Abstract
Eleven mammalian toll-like receptors (TLRs 1–11) have been identified to date and are known to play a crucial role in the regulation of immune responses; however, the factors that regulate TLR expression and function in vivo are poorly understood. Therefore, in the present study, we investigated the physiological regulation of TLR expression and function in humans. To examine the influence of diurnal rhythmicity on TLR expression and function, peripheral venous blood samples were collected from healthy volunteers (n = 8) at time points coinciding with the peak and nadir in the endogenous circulating cortisol concentration. While no diurnal rhythmicity in the expression of TLRs 1, 2, 4 or 9 was observed, the upregulation of costimulatory (CD80 and CD86) and antigen-presenting (MHC class II) molecules on CD14+ monocytes following activation with specific TLR ligands was greater (P < 0.05) in samples obtained in the evening compared with the morning. To examine the influence of physical stress on TLR expression and function, peripheral venous blood samples were collected from healthy volunteers (n = 11) at rest and following 1.5 h of strenuous exercise in the heat (34°C). Strenuous exercise resulted in a decrease (P < 0.005) in the expression of TLRs 1, 2 and 4 on CD14+ monocytes. Furthermore, the upregulation of CD80, CD86, MHC class II and interleukin-6 by CD14+ monocytes following activation with specific TLR ligands was decreased (P < 0.05) in samples obtained following exercise compared with at rest. These results demonstrate that TLR function is subject to modulation under physiological conditions in vivo and provide evidence for the role of immunomodulatory hormones in the regulation of TLR function.
An evolutionary conserved family of pattern recognition receptors called Toll receptors plays an essential role in detecting infection in Drosophila and mammals (Janeway & Medzhitov, 2002). A family of 11 mammalian toll-like receptors (TLRs 1–11) has been identified to date, each recognizing specific, highly conserved structures present on infectious microorganisms (Medzhitov, 2001). TLRs control the activation of innate immunity through the induction of antimicrobial activity (Aliprantis et al. 1999; Thoma-Uszynski et al. 2001) and the production of inflammatory cytokines (Medzhitov et al. 1997; Hemmi et al. 2000; Alexopoulou et al. 2001; Hayashi et al. 2001; Yamamoto et al. 2002). TLRs control the generation of adaptive immunity through the induction of antigen-presenting (MHC class II) and costimulatory molecules (CD80/86) (Medzhitov et al. 1997; Hemmi et al. 2000; Alexopoulou et al. 2001; Hayashi et al. 2001; Yamamoto et al. 2002), and specific cytokines (Pasare & Medzhitov, 2003) (interleukin (IL)-6) on antigen-presenting cells (APCs). The importance of the Toll signalling pathway in Drosophila and mammalian immunity is evident from gene targeting studies examining the Toll family of receptors and their cognate downstream signalling molecules (Lemaitre et al. 1996; Takeuchi et al. 2000; Schnare et al. 2001; Scanga et al. 2002; Seki et al. 2002). Furthermore, human studies provide evidence that the expression and activation of TLRs in vivo contributes to host defence against microbial pathogens, and the effective generation of specific antibodies following vaccination (Alexopoulou et al. 2002; Krutzik et al. 2003).
Despite tremendous advances in our understanding of the role of TLRs in host defence, and the specific signalling events initiated following TLR activation (Beutler, 2004), factors that regulate TLR expression and function are poorly understood. Cytokines exert considerable influence over the development of host immunity, and recently they have been shown to modulate the expression and activation of TLRs. For example, viral infection of human macrophages induces expression of TLR1, TLR2, TLR3 and TLR7 mRNA, a process that has been shown to be dependent upon the production of type 1 interferons (Miettinen et al. 2001), and recent data provide evidence for a differential regulation of TLR expression and activation by type 1 and type 2 cytokines (Krutzik et al. 2003).
Neuroendocrine hormones have a well-established role in modulating the immune system, and recent studies suggest a role for glucocorticoids (GCs) in regulating TLR expression, since dexamethasone (DEX), a synthetic glucocorticoid, was shown to induce TLR2 mRNA expression in epithelial cells (Imasato et al. 2002; Shuto et al. 2002). Furthermore, TLR2 and TLR4 gene expression in peripheral blood mononuclear cells (PBMCs) is enhanced by DEX treatment (Galon et al. 2002). In addition, given that GCs are potent modulators of NF-κB transcriptional activity, and that the activation of NF-κB is required for an increase in the expression of several TLR-controlled genes, it is likely that GCs play an important role in modulating TLR function in vivo.
In the present study, we have investigated the regulation of TLR expression and function under physiological conditions in vivo. Firstly, we have examined whether TLR expression and function is subject to circadian timing. Numerous physiological processes display circadian timing and, importantly, circadian variations in the endogenous circulating GC concentration influence many aspects of the immune system (Angeli et al. 1992; Dhabhar et al. 1994; Petrovsky & Harrison, 1997). Preliminary data suggest that TLR expression is influenced by GCs (Galon et al. 2002; Imasato et al. 2002); furthermore, the activation of NF-κB, a transcription factor critical to the induction of TLR responsive genes, is also inhibited by GCs. Therefore, we hypothesized that TLR expression and function would display circadian timing.
Secondly, we have examined the influence of 1.5 h of strenuous exercise in hot (34°C) environmental conditions on TLR expression and function. Strenuous exercise is known to influence numerous aspects of the immune system (Pedersen & Hoffman-Goetz, 2000), and an exercise-induced increase in the circulating concentration of several immunomodulatory hormones is believed to be central to the modulation of the immune system by exercise. We hypothesized that exercise would influence the expression and function of TLRs, possibly via increases in the circulating concentrations of stress hormones. To augment the exercise-induced stress hormone response, and thus maximize the likelihood of observing an effect of exercise on TLR expression and function, exercise in combination with heat stress was employed.
