Abstract
Various inhibitory pathways exist in the human brain which are crucial in modulating motor cortex output and they can be investigated non-invasively using transcranial magnetic stimulation. Interhemispheric inhibition (IHI) is one form of cortical inhibition. It can be elicited by stimulation of the opposite motor cortex at interstimulus intervals (ISIs) of 10 ms (IHI10) or 40 ms (IHI40) and inhibitions at these intervals are probably mediated by different mechanisms. Peripheral sensory stimulation can also inhibit the motor cortex. Median nerve stimulation produces long latency afferent inhibition (LAI) at ISI 200 ms. LAI inhibits another form of cortical inhibition known as long interval intracortical inhibition (LICI) and a study that examined the interaction between IHI10 and LICI hypothesized that they are mediated by an overlapping population of inhibitory neurones. We tested this hypothesis by examining the interaction between IHI10, IHI40 and LAI. With increasing test MEP amplitude LAI, IHI10 and IHI40 all decreased. There was no correlation between the strength of LAI, IHI10 and IHI40. In the presence of LAI, IHI10 was slightly but significantly reduced compared to IHI10 alone. There was no correlation between the reduction in IHI10 in the presence of LAI and the strength of LAI or IHI10. In the presence of LAI, IHI40 was significantly reduced compared to IHI40 alone. LAI produced a greater decrease in IHI40 than in IHI10. The decrease in IHI40 in the presence of LAI strongly correlated with the strength of LAI but not with the strength of IHI40. Reducing the strength of LAI, IHI10 and IHI40 still resulted in similar interaction between IHI10 and LAI but markedly decreased the effect of LAI on IHI40. We conclude that LAI and IHI10 do not directly inhibit each other but LAI probably inhibits IHI40. LICI is more likely to be related to IHI40 than to IHI10.
Transcranial magnetic stimulation (TMS) of the human motor cortex has been used to study different cortical inhibitory and excitatory pathways non-invasively (Hallett, 2000; Chen, 2000). These include cortico-cortical circuits as well as changes in cortical excitability induced by stimulation of other areas.
One form of cortico-cortical inhibition has been termed long interval intracortical inhibition (LICI) and is elicited by suprathreshold conditioning and test stimuli at interstimulus intervals (ISIs) of 50–200 ms. LICI may involve GABAB receptors (Siebner et al. 1998; Werhahn et al. 1999; Sanger et al. 2001). The motor cortex can also be inhibited by stimulation of the contralateral motor cortex or peripheral nerves. Interhemispheric inhibition (IHI) can be demonstrated by applying a conditioning stimulus (CS) to the contralateral motor cortex. This inhibits the size of the motor-evoked potential (MEP) produced by the test stimulus (TS) at ISIs between 8 and 50 ms (Ferbert et al. 1992; Hanajima et al. 2001; Chen et al. 2003). IHI at ISI of about 10 ms (IHI10) appears to be mediated by different mechanisms compared to IHI at ISI of about 40 ms (IHI40) (Chen et al. 2003; Gilio et al. 2003). The ipsilateral silent period (iSP) is another measure of IHI and involves the interruption of ongoing EMG activity of ipsilateral muscles. IHI40 appears to be related to the ipsilateral silent period (iSP) while IHI10 is not (Chen et al. 2003). Abnormalities in IHI10 and iSP have been demonstrated in various neurological and psychiatric conditions. For example, IHI10 is diminished in patients with schizophrenia (Daskalakis et al. 2002a) and iSP is reduced in patients with focal dystonia (Niehaus et al. 2001). IHI40 has not been examined in disease states. Peripheral nerve stimulation can also inhibit the contralateral motor cortex. Short latency afferent inhibition (Tokimura et al. 2000) can be elicited by median nerve stimulation (MNS) applied about 20 ms before a TS to the contralateral motor cortex, while long latency afferent inhibition (LAI) can be obtained at ISIs of about 200 ms (Chen et al. 1999; Sailer et al. 2002). LAI is reduced in patients with focal dystonia (Abbruzzese et al. 2001) and Parkinson's disease (Sailer et al. 2003).
Knowledge of how different inhibitory and facilitatory circuits interact may improve our understanding of the functional organization of the motor cortex and allow better interpretation of findings in disease states (Chen, 2004). Sailer et al. (2002) have investigated the interaction between LAI and cortico-cortical inhibitory circuits and found that LAI inhibits LICI. Daskalakis et al. (2002b) found that IHI10 was reduced in the presence of LICI.
Daskalakis et al. (2002b) suggested that a similar population of inhibitory neurones mediates LICI and IHI10. The goal of this study is to test this hypothesis by examining the interaction between LAI and IHI10. If the hypothesis is correct, then the interaction between LAI and IHI10 should be similar to the interaction between LAI and LICI. We also examined the interaction between LAI and IHI40 because there is little information on how IHI40 interacts with other cortical inhibitory circuits.
Methods
Subjects
We studied 15 healthy volunteers (9 males and 6 females, aged 35.1 ± 11.7 years (mean ±s.d.), range 22–60 years) in Expts 1 and 2, and 10 healthy volunteers (7 males and 3 females, aged 36.5 ± 13.7 years, range 21–61 years) in Expt 3. Three subjects participated in all three experiments. All subjects gave their written informed consent. The protocol was approved by the University Health Network Research Ethics Board in accordance with the Declaration of Helsinki on the use of human subjects in experiments.
Experimental set-up
This study involved three experiments. The first experiment (Expt 1) examined the effects of different test MEP amplitudes on IHI10, IHI40 and LAI. The second experiment (Expt 2) examined the effects of LAI on IHI10 and IHI40. The third experiment (Expt 3) examined the same interactions as Expt 2, but with less intense LAI, IHI10 and IHI40.
Left motor cortex stimulation
TMS was performed using a figure-of-eight coil (7 cm mid-diameter for each loop, P/N 9925-00) and two Magstim 200 (Magstim Company, Whitland, UK) stimulators connected by a BiStim module to allow us to deliver test stimuli (TS) at two different intensities through the same coil in the same experimental run. The magnetic coil was placed over the left motor cortex at the optimal position for eliciting motor-evoked potentials (MEPs) from the right first dorsal interosseus (FDI) muscle. The handle of the coil pointed backwards and was perpendicular to the presumed direction of the central sulcus, about 45 deg to the midsagittal line. The direction of the induced current was from posterior to anterior and was optimal to activate the motor cortex transsynaptically. The optimal position was marked on the scalp using a felt pen to ensure identical placement of the coil throughout the experiment. The motor threshold was defined as the lowest TMS intensity able to elicit MEPs of > 50 μV in at least 5 out of 10 trials with the muscles relaxed. It was expressed as a percentage of maximum stimulator output.
