Abstract
Non-adrenergic, non-cholinergic (NANC) inhibitory neurotransmission has been an area of intense interest in gut motor physiology, whereas excitatory NANC neurotransmission has received less attention. In order to further explore excitatory NANC neurotransmission, we performed conventional intracellular recordings from guinea-pig taenia caeci smooth muscle. Tissue was perfused with oxygenated Krebs solution at 35°C and nerve responses evoked by either oral or aboral nerve stimulation (NS) (4 square wave pulses, 0.3 ms duration, 20 Hz). Electrical activity was characterized by slow waves upon which one to three action potentials were superimposed. Oral NS evoked an inhibitory junction potential (IJP) at either the valley or peak of the slow wave. Application of nifedipine (1 μm) abolished slow waves and action potentials, but membrane potential flunctuations (1–3 mV) and IJPs remained unaffected. Concomitant application of apamin (300 nm), a small-conductance Ca2+-activated K+ channel blocker, converted the IJP to an EJP that was followed by slow IJP. Further administration of NG-nitro-l-arginine methyl ester (l-NAME, 200 μm), a nitric oxide synthase inhibitor, abolished the slow IJP without affecting the EJP, implying that the slow IJP is due to nitrergic innervation. The EJP was abolished by tetrodotoxin (1 μm), but was not significantly affected by atropine (3 μm) and guanethidine (3 μm) or hexamethonium (500 μm). Substance P (SP, 1 μm) desensitization caused slight attenuation of the EJP, but the EJP was abolished by desensitization with α,β-methylene ATP (50 μm), a P2 purinoceptor agonist that is more potent than ATP at the P2X receptor subtype, suramin (100 μm), a non-selective P2 purinoceptor antagonist, and pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS, 100 μm), a selective P2X purinoceptor antagonist. In contrast, the EJP was unaffected by MRS-2179 (2 μm), a selective P2Y1 receptor antagonist. Aboral NS evoked an apamin- and l-NAME-sensitive IJP, but virtually no NANC EJP. These data suggest the presence of polarized excitatory purinergic neurotransmission in guinea-pig taenia caeci, which appears to be mediated by P2X purinoceptors, most likely the P2X1 subtype.
As the taenia caeci is a readily accessible longitudinal smooth muscle on the surface of the colon of several species, it has been widely used to study the neural control of GI smooth muscle. A non-cholinergic, non-adrenergic (NANC) postsynaptic inhibitory junction potential (IJP) was first described in this tissue in guinea-pigs (Burnstock et al. 1963), which was a major breakthrough in our understanding of the mechanisms of GI smooth muscle regulation by autonomic nerves. It was later proven that the NANC IJP was mediated by ATP or related nucleotides (Burnstock et al. 1970; Sneddon et al. 1973). The discovery of a NANC IJP broke the classical paradigm of the autonomic nervous system in which only two substances, namely acetylcholine and noradrenaline, were thought to mediate all transmission between nerve and muscle, as well as between neurones. Since then, extensive studies have added more substances, such as nitric oxide (NO), vasoactive intestinal peptide, carbon monoxide and pituitary adenylyl cyclase-activating peptide, to the candidates that mediate a NANC IJP (Bennett, 1997).
The cholinergically mediated excitatory junction potential (EJP) has been extensively studied in smooth muscle cells of the GI tract (Bridgewater et al. 1995; Bennett, 1997). In contrast, the excitatory NANC neurotransmission has received less attention. Tachykinins, such as SP, have been shown to be involved in excitatory neurotransmission in a number of GI smooth muscles (Niel et al. 1983; Krysiak & Preiksaitis, 2001). Although ATP is well characterized as a NANC inhibitory neurotransmitter in GI smooth muscle, it also has an important role as an excitatory neurotransmitter in other smooth muscles (Burnstock & Holman, 1964; Burnstock et al. 1972; Holman et al. 1977; Hoyle & Burnstock, 1985; Kennedy, 1996). On the other hand, purinergic excitatory neurotransmission is less well defined in GI smooth muscle. Shuba & Vladimirova (1980) reported the presence of a NANC EJP in guinea-pig caecal smooth muscle that was only apparent when apamin was used to block the predominant IJP. Because they could also demonstrate that exogenous ATP evoked depolarization in the presence of apamin, they suggested that this NANC EJP may be purinergic in origin. Subsequently, others have confirmed dual actions (excitatory and inhibitory) of exogenous ATP in GI smooth muscle (Huizinga et al. 1981). Using the sucrose-gap technique, Zagorodnyuk & Maggi (1998) found evidence of a suramin- and PPADS-sensitive EJP in circular muscle of guinea-pig colon that was only demonstrable when both apamin and TEA were present in the bath. In addition, Bridgewater et al. (1995) noted atropine-resistent EJPs in guinea-pig taenia caeci, but it was unclear whether this might be due to a residual cholinergic effect, tachykinins, or some other unidentified neurotransmitter.
Although somewhat inconsistent, when taken together the aforementioned observations suggest that purinergic nerves are involved in NANC excitation of GI smooth muscle. However, the precise nature of this remains uncertain. In the aforementioned studies, polarity of the NANC EJP was not looked for, which could at least partly explain the inconsistent results. The goal of the current study therefore was to further characterize the putative purinergic excitatory neurotransmission in GI smooth muscle and determine whether this innervation is polarized.