Methods
Subjects
To examine whether TLR expression and activation was influenced by circadian rhythmicity, peripheral venous blood samples were obtained from eight (five males, three females) healthy volunteers (age 25 ± 1 years, body mass 68 ± 3 kg) at 07.00 h after an overnight fast and again at 17.00 h following a 3–4 h fast. To examine the effects of strenuous exercise on TLR expression and activation, 11 healthy, moderate-to-well endurance-trained males (age 25 ± 1 years, body mass 74 ± 2 kg, maximal oxygen uptake ![]() ; means ± s.e.m.) volunteered to participate in the study. All participants were nonsmokers, were not taking any medication, and had remained free of respiratory infection for 4 weeks prior to participation in the study. Subjects were informed as to the purposes and risks of the experiments before voluntarily giving their written and informed consent. The study was approved by the University of Birmingham Ethics Committee and conformed to the Declaration of Helsinki.
; means ± s.e.m.) volunteered to participate in the study. All participants were nonsmokers, were not taking any medication, and had remained free of respiratory infection for 4 weeks prior to participation in the study. Subjects were informed as to the purposes and risks of the experiments before voluntarily giving their written and informed consent. The study was approved by the University of Birmingham Ethics Committee and conformed to the Declaration of Helsinki.
Exercise protocol
At least 7 days prior to the experimental trial, each subject's maximal work rate (Wmax) was determined during an incremental exercise test to volitional exhaustion, as previously described (Jentjens et al. 2002). In addition, subjects completed a familiarization exercise trial at 55%Wmax for 1.5 h at 34°C. Subjects were instructed to abstain from alcohol, tobacco and exercise on the day prior to the experimental trials. On the day of the experimental trial, subjects reported to the Human Performance Laboratory at the University of Birmingham after an overnight fast. To avoid circadian rhythms in immunomodulatory hormones, all trials commenced at 07.00 h. An indwelling catheter was inserted into an antecubital vein of one arm, and a resting blood sample was drawn (Rest). Following 5 min of exercise at 40%Wmax, subjects commenced the 1.5 h of cycling exercise at 55%Wmax (∼65%![]() ). Further blood samples were obtained upon completion of the exercise (Post-exercise), and following 2 h of resting recovery (2 h Post-exercise). Subjects consumed a total of 1.5 l of flavoured water during the experimental trials. Furthermore, all trials were performed in the heat (34°C room temperature, 30% relative humidity).
). Further blood samples were obtained upon completion of the exercise (Post-exercise), and following 2 h of resting recovery (2 h Post-exercise). Subjects consumed a total of 1.5 l of flavoured water during the experimental trials. Furthermore, all trials were performed in the heat (34°C room temperature, 30% relative humidity).
At each time point during the experimental trials, whole blood (6 ml) was collected in sterile K3EDTA vacutainer tubes (Becton Dickinson, Oxford, UK). From this, total and differential leucocyte counts were determined, while the remainder was separated immediately by centrifugation (1500 g) for 10 min, and aliquots of plasma were stored at −20°C until analysis for adrenocorticotropic hormone (ACTH), cortisol, growth hormone and glucose. In addition, whole blood (7 ml) was collected in sterile lithium-heparin vacutainer tubes (Becton Dickinson, Oxford, UK), and kept at room temperature until the end of the trial for analysis of TLR expression and activation.
Determination of TLR expression
To determine cell surface expression of TLRs on circulating monocytes and neutrophils, whole blood was surface stained with CD14-FITC (Becton Dickinson Biosciences) and antihuman PE-conjugated TLR1 (clone GD2.F4), TLR2 (clone TL2.1) or TLR4 (clone HTA 125) (e-Bioscience), or the appropriate isotype control for TLR1 (mouse IgG1-PE), TLR2 (mouse IgG2a-PE) and TLR4 (mouse IgG2a-PE) (e-Bioscience). In contrast to the cell surface localization of TLR1, 2 and 4, TLR9 is localized intracellularly. Therefore, following surface staining with CD14-FITC, samples were permeabilized and stained with antihuman TLR9-PE (clone eB72-1665) or rat IgG2a isotype control antibodies (e-Bioscience). Samples were analysed on a flow cytometer (BD FACSCalibur) equipped with the CellQuest software package (BD Biosciences). Cells were gated according to side-scatter and CD14-FITC expression, and the change in the geometric mean fluorescence intensity (GMFI) between TLR1, 2, 4 and 9 antibodies and isotype controls was obtained to quantify TLR expression.
Assessment of IL-6 production following stimulation with TLR ligands
Whole blood (3 × 106 leucocytes ml−1) was added to RPMI 1640 medium supplemented with 2 mml-glutamine, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin. In addition, Brefeldin A (10 μg ml−1), a potent inhibitor of intracellular protein transport, was added to all samples. Samples were then stimulated with either 10 μg ml−1 zymosan (a specific TLR2 and 6 ligand) from Saccharomyces cerevisiae, 10 ng ml−1 lipopolysaccharide (LPS; a specific TLR4 ligand) purified from Escherichia coli (011:B4 strain) (InvivoGen), or they were unstimulated. Following incubation at 37°C for 6 h in a 5% CO2 humidified atmosphere, samples were surface stained with CD14-fluorescein isothiocyanate (FITC) (BD Biosciences). After permeabilization, samples were stained using intracellular PE-conjugated antibodies against human IL-6, or mouse IgG1-PE as a negative control. Samples were analysed on a flow cytometer (BD FACSCalibur) equipped with the CellQuest software package (BD Biosciences). Monocytes were gated according to side-scatter and CD14-FITC expression, and the GMFI of the IL-6-PE antibody was obtained to quantify intracellular cytokine expression.
