Abstract
Thermoregulatory cutaneous vasodilatation (VD) is attenuated in aged skin. While acetylcholine (ACh) plays a role in thermally mediated VD, the precise mechanisms through which ACh-mediated VD acts and whether those downstream mechanisms change with ageing are unclear. We tested the hypotheses that both nitric oxide (NO)- and prostanoid-mediated pathways contribute to exogenous ACh-mediated VD, and that both are attenuated with advanced age. Twelve young (Y: 23 ± 1 years) and 10 older (O: 69 ± 1 years) subjects underwent infusions of 137.5 μm ACh at four intradermal microdialysis sites: control (C, Ringer solution), NO synthase inhibited (NOS-I, 10 mml-NAME), cyclooxygenase inhibited (COX-I, 10 mm ketorolac) and NOS-I + COX-I. Red blood cell flux was monitored using laser-Doppler flowmetry, and cutaneous vascular conductance (CVC) was calculated (laser-Doppler flux/mean arterial pressure) and normalized to maximal CVC (%CVCmax) (28 mm sodium nitroprusside + local heating to 43°C). Baseline %CVCmax was increased in the O at COX-I sites (COX-I 16 ± 1, NOS-I + COX-I 16 ± 2 versus C 10 ± 1%CVCmax; P < 0.001) but not in the young, suggesting an age-related shift toward COX vasoconstrictors contributing to basal cutaneous vasomotor tone. There was no difference in peak %CVCmax during ACh infusion between age groups, and the response was unchanged by NOS-I (O: NOS-I 35 ± 5 versus C 38 ± 5%CVCmax; P = 0.84) (Y: NOS-I 41 ± 4 versus C 39 ± 4%CVCmax; P = 0.67). COX-I and NOS-I + COX-I attenuated the peak CVC response to ACh in both groups (COX-I O: 29 ± 3, Y: 22 ± 2%CVCmaxversus C; P < 0.001 both groups; NOS-I + COX-I O: 32 ± 3 versus Y: 29 ± 2%CVCmax; versus C; P < 0.001 both groups). ACh mediates cutaneous VD through prostanoid and non-NO-, non-prostanoid-dependent pathways. Further, older subjects have a diminished prostanoid contribution to ACh-mediated VD.
Skin blood flow is controlled by two branches of the sympathetic nervous system, a noradrenergic vasoconstrictor system and a cholinergic active vasodilator system (Grant & Holling, 1938). As core body temperature begins to rise, the initial increase in skin blood flow is mediated by a release of vasoconstrictor tone; upon reaching a specific threshold, sweating and reflex active vasodilatation are initiated, stimulating the co-release of acetylcholine (ACh) and an unknown vasodilator from sympathetic cholinergic nerves (Grant & Holling, 1938; Roddie et al. 1957; Kellogg et al. 1995, 1998). Nitric oxide (NO) appears to contribute approximately 30% to the total reflex vasodilatory response (Kellogg et al. 1998; Shastry et al. 1998). Vasoactive intestinal peptide has been identified as a potential cutaneous cholinergic vasodilator substance and is known to have a NO-dependent component (Bennett et al. 2003; Wilkins et al. 2004).
With advanced age, there is an attenuated reflex vasodilatory response (Kenney, 1988; Martin et al. 1995; Kenney et al. 1997). The initial rise in skin blood flow during hyperthermia is blunted in healthy older subjects, such that a greater rise in core temperature is required to observe significant reflex vasodilatation (Holowatz et al. 2003). Shibasaki et al. (2002) demonstrated that ACh may directly contribute to the initial rise in skin blood during hyperthermia in young healthy subjects. When acetylcholinesterases were inhibited by neostigmine administration, an augmented initial vasodilatory response was observed; however, this augmentation was abolished by the co-administration of neostigmine and a nitric oxide synthase (NOS) inhibitor. Furthermore, muscarinic receptor antagonism with atropine has no effect on skin blood flow when it is administered after skin blood flow has reached a plateau during established hyperthermia (Shastry et al. 2000). These results suggest that that ACh mediates a portion of the initiation of reflex cutaneous vasodilatation in young subjects, most likely through NO-dependent mechanisms.
The precise mechanisms of ACh-mediated vasodilatation in the cutaneous vasculature of young subjects remain unclear. It is generally hypothesized that ACh produces endothelium-dependent vasodilatation through NO-dependent, prostanoid-dependent, and non-NO-, nonprostanoid-dependent pathways. Furthermore, ACh can produce vasoconstriction by acting directly on the vascular smooth muscle (Collier & Vallance, 1990). Our understanding of the mechanisms of ACh-mediated vasodilatation is limited by the techniques used to deliver ACh to the cutaneous vasculature. Most studies investigating these mechanisms have used iontophoresis to deliver ACh to the skin, where the anodal current from this technique can cause cutaneous vasodilatation through prostanoid-dependent mechanisms (Grossmann et al. 1995; Khan et al. 1997; Noon et al. 1998; Durand 2002a et al. b, c). Moreover, iontophoretically initiated cutaneous vasodilatation varies as a function of the resistance of the vehicle used to dissolve and deliver ACh (Asberg et al. 1999; Droog & Sjoberg, 2003; Khan et al. 2004).
ACh-mediated vasodilatation in the cutaneous vasculature is also attenuated with advanced age (Algotsson et al. 1995). Studies in human forearm and cutaneous microvasculature demonstrate that there is a reduction in prostanoid-dependent vasodilatation with healthy ageing due to an increase in thromboxane vasoconstrictor activity and a decrease in prostacyclin-mediated vasodilator activity (Taddei et al. 1997; Buus et al. 2000; Heymes et al. 2000). Furthermore, we have previously shown a decrease in NO-mediated vasodilatation in aged skin (Minson et al. 2002). These data suggest that downstream pathways in ACh-mediated vasodilatation may be attenuated in aged skin. Therefore the purpose of the present study was to characterize ACh-mediated vasodilatation in the skin of young and older subjects. Specifically, by infusing exogenous ACh directly into the cutaneous vasculature via intradermal microdialysis along with specific antagonists, we sought to delineate the NO contribution and the prostanoid-dependent contribution to ACh-mediated vasodilatation. Finally, we hypothesized that older subjects would have attenuated ACh-mediated vasodilatation due to decreased NO- and prostanoid-dependent vasodilatation.
