisol increase could function as an inhibitor of IL-8 release, and thus explain the attenuation of IL-8 release at 3 h of exercise.
IL-8-induced angiogenesis is distinct from its ability to induce inflammation (Keane et al. 1997). IL-8 associates with the CXC receptors 1 and 2 (CXCR1 and CXCR2) (Belperio et al. 2000). CXCR2 is expressed by human microvascular endothelial cells, and is the receptor responsible for IL-8-induced angiogenesis (Addison et al. 2000). The finding that a high local IL-8 expression takes place in working muscle, which is accompanied by only a small and transient release, indicates that muscle-derived IL-8 exerts its effect locally. The IL-8 produced by the exercising limb might elicit its response by interacting with the CXCR2 receptor present in the capillary (endothelial tissue) (Addison et al. 2000; Heidemann et al. 2003). Given that IL-8 is a potent angiogenic factor in several tissues, we suggest that a possible role of the skeletal muscle-derived IL-8 is to stimulate neovascularization.
In conclusion, the present study demonstrates that pure concentric exercise induces a marked IL-8 mRNA and protein expression within muscle fibres, without changes in plasma concentration of IL-8.
Acknowledgments
We would like to thank the subjects for their participation. The excellent technical assistance of Ruth Rousing and Hanne Willumsen is gratefully acknowledged. The Copenhagen Muscle Research Centre was supported by grants from The University of Copenhagen, The Faculties of Science and Health Sciences at this University, The Copenhagen Hospital Corporation and the Danish National Research Foundation (Grant 504-14). The study was also supported by grants from The Danish Research Agency (22-01-0019), the Novo Nordisk Foundation and the Lundbeck Foundation.
References
- Addison CL, Daniel TO, Burdick MD, Liu H, Ehlert JE, Xue YY, Buechi L, Walz A, Richmond A, Strieter RM. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR + CXC chemokine-induced angiogenic activity. J Immunol. 2000;165:5269–5277. doi: 10.4049/jimmunol.165.9.5269. [DOI] [PubMed] [Google Scholar]
- Alvarez B, Quinn LS, Busquets S, Lopez-Soriano FJ, Argiles JM. TNF-alpha modulates cytokine and cytokine receptors in C2C12 myotubes. Cancer Lett. 2002;175:181–185. doi: 10.1016/s0304-3835(01)00717-0. 10.1016/S0304-3835(01)00717-0. [DOI] [PubMed] [Google Scholar]
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M. Chemokines in pathology and medicine. J Intern Med. 2001;250:91–104. doi: 10.1046/j.1365-2796.2001.00867.x. [DOI] [PubMed] [Google Scholar]
- Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines – CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- Bek EL, McMillen MA, Scott P, Angus LD, Shaftan GW. The effect of diabetes on endothelin, interleukin-8 and vascular endothelial growth factor-mediated angiogenesis in rats. Clin Sci (Lond) 2002;103(suppl. 48):424S–429S. doi: 10.1042/CS103S424S. [DOI] [PubMed] [Google Scholar]
- Belperio JA, Keane MP, Arenberg DA, Addison CL, Ehlert JE, Burdick MD, Strieter RM. CXC chemokines in angiogenesis. J Leukoc Biol. 2000;68:1–8. [PubMed] [Google Scholar]
- Brenner IK, Natale VM, Vasiliou P, Moldoveanu AI, Shek PN, Shephard RJ. Impact of three different types of exercise on components of the inflammatory response. Eur J Appl Physiol. 1999;80:452–460. doi: 10.1007/s004210050617. [DOI] [PubMed] [Google Scholar]
- Brooke MH, Kaiser KK. Three ‘myosin adenosine triphosphatase’ systems: the nature of their pH lability and sulfhydryl dependence. J Histochem Cytochem. 1970;18:670–672. doi: 10.1177/18.9.670. [DOI] [PubMed] [Google Scholar]
- Bruun JM, Pedersen SB, Kristensen K, Richelsen B. Opposite regulation of interleukin-8 and tumor necrosis factor-alpha by weight loss. Obes Res. 2002;10:499–506. doi: 10.1038/oby.2002.68. [DOI] [PubMed] [Google Scholar]