Methods
Tissue preparation and conventional intracellular recordings
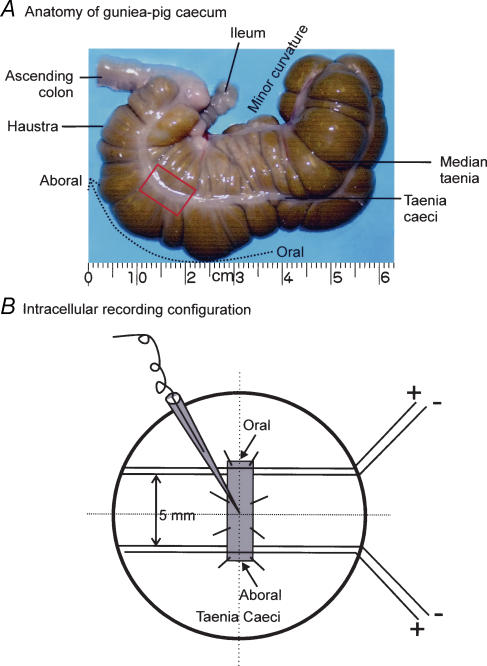
The protocol was approved by the Animal Care Committee of Queen's University. Guinea-pigs of either of sex and weighing between 50 and 100 g were killed by decollation after isoflurane anaesthesia. The abdominal cavity was then exposed via a mid-line incision and part of the taenia caecum was dissected free from the caecum. This muscle layer was visible as a distinct thickening of longitudinal muscle in the resultant tissue (Fig. 1A). Strips of taenia caeci were pinned on the bottom of a recording chamber covered by Sylgard (Dow Corning) and perfused at 2.1 ml min−1 with preoxygenated Krebs solution at 35°C (Zhang & Paterson, 2003). Orientation of strips was marked as depicted in Fig. 1A and B. Nerve stimulation using either one or four square wave pulses (20 Hz) with a duration of 0.3 ms and voltage of 70 V was delivered to the muscle preparation by two pairs of silver wires placed at oral and aboral ends of the tissue (Fig. 1B), while electrical activity was recorded using conventional intracellular electrodes as previously described (Zhang & Lang, 1994; Zhang & Paterson, 2001). Data depicted in Figs 2, 3, 4, 5, 6, 7, 8 are derived from oral stimulation, whereas data in Fig. 9 are derived from oral and aboral stimulations. Previously defined electrical parameters were used to quantitatively analyse the electrical properties of the smooth muscle (Zhang & Paterson, 2001).
Figure 1. Anatomy of taenia caecum and intracellular recording configuration.
A, photograph of guinea-pig caecum. The taenia caecum is clearly visible as a distinct thickening of longitudinal muscle, with the aboral end being the portion closest to the ascending colon. The framed area represents the portion dissected free for intracellular recording. B, intracellular recording set up. Two pairs of silver wires were placed at the oral and aboral ends of the tissue 5 mm far from each other. Intracellular recording was made in the middle of two pairs of stimulating electrodes. In all figures except Fig. 9, recordings depict results obtained with oral NS.
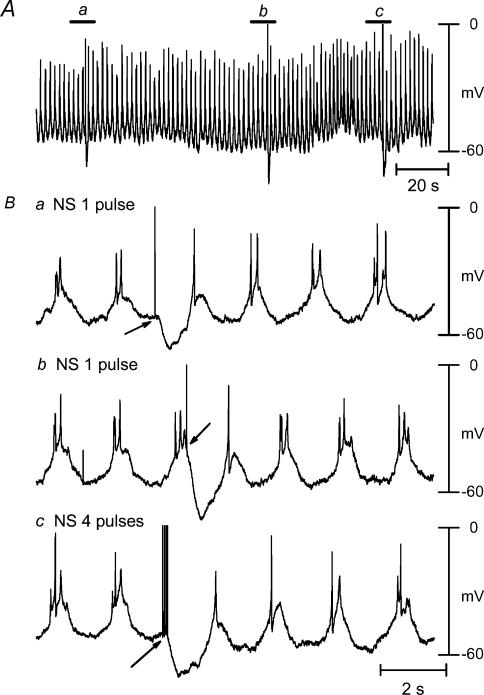
Figure 2. Properties of electrical activity recorded in smooth muscle of guinea-pig taenia caeci.
A, raw recordings of spontaneous action potentials. B, original recordings from panel A at expanded time scale. Ba and b, IJP evoked by 1 pulse of oral NS at either valley or peak, respectively, of slow wave. Bc, an IJP induced by 4 pulses. Arrows indicated nerve stimulation. Smooth muscle of taenia caeci was characterized by slow waves on which 1–3 spontaneous action potentials were superimposed.
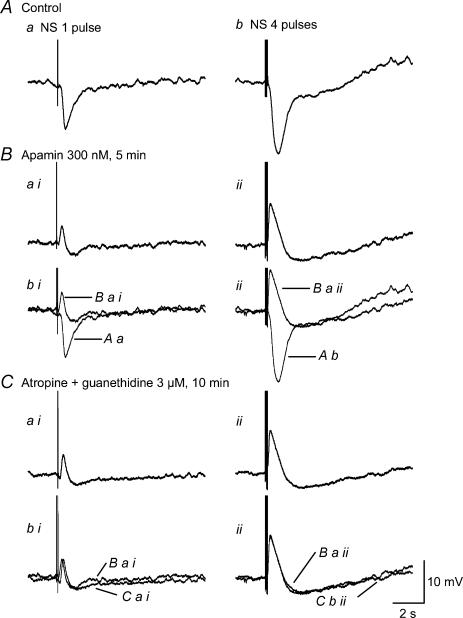
Figure 3. Pharmacological isolation of an EJP induced by oral NS resistant to atropine and guanethidine in the presence of nifedipine.
Aa, 1 pulse NS. Bb, 4 pulse NS. The stronger (4 pulse) NS produced a larger fast IJP, followed by a slow IJP and postinhibitory EJP. Bai and ii, junction potentials induced by NS of 1 and 4 pulses 5 min after application of apamin. Bbi and ii, junction potentials superimposed before (panel A) and after apamin. Cai and ii, junction potentials 10 min after atropine and guanethidine in the presence of nifedipine and apamin. Cbi and ii, overlapped junction potentials from panel Ba and panel Ca. Application of apamin converted the IJP to an EJP followed by a slow IJP, which was not affected by concomitant application of atropine and guanethidine.