Methods
Subjects
Studies were performed on 12 young (23 ± 1 years) and 10 older (69 ± 1 years) men and women. Experimental protocols were approved by the Institutional Review Board at The Pennsylvania State University and conformed to the guidelines set forth by the Declaration of Helsinki. Verbal and written consent was voluntarily obtained from all subjects before their participation. Each subject underwent a complete medical screening including blood chemistry, lipid profile evaluation (Quest Diagnostics Nichol Institute, Chantily, VA, USA), physical examination, and an assessment of peak oxygen uptake ![]() (Parvomedics, Salt Lake City, UT, USA). All subjects were screened for the presence of cardiovascular, dermatological and neurological disease. Subjects were normally active, normotensive, healthy nonsmokers who were not currently taking medications, including aspirin therapy, hormone replacement therapy or oral contraceptives. All young female subjects were studied in the early follicular phase of their menstrual cycle. All subjects were asked to abstain from all non-steroidal anti-inflammatory products for at least a week prior to participation in the experiment.
(Parvomedics, Salt Lake City, UT, USA). All subjects were screened for the presence of cardiovascular, dermatological and neurological disease. Subjects were normally active, normotensive, healthy nonsmokers who were not currently taking medications, including aspirin therapy, hormone replacement therapy or oral contraceptives. All young female subjects were studied in the early follicular phase of their menstrual cycle. All subjects were asked to abstain from all non-steroidal anti-inflammatory products for at least a week prior to participation in the experiment.
Instrumentation
Upon arrival to the laboratory, subjects were seated in a reclined position and instrumented with four intradermal microdialysis fibres (MD 2000, Bioanalytical Systems, IN, USA) (10 mm, 20 kDa cutoff membrane) in the skin on the ventral side of the forearm. Microdialysis sites were spaced at least 4.0 cm apart to insure no cross-reactivity of pharmacological agents being delivered to the skin. Placement of the microdialysis fibres was accomplished at each site by first inserting a 25 gauge needle through the unanaesthetized skin using sterile technique. The entry and exit points were ∼2.5 cm apart. The microdialysis fibre was then threaded through the lumen of the needle, and the needle was withdrawn, leaving the fibre in place. The microdialysis fibres were then taped in place and attached to the outlet port of four individual stopcocks. Lactated Ringers solution was perfused through the microdialysis fibres at a rate of 2.0 μl min−1 (Harvard microinfusion pump, South Natick, MA, USA).
Measurements
To obtain an index of skin blood flow, cutaneous red blood cell (RBC) flux was measured with a laser-Doppler flowmeter probe placed in a local heater (Moor Instruments, Temperature Monitor SH02, MoorLAB, UK) over each microdialysis fibre. All laser-Doppler probes were calibrated using Brownian standard solution before each experiment. Cutaneous vascular conductance was calculated as RBC flux divided by mean arterial pressure (MAP). Blood pressure was measured via brachial auscultation every 10 min during the protocol. MAP was calculated as diastolic blood pressure plus one-third of the pulse pressure.
Protocol
After placement of the microdialysis fibres, RBC flux over each microdialysis site was monitored to ensure that the initial hyperaemia caused by the insertion trauma had resolved before the protocol started. Microdialysis fibres were randomly assigned to receive 10 mmNG-nitro-l-arginine methyl ester (l-NAME; Calbiochem, San Diego, CA, USA) to inhibit NO production by NOS, or 10 mm ketorolac (Sigma) to nonspecifically inhibit cyclooxygenase (COX), thereby inhibiting all prostanoid and thromboxane products through COX. Both NOS and COX were inhibited in a third microdialysis site with 10 mml-NAME + 10 mm ketorolac at final dilution. Our laboratory previously showed that this dose of l-NAME is sufficient to maximally inhibit NO production in both subject groups (Minson et al. 2002). Pilot work was performed by infusing separated microdialysis fibres with 2.5, 5, 10, 15, 20 and 25 mm doses of ketorolac, and then infusing increasing doses of ACh (68.7, 137.5 and 550 μm). We observed a dose-dependent rise in skin blood flow with increasing doses of ACh. Further, doses of ketorolac greater than 10 mm did not further reduce the skin blood flow response to ACh; therefore 10 mm ketorolac was used to maximally inhibit production of vasoactive substances from COX. All pharmacological agents were dissolved in lactated Ringers solution. A fourth microdialysis fibre was perfused with lactated Ringers solution and served as a control site. These infusions were maintained throughout the protocol.
The microdialysis fibres were perfused with assigned pharmacological agents at a rate of 2.0 μl min−1 for at least 75 min before ACh infusion to ensure adequate NOS and COX inhibition. After 10 min of baseline measurements of RBC flux were obtained, each microdialysis fibre was perfused with 137.5 μm ACh (Sigma) for 1 min at a rate of 2.0 μl min−1. Extensive pilot studies were performed to determine the appropriate dose of ACh that corresponded with the magnitude of cutaneous vasodilatation that was observed at the initiation of reflex vasodilatation. Precise infusion volumes were obtained using Liquid Switch stopcocks (CMA Microdialysis, Solma, Sweden). Infusions at the different microdialysis sites were separated by at least 2 min. Following a return to baseline RBC flux and at least 20 min after the first ACh infusion, a second infusion of the same dose was repeated at all sites. RBC flux was again allowed to return to baseline values. Blood pressure was measured at baseline, peak and upon returning to baseline during each ACh infusion. After completion of both ACh infusions, 28 mm sodium nitroprusside (SNP; Nitropress, Abbot Laboratories, Chicago, IL, USA) was perfused through all microdialysis fibres to achieve maximal cutaneous vascular conductance (CVC) at all sites. Local heating of the skin to 43°C was conducted after SNP infusion to ensure maximal CVC had been obtained.
