Abstract
The vast majority of quantitative data examining the effects of breathing on venous return have been derived from anaesthetized or reduced animal preparations, making an extrapolation to an upright exercising human problematic due to the lack of a hydrostatic column and an absence of muscular contraction. Thus, this study is the first to quantitatively examine the effects of different breathing mechanics on venous return from the locomotor limbs both at rest and during calf contraction exercise in the semirecumbent human. When subjects inspired using predominantly their ribcage/accessory inspiratory muscles at rest (change in gastric pressure (ΔPGA) = < 2 cmH2O, change in oesophageal pressure (ΔPES) =∼−6 cmH2O; inspiratory time/total breath time (TI/TTOT) = 0.5), a slight facilitation of femoral venous return was observed during inspiration (65% of all flow occurred during inspiration), with a slight reduction in femoral venous return during the ensuing expiratory phase of the breath. However, when subjects inspired using a predominantly diaphragmatic breath at rest (ΔPGA= > 5 cmH2O, ΔPES=∼−6 cmH2O; TI/TTOT= 0.5), femoral venous return was markedly impeded (net retrograde flow of 11%) and significantly lower than that observed during ribcage breathing conditions (P < 0.01). During the ensuing expiratory phase of a diaphragmatic breath, there was a large resurgence of femoral venous blood flow. The pattern of modulation during ribcage and diaphragmatic breathing persisted during both mild (peak calf force = 7 kg) and moderate (peak calf force = 11 kg) levels of calf contraction. Despite the significant within-breath modulation of femoral venous return by breathing, net blood flow in the steady state was not altered by the breathing pattern followed by the subjects. Though popliteal blood flow appeared to be modulated by respiration at rest, this pattern was absent during mild calf contraction where popliteal outflow was phasic with the concentric phase of calf contraction. We conclude that respiratory muscle pressure production is the predominant factor modulating venous return from the locomotor limb both at rest and during calf contraction even when the veins of the lower limb are distended due to the presence of a physiologic hydrostatic column.
In upright humans, up to 70% of the circulating blood volume is below the heart, with the vast majority of this blood being stored in the venous capacitance vessels (Rowell, 1993). As the thin-walled veins readily distend with relatively small increases in transmural pressure (Rothe, 1983), compensatory mechanisms must be present to prevent the blood from pooling in the extremities and aid in the return of blood to the heart. Two of the most widely accepted of these mechanisms are the respiratory muscle pump and the skeletal muscle pump.
During inspiration at rest, the diaphragm descends, resulting in a lowering of intrathoracic pressure (PITP) and a resultant expansion of the lungs. This lowered PITP is also transmitted across the walls of the right atrium, which promotes right atrial filling (i.e. by lowering right atrial pressure and facilitating venous return during inspiration) and widens right atrial transmural pressure. However, an often unappreciated aspect of diaphragmatic descent in the resting human is the concomitant increase in abdominal pressure. Previous work has shown that increases in abdominal pressure as small as 5 cmH2O can result in the complete cessation of venous return from the lower limb, with the ‘classic’ inspiratory facilitation of lower limb venous return only occurring when inspiration is achieved by ribcage expansion (Willeput et al. 1984). However, these observations were made with subjects in the supine position, where venous filling pressure is relatively low (due to the absence of a hydrostatic column), and capacitance vessel distension is readily achieved with very small increases in venous filling pressure. Additionally, a major limitation to that study was that the haemodynamic measures were purely qualitative (i.e. velocimetric estimates of blood flow without correcting for changes in vessel cross-sectional area), and the effects of different breathing patterns on steady-state venous return from the lower limb were not examined. Thus, whether changes in breathing pattern per se (i.e. predominantly diaphragm versus inspiratory intercostals/neck muscle recruitment) can increase steady-state blood flow from the resting limb remains unknown.
During dynamic exercise, the rhythmic contraction of the peripheral skeletal muscles results in the compression of the intramuscular veins, and imparts a considerable amount of kinetic energy to the venous blood and facilitates its return to the heart. The skeletal muscle pump has been shown to be very effective at emptying the venous vessels, as more than 40% of the intramuscular blood volume can be translocated centrally with a single muscular contraction (Stewart et al. 2004). Furthermore, the vast majority of venous outflow during dynamic muscular exercise occurs during the concentric phase of contraction, providing further credence to the notion that the increases in intramuscular pressure provide an important source of energy for the blood returning to the heart during exercise (Folkow et al. 1970; Hogan et al. 2003).
This study addresses the following questions: is the respiratory modulation of venous return from the resting lower limb affected by the presence of a hydrostatic column (i.e. a semirecumbent body position)? How does the addition of peripheral locomotor limb muscular contraction affect the respiratory modulation of venous return from the lower limb? Can net venous return from the lower limb be increased over time by different types of breathing patterns?
Methods
General procedures
Thirty-five men were screened for ‘adequate’ venous images and velocities (see online supplement for specific inclusion and exclusion criteria). Following the screening visit, five men aged 25 ± 6 years and of normal weight (88 ± 8 kg) and height (184 ± 4 cm) served as subjects for this study after providing written informed consent. All subjects were normotensive and free from cardiovascular and pulmonary disease. All experimental procedures and protocols were approved by the University of Wisconsin-Madison Health Sciences Human Research Review Committee and conformed with the Declaration of Helsinki.
Subjects breathed through a mouthpiece connected to a non-rebreathing valve with nose occluded. Airflow rates, tidal volume (VT), mouth pressure (PM), and the end-tidal partial pressure of CO2 were measured using equipment and techniques previously described (Sheel et al. 2001). Gastric and oesophageal balloons (Ackrad, Cranford, NJ, USA) were placed in the stomach and lower one-third of the oesophagus for the estimation of abdominal (PGA) and intrathoracic (PES) pressure, respectively. Electromyogram recordings were obtained from surface electrodes placed on the right quadriceps and gastrocnemius to ensure quiescence of the upper thigh muscles and consistent activation of the calf muscles during contraction. Ribcage and abdominal excursions were measured using a direct current-coupled respiratory inductive plethysmograph (Respitrace; Ambulatory Monitoring, Ardsley, NY, USA). Mean arterial blood pressure (MAP) was measured beat-by-beat using the finger photoplethysmography technique (Finapres Model 2300; Ohmeda, Englewood, CO, USA), and drift corrected at 1 min intervals using an automated sphygmomanometer (Dinamap model 1846 SX/P; Critikon, Tampa, FL, USA) to determine MAP (one-third pulse pressure plus diastolic pressure).
Measurement of regional blood velocity
Femoral venous blood velocity was measured in the femoral vein, proximal to the vena profunda and distal to the saphenous vein, using an ultrasound Doppler system (Image Point Hx; Hewlett Packard, Andover, MA, USA). This location is both adequately superficial to provide a high quality cross-sectional image at an imaging frequency of 7.5–10 MHz (see video clip in online supplementary data) and distal to the main tributaries containing skin blood flow (which can be affected by changes in room temperature and behaviour). In a separate trial, venous blood velocity was measured in the popliteal vein on the posterior portion of the thigh, and immediately proximal to the knee joint. Although adequate longitudinal popliteal venous vessel images were available for all subjects, the nature of this vessel (thin walled, small diameter, steep angle of descent into the muscle) precluded the measurement of its cross-sectional area. In a separate trial, arterial blood velocity was measured in the superficial femoral artery using the same ultrasound Doppler system.
Measurement of blood vessel cross-sectional area, blood flow and regional vascular conductance
During a separate trial, venous cross-sectional images were continuously acquired, and vessel cross-sectional area was measured at end-expiration and end-inspiration at 1 min intervals using planimetry software incorporated into our ultrasound system. Importantly, this approach eliminates the use of assumptions regarding vessel geometry. Due to these continuous changes in vessel cross-sectional area that occur over the course of a breath, a linear interpolation technique was used to estimate the instantaneous venous cross-sectional area over the course of a breath. Arterial blood velocity and vessel diameter (d) were acquired during the same trial, and arterial cross-sectional area was calculated using π(d/2)2 from the longitudinal arterial vessel image at the point of peak arterial blood velocity at 1 min intervals. Instantaneous arterial and venous blood flow were calculated as the product of the blood velocity and the interpolated value for vessel cross-sectional area at 100 equally spaced points over the course of a breath. Arterial limb vascular conductance was calculated as mean femoral arterial blood flow (Qfa)/MAP.