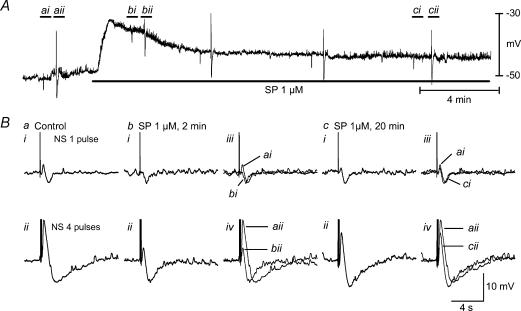
Figure 4. Effects of SP desensitization on the NANC EJP in the presence of nifedipine, apamin, atropine and guanethidine.
A, raw recordings of resting membrane potential. Application of SP produced significant membrane depolarization. However, continuous exposure to SP for up to 30 min resulted in slow recovery of resting membrane potential. Ba, control. Bb and Ca, 2 and 4 min after SP. iii and iv in panel Bb and Ca, junction potentials induced by oral NS of 1 and 4 pulses before, 2 and 20 min after administration of SP, respectively. The EJP amplitude was significantly inhibited and resting membrane potential was depolarized by application of SP for 2 min. However, the EJP amplitude slowly recovered to baseline despite continuous exposure to SP, suggesting that the EJP is resistant to SP desensitization.
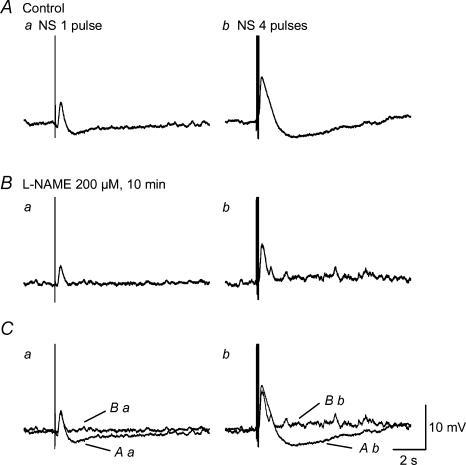
Figure 5. Inability of L-NAME to suppress EJP.
Aa and b, junction potentials induced by oral NS of 1 and 4 pulses in the presence of nifedipine, apamin, atropine, guanethidine and SP. B, 10 min after application of l-NAME. l-NAME abolished slow IJP and left EJP intact. C, superimposed junctional potentials before and after l-NAME.
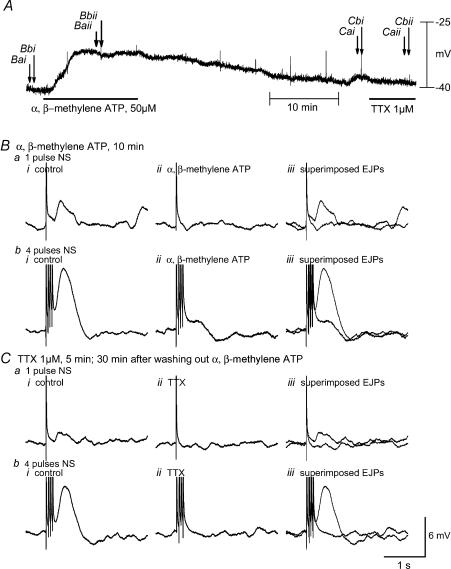
Figure 6. Effects of α,β-methylene ATP and TTX on EJP.
A, original recordings of resting membrane potential for 40 min α,β-methylene ATP produced significant membrane depolarization in the presence of nifedipine, apamin, atropine, guanethidine and SP. This effect reached a maximum in 5–10 min and stayed stable until wash out. The membrane potential gradually returned to the baseline 30 min after washing. Resting membrane potential was not affected by application of TTX. Ba and b, EJPs before and 10 min after α,β-methylene ATP. Biii, superimposed EJPs. Ca and b, EJPs before and 10 min after α,β-methylene ATP. Ciii, superimposed EJPs. The EJP induced by oral NS was markedly inhibited by application of α,β-methylene ATP and fully recovered 30 min after washing out. Subsequent application of TTX abolished the EJP.
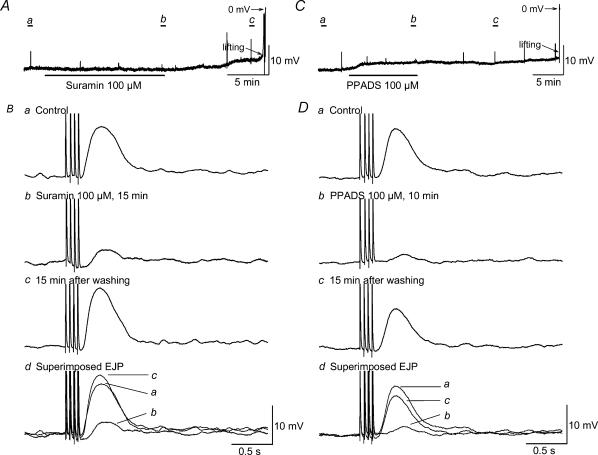
Figure 7. Inhibitory effects of suramin, a non-selective P2 purinoceptor antagonist, and PPADS, a selective P2X purinoceptor antagonist, on EJP induced by oral NS.
A and C, raw recordings of resting membrane potentials. Ba–c and Da–c, the EJP induced by 4 pulses or oral NS at expanded time scale before, during and after application of suramin and PPADS, respectively. Bd and Dd, superimposed EJPs. Resting membrane potential was not affected by suramin, but was significantly depolarized by PPADS. Suramin reached maximal inhibitory effects on the EJP in 15 min, while maximal PPADS effect occurred within 10 min. Effects of suramin and PPADS reversed within 15 min after washing out.