Data acquisition and analysis
Data were acquired using Labview software and National Instruments data acquisition system (Austin, TX, USA). The data were digitized, recorded and stored on a personal computer for further analysis. The CVC data were averaged over 30 s periods, and are presented as a percentage of maximal CVC (%CVCmax).
Statistical analyses
Student's t tests were used to determine significant differences between the young and the old groups for physical characteristics. A two-way repeated measures analysis of variance was conducted within each group to detect potential differences between infusions at the different drug treatment sites. A three-way repeated measures analysis of variance was conducted to detect differences between subject groups at the drug treatment sites over time (SAS statistical software, version 8.01). Planned comparison tests, including Tukey post hoc tests, were performed when appropriate to determine where differences between groups and drug treatment occurred. The level of significance was set at α= 0.05. Values are means ± s.e.m.
Results
The physical characteristics of the subjects are presented in Table 1. There was no difference between the groups for body mass index, but the older subject group had a significantly lower ![]() (P < 0.001). There was no difference between the groups for total cholesterol (P = 0.12); however, there was a significant age difference in both high-density and low-density lipoproteins (P = 0.01, P = 0.02, respectively). There was a significant difference for MAP between the groups (P = 0.002); however, MAP did not change over the duration of the protocol.
(P < 0.001). There was no difference between the groups for total cholesterol (P = 0.12); however, there was a significant age difference in both high-density and low-density lipoproteins (P = 0.01, P = 0.02, respectively). There was a significant difference for MAP between the groups (P = 0.002); however, MAP did not change over the duration of the protocol.
Table 1.
Subject characteristics
| Young | Older | |
|---|---|---|
| Sex (M, F) | 7, 5 | 5, 5 |
| Age (years) | 23 ± 1 | 69 ± 1* |
| BMI (kg m−2) | 23 ± 1 | 23 ± 1 |
|
|
37 ± 2 | 23 ± 1* |
| Total cholesterol (mg dl−1) | 160 ± 7 | 184 ± 11 |
| HDL (mg dl−1) | 36 ± 5 | 53 ± 4* |
| LDL (mg dl−1) | 75 ± 7 | 109 ± 10* |
| Resting MAP (mmHg) | 86 ± 2 | 95 ± 2* |
Values are means ± s.e.m.; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MAP, mean arterial pressure; ![]() , peak rate of oxygen uptake; BMI, body mass index.
, peak rate of oxygen uptake; BMI, body mass index.
Significant difference from younger subjects (P < 0.05).
No significant physiological differences in the %CVCmax responses to ACh were observed between the sexes for either the older or the younger subject group; therefore, the data from both sexes in each group were combined. When drug treatment sites were compared within age group over time, there were no difference in %CVCmax between infusion 1 and infusion 2 (P = 1.0) for both subject groups. Therefore, data from both infusions were combined.
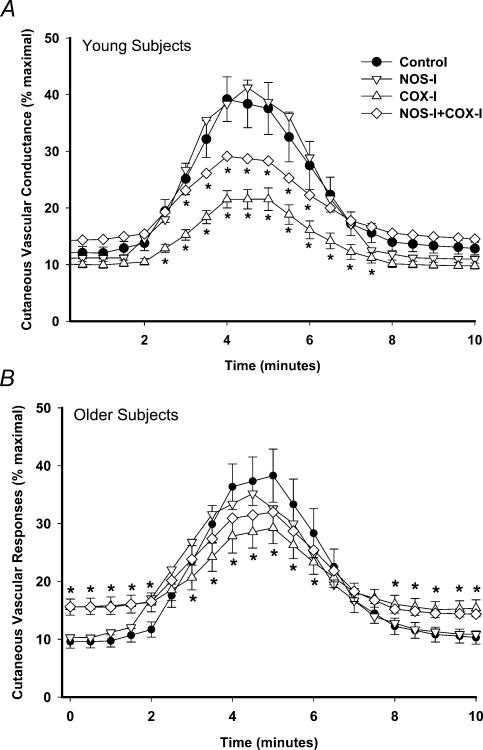
Group mean responses in all drug treatment sites over time to 137.5 μm ACh infusion are shown in Fig. 1A for the young subject group and Fig. 1B for the older subject group. There were no differences between the responses in the control and NOS-inhibited sites in either subject group. In the young subjects, COX inhibition caused a significant attenuation of the response in comparison to the control site (P < 0.001). Additionally, with NOS + COX inhibition, the response was attenuated compared to control (P < 0.001), but it was increased compared to COX inhibition alone (P < 0.001). In the older subjects COX inhibition and NOS + COX inhibition attenuated the %CVCmax response to ACh (P < 0.001), but there was no significant difference between these two drug treatment sites (P = 0.86).
Figure 1. Group mean responses in drug treatment sites to infusion of 137.5 μm acetylcholine (ACh).
A, young subjects (n = 12); B, older subjects (n = 10). • Control site, lactated Ringer infusion; ▿ nitric oxide synthase inhibited (NOS-I), 10 mmNG-nitro-l-arginine methyl ester (l-NAME); ▵ cylooxygenase inhibited (COX-I), 10 mm ketorolac; ⋄ NOS-I + COX-I, 10 mml-NAME+ 10 mm ketorolac. Standard error bars omitted for the NOS-I and NOS-I + COX-I sites for clarity. *Significant difference from the control site (P < 0.05).