Right motor cortex stimulation
The second coil was connected to a third Magstim 200 stimulator and was used to deliver the contralateral conditioning stimulus (CCS) for IHI. The coil was placed over the right motor cortex at the optimal position for eliciting MEPs from the left FDI muscle. The handle of the coil pointed forward and laterally about 45 deg to the midsagittal line. This orientation was chosen because in some subjects it was not possible to place both coils at the optimal position with the handle pointing backwards and laterally due to the size of the coils relative to the subject's head. Stimulus intensity was set at 75% of maximum stimulator output for Expts 1 and 2. These parameters are based on a previous study that found no difference in IHI with different directions of the induced current and with stimulus intensities between 75% and 90% of maximum stimulator output (Chen et al. 2003). In Expt 3, the stimulus intensity was reduced to 50% of maximum stimulator output.
EMG recording
Surface electromyogram (EMG) was recorded from the right and left FDI muscles with disposable disc electrodes in a tendon–belly arrangement. The subjects were relaxed for the duration of the study. EMG was monitored by speakers at high gain and on a computer screen to ensure that the muscle was at rest. Trials with voluntary muscle activity were rejected. The signal was amplified (Intronix Technologies Corporation Model 2024F, Bolton, Ontario, Canada), filtered (band pass 2 Hz to 2.5 kHz), digitized at 5 kHz (Micro 1401, Cambridge Electronics Design (CED), Cambridge, UK), and stored in a laboratory computer for offline analysis.
Median nerve stimulation
The median nerve was stimulated at the right wrist with standard bar electrodes (0.2 ms square wave constant current pulses). The cathode was positioned proximally. Stimulus intensity was adjusted to produce a slight thumb twitch (Chen et al. 1999; Abbruzzese et al. 2001; Sailer et al. 2002) for Expts 1 and 2. For Expt 3, the stimulus intensity was reduced to 75% of that required to produce a slight thumb twitch.
Study design
We tested LAI, IHI10, IHI40 and their interactions. Each trial consisted of a conditioning stimulus followed by a suprathreshold TS. Conditioning pulses were named according to their timings before the test pulse. For LAI, the conditioning stimulus was median nerve stimulation 200 ms before the TS (MNS200) (Chen et al. 1999; Sailer et al. 2002). Previous studies have shown that MNS200 inhibits test responses without changes in spinal excitability (Chen et al. 1999; Classen et al. 2000). For IHI, a CCS at 75% or 50% of maximal stimulator output was applied 10 ms (IHI10) and 40 ms (IHI40) prior to the TS. The timing of the pulses was controlled by the output features of the A/D converter (Micro 1401, CED).
Test pulse intensities were labelled according to target MEP amplitudes. For example, TS 0.2 mV refers to the minimum stimulus intensity that produced > 0.2 mV MEPs in at least 5 out of 10 trials. Stimulus intensities for target MEPs of 1 mV and 4 mV were labelled similarly (TS 1 mV and TS 4 mV, respectively).
Experiment 1: effects of test stimulus intensity on LAI, IHI10 and IHI40
The test conditions were TS preceded by MNS200 (to elicit LAI), CCS10 (to elicit IHI10) or CCS40 (to elicit IHI40) and TS alone. Each run consisted of 10 trials of each condition (40 trials) delivered 6 s apart in random order. Three test stimulus intensities adjusted to produce MEPs of 0.2, 1 and 4 mV were studied in separate runs.
Experiment 2: interactions between LAI, IHI10 and IHI40
The interactions between LAI and IHI were investigated by comparing the effects of LAI and IHI alone to that of applying them together. The test conditions are listed in Table 1.
Table 1.
Configuration of pulses for Expt 2
| Condition | MNS200 | CCS10 | CCS40 | Test |
|---|---|---|---|---|
| 2A | 1 mV | |||
| 2B | + | 1 mV | ||
| 2C | 75% | 1 mV | ||
| 2D | 75% | 1 mV | ||
| 2E | 1 mVMNS200 | |||
| 2F | + | 1 mV MNS200 | ||
| 2G | 75% | 1 mV MNS200 | ||
| 2H | + | 75% | 1 mV MNS200 | |
| 2I | 75% | 1 mV MNS200 | ||
| 2J | + | 75% | 1 mV MNS200 |
MNS200, right median nerve stimulation delivered 200 ms before test stimulus to elicit long latency afferent inhibition (LAI) with the intensity adjusted to produce a thumb twitch; CCS10, contralateral conditioning stimulus delivered to the right motor cortex 10 ms before the test stimulus to elicit IHI10; CCS40, contralateral conditioning stimulus delivered to the right motor cortex 40 ms before the test stimulus to elicit IHI40. Test stimulus was delivered to the left motor cortex. For Expt 3, the MNS200 pulse was reduced to 75% of that needed to produce a thumb twitch, and the CCS10 and CCS40 pulses were set at 50% of maximum stimulator output.
LAI and IHI were derived from the ratios of the MEP amplitude induced in one test condition compared to another test condition. For example, LAI for a test MEP of 1 mV was calculated from the MEP amplitude of condition 2B (MNS200 followed by test stimulus) divided by the MEP amplitude of condition 2A (test stimulus alone) and will be abbreviated as 2B/2A. Similar notations will be used throughout the paper. Conditions 2A to 2D were used to determine LAI (2B/2A), IHI10 (2C/2A) and IHI40 (2D/2A) for a test MEP of 1 mV. In conditions 2E to 2J, the intensity of the test pulse was adjusted to produce 1 mV MEPs in the presence of median nerve stimulation 200 ms earlier (termed ‘1 mVMNS200’). Thus, the MEP amplitude in condition 2F was 1 mV. This allowed us to match the MEP amplitude to produce a similar degree of corticospinal activation with and without a preceding MNS200. IHI10 in the presence of MNS200 (2H/2F) was compared to IHI10 without MNS200 matched for a test MEP amplitude of 1 mV (2C/2A) and test stimulus intensity (2G/2E) (both 1 mVMNS200). Similarly, IHI40 in the presence of MNS200 (2J/2F) was compared to IHI40 without MNS200 matched for a test MEP amplitude of 1 mV (2D/2A) and test stimulus intensity (2I/2E) (both 1 mVMNS200). Each run consisted of 10 trials of each condition (100 trials) delivered 6 s apart in random order.