Breathing pattern
During ribcage breathing conditions, subjects were instructed to inspire using predominantly their inspiratory intercostals and neck muscles, such that the inspiratory change in gastric pressure during inspiration was less than 2 cmH2O. During diaphragmatic breathing conditions, subjects were instructed to inspire such that the diaphragm descended and forced an outward excursion of the abdominal wall during inspiration, and a concomitant inspiratory increase in gastric pressure of ≥5 cmH2O. Breathing frequency was set at 15 breaths min−1 with a TI/TTOT= 0.50. The PGA and PES changes over the course of a breath were monitored closely throughout the study to ensure that the pressure waveform was uniform across breaths, the intrathoracic pressure excursions were comparable between ribcage and diaphragm breathing conditions, and that the breath timing and breathing patterns conformed to the instructions given to the subjects at the beginning of the study. Though tidal volume was not controlled specifically, subjects were encouraged to maintain similar tidal and end-expiratory lung volumes across ribcage and diaphragm breathing conditions.
Calf contraction
Subjects were placed in a semirecumbent position (∼45 deg recumbency) with knees extended and parallel to the floor. The subjects performed plantar flexion exercise, with the range of motion limited by two adjustable metal bars. Subjects were instructed to lightly touch the distal bar during plantar flexion, and maintain a consistent level of force production with each contraction. The force generated by the subject during calf contraction could be altered by the addition or removal of elastic bands (peak force = 7 or 11 kg). The calf was contracted at a frequency of 30 min−1 with a duty cycle of 0.50, which allowed for the synchronization of contraction with the onset of inspiration and expiration (i.e. one contraction cycle occurred during inspiration and one during expiration).
Protocol 1: effects of breathing pattern and calf contraction on femoral venous outflow from and arterial inflow to the lower limb
Subjects alternated between ribcage and diaphragm breathing for 5 min periods both with and without mild or moderate calf contraction. Each trial lasted 35 min, and trials were repeated three times for the measurement of femoral venous blood velocity, femoral venous cross-sectional area, and arterial blood velocity and diameter (see online supplement for greater detail).
Protocol 2: role of diaphragmatic descent per se in the modulation of venous return from the lower limb
Similar to the protocol of Willeput et al. (1984), during ribcage breathing the abdomen was manually compressed by one of the investigators both at rest and during mild calf contraction. Pressure was applied during ribcage breathing conditions so that the inspiratory PGA excursion during the compression trials approximated that observed during the diaphragm breathing trials.
Protocol 3: effects of breathing pattern and calf contraction on popliteal venous blood velocity at rest and during calf contraction
Popliteal blood velocity was measured on the posterior part of the limb immediately proximal to the calf both at rest and during mild calf contraction (i.e. prior to the entrance of the blood into the capacitance vessels of the upper thigh). We postulated that if we observed no respiratory modulation of popliteal venous blood velocity over the course of a breath, then the majority of the modulation of femoral venous return by respiration could be attributed to the emptying or filling of the upper thigh venous vessels.
Data analysis
Composite waveforms were generated over the course of each breath for each variable by sampling each variable at 100 equally spaced points over the course of a breath, and averaging these values for each 5 min condition. A repeated measures ANOVA with a post hoc Tukey test was used to detect differences in the mean and peak values of each variable during inspiration and expiration between conditions of ribcage and diaphragm breathing, both with and without different levels of calf contraction.
Results
Effects of breathing pattern on femoral venous blood flow at rest and during calf contraction
Effects of breathing pattern on femoral venous and arterial blood flow at rest
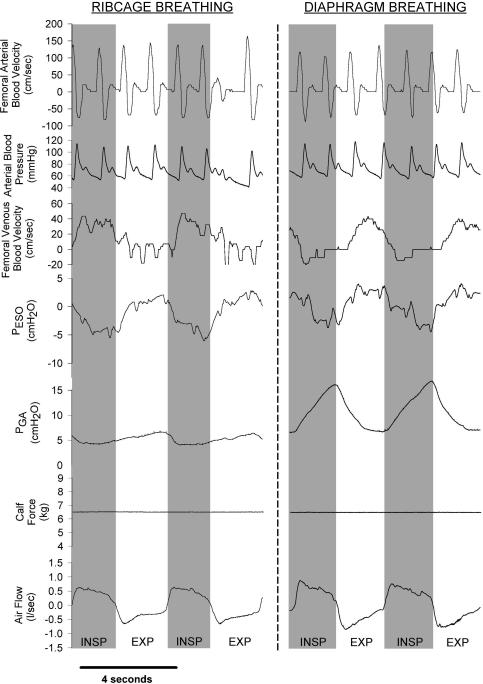
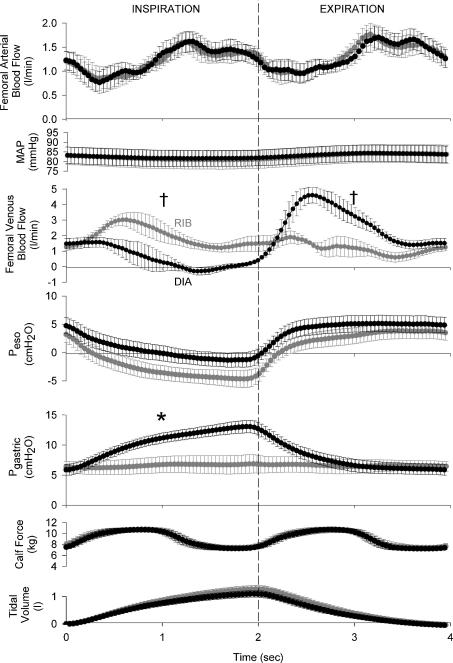
Raw data traces showing the qualitative effects of ribcage and diaphragm breathing on femoral arterial and femoral venous velocity from one representative subject are shown in Fig. 1, with the signal-averaged, quantitative data for all subjects shown in Fig. 2 and Table 1. Subjects were able to significantly alter their inspiratory abdominal pressure production between ribcage and diaphragm breathing conditions (ΔPGA= 0.6 ± 1.5 and 7.0 ± 0.7 cmH2O, respectively, P < 0.01) while producing similar intrathoracic pressure excursions (ΔPES=−6.8 ± 1.5 and −4.8 ± 1.3 cmH2O, respectively, P = n.s.).
Figure 1. Raw data traces showing arterial and venous blood velocity, mean arterial pressure, gastric (PGA) and oesophageal (PES) pressure, calf force and airflow during resting conditions.
Inspiration is denoted by the shaded areas and an upward deflection in airflow; the actual force being produced by the calf under these conditions is zero (baseline force is elevated due to the elastic cord pulling the foot against a metal bar positioned to achieve 90 degree ankle dorsiflexion). Note that arterial inflow remains unaffected by the type of breathing pattern utilized by the subject. In contrast, femoral venous blood flow is augmented only observed during inspiration with ribcage breathing, whereas femoral venous return is virtually abolished for the latter half of a diaphragmatic inspiration.
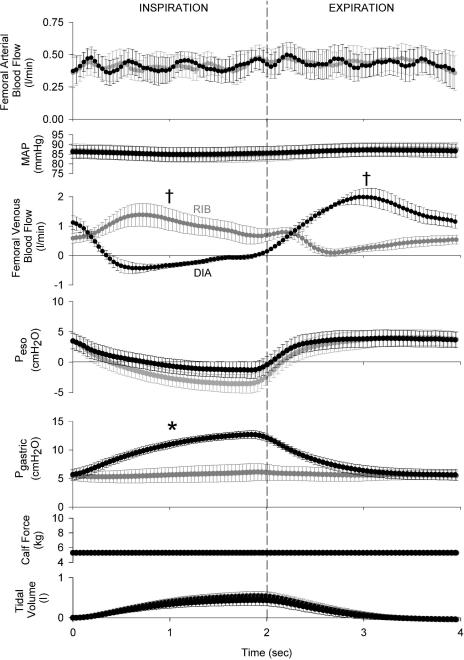
Figure 2. Effects of ribcage or diaphragm breathing patterns on femoral arterial inflow, mean arterial pressure (MAP) and femoral venous outflow signal-averaged over the course of a breath during resting conditions (n = 5, minimum of 200 breaths per subject per condition).
Though there is no discernable effect of breathing pattern on arterial inflow, femoral venous return is facilitated during a ribcage inspiration and impeded during a diaphragmatic inspiration, with these modulatory effects being reversed during the ensuing expiratory phase of the breath. Grey circles, ribcage breathing; black circles, diaphragm breathing; †P < 0.01 and *P < 0.05 for comparisons of ribcage versus diaphragm for mean inspiratory or expiratory values.
Table 1.