Assessment of costimulatory molecule expression following stimulation with TLR ligands
Whole blood (3 × 106 leucocytes ml−1) was added to RPMI 1640 medium supplemented with 2 mml-glutamine, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin. Samples were then stimulated with either 10 μg ml−1 zymosan, 10 ng ml−1 LPS, 25 μg ml−1 polyinosine-polycytidylic acid (Poly(I:C)) a synthetic dsRNA analogue (a specific TLR3 ligand) (InvivoGen), or were unstimulated. Following incubation at 37°C for either 6 or 24 h in a 5% CO2 humidified atmosphere, samples were surface stained with CD14-FITC, CD80-PE, HLA-DR-PerCP (BD Biosciences), CD86-PE (Santa Cruz Biotechnology) and appropriate isotype controls. Samples were analysed on a flow cytometer (BD FACSCalibur) equipped with the CellQuest software package (BD Biosciences). Monocytes were gated according to side-scatter and CD14-FITC expression, and the change in the GMFI between CD80-PE, CD86-PE and HLA-DR-PerCP antibodies and isotype controls was obtained to quantify cell surface expression of costimulatory molecules.
Circulating stress hormones and metabolites
Plasma cortisol (DRG Instruments, Germany), growth hormone (DRG Instruments) and ACTH (Biomerica, USA) concentrations were determined by enzyme-linked immunosorbent assay (ELISA). Plasma glucose concentration was determined enzymatically (Randox Laboratories Ltd, UK) on a semiautomatic analyser (COBAS MIRA plus; Roche, Switzerland).
Statistical analysis
A one-way (time) ANOVA was used to compare the means of data obtained from the exercise study. Where the ANOVA revealed a significant F ratio, differences were inspected with Tukey's honestly significant difference post hoc test. A Student's paired samples t test was used to compare the means of data obtained from the diurnal rhythm study. SPSS version 10 for Windows (SPSS, Inc., Chicago, IL, USA) was used to calculate these statistics. The level of statistical significance accepted to reject the null hypothesis was P < 0.05. Data in the text, tables and figures are means ± s.e.m.
Results
Diurnal rhythmicity of TLR expression and activation
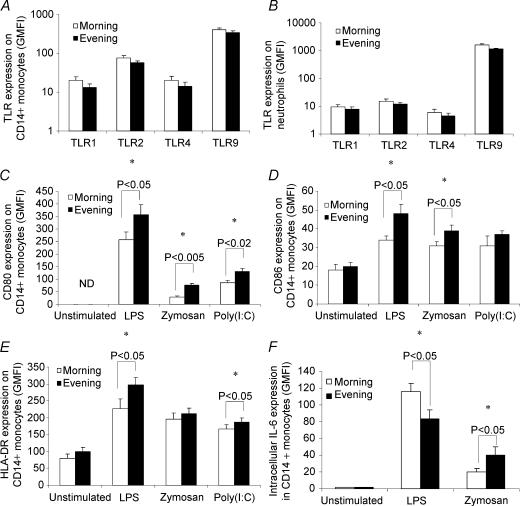
The endogenous circulating cortisol concentration typically reaches its daily peak soon after waking in the morning, and its nadir prior to sleep in the evening. Therefore, we obtained peripheral venous blood samples at 07.00 h in the morning (∼30 min after waking) and 17.00 h in the evening. As expected, we observed a significantly higher circulating cortisol concentration in samples obtained in the morning compared with the evening (morning, 802 ± 92 nmol l−1; evening, 323 ± 44 nmol l−1; means ± s.e.m., n = 8, P < 0.001, paired sample t test). We observed no statistically significant differences in the expression of TLR1, 2, 4 or 9 on CD14+ monocytes between samples obtained at 07.00 h (morning) and 17.00 h (evening) (Fig. 1A). Similarly, no differences in the expression of TLR1, 2 or 4 on neutrophils were observed; although there was a statistically significant difference (P < 0.05) between morning and evening samples for TLR9 expression (Fig. 1B). The relative levels of TLR expression we observed, i.e. TLR9 > TLR2 > TLR1 and TLR4, are consistent with previous studies (Renshaw et al. 2002; Marsik et al. 2003).
Figure 1. The effect of circadian rhythmicity on toll-like receptor (TLR) cell surface expression and activation.
Peripheral blood samples were obtained at 07.00 h and 17.00 h from eight healthy donors. A and B, samples were labelled with specific TLR monoclonal antibodies or isotype controls, and examined by flow cytometry. C–F, samples were incubated with media only (unstimulated), lipopolysaccharide (LPS; TLR4 ligand), zymosan (TLR2 and 6 ligand) or polyinosine-polycytidylic acid (poly(I:C); TLR3 ligand) for either 6 (D, E and F) or 24 h (C), and expression of costimulatory molecules and intracellular cytokines was examined by flow cytometry. All data represent means ± s.e.m.*Statistically significant difference as determined by paired-samples t test. ND, not detected.
The upregulation of costimulatory molecules, and the production of cytokines following activation, are critical aspects of TLR function. To assess TLR function we incubated whole-blood samples with either zymosan, poly(I:C), LPS, or culture media only, for either 6 or 24 h, and quantified the cell surface expression (GMFI) of CD80/86 and MHC II on CD14+ monocytes. TLR activation caused an upregulation in the surface expression of CD80, CD86 and MHC II compared with unstimulated samples (Fig. 1C, D and E). However, the expression of CD80 (Fig. 1C), CD86 (Fig. 1D) and MHC II on CD14+ monocytes (Fig. 1E) following activation with zymosan, LPS and poly(I:C) was significantly greater in samples obtained in the evening compared with the morning.
