Abstract
In view of the important protective role of the fetal membranes, wound sealing, tissue regeneration, or wound healing could be life saving in cases of preterm premature rupture of the membranes. Although many investigators are studying the causes of preterm premature rupture of membranes, the emphasis has not been on the wound healing capacity of the fetal membranes. In this review, the relevant literature on the pathophysiologic condition that leads to preterm premature rupture of membranes will be summarized to emphasize a continuum of events between rupture and repair. We will present the current knowledge on fetal membrane wound healing and discuss the clinical implications of these findings. We will critically discuss recent experimental interventions in women to seal or heal the fetal membranes after preterm premature rupture of membranes.
Keywords: Preterm premature rupture of membranes, Pregnancy outcome
Intact fetal membranes during pregnancy are important to the maintenance of amniotic fluid homeostasis and in the defense against ascending infection. Although the association with preterm premature rupture of membranes (PROM) and adverse pregnancy outcomes have been well-described, data regarding the capacity of the fetal membranes for healing and repair remain limited. Multiple potential therapies have been advocated for the treatment of preterm PROM with some success, especially in cases of postprocedural fetal membrane rupture. However, before incorporation in clinical practice, potential therapies should be studied further in animal models and human fetal membrane explants to determine the efficacy and optimal treatment regimens.
The amnion, chorion, and contiguous maternal decidua form the human fetal membranes in the second and third trimester of pregnancy. The membranes form an adjustable bio-container that provides the fetus with a limited space that is filled with amniotic fluid that allows movement. They also protect the growing fetus against infection, trauma, and toxins. The strength of the tissue is provided predominantly by the collagen in the amnion, although the amnion and chorion together are stronger than either layer alone.1
Preterm PROM is the rupture of the amnion and chorion before 37 completed weeks of gestation before the onset of labor. This complicates 1% to 4% of all pregnancies. Especially at very early gestational ages, it is often associated with adverse perinatal outcome; many surviving infants have permanent morbidities as a result of preterm birth. There are multiple clinical risk factors for preterm PROM that include previous preterm birth, previous preterm PROM, genitourinary tract infections, behavioral factors, and obstetric complications, such as polyhydramnios and antenatal hemorrhage.2
Cause of membrane rupture
Preterm PROM likely arises from multiple pathologic pathways. Most commonly, chronic inflammation and/or infection increases the production of hormones and cytokines in the uterus, membranes, and/or placenta, which results in preterm contractions, membrane weakening, and preterm PROM.3 It has been suggested that repeated stretching of the amniochorion induces a phenomenon that is known as “strain hardening,” which makes the membranes less elastic and more susceptible to preterm PROM.4 A localized alteration in the amnion and chorion has been identified adjacent to the rupture site,5 and generalized placental and decidual relaxin levels have been shown to be increased in noninfected fetal membranes with preterm PROM.6 Relaxin increases local levels of metalloproteinases and tissue plasminogen activator that results in extracellular matrix degradation and rupture.7 A generalized loss of collagen in the fetal membranes has been associated with a short interval between preterm PROM and delivery.8 A deficiency of vitamin C has long been postulated to play a role in preterm PROM because ascorbic acid functions at many levels in the biosynthesis of collagen and in the immunologic response to infection.9 Maternal stress has an important effect on the autocrine/paracrine systems that control labor and may play a role in preterm PROM.10 Finally, fetal membrane rupture can occur as a result of trauma and after fetoscopy or amniocentesis. Preterm PROM that results from an invasive prenatal intervention is usually referred to as “iatrogenic” preterm PROM in contrast to “spontaneous” preterm PROM.11 During the last decade, developments in the fields of fetoscopy and open fetal surgery have resulted in the development of new therapeutic options in selected cases of complicated monochorionic pregnancies, congenital diaphragmatic hernia, myelomeningocele, and lower urinary tract obstruction, among others.12 These developments have revived interest in the problem of iatrogenic preterm PROM. At present, there is a limited role for open fetal surgery, the risks and benefits of which continue to be elucidated in randomized prospective clinical trials. Fetoscopy is being used increasingly because it is less invasive and because most fetoscopic procedures currently can be carried out with a single intrauterine entry. Despite these improvements, 5% to 30% of fetoscopic procedures are still complicated by preterm PROM.13 This limits the clinical use of the technique and is a major obstacle to the further development of this field. It is important therefore to develop strategies to seal the membrane defect or to stimulate the spontaneous repair mechanisms of the fetal membranes at the time of the procedure.
Although there is considerable information regarding the mechanisms that lead to preterm PROM, little is known about the growth and healing capacity of the fetal membrane. Further, current literature that suggests a correlation between inflammation and the cause of membrane rupture may be confounded by the presence of an inflammatory response subsequent to membrane rupture that is associated with wound healing. For example, it appears that collagen turnover and content are determined by the level of transcription and the subsequent activation of the collagenolytic metalloproteinases. These enzymes have been the focus of attention for several years as a cause of preterm PROM. Multiple studies have reported increased levels of the genes, proteins, and/or activation of metalloproteinases in the fetal membranes after preterm PROM,14 but few authors have considered the length of the latency period of the tissues that are used in their studies. With the use of highly selected fetal membranes, no increase in the genes for any of the major metalloproteinases was found by macroarray and by confirmatory Northern analysis.15 None of the genes for the metalloproteinases, other than metalloproteinase-11 that was 2-fold up-regulated, were expressed differentially. Northern analysis of metalloproteinase-8 and -9 confirmed the array results. The increase of metalloproteinase gene expression that has been reported by other studies may be the result of the rupture and part of the tissue response to the injury rather than a cause of the rupture per se (Figure 1).15 Alternatively, the metalloproteinases could be a cause of some cases of rupture, at the same time as being an integral part of the subsequent repair process. However, other studies also have shown a lack of increase in metalloproteinase-9 protein/activation in preterm PROM.16
Figure 1.