Group mean femoral arterial (Qfa) and femoral venous blood flow (Qfv) responses to diaphragm and ribcage breathing at rest and during calf exercise (n = 5)
| Qfa (l min−1) | Qfv (l min−1) | MAP (mmHg) | Qfa (% of Rest) | Qfv (% of Rest) | Qfa,I/Qfa,TOT (%) | Qfv,I/Qfv,TOT (%) | CSAfv (end-exp) | CSAfv (end-insp) | Femoral arterial conductance (ml mmHg−1 min−1) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Rest | ||||||||||
| Ribcage | 0.42 (±0.10) | 0.73 (±0.13) | 84 (±5) | — | — | 48 (±1) | 65 (±8) | 1.32 (±0.17) | 1.25 (±0.16) | 5 (±1) |
| Diaphragm | 0.42 (±0.10) | 0.64 (±0.11) | 84 (±5) | — | — | 49 (±1) | −11 (±10)‡§ | 1.34 (±0.17) | 1.50 (±0.16) | 5 (±1) |
| Mild calf contraction | ||||||||||
| Ribcage | 0.89 (±0.10)* | 1.10 (±0.17)* | 87 (±6) | 235 (±38)* | 168 (±23)* | 47 (±1) | 57 (±7) | 1.37 (±0.19) | 1.28 (0.16) | 11 (1)* |
| Diaphragm | 0.88 (±0.09)* | 1.04 (±0.17)* | 85 (±5) | 232 (±37)* | 173 (±22)* | 48 (±1) | 13 (±5)§‡ | 1.34 (±0.15) | 1.52 (±0.16)¶ | 11 (±2)* |
| Moderate calf contraction | ||||||||||
| Ribcage | 1.26 (±0.17)*† | 1.57 (±0.19)*† | 83 (±4) | 319 (±34)*† | 239 (±27)*† | 48 (±1) | 60 (±4) | 1.35 (±0.24) | 1.24 (±0.20) | 15 (±1)*† |
| Diaphragm | 1.27 (±0.17)*† | 1.57 (±0.17)*† | 82 (±5) | 330 (±56)*† | 259 (±20)*† | 48 (±1) | 18 (±5)*†‡§ | 1.38 (±0.15) | 1.60 (±0.18)¶ | 15 (±1)*† |
Qfa, mean femoral arterial blood flow; Qfv, mean femoral venous blood flow; MAP, mean arterial blood pressure; Qfa,I/Qfa,TOT, inspiratory Qfa divided by the total Qfa over the course of a breath; Qfv,I/Qfv,TOT, inspiratory Qfv divided by the total Qfv over the course of a breath; CSAfv, femoral venous cross-sectional area.
Significant change from resting conditions during same breathing pattern, P < 0.05;
significantly different from mild calf contraction conditions during same breathing pattern, P < 0.05;
significantly different from ribcage breathing conditions, P < 0.05;
significant difference between flow during inspiration and flow during expiration, P < 0.001;
significantly different from end-expiratory CSAfv during same breathing pattern, P < 0.05.
Both femoral arterial inflow and vascular conductance were relatively unaffected by the type of breathing pattern followed by the subjects, as differences in mean blood flow during inspiration and expiration with diaphragm and ribcage breathing were not detected under either breathing condition (see Table 1). However, this was not the case for femoral venous return. During a diaphragmatic breath, femoral venous return was abolished during the latter three-quarters of inspiration, with a resurgence of blood flow during expiration (P < 0.01, inspiration versus expiration, see Table 1). In contrast, during a ribcage inspiration, femoral venous blood flow was always positive and there was a slight but non-significant facilitation of femoral venous return relative to the ensuing expiratory phase of the breath. Thus, during a diaphragmatic inspiration, mean femoral venous return was at or near zero flow, and was significantly lower than that observed during a ribcage inspiration (P < 0.01, see Fig. 2). During the ensuing expiratory phase of a diaphragmatic breath, mean femoral venous blood flow was significantly higher than that observed during the expiratory phase of ribcage breathing (P < 0.01, see Fig. 2). However, in the steady-state (i.e. over the course of >50 complete respiratory cycles), neither femoral arterial inflow nor femoral venous outflow was different between diaphragm and ribcage breathing (see Table 1).
Effects of breathing pattern on femoral venous and arterial blood flow during mild intensity calf contraction
The addition of mild intensity calf contraction (peak force =∼7 kg) increased mean Qfa and mean femoral venous blood flow (Qfv) by 234 ± 36 and 172 ± 22% over resting conditions, respectively, during both ribcage and diaphragm breathing (P < 0.01, see Table 1).
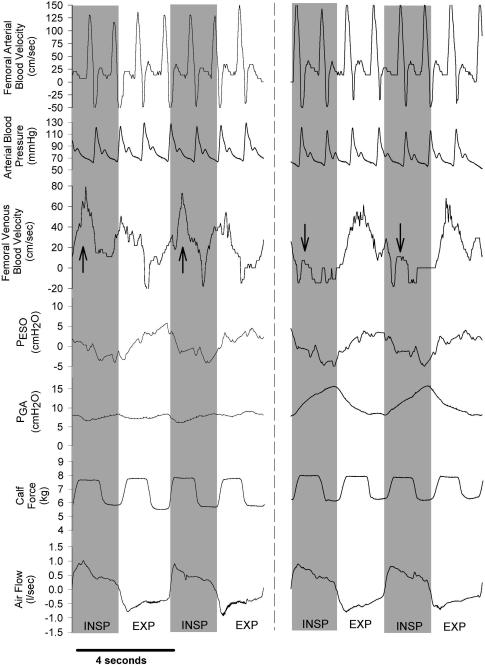
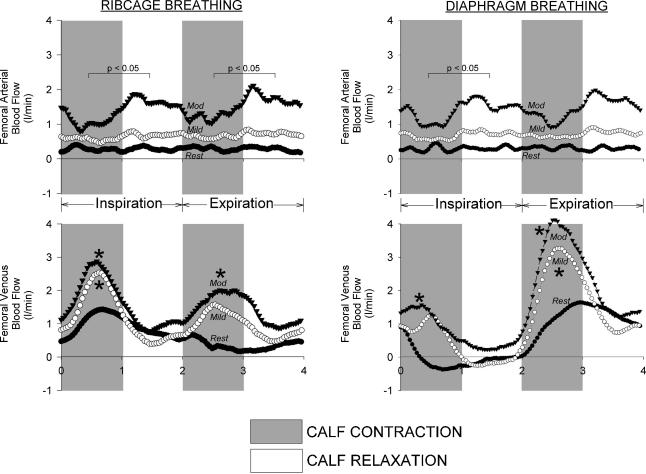
Raw data traces showing qualitative differences in femoral arterial and femoral venous blood velocity during diaphragm and ribcage breathing from a representative subject are depicted in Fig. 3, with the signal-averaged, quantitative data for all subjects shown in Fig. 4. The intrathoracic and abdominal pressure excursions during ribcage and diaphragm breathing were similar to those observed under resting conditions. Significant within-breath modulation of femoral venous blood flow persisted despite the presence of rhythmic calf contraction forcing blood centrally. Specifically, during diaphragmatic breathing, though femoral venous blood flow was slightly elevated with each calf contraction at the onset of a diaphragmatic inspiration, anterograde blood flow was virtually abolished during the latter half of the diaphragmatic inspiration, and the vast majority of femoral venous return occurred during the ensuing expiration (87 ± 5% of total flow, P < 0.01 inspiration versus expiration). During a ribcage inspiration, femoral venous blood flow was slightly augmented (57 ± 7% of blood flow occurred during inspiration; P = n.s., inspiration versus expiration, see Table 1), with the inspiratory increases in blood flow being particularly pronounced during the calf contraction phase.
Figure 3. Raw data traces showing arterial and venous blood velocity, MAP, PES and PGA, calf force and airflow during mild contraction conditions. Inspiration is again denoted by the shaded areas and a downward deflection in the airflow trace.
Note that arterial inflow remains again unaffected by the type of breathing pattern utilized by the subject, and is also relatively unaffected by the calf contraction cycle. However, significant respiratory modulation of femoral venous outflow persists in the face of mild calf contraction during both ribcage and diaphragm breathing, as the phasic increases in venous return associated with the calf muscle pump are most pronounced during a ribcage inspiration (denoted by upward arrows). In contrast, during a diaphragmatic inspiration, anterograde femoral venous blood flow occurs exclusively during the calf contraction phase (denoted by downward arrows).
Figure 4. Effects of ribcage or diaphragm breathing patterns on femoral arterial inflow, MAP and femoral venous outflow signal-averaged over the course of a breath during mild calf contraction conditions (minimum of 120 breaths per subject per condition).
Note that the respiratory modulation of femoral venous return persists despite the addition of the calf muscle pump, as mean inspiratory femoral venous return is significantly lower during diaphragm breathing conditions when compared to ribcage breathing, with this pattern being reversed during expiration. Grey circles, ribcage breathing; black circles, diaphragm breathing; †P < 0.01 and *P < 0.05 for comparisons of ribcage versus diaphragm for mean inspiratory or expiratory values.
Despite the persistence of the substantial, directionally opposite within-breath modulation of femoral venous return by the two breathing patterns during mild calf contraction, steady-state femoral venous return was unaffected by the type of breathing pattern followed (i.e. ribcage versus diaphragm breathing, see Table 1). Additionally, steady-state femoral arterial inflow was unaffected by the type of breathing pattern followed by the subjects (see Table 1).