A previous study found that MNS may change the excitability of the ipsilateral motor cortex (Chen et al. 1999). Therefore, we determined the effects of right MNS on the ipsilateral (right) motor cortex by examining the MEPs from the left FDI muscle evoked by the CCS10 and CCS40 pulses. To determine the effect of ipsilateral MNS 190 ms before motor cortex stimulation, we compared the MEPs produced by MNS–CCS10 (2H) to the MEPs produced by CCS10 alone (2C and 2G). Similarly, the effect of ipsilateral MNS 160 ms before motor cortex stimulation was measured by comparing the MEPs produced by MNS–CCS40 (2J) to the MEPs produced by CCS40 alone (2D and 2I).
Experiment 3: interactions between LAI, IHI10 and IHI40 at submaximal inhibition
While Expt 2 examined the interactions between LAI and IHI near maximal inhibition, this experiment was designed to examine their interaction at submaximal levels of inhibition. The experimental design and test conditions used were identical to those of Expt 2 (Table 1) except that the stimulus intensity for MNS was reduced to 75% of that required to produce a thumb twitch and the stimulus intensity for CCS10 and CCS40 was reduced to 50% of stimulator output.
Data analysis
The peak-to-peak MEP amplitude for each trial was analysed offline. The MEP amplitude for each trial was expressed as a ratio of the mean unconditioned MEP amplitude for each subject. Ratios less than one indicate inhibition, and ratios greater than one indicate facilitation. To determine the change in IHI10 in the presence of LAI, the ‘change in IHI10’ was defined as the IHI10 in the presence of LAI (2H/2F) minus IHI10 alone (2C/2A, matched for MEP amplitude; or 2G/2E, matched for test stimulus intensity). Therefore, a positive value in the ‘change in IHI10’ indicates a reduction in IHI10 in the presence of LAI. Similar to IHI10 and LAI, the unit of this measurement is a fraction of the MEP amplitude produced by the test pulse alone. Similarly, the ‘change in IHI40’ was defined as IHI40 in the presence of LAI (2J/2F) minus IHI40 alone (2D/2A, matched for MEP amplitude; 2I/2E, matched for test stimulus intensity).
Statistical analysis
Values are expressed as means ± standard deviation (s.d.). For Expt 1, repeated-measures ANOVA and Fisher's protected least significant difference (PLSD) post hoc test was used to determine the effects of different test MEP amplitudes on LAI, IHI10 and IHI40. Correlations between LAI and IHI10 or IHI40 were tested by Pearson product-moment correlation coefficients.
For Expts 2 and 3, the effect of LAI on IHI10 was assessed by comparing IHI10 in the presence of LAI (2H/2F) to IHI10 alone matched for TS amplitude (2C/2A; TS 1 mV) and TS intensity (2G/2E; TS 1 mVMNS200) using repeated-measures ANOVA and Fisher's PLSD post hoc test. The effect of LAI on IHI40 was tested in a similar manner. The paired t test was used to compare the change in IHI10 to the change in IHI40 within each experiment. Changes in IHI10 and IHI40 in Expts 2 and 3 were compared with the unpaired t test.
For Expt 2, the paired t test was used to compare the MEP amplitude in the left FDI muscle evoked by CCS10 alone (2C and 2G) to that conditioned by ipsilateral MNS 190 ms earlier (2H) and the MEP evoked by CCS40 alone (2D and 2I) to that conditioned by ipsilateral MNS 160 ms earlier (2J).The significance level was set at P < 0.05.
Results
Experiment 1: effects of test stimulus intensity on LAI, IHI10 and IHI40
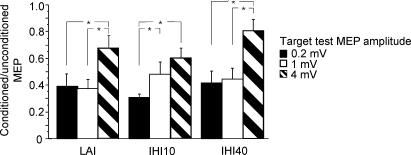
Fifteen subjects participated in this study. Data from one participant was excluded because we were unable to achieve MEPs greater than 1.5 mV due to high motor threshold. The motor threshold was 42.8 ± 5.8% of stimulator output. TS intensities were 46.4 ± 6.9% for the 0.2 mV condition, 52.9 ± 9.6% for the 1 mV condition and 70.0 ± 17.2% for the 4 mV condition. The MEP amplitude for the TS alone was 0.33 ± 0.11 mV for TS 0.2 mV, 1.18 ± 0.29 mV for TS 1 mV, and 3.50 ± 0.88 mV for TS 4 mV. The results are shown in Fig. 1. Repeated measures ANOVA showed that increasing TS intensity from 0.2 mV to 4 mV resulted in a significant decrease in LAI (P < 0.0004), IHI10 (P < 0.0022) and IHI40 (P < 0.0013). Post hoc testing demonstrated that LAI and IHI40 were significantly greater at TS 0.2 mV and TS 1 mV intensity than at the TS 4 mV intensity. IHI10 was significantly greater at TS 0.2 mV intensity than at TS 1 mV intensity or TS 4 mV intensity. There was no significant correlation among these measures of inhibition at each TS intensity.
Figure 1. Effects of different test MEP amplitudes on LAI, IHI10 and IHI40.
The y-axis shows the ratio of conditioned MEP to unconditioned MEP. Values less than 1 represent inhibition. For all three forms of inhibition, inhibition decreases significantly with increasing test MEP amplitude (repeated measures ANOVA). *Significant differences with post hoc testing. Error bars represent standard error of the mean.
Experiment 2: interactions between LAI and IHI10 and between LAI and IHI40
Fifteen subjects participated in this study. One subject was excluded because we were unable to achieve a similar degree of corticospinal activation with and without MNS. For the remaining 14 subjects, TS intensities were 53.4 ± 9.5% of stimulator output to elicit a 1 mV test MEP and 62.6 ± 13.2% to elicit a test MEP of 1 mV in the presence of MNS (termed 1 mVMNS200). The test MEP amplitude for TS 1 mV was 1.45 ± 0.68 mV (Table 1, condition 2A) and 2.9 ± 1.5 mV for TS 1 mVMNS200 (2E). When a TS of 1 mVMNS200 was preceded by MNS, the test MEP amplitude was 1.40 ± 0.67 mV (2F). Therefore, test MEP amplitudes for conditions 2A and 2F were matched.