- Bruun JM, Verdich C, Toubro S, Astrup A, Richelsen B. Association between measures of insulin sensitivity and circulating levels of interleukin-8, interleukin-6 and tumor necrosis factor-alpha. Effect of weight loss in obese men. Eur J Endocrinol. 2003;148:535–542. doi: 10.1530/eje.0.1480535. [DOI] [PubMed] [Google Scholar]
- Chan MH, Carey AL, Watt MJ, Febbraio MA. Cytokine gene expression in human skeletal muscle during concentric contraction: evidence that IL-8, like IL-6, is influenced by glycogen availability. Am J Physiol Regul Integr Comp Physiol. 2004;287:R322–327. doi: 10.1152/ajpregu.00030.2004. [DOI] [PubMed] [Google Scholar]
- Chang MM, Juarez M, Hyde DM, Wu R. Mechanism of dexamethasone-mediated interleukin-8 gene suppression in cultured airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L107–115. doi: 10.1152/ajplung.2001.280.1.L107. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform–extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- De Rossi M, Bernasconi P, Baggi F, de Waal MR, Mantegazza R. Cytokines and chemokines are both expressed by human myoblasts: possible relevance for the immune pathogenesis of muscle inflammation. Int Immunol. 2000;12:1329–1335. doi: 10.1093/intimm/12.9.1329. 10.1093/intimm/12.9.1329. [DOI] [PubMed] [Google Scholar]
- Feoktistov I, Goldstein AE, Biaggioni I. Role of p38 mitogen-activated protein kinase and extracellular signal-regulated protein kinase kinase in adenosine A2B receptor-mediated interleukin-8 production in human mast cells. Mol Pharmacol. 1999;55:726–734. [PubMed] [Google Scholar]
- Heidemann J, Ogawa H, Dwinell MB, Rafiee P, Maaser C, Gockel HR, Otterson MF, Ota DM, Lugering N, Domschke W, Binion DG. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278:8508–8515. doi: 10.1074/jbc.M208231200. 10.1074/jbc.M208231200. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Maclean D, Radegran G, Saltin B, Bangsbo J. Adenosine concentrations in the interstitium of resting and contracting human skeletal muscle. Circulation. 1998;98:6–8. doi: 10.1161/01.cir.98.1.6. [DOI] [PubMed] [Google Scholar]
- Henson DA, Nieman DC, Nehlsen-Cannarella SL, Fagoaga OR, Shannon M, Bolton MR, Davis JM, Gaffney CT, Kelln WJ, Austin MD, Hjertman JM, Schilling BK. Influence of carbohydrate on cytokine and phagocytic responses to 2 h of rowing. Med Sports Exerc. 2000;32:1384–1389. doi: 10.1097/00005768-200008000-00005. 10.1097/00005768-200008000-00005. [DOI] [PubMed] [Google Scholar]
- Hildebrandt AL, Neufer PD. Exercise attenuates the fasting-induced transcriptional activation of metabolic genes in skeletal muscle. Am J Physiol Endocrinol Metab. 2000;278:E1078–1086. doi: 10.1152/ajpendo.2000.278.6.E1078. [DOI] [PubMed] [Google Scholar]
- Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M. Multiple control of interleukin-8 gene expression. J Leukoc Biol. 2002;72:847–855. [PubMed] [Google Scholar]
- Keane MP, Arenberg DA, Lynch JP, III, Whyte RI, Iannettoni MD, Burdick MD, Wilke CA, Morris SB, Glass MC, DiGiovine B, Kunkel SL, Strieter RM. The CXC chemokines, IL-8 and IP-10, regulate angiogenic activity in idiopathic pulmonary fibrosis. J Immunol. 1997;159:1437–1443. [PubMed] [Google Scholar]
- Kjaer M, Bangsbo J, Lortie G, Galbo H. Hormonal response to exercise in humans: influence of hypoxia and physical training. Am J Physiol Regul Integr Comp Physiol. 1988;254:R197–203. doi: 10.1152/ajpregu.1988.254.2.R197. [DOI] [PubMed] [Google Scholar]
- Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- Malm C, Nyberg P, Engstrom M, Sjodin B, Lenkei R, Ekblom B, Lundberg I. Immunological changes in human skeletal muscle and blood after eccentric exercise and multiple biopsies. J Physiol. 2000;529:243–262. doi: 10.1111/j.1469-7793.2000.00243.x. 10.1111/j.1469-7793.2000.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucci P, Durand F, Lebel B, Bousquet J, Prefaut C. Interleukins 1-beta, -8, and histamine increase in highly trained, exercising athletes. Med Sports Exerc. 2000;32:1094–1100. doi: 10.1097/00005768-200006000-00009. 10.1097/00005768-200006000-00009. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Davis JM, Henson DA, Walberg-Rankin J, Shute M, Dumke CL, Utter AC, Vinci DM, Carson JA, Brown A, Lee WJ, McAnulty SR, McAnulty LS. Carbohydrate ingestion influences skeletal muscle cytokine mRNA and plasma cytokine levels after a 3-h run. J Appl Physiol. 2003;94:1917–1925. doi: 10.1152/japplphysiol.01130.2002. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Henson DA, McAnulty SR, McAnulty L, Swick NS, Utter AC, Vinci DM, Opiela SJ, Morrow JD. Influence of vitamin C supplementation on oxidative and immune changes after an ultramarathon. J Appl Physiol. 2002;92:1970–1977. doi: 10.1152/japplphysiol.00961.2001. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Henson DA, Smith LL, Utter AC, Vinci DM, Davis JM, Kaminsky DE, Shute M. Cytokine changes after a marathon race. J Appl Physiol. 2001;91:109–114. doi: 10.1152/jappl.2001.91.1.109. [DOI] [PubMed] [Google Scholar]
- Norrby K. Interleukin-8 and de novo mammalian angiogenesis. Cell Prolif. 1996;29:315–323. doi: 10.1111/j.1365-2184.1996.tb01583.x. [DOI] [PubMed] [Google Scholar]
- Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK. Chemokines are elevated in plasma after strenuous exercise in humans. Eur J Appl Physiol. 2001;84:244–245. doi: 10.1007/s004210170012. 10.1007/s004210170012. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Ostrowski K, Rohde T, Bruunsgaard H. The cytokine response to strenuous exercise. Can J Physiol Pharmacol. 1998;76:505–511. doi: 10.1139/cjpp-76-5-505. 10.1139/cjpp-76-5-505. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Ordway GA, Saltin B, Neufer PD. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am J Physiol Endocrinol Metab. 2000;279:E806–814. doi: 10.1152/ajpendo.2000.279.4.E806. [DOI] [PubMed] [Google Scholar]
- Qu Z, Andersen JL, Zhou S. Visualisation of capillaries in human skeletal muscle. Histochem Cell Biol. 1997;107:169–174. doi: 10.1007/s004180050101. 10.1007/s004180050101. [DOI] [PubMed] [Google Scholar]
- Radegran G. Ultrasound Doppler estimates of femoral artery blood flow during dynamic knee extensor exercise in humans. J Appl Physiol. 1997;83:1383–1388. doi: 10.1152/jappl.1997.83.4.1383. [DOI] [PubMed] [Google Scholar]
- Saghizadeh M, Ong JM, Garvey WT, Henry RR, Kern PA. The expression of TNF alpha by human muscle. Relationship to insulin resistance. J Clin Invest. 1996;97:1111–1116. doi: 10.1172/JCI118504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorichter S, Mair J, Koller A, Gebert W, Rama D, Calzolari C, Artner-Dworzak E, Puschendorf B. Skeletal troponin I as a marker of exercise-induced muscle damage. J Appl Physiol. 1997;83:1076–1082. doi: 10.1152/jappl.1997.83.4.1076. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Nakaji S, Yamada M, Liu Q, Kurakake S, Okamura N, Kumae T, Umeda T, Sugawara K. Impact of a competitive marathon race on systemic cytokine and neutrophil responses. Med Sports Exerc. 2003;35:348–355. doi: 10.1249/01.MSS.0000048861.57899.04. [DOI] [PubMed] [Google Scholar]
- Viru A, Karelson K, Smirnova T. Stability and variability in hormonal responses to prolonged exercise. Int J Sports Med. 1992;13:230–235. doi: 10.1055/s-2007-1021259. [DOI] [PubMed] [Google Scholar]
- Yu Y, De Waele C, Chadee K. Calcium-dependent interleukin-8 gene expression in T84 human colonic epithelial cells. Inflamm Res. 2001;50:220–226. doi: 10.1007/s000110050747. 10.1007/s000110050747. [DOI] [PubMed] [Google Scholar]
- Zhao B, Stavchansky SA, Bowden RA, Bowman PD. Effect of interleukin-1beta and tumor necrosis factor-alpha on gene expression in human endothelial cells. Am J Physiol Cell Physiol. 2003;284:C1577–1583. doi: 10.1152/ajpcell.00243.2002. [DOI] [PubMed] [Google Scholar]