Effects of breathing pattern on femoral venous and arterial blood flow during moderate intensity contraction
The addition of moderate calf contraction (peak force =∼11 kg) significantly increased Qfa and Qfv from both resting conditions (average increase of 325 ± 44% and 250 ± 23% over resting conditions, respectively, P < 0.01 for both) and mild calf contraction conditions (average increase of 50 ± 29 and 55 ± 22%, respectively, P < 0.05 for both).
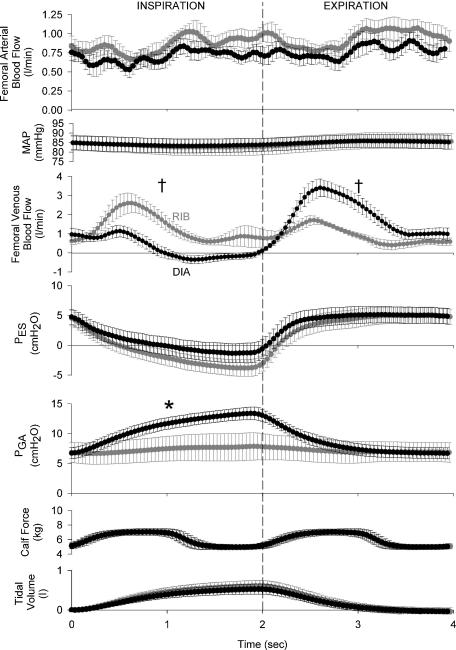
The signal-averaged, quantitative differences in femoral arterial and venous blood flow between ribcage and diaphragm breathing for all subjects are shown in Fig. 5. Similar to both resting and mild calf contraction conditions, the within-breath respiratory modulation of femoral arterial inflow and MAP is negligible during both ribcage and diaphragm breathing (see Fig. 5). However, femoral arterial inflow was now transiently reduced (P < 0.05) during the concentric portion of the calf contraction cycle during both inspiration and expiration (see Figs 5 and 6). Similar to resting and mild calf contraction conditions, femoral venous return during a diaphragmatic inspiration was significantly lower than that during the ensuing expiratory phase of a diaphragmatic breath (see Table 1). It is worthy to note that only the moderate levels of calf contraction (versus rest or mild calf contraction) were able to significantly increase mean femoral venous blood flow during a diaphragmatic inspiration above resting conditions (mean inspiratory Qfv at rest =−0.06 ± 0.09 l min−1, mean inspiratory Qfv during moderate calf contraction = 0.59 ± 0.19 l min−1, P < 0.05). However, mean femoral venous blood flow during a diaphragmatic inspiration was still significantly lower than that observed during a ribcage inspiration (P < 0.01, see Fig. 5), and mean femoral venous blood flow during a diaphragmatic expiration remained significantly higher than that observed during a ribcage expiration (P < 0.01; see Fig. 5). Similar to both resting and mild calf contraction conditions, neither steady-state femoral arterial inflow nor steady-state femoral venous outflow was affected by the type of breathing pattern performed by the subjects (see Table 1).
Figure 5. Effects of ribcage or diaphragm breathing patterns on femoral arterial inflow, MAP and femoral venous outflow signal averaged over the course of a breath during moderate calf contraction conditions (minimum of 120 breaths per subject per condition).
Similar to both resting and mild calf contraction conditions, the respiratory modulation of femoral arterial inflow and mean arterial pressure is negligible, though arterial inflow is now impeded due to the local compression of the arteries during the calf contraction (P < 0.05, nadir femoral arterial blood flow during the calf contraction phase versus calf relaxation phase, also see Fig. 6). Similar to mild calf contraction conditions, significant within-breath modulation of femoral venous blood flow persists during moderate calf contraction, as mean inspiratory and mean expiratory femoral venous blood flows are significantly different during the two breathing conditions. Grey circles, ribcage breathing; black circles, diaphragm breathing; †P < 0.01 and *P < 0.05 for comparisons of ribcage versus diaphragm for mean inspiratory or expiratory values.
Figure 6. Effects of ribcage breathing and diaphragm breathing on femoral arterial inflow and femoral venous outflow at rest and during mild and moderate calf contraction (traces represent the signal averaging of over 120 breaths per condition per subject).
Note that the only modulation of arterial inflow is during moderate levels of calf contraction where increases in intramuscular pressure during the concentric portion of calf contraction are great enough to compress the arteries and increase the impedance to arterial inflow. Additionally, only moderate levels of contraction were able to increase the mean femoral venous return during a diaphragmatic inspiration. These observations support the notion that increases in abdominal pressure are a powerful modulator of venous return from the lower limb, even during dynamic calf exercise. *P < 0.05 compared to resting conditions during the same breathing pattern (i.e. ribcage or diaphragm) and the same phase of the breath (i.e. inspiration or expiration).
Reproducibility of the within-breath modulation of femoral venous blood flow
The intraclass correlation coefficients for the waveform of the femoral venous blood flow trace over the course of a breath during ribcage and diaphragm breathing, along with the reproducibility of the differences between the two waveforms are included in the online supplementary data. Briefly, the waveforms for ribcage and diaphragm breathing were highly reproducible for each subject within each visit both at rest and during various levels of calf contraction, as evidenced by an average intraclass correlation coefficient higher than 0.85 for both breathing conditions (P < 0.01; range = 0.82–0.96). The ‘difference’ waveform (i.e. the difference between ribcage and diaphragm breathing at 100 equally spaced points over the course of a breath) was slightly less reproducible within and between each visit, but retained a highly significant intraclass correlation coefficient for each subject during all resting and calf contracting conditions (P < 0.01; range = 0.82–0.93).
Effects of manual abdominal compression during ribcage breathing on femoral venous blood flow over the course of a breath at rest and during calf contraction
During resting and mild calf contraction conditions, the manual compression of the abdomen during ribcage breathing conditions resulted in significant increases in both peak and mean abdominal pressure compared to ribcage breathing without abdominal compression, and were not significantly different from the abdominal pressures generated during diaphragmatic breathing (see Table 2). The magnitude of the oesophageal pressure excursion was not significantly affected by the addition of manual abdominal compression when compared to ribcage or diaphragm breathing alone (see Table 2). The pattern of within-breath modulation of femoral venous blood flow with the addition of manual abdominal compression to ribcage breathing at rest and during mild calf contraction was nearly identical to that observed during diaphragmatic breathing.
Table 2.
Group mean femoral venous blood velocity responses from protocol 2 during ribcage, diaphragm and ribcage with manual abdominal compression breathing conditions at rest and during mild calf exercise (n = 5)
|
|
∫Vfv,I/∫Vfv,TOT (%) | ΔPES,I (cmH2O) | ΔPGA,I (cmH2O) | MAP (mmHg) | |
|---|---|---|---|---|---|
| Rest | |||||
| Ribcage | 13.0 ± 4.7 | 115 ± 44 | –7.9 ± 1.3 | –2.4 ± 1.7 | 94 ± 3 |
| Diaphragm | 10.2 ± 2.4 | 6 ± 9 | –5.4 ± 1.3 | 5.6 ± 1.9 | 94 ± 3 |
| Ribcage + abdominal compression | 9.8 ± 3.3 | 6 ± 21† | –8.1 ± 1.2 | 3.2 ± 1.0* | 93 ± 3 |
| Mild calf contraction | |||||
| Ribcage | 18.0 ± 2.4 | 69 ± 10 | –8.1 ± 1.5 | –1.69 ± 1.3 | 94 ± 5 |
| Diaphragm | 15.3 ± 2.1 | 18.9 ± 1.6 | –6.5 ± 1.3 | 6.3 ± 1.8† | 96 ± 4 |
| Ribcage + abdominal compression | 17.5 ± 2.9 | 31.6 ± 10.4† | –7.8 ± 1.7 | 3.55 ± 1.4† | 97 ± 5 |
![]() , mean femoral venous blood velocity over the course of a breath; ∫Vfv,I/∫Vfv,TOT, integrated femoral venous blood velocity during inspiration divided by integrated blood velocity over the course of a breath; ΔPES,I inspiratory oesophageal pressure excursion; ΔPGA,I, inspiratory gastric pressure excursion.
, mean femoral venous blood velocity over the course of a breath; ∫Vfv,I/∫Vfv,TOT, integrated femoral venous blood velocity during inspiration divided by integrated blood velocity over the course of a breath; ΔPES,I inspiratory oesophageal pressure excursion; ΔPGA,I, inspiratory gastric pressure excursion.
Significantly different from ribcage breathing at rest, P < 0.05;
significantly different from ribcage breathing during mild calf contraction, P < 0.05.