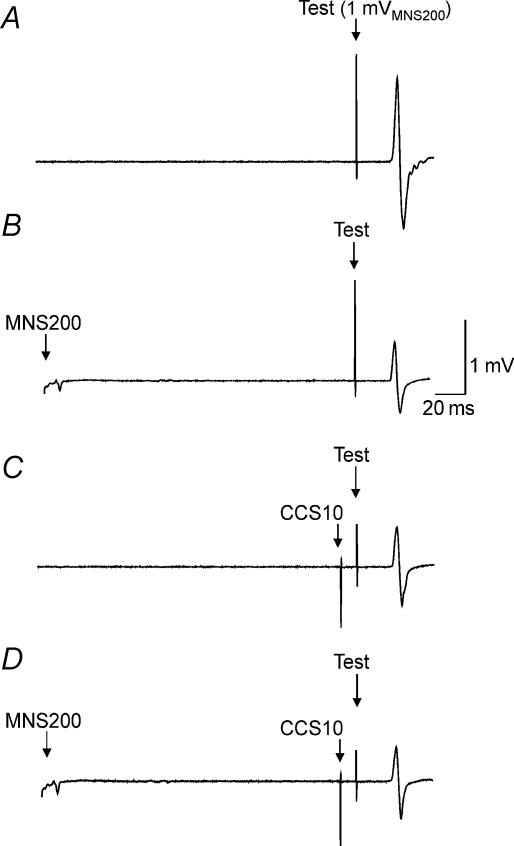
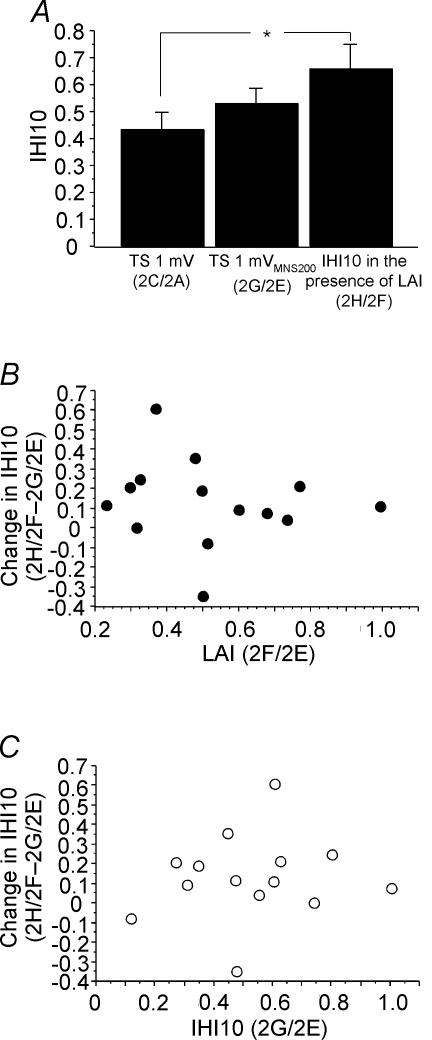
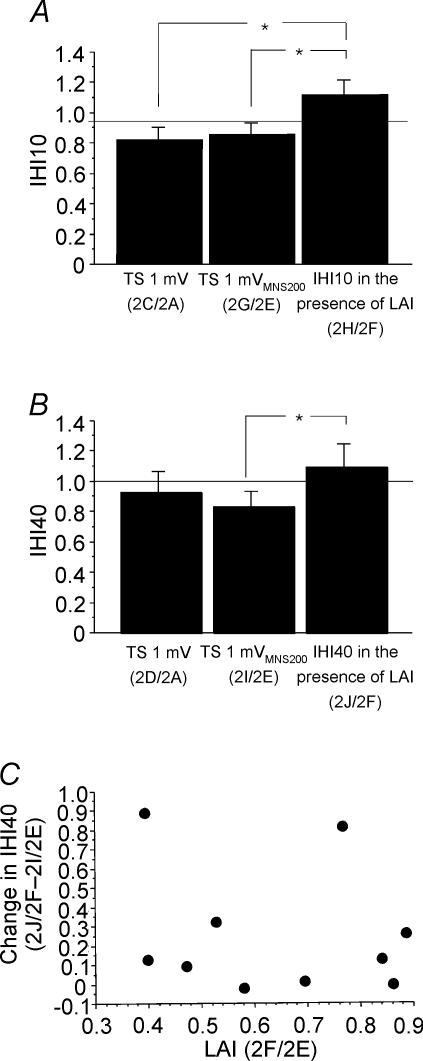
Figure 2 shows the effects of combining LAI and IHI10 in one representative subject. Compared to TS alone (Fig. 2A), a preceding MNS200 (Fig. 2B) or CCS10 (Fig. 2C) inhibited the test response. Combining LAI and IHI10 did not result in further inhibition. Figure 3A shows IHI10 for a 1 mV test MEP (2C/2A, Table 1), for a 1 mVMNS200 test MEP (2G/2E) and IHI10 in the presence of LAI (2H/2F) for all subjects. Repeated measures ANOVA showed significant effects of test conditions (P = 0.006). Post hoc testing showed that in the presence of LAI, IHI10 was significantly lower when matched for test MEP amplitude (P = 0.0015) (Fig. 3A, left and right columns) but not when matched for test stimulus intensity (Fig. 3A, centre and right columns). The degree of inhibition of IHI10 (condition 2H/2F – 2G/2E) did not correlate with the strength of LAI (2F/2E) or IHI10 (2G/2E) when matched for test stimulus intensity (Fig. 3B and C). It should be noted that two subjects did not demonstrate the inhibitory effect of LAI on IHI10 but showed increased inhibition when the two inhibitory effects were combined (Fig. 3B and C). Similarly, change in IHI10 (condition 2H/2F – 2C/2A) did not correlate with the strength of LAI (2B/2A, P = 0.80, r= 0.07) or IHI10 (2C/2A, P = 0.35, r= 0.27) when matched for test MEP amplitude.
Figure 2. Effects of LAI on IHI10 in a single subject.
Each trace represents an average of 10 trials in Expt 2. The test stimulus intensity was identical in all traces. Top trace (A) shows MEP evoked by the test stimulus alone of 1 mVMNS200 (condition 2E; Table 1). The middle traces show MEPs for LAI alone (B; condition 2F) and IHI10 alone (C; condition 2G). In both conditions the MEP amplitudes were decreased compared to the test stimulus alone. The effects of combining IHI10 and LAI are shown in the bottom trace (D; condition 2H). There is no further inhibition compared to LAI alone or IHI10 alone.
Figure 3. Effects of LAI on IHI10.