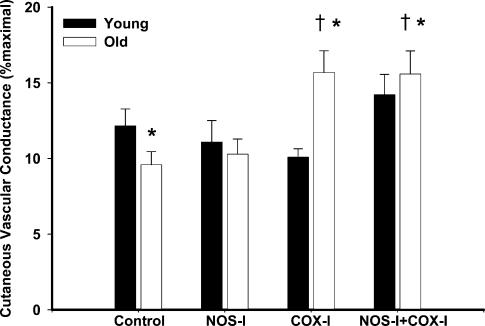
Group mean data for baseline %CVCmax in all of the drug treatment sites in young and older subjects are shown in Fig. 2. There was no difference between groups for baseline values at the control site or the NOS-inhibited site (O: 10 ± 1 versus Y: 12 ± 1%CVCmax, P = 0.18) and (O: 10 ± 1 versus Y: 11 ± 1%CVCmax, P: = 0.34), respectively. There was a significant increase in baseline %CVCmax above the control site in the older subject group in the COX-inhibited site that was not seen in the younger subject group (O: 16 ± 1 versus Y: 10 ± 1%CVCmax, P < 0.001; within group versus control O: P < 0.001, Y: P = 0.25). NOS + COX inhibition similarly resulted in an increase in baseline %CVCmax above control values in only the older subject group (O: 16 ± 2 versus Y: 14 ± 1%CVCmax, P < 0.001; within group versus control O: P < 0.001, Y: P = 0.19).
Figure 2. Baseline %CVCmax in all drug treatment sites before infusion of 137.5 μm ACh.
%CVCmax, percentage maximal cutaneous vascular conductance. Filled bars, young subjects (n = 12); open bars, older subjects (n = 10). Treatment with ketorolac alone (COX-I) and ketorolac +l-NAME (COX-I + NOS-I) significantly increased baseline in the older subjects. *P < 0.05, significant difference between groups; †P < 0.005, significant versus control site older subject group; ‡P < 0.005, significant versus control site young subject group.
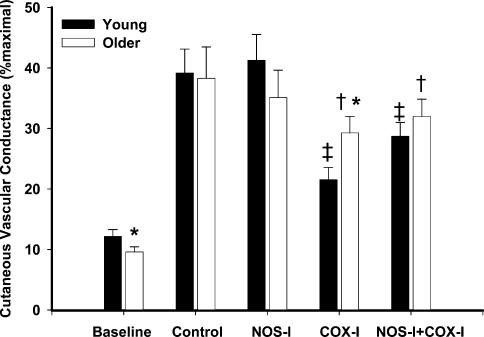
Group mean data for peak %CVCmax responses to infusion of 137.5 μm ACh in both subject groups are shown in Fig. 3. There was no difference between the groups for the peak responses at the control site (O: 38 ± 5 versus Y: 39 ± 4%CVCmax, P = 0.74). Furthermore, there were no significant differences between the control and the NOS inhibited sites within or between groups (O: 35 ± 5 versus Y: 41 ± 4%CVCmax, P = 0.16; within group versus control O: P = 0.84, Y: P = 0.67). COX inhibition significantly attenuated the peak responses to ACh in both subject groups compared to the control site (O: 29 ± 3 versus Y: 22 ± 2%CVCmax, P < 0.001). However, the older subject group exhibited attenuation, but to a lesser degree, in peak %CVCmax when COX was inhibited when compared to the young subject group (P < 0.001). Inhibiting both NOS + COX decreased the peak responses in both subject groups versus control (O: 32 ± 3, Y: 29 ± 2%CVCmax, P < 0.001). Peak responses during NOS + COX inhibition were increased compared to COX inhibition alone (P < 0.001) in the young subject group but not in the older subject group (P = 0.68).
Figure 3. Peak %CVCmax responses in all drug treatment sites to infusion of 137.5 μm ACh.
Filled bars, young subjects (n = 12); open bars, older subjects (n = 10). Peak %CVCmax was attenuated in both subject groups in sites treated with ketorolac (COX-I), but not in sites treated with l-NAME (NOS-I) alone,*P < 0.05, significant difference between groups; †P < 0.005, significant versus control site older subject group; ‡P < 0.005, significant versus control site young subject group.
Discussion
The major findings of the present study were that in young and older subjects, cutaneous vasodilatation to exogenous ACh is mediated by prostanoid-dependent and non-NO-, non-prostanoid-dependent pathways. NO does not directly contribute to ACh-mediated cutaneous vasodilatation in either age group. The older subjects exhibited an increase in baseline %CVCmax in sites where COX was inhibited, suggesting that there is an increase in COX pathway vasoconstrictor products that contribute to basal cutaneous vasomotor tone. Finally, there is an attenuated prostanoid-dependent contribution in the vasodilatory response to exogenous ACh as skin ages.
Mechanisms of ACh-mediated vasodilatation in young skin
Our findings implicate prostanoid-dependent as well as non-NO-, non-prostanoid-dependent contributions to exogenous ACh-mediated vasodilatation, but they indicate no direct role for NO. This in vivo finding supports in vitro data from Buus et al. (2000) who concluded that while the l-arginine/NO pathway is present in human isolated subcutaneous vessels, the NO pathway contributes only minimally to ACh-mediated vasodilatation. Studies using iontophoresis application of ACh have also demonstrated a limited role for NO in the cutaneous response to ACh (Khan et al. 1997; Noon et al. 1998). As validated by the data presented here, those authors attributed much of the observed cutaneous vasodilatation to prostanoid-dependent pathways.
In contrast to our finding for a limited direct role for NO in ACh-mediated vasodilatation, Boutsiouki et al. (2004) recently recovered NO byproducts in microdialysis dialysate in response to ACh infusion. Dialysate recovery of NO metabolites was subsequently abolished using 5 mml-NAME, but NOS inhibition only partially attenuated the vasodilator response by ∼30% (Boutsiouki et al. 2004), suggesting the involvement of other vasodilator pathways. Moreover, direct measurements of cutaneous NO in vivo during 160 mm ACh infusion have demonstrated limited measurable increases (Kellogg et al. 2003). Our data do not support a direct role for NO; however, it is possible that a small NO-dependent vasodilatation may be observed with higher doses of ACh such as those that were used in the Boutsiouki study (Boutsiouki et al. 2004). One possible mechanistic explanation for a limited direct role for NO in ACh-mediated vasodilatation is that NO may be quenched by superoxide anions produced from the COX pathway before acting on the vascular smooth muscle (Marcelin-Jimenez & Escalante, 2001; Bratz & Kanagy, 2004). Additionally, animal studies have shown that the NOS and the COX pathways interact in a complex manner capable of modulating enzymatic cross talk (Bratz & Kanagy, 2004), such that inhibiting one pathway may augment the vasodilator contributions of other pathways. In this construct, it may be possible to recover NO metabolites from microdialysis dialysate, but observe little or no direct contribution of NO and a significant COX contribution to ACh-mediated vasodilatation.