Effects of breathing pattern on popliteal venous blood velocity over the course of a breath at rest and during calf contraction
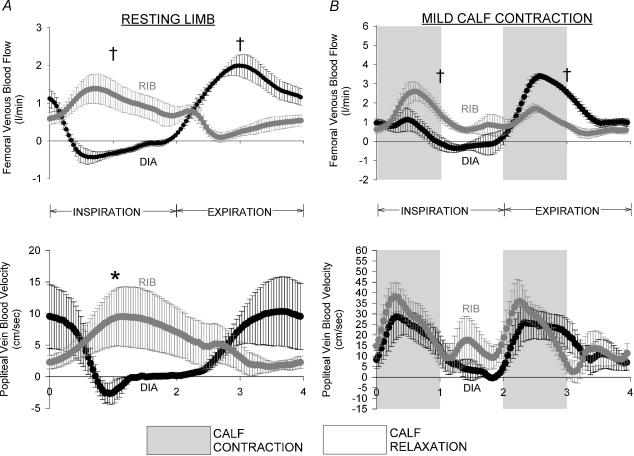
During ribcage and diaphragm breathing, we observed a pattern of respiratory modulation of popliteal venous blood velocity qualitatively similar to that observed in the common femoral vein, though the popliteal venous blood velocity waveform was significantly shifted rightward relative to the femoral venous blood velocity waveform (P < 0.05, popliteal versus femoral, see Table 3). During diaphragmatic breathing, within-breath modulation of popliteal venous blood velocity was evidenced by a significant difference between the nadir blood velocity during inspiration and the peak blood velocity during expiration (P < 0.05). Also, mean popliteal venous blood velocity during a diaphragmatic inspiration was significantly lower than that observed during a ribcage inspiration, though we did not detect any significant differences between the two breathing patterns during expiration (P < 0.05, see Fig. 7A).
Table 3.
Group mean popliteal venous blood velocity responses to diaphragm and ribcage breathing at rest and during mild calf exercise (n = 5).
| VPV (cm s−1) | VPV (% of Rest) | ∫VPV,I/∫VPV,TOT (%) | VPV Offset (s) | |
|---|---|---|---|---|
| Rest | ||||
| Ribcage | 5.4 ± 2.7 | — | 67 ± 7* | 0.41 ± 0.14‡ |
| Diaphragm | 3.9 ± 1.8 | — | 24 ± 5 | 0.48 ± 0.14‡ |
| Mild calf contraction | ||||
| Ribcage | 18.7 ± 5.7 | 503 ± 269 | 54 ± 2 | –0.27 ± 0.06‡ |
| Diaphragm | 14.6 ± 3.8 | 590 ± 337 | 46 ± 2† | –0.11 ± 0.10 |
VPV, mean popliteal venous blood velocity over the course of a breath; VPV,I/VPV,TOT, integrated inspiratory popliteal venous blood velocity divided by the integrated popliteal venous blood velocity over the course of a breath; VPV Offset, time delay between the peak or nadir popliteal venous blood velocity and the peak or nadir femoral venous blood velocity for ribcage and diaphragm breathing, respectively. The magnitude of the inspiratory oesophageal or gastric pressure excursion and MAP over time did not differ significantly from the breathing conditions in protocol 1.
Significant difference between ribcage and diaphragm breathing patterns, P < 0.05;
significantly different from resting conditions during the same breathing pattern, P < 0.05;
significant delay between popliteal venous velocity peak/nadir and femoral venous velocity peak/nadir during same breathing and calf contraction conditions, P < 0.05.
Figure 7. Effects of ribcage and diaphragm breathing patterns on blood flow measured at points proximal (i.e. femoral venous blood flow) and distal (i.e. popliteal venous blood velocity) to the capacitance vessels of the upper thigh.
A, effects of ribcage and diaphragm breathing on femoral venous blood flow and popliteal blood velocity over the course of a breath at rest. Note the significant differences between ribcage and diaphragm breathing in femoral venous blood flow during inspiration and expiration, though only mean inspiratory popliteal blood flow is only significantly different between the two breathing conditions. The lack of significance during expiration may be due in part to the significant rightward shift in the popliteal blood velocity trace in relation to the femoral venous blood flow trace or the increased variability of the popliteal venous blood flow trace during ribcage breathing. B, effects of ribcage and diaphragm breathing on femoral venous blood flow and popliteal blood velocity over the course of a breath during mild contraction. Note that increases in blood velocity occur in synchrony with the concentric portion of the calf contraction cycle, and the popliteal velocity waveform is nearly identical during inspiration and expiration with both ribcage and diaphragm breathing, despite a significant within-breath modulation of femoral venous outflow. Grey circles, ribcage breathing; black circles, diaphragm breathing; †P < 0.01 and *P < 0.05 for comparisons of ribcage versus diaphragm for mean inspiratory or expiratory values.
When calf contraction was present, significant modulation of femoral venous blood flow by respiration was not present in the popliteal venous blood velocity traces (though respiratory modulation was evident in the common femoral vein). Specifically, the addition of mild calf contraction resulted in increases in popliteal blood velocity which occur concomitant with the concentric portion of the calf contraction phase, and decreases in popliteal venous blood velocity during the calf relaxation phase. There were no significant differences between mean inspiratory or mean expiratory popliteal blood velocity during either inspiration or expiration (P = n.s., inspiration versus expiration for both breathing conditions, see Fig. 7B and Table 3), and there were no significant differences in the velocity traces between ribcage and diaphragm breathing. It is also important to note that the popliteal venous blood velocity trace is now shifted leftward (i.e. the peak femoral venous outflow associated with calf contraction now occurs after the peak popliteal venous blood velocity), though this difference only reached significance during ribcage breathing conditions.
Discussion
The main findings of this study can be summarized as follows: (1) in the semirecumbent human, there was significant modulation of femoral venous return from the lower limb by respiration at rest, and to a lesser (but still significant) extent during various intensities of calf contraction exercise; (2) the nature of the within-breath modulation of femoral venous outflow was critically dependent upon abdominal pressure dynamics over the course of a breath; and (3) despite profound within-breath modulation, net femoral venous return over time (i.e. steady-state) was not affected by the type of breathing pattern followed by the subjects; and (4) the magnitude of the modulation of femoral venous return during calf exercise was likely to be affected by the compliance of the upper thigh capacitance vessels, as we did not observe any within-breath modulation of popliteal venous blood flow during calf contraction.
Mechanical effects of breathing on venous return from the lower limb at rest
Within-breath modulation
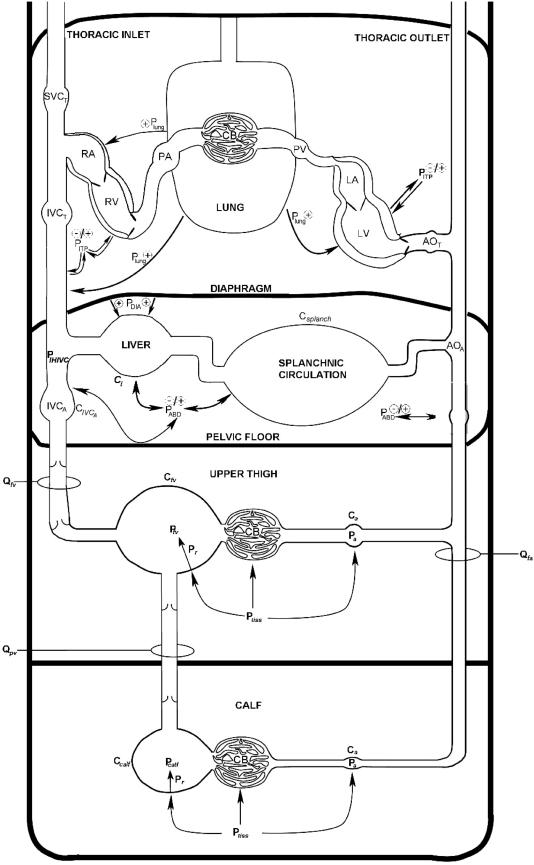
In this study, we observed a significant modulation of femoral venous return over the course of a diaphragmatic breath, such that femoral venous blood flow was significantly reduced during the inspiratory phase of a diaphragmatic breath, with a resurgence of blood flow during the expiratory phase of the breath. Our finding that the manual compression of the abdomen during a ribcage inspiration resulted in a qualitatively (and quantitatively) similar pattern of modulation to that observed during a diaphragmatic inspiration supports the notion that it is the inspiratory change in abdominal pressure per se that dictates the modulation of femoral venous blood flow over the course of a breath (Doppman et al. 1966; Willeput et al. 1984), and not the impingement of the inferior vena cava at its diaphragmatic foramen as a result of concentric diaphragmatic contraction (Norhagen, 1964). These findings in the semirecumbent human are qualitatively similar to those of Willeput (1984), who reported similar reductions in femoral venous blood velocity during a diaphragmatic inspiration or manual abdominal compression in the resting, supine human. However, the addition of a hydrostatic column in our study was likely to result in a much greater degree of venous distention and increased the stressed volume of the capacitance vessels (i.e. an increase in femoral venous recoil pressure in the calf (Pr,fv/calf) and decrease in femoral venous compliance (Cfv) in Fig. 8) within the lower limb, both of which would favour the return of venous blood to the heart (see Appendix). The fact that the respiratory modulation of femoral venous blood flow persisted under these conditions emphasizes the dominance of abdominal pressure in determining abdominal inferior vena cava (IVCA) resistance (see Fig. 8), and the consequent modulation of femoral venous return from the lower limb.