Hypothesis on the relationship between the causes and effects in preterm PROM. The primary cause of rupture may be present from early gestation and may involve genes the products of which can modify the growth and/or the structure of the extracellular matrix (ECM). An inflammatory response may be a consequence of these changes in the extracellular matrix. A rapid effect of the injury is shown, which increases over the latency period and is of unknown duration. Reproduced from the American Journal of Obstetrics and Gynecology, vol 187, Tashima LS, Yamamoto SY, Yasuda M, Millar LK, Bryant-Greenwood GD, Decidual relaxins: gene and protein up-regulation in preterm premature rupture of the membranes by complimentary cDNA arrays and quantitative immunocytochemistry, p. 285-97, 2002, with permission from Elsevier.
Fetal membrane healing
Because the fetal membranes are not innervated and only very poorly vascularized in the human being, a typical wound healing response that includes inflammation, scar formation, and tissue regeneration as described in the skin17 and many other organs is unlikely to occur.
Clinical evidence regarding the healing potential of the fetal membranes is largely related to rupture after amniocentesis. Older case reports mention the persistence of defects in the fetal membranes several weeks after invasive procedures.18 Most cases of post-amniocentesis amniorrhexis are self-limited, however, and resolve spontaneously, which leads to favorable pregnancy outcomes.19 Occasionally, patients with spontaneous preterm PROM cease to leak amniotic fluid. These patients also have good pregnancy outcomes as they are delivered at a mean gestational age of 38 weeks.20 This suggests that the fetal membranes have the capacity to seal a created or spontaneously occurring defect. However, the leakage could be concealed, or the membranes could reseal through retraction, sliding, contraction, and scarring in the myometrial and decidual layers of the uterus, rather than involving an active healing mechanism at the level of the fetal membranes.21
Fetal membrane healing in vitro
Cell monolayers that are obtained from an amnion-derived cell line (FL [ATCC, CCL-62]) were found to be capable of repairing a central microsurgical defect, with 75% to 80% of the defect being repaired within 24 hours.22 Using a comparable cell line (WISH [ATCC, CCL-25]), we found repair to be stimulated by increasing levels of epidermal growth factor and insulin-like growth factor-I in the culture medium.23 In amniocytes that are obtained from digested fresh human fetal membranes, the repair capacity of these monolayers was found to be gestational age dependent, with cells that are obtained at earlier gestational ages showing higher proliferation rates and faster closure of the central defect.24 In a similar set-up Bilic et al25 found this difference between term and preterm tissues to be present only for mesenchymal cells from the amnion.
Studies of surgical trauma in full-thickness cultured human fetal membrane explants show only a limited repair mechanism to be present. Despite evidence of epithelial cell proliferation and migration and survival of the explants in culture for a period of 12 days, the overall size of the defects remains unchanged.26 This model offers the advantage of including all layers of the fetal membranes; however, in vitro tissue explants become increasingly unhealthy over time, and this likely affects their ability to heal.
Fetal membrane healing in animal models
The first animal model that was used to study the wound healing response in the fetal membranes was the rat.27 Rat fetal membranes that are punctured with a very fine needle were examined grossly and histologically. There was a significant reduction in the wound size over time, mostly because of contraction of the wound. Subtle histologic changes were documented and included thickening, membrane fusion, adhesion, and clot formation. The membrane integrity was not restored for 5 days after the membrane puncture, and no active tissue proliferation was documented. In a rabbit model for iatrogenic preterm PROM, 40% of the rabbits had restored membrane integrity 1 week after iatrogenic preterm PROM. Levels of metalloproteinase-2 and -9 and tissue inhibitors of the metalloproteinases were all increased in the amniotic fluid 1 week after the rupture, which suggests a mechanism of active fetal membrane remodeling that involves gelatinase activation.28 Evidence that have been obtained in sheep and rhesus monkeys confirm that the fetal membranes have a very limited ability to heal (Figure 2).29 Figure 2 shows a fetoscopic access port site in the fetal membrane of a rhesus monkey 6 weeks after fetoscopy. The defect remained open without evidence of healing. In an important study on the subject, Gratacós et al performed microscopic examination of 19 fetoscopic membrane defects. No evidence of spontaneous healing was found, and proliferation was limited to the chorion.30
Figure 2.
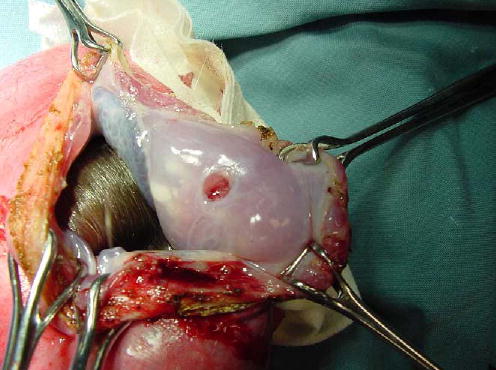
Fetoscopic access port site in a rhesus monkey 6 weeks after fetoscopy. The defect in the amnion is well-defined and round and does not show evidence of wound healing, although the wound edges appear to be thickened.
Potential treatments for preterm PROM
Tissue sealants have been used since the early 1900s for a variety of purposes but have become particularly important in maintaining surgical hemostasis and to stimulate wound healing.31 In 1979, Genz et al32 was the first to report 2 cases of fibrin sealing in women with preterm PROM. Since then, a variety of studies have been performed in fetal membrane explants, animal models, and humans with preterm PROM to determine the efficacy of sealants in the repair of fetal membrane defects.