Using flow cytometry, we also determined the intracellular expression (GMFI) of IL-6 in CD14+ monocytes following 6 h of incubation with LPS and zymosan. Interestingly, although IL-6 expression within CD14+ monocytes following zymosan stimulation was higher (P < 0.05) in evening compared with morning samples (consistent with the CD80/86 and MHC class II expression data), intracellular IL-6 expression following LPS stimulation was higher (P < 0.05) in morning compared with evening samples (Fig. 1F). These data indicate a role of physiological alterations in immunomodulatory hormones in the regulation of TLR activation.
The effects of strenuous exercise on TLR expression and activation
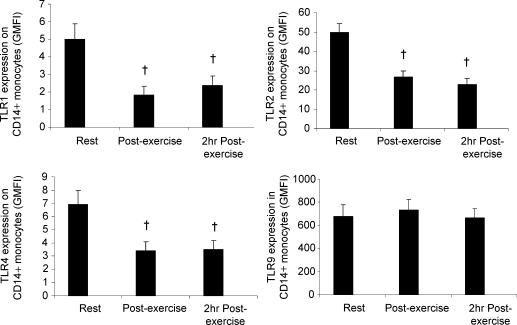
To examine whether an acute physiological stressor influences the expression and activation of TLRs, we obtained peripheral blood samples from 11 healthy volunteers before, immediately after, and following 2 h of resting recovery from 90 min of strenuous exercise in the heat (34°C). Compared with values obtained at rest, exercise resulted in a significant decrease in the surface expression of TLR1 (P < 0.005), TLR2 (P < 0.005) and TLR4 (P < 0.005) on CD14+ monocytes; furthermore, this decrease was maintained at 2 h postexercise (Fig. 2A, B and C). No effect of exercise was observed on TLR9 expression (Fig. 2D).
Figure 2. The effect of exercise on TLR expression on CD14+ monocytes.
Peripheral blood samples were obtained from 11 healthy volunteers before, immediately after, and following 2 h of resting recovery from 90 min of exercise at 55% maximal work rate (Wmax) in the heat (34°C). A, B, C and D, samples were labelled with specific TLR monoclonal antibodies or isotype controls, and examined by flow cytometry. All data represent means ± s.e.m.†Statistically significant difference (P < 0.005) from pre-exercise. GMFI, geometric mean fluorescence intensity.
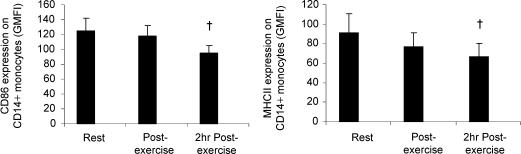
Next, to examine whether exercise influences the activation of TLRs, we assessed the upregulation of costimulatory molecules (CD80/86) and MHC II following stimulation with known TLR ligands in samples obtained at rest and following exercise. Whole-blood samples were incubated with either zymosan, poly(I:C), LPS, or culture media only, for either 6 or 24 h, and the cell surface expression (GMFI) of CD80/86 and MHC II on CD14+ monocytes was quantified. In the unstimulated samples there was a decrease in the surface expression of CD86 (P < 0.05) and MHC II (P < 0.05) on CD14+ monocytes in samples obtained following exercise compared with the resting samples (Fig. 3A and B). CD80 was not detectable in unstimulated samples obtained at any time point (data not shown).
Figure 3. The effect of exercise on CD86 and MHC class II expression on CD14+ monocytes.
Peripheral blood samples were obtained from 11 healthy volunteers before, immediately after, and following 2 h of resting recovery from 90 min of exercise at 55%Wmax in the heat (34°C). A and B, samples were incubated with culture media only for 6 h, and the expression of CD86 and MHC class II was examined by flow cytometry. All data represent means ± s.e.m.†Statistically significant difference (P < 0.05) from pre-exercise.
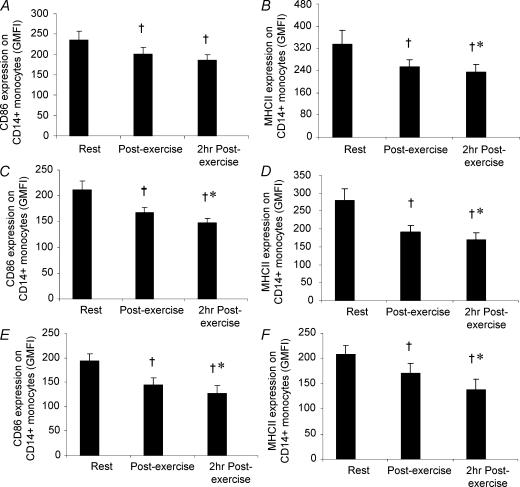
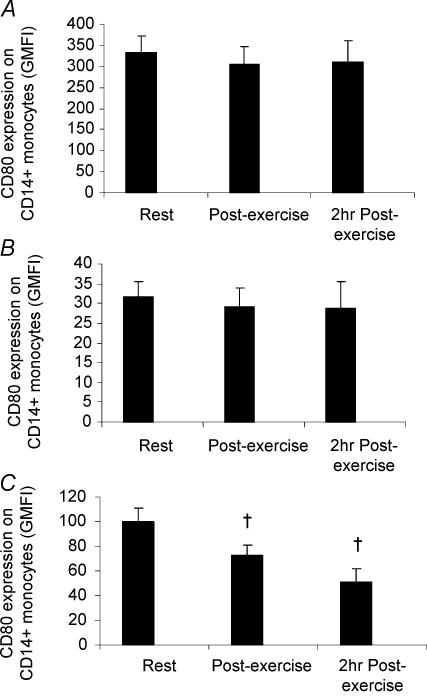
TLR activation with zymosan, LPS or poly(I:C) caused an upregulation in the surface expression of CD80, CD86 and MHC II compared with unstimulated samples. However, the upregulation of CD86 and MHC II expression on CD14+ monocytes following 6 h of stimulation with zymosan, LPS or poly(I:C) was significantly lower in samples obtained following exercise compared with the resting samples (Fig. 4A–F). The upregulation of CD80 expression on CD14+ monocytes following 24 h of stimulation with zymosan or LPS was not statistically significantly different between samples obtained at rest and following exercise (Fig. 5A and B). However, the upregulation of CD80 expression following stimulation with poly(I:C) was significantly lower in samples obtained following exercise compared with the resting samples (Fig. 5C).