Figure 8. Schematic showing the sites of blood flow/velocity measurement and potential variables affecting the respiratory modulation of femoral and popliteal venous return.
For further explanation, please see Discussion and Appendix. LA, left atrium; LV left ventricle; AOT thoracic aorta; AOA, abdominal aorta; Csplanch, splanchnic vasculature compliance; Cl, liver compliance; Qfa, femoral arterial blood flow; Ca, arterial compliance; CB, capillary bed; Qfv, femoral venous blood flow; Cv, venous compliance; Ptiss, extravascular tissue pressure; Pr, recoil pressure due to elastic properties of vessel; QPV, popliteal venous blood flow (estimated by VPV in this investigation); IVCA, abdominal inferior vena cava, CIVCA, IVCA compliance; IVCT, thoracic inferior vena cava; SVC, superior vena cava; RA, right atrium; RV, right ventricle; PA, pulmonary artery; PV, pulmonary vein; PABD, abdominal pressure; PDIA, diaphragmatic pressure; PITP, intrathoracic pressure; Plung, lung surface pressure.
During ribcage breathing conditions, the pattern of within-breath modulation was reversed compared to diaphragm breathing, such that we observed a slight facilitation of femoral venous blood flow during inspiration (with mean inspiratory blood flow being significantly higher than that observed during diaphragm breathing), and a reduction in femoral venous blood flow during the ensuing expiratory phase of a ribcage breath. Interestingly, our data suggest that during ribcage breathing, the facilitation of femoral venous blood flow due to reductions in intrathoracic pressure (which ultimately reduce right atrial pressure (PRA) and infrahepatic inferior vena caval pressure (PIHIVC) in Fig. 8), per se, is actually quite modest, as we did not find significant differences in mean blood flow between the inspiratory and expiratory phases of a ribcage breath, and actually observed a dissociation between the inspiratory reductions in intrathoracic pressure and change in anterograde femoral venous blood flow during the latter phases of inspiration.
These observations during ribcage breathing differ from the findings of Makin (1969) whose data suggest that marked increases in femoral venous blood velocity occur throughout all of a predominantly ribcage inspiration. However, the exaggerated inspiratory efforts performed by their subjects may have widened the Pfv to PIHIVC gradient enough to facilitate venous return over course of a much shorter inspiratory time (Makin, 1969), and concomitant reductions in abdominal pressure may have facilitated the filling of the abdominal inferior vena cava or prevented increases in IVCA resistance by preventing its collapse (Willeput et al. 1984). Thus, the approaches taken in previous studies have likely precluded the determination of the true contribution of the resting inspiratory intrathoracic pressure excursion to the facilitation of femoral venous return from the lower limb, and our relatively unchanged abdominal pressure (inspiratory change in abdominal pressure (ΔPABD) =∼0.6 cmH2O) and longer inspiratory times are likely to be the contributors to the qualitative differences in the within-breath changes in femoral venous return between previous studies (Makin, 1969; Willeput et al. 1984) and the present investigation.
Why does femoral venous blood flow decrease during the latter half of a ribcage inspiration? We believe the two most likely mechanisms limiting the facilitation of femoral venous return during ribcage breathing are as follows. (a) Reductions in upstream driving pressure (Pfv in Fig. 8) due to reductions in upper thigh venous recoil (Pr, the recoil due to the elastic properties of the vessels) from the transient inspiratory translocation of blood centrally (Permutt & Caldini, 1978). (b) Inspiratory increases in downstream resistance (at the point of IVCA in Fig. 8) due to the collapse of the IVCA (Holt, 1941; Guyton & Adkins, 1954; Permutt & Riley 1963). Our observation that femoral venous cross-sectional area was decreased slightly during inspiration in the present study provides indirect evidence for the former phenomenon, as increases in downstream resistance due to inferior vena caval collapse would be expected to increase femoral venous cross-sectional area during the latter portions of inspiration.
Net effects of breathing pattern on steady-state venous blood flow
Perhaps the greatest difference between the present study and those published previously is that our data are the first to quantify femoral venous blood flow accurately (versus femoral venous blood velocity), and show no net effect of breathing pattern per se on steady-state femoral venous blood flow. Thus, any obstruction of venous return during a diaphragmatic inspiration was balanced by an equal and opposite resurgence of venous return during the ensuing expiratory phase of the breath, and vice versa during ribcage breathing. In the present study, we directly measured changes in vessel cross-sectional area at end-expiration and end-inspiration using a computerized planimeter, which allowed for the calculation of instantaneous blood flow over the course of a breath after the estimation of instantaneous venous cross-sectional area with a linear interpolation approach. We also kept the magnitude of the inspiratory intrathoracic pressure excursion very similar and the breath timing identical between ribcage and diaphragm breathing conditions, and measured blood flow under each condition for a minimum of 5 min on a minimum of four separate visits. Previous studies suggesting a net effect of breathing pattern on blood flow over time in the human have either measured blood velocity (Makin, 1969; Willeput et al. 1984; Kwon et al. 2003) (an inappropriate surrogate for blood flow in veins) or have only measured blood flow during non-steady-state conditions (e.g. during a transition from diaphragm breathing to ribcage breathing, for two to three breaths, etc.), where transient shifts in blood volume to or from the lower limb could lead one to the incorrect conclusion that steady-state blood flow has increased.
The skeletal muscle pump versus the respiratory muscle pump
Many studies have examined the effects of skeletal muscle contraction on venous outflow from the muscular bed in both humans and animals, and virtually all studies (including the present investigation) have found that rhythmic contractions force blood centrally in phase with the concentric portion of the limb muscle contraction cycle, with this effect being most pronounced when the contracting muscle is below heart level (Folkow et al. 1970; Hogan et al. 2003). However, the few studies that have examined the interaction between the skeletal muscle pump and the respiratory muscle pump have made inaccurate assumptions regarding vessel cross-sectional area (Osada et al. 2002), used femoral venous velocity as a surrogate for venous blood flow (Makin, 1969; Kwon et al. 2003), performed limb muscle contraction against little or no resistance (Osada et al. 2002; Kwon et al. 2003), or have not measured and rigorously controlled changes in respiratory muscle pressure production (Makin, 1969; Osada et al. 2002; Kwon et al. 2003).
During diaphragm breathing, only moderate levels of calf contraction were able to significantly increase both femoral venous return during a diaphragmatic inspiration (though Qfv during the ensuing expiratory phase of the breath remained significantly higher) and increase Qfv during the expiratory portion of a ribcage breath. This observation would suggest that the skeletal muscle pump is relatively ineffective at competing with the respiratory muscle pump at lower levels of locomotor muscle contraction. However, it is important to note that in our study, the limb skeletal muscle pump and respiratory muscle pump are separated by the venous vessels of the upper thigh, and even though these vessels are relatively distended due to the presence of a hydrostatic column (i.e. a decrease in Cfv relative to the supine body position, see Fig. 8), transient changes in the volume of blood within the upper thigh are likely to play an important role in the respiratory modulation of femoral venous return from the resting and contracting limb (see below).
The role of upper thigh venous capacitance in the respiratory modulation of femoral venous return
The magnitude of the respiratory modulation of femoral venous return we observed at rest and during calf contraction exercise with both breathing patterns led us to broach the question: what is the role of the upper thigh capacitance vessels in the modulation of venous return from the lower limb? To examine this directly, we examined the within-breath changes in blood velocity in the popliteal vein – a vessel which is immediately distal to the capacitance vessels of the upper thigh and provides the majority of drainage from the musculature of the calf.
At rest, during both diaphragmatic and ribcage breathing patterns, the popliteal venous velocity waveform was morphologically similar to that observed in the femoral vein, as the mean popliteal blood velocity during a diaphragmatic inspiration was significantly lower than that observed during a ribcage inspiration. The relatively short delay in the response of the popliteal velocity waveforms relative to the femoral venous blood flow waveforms suggests that the stressed volume of the venous vessels within the upper thigh increases rapidly – a phenomenon that is likely to be attributable to the lower limb venous distension associated with a semirecumbent body position (i.e. a hydrostatic column). We are not aware of any previous studies demonstrating such an effective transmission of respiratory modulation of venous return throughout the venous vasculature of the lower limb, and postulate that such an effect would be minimized (or even eliminated) in the supine body position due to the reductions in stressed volume and increases in venous compliance (Cfv) of the upper thigh venous vessels associated with the loss of a hydrostatic column.