Data from 14 subjects. A: left column represents IHI10 alone for a 1 mV test MEP (2C/2A, Table 1); centre column represents IHI10 for a 1 mVMNS200 test MEP (2G/2E); right column represents IHI10 in the presence of LAI (2H/2F). The left and right columns are matched for test MEP amplitude (both ∼1 mV) and the centre and right columns are matched for test stimulus intensity (both 1 mVMNS200). *Significant changes shown by post hoc testing. Error bars represent standard errors of the mean. B, the relationship between LAI and the change in IHI10 revealed no significant correlation. Change in IHI10 is defined as IHI10 in the presence of LAI minus IHI10 alone, and is equivalent to the data from the right column minus the centre column of A. A positive value indicates that IHI10 decreased in the presence of LAI. C, relationship between IHI10 and change in IHI10. The change in IHI10 did not correlate with the strength of IHI10.
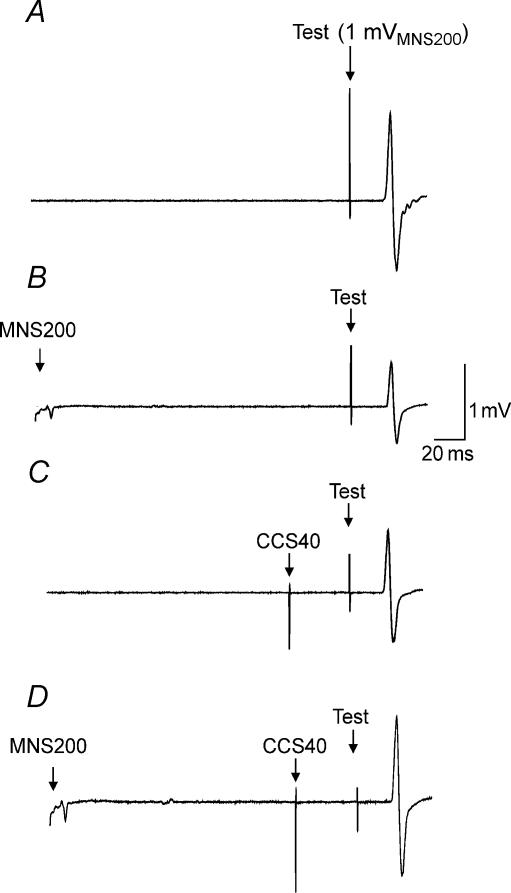
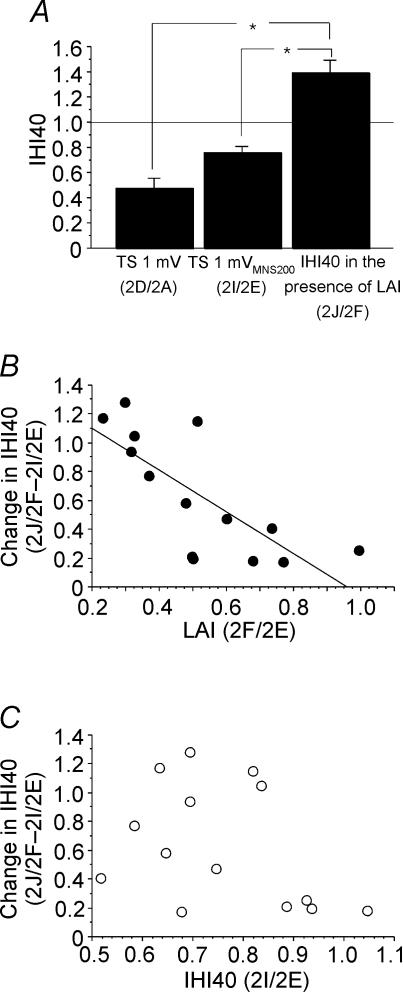
Figure 4 shows the effects of combining LAI and IHI40 for one representative subject. Compared to TS alone (Fig. 4A), a preceding MNS200 (Fig. 4B) or CCS40 (Fig. 4C) inhibited the test response. Combining LAI and IHI40 (Fig. 4D) not only did not result in further inhibition, but lead to a slight facilitation compared to IHI40 alone (Fig. 4B) or LAI alone (Fig. 4C). Figure 5A shows IHI40 for 1 mV test MEP (2D/2A, Table 1), for 1 mVMNS200 test MEP (2I/2E) and IHI40 in the presence of LAI (2J/2F) from all subjects. ANOVA showed significant effects of test conditions (P < 0.0001). Post hoc testing demonstrated that in the presence of LAI, IHI40 was significantly lower whether matched for test MEP amplitude (Fig. 5A, left and right columns, P < 0.0001) or test stimulus intensity (Fig. 5A, centre and right columns, P < 0.0001). When matched for test stimulus intensity, the decrease in IHI40 in the presence of LAI (condition 2J/2F – 2I/2E) correlated with the strength of LAI (2F/2E, P = 0.0022, r= 0.75, Fig. 5B) but not with the strength of IHI40 (2I/2E, Fig. 5C). In the presence of LAI, the decrease in IHI40 (0.63 ± 0.18, Fig. 5B and C) was significantly greater than the decrease in IHI10 (0.13 ± 0.22, Fig. 3B and C) (P = 0.0008, paired t test). Similarly, when matched for test MEP amplitude, the decrease in IHI40 in the presence of LAI (condition 2J/2F – 2D/2A) correlated with the strength of LAI (2B/2A, P = 0.005, r= 0.70) but not with the strength of IHI40 (2I/2E, P = 0.28, r= 0.31).
Figure 4. Effects of LAI on IHI40 in a single subject.
Each trace represents the average of 10 trials in Expt 2. The test stimulus intensity (1 mVMNS200) was identical in all traces. Top trace (A) shows the MEP evoked by test stimulus alone (condition 2E, Table 1). Both LAI alone (B; condition 2F) and IHI40 alone (C; condition 2I) decreased the MEP amplitude compared to the test stimulus alone. Combining IHI40 and LAI (D; condition 2J) resulted in no further inhibition but slight facilitation compared to B or C.
Figure 5. Effects of LAI on IHI40.
Data from 14 subjects. To produce a similar degree of corticospinal activation with and without LAI, test MEP amplitude was matched by increasing the TS when followed by median nerve stimulation. A, IHI40 in the presence of LAI was compared to IHI40 alone matched for TS amplitude (TS 1 mV) and TS intensity (TS 1 mVMNS200). The left column represents IHI40 alone for a 1 mV test MEP (2D/2A, Table 1); the centre column represents IHI40 for a 1 mVMNS200 test MEP (2I/2E); the right column represents IHI40 in the presence of LAI (2J/2F). The left and right columns are matched for test MEP amplitude (both ∼1 mV) and the centre and right columns are matched for test stimulus intensity (both 1 mVMNS200). *Significant changes shown by post hoc testing. Error bars represent standard errors of the mean. B, the relationship between LAI and the change in IHI40 demonstrated that the change in IHI40 correlated with the strength of LAI (P = 0.0022, r= 0.75). Change in IHI40 is defined as IHI40 in the presence of LAI minus IHI40 alone, and is equivalent to the right column minus the centre column of A. A positive value indicates that IHI40 decreased in the presence of LAI. C, the relationship between IHI40 and change in IHI40 demonstrated that the change in IHI40 did not correlate significantly with the strength of IHI40.
