Abstract
Outer hair cells (OHCs) in the mammalian organ of Corti display electromotility, which is thought to provide the local active mechanical amplification of the cochlear response. Prestin is the key molecule responsible for OHC electromotility. Several compounds, including cGMP, have been shown to influence OHC electromotility. There are two potential cAMP/cGMP-dependent protein kinase phosphorylation sites on prestin. Whether these sites are involved in cGMP-dependent reactions is as yet unknown. In this study, prestin cDNA was transiently transfected into TSA 201 cells. Cells that expressed prestin were selected to measure non-linear capacitance (NLC), a signature of outer hair cell motility. We applied cGMP and cAMP analogues and a protein kinase G (PKG) antagonist to the cells. Furthermore, nine mutations at putative phosphorylation sites of prestin were produced. The neutral amino acid alanine replaced serine/threonine at phosphorylation sites to change the conserved phosphorylation motif in order to mimic the dephosphorylated state of prestin, whereas replacement with the negatively charged aspartic acid mimicked the phosphorylated state. The properties of such modified prestin-expressing cells were examined, through measurement of NLC and with confocal microscopy. Our data demonstrate that cGMP is significantly more influential than cAMP in modifying the non-linear, voltage-dependent charge displacement in prestin-transfected cells. The electrical properties of the single and double mutations further indicate a possible interaction between the two PKG target sites. One of these sites may influence the membrane targeting process of prestin. Finally, a new topology map of prestin is proposed.
In the mammalian cochlea there are two mechanical processes operating co-operatively, the ‘passive’ travelling-wave system (von Békésy, 1960) and an ‘active’ local amplification process (Davis, 1983). The active amplifier mechanism is provided by outer hair cells (OHCs). Hearing sensitivity is degraded by 40–50 dB in the absence of OHCs (Ryan & Dallos, 1975) and frequency selectivity is also impaired (Dallos & Harris, 1978; Harrison & Evans, 1979). OHCs can change their lengths either on a microsecond (fast motility; Brownell et al. 1985) or second (slow motility; Zenner et al. 1985) time scale. The work reported here pertains to the modulation of fast length changes, occurring at acoustic frequencies (Dallos & Evans, 1995). Sound-evoked deflection of OHC stereocilia leads to transmembrane potential changes (Flock, 1965; Hudspeth & Corey, 1977) that drive fast somatic shape changes (electromotility) (Brownell et al. 1985; Kachar et al. 1986; Ashmore, 1987). The OHC fast electromotility is a membrane voltage-controlled cellular length change (Santos-Sacchi & Dilger, 1988), which is assumed to amplify the sound-evoked vibrations in the cochlea and thereby serve as the cellular basis of the cochlear amplifier (e.g. Dallos, 1992). It has been assumed that electromotility is produced by the aggregate action of membrane-bound motor proteins; these have been identified as prestin (Zheng et al. 2000).
Electromotility does not require any secondary compounds such as ATP or calcium for force generation (Kachar et al. 1986; Holley & Ashmore, 1988) even though calcium and other chemicals can modify it. OHCs receive prominent cholinergic efferent innervation (e.g. Eybalin, 1993). Application of acetylcholine (ACh) to OHCs increases the amplitude of fast motility (Sziklai et al. 1996) and decreases the axial stiffness of the cells (Dallos et al. 1997). The change in stiffness was proposed to be due to the phosphorylation of both the motor and cytoskeletal proteins (He et al. 2003). Both the ACh and cGMP effects can be inhibited by specific protein kinase-G (PKG) inhibitors, suggesting that a cGMP–PKG cascade modulates fast motility (Szönyi et al. 1999).
The involvement in the ACh effect of several other phosphorylation pathways had been suggested (Kalinec et al. 2000; Sziklai et al. 2001; Zhang et al. 2003). Furthermore, the motor protein changes its voltage sensitivity when general phosphatases or dephosphatases are applied (Frolenkov et al. 2000, 2001). As the motor proteins and the cytoskeleton are physically interlinked (Flock et al. 1986; Holley, 1991; Holley et al. 1992), modification of either will influence their combined behaviour.
The motor protein prestin has several potential phosphorylation sites including two locations for phosphorylation by cAMP/cGMP-dependent protein kinase, predicted by PROSITE search (Bairoch & Apweiler, 1997). Whether or not these sites are direct targets of the cGMP–PKG pathway is not known. To address such questions, we established a heterologous system in which wild-type or various cAMP/cGMP-dependent protein kinase phosphorylation-site mutant cDNAs were transfected into a TSA cell line. We used a non-hydrolysable cGMP analogue, DBcGMP (Sato & Kawatani, 1998) and the PKG antagonist, 8-Rp-pCPT-cGMPS (Lohmann et al. 1997) to study the effect of cGMP on the function of prestin. To clarify the specificity of the cGMP effect, DBcAMP was also tested in the same experimental conditions.
Methods
Prestin DNA constructs and generation of mutant forms of prestin
Gerbil Prestin (gPrestin) was cloned into the vector pcDNA3.1 (Zheng et al. 2000). A QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA) was used to generate wild-type (wt) prestin and the following associated mutants: cluster 1, ‘C1’ (K233Q, K235Q, R236Q), S238A, T560A, S238D, T560D and the double mutants S238A/T560A and S238D/T560D. The nucleotide exchanges were confirmed by DNA sequencing; cluster mutants 3 and 4, ‘C3’ (K557Q, R558Q, K559Q) and ‘C4’ (R571Q, R572Q, K577Q), generated from rat prestin cDNA (provided by Dr D. Oliver, University of Freiberg, Germany; Oliver et al. 2001). These two constructs had green fluorescent protein (GFP) tags on the N-terminal of prestin. Instead of changing phosphorylation sites T or S directly, C1 and C3 abolish potential cAMP/cGMP-dependent protein kinase phosphorylation sites by replacing the conserved R or K amino acids with non-charged amino acids. C4 is a corresponding control mutant in which K and R in non-phosphorylation sites are changed to non-charged amino acids.
Cell culture and transfection
TSA201 cells, derived from human embryonic kidney cells (HEK293), or OK (opossum kidney) cells were transiently cotransfected with GFP and prestin and its associated mutant constructs using the Effectene (Qiagen, Valencia, CA, USA) or Exgen 500 (Fermentas, Hanover, MD, USA) reagents (Zheng et al. 2001). Either 0.4 μg (Effectene) or 1 μg (Exgen 500) of prestin cDNA was used for one 35-mm culture dish. Based on our previous experience, the concentration ratio for GFP and prestin was 1 : 10. This ratio allowed the cells to show just enough green fluorescent light to allow visual identification of transfected cells.
For TSA201 cell culturing, Dulbecco's modified Eagle's medium (DMEM) was supplemented with 5% fetal bovine serum, in addition to 100 U ml−1 penicillin, and 100 μg ml−1 streptomycin. For OK cells, Eagle's minimum essential medium (MEM) was used, supplemented with 10% fetal bovine serum, 1 mm sodium pyruvate, 1X non-essential amino acid (Gibco, Carlsbad, CA, USA), 0.075% (9.2 mm) sodium bicarbonate, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin. Cells were dissociated 48 h after transfection with non-enzymatic cell-dissociation solution (Sigma).
Drugs
DBcGMP (N2,2′-O-dibutyrylguanosine 3′,5′-cyclic monophosphate sodium salt hydrate) was purchased from Sigma. DBcAMP (adenosine 3′, 5′-cyclic monophosphate N6, O2′-dibutyryl-, sodium salt) and 8-Rp-pCPT-cGMPS (guanosine 3′,5′-cyclic monophosphorothionate, 8-(4-chlorophenylthio)-, Rp-isomer, triethylammonium salt) were purchased from Calbiochem. All stock solutions were stored at −20°C and used within a month after being dissolved in water.
Drug delivery
For any one set of transfections several measurement dishes were prepared after dissociation. The drug was applied to the cells in three different ways. (1) Pre-incubation: cells were incubated with DBcGMP (various doses), DBcAMP (200 μm) or with 8-Rp-pCPT-cGMPS (100 μm) in their culture (DMEM) solution for 45 min at 36.7°C. (2) The drug – with various doses dissolved in intracellular solution – was also placed in the patch pipette (incubation plus intracellular application). (3) Intracellular application: in a limited number of cases no pre-incubation was used, the drug was present only in the patch pipette.