Use of tissue sealants in vitro in fetal membrane explants
Fibrin glue has been used as a sealant for in vitro wounded fetal membrane explants. Harmanli et al33 measured the tensile strength of the tissue before and after wounding and fibrin stabilization of the wound. Puncture of the tissue decreased the tensile strength. After the application of fibrin glue, the rupture tension increased, which suggests that the application of fibrin glue potentially could improve the structural and functional integrity of artificially ruptured fetal membranes. In another study, patches from term membranes were stretched over a modified syringe and secured to the tube with elastic rings.34,35 The syringe was oriented vertically, and the pieces of membrane were punctured with a 20-gauge needle. The syringe was filled with either platelet-rich plasma, plasma without platelets, or saline solution. There was a diminution of flow when plasma or saline control solutions were used. In 42 of 46 experiments with varying doses of platelet-rich plasma, sealing occurred, and fluid leakage ceased. Scanning electron microscopy showed a plug of platelets and fibrin sealing the hole, which demonstrates that platelet-rich plasma is capable of sealing a needle defect in the fetal membranes.
A similar model was used to evaluate the ability of 7 different sealant preparations to stop fluid leakage through wounded explants. Platelets alone failed to seal the puncture site. Tisseel (Baxter Co, Lessen, Belgium), a commercially available preparation that contains thrombin, fibrinogen, aprotinin, and calcium, and cryoprecipitate/thrombin preparations led to more prolonged sealing of punctured amniotic membranes than platelets alone.36 Although this model represents an over-simplification of the situation in vivo, it provides crucial information on the properties of potential sealants for fetal membrane defects. Additionally, this model was useful in the evaluation of the importance of other factors in iatrogenic preterm PROM, such as the angle of needle introduction, the duration of the puncture, and fluid pressure on the persistence of fluid leakage.37
In vitro laser (Nd:YAG) treatment has been used to attempt welding of human fetal membrane explantations.38 Term fetal membrane explants were cut into 1-cm2 pieces and a polytetrafluoroethylene material (Gore-Tex Co, Gore and Associates; Flagstaff, AZ) was grafted onto the explants. Successful laser welding occurred with 1 to 7 W of laser energy for 15 seconds, although the methods of assessment of the resulting anastomotic integrity (manual traction) were subjective. Standardization of laser welding is difficult because the efficacy of laser welding depends on the tissue temperature that is reached; the ultimate tissue temperature that is generated by the laser is unpredictable because of variations in local tissue factors that include thermal conductivity and absorption. Additionally, there is a substantial risk for thermal injury to the membranes with laser welding that represents a major limitation for this technique.
Stress and leak point pressure of term human fetal membranes after closure with suture (3 interrupted, 6–0 Prolene stitches), laser welding (either a 1.32-μm Nd:YAG laser at 2 W or a 810-nm diode laser at 0.2 W), or Synthaseal (Promethean Surgical Devices, Mendon, MA) was evaluated in vitro.39 Synthaseal is a single component synthetic that infiltrates tissue and polymerizes the formation of a biocompatible hydrogel. The anastomotic stress and leak pressure of repaired fetal membranes was higher with Synthaseal, compared with sutures. Laser welding with albumin solders was unsuccessful. Histologic examination of these specimens showed that laser-fused membranes had marked thermal artifact at the site of welding, which extended outward almost to the edge of the section. Sections of membranes that were fused by Synthaseal showed compression and contraction of the tissue with crush artifact of the cells, predominantly in the chorion layer. Despite this, the integrity of the amniotic epithelium appeared intact. Unfortunately, the explants were not cultured, and the interactions between the membranes and the sealant over time were not studied. This could be important because the repair of cultured fetal membrane explants is delayed in time, with clear tissue outgrowth present only after 10 days of culture.26
In these models, membrane puncture with a needle or scalpel creates a clean, localized defect in the membranes. It therefore represents a good simulation of iatrogenic preterm PROM. Results from these studies with wounded or cut fetal membrane explants cannot be extrapolated to the clinical setting of spontaneous preterm PROM. In the latter, the defect usually is larger, often poorly demarcated, located in the cervical region of the uterus40; infection and inflammation often play an important etiologic role (Table I). However, such models are important to identify potentially safe and effective tissue sealants for fetal membrane repair and allow optimization of the dose and composition of tissue sealant before in vivo use.
Table I.