Figure 4. The effect of exercise on CD86 and MHC class II expression on CD14+ monocytes.
Peripheral blood samples were obtained from 11 healthy volunteers before, immediately after, and following 2 h of resting recovery from 90 min of exercise at 55%Wmax in the heat (34°C). A–F, samples were incubated with LPS (TLR4 ligand; A and B), zymosan (TLR2 and 6 ligand; C and D) or poly(I:C) (TLR3 ligand; E and F) for 6 h, following which the expression of CD86 and MHC class II was examined by flow cytometry. All data represent means ± s.e.m.†Statistically significant difference (P < 0.05) from pre-exercise. *Statistically significant difference (P < 0.05) from post-exercise.
Figure 5. The effect of exercise on CD80 expression on CD14+ monocytes.
Peripheral blood samples were obtained from nine healthy volunteers before, immediately after, and following 2 h of resting recovery from 90 min of exercise at 55%Wmax in the heat (34°C). A–C, samples were incubated with LPS (TLR4 ligand; A), zymosan (TLR2 and 6 ligand; B) or poly(I:C) (TLR3 ligand; C) for 24 h, following which the expression of CD80 was examined by flow cytometry. All data represent means ± s.e.m.†Statistically significant (P < 0.01) difference from pre-exercise during the placebo trial.
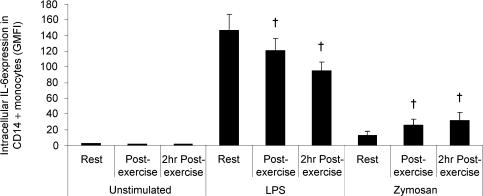
To further assess the effect of exercise on TLR function, we determined the intracellular expression of IL-6 following stimulation with known TLR ligands in samples obtained at rest and following exercise. Whole-blood samples were incubated for 6 h with zymosan, LPS, or culture media only (unstimulated), and the intracellular expression (GMFI) of IL-6 within CD14+ monocytes was quantified. Although the intracellular expression of IL-6 was barely detectable in unstimulated monocytes, incubation with zymosan or LPS, as expected, resulted in a marked increase in the expression of IL-6 (Fig. 6). However, the increase in monocyte intracellular IL-6 expression in response to LPS stimulation was significantly reduced in the samples obtained following exercise compared with resting samples (Fig. 6). In contrast to the effects of exercise on IL-6 production in monocytes stimulated with LPS, intracellular IL-6 expression in monocytes stimulated with zymosan was significantly increased in samples obtained following exercise compared with samples obtained at rest (Fig. 6).
Figure 6. The effect of exercise on intracellular interleukin (IL)-6 expression in CD14+ monocytes.
Peripheral blood samples were obtained from 10 healthy volunteers before, immediately after, and following 2 h of resting recovery from 90 min of exercise at 55%Wmax in the heat (34°C). Samples were incubated with LPS (TLR4 ligand), zymosan (TLR2 and 6 ligand), or with culture media only for 6 h, following which monocyte intracellular IL-6 expression was examined by flow cytometry. All data represent means ± s.e.m.†Statistically significant (P < 0.01) difference from pre-exercise.
The effect of exercise on plasma hormone and glucose concentrations
Exercise resulted in a significant elevation in the circulating concentrations of ACTH and growth hormone (Table 1). Plasma cortisol concentrations were similar in post-exercise samples compared with samples obtained at rest; however, the cortisol concentration was significantly lower in samples obtained at 2 h post-exercise compared with samples obtained at rest and post-exercise (Table 1). Exercise had no significant effect on the plasma glucose concentration (Table 1).
Table 1.
The effect of exercise on circulating hormone and glucose concentrations
| Pre-exercise | Post-exercise | 2 h post-exercise | |
|---|---|---|---|
| Cortisol (nmol l−1) | 560 (19) | 615 (46) | 412 (24)*† |
| ACTH (pg ml−1) | 37 (8) | 116 (22)* | 9 (3) |
| GH (μIU ml−1) | 0 (0) | 18 (3)* | 1 (0) |
| Glucose (mmol l−1) | 4.6 (0.1) | 4.7 (0.2) | 4.5 (0.1) |
Peripheral blood samples were obtained from 11 healthy volunteers before, immediately after and following 2 h of resting recovery from 90 min of exercise at 55% maximal work rate in the heat (34°C). All data represent the means ± s.e.m.
Statistically significant (P < 0.01) difference from pre-exercise.
Statistically significant (P < 0.01) difference from post-exercise.