Electrophysiology
It is a commonly accepted idea that non-linear capacitance (NLC) can serve as a surrogate electrophysiological measure of electromotility (Ashmore, 1990, 1992; Santos-Sacchi, 1991). Therefore, we used NLC as an assay for the electromechanical effects produced by the molecular motor. A complete description of NLC measurements was given by Huang & Santos-Sacchi (1993) and a brief overview is given by Dallos & Fakler (2002). All experiments were performed at room temperature (24–26°C). For electrophysiology experiments, cells from one transfected dish were transferred to several poly-d-lysine-covered dishes (Becton Dickinson). Whole-cell voltage-clamp recordings were made with an Axopatch 200B amplifier (Axon Instruments). Recording glass pipettes (Borosilicate glass, Fisher) had open tip resistances between 1.5 and 2.4 MΩ. They were filled with an internal solution consisting of (mm): CsCl 130, MgCl2 2, EGTA 10 and Hepes 10 at pH 7.2. The external medium (ionic blocking solution) had the following composition (mm): NaCl 99, TEA-Cl 20, CoCl2 2, MgCl2 1.47 CaCl2 1 and Hepes 10, and 4.6 g glucose; pH 7.2. Osmolarity was adjusted to 300 ± 4 mosmol l−1 with glucose. For data collection, single fluorescing cells without membrane disruption or any apoptotic signs were selected. As a sign of good condition, all cells had to possess a membrane resistance of at least 100 MΩ, and a linear capacitance of at least 5 pF. Experiments started 45 min after dissociation and finished within 3 h. In this time period, the electrophysiological properties of the cells did not change (data not shown) and we were able to make measurements from approximately 25–30 cells. After gigaseal formation between the cell and the recording pipette, the stray capacitance was manually compensated. Whole-cell recording was established by pulses of suction. Cells were clamped at 0 mV and the pipette pressure was adjusted to zero. Whole-cell series resistance was less than 10 MΩ. Voltage-dependent capacitance was measured after the whole-cell configuration was established, within ∼5 s, using a Windows-based whole-cell voltage-clamp program, jClamp (SciSoft, CT, USA). To obtain the voltage dependence of the membrane capacitance, a continuous high-resolution, two-sine voltage stimulus protocol was used. The peak amplitudes were 20 mV at 976.5 and 1953 Hz fundamental frequencies. The sine-waves were superimposed on a variable DC bias voltage, usually ranging from −120 mV to +120 mV. Exception was for cases when the estimated peak non-linear capacitance (V1/2) was shifted to the extreme hyperpolarized side. In such cases, the voltage range was extended.
Data collection
Capacitance data were fitted to the first derivative of a two-state Boltzmann function (Santos-Sacchi, 1991; Huang & Santos-Sacchi, 1993).
 |
(1) |
Where Qmax is the maximum charge moved through the membrane's electrical field, V1/2 is the voltage at half-maximum charge transfer (peak of the capacitance curve), V is the membrane potential, and α is the slope factor of the voltage dependence, and Clin is the residual linear membrane capacitance. The slope factor, is dependent on temperature and the valence of the charge moved. Maximum voltage-dependent capacitance (Cv) occurs at V1/2. Inasmuch as variation in cell size caused differences in the maximal charge transfer, the Qmax was divided by linear capacitance (Clin) and designated as charge density (Oliver & Fakler, 1999). The unit of charge density is fC pF−1. The non-linear capacitance component is obtained as the difference between measured capacitance (Cv) and linear cell capacitance (Clin). The software Igor Pro (WaveMetrics, Lake Oswego, OR, USA) was used for data analysis and curve fitting.
Immunofluorescence experiments
The transiently transfected cells were fixed with 1% formaldehyde in PBS for 10 min at room temperature. The cells were incubated with PBS containing anti-C-prestin (Matsuda et al. 2004), and/or anti-Golgin 97, a Golgi marker (Molecular Probes, 1 : 200 dilution), or anti-Na+–K+ ATPase, a plasma membrane marker (Upstate Biotechnology, Switzerland; 10 ng ml−1), 0.1% saponin and 5% bovine serum albumin (BSA). After washing with PBS, the samples were then incubated with the corresponding second antibody, anti-mouse IgG or anti-rabbit IgG, conjugated with different fluorescent labels in PBS containing 5% BSA, 0.1% saponin and 10% normal donkey serum or goat serum. The samples were mounted on glass slides with mounting solution (Fluoromount-G) and observed using a Leica confocal system with a standard configuration DMRXE7 microscope (63 × magnification, 1.32 NA).
Statistics
The effects of cyclic nucleotides can occur on time scales ranging from milliseconds to seconds (Beavo et al. 2002), potentially faster than one can establish whole-cell recording configuration. Because of this time constraint, the control cells, without drug treatment, were taken from a different dish, but from the same transfection. In other words, in these experiments we are comparing two populations of cells for any experimental condition, inasmuch as no individual cell could serve as its own control.
Each experimental group contained at least 11 cells and most experiments were repeated (a second group of at least 11 cells). A conclusion was reached if the two results corroborated one another and if both showed statistically significant effects. Charge density tended to change with the age of the cell line, and also showed variability dependent on transfection efficiency. Thereby, to evaluate the drug effects on the charge density, we used the data obtained from one set of transfection. Cultures of a TSA 201 cell line, with 5–30 passages after thawing from liquid nitrogen, were used for transient transfection. Our principal criterion for accepting a recording was a signal-to-noise ratio of two. In other words, the peak non-linear capacitance had to be at least twice that the peak-to-peak noise in the recording. Along with this criterion, we required at least 2 fC pF−1 charge density from control cells. Other electrical properties of prestin, slope factor (α) and V1/2, did not show large variability in the control cell population. When the electrical characteristics of mutants were investigated, our total population of wild-type prestin data (n = 211) served as a control. For statistical evaluation the parametric t test was used and data are presented as means and standard deviations.
Results
Effect of cyclic nucleotides on voltage-dependent capacitance
It is generally thought that one effect of a second messenger is to modify the phosphorylation state of a functional protein and thereby alter its cellular function. The motor protein prestin has two potential cAMP/cGMP-dependent phosphorylation sites, predicted by PROSITE search (Bairoch et al. 1997). Therefore, we tested the possibility that application of DBcGMP or DBcAMP may change the functional characteristics of the motor protein. Prestin-expressing cells were incubated with the agonist DBcGMP (200 μm) for 45 min and the recording patch pipette also included the cyclic nucleotide. As shown in Fig. 1A, DBcGMP-treated prestin-transfected cells had larger NLC than controls from the same transfection batch. Their derived average maximum charge density of 16.4 ± 5 fC pF−1, was more than twice that of corresponding untreated prestin-expressing TSA cells (control). The average maximum charge density of the control cells was 7.4 ± 4 fC pF−1. The change in charge density due to application of DBcGMP (122%) was statistically significant (P < 0.001). Similar experiments were conducted on prestin-expressing cells with DBcAMP stimulation. At the same dose (200 μm), there was no difference in charge density between DBcAMP-treated and untreated prestin-expressing cells (P > 0.6) (Fig. 1B). These data suggest that, with the dosage used, cGMP, but not cAMP, can increase electrically induced charge displacement in prestin-expressing mammalian cells.
Figure 1. Effects of DBcGMP, DBcAMP and 8-Rp-pCPT-cGMPS on non-linear capacitance.
Effects of cyclic nucleotide agonists DBcGMP (A) and DBcAMP (B), and PKG blocker 8-Rp-pCPT-cGMPS (C) on NLC. The drugs were applied in the bath and also intracellularly through the patch pipette. Control data (○) and the effect of drugs (•). NLC equals Cv–Clin. The daily control maximum NLC values had been normalized to unity in all three parts of the figure. The relative drug effect is shown compared to its own daily control. It is emphasized that the only normalization is to set the maximum NLC value of the control measurements to unity. Symbols represent data from the individual cells that best approximate the average for the population. For the cells included in this graph the following values were obtained by curve fitting of eqn (1) (solid lines). The values for DBcGMP are: Qmax, 168.5 fC; α, 37.6 mV; V1/2, −66.8 mV. Control for DBcGMP: Qmax, 88.1 fC; α, 34.4 mV; V1/2, −72.0 mV. DBcAMP: Qmax, 85.5 fC; α, 37.9 mV, V1/2, −68.2 mV. Control for DBcAMP: Qmax, 82.9 fC; α, 36.7 mV, V1/2, −72.0 mV. 8-Rp-pCPT-cGMPS: Qmax, 65 fC, α, 43 mV, V1/2, −74.6 mV. Control for 8-Rp-pCPT-cGMPS: Qmax, 122.4 fC, α, 34.6 mV, V1/2, −69.3 mV.