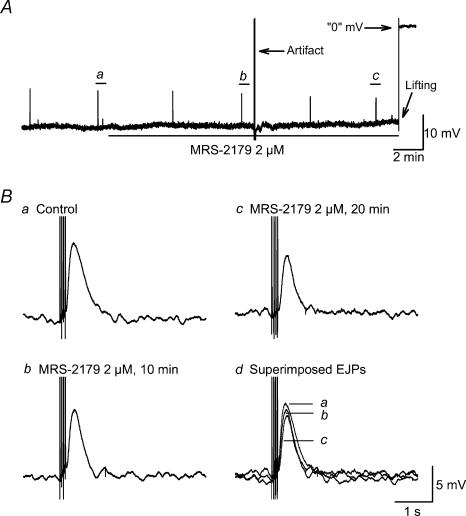
Figure 8. The purinergic EJP induced by oral NS was not significantly affected by MRS-2179, a specific P2Y1 antagonist.
A, raw recording of membrane electrical activity for up to 20 min after application of MRS-2179. Ba–c, expanded time scale depiction of EJPs from panel A in control, 10 min and 20 min after MRS-2179. Bd, superimposed EJPs.
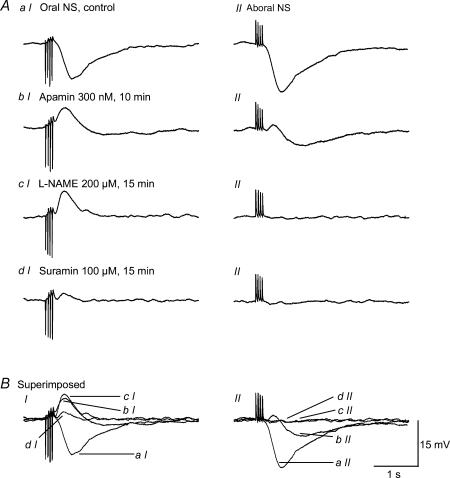
Figure 9. An example of junction potentials recorded in the same cell in the presence of atropine, guanethidine and SP, as induced by either oral (panel i) or aboral (panel ii) NS.
A, junction potentials produced by oral nerve stimulation in control (a), and after cumulative application of apamin (b), l-NAME (c) and suramin (d). B, overlay of junction potentials corresponding to panel A. Note that the purinergic EJP is polarized, whereas the NANC IJP is not.
Drugs
All drugs were purchased from Sigma, except isoflurane (Baxter, Canada). The following drugs were used: nifedipine, atropine, guanethidine, apamin, hexamethonium, substance P (SP), l-NAME, α,β-methylene ATP, TTX, suramin, pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS) and MRS-2179. Nifedipine was dissolved in DMSO, and the others in distilled and de-ionized water. These were diluted to final concentrations with Krebs solution. Final concentration of DMSO in Krebs solution was no more than 1%, which did not produce any effect on the electrical activity of the tissue.
Statistical analysis
Data are shown as means ± s.e.m.; n refers to number of animals. Only recordings in which a full protocol was completed in the same cell are included in the statistical analysis. Pre- and postdrug comparisons were made using Student's paired t test, and a P-value of < 0.05 was considered statistically significant.
Results
General membrane electrical properties
Longitudinal smooth muscle of taenia caeci was characterized by spontaneous electrical activity consisting of slow waves with a resting membrane potential (RMP) of −53.8 ± 1.7 mV, a half-amplitude duration of 559 ± 41 ms and a frequency of 34.3 ± 1.7 min−1 (n = 9). Usually, one to three spike-like action potentials (AP) with amplitude of 30.3 ± 3.3 mV (n = 9) were superim-posed on the slow waves (Fig. 2A). Nerve stimulation with one and four square pulses produced an IJP with an amplitude of 13.3 ± 1.1 mV and 19.2 ± 1.9 mV, and a half-amplitude duration of 536 ± 20 ms and 851 ± 31 ms (n = 9), respectively (Fig. 2Bc). Application of nifedipine (1 μm), an L-type Ca2+ channel antagonist, depolarized RMP to −47.1 ± 1.8 mV (n = 6, P < 0.05) and abolished slow waves and spontaneous AP. The blockade of slow waves and AP by nifedipine unmasked spontaneous fluctuations of the RMP with an amplitude of 1–3 mV (Fig. 3Aa), which is similar to that recorded in other species (Zhang & Paterson, 2002). However, the nerve stimulation of one and four pulses in the presence of nifedipine evoked an IJP with an amplitude of 12.5 ± 1.8 mV and 20.3 ± 1.6 mV, and a half-amplitude duration of 584 ± 36 ms and 858 ± 41 ms (n = 6) that was not significantly different from the IJP recorded in the presence of nifedipine. The IJP complex consisted of a fast IJP, a slow ‘shoulder’-like IJP and postinhibitory EJP (Fig. 3Ab).
Pharmacological isolation of EJP resistant to atropine, guanethidine and SP desensitization
Nifedipine (1 μm) was routinely used to immobilize spontaneous muscle contraction and stabilize the intracellular impalements in the remaining experiments. RMP was significantly depolarized from −46.5 ± 1.7 mV to −40.3 ± 2.2 (n = 6, P < 0.05) by administration of apamin (300 nm, 5 min), a small-conductance Ca2+-activated K+ channel (SK) antagonist, suggesting that SK channels contribute to the RMP. Apamin also converted the IJP complex induced by one and four pulses to an EJP with an amplitude of 4.1 ± 0.9 mV and 10.0 ± 1.9 mV, and a half-amplitude duration of 239 ± 12 ms and 447 ± 38 ms, followed by a slow IJP with an amplitude of 2.8 ± 0.6 mV and 4.9 ± 0.7 mV, and a half-amplitude duration of 715 ± 61 ms and 1521 ± 217 ms (n = 6) (Fig. 3B), respectively.