Major characteristics of spontaneous compared to iatrogenic preterm PROM
| Characteristic | Spontaneous preterm PROM | Iatrogenic preterm PROM |
|---|---|---|
| Cause | Multifactorial, often infectious | Invasive intrauterine procedure (amniocentesis, fetoscopy) |
| Defect size | Large | Depends on size of instrument used |
| Defect type | Often poorly delineated | Sharply delineated |
| Location of defect | Over cervical ostium | At insertion site (often anterior or fundal) |
| Spontaneous resealing | Infrequent | Frequent |
| Occurrence in gestation | More common at advanced gestational age (usually 3rd trimester) | Soon after invasive procedure (often 2nd trimester) |
| Incidence | 1%–4% | 1%–2% after amniocentesis; 5%–30% after fetoscopy |
The use of tissue sealants in fetal membrane repair: Animal models
Animal models have been used widely to investigate potential tissue sealants. In 1 study, New Zealand rabbits underwent laparotomy at 23 to 24 days of gestation (term, 31 days); a 5-mm excision was made in the amnion. A Biosis (small intestinal submucosa; Cook Ob/Gyn, Spencer, IN) disc was welded to the edges of the amnion with a Nd:YAG laser; control sacs were left unrepaired. Four of 26 animals showed histologic evidence of graft welding; in 2 of these cases, amnion growth into the graft was observed. Multiple technical limitations with this model were noted; however, it demonstrated that laser welding was a possibility in rabbit membranes.41
We have used New Zealand rabbits extensively to develop fetal membrane closure techniques after hysteroamniotomy and fetoscopy.42,43 In our most successful study, operative fetoscopy was performed at 22 days of gestation through a 14-gauge needle. After withdrawal of the needle, the fetal membranes access site was left unclosed, covered with a 0.3-mL extracellular matrix (Matrigel; BD Labware, Bedford, MA), or plugged with a collagen plug (Colgen; International Phar, Paris, France). The use of collagen plugs and myometrial suture resulted in functional restoration of membrane integrity at term in 82% of cases, compared to 40% closure in unsealed animals. More importantly, it prevented oligohydramnios and pulmonary hypoplasia in these animals.42 When a bioactive membrane that contained growth-stimulating cytokines was used in the same model, the sealing rates were comparable; however, increased polymorphonuclear infiltration was observed frequently, and 1 fetus had an intrauterine adhesion that resembled an amniotic band.43 The efficacy and safety of placing a collagen plug in the fetoscopic access port site was assessed in 9 sheep. All sheep maintained normal amniotic fluid levels throughout the study period. Histologic evidence showed good integration of the collagen material in the fetal membrane, but the membrane defects were not healed. Levels of metalloproteinase-2 and -9 and tissue inhibitors of the metalloproteinases in amniotic and allantoic fluids were increased, which again were suggestive of an active healing process that is regulated by gelatinases.28
These animal studies show a potential place for biologic substitutes in the sealing of fetal membrane defects that are created by hysterotomy or fetoscopy but await further histologic evaluation and a confirmation of safety and feasibility in pregnant primates, whose size and anatomy closely resemble the human situation.
Use of tissue sealants human fetal membranes repair: Iatrogenic preterm PROM
In 8 cases of iatrogenic preterm PROM and 14 cases of amnion-chorion separation after amniocentesis or fetoscopy between 16 and 24 weeks if gestation, the uterine cavity was punctured with a 22-gauge needle, and various volumes of platelets and cryoprecipitate were injected intra-amniotically.44 In 9 cases, stoppage of amniotic fluid leakage was documented, and 18 of the 32 fetuses (56%) survived. Other authors have reported successful cases with the same or a slightly modified technique (Table II).45–48 This “amniopatch” therapy mimics the “blood patch” that is used in cases of spinal headache after iatrogenic cerebrospinal fluid leakage. It is experimentally supported by in vitro experiments that show that platelets adhere to wounded amnion and form a plug that is stabilized subsequently by cryoprecipitate.35 Two of the 6 cases in the original report were complicated by sudden intrauterine fetal death.44 Severe bradycardia and hypotension have been observed after platelet transfusion; thus, it has been hypothesized that severe hemodynamic changes that result from platelet activation could have caused the fetal deaths. Furthermore, because spontaneous sealing is not infrequent after iatrogenic preterm PROM, the efficacy of such a procedure cannot be ascertained in the absence of an adequate control group. Given these serious complications and the results of in vitro work by Reddy et al35 that showed that platelets alone were an ineffective fetal membrane sealant, platelets alone should not be used in women with preterm PROM. Further research is needed to determine the safety and efficacy of other blood products or combinations in the treatment of iatrogenic preterm PROM.
Table II.
Reports on fetal membrane sealing strategies after iatrogenic preterm PROM
| Study | N | Gestationalage at preterm PROM (wk) | Gestational age at treatment (wk) | Intervention | Outcome | Complication |
|---|---|---|---|---|---|---|
| Quintero (2001)43 | 22 | 15.6–24.0 | 18.7 | Intra-amniotic instillation of various volumes and concentrations of maternal platelets and cryoprecipitate | Membrane sealing in 10/22 cases; 18/32 survivors | 1 survivor surgery for residual bands; 2 sudden intrauterine fetal deaths |
| Young et al (2000)45 | 1 | 17.3 | 20.6 | Fetoscopic application of maternal platelets and fibrinogen/thrombin | Delivery at 32.3 weeks | – |
| Young et al (2004)48 | 3 | 15–23 | 16–24 | Fetoscopic application of maternal platelets and fibrinogen/thrombin | Delivery of viable infants at 26, 32, and 34 weeks; 1 TOP after unsuccessful procedure | – |
| Sener et al (1997)44 | 1 | 16.2 | 17.0 | Extra-amniotic instillation of 4 mL of maternal blood | Spontaneous birth at 37.0 weeks | – |
| O’Brien et al (2002)57 | 1 | 17.0 | 19 | Transabdominal instillation of gelatin sponge at 19 and 21 weeks, McDonald cerclage at 21 weeks | Delivery at 36.0 weeks | Unilateral club foot |
| Lewi et al (2004)47 | 2 | 17.2 and 22 | 18.5 and 23 | Intra-amniotic instillation of maternal platelets and cryoprecipitate | Uneventful neonatal outcome in the survivors | Chorioamnionitis |
TOP, Termination of pregnancy.