We found that a significant portion of ACh-mediated vasodilatation could not be abolished with either COX or NOS inhibition; by process of elimination, this suggests a non-NO-, non-prostanoid-dependent pathway contributes to ACh-mediated vasodilatation in the skin in humans. The remaining vasodilatation in response to ACh infusion could be attributed to endothelial-derived hyperpolarization factors (EDHF), which are most likely to be products of the arachidonic acid pathway. Although the identity of EDHF is unknown, metabolites of the cytochrome p450 enzymes and epoxyeicosatrienoic acids are good candidates as potenital EDHFs (Hecker et al. 1994; Mombouli & Vanhoutte, 1997; Hatoum et al. 2005). We found a significant portion of vasodilatation could not be blocked in the presence of COX inhibitors or with simultaneous NOS and COX inhibition (Figs 1 and 3), suggesting that EDHF-type substances may be more abundant in the skin when COX is inhibited, due to an increase in the availability and metabolism of arachidonic acid. One unexpected finding in the present study was that the cutaneous vascular response to ACh was actually higher when both NOS and COX were inhibited compared to COX inhibition alone. The most likely explanation for this response is an upregulation of the EDHF pathway(s) when all other vasodilatory pathways are inhibited, which further suggests the possibility of cross talk and redundant mechanisms at play in these pathways. Additional research is needed with specific inhibitors of arachidonic acid metabolism to determine possible EDHFs at play in this response. However, it is likely that there are many EDHFs with varied distribution within the branches of the cutaneous vasculature, as well as redundancy in dilator mechanisms between NO, prostanoids and EDHFs (Osanai et al. 2000). Exogenous infusion of ACh into the skin through microdialysis may prove to be a useful tool in future in vivo research on the identity of EDHFs in the cutaneous vasculature.
Age-related changes in ACh-mediated vasodilatation
Our findings indicate that aged human skin exhibits altered contributions of COX products to both tonic cutaneous blood flow and exogenous ACh-mediated vasodilatation. In the older subjects, baseline %CVCmax was significantly lower in comparison to the younger subjects in the control site, but was elevated in sites where COX was inhibited. One likely explanation for this response is that COX isoenzymes can produce both vasoconstrictor (PGE2, thromboxane A2) and well as vasodilator (PGI2) substances, and with advanced age there is a shift in the balance between COX vasoconstrictor and vasodilator products to favour vasoconstriction (Taddei et al. 1997; Matz et al. 2000a, b). Specifically, there may be alterations in the expression of the COX isoenzymes (Heymes et al. 2000). Data from the forearm circulation, where both NO- and prostanoid-dependent pathways contribute to ACh-mediated vasodilatation, suggests increases in COX-derived endothelial vasoconstrictor products in subjects with pathology-induced endothelial dysfunction (Taddei et al. 1997). This age-related shift toward increased vasoconstriction through alteration in COX products as well as COX isoenzyme expression may help to explain the baseline responses in the older subject group in the present study. Our data suggest that there is an increase in vasoconstrictor COX products that contribute to tonic cutaneous vascular tone in aged skin. Furthermore, peak %CVCmax responses to infusions of acetylcholine in COX-inhibited sites were augmented in older subjects compared to the young subjects, suggesting the nonspecific COX inhibition antagonized an upregulated COX-mediated vasoconstriction. Taken together, these data suggest that with advanced age there is a shift toward increased vasoconstrictor products of COX and a decrease in COX-mediated vasodilatation in the cutaneous vasculature.
We originally hypothesized we would find an attenuated vasodilatory response to exogenous ACh in the older subjects. Healthy ageing, in the absence of overt pathology, is associated with mild endothelial dysfunction (Singh et al. 2002), and a decreased vasodilatory response to ACh. Surprisingly, we did not find significant differences in the responses to acetylcholine between the subject groups at the control site. Although, we did not observe a significant difference in terms of %CVCmax, aged skin exhibits reduced maximal CVC (Martin et al. 1995) which may be masking the expected reduction in endothelium-dependent vasodilatation with ageing. That is, our choice to scale CVC as a percentage of maximal conductance in order to compare between experimental sites may be concealing larger potential differences between subject groups. We did, however, observe alteration in the downstream pathways mediating ACh-mediated vasodilatation in aged skin including an attenuated vasodilator prostanoid contribution.
ACh contributions to attenuated reflex vasodilatation with ageing
With advanced age there is attenuated reflex vasodilatation, including a substantial reduction in the initial rise in skin blood flow. We had previously attributed this attenuation in older subjects to a decreased ability of aged skin to respond to ACh (Holowatz et al. 2003). However, in the present study we found that healthy older subjects did not exhibit a significant reduction in cutaneous vasodilatation to this dose of exogenous ACh. Shibasaki et al. (2002) have implicated ACh mediating vasodilatation through NO-dependent mechanisms in the initial rise in skin blood flow during hyperthermia. Those authors observed an augmented initial rise in skin blood flow during acetylcholinesterase inhibition, but an attenuated response with concurrent NOS inhibition, suggesting that ACh contributes to the initial rise in skin blood flow during hyperthermia through NO-dependent mechanisms (Shibasaki et al. 2002). However, recent evidence has established a role for a histamine 1 (H1)-receptor-mediated component to the initial rise in reflex vasodilatation which is partially dependent on NO (Wong et al. 2004). Therefore, it is possible that ACh does not work directly through NO-dependent mechanisms during reflex cutaneous vasodilatation and, instead, what Shibasaki et al. (2002) attributed to NO-dependent vasodilatation through ACh may have been a H1 NO-dependent contribution. In other words, concurrent acethylcholinesterase and NOS inhibition would inhibit any NO-dependent vasodilatation whether it was mediated from ACh- or histamine-dependent mechanisms. In light of the evidence from Wong et al. (2004) and the data from our current study, it is possible that ACh mediates vasodilatation during the initial rise in skin blood flow during hyperthermia through non-NO-dependent mechanisms. An alternative hypothesis in terms of attenuated reflex cutaneous vasodilatation with ageing is that older subjects may have a reduced H1-receptor-mediated NO-contribution to the initial rise in skin blood flow. The contribution of H1-receptor-mediated reflex vasodilatation in aged skin, as well as the downstream ACh contributions, to the initial rise in skin blood flow during hyperthermia needs further investigation.