During calf contraction conditions, we did not observe any respiratory modulation of the popliteal venous blood velocity trace during either ribcage or diaphragm breathing conditions, even though significant respiratory modulation of femoral venous outflow persisted throughout all levels of calf contraction. Instead, increases in popliteal venous blood velocity occurred in synchrony only with calf contraction. This finding is perhaps not surprising given the observations that pressure within the soleus and gastrocnemius (Ptiss in Fig. 8) can exceed 30 cmH2O during muscular contraction (Henderson et al. 1935; Wells, 1938; Pollack & Wood, 1949), and up to 40% of the calf's blood volume can be ejected with a single contraction (Stewart et al. 2004). We believe the lack of respiratory modulation of popliteal venous blood velocity during calf contraction provides our most compelling evidence for the importance of upper thigh venous vessel compliance in the modulation of venous return from the lower limb during exercise. However, one must keep in mind that a limitation of our current approach of measuring popliteal venous velocity and not blood flow is that we cannot definitively state the magnitude of the upper thigh capacitance (see Methods for further detail on difficulties associated with measuring popliteal vessel cross-sectional area). During diaphragm breathing, however, our approach would actually underestimate the magnitude of the upper thigh capacitance, since femoral venous pressure is likely to increase during inspiration, resulting in increases in popliteal venous pressure, distention of the popliteal vein (thereby increasing vessel cross-sectional area), and an increase in of the amount of blood ejected into the upper thigh vessels per calf contraction.
Implications for femoral venous return during whole-body exercise
In extrapolating our findings to what may occur during whole-body exercise, it is important to take into consideration the fact that the muscle mass recruited in response to the isolated calf contraction mode of exercise used in this study differs markedly from that observed during cycle ergometry or walking/running. Specifically, the addition of quadriceps contraction is likely to play an important role minimizing the respiratory-related fluctuations in locomotor limb venous return by ejecting a greater volume of blood from the lower limb with each concentric contraction, increasing total locomotor limb blood inflow (via reductions in arterial resistance – see Fig. 8 and Appendix), and by reducing the capacitance and compliance of the venous vessels within the upper thigh (thereby decreasing its time constant for venous drainage, τ, which will also improve the effectiveness of the distally located calf muscle pump; see Fig. 8 and Appendix for mathematical explanation).
An important point to note is that unlike the breathing mechanics followed by the subjects in the present study, during whole-body exercise, expiration becomes active even at very low workloads (i.e. < 30% maximum rate of oxygen uptake), which also results in slight reductions in end-expiratory lung volume (Henke et al. 1988). Preliminary observations in our laboratory, using the same model of calf exercise presented here, suggest that the addition of active expiration to diaphragmatic breathing reverses the within-breath pattern of modulation of Qfv reported here and also reduces net femoral venous blood flow in the steady state (Miller et al. 2004). For further discussion on how the respiratory modulation of Qfv may affect inferior vena caval blood flow, the reader is referred to our Appendix section and our online supplementary Discussion.
Summary
In summary, our findings show that in the semirecumbent human the pressures produced by the respiratory muscles can have a profound modulatory effect on venous return from the lower limb over the course of a breath, with the qualitative nature of this modulation being critically dependent upon abdominal pressure dynamics. The modulatory effect of respiration persisted in the presence of both mild and moderate calf contraction. Despite this profound within-breath modulation, we did not find an effect of breathing pattern on steady-state venous return from either the resting or contracting locomotor limb, though expiration remained passive in all of the present experiments. Our data also suggest that the respiratory modulation of venous return from the lower limb is dependent not only on the pressures produced by the respiratory and skeletal muscle pumps, but is also critically dependent upon the capacitance and compliance of the venous vasculature separating them.
Acknowledgments
This work was supported by grants T32-HL007654 (J.D.M.) and RO1-HL015469 (J.D., D.F.P., A.J.J. and J.A.D.) from the National Heart, Lung and Blood Institute, as well as from a Grant-in-Aid from the American Heart Association (J.D. and J.A.D.).
Appendix
Mathematical basis for respiratory modulation of femoral venous return from the resting locomotor limb
Haemodynamic effects of respiration with Zone 3 abdominal vascular conditions (Pfv > PIHIVC > PABD) (ribcage breathing)
In an attempt to predict the effects of respiration on venous return under a variety of physiological conditions, we have considered the circulation of the inferior vena cava, thigh and calf as three capacitors in series (see Fig. 8), and combined the mathematical modelling approaches of Permutt & Caldini (1978) and Takata et al. (1990). We have chosen to begin the resting analysis at the point of the inciting stimulus at rest, i.e. a change in inferior vena caval blood flow. As such, when the inferior vena cava (IVC) is patent (e.g. Zone 3 abdominal vascular conditions), the change in IVC outflow can be given by the equation:
 |
(A1) |
Where CIVC is the compliance of the IVC; dPdriving/dt is the rate of change of driving pressure for blood flow across the IVCA; ΔVIVC is the change in IVC blood volume; τIVC is the time constant of the IVC (i.e. the time needed to drain all of its blood volume in a given vascular state); RIVC,out is the resistance to outflow from the IVC; dPfv/dt is the rate of change of femoral venous blood pressure (i.e. the upstream component of driving pressure); and dPIHIVC/dt is the rate of change of infrahepatic vena caval (IHIVC) blood pressure (i.e. the downstream component of driving pressure).
During ribcage breathing conditions, the negative intrathoracic pressure excursion during inspiration reduces right atrial pressure, which in turn will reduce PIHIVC and augment the pressure gradient across the IVCA. Flow past the point of the IHIVC will increase due to a translocation of blood centrally from both the abdominal inferior vena cava (as a function of CIVC) and from the upper thigh (as a function of Cfv). As arterial inflow to the upper thigh is constant, the change in upstream driving pressure across the IVCA is given by the following equation:
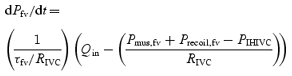 |
(A2) |
where:
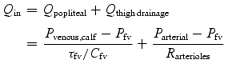 |
(A3) |
Close examination of eqn (A2) will reveal that as PIHIVC decreases, blood flow out of the thigh will increase, and at a constant arterial inflow and RIVC, Pfv will decrease in inverse proportion to the compliance of the thigh, thus reducing the effective driving pressure. Such an explanation fits with our observation that the Qfv profile during a ribcage inspiration at rest is biphasic (i.e. an initial increase in Qfv followed by a progressive decline).
In measuring popliteal blood velocity, though, we observed a delayed increase in VPV during a ribcage inspiration, which strongly suggests that blood flow into the upper thigh is not a constant. Thus, the change in QPV at a constant calf resistance will be a function of the driving pressure (Pcalf−Pfv), with the upstream component (Pcalf) being predicted by the equation:
 |
(A4) |
Haemodynamic effects of respiration with Zone 1 (PIHIVC < Pfv < PABD) or Zone 2 (PIHIVC < PABD < Pfv) abdominal vascular conditions
An important caveat in using the above analysis is that the critical closing pressure of the inferior vena cava cannot be exceeded (i.e. Pcrit,IVC). During our diaphragmatic breathing conditions, this condition was presumably violated, as Qfv was zero (or even slightly negative) for the last half of inspiration, implying that PABD exceeded Pcrit,IVC during this period of time. Under these conditions PIHIVC is no longer the effective downstream pressure for QIVC, and Qfv can only increase if Pthigh exceeds the effective backpressure to flow through the inferior vena cava (Pback,IVC), which is defined by the sum of PABD and Pcrit,IVC. Under these conditions, flow through the inferior vena cava in response to an increase in upstream pressure can be given by the following equation:
| (A5) |
Now, when dPback,IVC/dt exceeds dPfv/dt, flow through the IVC will cease, and we can see from eqn (A2) that Pfv will now increase in a manner that is proportional to Qin and inversely proportional to Cfv. Once again, the increase in Pfv will be buffered by the reduction in the Qpopliteal component of Qin in eqn (A3).
Mathematical basis for the respiratory modulation of femoral venous return from the locomotor limb during calf contraction exercise
In attempting to predict the effects of calf contraction exercise on femoral venous return, we can use the same mathematical concepts outlined above, though we find it most intuitive to now begin with the contracting calf. In examining the effects of calf contraction on the components of dPcalf/dt in eqn (A4), we know that the largest changes induced by plantar flexion will be reductions in τcalf (due to a decreased calf compliance) and increases in Ptiss,calf and Qin. Thus, the net effect will be an increase in dPcalf/dt during the concentric portion of the calf contraction phase, which will serve to force a volume of blood centrally that is proportional to the initial volume of the capacitance vessels within calf times the quotient of the change in transmural pressure across those vessels and their compliance.