Use of tissue sealants in vivo in human fetal membranes: Spontaneous preterm PROM
In 3 women with spontaneous preterm PROM between 16 and 26 weeks of gestation without clinical evidence of infection, fetoscopy was performed to visualize the rupture site.40 In all 3 cases, the rupture was over the internal os. A longer lapse between preterm PROM and endoscopy was associated with a larger, less-defined, torn, or rolled membrane defect, which suggests a tissue response to the rupture. Multiple authors over the last 20 years have presented data on the use of fibrin sealants in small groups of women with preterm PROM.31,48–57 As summarized in Table III, these case series used different methods, enrolled patients at varying gestational ages, had different criteria for successful outcomes, and are therefore difficult to compare or to pool. In 1 of the largest series, the authors attempted fibrin adhesion in 26 women between 18 and 36 weeks of gestation with preterm PROM.48 McDonald cerclage was placed to prevent amniotic fluid leakage. The fibrinogen solution (Tissucol, Baxter Co) was clotted with 500 IU/ mL thrombin that was injected through the cervical os in a duplo-jet double syringe. If the patient was not completely dry after 24 hours, then another sealing was performed. This was repeated every 24 hours for up to 6 attempts. The pregnancies were prolonged 3 to 172 days after the application of the sealant without any documented intrauterine infection. However, the perinatal mortality rate was still high (70%). Unfortunately, this study lacked a control group, so the efficacy of the technique cannot be determined. Recently, a case report described a new potential therapy for preterm PROM. An “amniograft” was placed over an endoscopically visualized fetal membrane defect.55 The collagen graft was fixed to the membranes with fibrin glue, after laser welding proved unsuccessful. After the procedure, only a slight expansion of the amniotic cavity was demonstrable ultrasonographically, and the patient reported leakage of fluid from 14 days after surgery until delivery at 30 weeks estimated gestational age. In this case, a minilaparotomy was required to tack the fetal membranes to the anterior uterine wall, which caused substantial maternal morbidity that was associated with the procedure. This type of attempt to seal the fetal membranes should not be repeated at this time, given the lack of understanding of the pathologic condition that caused preterm PROM and of the repair mechanisms that are intrinsic to the tissue.
Table III.
Reports on strategies for fetal membrane sealing after spontaneous preterm PROM
| Study | N | Gestational age at preterm PROM (wk) | Gestational age at treatment (wk) | Intervention | Outcome | Complication |
|---|---|---|---|---|---|---|
| Genz et al (1979)32 | 24 | 17–32 | 17–32 | Cervical application of factor XIII and thrombin + aprotinin | 50% survival | – |
| Anger (1988)50 | 4 | 26–29 | 26–34 | Intracervical instillation of fibrin glue | 100% survival | Chorioamnionitis at 28 weeks (1 case) |
| Passloer (1989)54 | 2 | 24.5 and 29 | 26 and 29 | Intracervical applications of fibrin glue with or without cerclage | Delivery at 29 and 39 weeks | Respiratory distress syndrome and severe prematurity (1 case) |
| Baumgarten and Moser (1986)49 | 26 | 16–36 | 16–36 | Intracervical instillation of fibrin glue and cerclage | 30% survival | – |
| Delzanno and Gaudiano (1994)55 | 1 | 21 | 21.5 | Intracervical application of fibrin glue | Delivery at term | – |
| Uchide et al (1994)51 | 1 | 24 | 24 | Intracervical instillation of fibrin glue and cerclage | Delivery at 31 weeks | – |
| Sciscione et al (2001)52 | 12 | 19.4 (range, 13–23) | 20.5 (range, 17–23) | Intracervical application of fibrin glue | 7/13 survivors | Chorioamnionitis (1 case), mild pulmonary hypoplasia (1 case) |
| O’Brien et al (2002)57 | 14 | 17.9 (range, 13–21) | 20.1 | Intra-amniotic (12 cases) or transcervical (2 cases) instillation of gelatin sponge and amnioinfusion | 6/14 survivors | Intrauterine fetal death (2 cases), club foot (3 cases), torticollis and hip dysplasia (2 cases); maternal pulmonary edema (2 cases) |
| Quintero (2001)43 | 12 | 16–24 | 16–24 | Intra-amniotic instillation of maternal platelets and cryoprecipitate | Failure to stop amniotic fluid leakage in all cases | – |
| Quintero et al (2002)56 | 1 | 16.4 | 17.0 | Endoscopically placed collagen graft over fetal membrane defect | Relapse of fluid leakage after 2 weeks, delivery at 30.5 weeks | Bilateral hip dysplasia |
| Young et al (2004)58 | 4 | 15–23 | 16–24 | Fetoscopic application of maternal platelets and fibrinogen/thrombin | Immediate PROM (2 cases); intrauterine fetal death (1 case); viable infant (1 case) | Severe respiratory distress syndrome in the only survivor |
Although these case reports and published series are exciting as potential new therapies to treat iatrogenic and spontaneous preterm PROM, at present there is no evidence that these treatments are truly safe or efficacious. In general, the number of patients who are enrolled in these studies are inadequate to determine the safety of the procedures, and efficacy cannot be determined because of the lack of control groups.
Comment
Further research should attempt to separate the fundamental defects that cause preterm PROM from the intrinsic repair mechanisms. This dissection will allow greater insights into the causes of preterm PROM, while helping us to optimize potential interventions to repair, seal, or heal the membrane defect in vivo. Strategies to heal defects in the fetal membranes after iatrogenic or spontaneous preterm PROM may enable us to improve the poor outcomes that result from this condition. Multiple fetal membrane sealants have been advocated over the last 30 years to treat or seal fetal membrane defects; however, at present, neither the safety nor the efficacy of these treatments has been determined. Future therapies should be first studied in depth in vitro with cell lines and explants and in animal models before implementation of these therapies in humans. Once treatment regimens have been optimized and safety issues have been addressed, efficacy should be evaluated in well-designed, controlled trials. Meanwhile, prevention of iatrogenic preterm PROM and its complications is currently best performed at the time of amniocentesis and operative fetoscopy by reducing the number and size of the created defects in the fetal membranes.
Footnotes
This review discusses human fetal membrane healing by focusing on cause, clinical consequences, and potential therapies to prevent and treat preterm premature rupture of membranes.