As noted in the Methods, drug-effects were compared between separate groups of cells derived from the same transfection batch. As an example, the deviation of charge density from one dish, containing wild-type prestin-expressing cells (12.1 ± 3 fC pF−1), to another (11.5 ± 5 fC pF−1) from the same transfection was not significant (P > 0.8).
We wanted to evaluate possible confounding factors that might arise from dialysis of the cell upon establishing whole-cell recording conditions, or due to a cGMP-triggered fast vesicular transport of prestin to the cell membrane due to delivery of cGMP from the pipette. Either of these mechanisms have the potential to influence our data. Consequently, we monitored NLC function during a 5-min time period after establishing whole-cell configuration. Only the recording pipette contained DBcGMP. The first measurement was taken at ∼5 s after membrane rupture, subsequent measurements were made 2 and 5 min later. No significant changes in charge density (derived from NLC functions) were seen whether the pipette contained a drug (P > 0.6) or not (P > 0.8). The absence of significant changes in NLC during the first 5 min suggests that our results are not affected by dialysis or fast translocation of prestin to the membrane.
Prestin function is modified by PKG agonist and antagonist
To determine whether PKG is responsible for the effects of cGMP, a blocker (8-Rp-pCPT-cGMPS; Lohmann et al. 1997) of the PKG regulatory domain was used. On the PKG regulatory subunit there are four cGMP binding sites, the activation of which leads to phosphorylation of the serine and threonine residues of the substrate proteins (Hood & Granger, 1998). The commonly used specific catalytic domain inhibitor (KT5823) has not been tested because it must be dissolved in DMSO. DMSO (0.1%) alone induces significant reduction in the charge density parameter of the NLC function (P < 0.05; data not shown).
As shown by the representative example included in Fig. 1C, application of 8-Rp-pCPT-cGMPS decreased NLC. In this experiment, the average charge density of prestin-expressing cells (control) was 13 ± 5 fC pF−1. With the application of 8-Rp-pCPT-cGMPS, the charge density decreased to 8.1 ± 4 fC pF−1 (38% decrease; P < 0.01). That 8-Rp-pCPT-GMPS alone can decrease the charge displacement of prestin suggests that the basal level of cGMP – the naturally present compound in this cell line (Bischof et al. 1997) – already has a modifying function on the motor protein and PKG is involved in this modification.
To further investigate the modifying role of cGMP in the function of prestin, different doses of DBcGMP, ranging from 50 to 600 μm, were tested. At doses up to 200 μm DBcGMP, the charge density increased, as shown in Fig. 2A. At higher doses, beyond 400 μm, the effect levelled off. Least-square fitting with the Hill equation yields a Hill coefficient of 8.4 and a Kd of 180 μm.
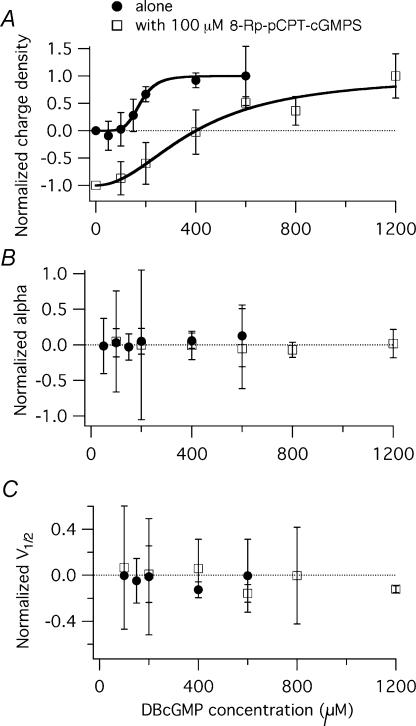
Figure 2. Dose–response relations in wild-type prestin-transfected TSA cells.
The three parameters of curve fitting of NLC functions are charge density (A), α (B) and V1/2 (C). DBcGMP application alone (•) and DBcGMP application in the presence of 100 μm 8-Rp-pCPT-cGMPS (□) are shown separately. At any concentration, data are presented as percentage of the appropriate set of control data and normalized to maximum response (±s.d., shown as percentage of the control s.d.). Experimental and control data were obtained from same-day transfection batches. Systematic effects due to DBcGMP application occur only for the charge density parameter. Results for DBcGMP application are fitted with the Hill equation: cn/(cn+Kdn). Where c is the concentration and n is the Hill coefficient. Least-square fit values are: n = 8.4 and Kd = 180 μm. Data for the combined 8-Rp-pCPT-cGMPS–DBcGMP application are also shown normalized for the daily control. A modified form of the Hill equation, having asymptotes at −1 and +1, is used to fit the data. Parameters of the least-square fit: n = 2, Kd = 401.
The comparative antagonism between DBcGMP and 8-Rp-pCPT-cGMPS on affecting translocated charges in prestin-expressing cells was examined by testing the inhibitory dose of 100 μm 8-Rp-pCPT-cGMPS against different stimulator concentrations (200, 400, 600, 800 and 1200 μm) of DBcGMP (these data are included in Fig. 2). The inhibitor was placed in the culture medium for 45 min and the activator was added to the pipette solution. The effect was evaluated by comparison between treated (8-Rp-pCPT-cGMPS + DBcGMP) and untreated prestin-expressing groups of cells from the same transfection batch. Compared to untreated prestin-expressing cells, 100 μm 8-Rp-pCPT-cGMPS decreases the charge density produced by up to approximately 400 μm of simultaneously delivered DBcGMP stimulation. However, the inhibitory effect was abolished when the DBcGMP dose was increased above 600 μm and the stimulator was able significantly to increase the charge density at doses of 800 and 1200 μm. Therefore, it is observed that prestin can be regulated through either a PKG stimulator or inhibitor. Besides charge density, other parameters of NLC were also monitored (Fig. 2B and C); these will be considered later.
Directed mutagenesis of targeted PKG sites
Response of mutants to DBcGMP
As demonstrated in Fig. 1A and B, DBcGMP (but not DBcAMP at the dose used) can significantly increase non-linear charge displacement, as measured by NLC in prestin-expressing cells. The cGMP–PGK cascade is known to regulate many biological functions. It is understood that cGMP-dependent protein kinase phosporylation sites have the consistent motif: [RK] (2)-×-[ST], where the S or T is the phosphorylation site (Bairoch et al. 1997). There are two such cGMP-dependent protein kinase phosporylation sites in the prestin protein, represented by the motifs: KRYS and KRKT, at the S238 and T560 positions, respectively. In order to ascertain the mode of action of cGMP–PKG upon prestin, mutant proteins were made in which the potential phosphorylation sites serine or threonine was replaced with the non-phosphorylatable neutral alanine at the 238 and/or the 560 positions. Thus the following mutants were made: S238A, T560A and S238A/T560A. Wild-type prestin or its associated PKG mutants were transiently transfected into TSA cells. Responses to DBcGMP application were measured by monitoring NLC. As shown in Fig. 3A–C, none of these phosphorylation-site mutants responded to the drug application. The replacement of serine or threonine with the neutral alanine at either or both 238 or 560 positions abolished the effect of DBcGMP.
Figure 3. Changes of non-linear capacitance functions after DBcGMP application for various prestin mutants.