GH, gth hormone.
Discussion
The discovery of a human homologue of the Drosophila Toll protein, and the subsequent identification and characterization of a family of mammalian TLRs, have provided new insights into the development of innate and adaptive immunity. Activation of TLRs leads to the induction of antimicrobial activity and the production of inflammatory cytokines, events central to innate defence, and the upregulation of costimulatory molecules and cytokines, which provide secondary signals critical to the initiation and development of adaptive immunity (Takeda et al. 2003). In these experiments, we have examined the physiological regulation of TLR expression and activation in vivo in humans by analysing the influence of circadian rhythmicity and strenuous exercise.
The expression of TLR1, TLR2 and TLR4 on monocytes was markedly reduced in samples obtained immediately after, and following 2 h of resting recovery from 1.5 h of strenuous exercise, in comparison with samples obtained at rest; no effect of exercise on TLR9 expression was observed. In contrast, we observed no diurnal variation in the expression of TLR1, TLR2, TLR4 or TLR9 on monocytes, and no change in the expression of TLR1, TLR2 or TLR4 on neutrophils. It has been demonstrated previously that GCs modulate TLR expression in vitro (Galon et al. 2002). However, in the present study we observed no circadian rhymicity in TLR expression despite large changes in the endogenous cortisol concentration, and a significant decrease in TLR expression following strenuous exercise despite only a moderate change in circulating cortisol concentrations. Taken together, these data suggest that GCs do not play a major role in regulating TLR expression under physiological conditions in vivo. While exercise, surgery (Dybdahl et al. 2002), endotoxaemia (Marsik et al. 2003) and ageing (Renshaw et al. 2002) have all been associated with changes in TLR expression, the factors responsible are unclear, and while our data provide evidence against a role for GCs in regulating TLR expression, a variety of factors are known to regulate TLR expression. In particular, several cytokines have been shown to regulate the expression of TLRs in vitro (Staege et al. 2000; Miettinen et al. 2001; Krutzik et al. 2003), and increases in the concentrations of specific cytokines have been associated with changes in TLR expression in vivo in human disease conditions (Krutzik et al. 2003; Seibl et al. 2003). Interestingly, exercise induces a large and sustained increase in the circulating concentration of several cytokines (Febbraio & Pedersen, 2002), and it is possible that changes in the circulating cytokine environment may contribute to alterations in the expression of TLRs following exercise.
The upregulation of the accessory signals CD80, CD86 and MHC class II following TLR activation is critical to the generation of adaptive immune responses. Here, we have found that the upregulation of the costimulatory molecules CD80 and CD86 and the antigen-presenting molecule MHC class II on CD14+ monocytes following treatment with a variety of TLR ligands was significantly greater in samples obtained in the evening compared with the morning. Furthermore, the upregulation of CD80, CD86 and MHC class II expression on CD14+ monocytes following TLR activation was significantly lower in samples obtained immediately after, and following 2 h of resting recovery from 1.5 h of strenuous exercise, in comparison with samples obtained at rest. Importantly, NF-κB activation is required for the upregulation of CD80, CD86 and MHC class II expression (Yoshimura et al. 2001), and GCs potently negatively regulate NF-κB activity (Almawi et al. 2002). Although correlative, our data, which demonstrate diurnal rhymicity in the upregulation of CD80, CD86 and MHC class II expression, provide evidence that GCs modulate TLR function under physiological conditions in vivo. Interestingly, we observed a significant decrease in the upregulation of CD80, CD86 and MHC class II molecules following TLR activation, despite only modest changes in the circulating cortisol concentration. These data strongly suggest that exercise-induced alterations in the circulating cortisol concentration are not responsible for the suppression in TLR function that we observed following exercise. Given that prolonged strenuous exercise results in a marked elevation in the circulation concentration of several cytokines, and that cytokines have been shown to have potent effects on monocyte TLR function in vitro (Krutzik et al. 2003), the exercise-induced alteration in the cytokine environment represents a potential mechanism by which exercise may affect TLR function.
In addition to its well-characterized role in the development of the inflammatory response, it was recently shown that IL-6 production following activation of TLRs is critical to the development of adaptive immune responses (Pasare & Medzhitov, 2003). Similar to the effects of exercise on the upregulation of CD80, CD86 and MHC class II expression, LPS-stimulated monocyte intracellular IL-6 expression was significantly lower in samples obtained immediately after, and following 2 h of resting recovery from 1.5 h of strenuous exercise in comparison with samples obtained at rest. However, intracellular IL-6 expression following zymosan treatment was significantly higher in samples obtained following exercise compared with samples obtained at rest. Given that DEX treatment at physiological levels causes suppression in both LPS- and zymosan-stimulated monocyte intracellular IL-6 production in vitro (G.I. Lancaster, Q. Khan, P. Drysdale, F. Wallace, A.E. Jeukendrup, M.T. Drayson & M. Gleeson, unpublished observations), these data argue against a role for exercise-induced elevations in the circulating cortisol concentration in the modulation of monocyte IL-6 production following exercise. Elucidation of the factors responsible for this differential effect of exercise on monocyte IL-6 production awaits further research.