Limitations
ACh produces vasodilatation through endothelium-dependent mechanisms, as well as through neurogenic mechanisms, such as the axon reflex (Berghoff et al. 2002), although the precise mechanisms of the axon reflex and a potential role for ACh remain unclear. Many studies examining ACh-mediated vasodilatation have used iontophoresis as a way to deliver ACh to the skin, where anodal current from this technique alone has been shown to cause an axon reflex that is sensitive to systemic COX inhibition using acetylsalicylic acid (Morris & Shore, 1996; Berghoff et al. 2002; Durand et al. 2002a b, c). Moreover, other studies examining the ACh-induced axon reflex have used direct subcutaneous injections of ACh (Douglas & Ritchie, 1960). In this instance, the trauma from the injection can alone stimulate neurogenic axon reflexes. Boutsiouki et al. (2004) recently found that ACh delivered through a microdialysis fibre produced localized vasodilatation in response to low doses of ACh, and a more widespread flare response with an accompanying itch sensation at doses above 6.25 mm. Our dose of ACh was very small in comparison to the study by Boutsiouki et al. (2004), and we did not observe a flare response, nor did any of our subjects report feeling an itching sensation with ACh infusion. Our data are limited in that they do not allow us to differentiate between an axon reflex and endothelium-dependent vasodilatation. However, we attempted to minimize the potential for neurogenic vasodilatation by using the skin-specific technique of microdialysis instead of iontophoresis, and by delivering a low dose of ACh.
Methodological steps were taken in the present study to use sufficient doses of l-NAME and ketorolac to maximally inhibit the cutaneous vasodilatory response to stimulators of the NO and COX pathways. Inhibition of the NOS pathway has been demonstrated using 10 mml-NAME which maximally inhibits NO production in response to local heating of the skin of both young and aged subject groups (Minson et al. 2002). With regard to the dose of ketorolac used in this study, increasing the concentration of the COX antagonist did not further decrease the skin blood flow response to ACh. Additionally, our dose of ketorolac per volume of tissue was higher than that commonly given during arterial infusion studies (Dinenno & Joyner, 2004; Schrage et al. 2004). Even though we are confident that we would not have seen a different skin blood flow response to ACh with higher doses of antagonists in either pathway investigated, we cannot be certain that we maximally inhibited both the NO and the COX pathways. Therefore, we cannot rule out a larger role for NO or COX products in the cutaneous vascular response to ACh.
Summary
In summary, we found that NO did not directly contribute to ACh-mediated vasodilatation in either age group. Furthermore, prostanoid-dependent and non-NO-, non-prostanoid-dependent pathways contribute to cutaneous ACh-mediated vasodilatation. Our data suggest that older subjects exhibit alterations in COX vasoactive products to favour vasoconstriction. This shift may contribute to basal cutaneous vascular tone and to the attenuated vasodilator prostanoid contribution to ACh-mediated vasodilatation.
Acknowledgments
We are grateful for the outstanding technical and data collection assistance of Jane Pierzga, David Degroot and Meghan McElrone. We are appreciative of the all of the dedicated volunteers that participated in this study. Support for this study was provided by a grant from the National Institute on Ageing (R01-AG-07004-14), and additional support was provided by the General Clinical Research Center at The Pennsylvania State University, National Institute of Health (M01 RR 10732).
