Abstract
Developing embryos of Brassica napus accumulate both triacylglycerols and proteins as major storage reserves. To evaluate metabolic fluxes during embryo development, we have established conditions for stable isotope labeling of cultured embryos under steady-state conditions. Sucrose supplied via the endosperm is considered to be the main carbon and energy source for seed metabolism. However, in addition to 220 to 270 mm carbohydrates (sucrose, glucose, and fructose), analysis of endosperm liquid revealed up to 70 mm amino acids as well as 6 to 15 mm malic acid. Therefore, a labeling approach with multiple carbon sources is a precondition to quantitatively reflect fluxes of central carbon metabolism in developing embryos. Mid-cotyledon stage B. napus embryos were dissected from plants and cultured for 15 d on a complex liquid medium containing 13C-labeled carbohydrates. The 13C enrichment of fatty acids and amino acids (after hydrolysis of the seed proteins) was determined by gas chromatography/mass spectrometry. Analysis of 13C isotope isomers of labeled fatty acids and plastid-derived amino acids indicated that direct glycolysis provides at least 90% of precursors of plastid acetyl-coenzyme A (CoA). Unlabeled amino acids, when added to the growth medium, did not reduce incorporation of 13C label into plastid-formed fatty acids, but substantially diluted 13C label in seed protein. Approximately 30% of carbon in seed protein was derived from exogenous amino acids and as a consequence, the use of amino acids as a carbon source may have significant influence on the total carbon and energy balance in seed metabolism. 13C label in the terminal acetate units of C20 and C22 fatty acids that derive from cytosolic acetyl-CoA was also significantly diluted by unlabeled amino acids. We conclude that cytosolic acetyl-CoA has a more complex biogenetic origin than plastidic acetyl-CoA. Malic acid in the growth medium did not dilute 13C label incorporation into fatty acids or proteins and can be ruled out as a source of carbon for the major storage components of B. napus embryos.
Plant oils represent the largest renewable resource of highly reduced carbon chains and there is interest in increasing their production by oilseed crops. Brassica napus (canola, oilseed rape) is a major oil crop and a multitude of literature focuses on the biochemistry and physiology of oil accumulation in developing seeds of B. napus (see Singal et al., 1987; Murphy and Cummis, 1989; Kang and Rawsthorne, 1994; Eastmond and Rawsthorne, 1998; King et al., 1998). Although the biochemical pathways leading from Suc to oil storage are largely understood, a number of questions remain regarding, for example, the subcellular organization of reactions, and the origin of acetyl-CoA, reducing power, and ATP for fatty acid synthesis. These questions are particularly difficult to address using standard in vitro or organellar biochemical analyses that often result in loss of key activities. In addition, due to several reasons including dilution of isotopes by internal metabolite pools, many classical radioisotope tracer experiments may have led to major misinterpretations of in vivo metabolic fluxes.
New stable isotope labeling methods have been developed to dissect a number of aspects of in vivo intermediary metabolism (Szyperski, 1998; Eisenreich and Bacher, 2000). Most frequently, 13C-labeled substrates are fed to cells followed by NMR or gas chromatography/mass spectrometry (GC/MS) analysis of the products of this metabolism. Because the carbon atoms incorporated into amino acids and fatty acids can be traced back to the structures of a number of central intermediates, different pathways of intermediary metabolism can be said to differently “imprint” the pattern of 13C recorded in end products of metabolism. In addition, under metabolic and isotopic steady-state conditions, fluxes through the central carbon metabolic network can be quantified. So far, the more evolved techniques of metabolic flux analysis with stable isotope labeling have been applied almost exclusively to microorganisms or plant cell cultures, where physiological and growth conditions can be controlled very exactly. If the steady-state condition is fulfilled, the labeling pattern in amino acids and other products gives important information on carbon fluxes in the central carbon metabolism during growth. In principal, these techniques can be extended toward growth of a plant organ on a general 13C-labeled carbon source under conditions similar to those found in planta. Recently, Glawischnig et al. (2001) reported initial results in this direction by labeling of maize (Zea mays) kernels.