In human blood, two monocyte populations have been identified, the CD14++CD16−HLA-DR+ classical monocytes and the CD14+CD16+HLA-DR++ pro-inflammatory monocytes (Ziegler-Heitbrock, 1996). It could be argued that the effects we observed of diurnal rhythmicity and exercise on TLR expression and function are due to the differential mobilization of monocyte subsets to the circulation. Indeed the CD14+CD16+ pro-inflammatory monocytes have a higher surface expression of TLR2, and produce a greater amount of tumour necrosis factor (TNF)-α following treatment with LPS or Pam3Cys (TLR2 ligand) compared with the CD14+CD16− classical monocytes (Belge et al. 2002). However, CD14+CD16+ monocytes are mobilized to the circulation to a greater extent than CD14+CD16− monocytes during exercise (Steppich et al. 2000), indicating that the effects of diurnal rhythmicity and exercise on TLR expression and function that we have observed are not due the mobilization of phenotypically distinct monocyte subsets.
In the present study, we have demonstrated that TLR function displays a diurnal rhythmicity, and that prolonged strenuous exercise causes suppression of both TLR expression and function. Our data (diurnal rhythm study) provide the first evidence that immunomodulatory hormones may influence TLR function in vivo. Furthermore, our data also demonstrate that strenuous exercise is a potent modulator of TLR expression and function, and, furthermore, that this effect appears to be independent of changes in immunomodulatory hormones. Further studies are required to confirm the possible influences of hormones and cytokines in the modulation of TLR function in vivo. Finally, epidemiological data indicate that individuals engaged in intensive exercise training have an increased susceptibility to upper respiratory tract infections (Peters & Bateman, 1983; Fitzgerald, 1988; Nieman et al. 1990; Peters et al. 1993). It is possible that the exercise-induced suppression in TLR expression and function that we have observed in the present study may provide a mechanistic basis for these observations. Given the crucial roles of the upregulation of costimulatory molecules, and the production of cytokines by APCs following the activation of TLRs, to the generation of adaptive immune responses (Schnare et al. 2001), future work will be required to examine the clinical importance of changes in TLR expression and function to the generation of in vivo immune responses.
Acknowledgments
The authors gratefully acknowledge the financial support of GlaxoSmithKline consumer healthcare for providing funding for the present study.
References
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Thomas V, Schnare M, Lobet Y, Anguita J, Schoen RT, Medzhitov R, Fikrig E, Flavell RA. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat Med. 2002;8:878–884. doi: 10.1038/nm732. [DOI] [PubMed] [Google Scholar]
- Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–739. doi: 10.1126/science.285.5428.736. 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- Almawi WY, Abou Jaoude MM, Li XC. Transcriptional and post-transcriptional mechanisms of glucocorticoid antiproliferative effects. Hematol Oncol. 2002;20:17–32. doi: 10.1002/hon.684. 10.1002/hon.684. [DOI] [PubMed] [Google Scholar]
- Angeli A, Gatti G, Sartori ML, Masera RG. Chronobiological aspects of the neuroendocrine-immune network. Regulation of human natural killer (NK) cell activity as a model. Chronobiologia. 1992;19:93–110. [PubMed] [Google Scholar]
- Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, Espevik T, Ziegler-Heitbrock L. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536–3542. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430:257–263. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, Stein M, McEwen BS, Spencer RL. Diurnal and acute stress-induced changes in distribution of peripheral blood leukocyte subpopulations. Brain Behav Immun. 1994;8:66–79. doi: 10.1006/brbi.1994.1006. 10.1006/brbi.1994.1006. [DOI] [PubMed] [Google Scholar]
- Dybdahl B, Wahba A, Lien E, Flo TH, Waage A, Qureshi N, Sellevold OF, Espevik T, Sundan A. Inflammatory response after open-heart surgery: release of heat-shock protein 70 and signaling through toll-like receptor-4. Circulation. 2002;105:685–690. doi: 10.1161/hc0602.103617. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Pedersen BK. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J. 2002;16:1335–1347. doi: 10.1096/fj.01-0876rev. 10.1096/fj.01-0876rev. [DOI] [PubMed] [Google Scholar]
- Fitzgerald L. Exercise and the immune system. Immunol Today. 1988;9:337–339. doi: 10.1016/0167-5699(88)91332-1. 10.1016/0167-5699(88)91332-1. [DOI] [PubMed] [Google Scholar]
- Galon J, Franchimont D, Hiroi N, Frey G, Boettner A, Ehrhart-Bornstein M, O'Shea JJ, Chrousos GP, Bornstein SR. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J. 2002;16:61–71. doi: 10.1096/fj.01-0245com. 10.1096/fj.01-0245com. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- Imasato A, Desbois-Mouthon C, Han J, Kai H, Cato AC, Akira S, Li JD. Inhibition of p38 MAPK by glucocorticoids via induction of MAPK phosphatase-1 enhances nontypeable Haemophilus influenzae-induced expression of toll-like receptor 2. J Biol Chem. 2002;277:47444–47450. doi: 10.1074/jbc.M208140200. 10.1074/jbc.M208140200. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Jentjens RL, Wagenmakers AJ, Jeukendrup AE. Heat stress increases muscle glycogen use but reduces the oxidation of ingested carbohydrates during exercise. J Appl Physiol. 2002;92:1562–1572. doi: 10.1152/japplphysiol.00482.2001. [DOI] [PubMed] [Google Scholar]