The contribution of cholinergic and adrenergic innervation to the EJP was tested by concurrent application of atropine (3 μm) and guanethidine (3 μm). Parameters of EJP produced by one and four pulses were 4.6 ± 1.1 mV and 10.5 ± 2.0 mV of amplitude, and 258 ± 26 ms and 452 ± 37 ms of half-amplitude duration in control, versus 3.1 ± 0.6 mV and 9.9 ± 2.1 mV of amplitude, and 228 ± 8 ms and 407 ± 50 ms of half-amplitude duration 10 min after application (n = 6, P > 0.05), while that of the followed IJP were 2.7 ± 0.6 mV and 4.8 ± 0.7 mV, and 361 ± 83 ms and 1385 ± 279 ms in control, versus 3.1 ± 0.4 mV and 5.1 ± 0.9 mV, and 1009 ± 193 ms and 1949 ± 384 ms 10 min after atropine and guanethidine (n = 6, P > 0.05) (Fig. 3C). RMP was also not significantly affected by the combination of atropine and guanethidine.
SP has been reported as a potential candidate for the NANC EJP in circular smooth muscle of guinea-pig ileum (Niel et al. 1983; Niel & Miolan, 1990). Application of SP (1 μm) caused marked depolarization of the RMP that peaked in 1–2 min. Continuous exposure led to desensitization and gradual recovery of RMP (Fig. 4). After 2 min of SP exposure, the EJP induced by four pulses was significantly decreased. However, constant exposure up to 20 min resulted in the recovery of the EJP to baseline values.
It is well-known that nitrergic innervation mediates the slow IJP in circular smooth muscle of gastrointestinal tract in several species (Bridgewater et al. 1995; Ward et al. 1996; Bennett, 1997; Zhang & Paterson, 2002). We confirmed that l-NAME (200 μm), a nitric oxide synthase inhibitor, abolished the slow IJP without affecting the EJP (7.1 ± 1.1 mV versus control of 7.6 ± 1.5 mV, n = 10, P > 0.05) 10 min after application (Fig. 5), implying that the slow IJP is due to nitrergic innervation. However, RMP was depolarized from −38.6 ± 1.7 mV to −34.4 ± 1.9 mV by l-NAME (n = 10, P < 0.05), suggesting that myogenic nitric oxide synthesis may be involved in maintenance of RMP in this tissue (Salapatek et al. 1998).
Pharmacological properties of the NANC EJP resistant to SP desensitization
TTX (1 μm), a specific Na+ channel blocker, completely abolished the NANC EJP (Fig. 6A and C). As the EJP was successfully isolated by concomitant application of apamin, atropine, guanethidiine, SP and l-NAME in addition to nifedipine, the following experiments were conducted in the presence of the aforementioned drugs. α,β-Methylene ATP (50 μm), a P2 purinoceptor agonist that is more potent than ATP at the P2X receptor subtype, depolarized RMP to −29.3 ± 1.7 mV from −36.5 ± 1.6 mV (n = 7, P < 0.05), which reached a maximum in 5–10 min and showed no sign of desensitization. The depolarization effect was reversed 15–30 min after washing out. Within 10 min of α,β-methylene ATP application, the EJP evoked by one pulse was abolished, whereas the EJP evoked by four pulses was significantly inhibited to 1.5 ± 0.2 mV from 7.3 ± 1.1 mV (n = 7, P < 0.05) (Fig. 6A and B). Suramin (100 μm), a P2 purinoceptor antagonist, did not significantly affect RMP (−37.0 ± 1.8 versus control of −36.0 ± 1.5, n = 8, P > 0.05), but PPADS (100 μm), a selective P2X purinoceptor antagonist, significantly depolarized RMP (−32.2 ± 1.4 mV versus control of −38.1 ± 1.5 mV, n = 9, P < 0.05) (Fig. 7A and C). The EJP evoked by four pulses was maximally inhibited by suramin in 15 min (3.7 ± 1.1 versus control of 11.1 ± 1.4 mV, n = 8, P < 0.05) and by PPADS in 10 min (1.3 ± 0.4 versus control of 10.0 ± 1.2 mV, n = 9, P < 0.05). The inhibitory effects of suramin and PPADS on EJP fully recovered 15 min after washing out (Fig. 7B and D). Resting MP and amplitude of EJP were not significantly altered by application of MRS-2179 (2 μm), a specific P2Y1 receptor antagonist of (MP −33.2 ± 2.1 mV and EJP amplitude 7.8 ± 0.9 versus control of −32.6 ± 1.8 mV and 8.9 ± 1.2 mV, n = 4, P > 0.05) (Fig. 8).
To determine whether the effects of P2X antagonism might relate to an effect on ganglionic neurotransmission, we conducted additional experiments (4 cells from 2 animals) in which hexamethonium in concentrations of 100 and 500 μm was added to the bath in the presence of apamin, atropine, guanethidine, SP, l-NAME and nifedipine. This failed to significantly influence the EJP amplitude (9.0 ± 1.0 mV in control versus 7.7 ± 0.7 mV and 7.8 ± 1.6 mV 15 min following 100 μm and 500 μm hexamethonium, respectively).
Polarization of excitatory purinergic innervation
When comparing oral versus aboral NS in the same cell, we consistently recorded large purinergic EJPs with oral NS, whereas aboral NS produced a negligible excitatory purinergic response (Fig. 9). However, the apamin- and l-NAME-sensitive IJP provoked by aboral NS were comparable to that induced by oral NS, suggesting that inhibitory purinergic and nitrergic innervation is bi-directional. Such a result was identical in the four animals consecutively studied.