Each curve represents the individual cell that approximately describes the mean of the population for the given experimental condition: S238A (A); T560A (B); S238A/T560A (C); C1 (D); C3 (E); and C4 (F) mutants. The drug was placed in the culture medium for 45 min and was present in the intracellular pipette solution as well. For the cells included in this graph the following values were obtained by curve fitting; probability values pertain to charge density measures. S238A: Qmax, 149/141 fC; α, 30.8/32.4 mV; V1/2, −51.4/−51.4 mV (P > 0.7); T560A: Qmax, 115/113 fC; α, 30.5/29.8 mV; V1/2, −63.7/−66.9 mV (P = 0.8); S238A/T560A: Qmax, 83/123 fC; α, 32.5/38.8 mV; V1/2, −38.5/−40.6 mV (P = 0.6); C1: Qmax, 263/215 fC; α, 32.9/29.8 mV; V1/2, −132.3/−126.8 mV (P = 0.7); C3: Qmax, 68/76 fC; α, 39.6/35 mV; V1/2, −63.3/−70.2 mV (P = 0.8); C4: Qmax, 90/55 fC; α, 32.1/37.5 mV; V1/2, −64/−73.6 mV (P < 0.05).
In theory, substitution of the non-phosphorylatable neutral amino acid alanine for S or T in the motif, or interruption of the conserved motif, mimicks an unphosphorylated state (van Balkom et al. 2002). Therefore, we also mutated the cGMP-dependent protein kinase phosphorylation sites by the replacement of the conserved positively charged amino acids arginine (R) or lysine (K) within the phosphorylation motif with the neutral amino acid glutamine (Q). The mutation cluster ‘C1’ (K233Q, K235Q, R236Q) (Fig. 3D) interrupts the conserved sequence recognized by the cGMP-dependent protein kinase at the S238 site, while the cluster ‘C3’ (K557Q, R558Q, K559Q) (Fig. 3E) alters the phosphorylation motif at T560. Cells transfected with cluster-mutant prestin C1 or C3, just as the point-mutants S238A and T560A, lost their ability to show the cGMP-mediated increase in NLC.
To be sure that the results seen did not arise from some unpredictable conformational change, which might eliminate the cGMP effect, we also measured the C4 mutant response to DBcGMP stimulation (Oliver et al. 2001). The cluster mutant C4 (R571Q, R572Q, K577Q) (Fig. 3F) was created as a double negative control. The positive amino acid sites 571/572/577 are not involved in the cGMP-dependent PKG phosphorylation process. In C4, we changed these positively charged amino acids into non-charged amino acids, similar to the modifications made in the C1 and C3 mutants. However, unlike the C1 and C3 mutants, C4 maintains the original PKG motifs. As shown in Fig. 3F, this mutant displayed all characteristics of the response of wild-type prestin to the drug application. The average charge density of C4 increased from 3.5 ± 1 fC pF−1 to 6.7 ± 4 fC pF−1 (P < 0.05), which is similar to the pattern seen for wild-type prestin in response to DBcGMP. As noted above, different transfection batches yield variable absolute values of NLC parameters. However, in comparison to the internal (same batch) control, relative changes due to manipulations are similar. This evidence suggests that the failure to respond to DBcGMP of the S238A and T560A neutral mutants, as well as the C1 and C3 mutants, is not some secondary consequence of protein misfolding.
The effects due to the cGMP analogue and the PKG blocker on wild-type prestin, and the lack of effect of DBcGMP after the elimination of the PKG phosphorylation sites, suggests that the cGMP–PKG pathway plays a major role in modifying the function of prestin, and that S238 and T560 are the cGMP-dependent phosphorylation sites.
Charge density and membrane insertion
It is also commonly thought that substitution of a negatively charged amino acid, placed at either the S or T sites, can simulate the fully phosphorylated form of the protein (Potter & Hunter, 1999). Therefore, three new mutants were produced, where the S and/or T was replaced with aspartic acid (D) (S238D, T560D and S238D/T560D). As shown in Fig. 4C, simulated phosphorylation (substitution of D) at the 238 site produced the same charge density as the wild-type measurements. In contrast, the mimicked phosphorylation of the 560 position (T560D or S238D/T560D) resulted in much lower maximum charge density values, so that, due to the poor signal-to-noise ratio, evaluation of capacitance functions was not possible.
Figure 4. Prestins subcellular localization.
A, immunofluorescence images of OK cells transiently transfected with prestin and its associated mutant proteins. Prestin is visualized with anti-C-prestin tagged with fluorescein isothiocyanate (FITC; green). Na+–K+-ATPase (rhodamine, red) and anti-golgin 97 (rhodamine, red) were used as the plasma membrane and Golgi markers. Yellow images are superimposed from green and red images, indicating the colocalization of prestin with Na+–K+-ATPases or Golgi markers. At the left side of the images we indicate the three different expression classes associated with different prestin constructs. Insets for each construct are the enlarged portions taken from the position of the cells indicated by arrows. All images were obtained with confocal microscopy. The calibration bar in all images represents 20 μm, except in the enlarged portions, where it is 5 μm. B, results of the evaluation of different expression patterns for different mutants: Class I, dense, punctate or linear staining in the perinuclear region of the cell; Class II, dense vesicular staining throughout the cell, without plasma membrane delineation; Class III, clear plasma membrane localization (wild-type-like). The cells were subjectively graded by blinded classification of ∼100 cells in each group. C, examples of NLC functions obtained from the S238D, T560D and the double mutant. Only the S238D mutation had NLC function suitable for curve fitting (Qmax, 170.7 fC; α, 44.9 mV; V1/2, −108.7 mV).
One possibility for an ensemble of prestin proteins to perform detectable but reduced function is if its ability for membrane insertion is reduced. To examine this possibility, we studied the cellular expression patterns of transiently expressed prestin and its associated mutant proteins. Immunofluorescence staining with anti-C-prestin antibodies of different organelles was studied in opossum kidney (OK) cells with confocal microscopy. OK cells were used instead of TSA cells, because their morphology allowed for better determination of intracellular localization of the antibodies. NLC measurements in TSA cells and OK cells, transfected with wild-type prestin, were similar (data not shown). For any given transfection, we observed different prestin expression patterns in different cells in the same culture dish. In order to evaluate these patterns, we subjectively grouped the cells into three different classes for each experiment (∼100 cells each) using blinded classification of cell types by examining immunofluorescence images from at least two transfection experiments for each mutant form of prestin. Class I cells were characterized by dense, punctate or linear staining in the perinuclear region of the cell. Class II cells were defined as those displaying dense vesicular staining throughout the cell, without plasma membrane delineation. Class III cells, defined as wild-type, demonstrated clear plasma membrane localization. It is worth mentioning that the efficiency of transfection among wild-type prestin and its associated mutants was similar; around 40–60% of the cells were transfected. As shown in Fig. 4B, the majority of cells transfected with T560D and S238D/T560D displayed the Class I and II phenotypes, with less than ∼10% showing signs of prestin-staining in the plasma membrane. In contrast, more than 70% of cells expressing either T560A or wild-type prestin, produced the Class III pattern. In order to determine whether the prestin mutant proteins were truly inserted into the plasma membrane, we examined prestin colocalization with Na+–K+ ATPase, a plasma membrane protein made naturally by OK cells. As shown in Fig. 4A, in a limited number of cells (designated as Class III) found for mutants T560D, or S238D/T560D, green prestin labelling demonstrates near complete colocalization in the membrane with red staining of Na+–K+ ATPase, similar to that seen for wild-type gPrestin. In contrast, S238D/T560D mutant proteins producing Class II cells did not shown such colocalization. The enlarged window in Fig. 4A shows that the Na+–K+ ATPase staining (red) was localized peripheral to the S238D/T560D protein (green), indicating that in this case the S238D/T560D protein was not inserted into the plasma membrane, even though it may have been apposed to it. Nearly 30% of T560D and S238D/T560T-expressing cells were type I cells, in which prestin mutant proteins were stocked in the Golgi apparatus as shown in Fig. 4A (upper lane) and Fig. 4B. These results suggest that replacement of T with D at the 560 site significantly impairs the ability of the mutant protein (T560D or S238D/T560D) to insert in the plasma membrane. It is unclear whether this results from the mutant prestin being in a misfolded state, or if the 560 site plays a crucial role in the membrane targeting process.