- Krutzik SR, Ochoa MT, Sieling PA, Uematsu S, Ng YW, Legaspi A, Liu PT, Cole ST, Godowski PJ, Maeda Y, Sarno EN, Norgard MV, Brennan PJ, Akira S, Rea TH, Modlin RL. Activation and regulation of Toll-like receptors 2 and 1 in human leprosy. Nat Med. 2003;9:525–532. doi: 10.1038/nm864. 10.1038/nm864. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- Marsik C, Mayr F, Cardona F, Derhaschnig U, Wagner OF, Jilma B. Endotoxaemia modulates Toll-like receptors on leucocytes in humans. Br J Haematol. 2003;121:653–656. doi: 10.1046/j.1365-2141.2003.04350.x. 10.1046/j.1365-2141.2003.04350.x. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Janeway CA. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- Miettinen M, Sareneva T, Julkunen I, Matikainen S. IFNs activate toll-like receptor gene expression in viral infections. Genes Immun. 2001;2:349–355. doi: 10.1038/sj.gene.6363791. 10.1038/sj.gene.6363791. [DOI] [PubMed] [Google Scholar]
- Nieman DC, Johanssen LM, Lee JW, Arabatzis K. Infectious episodes in runners before and after the Los Angeles Marathon. J Sports Med Phys Fitness. 1990;30:316–328. [PubMed] [Google Scholar]
- Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T-cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev. 2000;80:1055–1081. doi: 10.1152/physrev.2000.80.3.1055. [DOI] [PubMed] [Google Scholar]
- Peters EM, Bateman ED. Ultramarathon running and upper respiratory tract infections. An epidemiological survey. S Afr Med J. 1983;64:582–584. [PubMed] [Google Scholar]
- Peters EM, Goetzsche JM, Grobbelaar B, Noakes TD. Vitamin C supplementation reduces the incidence of postrace symptoms of upper-respiratory-tract infection in ultramarathon runners. Am J Clin Nutr. 1993;57:170–174. doi: 10.1093/ajcn/57.2.170. [DOI] [PubMed] [Google Scholar]
- Petrovsky N, Harrison LC. Diurnal rhythmicity of human cytokine production: a dynamic disequilibrium in T helper cell type 1/T helper cell type 2 balance? J Immunol. 1997;158:5163–5168. [PubMed] [Google Scholar]
- Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169:4697–4701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- Scanga CA, Aliberti J, Jankovic D, Tilloy F, Bennouna S, Denkers EY, Medzhitov R, Sher A. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J Immunol. 2002;168:5997–6001. doi: 10.4049/jimmunol.168.12.5997. [DOI] [PubMed] [Google Scholar]
- Schnare M, Barton GM, Holt AC, Takeda K, Akira S, Medzhitov R. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. doi: 10.1038/ni712. 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- Seibl R, Birchler T, Loeliger S, Hossle JP, Gay RE, Saurenmann T, Michel BA, Seger RA, Gay S, Lauener RP. Expression and regulation of Toll-like receptor 2 in rheumatoid arthritis synovium. Am J Pathol. 2003;162:1221–1227. doi: 10.1016/S0002-9440(10)63918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki E, Tsutsui H, Tsuji NM, Hayashi N, Adachi K, Nakano H, Futatsugi-Yumikura S, Takeuchi O, Hoshino K, Akira S, Fujimoto J, Nakanishi K. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J Immunol. 2002;169:3863–3868. doi: 10.4049/jimmunol.169.7.3863. [DOI] [PubMed] [Google Scholar]
- Shuto T, Imasato A, Jono H, Sakai A, Xu H, Watanabe T, Rixter DD, Kai H, Andalibi A, Linthicum F, Guan YL, Han J, Cato AC, Lim DJ, Akira S, Li JD. Glucocorticoids synergistically enhance nontypeable Haemophilus influenzae-induced Toll-like receptor 2 expression via a negative cross-talk with p38 MAP kinase. J Biol Chem. 2002;277:17263–17270. doi: 10.1074/jbc.M112190200. 10.1074/jbc.M112190200. [DOI] [PubMed] [Google Scholar]
- Staege H, Schaffner A, Schneemann M. Human toll-like receptors 2 and 4 are targets for deactivation of mononuclear phagocytes by interleukin-4. Immunol Lett. 2000;71:1–3. doi: 10.1016/s0165-2478(99)00168-6. 10.1016/S0165-2478(99)00168-6. [DOI] [PubMed] [Google Scholar]
- Steppich B, Dayyani F, Gruber R, Lorenz R, Mack M, Ziegler-Heitbrock HW. Selective mobilization of CD14+ CD16+ monocytes by exercise. Am J Physiol Cell Physiol. 2000;279:C578–586. doi: 10.1152/ajpcell.2000.279.3.C578. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol. 2000;165:5392–5396. doi: 10.4049/jimmunol.165.10.5392. [DOI] [PubMed] [Google Scholar]
- Thoma-Uszynski S, Stenger S, Takeuchi O, Ochoa MT, Engele M, Sieling PA, Barnes PF, Rollinghoff M, Bolcskei PL, Wagner M, Akira S, Norgard MV, Belisle JT, Godowski PJ, Bloom BR, Modlin RL. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science. 2001;291:1544–1547. doi: 10.1126/science.291.5508.1544. 10.1126/science.291.5508.1544. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T, Hoshino K, Takeuchi O, Kobayashi M, Fujita T, Takeda K, Akira S. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature. 2002;420:324–329. doi: 10.1038/nature01182. 10.1038/nature01182. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Bondeson J, Brennan FM, Foxwell BM, Feldmann M. Role of NFkappaB in antigen presentation and development of regulatory T cells elucidated by treatment of dendritic cells with the proteasome inhibitor PSI. Eur J Immunol. 2001;31:1883–1893. doi: 10.1002/1521-4141(200106)31:6<1883::aid-immu1883>3.0.co;2-v. 10.1002/1521-4141(200106)31:6<1883::AID-IMMU1883>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock HW. Heterogeneity of human blood monocytes: the CD14+CD16+ subpopulation. Immunol Today. 1996;17:424–428. doi: 10.1016/0167-5699(96)10029-3. 10.1016/0167-5699(96)10029-3. [DOI] [PubMed] [Google Scholar]