For changes in the motor cortex ipsilateral to median nerve stimulation, 3 of the 15 subjects were excluded because no reliable MEP was produced in the left FDI muscle. Paired t test showed that right MNS delivered 190 ms before the CCS10 pulse to the right motor cortex did not significantly change the MEP amplitude in the left FDI muscle (2.94 ± 1.30 mV for CCS10 alone, 2.66 ± 1.38 mV for CCS10 preceded by MNS 190 ms earlier). Similarly, right MNS delivered 160 ms before the CCS40 pulse to the right motor cortex did not significantly change the MEP amplitude in the left FDI muscle (2.91 ± 1.39 mV for CCS40 alone, 2.67 ± 1.39 mV for CCS40 preceded by MNS 160 ms earlier).
Experiment 3: interactions between LAI, IHI10 and IHI40 at submaximal inhibition
Ten subjects participated. In one subject, no IHI10 or IHI40 was obtained with right motor cortex stimulation at 50% stimulator output, and the stimulus was increased to 60% stimulator output. TS intensities were 64.2 ± 10.9% of stimulator output to elicit a 1 mV test MEP and 70.7 ± 13.3% to elicit a test MEP of 1 mV in the presence of MNS (termed 1 mVMNS200). The test MEP amplitude for TS 1 mV was 0.89 ± 0.27 mV (Table 1: condition 2A) and for TS 1 mVMNS200 was 1.63 ± 0.63 mV (2E). When a TS 1 mVMNS200 was preceded by MNS, the test MEP amplitude was 0.98 ± 0.32 mV (2F). Therefore, test MEP amplitudes for conditions 2A and 2F were matched.
Figure 6A shows IHI10 for a 1 mV test MEP (2C/2A, Table 1), for a 1 mVMNS200 test MEP (2G/2E) and IHI10 in the presence of LAI (2H/2F) for all subjects. Repeated measures ANOVA showed significant effects of test conditions (P = 0.035). Post hoc testing demonstrated that in the presence of LAI, IHI10 was significantly lower whether matched for test MEP amplitude (Fig. 6A, left and right columns, P = 0.018) or test stimulus intensity (Fig. 6A, centre and right columns, P = 0.034). The reduction in IHI10 did not correlate with the strength of LAI or IHI10. Figure 6B shows IHI40 for a 1 mV test MEP (2D/2A, Table 1), for a 1 mVMNS200 test MEP (2I/2E) and IHI40 in the presence of LAI (2J/2F) from all subjects. ANOVA showed only a trend for significant effects of test conditions (P = 0.074). Post hoc testing demonstrated that in the presence of LAI, IHI40 was significantly lower when matched for test stimulus intensity (Fig. 6B, centre and right columns, P = 0.026). The other comparisons were not significant. The decrease in IHI40 in the presence of LAI did not correlate with the strength of LAI whether matched for test stimulus intensity (2J/2F – 2I/2E versus 2F/2E, P = 0.89, r= 0.05, Fig. 6C) or test MEP amplitude (2J/2F – 2D/2A versus 2B/2A, P = 0.19, r= 0.46). The decrease in IHI40 in the presence of LAI also did not correlate with the strength of IHI40.
Figure 6. Interactions between weak LAI, IHI10 and IHI40 (Expt 3).
Data from 10 subjects. A: left column represents IHI10 alone for a 1 mV test MEP (2C/2A, Table 1); centre column represents IHI10 for a 1 mVMNS200 test MEP (2G/2E); right column represents IHI10 in the presence of LAI (2H/2F). The left and right columns are matched for test MEP amplitude (both ∼1 mV) and the centre and right columns are matched for test stimulus intensity (both 1 mVMNS200). B: left column represents IHI40 alone for a 1 mV test MEP (2D/2A, Table 1); centre column represents IHI40 for a 1 mVMNS200 test MEP (2I/2E); right column represents IHI40 in the presence of LAI (2J/2F). The left and right columns are matched for test MEP amplitude (both ∼1 mV) and the centre and right columns are matched for test stimulus intensity (both 1 mVMNS200). *Significant changes shown by post hoc testing. C, relationship between LAI and change in IHI40. Change in IHI40 is defined as IHI40 in the presence of LAI minus IHI40 alone, and is equivalent to the right column minus the centre column of B. A positive value indicates that IHI40 decreased in the presence of LAI. There was no significant correlation.
In the presence of LAI, there was no significant difference in the reduction of IHI10 (0.26 ± 0.39) and IHI40 (0.26 ± 0.33).
Compared to Expt 2, there was considerably less IHI10 alone (Fig. 3A) and IHI40 alone (Fig. 5A) due to reduction in the intensity of the conditioning stimulus. LAI for a 1 mV MEP (2B/2A) was significantly greater (P = 0.002, unpaired t test) in Expt 2 (0.28 ± 0.18) compared to Expt 3 (0.75 ± 0.45). LAI for the higher test stimulus of 1 mVMNS200 intensity (2F/2E) was also greater in Expt 2 (0.52 ± 0.21) than in Expt 3 (0.64 ± 0.19) but the differences were not significant. The reduction in IHI10 in the presence of LAI (Expt 2: 0.13 ± 0.22, Fig. 3B and C; Expt 3: 0.26 ± 0.39) was not significantly different between the two experiments (unpaired t test). However, there was significantly less reduction in IHI40 in the presence of LAI (P = 0.03, unpaired t test) in this experiment (0.26 ± 0.33) compared to Expt 2 (0.63 ± 0.18, Fig. 5B and C).
Discussion
Effects of different test stimulus intensities
We examined how LAI interacts with IHI10 and IHI40. Increasing the TS intensity decreases LAI, IHI10 and IHI40. The findings for LAI (Sailer et al. 2002) and IHI10 (Ferbert et al. 1992; Daskalakis et al. 2002b) are similar to previous studies while the effect of test stimulus intensity on IHI40 has not been previously reported. Thus, all three inhibitory phenomena tested have a relatively greater effect on motor cortical neurones activated at low intensities than those activated at high intensities.