As a final step, we were interested to see how the 560 site responds to the application of DBcGMP once the S238 site has been fully phosphorylated by the substitution of aspartic acid. In other words, we tested the responsiveness of the mutant S238D to the application of DBcGMP (Fig. 5). After drug application, the charge density increased (P < 0.01). These data indicate that, once prestin was located in the plasma membrane, phosphorylation of the T560 site through a cGMP-dependent reaction could increase charge density. Conversely, the S/T ↠ A substitution at either site eliminates the influence of cGMP. This finding intimates co-operativity between the two PKG target sites. Since the expression level at the plasma membrane for both the T560D and the double–D mutation was very low, as derived from either confocal microscopy or attempted NLC measurements, their responsiveness to application of DBcGMP was impractical to evaluate.
Figure 5. DBcGMP increased the capacitance function for the S238D mutant.
The relative drug effect is shown compared to its own daily control. Symbols represent data from the individual cells that best approximate the average for the population. For the cells included in this graph the following values were obtained by curve fitting of eqn (1). Drug/control: Qmax, 141/79 fC; α, 43/45 mV; V1/2, −91/−98 mV.
Slope factor
As one can surmise from inspection of Fig. 1, application of neither DBcGMP nor 8-Rp-pCPT-cGMPS produces a significant change in slope factor (α). This impression is reinforced by examining the effects on α of applying different doses of DBcGMP, either alone or in the presence of 8-Rp-pCPT-cGMPS. As seen in Fig. 2B, changes in α are small (± 4 mV) and show no consistent trend.
As seen in Fig. 6A, the wild-type group average of α peaks around 34 ± 5 mV. When either the 238 (32 ± 2 mV) or 560 (32.5 ± 2 mV) sites are mutated to alanine, the change in α is insignificant. In contrast, full phosphorylation of the 238 site (change to aspartic acid) has profound influence; α increases (45.4 ± 5 mV; P < 0.001).
Figure 6. Parameter (α and V1/2) variation for all mutations.
Reference is the average value of the given parameter for the entire control population (n = 211; ±s.d.) for the control population. A, variation of α with mutation. Note that S238D had much higher α value (45.5 ± 5) than other mutations. B, variation of V1/2 with mutation. Several of the mutations present significant shifts. The shift for the double mutation seems to be the accumulation of shifts from the corresponding single mutations. The C1 mutation had been excluded from the V1/2 graph, as it displayed a very large shift (−134 ± 6 mV). We do not show charge density in a separate panel, because all values were within the range of ± 1 standard deviation for the wild-type population.
Peak voltage (V1/2)
Application of DBcGMP to cells transfected with wild-type prestin did not consistently shift the voltage at half-maximal charge movement of prestin (for an example of a small, +6 mV shift see Fig. 1A). As seen in the dose–response curves of Fig. 2C, shifts in V1/2 were less than 10% and did not show a consistent direction. Similarly, applying the inhibitor (8-Rp-pCPT-cGMPS) to prestin-expressing TSA cells caused a small hyperpolarization-directed shift in some batches (see Fig. 1C for example, where the shift is −5.3 mV). However, as seen in Fig. 2C, the interaction between 8-Rp-pCPT-cGMPS and DBcGMP did not produce shifts in V1/2 of a consistent nature.
In order to see how the voltage peak is influenced after the modification of the phosphorylation sites, V1/2 data had been obtained in TSA cells transfected with mutant forms of prestin (Fig. 6B). When S238 was changed to alanine, the V1/2 shifted in the depolarization direction in comparison to the wild-type. T560A showed consistent, but very small shift, within the range of wild-type data. Interestingly, the voltage shift appears to be facilitated with the dephosphorylation of both sites; the shift of S238A/T560A is about +30 mV. The mimicked phosphorylation of the 238 site (i.e. S238D) produced −30 mV shift. Dephosphorylation (S238A) of the same site produced an opposite shift (+ 20 mV). All the above-mentioned voltage shifts were significant (P < 0.001).
As mentioned before, the mutants T560D and S238D/T560D had less than 10% expression in the membrane, producing low signal-to-noise ratio of capacitance measurements. Consequently, no curve fitting and parameter estimation was attempted for these mutants.
Topology map
According to a previously proposed topology model of prestin (Zheng et al. 2001; Oliver et al. 2001), S238 is located in the third extracellular loop. Our results demonstrate that the S238 site is a cGMP-dependent phosphorylation site. As neither a PKG kinase nor ATP (required for phosphorylation) was present in the extracellular medium, it is unlikely that S238 could be located in an extracellular loop. Therefore, the loop where S238 is located should be facing the cytoplasm, instead of being extracellular. Based on this information, a new topology model is proposed (Fig. 7). In this model, previously assumed transmembraneous helices 5 and 6 are now inserted into the membrane, but do not cross it, forming re-entrant loops. Similar configurations are found in numerous cases; for example, in various transporter and exchanger proteins (Seal et al. 2000; Iwamoto et al. 2000; Grunewald et al. 2002). The N-glycosylation sites are in the second extracellular loop (Matsuda et al. 2004), while the positions of the fourth and fifth intracellular loops are determined by inserted HA tags (Oliver et al. 2001). The T560 site is on the C-terminus, which is located on the cytoplasmic side (Zheng et al. 2001). The S238 site is more closely related to the plasma membrane than the T560 site. The conserved ‘SLC26A transporter signature’ is present in the second transmembrane domain (us.expasy.org/prosite), while a STAS motif (Aravind & Koonin, 2000) and positive and negative clusters of amino acids are located in the C-terminus tail. Although the functions of these domains are not fully understood, there is some evidence suggesting that the C-terminus can interact with other proteins (Ko et al. 2002, 2004).
Figure 7. Modified, new topology map of prestin.
What was previously suggested to be the third extracellular loop (as in Zheng et al. 2001; Oliver et al. 2001) – where the S238 site is located – had been changed so that it should face the cytoplasm. This alteration necessitated changing the fifth and sixth helices, which are now inserted into the membrane, without crossing it. The T560 site is on the C-terminus, which is located on the cytoplasmic side.
Discussion
Evidence for cGMP effect in a heterologous expression system
In the present paper, we show that DBcGMP can modify the function of the motor protein of OHCs. The effectiveness of affecting the electromotility function via the cGMP–PKG cascade, as opposed to cAMP–PKA, was suggested earlier by Szönyi et al. (1999). We were able to confirm their observations, because the motor protein failed to respond to DBcAMP at the same 200 μm test dose as DBcGMP. Higher concentrations of cAMP were not tested to avoid any cross reaction with the GMP/PKG cascade (Pelligrino & Wang, 1998).
The DBcGMP effect on prestin had been confirmed by three different means. First, the application of a cGMP analogue was able significantly to increase the electrically evoked charge displacement of prestin in TSA 201 cells. Second, a PKG blocker decreased the NLC of prestin. Third, the cGMP-effect was abolished when putative PKG target motifs at the S238 or T560 sites were interrupted by either placing alanine at those positions, or by an alteration of the phosphorylation motif. The evidence clearly suggests that the cGMP–PKG pathway plays a major role in modifying the function of prestin, and S238 and T560 are cGMP-dependent phosphorylation sites.
Is prestin directly phosphorylated or are there companion molecules involved?
Recording of increased NLC from wild-type prestin after DBcGMP application can occur due to three causes. (1) The cGMP–PKG cascade modifies the function of prestin that is already inserted into the membrane. (2) The cascade facilitates the fast translocation of these molecules into the plasma membrane, thereby increasing the total number of prestin molecules in the plasma membrane (similar to the telokin protein reported by Komatsu et al. (2002)). (3) The phosphorylation of some other protein might influence the NLC function of prestin. As several prestin mutants failed to increase their response to the cGMP analogue, this third possibility is unlikely.
Whether the increased charge density occurs due to more prestin insertion, or action on the same amount of protein but with better performance, is still unknown. The possibility of cGMP-triggered vesicular transport cannot be ruled out a priori. However, the experiment in which competitive antagonism was shown between 8-Rp-pCPT-cGMPS and DBcGMP argues against fast vesicular transport as a plausible mechanism. To explain, in these experiments there was no time for a fast translocation to take place as after establishing whole cell recording – which usually takes less than 5 s in our hands – the full DBcGMP effect could be seen. According to reports, ‘fast translocation’ occurs on the minute time scale (Komatsu et al. 2002).
Interaction between the 238 and 560 sites
When one of prestin's phosphorylation sites was made to mimic full phosphorylation (S238D), increased charge density value was still seen after DBcGMP application. This finding indicates that the functional consequence of phosphorylation (increased charge density) can be mediated by the 560 site, inasmuch as the S238 site was already fully phosphorylated. Conversely, the S/T ↠ A substitution at either site eliminates the influence of cGMP. This finding intimates co-operativity between the two PKG target sites.