Supported by the Biomed 2 Programme of the European Commission (EUROFOETUS, BMH4-CT-97-2383), co-financed by the Vlaamse Gemeenschap (COF 98/012) and Fonds voor Wetenschappellijk Onderzoek Vlaanderen (G.0153.00; R.D., J.A.D.) and by the National Institutes of Health grant No. HD 24314 (G.B-G) and grants to the University of Hawaii and Kapiolani Medical Center under the Research Centers in Minority Institutions Program of the National Center for Research Resources (No. RR-03061 and RR-11091; L.K.M., G.G-B.).
References
- 1.Bryant-Greenwood GD. The extracellular matrix of the human fetal membranes: structure and function. Placenta. 1998;19:1–11. doi: 10.1016/s0143-4004(98)90092-3. [DOI] [PubMed] [Google Scholar]
- 2.Mercer BM. Preterm premature rupture of the membranes. Obstet Gynecol. 2003;101:178–93. doi: 10.1016/s0029-7844(02)02366-9. [DOI] [PubMed] [Google Scholar]
- 3.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 4.Lavery JP, Miller CE, Knight RD. The effect of labor on the rheologic response of chorioamniotic membranes. Obstet Gynecol. 1982;60:87–92. [PubMed] [Google Scholar]
- 5.McLaren J, Malak TM, Bell SC. Structural characteristics of term human fetal membranes prior to labour: identification of an area of altered morphology overlying the cervix. Hum Reprod. 1999;14:237–41. doi: 10.1093/humrep/14.1.237. [DOI] [PubMed] [Google Scholar]
- 6.Millar LK, Boesche MH, Yamamoto SY, Killeen J, DeBuque L, Chen R, et al. A relaxin-mediated pathway to preterm premature rupture of the fetal membranes that is independent of infection. Am J Obstet Gynecol. 1998;179:126–34. doi: 10.1016/s0002-9378(98)70262-5. [DOI] [PubMed] [Google Scholar]
- 7.Qin X, Garibay-Tupas J, Chua PK, Cachola L, Bryant-Greenwood GD. An autocrine/paracrine role of human decidual relaxin: I, interstitial collagenase (matrix metalloproteinase-1) and tissue plasminogen activator. Biol Reprod. 1997;56:812–20. doi: 10.1095/biolreprod56.4.800. [DOI] [PubMed] [Google Scholar]
- 8.Vadillo-Ortega F, Gonzalez-Avila G, Karchmer S, Cruz NM, Ayala-Ruiz A, Lama MS. Collagen metabolism in premature rupture of membranes. Obstet Gynecol. 1990;75:84–8. [PubMed] [Google Scholar]
- 9.Wideman GL, Baird GH, Bolding OT. Ascorbic acid deficiency and premature rupture of the fetal membranes. Am J Obstet Gynecol. 1984;88:592–5. doi: 10.1016/0002-9378(64)90885-3. [DOI] [PubMed] [Google Scholar]
- 10.Hobel CJ, Dunkel-Schetter C, Roeschs SC. Maternal stress as a signal to the fetus. Prenat Neonat Med. 1998;3:116–20. [Google Scholar]
- 11.Deprest JA, Gratacós E. Obstetrical endoscopy. Curr Opin Obstet Gynecol. 1999;11:195–203. doi: 10.1097/00001703-199904000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Farmer D. Fetal surgery. BMJ. 2003;326:461–2. doi: 10.1136/bmj.326.7387.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrison MR. Surgically correctable fetal disease. Am J Surg. 2000;180:335–42. doi: 10.1016/s0002-9610(00)00490-6. [DOI] [PubMed] [Google Scholar]
- 14.Fortunato SJ, Meron R. Distinct molecular events suggest different pathways for preterm labor and premature rupture of membranes. Am J Obstet Gynecol. 2001;184:1399–405. doi: 10.1067/mob.2001.115122. [DOI] [PubMed] [Google Scholar]
- 15.Tashima LS, Yamamoto SY, Yasuda M, Millar LK, Bryant-Greenwood GD. Decidual relaxins: gene and protein up-regulation in preterm premature rupture of the membranes by complimentary cDNA arrays and quantitative immunocytochemistry. Am J Obstet Gynecol. 2002;187:285–97. doi: 10.1067/mob.2002.125763. [DOI] [PubMed] [Google Scholar]
- 16.Draper D, McGregor J, Hall J, Jones W, Beutz M, Heine RP, et al. Elevated protease activities in human amnion and chorion correlate with preterm premature rupture of membranes. Am J Obstet Gynecol. 1995;173:1506–12. doi: 10.1016/0002-9378(95)90640-1. [DOI] [PubMed] [Google Scholar]
- 17.Singer AJ, Clark RAF. Cutaneous wound healing. N Engl J Med. 1999;341:738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 18.Rocker I, Laurence KM. Defect in fetal membranes after fetoscopy. Lancet. 1978;2:716. doi: 10.1016/s0140-6736(78)90827-9. [DOI] [PubMed] [Google Scholar]
- 19.Borgida AF, Mills AA, Feldman DM, Rodis JF, Egan JF. Outcome of pregnancies complicated by ruptured membranes after genetic amniocentesis. Am J Obstet Gynecol. 2000;183:937–9. doi: 10.1067/mob.2000.108872. [DOI] [PubMed] [Google Scholar]
- 20.Johnson JWC, Egerman RS, Moorhead J. Cases with ruptured membranes that “reseal”. Am J Obstet Gynecol. 1990;163:1024–32. doi: 10.1016/0002-9378(90)91117-u. [DOI] [PubMed] [Google Scholar]