Developing embryos of B. napus take up nutrients from the liquid endosperm, which surrounds them (Fowler and Downey, 1970). After the mid-cotyledon stage, rapid accumulation of storage lipid and proteins occurs (Norton and Harris, 1975; Pomeroy et al., 1991), resulting in mature seeds with 40% to 50% (w/w) oil and 30% (w/w) protein (Murphy and Cummis, 1989). During storage product accumulation, Suc at high concentration is the predominant sugar in the seed and has been considered the main carbon source for the embryo (Norton and Harris, 1975; King et al., 1997). However, in addition to sugars, considerable amounts of amino acids and organic acids have been reported to be present in the endosperm liquid of Phaseolus vulgaris (Smith, 1973) and, as reported in this paper, are also present in the endosperm liquid of B. napus. Therefore, in addition to Suc, the embryo may take up at least a part of carbon in the form of amino acids and/or organic acids. The uptake and influence of amino acids is also suggested by the presence of amino acid transporters in developing embryos of Arabidopsis (Hirner et al., 1998) and by the finding that protein content is correlated with the concentration ratio of amino acids to sugars in the phloem sap between different genotypes of B. napus (Lohaus and Moellers, 2000). In addition, it has been shown for rapeseed plants that with the beginning of seed filling, nitrogen is moved from roots, stem, and leaves into the pods, while the uptake of NO3− into plants comes to an end (Rossato et al., 2001). This suggests that most or all the nitrogen is imported into the developing embryo in the form of amino acids.
During oil and protein accumulation in the developing embryo, both fatty acid and protein biosynthesis have high demands for precursor molecules and cofactors (NADH, NADPH, and ATP). If amino acids are taken up from the liquid endosperm, the demand of precursors and cofactors for protein synthesis will be much lower than if the amino acids must be synthesized de novo from Suc and nitrate. Thus, the carbon economy of the developing embryo is dependent on which sources of carbon, nitrogen, and energy are used. As a consequence, unless all natural carbon sources are considered in the composition of the growth medium for labeling experiments, even very precise measurements of carbon flux ratios in intermediary metabolism would be of reduced significance. Therefore, in this study, we present the analysis of organic constituents of EL and the design of a liquid growth medium that mimics the in planta liquid environment. With this medium, embryos can be grown on different isotopically labeled compounds and accumulate milligram amounts of labeled storage lipids and storage proteins. By supplying uniformly 13C-labeled Glc with either unlabeled amino acids or malate and subsequent measurement of the 13C enrichment in fatty acids and amino acids by GC/MS, we have determined the contribution of the different carbon sources to fatty acid and storage protein biosynthesis. In addition to the measurement of 13C enrichment, the fractional 13C labeling of fatty acids and proteinogenic amino acids can be investigated by GC/MS to provide additional quantitative information on fluxes through alternative pathways of central carbon metabolism in developing B. napus embryos.
RESULTS AND DISCUSSION
Analysis of EL for Sugars, Amino Acids, and Organic Acids
To understand which carbon sources are available in planta to developing B. napus embryos, we analyzed the endosperm liquid after dissection of seeds of greenhouse-grown B. napus plants.
Sugars
As shown in Table I, endosperm liquid of seeds at the beginning of oil accumulation (embryos of 0.1–0.5 mg fresh weight, mid-cotyledon stage, 20 DAF) contains Glc and Fru as the main sugars and at similar concentrations. As development proceeds, the concentration of Glc and Fru decreases severalfold, whereas that of Suc increases about 10-fold (Table I) such that for seeds in the late cotyledon stage (>3 mg fresh weight, late cotyledon, 26 DAF), Suc dominates over Glc and Fru. The change in the ratio of hexoses to Suc is similar to that observed earlier by King et al. (1997) in whole seeds of B. napus as well as by Hill and Rawsthorne (2000) in endosperm liquid. After 26 DAF, the growth of the embryo comes to an end and the embryo takes up most of the volume inside the seed coat. In this stage, the embryos have maximal oil accumulation (Murphy and Cummis, 1989). Similarly, in Vicia faba, a shift from high hexose to Suc ratio to a high Suc to hexose ratio is believed to govern the developmental process from cell division to cell expansion and accumulation of storage compounds (Wobus and Weber, 1999).
Table I.