The T560D and the double negative-charge (DD) mutations of prestin showed less than 10% membrane insertion of the proteins. It is unclear whether this results from the mutant prestin being in a misfolded state, or if the 560 site plays a crucial role in the membrane targeting process. Phosphorylation of specific amino acids needs to occur at appropriate time points during the maturation of the protein. For example, phosphorylation must take place after aquaporin-2 has left the endoplasmic reticulum, but prior to delivery to the plasma membrane (van Balkom et al. 2002; Procino et al. 2003). Conversely, there are also some examples indicating that cGMP-dependent phosphorylation occurs after membrane insertion (Vaandrager et al. 1998). Clearly, the roles of phosphorylation in regulating a protein's functions, including its ability to insert into the cell membrane, are complex and not fully understood. The effects of cGMP-dependent phosphorylation at the T560 site on membrane-targeting, while clearly present, need further investigation.
Slope factor
The gain factor, 1/α, characterizes the voltage dependence of the capacitance function, and is determined by the interaction between the properties of the putative anion binding site and the anion being bound (Oliver et al. 2001). For example, Oliver et al. (2001) demonstrated that halides and carboxylic acids could substitute for Cl− in eliciting NLC. The slope factor was related to the chain length of the carboxylic acids. In general, the larger the value of α, the larger voltage is necessary to translocate a unit charge across the cell membrane. As shown above (Fig. 2B), application of DBcGMP or interaction between DBcGMP and 8-Rp-pCPT-cGMPS had an extremely modest, non-systematic, effect on α, amounting to at most a few millivolts. In contrast, mutations of the molecule that in theory simulate full phosphorylation had larger effects, changing α by as much as 10 mV (Fig. 6A). The different values of α observed for the S238 mutant cannot be simply explained by an increase/decrease of charged residues corresponding to phosphorylation/dephosphorylation or by the replacement of S/T with the negatively charged amino acid D. The 238 site is close to regions where positively charged amino acid groups are located. The cluster mutations C1 and C3 were made to substitute neutral amino acids for these positive residues (Oliver et al. 2001; and present data). It is interesting that the slope factors of the C1 and C3 mutants were not significantly different from those of wild-type prestin. Thus the tentative conclusion is reached that it is unlikely that the behaviour of the chloride-binding site would be determined by these positively charged amino acid groups. Although we do not understand how S238D mutation changes α, or know where the actual chloride-binding site is on the prestin molecule, it is possible that the S238 site could relate to the binding region or modify binding efficiency.
Operating region (V1/2)
We recall that forcing target proteins in OHCs to be in their phosphorylated state by adding okadaic acid – a non-specific inhibitor of native phosphatases – shifted the V1/2 to the hyperpolarization direction by ∼20 mV (Frolenkov et al. 2000, 2001). Our mutation, where the phosphorylated state of prestin was mimicked (S238D), displayed similar directional shift with somewhat higher value, ∼30 mV. To create dephosphorylation of proteins associated with the motor process, W-7 and trifluoperazine – commonly used promoters of protein dephosphorylation – had been used previously in OHCs (Frolenkov et al. 2000). The result was a voltage shift in the depolarization direction (∼30 mV), which is similar to our finding with the S238A and the S238A/T560A mutants. To restate, achieving full phosphorylation (dephosphorylation) of the S238 site by its mutation, or the application of phosphorylation (dephosphorylation) promoting agents, produce complementary and significant V1/2 shifts in the hyperpolarization (depolarization) directions. In contrast, producing phosphorylation (dephosphorylation), by application of DBcGMP (8-Rp-pCPT-cGMPS), moves V1/2 at most minimally, and not in a consistent manner. One may conclude that the results of Frolenkov et al. (2000) can be due to the effect of the agents used on prestin or prestin-related proteins, but not exclusively on the PKG sites. Aside from the two PKG phosphorylation sites, prestin possesses five potential PKC motifs and one potential tyrosine phosphorylation motif. General agents that antagonize phosphatases or produce dephosphorylation will affect all phosphorylation processes of the molecule. As a result, their overall effect will be more pronounced, and can drive variables in the opposing direction, than if only specific sites are involved. Consequently, the larger voltage shifts seen in the experiments of Frolenkov et al. (2000) need not be in conflict with our DBcGMP dose–response data. To further amplify these points, we note that Frolenkov and colleagues made their measurements in OHCs. This has two consequences. First, inasmuch as charge density is considerably in excess in the native cell versus in a transiently transfected one, all effects seen might be greater in the former. Second, prestin has reciprocal (piezoelectric) performance (Santos-Sacchi et al. 2001; Ludwig et al. 2001). Phosphorylation and dephosphorylation in OHCs affect not only the molecule but cytoskeletal elements as well (Dallos et al. 1997; He et al. 2003). Recent evidence shows that Rho GTPases can modify the length changes of OHCs without affecting the motor molecules (Kalinec et al. 2000; Zhang et al. 2003). Changes in the mechanical state of the cytoskeleton will influence the mechanical state of prestin, which, due to its reciprocal behaviour, will affect charge displacement (Gale & Ashmore, 1994; Kakehata & Santos-Sacchi, 1995). Such influence is not expected to be present in TSA cells, which have very poorly developed cytoskeletal network (data not shown).
Mutations of the targeted PKG-dependent phosphorylation sites significantly shifted the voltage sensitivity. The S238 site was particularly sensitive to mutations, producing −30 mV (hyperpolarization-directed) shift for the S ↠ D substitution and +20 mV (depolarization-directed) for the S ↠ A change. Interestingly, the S238A/T560A mutation produced a cumulative shift of +30 mV (with T560A contributing ∼10 mV; see Fig. 6B). All these mutation-related shifts are vastly in excess to those that can be produced by phosphorylation via application of DBcGMP, or dephosphorylation via application of 8-Rp-pCPT-cGMPS. To explain, using change in charge density as a measure of degree of phosphorylation (Fig. 2A), the DBcGMP effect does saturate, implying that the effects of cGMP and native phosphatases are in equilibrium. Even at saturation though, the V1/2 shift is essentially non-existent, to be compared to that seen with simulated full phosphorylation of the 238 site. Conversely Fig. 2A, when 8-Rp-pCPT-cGMPS is applied, implies asymptotic behaviour in the charge density measure. Yet, there is no corresponding shift in V1/2, as one might expect from the result obtained from mutation S238A/T560A (Fig. 6B). Thus one is tempted to conclude that, in spite of the common assumption (Potter et al. 1999) that the result of the S/T ↠ A or S/T ↠ D substitutions is the stimulation of full dephosphorylation or phosphorylation, they in fact do more. It is possible that such modifications of the protein alter its function in other ways, so that its voltage sensitivity is significantly modified.
We conclude, with the above caveat in mind, that phosphorylation of prestin via the cGMP–PKG cascade significantly modulates the amount of charge moved, but only insignificantly, if at all, affects other parameters (α, V1/2) of the electromotile process. Thus the cGMP cascade can exert a straightforward modulatory effect upon prestin-mediated electromotility and is probably a significant component of slow efferent modulation of the state of OHCs. Finally, we note that the establishment of S238 as a site of phosphorylation, dictates a revision of prestin's previously suggested topology (Zheng et al. 2001; Oliver et al. 2001). Inasmuch as S238 needs to be in a cytoplasmic location, helices 5 and 6, previously considered membrane-spanning, are now assumed to form re-entrant loops.
Acknowledgments
We thank Professor Péter Csermely of the Faculty of Medicine Institute of Medical Chemistry, Semmelweis University, Budapest, Hungary and our colleague, Dr Enrique Navarrete for their contributions to this study. We also thank Dr W. Russin at the Biological Imaging Facility of Northwestern University for his help in image processing, and Dr D. Oliver for providing the C3 and C4 constructs. This work was supported by National Institutes of Health Grant DC00089.