- 21.Behzad F, Dickinson MR, Charlton A, Aplin JD. Brief communication: sliding displacement of amnion and chorion following controlled laser wounding suggests a mechanism for short-term sealing of ruptured membranes. Placenta. 1994;15:775–8. doi: 10.1016/0143-4004(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 22.Quintero RA, Carreno CA, Yelian F, Evans M. Repair kinetics of amnion cells after microsurgical injury. Fetal Diagn Ther. 1996;11:348–56. doi: 10.1159/000264340. [DOI] [PubMed] [Google Scholar]
- 23.Devlieger R, Verbist L, Pijnenborg R, Deprest J. Growth factors in fetal membrane healing: evaluation using confluent cultures of amnion derived epithelial WISH cells [abstract] J Soc Gynecol Investig. 2002;9:219A. [Google Scholar]
- 24.Devlieger R, Gratacós E, Verbist L, Pijnenborg R, Deprest J. Gestational age dependent repair kinetics of human amnion cells in vitro [abstract] J Soc Gynecol Investig. 2001;9:220A. doi: 10.1159/000255956. [DOI] [PubMed] [Google Scholar]
- 25.Bilic G, Ochsenbein-Kolble N, Hall H, Huch R, Zimmerman R. In vitro lesion repair by human amnion epithelial and mesenchymal cells. Am J Obstet Gynecol. 2004;190:87–92. doi: 10.1016/j.ajog.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Devlieger R, Gratacós E, Wu J, Verbist L, Pijnenborg R, Deprest JA. An organ-culture for in vitro evaluation of fetal membrane healing capacity. Eur J Obstet Gynecol Reprod Biol. 2000;92:145–50. doi: 10.1016/s0301-2115(00)00439-5. [DOI] [PubMed] [Google Scholar]
- 27.Sopher D. The response of rat fetal membranes to injury. Ann R Coll Surg Engl. 1972;5:240–9. [PMC free article] [PubMed] [Google Scholar]
- 28.Devlieger R, Deprest JA, Gratacós E, Pijnenborg R, Leask R, Riley SC. Matrix metalloproteinases -2 and -9 and their endogenous tissue inhibitors in fetal membrane repair following fetoscopy in a rabbit model. Mol Hum Reprod. 2000;6:479–85. doi: 10.1093/molehr/6.5.479. [DOI] [PubMed] [Google Scholar]
- 29.Devlieger R, Riley SC, Verbist L, Leask R, Pijnenborg R, Deprest JA. Matrix metalloproteinases-2 and -9 and their endogenous tissue inhibitors in tissue remodeling after sealing of the fetal membranes in a sheep model of endoscopic surgery. J Soc Gynecol Investig. 2002;9:137–45. [PubMed] [Google Scholar]
- 30.Gratacós E, Sanin-Blair J, Lewi L, Toran N, Verbist L, Cabero L, et al. A histological study of fetoscopic membrane defects to document membrane healing. Placenta. 2006;27:452–6. doi: 10.1016/j.placenta.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Redl H, Schlag G. Properties of different tissue sealants with special emphasis of fibrinogen-based preparations. In: Redl H, Schlag G, editors. Fibrin sealant in operative medicine. Berlin: Springer; 1991. p. 27–38.
- 32.Genz HJ, Gerlach H, Metzger H. Behandlung des vorzeitigen Blasensprungs durch fibrinklebung. Med Welt. 1979;42:1557. [PubMed] [Google Scholar]
- 33.Harmanli OH, Wapner RJ, Lontz JF. Efficacy of fibrin glue for in vitro sealing of human chorioamniotic membranes. J Reprod Med. 1998;43:986–90. [PubMed] [Google Scholar]
- 34.Louis-Sylvestre C, Rand J, Gordon R, Salafia C, Berkowitz R. In vitro studies of the interactions between platelets and amniotic membranes: a potential treatment for preterm premature rupture of the membranes. Am J Obstet Gynecol. 1998;178:287–93. doi: 10.1016/s0002-9378(98)80014-8. [DOI] [PubMed] [Google Scholar]
- 35.Reddy UM, Shah SS, Nemiroff RL, Ballas SK, Hyslop T, Chen J, et al. In vitro sealing of punctured fetal membranes: potential treatment for mid trimester premature rupture of membranes. Am J Obstet Gynecol. 2001;185:1090–3. doi: 10.1067/mob.2001.117685. [DOI] [PubMed] [Google Scholar]
- 36.Devlieger R, Gratacós E, Ardon H, Vanstraelen S, Deprest J. Factors influencing the flow rate through a surgical defect in human fetal membranes. Prenat Diagn. 2002;22:201–5. doi: 10.1002/pd.284. [DOI] [PubMed] [Google Scholar]
- 37.Mendoza GA, Acuna E, Allen M, Arroyo J, Quintero RA. In vitro laser welding of amniotic membranes. Lasers Surg Med. 1999;24:315–8. doi: 10.1002/(sici)1096-9101(1999)24:5<315::aid-lsm1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 38.Petratos PB, Baergen RN, Bleustein CB, Felsen D, Poppas DP. Ex vivo evaluation of human fetal membrane closure. Lasers Surg Med. 2002;30:48–52. doi: 10.1002/lsm.1140. [DOI] [PubMed] [Google Scholar]
- 39.Quintero RA, Morales WJ, Kalter CS, Allen M, Mendoza G, Angel J, et al. Transabdominal intra-amniotic endoscopic assessment of previable premature rupture of membranes. Am J Obstet Gynecol. 1998;179:71–6. doi: 10.1016/s0002-9378(98)70252-2. [DOI] [PubMed] [Google Scholar]
- 40.Quintero RA, Mendoza GA, Allen M, Arroyo J, Bornick PW, Morlaes WJ, et al. In vivo laser welding of collagen-based graft material to the amnion in a rabbit model of ruptured membranes. Prenat Neonat Med. 1999;4:453–6. [Google Scholar]