Concentration of several organic constituents in the endosperm liquid
| Av. FW/embryo | 0.2 mg | 0.4 mg | 1.2 mg | 2.8 mg | 3.6 mg |
|---|---|---|---|---|---|
| mm | |||||
| Glc | 129 ± 9 | 101 | 59 | 40 ± 11 | 25 |
| Suc | 9 ± 8 | 20 | 78 | 76 ± 5 | 88 |
| Sugarsa | 276 | 242 | 274 | 232 | 226 |
| Malate | 6 ± 0.1 | 7 | 10 ± 1 | 15 | 15 ± 2 |
| Gln | 20 ± 1 | – | – | 36 ± 1 | – |
| Asn | – | – | 1 | – | |
| Glu | 5 ± 1 | – | – | 11 ± 1 | – |
| Ala | 5 ± 1 | – | – | 7 ± 1 | – |
| Sum of measured amino acids | 39 | – | – | 70 | – |
Endosperm liquid was collected from seeds at different developmental stages (0.2 to 3.6 mg average fresh wt embryo−1). The stage of maximal oil accumulation is represented by 2.8 and 3.6 mg fresh wt (Murphy and Cummis, 1989). Gln, Glu, and Ala were the predominant amino acids with all other amino acids in concentrations less than 3 mm. sd is given with three replicates.
Sum of Glc, Fru, and Suc. Suc was converted to hexose equivalents. According to thin-layer chromatography (TLC) analysis, the concentration of Fru was assumed to be equal to the Glc concentration.
Amino Acids
TLC of endosperm liquid and staining with ninhydrin revealed that in all stages of embryo development, Gln is the main amino acid constituent of the endosperm liquid. The concentration of l-Gln was determined enzymatically and ranged between 20 (mid-cotyledon stage) and 36 mm at late cotyledon stage (Table I). In addition, the concentrations of 16 other proteinogenic amino acids were determined at mid- and late cotyledon stage and the sum totaled 40 and 70 mm, respectively, with Gln, Glu, and Ala as the dominating components (Table I). Thus, the amino acids in the endosperm liquid represent substantial possible sources of carbon and reduced nitrogen for seed storage product biosynthesis. In contrast to the endosperm liquid, in phloem sap of B. napus, Gln, Glu, Ser, Thr, and Asp have been found as the major amino acids (Lohaus and Moellers, 2000).
Malic Acid
By TLC of endosperm liquid, malic acid was found in all stages of seed development as the principal carboxylic acid. This result was confirmed by GC/MS of a derivatized acidic fraction of endosperm liquid. The concentration of malic acid, as quantified enzymatically, increased during development from 6 to 15 mm (Table I).
Design of the Culture Medium Composition and Labeling Experiments
Based on the above analysis, we developed a culture medium to mimic the composition of the endosperm liquid and to allow stable isotope labeling. In all experiments, the volume of liquid medium per embryo provided approximately 10-fold excess of carbon and nitrogen sources as related to the expected yield of oil and protein. Glc and Suc were provided at 40 and 80 mm, respectively, to mimic the composition during the most active oil and protein synthesis stage (Table I).
General Procedure of Labeling Experiments
Embryos were grown on media under day length and low-light conditions simulating in planta growth. Media contained the following carbon sources: sugars (40 mm Glc and 80 mm Suc [S medium]); sugars and amino acids (SA medium); or sugars, amino acids, and malate (SMA medium). For labeling experiments, 13C-labeled Glc (99% 13C enrichment) was mixed with unlabeled Glc and Suc in the molar ratio of 20:20:80, which is a 10:20:160 molar ratio based on hexose units, and results in a 10% isotopic enrichment in hexose units. In an additional experiment, 13C-labeled Suc was supplied (see below).
After 15 d of growth, the (fractional) 13C enrichment in fatty acids and amino acids (after protein hydrolysis) was analyzed by GC/MS as outlined by the scheme shown in Figure 1.
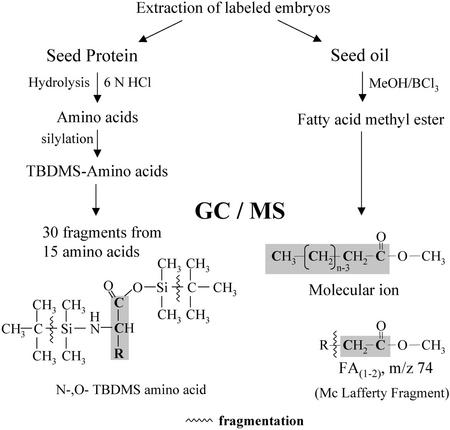
Figure 1.