Discussion
The current electrophysiological data demonstrates that the nerve-mediated response in guinea-pig taenia caeci smooth muscle can be pharmacologically discriminated into at least four components, namely a fast IJP, a slow IJP, a postinhibitory EJP, and a NANC EJP. Furthermore, we provide evidence for polarization of excitatory purinergic innervation in GI smooth muscle.
Cholinergic nerves appear to provide the predominant excitatory innervation of smooth muscle (Bennett et al. 1966; Bridgewater et al. 1995; Spencer & Smith, 2001). However, NANC excitatory innervation, and in particular tachynergic innervation, also plays a significant role (Maggi et al. 1992). As early as 1964, Burnstock & Holman (1964) recorded an atropine-resistant EJP in muscle cells of guinea-pig vas deferens, which was subsequently proven to be mediated by ATP or a related nucleotide. Subsequently, an important role for purinergic excitatory neurotransmission in vascular smooth muscle was demonstrated (Kennedy, 1996; McLaren et al. 1998). To date there has been relatively little evidence supporting a role for excitatory purinergic innervation in the gut.
We consistently recorded EJPs resistant to atropine, guanethidine and SP desensitization in the smooth muscle of the guinea-pig taenia caeci, but required apamin in the bath to unmask these EJPs. Application of apamin converted the IJP complex to an EJP, followed by a slow IJP and a postinhibitory EJP (Fig. 3Ab). The abolition of the fast IJP by apamin is consistent with previous reports suggesting that ATP mediates this response (Xue et al. 1999; Spencer & Smith, 2001). The fact that the unmasked EJP was resistant to atropine (Fig. 3C), SP desensitization (Fig. 4) and l-NAME suggests that it is not mediated by cholinergic, peptidergic and nitrergic innervation. However, l-NAME completely abolished the slow IJP, in keeping with it being mediated by nitrergic nerves.
In order to determine whether purinoceptors mediated the unmasked NANC EJP, we used purinoceptor agonists and antagonists in the presence of nifedipine, atropine, quanethidine, SP and l-NAME. Administration of α,β-methylene ATP, a P2 purinoceptor agonist that is more potent than ATP at the P2X receptor subtype, produced membrane depolarization (∼7 mV), which mimicked the membrane potential alteration evoked by nerve stimulation (Fig. 6A). α,β-Methylene ATP also abolished atropine-resistant EJPs induced by one and four pulses of electrical stimuli (Fig. 6B). Without apamin pretreatment, α,β-methylene ATP (50 μm) produced a significant membrane hyperpolarization and strongly inhibited the fast IJP (data not shown). These inhibitory effects were completely reverse 15 min after wash out of α,β-methylene ATP.
Because abolition of the EJP could be attributed to the membrane depolarization induced by α,β-methylene ATP, we also examined the effects of different purinoceptor antagonists on the EJP. Suramin, a non-selective P2 receptor antagonist, had no effect on RMP, but markedly suppressed the EJP (Fig. 7A and B). PPADS, a putative selective P2X receptor antagonist, significantly depolarized the RMP and almost completely eradicated the EJP (Fig. 7C and D). The pharmacological effect of these two antagonists in the current study is very similar to what has been reported in guinea-pig vas deferens, where sympathetic nerve stimulation also induces an EJP (Sneddon, 1992). The reason for the RMP depolarization induced by PPADS is unclear. McLaren et al. (1994) reported that in guinea-pig vas deferens, the depolarization caused by PPADS was not affected by pretreatment with suramin, suggesting that this was not due to partial agonist properties of the drug. Whether PPADS has non-specific effects on one or more membrane ion channels remains to be determined. It has been reported that in smooth muscle of guinea-pig small intestine, MRS-2179, a specific P2Y1 receptor antagonist, blocks more than 90% of the apamin-sensitive IJP as well as the hyperpolarization induced by bath application of ATP, which supports the involvement of P2Y1 receptors in the apamin-sensitive IJP (Wang et al. 2004). The lack of effect of MRS-2179 on the purinergic EJP in the current experiments (Fig. 8) suggests that P2Y1 receptors are not involved in this response. Recently, immunohistochemical evidence for P2X1 receptor expression in guinea-pig GI smooth muscles (but not interstitial cells of Cajal) has been reported (Van Nassauw et al. 2003). This morphological evidence, when combined with our functional data, provides strong support for postjunctional involvement of P2X receptors in excitatory responses in GI smooth muscle.
Although purinergic neurotransmission is known to occur between neurones in the enteric nervous system, it is highly unlikely that the effects of P2X blockade seen in the current study are due to an effect on ganglionic neurotransmission. Firstly, hexamethonium in high concentrations did not inhibit the EJP, suggesting that the observed response does not involve ganglionic neurotransmission. Furthermore, the EJP was recorded in the presence of atropine, guanethidine, l-NAME and substance P tachyphylaxis, and therefore if the effect of P2X blockade was at the level of the ganglion, one would have to postulate the presence of some other neurotransmitter being released onto the muscle to produce the EJP.
Nerve-mediated postsynaptic responses in guinea-pig taenia caeci smooth muscle have been extensively studied, but the focus has been on the NANC IJP and cholinergic EJP. Nevertheless, NANC EJPs have been described in this tissue, but the results have been inconsistent. Similar to our observations, Shuba & Vladimirova (1980) found that apamin unmasked a NANC EJP in guinea-pig caecal smooth muscle, and proposed that this may be purinergic in origin because exogenous ATP also evoked depolarization in the presence of apamin. Bridgewater et al. (1995) noted occasional small, atropine-resistant EJPs in some preparations, particularly following apamin treatment, but did not further characterize this response. They also demonstrated atropine-sensitive EJPs, but could only evoke these in about 15% of trials. Using the sucrose gap technique, Zagorodnyuk & Maggi (1998) also demonstrated suramin- and PPADS-sensitive EJPs, but only in the presence of both apamin and TEA, putative small and large conductance K+ channel blockers, respectively. In the present study, we were unable to find evidence of atropine-sensitive EJPs. Furthermore, Ward et al. (1996) reported that a nitrergic IJP could only be demonstrated in guinea-pig taenia caeci in the absence of atropine. However, we found clear evidence of a l-NAME-sensitive slow IJP in the presence of atropine (Fig. 5C).