- 41.Gratacó s E, Wu J, Yesildaglar N, Devlieger R, Pijnenborg R, Deprest JA. Successful sealing of fetoscopic access sites with collagen plugs in the rabbit model. Am J Obstet Gynecol. 2000;182:142–6. doi: 10.1016/s0002-9378(00)70503-5. [DOI] [PubMed] [Google Scholar]
- 42.Devlieger R, Ardon H, Verbist L, Gratacós E, Pijnenborg R, Deprest JA. Increased polymorphonuclear infiltration and iatrogenic amniotic band after closure of fetoscopic access sites with a bioactive membrane in the rabbit at mid gestation. Am J Obstet Gynecol. 2003;188:844–8. doi: 10.1067/mob.2003.213. [DOI] [PubMed] [Google Scholar]
- 43.Quintero RA. New horizons in the treatment of preterm premature rupture of membranes. Clin Perinatol. 2001;28:861–75. doi: 10.1016/s0095-5108(03)00083-6. [DOI] [PubMed] [Google Scholar]
- 44.Sener T, Ozalp S, Hassa H, Yalcin OT, Polay S. Maternal blood clot patch therapy: a model for postamniocentesis amniorrhea. Am J Obstet Gynecol. 1997;177:1535–6. doi: 10.1016/s0002-9378(97)70104-2. [DOI] [PubMed] [Google Scholar]
- 45.Young BK, Roque H, Abdelhak YE, Poiolek D, Demopulos R, Lockwood CJ. Minimally invasive endoscopy in the treatment of preterm premature rupture of membranes by application of fibrin sealant. J Perinat Med. 2000;28:326–30. doi: 10.1515/JPM.2000.042. [DOI] [PubMed] [Google Scholar]
- 46.Quintero RA, Morales WJ, Allen M, Bornick P, Arroyo J, LeParc G. Treatment of iatrogenic previable premature rupture of membranes with intra-amniotic injection of platelets and cryoprecipitate (amniopatch): preliminary experience. Am J Obstet Gynecol. 1999;181:744–9. doi: 10.1016/s0002-9378(99)70522-3. [DOI] [PubMed] [Google Scholar]
- 47.Lewi L, Van Schoubroeck D, Van Ranst M, Bries G, Emonds MP, Arabin B, et al. Successful patching of iatrogenic rupture of the fetal membranes. Placenta. 2004;25:352–6. doi: 10.1016/j.placenta.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 48.Young BK, Roman AS, MacKenzie AP, Stephenson CD, Minior V, Rebarber A, et al. The closure of iatrogenic membrane defects after amniocentesis and endoscopic intrauterine procedures. Fetal Diagn Ther. 2004;19:296–300. doi: 10.1159/000076715. [DOI] [PubMed] [Google Scholar]
- 49.Baumgarten K, Moser S. The technique of fibrin adhesion for premature rupture of the membranes during pregnancy. J Perinat Med. 1986;14:43–9. doi: 10.1515/jpme.1986.14.1.43. [DOI] [PubMed] [Google Scholar]
- 50.Anger H. Saldatura con fibrina in caso di rottura precoce delle membrane. La Ricerca Clin Lab. 1988;18:105–8. doi: 10.1007/BF02918871. [DOI] [PubMed] [Google Scholar]
- 51.Uchide K, Terada S, Hamasake H, Suzuki N, Akasofu K. Intra-cervical fibrin instillation as an adjuvant to treatment for second trimester rupture of membranes. Arch Gynecol Obstet. 1994;255:95–8. doi: 10.1007/BF02391804. [DOI] [PubMed] [Google Scholar]
- 52.Sciscione AC, Manley JS, Pollock M, Maas B, Shlossman PA, Mulla W, et al. Intracervical fibrin sealants: a potential treatment for early preterm premature rupture of the membranes. Am J Obstet Gynecol. 2001;184:368–73. doi: 10.1067/mob.2001.111796. [DOI] [PubMed] [Google Scholar]
- 53.O’Brien JM, Barton JR, Milligan DA. An aggressive interventional protocol for early mid trimester premature rupture of the membranes using gelatin sponge for cervical plugging. Am J Obstet Gynecol. 2002;187:1143–6. doi: 10.1067/mob.2002.127124. [DOI] [PubMed] [Google Scholar]
- 54.Passloer HJ. Problems and practical application of fibrin gluing in early premature rupture of the fetal membranes: 4 clinical single cases using various procedures. Geburtshilfe Frauenheilkd. 1989;49:1067–9. doi: 10.1055/s-2008-1036289. [DOI] [PubMed] [Google Scholar]
- 55.Delzanno G, Gaudiano L. Use of fibrin glue in premature rupture of membranes. Minerva Ginecol. 1994;46:495–7. [PubMed] [Google Scholar]
- 56.Quintero RA, Morales WJ, Bornick PW, Allen M, Garabelis N. Surgical treatment of spontaneous rupture of membranes: the amniograft-first experience. Am J Obstet Gynecol. 2002;186:155–7. doi: 10.1067/mob.2002.119185. [DOI] [PubMed] [Google Scholar]
- 57.O’Brien JM, Milligan DA, Barton JR. Gelatin sponge embolization: a method for the management of iatrogenic preterm premature rupture of the membranes. Fetal Diagn Ther. 2002;17:8–10. doi: 10.1159/000047997. [DOI] [PubMed] [Google Scholar]
- 58.Young BK, Mackenzie AP, Roman AS, Stephenson CD, Minior V, Rebarber A, et al. Endoscopic closure of fetal membrane defects: comparing iatrogenic versus spontaneous rupture cases. J Matern Fetal Neonatal Med. 2004;16:235–40. doi: 10.1080/14767050400014774. [DOI] [PubMed] [Google Scholar]