References
- Aravind L, Koonin EV. The STAS domain – a link between anion transporters and antisigma-factor antagonists. Curr Biol. 2000;10:53–55. doi: 10.1016/s0960-9822(00)00335-3. 10.1016/S0960-9822(00)00335-3. [DOI] [PubMed] [Google Scholar]
- Ashmore JF. A fast motile response in guinea-pig outer hair cells: the cellular basis of the cochlear amplifier. J Physiol. 1987;388:323–347. doi: 10.1113/jphysiol.1987.sp016617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore JF. Forward and reverse transduction in the mammalian cochlea. Neurosci Res Suppl. 1990;12:S39–S50. doi: 10.1016/0921-8696(90)90007-p. 10.1016/0921-8696(90)90007-P. [DOI] [PubMed] [Google Scholar]
- Ashmore JF. Mammalian hearing and the cellular mechanisms of the cochlear amplifier. Soc Gen Physiol Ser. 1992;47:395–412. [PubMed] [Google Scholar]
- Bairoch A, Apweiler R. The SWISS-PROT protein sequence database: its relevance to human molecular medical research. J Mol Med. 1997;75:312–316. [PubMed] [Google Scholar]
- Beavo JA, Laurence L, Brunton LL. Cyclic nucleotide research-still expanding after half century. Nature. 2002;3:710–718. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- Bischof G, Serwold TF, Machen TE. Does nitric oxide regulate capacitative Ca influx in HEK 293 cells? Cell Calcium. 1997;2:135–142. doi: 10.1016/s0143-4160(97)90037-3. [DOI] [PubMed] [Google Scholar]
- Brownell WE, Bader CR, Bertrand D, de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985;227:194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- Dallos P. The active cochlea. J Neurosci. 1992;12:4575–4585. doi: 10.1523/JNEUROSCI.12-12-04575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P, Evans BN. High-frequency motility of outer hair cells and the cochlear amplifier. Science. 1995;267:2006–2009. doi: 10.1126/science.7701325. [DOI] [PubMed] [Google Scholar]
- Dallos P, Fakler B. Prestin, a new type of motor protein. Nat Rev Mol Cell Biol. 2002;2:104–111. doi: 10.1038/nrm730. 10.1038/nrm730. [DOI] [PubMed] [Google Scholar]
- Dallos P, Harris D. Properties of auditory nerve responses in absence of outer hair cells. J Neurophysiol. 1978;41:365–383. doi: 10.1152/jn.1978.41.2.365. [DOI] [PubMed] [Google Scholar]
- Dallos P, He DZZ, Lin X, Sziklai I, Mehta S, Evans BN. Acetylcholine, outer hair cell electromotility, and the cochlear amplifier. J Neurosci. 1997;17:2212–2226. doi: 10.1523/JNEUROSCI.17-06-02212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis H. An active process in cochlear mechanics. Hear Res. 1983;9:79–90. doi: 10.1016/0378-5955(83)90136-3. 10.1016/0378-5955(83)90136-3. [DOI] [PubMed] [Google Scholar]
- Eybalin M. Neurotransmitters and neuromodulators of the mammalian cochlea. Physiol Rev. 1993;73:309–373. doi: 10.1152/physrev.1993.73.2.309. [DOI] [PubMed] [Google Scholar]
- Flock Å. Transducing mechanisms in lateral line canal organ receptors. Cold Spring Harb Symp Quant Biol. 1965;30:133–145. doi: 10.1101/sqb.1965.030.01.016. [DOI] [PubMed] [Google Scholar]
- Flock Å, Flock B, Ulfendahl M. Mechanisms of movement in outer hair cells and a possible structural basis. Arch Otolaryngol. 1986;243:83–90. doi: 10.1007/BF00453755. [DOI] [PubMed] [Google Scholar]
- Frolenkov GI, Mammano F, Belyantseva IA, Coling D, Kachar B. Two distinct Ca2+-dependent signaling pathways regulate the motor output of cochlear outer hair cells. J Neurosci. 2000;20:5940–5948. doi: 10.1523/JNEUROSCI.20-16-05940.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolenkov GI, Mammano F, Kachar B. Action of 2,3-butanedione monoxime on capacitance and electromotility of guinea-pig cochlear outer hair cells. J Physiol. 2001;531:667–676. doi: 10.1111/j.1469-7793.2001.0667h.x. 10.1111/j.1469-7793.2001.0667h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale JE, Ashmore JF. Charge displacement induced by rapid stretch in the basolateral membrane of the guinea-pig outer hair cell. Proc R Soc Lond B Biol Sci. 1994;255:243–249. doi: 10.1098/rspb.1994.0035. [DOI] [PubMed] [Google Scholar]
- Grunewald M, Menaker D, Kanner BI. Cysteine-scanning mutagenesis reveals a conformationally sensitive reentrant pore-loop in the glutamate transporter GLT-1. J Biol Chem. 2002;277:26074–26080. doi: 10.1074/jbc.M202248200. 10.1074/jbc.M202248200. [DOI] [PubMed] [Google Scholar]
- Harrison RV, Evans EF. Cochlear fiber responses in guinea pigs with well defined cochlear lesions. Scand Audiol Suppl. 1979;9:83–92. [PubMed] [Google Scholar]
- He DZZ, Jia S, Dallos P. Prestin and the dynamic stiffness of cochlear outer hair cells. J Neurosci. 2003;23:9089–9096. doi: 10.1523/JNEUROSCI.23-27-09089.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley M. High frequency force generation in outer hair cells from the mammalian ear. Bioessays. 1991;13:115–120. doi: 10.1002/bies.950130304. [DOI] [PubMed] [Google Scholar]
- Holley MC, Ashmore JF. On the mechanism of a high-frequency force generator in outer hair cells isolated from the guinea pig cochlea. Proc R Soc Lond B Biol Sci. 1988;232:413–429. doi: 10.1098/rspb.1988.0004. [DOI] [PubMed] [Google Scholar]
- Holley MC, Kalinec F, Kachar B. Structure of the cortical cytoskeleton in mammalian outer hair cells. J Cell Sci. 1992;102:569–580. doi: 10.1242/jcs.102.3.569. [DOI] [PubMed] [Google Scholar]
- Hood J, Granger HJ. Protein kinase G mediates vascular endothelial growth factor-induced Raf-1 activation and proliferation in human endothelial cells. J Biol Chem. 1998;273:23504–23508. doi: 10.1074/jbc.273.36.23504. 10.1074/jbc.273.36.23504. [DOI] [PubMed] [Google Scholar]
- Huang G, Santos-Sacchi J. Mapping the distribution of the outer hair cell motility voltage sensor by electrical amputation. J Biophys. 1993;65:2228–2236. doi: 10.1016/S0006-3495(93)81248-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth AJ, Corey DP. Sensitivity, polarity, and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proc Natl Acad Sci U S A. 1977;74:2407–2411. doi: 10.1073/pnas.74.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto T, Uehara A, Imanaga I, Shigekawa M. The Na+/Ca2+ exchanger NCX1 has oppositely oriented reentrant loop domains that contain conserved aspartic acids whose mutation alters its apparent Ca2+ affinity. J Biol Chem. 2000;275:38571–38580. doi: 10.1074/jbc.M003788200. 10.1074/jbc.M003788200. [DOI] [PubMed] [Google Scholar]
- Kachar B, Brownell WE, Altschuler R, Fex J. Electrokinetic shape changes of cochlear outer hair cells. Nature. 1986;322:365–368. doi: 10.1038/322365a0. 10.1038/322365a0. [DOI] [PubMed] [Google Scholar]
- Kakehata S, Santos-Sacchi J. Membrane tension directly shifts voltage dependence of outer hair cell motility and associated gating charge. J Biophys. 1995;68:2190–2197. doi: 10.1016/S0006-3495(95)80401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinec F, Zhang M, Urrutia R, Kalinec G. Rho GTPases mediate the regulation of cochlear outer hair cell motility by acetylcholine. J Biol Chem. 2000;275:28000–28005. doi: 10.1074/jbc.M004917200. [DOI] [PubMed] [Google Scholar]