References
- Algotsson A, Nordberg A, Winblad B. Influence of age and gender on skin vessel reactivity to endothelium-dependent and endothelium-independent vasodilators tested with iontophoresis and a laser Doppler perfusion imager. J Gerontol A Biol Sci Med Sci. 1995;50:M121–127. doi: 10.1093/gerona/50a.2.m121. [DOI] [PubMed] [Google Scholar]
- Asberg A, Holm T, Vassbotn T, Andreassen AK, Hartmann A. Nonspecific microvascular vasodilation during iontophoresis is attenuated by application of hyperosmolar saline. Microvasc Res. 1999;58:41–48. doi: 10.1006/mvre.1999.2153. 10.1006/mvre.1999.2153. [DOI] [PubMed] [Google Scholar]
- Bennett LA, Johnson JM, Stephens DP, Saad AR, Kellogg DL., Jr Evidence for a role for vasoactive intestinal peptide in active vasodilatation in the cutaneous vasculature of humans. J Physiol. 2003;552:223–232. doi: 10.1113/jphysiol.2003.042135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghoff M, Kathpal M, Kilo S, Hilz MJ, Freeman R. Vascular and neural mechanisms of ACh-mediated vasodilation in the forearm cutaneous microcirculation. J Appl Physiol. 2002;92:780–788. doi: 10.1152/japplphysiol.01167.2000. [DOI] [PubMed] [Google Scholar]
- Boutsiouki P, Georgiou S, Clough GF. Recovery of nitric oxide from acetylcholine-mediated vasodilatation in human skin in vivo. Microcirculation. 2004;11:249–259. doi: 10.1080/10739680490425958. 10.1080/10739680490425958. [DOI] [PubMed] [Google Scholar]
- Bratz IN, Kanagy NL. Nitric oxide synthase-inhibition hypertension is associated with altered endothelial cyclooxygenase function. Am J Physiol Heart Circ Physiol. 2004;287:H2394–2401. doi: 10.1152/ajpheart.00628.2004. [DOI] [PubMed] [Google Scholar]
- Buus NH, Simonsen U, Pilegaard HK, Mulvany MJ. Nitric oxide, prostanoid and non-NO, non-prostanoid involvement in acetylcholine relaxation of isolated human small arteries. Br J Pharmacol. 2000;129:184–192. doi: 10.1038/sj.bjp.0703041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier J, Vallance P. Biphasic response to acetylcholine in human veins in vivo: the role of the endothelium. Clin Sci (Lond) 1990;78:101–104. doi: 10.1042/cs0780101. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Joyner MJ. Combined NO and PG inhibition augments alpha-adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol Heart Circ Physiol. 2004;287:H2576–2584. doi: 10.1152/ajpheart.00621.2004. 10.1152/ajpheart.00621.2004. [DOI] [PubMed] [Google Scholar]
- Douglas WW, Ritchie JM. The excitatory action of acetylcholine on cutaneous non-myelinated fibres. J Physiol. 1960;150:501–514. doi: 10.1113/jphysiol.1960.sp006401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droog EJ, Sjoberg F. Nonspecific vasodilatation during transdermal iontophoresis-the effect of voltage over the skin. Microvasc Res. 2003;65:172–178. doi: 10.1016/s0026-2862(03)00002-5. 10.1016/S0026-2862(03)00002-5. [DOI] [PubMed] [Google Scholar]
- Durand S, Fromy B, Bouye P, Saumet JL, Abraham P. Current-induced vasodilation during water iontophoresis (5 min, 0.10 mA) is delayed from current onset and involves aspirin sensitive mechanisms. J Vasc Res. 2002a;39:59–71. doi: 10.1159/000048994. 10.1159/000048994. [DOI] [PubMed] [Google Scholar]
- Durand S, Fromy B, Bouye P, Saumet JL, Abraham P. Vasodilatation in response to repeated anodal current application in the human skin relies on aspirin-sensitive mechanisms. J Physiol. 2002b;540:261–269. doi: 10.1113/jphysiol.2001.013364. 10.1113/jphysiol.2001.013364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand S, Fromy B, Koitka A, Tartas M, Saumet JL, Abraham P. Oral single high-dose aspirin results in a long-lived inhibition of anodal current-induced vasodilatation. Br J Pharmacol. 2002c;137:384–390. doi: 10.1038/sj.bjp.0704868. 10.1038/sj.bjp.0704868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant R, Holling H. Further observations on the vascular responses of the human limb to body warming: evidence for sympathetic vasodilator nerves in the normal subject. Clin Sci (Lond) 1938;3:273–285. [Google Scholar]
- Grossmann M, Jamieson MJ, Kellogg DL, Jr, Kosiba WA, Pergola PE, Crandall CG, et al. The effect of iontophoresis on the cutaneous vasculature: evidence for current-induced hyperemia. Microvasc Res. 1995;50:444–452. doi: 10.1006/mvre.1995.1070. 10.1006/mvre.1995.1070. [DOI] [PubMed] [Google Scholar]
- Hatoum OA, Binion DG, Miura H, Telford G, Otterson MF, Gutterman DD. Role of hydrogen peroxide in Ach-induced dilation of human submucosal intestinal microvessels. Am J Physiol Heart Circ Physiol. 2005;288:H48–54. doi: 10.1152/ajpheart.00663.2004. 10.1152/ajpheart.00663.2004. [DOI] [PubMed] [Google Scholar]
- Hecker M, Bara AT, Bauersachs J, Busse R. Characterization of endothelium-derived hyperpolarizing factor as a cytochrome P450-derived arachidonic acid metabolite in mammals. J Physiol. 1994;481:407–414. doi: 10.1113/jphysiol.1994.sp020449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymes C, Habib A, Yang D, Mathieu E, Marotte F, Samuel J, et al. Cyclo-oxygenase-1 and -2 contribution to endothelial dysfunction in ageing. Br J Pharmacol. 2000;131:804–810. doi: 10.1038/sj.bjp.0703632. 10.1038/sj.bjp.0703632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holowatz LA, Houghton BL, Wong BJ, Wilkins BW, Harding AW, Kenney WL, et al. Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. Am J Physiol Heart Circ Physiol. 2003;284:H1662–1667. doi: 10.1152/ajpheart.00871.2002. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Crandall CG, Liu Y, Charkoudian N, Johnson JM. Nitric oxide and cutaneous active vasodilation during heat stress in humans. J Appl Physiol. 1998;85:824–829. doi: 10.1152/jappl.1998.85.3.824. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Pergola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, et al. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res. 1995;77:1222–1228. doi: 10.1161/01.res.77.6.1222. [DOI] [PubMed] [Google Scholar]