Interaction between LAI and IHI10
When we applied strong LAI and IHI10 together in Expt 2, IHI10 was significantly decreased in the presence of LAI when matched for test MEP amplitude but not when matched for test pulse intensity. One possible explanation is that although the test MEP for conditions 2C (1 mV) and 2H (MNS200–1 mVMNS200) have the same amplitude, the test MEPs in condition 2H are predominantly mediated by high threshold neurones since LAI mainly inhibits low threshold neurones (Fig. 1). Another potential mechanism is that IHI10 predominantly inhibits late I waves (Di Lazzaro et al. 1999). If LAI also inhibits late I waves, the increase in MEP amplitude produced by the stronger test stimulus (e.g. condition 2H, MNS200–1 mVMNS200) may be mainly due to stronger activation of earlier I waves and D waves. With either of these potential mechanisms, the test MEP in condition 2H may be less susceptible to IHI10 resulting in an apparent decrease in IHI10 in the presence of LAI when matched for test MEP amplitude. A saturation or an occlusion effect is also possible. In this situation, one inhibitory mechanism would cause near-maximum inhibition so that the second inhibitory mechanism has little or no further inhibitory effect. Against this mechanism is the finding from Expt 3 that with reduced strength of IHI10 and LAI, there was no reduction in the degree of inhibition of IHI10 by LAI. However, occlusion cannot be ruled out because low intensity IHI10 and LAI may target the same population of cortical neurones (for example, those producing late I waves). The relatively weak effect of LAI on IHI10 and the absence of a correlation between the degree of interaction and the strength of LAI or IHI10 (Fig. 3B and C) argue against LAI or IHI10 directly inhibiting each other. Another possibility is that LAI may influence the ipsilateral motor cortex by reducing the effect of the conditioning pulse for IHI10. However, we found that MNS did not significantly change the MEP evoked by the conditioning pulse (CCS10) for IHI10 and there was also no correlation between the effect of MNS on the MEP evoked by CCS10 and the change in IHI10, making it unlikely that the change in IHI10 is due to the effect of MNS on the ipsilateral motor cortex. Thus, our findings suggest that LAI and IHI10 do not directly inhibit each other, but their interactions can potentially be explained by these inhibitory phenomena acting on a similar population of cortical neurones.
LAI inhibits IHI40
The interaction between LAI and IHI40 is different from the interaction between LAI and IHI10. Applying strong LAI and IHI40 together decreased IHI40 and the effect is much more prominent than the interaction between LAI and IHI10 (Figs 3 and 5). Moreover, the effect is present in all subjects (Fig. 5B and C) and was significant when matched for both test MEP amplitudes and test pulse intensities. A saturation or occlusion effect is unlikely to explain this interaction. In 12 of the 14 subjects, the presence of LAI changed the effect of IHI40 from inhibition to facilitation (Fig. 4) and a saturation effect cannot explain facilitation. In addition, the saturation hypothesis predicts that the effect will be greater with a larger baseline LAI or IHI40, but the interaction between LAI and IHI40 correlated with the strength of LAI but not with the strength of IHI40. We also found no evidence that MNS changed the excitability of the ipsilateral motor cortex thereby reducing the effect of the CCS40 pulse. Thus, our findings suggest that LAI and IHI40 have direct inhibitory interactions. Since the reduction in IHI40 in the presence of LAI strongly correlated with the strength of LAI (Fig. 5B) but not with the strength of IHI40, it is much more likely that LAI inhibits IHI40 than IHI40 inhibits LAI.
With weaker LAI and IHI40 in Expt 3, the effect of LAI on IHI40 was significantly reduced. The weaker IHI40 itself in Expt 3 (weak LAI, IHI10 and IHI40) compared to Expt 2 (strong LAI, IHI10 and IHI40) is unlikely to account for this result because there is a similar degree of reduction for IHI10 but there was no reduction in the interaction between LAI and IHI10. Thus, the reduction in IHI40 induced by LAI is dependent on the strength of LAI. These findings are consistent with our suggestion that LAI inhibits IHI40. The absence of a significant correlation between change in IHI40 in the presence of LAI and LAI alone in Expt 3 is likely because the effect size is much smaller with weaker LAI and IHI40.
IHI may be mediated by an overlapping population of neurones as LICI
Daskalakis et al. (2002b) have hypothesized that LICI and IHI10 may be mediated by an overlapping population of inhibitory neurones. Evidence supporting this hypothesis includes the result that both LICI and IHI10 preferentially affect lower threshold motor cortex neurones (Daskalakis et al. 2002b), both require suprathreshold conditioning stimuli to produce inhibitory effects in the motor cortex (Kujirai et al. 1993; Ziemann et al. 1996; Wassermann et al. 1996; Chen et al. 1998; Daskalakis et al. 2002b), and both inhibit SICI (Sanger et al. 2001; Daskalakis et al. 2002b). If an overlapping population of neurones mediates LICI and IHI, then the interactions between LAI and LICI should be the same as the interactions between LAI and IHI. Sailer et al. (2002) examined the interaction between LAI and LICI. When LAI and LICI are applied together, their inhibitory effects are less than their expected additive effect when matched for test MEP amplitude or TS intensity. This reduction correlated with the strength of LAI but not with the strength of LICI, suggesting that LAI inhibits LICI. Our results showed that the interaction between LAI and IHI40 is similar to the interaction between LAI and LICI, but different from the interaction between LAI and IHI10. Thus, LICI is probably more related to IHI40 than to IHI10. This is consistent with the findings of previous studies. Voluntary muscle activity reduces IHI8 (Chen et al. 2003) but has little effect on LICI (Valls-Soléet al. 1992; Wassermann et al. 1996) and IHI40 (Chen et al. 2003). LICI is probably related to the contralateral silent period (Wassermann et al. 1996) and IHI40 may be related to the iSP, whereas IHI elicited at an ISI of 8 ms is not related to the iSP (Chen et al. 2003).
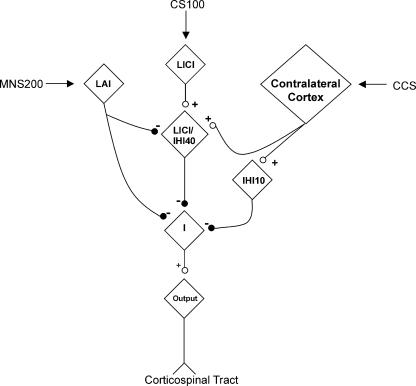
A possible mechanism of how LAI, LICI, IHI10 and IHI40 interact is shown in Fig. 7. It is hypothesized that IHI10 and IHI40 are due to excitatory input from the contralateral motor cortex acting on inhibitory neurones since there are no known long-range inhibitory neurones that cross the corpus callosum. LICI and IHI40 share a population of inhibitory neurones that is inhibited by LAI and separate from neurones mediating IHI10. The model is consistent with the existence of different classes of GABAergic inhibitory neurones in the cortex that have different connectivity and interaction with pyramidal neurones (Xiang et al. 2002), and have distinct pharmacological properties (Xiang et al. 1998). The model will need to be tested and refined in future studies.