- Ko SB, Shcheynikov N, Choi JY, Luo X, Ishibashi K, Thomas PJ, Kim JY, Kim KH, Lee MG, Naruse S, Muallem S. A molecular mechanism for aberrant CFTR-dependent HCO3 transport in cystic fibrosis. EMBO J. 2002;21:5662–5672. doi: 10.1093/emboj/cdf580. 10.1093/emboj/cdf580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko SB, Zeng W, Dorwart MR, Luo X, Kim KH, Millen L, Goto H, Naruse S, Soyombo A, Thomas PJ, Muallem S. Gating of CFTR by the STAS domain of SLC26 transporters. Nat Cell Biol. 2004;6:343–350. doi: 10.1038/ncb1115. 10.1038/ncb1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu S, Miyazaki K, Tuft RA, Ikebe M. Translocation of telokin by cGMP signaling in smooth muscle cells. Am J Physiol Cell Physiol. 2002;283:C752–C761. doi: 10.1152/ajpcell.00501.2001. [DOI] [PubMed] [Google Scholar]
- Lohmann SM, Vaandrager AB, Smolenski A, Walter U, De Jonge HR. Distinct and specific functions of cGMP-dependent protein kinases. Trends Biochem Sci. 1997;22:307–312. doi: 10.1016/s0968-0004(97)01086-4. 10.1016/S0968-0004(97)01086-4. [DOI] [PubMed] [Google Scholar]
- Ludwig J, Oliver D, Frank G, Klöcker N, Gummer AW, Fakler B. Reciprocal electromechanical properties of rat prestin: the motor molecule from rat outer hair cells. Proc Natl Acad Sci U S A. 2001;98:4178–4183. doi: 10.1073/pnas.071613498. 10.1073/pnas.071613498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K, Zheng J, Du GG, Klöcker N, Madison LD, Dallos P. N-linked glycosylation sites of the motor protein prestin: effects on membrane targeting and electrophysiological function. J Neurochem. 2004;89:928–938. doi: 10.1111/j.1471-4159.2004.02377.x. [DOI] [PubMed] [Google Scholar]
- Oliver D, Fakler B. Expression density and functional characteristics of the outer hair cell motor protein are regulated during postnatal development in rat. J Physiol. 1999;519:791–800. doi: 10.1111/j.1469-7793.1999.0791n.x. 10.1111/j.1469-7793.1999.0791n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D, He DZZ, Klöcker N, Ludwig J, Schulte U, Waldegger S, Ruppersberg JP, Dallos P, Fakler B. Intracellular anions as the voltage sensor of prestin, the outer hair cell motor protein. Science. 2001;292:2340–2343. doi: 10.1126/science.1060939. 10.1126/science.1060939. [DOI] [PubMed] [Google Scholar]
- Pelligrino DA, Wang Q. Cyclic nucleotide crosstalk and the regulation of cerebral vasodilation. Prog Neurobiol. 1998;56:1–18. doi: 10.1016/s0301-0082(98)00009-4. 10.1016/S0301-0082(98)00009-4. [DOI] [PubMed] [Google Scholar]
- Potter LR, Hunter T. Identification and characterization of the phosphorylation sites of the guanylyl cyclase-linked natriuretic peptide receptors A and B. Methods. 1999;19:506–520. doi: 10.1006/meth.1999.0893. 10.1006/meth.1999.0893. [DOI] [PubMed] [Google Scholar]
- Procino G, Carmosino M, Marin O, Brunati AM, Contri A, Pinna LA, Mannucci R, Nielsen S, Kwon TH, Svelto M, Valenti G. Ser-256 phosphorylation dynamics of Aquaporin 2 during maturation from the ER to the vesicular compartment in renal cells. FASEB J. 2003;17:1886–1888. doi: 10.1096/fj.02-0870fje. [DOI] [PubMed] [Google Scholar]
- Ryan A, Dallos P. Effect of absence of cochlear outer hair cells on behavioural auditory threshold. Nature. 1975;253:44–46. doi: 10.1038/253044a0. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J. Reversible inhibition of voltage-dependent outer hair cell motility and capacitance. J Neurosci. 1991;11:3096–3110. doi: 10.1523/JNEUROSCI.11-10-03096.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Sacchi J, Dilger JP. Whole cell currents and mechanical responses of isolated outer hair cells. Hear Res. 1988;35:143–150. doi: 10.1016/0378-5955(88)90113-x. 10.1016/0378-5955(88)90113-X. [DOI] [PubMed] [Google Scholar]
- Santos-Sacchi J, Shen W, Zheng J, Dallos P. Effects of membrane potential and tension on prestin, the outer hair cell lateral membrane motor protein. J Physiol. 2001;531:661–666. doi: 10.1111/j.1469-7793.2001.0661h.x. 10.1111/j.1469-7793.2001.0661h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Kawatani M. Dibutyryl cGMP raises cytosolic concentrations of Ca2+ in cultured nodose ganglion neurons of the rabbit. Brain Res. 1998;813:203–206. doi: 10.1016/s0006-8993(98)01021-x. 10.1016/S0006-8993(98)01021-X. [DOI] [PubMed] [Google Scholar]
- Seal RP, Leighton BH, Amara SG. A model for the topology of excitatory amino acid transporters determined by the extracellular accessibility of substituted cysteines. Neuron. 2000;25:695–706. doi: 10.1016/s0896-6273(00)81071-5. 10.1016/S0896-6273(00)81071-5. [DOI] [PubMed] [Google Scholar]
- Sziklai I, He DZZ, Dallos P. Effect of acetylcholine and GABA on the transfer function of electromotility in isolated outer hair cells. Hear Res. 1996;95:87–99. doi: 10.1016/0378-5955(96)00026-3. 10.1016/0378-5955(96)00026-3. [DOI] [PubMed] [Google Scholar]
- Sziklai I, Szönyi M, Dallos P. Phosphorylation mediates the influence of acetylcholine upon outer hair cell electromotility. Acta Otolaryngol. 2001;121:153–156. doi: 10.1080/000164801300043280. 10.1080/000164801300043280. [DOI] [PubMed] [Google Scholar]
- Szönyi M, He DZZ, Ribari O, Sziklai I, Dallos P. Cyclic GMP and outer hair cell electromotility. Hear Res. 1999;137:29–42. doi: 10.1016/s0378-5955(99)00127-6. 10.1016/S0378-5955(99)00127-6. [DOI] [PubMed] [Google Scholar]
- Vaandrager AB, Smolenski A, Tilly BC, Houtsmuller AB, Ehlert EM, Bot AG, Edixhoven M, Boomaars WE, Lohmann SM, de Jonge HR. Membrane targeting of cGMP-dependent protein kinase is required for cystic fibrosis transmembrane conductance regulator Cl− channel activation. Proc Natl Acad Sci U S A. 1998;95:1466–1471. doi: 10.1073/pnas.95.4.1466. 10.1073/pnas.95.4.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Balkom BW, Savelkoul PJ, Markovich D, Hofman E, Nielsen S, van der Sluijs P, Deen PM. The role of putative phosphorylation sites in the targeting and shuttling of the aquaporin-2 water channel. J Biol Chem. 2002;277:41473–41479. doi: 10.1074/jbc.M207525200. 10.1074/jbc.M207525200. [DOI] [PubMed] [Google Scholar]
- von Békésy G. Experiments in Hearing. New York: McGraw-Hill; 1960. [Google Scholar]
- Zenner HP, Zimmermann U, Schmitt U. Reversible contraction of isolated mammalian cochlear hair cells. Hear Res. 1985;18:127–133. doi: 10.1016/0378-5955(85)90004-8. 10.1016/0378-5955(85)90004-8. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kalinec GM, Urrutia R, Billadeau DD, Kalinec F. Rock-dependent and Rock-independent control of cochlear outer hair cell electromotility. J Biol Chem. 2003;278:35644–35650. doi: 10.1074/jbc.M301668200. 10.1074/jbc.M301668200. [DOI] [PubMed] [Google Scholar]
- Zheng J, Long KB, Shen W, Madison LD, Dallos P. Prestin topology: localization of protein epitopes in relation to the plasma membrane. Neuroreport. 2001;12:1929–1935. doi: 10.1097/00001756-200107030-00032. 10.1097/00001756-200107030-00032. [DOI] [PubMed] [Google Scholar]
- Zheng J, Shen W, He DZZ, Long KB, Madison LD, Dallos P. Prestin is the motor protein of cochlear outer hair cells. Nature. 2000;405:149–155. doi: 10.1038/35012009. 10.1038/35012009. [DOI] [PubMed] [Google Scholar]