The reasons for the discrepancies between these studies and the current work are unclear. We were initially concerned that the atropine we were using had lost its potency, but this proved not to be the case as the same batch had the expected effects when tested in separate muscle strip studies. Another possibility relates to differences in strains or ages of guinea-pigs used for the experiments. We used young guinea-pigs weighing between 50 and 100 g, whereas it appears most other studies used adult animals. It is possible that development of excitatory purinergic innervation precedes that of cholinergic innervation. Finally, the intrinsic innervation of the GI tract is typically polarized, yet in the studies cited above, the site of stimulation and recording was not specified. We found that a purinergic EJP was only consistently evident when recordings were made aboral to the point of nerve stimulation. On the other hand, the nitrergic and purinergic IJPs were no different whether evoked by oral or aboral nerve stimulation.
We can only speculate on the physiological relevance of this polarized excitatory purinergic EJP in taenia caeci. Although the principal innervation of this muscle is inhibitory, it is interesting that activation of purinoceptors is capable of evoking both inhibition and excitation, suggesting that the excitatory component may serve to limit the predominant effects of inhibition. The fact that this occurs aboral to the point of stimulation might reflect the taenia caeci's function as a storage organ, in that delay of transit to the ascending colon serves to allow time for fluid to be reabsorbed from the faecal effluent.
In summary, we provide electrophysiological and pharmacological evidence for polarization of a purinergic EJP in guinea-pig taenia caeci smooth muscle that only became apparent when the predominant IJP was antagonized with apamin and nerve fibres were stimulated oral to a recording site. This EJP is resistant to atropine, quanethidine, SP desensitization and MRS-2179, but is markedly inhibited by suramin, PPADS and desensitization to α,β-methylene ATP, in keeping with mediation by P2X receptors, most likely the P2X1 subtype.
Acknowledgments
Thanks to Drs A. F. Brading at Oxford and R. J. Lang at Monash for reviewing this work and for their constructive suggestions. Supported by grant #9978 from the Canadian Institutes of Health Research.
References
- Bennett M. Non-adrenergic non-cholinergic (NANC) transmission to smooth muscle: 35 years on. Prog Neurobiol. 1997;52:159–195. doi: 10.1016/s0301-0082(97)00012-9. [DOI] [PubMed] [Google Scholar]
- Bennett M, Burnstock G, Holman M. Transmission from intramural inhibitory nerves to the smooth muscle of the guinea-pig taenia coli. J Physiol. 1966;182:541–558. doi: 10.1113/jphysiol.1966.sp007836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgewater M, Cunnane T, Brading A. Characteristic features of inhibitory junction potentials evoked by single stimuli in the guinea-pig isolated taenia caeci. J Physiol. 1995;485:145–155. doi: 10.1113/jphysiol.1995.sp020719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Campbell G, Bennett M, Holman M. Inhibition of the smooth muscle of the taenia coli. Nature. 1963;200:581–582. doi: 10.1038/200581a0. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Campbell G, Satchell D, Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol. 1970;40:668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Holman M. An electrophysiological investigation of the actions of some autonomic blocking drugs on transmission in the guinea-pig vas deferens. Br J Pharmacol. 1964;23:600–612. doi: 10.1111/j.1476-5381.1964.tb01613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Satchell G, Smythe A. A comparison of the excitatory and inhibitory effects of non-adrenergic, non-cholinergic nerve stimulation and exogenously applied ATP on a variety of smooth muscle preparations from different vertebrate species. Br J Pharmacol. 1972;46:234–242. doi: 10.1111/j.1476-5381.1972.tb06868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman M, Taylor G, Tomita T. Some properties of the smooth muscle of mouse vas deferens. J Physiol. 1977;266:751–764. doi: 10.1113/jphysiol.1977.sp011791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle V, Burnstock G. Atropine-resistant excitatory junction potentials in rabbit bladder are blocked by α,β-methylene ATP. Eur J Pharmacol. 1985;114:239–240. doi: 10.1016/0014-2999(85)90635-1. 10.1016/0014-2999(85)90635-1. [DOI] [PubMed] [Google Scholar]
- Huizinga J, Pielkenrood J, Den Hertog A. Dual action of high energy adenine nucleotides in comparison with responses evoked by other adenine derivatives and intramural nerve stimulation on smooth muscle. Eur J Pharmacol. 1981;74:175–180. doi: 10.1016/0014-2999(81)90528-8. 10.1016/0014-2999(81)90528-8. [DOI] [PubMed] [Google Scholar]
- Kennedy C. ATP as a cotransmitter in perivascular sympathetic nerves. J Auton Pharmacol. 1996;16:337–340. doi: 10.1111/j.1474-8673.1996.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Krysiak P, Preiksaitis H. Tachykinins contribute to nerve-mediated contractions in the human esophagus. Gastroenterol. 2001;120:39–48. doi: 10.1053/gast.2001.20910. [DOI] [PubMed] [Google Scholar]