- Kellogg DL, Jr, Zhao JL, Friel C, Roman LJ. Nitric oxide concentration increases in the cutaneous interstitial space during heat stress in humans. J Appl Physiol. 2003;94:1971–1977. doi: 10.1152/japplphysiol.00826.2002. [DOI] [PubMed] [Google Scholar]
- Kenney WL. Control of heat-induced cutaneous vasodilatation in relation to age. Eur J Appl Physiol. 1988;57:120–125. doi: 10.1007/BF00691250. 10.1007/BF00691250. [DOI] [PubMed] [Google Scholar]
- Kenney WL, Morgan AL, Farquhar WB, Brooks EM, Pierzga JM, Derr JA. Decreased active vasodilator sensitivity in aged skin. Am J Physiol Heart Circ Physiol. 1997;272:H1609–1614. doi: 10.1152/ajpheart.1997.272.4.H1609. [DOI] [PubMed] [Google Scholar]
- Khan F, Davidson NC, Littleford RC, Litchfield SJ, Struthers AD, Belch JJ. Cutaneous vascular responses to acetylcholine are mediated by a prostanoid-dependent mechanism in man. Vasc Med. 1997;2:82–86. doi: 10.1177/1358863X9700200202. [DOI] [PubMed] [Google Scholar]
- Khan F, Newton DJ, Smyth EC, Belch JJ. Influence of vehicle resistance on transdermal iontophoretic delivery of acetylcholine and sodium nitroprusside in humans. J Appl Physiol. 2004;97:883–887. doi: 10.1152/japplphysiol.00373.2004. 10.1152/japplphysiol.00373.2004. [DOI] [PubMed] [Google Scholar]
- Marcelin-Jimenez G, Escalante B. Functional and cellular interactions between nitric oxide and prostacyclin. Comp Biochem Physiol C Toxicol Pharmacol. 2001;129:349–359. doi: 10.1016/s1532-0456(01)00210-1. 10.1016/S1532-0456(01)00210-1. [DOI] [PubMed] [Google Scholar]
- Martin HL, Loomis JL, Kenney WL. Maximal skin vascular conductance in subjects aged 5–85 years. J Appl Physiol. 1995;79:297–301. doi: 10.1152/jappl.1995.79.1.297. [DOI] [PubMed] [Google Scholar]
- Matz RL, de Sotomayor MA, Schott C, Stoclet JC, Andriantsitohaina R. Vascular bed heterogeneity in age-related endothelial dysfunction with respect to NO and eicosanoids. Br J Pharmacol. 2000a;131:303–311. doi: 10.1038/sj.bjp.0703568. 10.1038/sj.bjp.0703568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matz RL, Schott C, Stoclet JC, Andriantsitohaina R. Age-related endothelial dysfunction with respect to nitric oxide, endothelium-derived hyperpolarizing factor and cyclooxygenase products. Physiol Res. 2000b;49:11–18. [PubMed] [Google Scholar]
- Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol. 2002;93:1644–1649. doi: 10.1152/japplphysiol.00229.2002. [DOI] [PubMed] [Google Scholar]
- Mombouli JV, Vanhoutte PM. Endothelium-derived hyperpolarizing factor(s): updating the unknown. Trends Pharmacol Sci. 1997;18:252–256. 10.1016/S0165-6147(97)90633-7. [PubMed] [Google Scholar]
- Morris SJ, Shore AC. Skin blood flow responses to the iontophoresis of acetylcholine and sodium nitroprusside in man: possible mechanisms. J Physiol. 1996;496:531–542. doi: 10.1113/jphysiol.1996.sp021704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noon JP, Walker BR, Hand MF, Webb DJ. Studies with iontophoretic administration of drugs to human dermal vessels in vivo: cholinergic vasodilatation is mediated by dilator prostanoids rather than nitric oxide. Br J Clin Pharmacol. 1998;45:545–550. doi: 10.1046/j.1365-2125.1998.00718.x. 10.1046/j.1365-2125.1998.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osanai T, Fujita N, Fujiwara N, Nakano T, Takahashi K, Guan W, et al. Cross talk of shear-induced production of prostacyclin and nitric oxide in endothelial cells. Am J Physiol Heart Circ Physiol. 2000;278:H233–238. doi: 10.1152/ajpheart.2000.278.1.H233. [DOI] [PubMed] [Google Scholar]
- Roddie IC, Shepherd JT, Whelan RF. The contribution of constrictor and dilator nerves to the skin vasodilatation during body heating. J Physiol. 1957;136:489–497. doi: 10.1113/jphysiol.1957.sp005775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrage WG, Dietz NM, Eisenach JH, Joyner MJ. Agonist-dependent variablity of contributions of nitric oxide and prostaglandins in human skeletal muscle. J Appl Physiol. 2005 doi: 10.1152/japplphysiol.00966.2004. (in press) [DOI] [PubMed] [Google Scholar]
- Shastry S, Dietz NM, Halliwill JR, Reed AS, Joyner MJ. Effects of nitric oxide synthase inhibition on cutaneous vasodilation during body heating in humans. J Appl Physiol. 1998;85:830–834. doi: 10.1152/jappl.1998.85.3.830. [DOI] [PubMed] [Google Scholar]
- Shastry S, Minson CT, Wilson SA, Dietz NM, Joyner MJ. Effects of atropine and l-NAME on cutaneous blood flow during body heating in humans. J Appl Physiol. 2000;88:467–472. doi: 10.1152/jappl.2000.88.2.467. [DOI] [PubMed] [Google Scholar]
- Shibasaki M, Wilson TE, Cui J, Crandall CG. Acetylcholine released from cholinergic nerves contributes to cutaneous vasodilation during heat stress. J Appl Physiol. 2002;93:1947–1951. doi: 10.1152/japplphysiol.00036.2002. [DOI] [PubMed] [Google Scholar]
- Singh N, Prasad S, Singer DR, MacAllister RJ. Ageing is associated with impairment of nitric oxide and prostanoid dilator pathways in the human forearm. Clin Sci (Lond) 2002;102:595–600. [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. Cyclooxygenase inhibition restores nitric oxide activity in essential hypertension. Hypertension. 1997;29:274–279. doi: 10.1161/01.hyp.29.1.274. [DOI] [PubMed] [Google Scholar]
- Wilkins BW, Chung LH, Tublitz NJ, Wong BJ, Minson CT. Mechanisms of vasoactive intestinal peptide-mediated vasodilation in human skin. J Appl Physiol. 2004;97:1291–1298. doi: 10.1152/japplphysiol.00366.2004. 10.1152/japplphysiol.00366.2004. [DOI] [PubMed] [Google Scholar]
- Wong BJ, Wilkins BW, Minson CT. H1 but not H2 histamine receptor activation contributes to the rise in skin blood flow during whole body heating in humans. J Physiol. 2004;560:941–948. doi: 10.1113/jphysiol.2004.071779. 10.1113/jphysiol.2004.071779. [DOI] [PMC free article] [PubMed] [Google Scholar]