Figure 7. Proposed model for LAI, LICI/IHI40 and IHI10 interactions.
IHI10 and IHI40 result from excitatory input from the contralateral motor cortex acting on inhibitory neurones. LICI and IHI40 share a population of inhibitory neurones that is inhibited by LAI. These neurones are separate from those mediating IHI10. ‘I’ represents the group of neurones mediating indirect (I) waves. The model is not proven and will need to be tested and refined in future studies.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research, Canada Foundation for Innovation, Ontario Innovation Trust, the University Health Network Krembil Family Chair in Neurology and the Institute of Medical Sciences at the University of Toronto.
References
- Abbruzzese G, Marchese R, Buccolieri A, Gasparetto B, Trompetto C. Abnormalities of sensorimotor integration in focal dystonia: a transcranial magnetic stimulation study. Brain. 2001;124:537–545. doi: 10.1093/brain/124.3.537. [DOI] [PubMed] [Google Scholar]
- Chen R. Studies of human motor physiology with transcranial magnetic stimulation. Muscle Nerve. 2000;23:S26–S32. doi: 10.1002/1097-4598(2000)999:9<::aid-mus6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res. 2004;154:1–10. doi: 10.1007/s00221-003-1684-1. [DOI] [PubMed] [Google Scholar]
- Chen R, Corwell B, Hallett M. Modulation of motor cortex excitability by median nerve and digit stimulation. Exp Brain Res. 1999;129:77–86. doi: 10.1007/s002210050938. [DOI] [PubMed] [Google Scholar]
- Chen R, Tam A, Bütefisch C, Corwell B, Ziemann U, Cohen LG. Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol. 1998;80:2870–2881. doi: 10.1152/jn.1998.80.6.2870. [DOI] [PubMed] [Google Scholar]
- Chen R, Yung D, Li J-Y. Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol. 2003;89:1256–1264. doi: 10.1152/jn.00950.2002. [DOI] [PubMed] [Google Scholar]
- Classen J, Steinfelder B, Liepert J, Stefan K, Celnik P, Cohen LG, Hess A, Kunesch E, Chen R, Benecke R, Hallett M. Cutaneomotor integration in humans is somatotopically organized at various levels of the nervous system and is task dependent. Exp Brain Res. 2000;130:48–59. doi: 10.1007/s002210050005. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Chen R, Fitzgerald PB, Zipursky RB, Kapur S. Evidence for impaired cortical inhibition in schizophrenia using transcranial magnetic stimulation. Arch Gen Psychiatry. 2002a;59:347–354. doi: 10.1001/archpsyc.59.4.347. 10.1001/archpsyc.59.4.347. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol. 2002b;543:317–326. doi: 10.1113/jphysiol.2002.017673. 10.1113/jphysiol.2002.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC. Direct demonstration of interhemispheric inhibition of the human motor cortex produced by transcranial magnetic stimulation. Exp Brain Res. 1999;124:520–524. doi: 10.1007/s002210050648. 10.1007/s002210050648. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992;453:525–546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilio F, Rizzo V, Siebner HR, Rothwell JC. Effects on the right motor hand-area excitability produced by low-frequency rTMS over human contralateral homologous cortex. J Physiol. 2003;551:563–573. doi: 10.1113/jphysiol.2003.044313. 10.1113/jphysiol.2003.044313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation and the human brain. Nature. 2000;406:147–150. doi: 10.1038/35018000. 10.1038/35018000. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Machii K, Mochizuki H, Terao Y, Enomoto H, Furubayashi T, Shiio Y, Uesugi H, Kanazawa I. Interhemispheric facilitation of the hand motor area in humans. J Physiol. 2001;531:849–859. doi: 10.1111/j.1469-7793.2001.0849h.x. 10.1111/j.1469-7793.2001.0849h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehaus L, Alt-Stutterheim K, Roricht S, Meyer BU. Abnormal postexcitatory and interhemispheric motor cortex inhibition in writer's cramp. J Neurol. 2001;248:51–56. doi: 10.1007/s004150170269. 10.1007/s004150170269. [DOI] [PubMed] [Google Scholar]
- Sailer A, Molnar GF, Cunic DI, Chen R. Effect of peripheral sensory input on cortical inhibition in humans. J Physiol. 2002;544:617–629. doi: 10.1113/jphysiol.2002.028670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer A, Molnar GF, Paradiso G, Lang AE, Chen R. Short and long latency afferent inhibition in Parkinson's disease. Brain. 2003;126:1883–1894. doi: 10.1093/brain/awg183. [DOI] [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol. 2001;530:307–317. doi: 10.1111/j.1469-7793.2001.0307l.x. 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner HR, Dressnandt J, Auer C, Conrad B. Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle Nerve. 1998;21:1209–1212. doi: 10.1002/(sici)1097-4598(199809)21:9<1209::aid-mus15>3.0.co;2-m. 10.1002/(SICI)1097-4598(199809)21:9<1209::AID-MUS15>3.3.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol. 2000;523:503–513. doi: 10.1111/j.1469-7793.2000.t01-1-00503.x. 10.1111/j.1469-7793.2000.t01-1-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Solé J, Pascual-Leone A, Wassermann EM, Hallett M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol. 1992;85:355–364. doi: 10.1016/0168-5597(92)90048-g. 10.1016/0168-5597(92)90048-G. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Samii A, Mercuri B, Ikoma K, Oddo D, Grill SE, Hallett M. Responses to paired transcranial magnetic stimuli in resting, active, and recently activated muscle. Exp Brain Res. 1996;109:158–163. doi: 10.1007/BF00228638. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol. 1999;517:591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Z, Huguenard JR, Prince DA. Cholinergic switching within neocortical inhibitory networks. Science. 1998;281:985–988. doi: 10.1126/science.281.5379.985. 10.1126/science.281.5379.985. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Huguenard JR, Prince DA. Synaptic inhibition of pyramidal cells evoked by different interneuronal subtypes in layer v of rat visual cortex. J Neurophysiol. 2002;88:740–750. doi: 10.1152/jn.2002.88.2.740. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol. 1996;496:873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]