- Maggi C, Theodorsson E, Santicioli P, Giuliani S. Tachykinins and calcitonin gene-related peptide as co-transmitters in local motor responses produced by sensory nerve activation in the guinea-pig isolated renal pelvis. Neuroscience. 1992;46:549–559. doi: 10.1016/0306-4522(92)90143-p. 10.1016/0306-4522(92)90143-P. [DOI] [PubMed] [Google Scholar]
- McLaren G, Burke K, Buchanan K, Sneddon P, Kennedy C. Evidence that ATP acts at two sites to evoke contraction in the rat isolated tail artery. Br J Pharmacol. 1998;124:5–12. doi: 10.1038/sj.bjp.0701772. 10.1038/sj.bjp.0701772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren G, Lambrecht G, Mutschler E, Baumert H, Sneddon P, Kennedy C. Investigation of the actions of PPADS, a novel P2x-purinoceptor antagonist, in the guinea-pig isolated vas deferens. Br J Pharmacol. 1994;111:913–917. doi: 10.1111/j.1476-5381.1994.tb14825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niel J, Bywater R, Taylor G. Effect of substance P on non-cholinergic fast and slow post-stimulus depolarization in the guinea-pig ileum. J Auton Nerv Syst. 1983;9:573–584. doi: 10.1016/0165-1838(83)90114-5. 10.1016/0165-1838(83)90114-5. [DOI] [PubMed] [Google Scholar]
- Niel J, Miolan J. Involvement of a cholinergic mechanism in the sustained depolarization and contraction of the lower oesophageal sphincter muscle cells in the cat. Neuroscience. 1990;36:803–809. doi: 10.1016/0306-4522(90)90023-w. 10.1016/0306-4522(90)90023-W. [DOI] [PubMed] [Google Scholar]
- Salapatek A, Wang Y, Mao Y, Lam A, Daniel E. Myogenic nitric oxide synthase activity in canine lower oesophageal sphincter: morphological and functional evidence. Br J Pharmacol. 1998;123:1055–1064. doi: 10.1038/sj.bjp.0701701. 10.1038/sj.bjp.0701701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuba M, Vladimirova I. Effect of apamin on the electrical responses of smooth muscle to adenosine 5′-triphosphate and to non-adrenergic, non-cholinergic nerve stimulation. Neuroscience. 1980;5:853–859. doi: 10.1016/0306-4522(80)90154-2. 10.1016/0306-4522(80)90154-2. [DOI] [PubMed] [Google Scholar]
- Sneddon P. Suramin inhibits excitatory junction potentials in guinea-pig isolated vas deferens. Br J Pharmacol. 1992;107:101–103. doi: 10.1111/j.1476-5381.1992.tb14469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon D, Smythe A, Satchell D, Burnstock G. An investigation of the identity of the transmitter substance released by non-adrenergic, non-cholinergic excitatory nerves supplying the small intestine of some lower vertebrate. Comp General Pharmacol. 1973;4:53–60. 10.1016/0010-4035(73)90021-9. [Google Scholar]
- Spencer N, Smith T. Simultaneous intracellular recordings from longitudinal and circular muscle during the peristaltic reflex in guinea-pig distal colon. J Physiol. 2001;533:787–799. doi: 10.1111/j.1469-7793.2001.00787.x. 10.1111/j.1469-7793.2001.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nassauw L, Jonge F, Timmermans J, Burnstock G. Presence of the P2x1 receptor in the guinea-pig gastrointestinal tract. Proceedings of the 19th International Symposium on Gastrointestinal Motility; Barcelona, Spain. 2003. p. 72. [Google Scholar]
- Wang G, Hu H, Wang X, Gao N, Liu S, Fang X, Xia Y, Wood J. Purinergic inhibitory neuromuscular transmission mediated by the P2Y1 receptor in guinea-pig small intestine. Gastroenterology. 2004;126(Suppl. 2):A-275. doi: 10.1152/ajpgi.00450.2006. [DOI] [PubMed] [Google Scholar]
- Ward S, Dalziel H, Khoyi M, Westfall A, Sanders K, Westfall D. Hyperpolarization and inhibition of contraction mediated by nitric oxide released from enteric inhibitory neurones in guinea-pig taenia coli. Br J Pharmacol. 1996;118:49–56. doi: 10.1111/j.1476-5381.1996.tb15365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue L, Farrugia G, Sarr M, Szurszewski J. ATP is a mediator of the fast inhibitory junction potential in human jejunal circular smooth muscle. Am J Physiol. 1999;276:G1373–G1379. doi: 10.1152/ajpgi.1999.276.6.G1373. [DOI] [PubMed] [Google Scholar]
- Zagorodnyuk V, Maggi C. Pharmacological evidence for the existence of multiple P2 receptors in the circular muscle of guinea-pig colon. Br J Pharmacol. 1998;123:122–128. doi: 10.1038/sj.bjp.0701558. 10.1038/sj.bjp.0701558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lang R. Effects of intrinsic prostaglandins on the spontaneous contractile and electrical activity of the proximal renal pelvis of the guinea-pig. Br J Pharmacol. 1994;113:431–438. doi: 10.1111/j.1476-5381.1994.tb17007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Paterson W. Nitric oxide contracts longitudinal smooth muscle of opossum oesophagus via excitation–contraction coupling. J Physiol. 2001;536:133–140. doi: 10.1111/j.1469-7793.2001.00133.x. 10.1111/j.1469-7793.2001.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Paterson W. Role of Ca2+-activated Cl− channels and MLCK in opossum esophageal smooth muscle. Am J Physiol. 2002;283:G104–G114. doi: 10.1152/ajpgi.00052.2002. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Paterson W. Role of sarcoplasmic reticulum in control of membrane potential and nitrergic response in opossum lower esophageal sphincter. Br J Pharmacol. 2003;140:1097–1107. doi: 10.1038/sj.bjp.0705537. 10.1038/sj.bjp.0705537. [DOI] [PMC free article] [PubMed] [Google Scholar]