If we assume that our popliteal velocity measures are a somewhat accurate estimate of blood flow out of the calf, or Qpopliteal in eqn (A3), our data would suggest that there is no respiratory modulation of Qpopliteal with calf contraction. Since we also know that Qin in eqn (A3) is not modulated by respiration, we can now show using eqn (A5) that Pfv(i.e. the effective upstream driving pressure for flow through the inferior vena cava) during the calf contraction phase is increased in proportion to the product of Cfv and ΔVfv (i.e. the change in thigh volume). As more than 70% of Qfv during diaphragmatic breathing occurred during expiration under all calf contraction conditions in the present investigation, the relatively large capacitance and compliance of the femoral venous bed is likely to be the dominant factor determining the modulation of femoral venous return under our exercise conditions (as Routflow as the dominant factor would result in either within-breath modulation of QPV during calf contraction conditions or no respiratory modulation of Qfv).
Finally, the rate at which Pfv changes at any given Cfv will be determined ultimately by the rate of blood flow translocation from the calf to the thigh, and using eqn (A5) we can readily see that the dPfv/dt is the primary determinant of QIVC (i.e. the final capacitance bed prior to PIHIVC) at any given PIHIVC or Pback,IVC, depending upon the abdominal vascular zone conditions.
Mathematical basis for respiratory modulation of femoral venous return from the locomotor limb during whole-body exercise
When attempting to predict the respiratory modulation of femoral venous return during whole-body exercise, we believe the components of eqn (A2) are of paramount importance to consider. First, the contraction of the quadriceps will markedly decrease τfv, thereby decreasing Cfv– this alone would be expected to decrease the respiratory modulation of femoral venous return by augmenting dPfv/dt in response to any increase in outflow resistance. Secondly, quadriceps contraction will markedly increase Qin, allowing for a more rapid increase in Pfv in response to any step increase in outflow resistance or Pback,IVC. Third, the rhythmic increases in Ptiss,fv associated with dynamic exercise will force a larger volume of blood centrally with each contraction, thereby increasing the flux through the IVC with each contraction. Finally, the increased respiratory muscle pressure excursions will augment the changes in PIHIVC and Pback,IVC, both of which will serve to augment the respiratory modulation of femoral venous return. Such predictions fit with the observations of Wexler et al. (1968) who demonstrated that respiratory modulation of IVC blood velocity persists during light intensity cycling exercise in supine humans (see online supplementary discussion section for further detail).
Supplementary Material
The online version of this paper can be accessed at:
DOI: 10.1113/jphysiol.2004.076422 http://jp.physoc.org/cgi/content/full/jphysiol.2004.076422/DC1 and contains supplementary material showing:
(1) additional information regarding the methods employed in this study, including a video clip of representative longitudinal and cross-sectional images of the femoral vein: (2) supplementary data regarding the reproducibility of the measures of venous blood flow, and (3) a supplementary discussion section entitled ‘Modulation of venous return from the lower limb: Implications for inferior vena caval return’.
This material can also be found at: http://www.blackwellpublishing.com/products/journals/suppmat/tjp/tjp753/tjp753sm.htm
References
- Doppman J, Rubinson RM, Rockoff SD, Vasko JS, Shapiro R, Morrow AG. Mechanism of obstruction of the infradiaphragmatic portion of the inferior vena cava in the presence of increased intra-abdominal pressure. Invest Radiol. 1966;1:37–53. doi: 10.1097/00004424-196601000-00027. [DOI] [PubMed] [Google Scholar]
- Folkow B, Gaskell P, Waaler BA. Blood flow through limb muscles during heavy rhythmic exercise. Acta Physiol Scand. 1970;80:61–72. doi: 10.1111/j.1748-1716.1970.tb04770.x. [DOI] [PubMed] [Google Scholar]
- Guyton AC, Adkins LH. Quantitative aspects of the collapse factor in relation to venous return. Am J Physiol. 1954;177:523–527. doi: 10.1152/ajplegacy.1954.177.3.523. [DOI] [PubMed] [Google Scholar]
- Henderson Y, Oughterson AW, Greenberg LA, Searle CP. Muscle tonus, intramuscular pressure, and the venopresor mechanism. Am J Physiol. 1935;114:261–268. [Google Scholar]
- Henke KG, Sharratt M, Pegelow D, Dempsey JA. Regulation of end-expiratory lung volume during exercise. J Appl Physiol. 1988;64:135–146. doi: 10.1152/jappl.1988.64.1.135. [DOI] [PubMed] [Google Scholar]
- Hogan MC, Grassi B, Samaja M, Stary CM, Gladden LB. Effect of contraction frequency on the contractile and noncontractile phases of muscle venous blood flow. J Appl Physiol. 2003;95:1139–1144. doi: 10.1152/japplphysiol.00226.2003. [DOI] [PubMed] [Google Scholar]
- Holt JP. The collapse factor in the measurement of venous pressure. Am J Physiol. 1941;134:292–299. [Google Scholar]
- Kwon OY, Jung DY, Kim Y, Cho SH, Yi CH. Effects of ankle exercise combined with deep breathing on blood flow velocity in the femoral vein. Aust J Physiother. 2003;49:253–258. doi: 10.1016/s0004-9514(14)60141-0. [DOI] [PubMed] [Google Scholar]
- Makin GS. Velocity in the femoral vein. The effect of respiration exercise and tilt on blood velocity in the femoral vein as detected by an ultrasonic technique. Angeiologie. 1969;21:319–328. [PubMed] [Google Scholar]
- Miller J, Pegelow D, Dempsey J. Skeletal muscle pump vs. respiratory muscle pump: active expiration reverses respiratory modulation of venous return. Med Sci Sports Exerc. 2004;36:S225. 10.1097/00005768-200405001-01076. [Google Scholar]
- Norhagen A. Selective angiography of the hepatic veins experimental investigation of basal circulatory dynamics. Acta Radiol. 1964;221:1–121. [Google Scholar]
- Osada T, Katsumura T, Hamaoka T, Murase N, Naka M, Shimomitsu T. Quantitative effects of respiration on venous return during single knee extension-flexion. Int J Sports Med. 2002;23:183–190. doi: 10.1055/s-2002-23177. 10.1055/s-2002-23177. [DOI] [PubMed] [Google Scholar]
- Permutt S, Caldini P. Regulation of cardiac output by the circuit: venous return. In: Baan J, Noordergraaf A, Raines J, editors. International Conference of Cardiovascular System Dynamics; Valley Forge PA USA: MIT Press; 1978. pp. 465–479. [Google Scholar]
- Permutt S, Riley RL. Hemodynamics of collapsible vessels with tone: the vascular waterfall. J Appl Physiol. 1963;18:924–932. doi: 10.1152/jappl.1963.18.5.924. [DOI] [PubMed] [Google Scholar]
- Pollack AA, Wood EH. Venous pressure in the saphenous vein at the ankle in man during exercise and changes in posture. J Appl Physiol. 1949;1:649–662. doi: 10.1152/jappl.1949.1.9.649. [DOI] [PubMed] [Google Scholar]
- Rothe CF. Reflex control of veins and vascular capacitance. Physiol Rev. 1983;63:1281–1342. doi: 10.1152/physrev.1983.63.4.1281. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human Cardiovascular Control. New York: Oxford University Press; 1993. Passive effects of gravity; pp. 3–36. [Google Scholar]
- Sheel AW, Derchak PA, Morgan BJ, Pegelow DF, Jacques AJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex reduction in resting leg blood flow in humans. J Physiol. 2001;537:277–289. doi: 10.1111/j.1469-7793.2001.0277k.x. 10.1111/j.1469-7793.2001.0277k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JM, Medow MS, Montgomery LD, McLeod K. Decreased skeletal muscle pump activity in patients with postural tachycardia syndrome and low peripheral blood flow. Am J Physiol Heart Circ Physiol. 2004;286:H1216–1222. doi: 10.1152/ajpheart.00738.2003. 10.1152/ajpheart.00738.2003. [DOI] [PubMed] [Google Scholar]
- Takata M, Wise RA, Robotham JL. Effects of abdominal pressure on venous return: abdominal vascular zone conditions. J Appl Physiol. 1990;69:1961–1972. doi: 10.1152/jappl.1990.69.6.1961. [DOI] [PubMed] [Google Scholar]
- Wells HS. Tissue pressure (intracutaneous, subcutaneous, and intramuscular) as related to venous pressure, capillary filtration, and other factors. J Clin Invest. 1938;17:489–499. doi: 10.1172/JCI100976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler L, Bergel DH, Gabe IT, Makin GS, Mills CJ. Velocity of blood flow in normal human venae cavae. Circ Res. 1968;23:349–359. doi: 10.1161/01.res.23.3.349. [DOI] [PubMed] [Google Scholar]
- Willeput R, Rondeux C, De Troyer A. Breathing affects venous return from legs in humans. J Appl Physiol. 1984;57:971–976. doi: 10.1152/jappl.1984.57.4.971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.