Overview of the analytical techniques used for the measurement of 13C labeling in amino acids and fatty acids (see also “Materials and Methods”). After extraction of labeled embryos, the seed protein was hydrolyzed and the amino acids derivatized to their N,O-t-butyl-dimethylsilyl (TBDMS) derivatives. By GC/MS, the amino acid molecule is represented by the fragment M-57. For most amino acids, additional fragments were measured that represent parts of the amino acid molecule. The abundances of mass isotope isomers (isotopomers) of a measured fragment (m0, m1, m2… mn) were corrected for isotopomer content in the derivatization reagent and for heteroatoms (1H, 13C, 15N, 17O, 18O, 29Si, and 30Si) as well as for natural 13C in the derivatized molecule fragment. Finally, the relative abundance of mass isotopomers (13C1, 13C2, 13C3… 13Cn) was obtained. After transmethylation of seed oil, fatty acid methyl esters were analyzed by GC/MS. The molecular ion and the McLafferty fragment (m/z 74) were measured and the relative abundance of mass isotopomers was obtained as described for the amino acids.
Formation of Storage Products in Cultured Embryos Reflects Seed Development in Planta
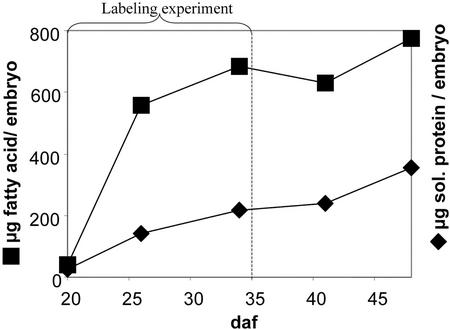
As shown in Figure 2, the main accumulation of fatty acids of B. napus embryos in culture occurs during the first 2 weeks of culture (20–35 DAF). In a labeling experiment, the amount of newly formed labeled fatty acids and protein were more than 10-fold greater than the initial biomass. After 45 d of growth, Suc and Glc were still abundant in the growth medium at similar concentrations as added initially, indicating constant nutrient supply. In some experiments, amino acids and/or malate were included into the media (SA, SM, and SMA media, see “Materials and Methods”). No substantial differences in overall growth of embryos were found between S, SA, SM, and SMA media. In general, the cultured embryos developed similar to in planta embryos. In average, 4 mg fresh weight (2 mg dry weight) and 20% fatty acids/fresh weight was obtained, which is similar to the data given for late cotyledon embryos (26 DAF) grown in planta (Pomeroy et al., 1991). In embryos of the rapeseed cv Reston (a high-erucic acid line), the accumulation of seed oil is characterized by a sharp increase in C20:1 and C22:1 (Pomeroy et al., 1991). The final fatty acid composition of the embryos grown in our medium is similar to literature values for embryos grown in siliques (Table II). The content of soluble protein increased continuously over more than 30 d (Fig. 2), which is also the case for B. napus embryos grown in planta (Eastmond and Rawsthorne, 2000). In summary, after growth of B. napus cv Reston embryos on S medium, gain of fresh weight and protein as well as content and composition of fatty acids were similar to embryos developing in planta. We conclude that the development of embryos under these culture conditions mimics development in siliques of intact plants. The major increase in fatty acid content during the 15-d growth period defines the main phase of storage deposition in seed development, during which we assume a condition of metabolic steady state (see “Materials and Methods”).
Figure 2.
Gain of lipid (micrograms fatty acid per embryo) and soluble protein (micrograms protein per embryo) of B. napus cv Reston embryos cultured in liquid medium. The embryos were taken into culture about 20 DAF, which is mid-cotyledon stage, and were cultivated up to 30 d. The duration of labeling experiments was 15 d, as indicated. Each data point is the average from three embryos.
Table II.
Fatty acid composition in embryos of B. napus cv Reston
| Fatty Acid | 20 DAF | 14 d in Culture | Greenhouse Grown
|
|
|---|---|---|---|---|
| Late cotyledon (26 DAF)a | Very late cotyledon (32 DAF)a | |||
| C16:0 | 10.2 | 6.3 ± 1.3 | 5.6 | 4.0 |
| C18:0 | 9.5 | 1.8 ± 0.2 | 1.9 | 1.2 |
| C18:1 | 42.8 | 15.3 ± 3.3 | 31.8 | 27.0 |
| C18:2 | 20.4 | 18.4 ± 2.1 | 24.0 | 17.8 |
| C18:3 | 8.7 | 14.2 ± 1.4 | 9.3 | 9.1 |
| C20:0 | 2.2 | 1.0 ± 0.2 | 1.0 | 0.7 |
| C20:1 | 3.4 | 9.4 ± 1.0 | 11.9 | 12.2 |
| C22:0 | 0.7 | 1.1 ± 0.2 | 0.2 | 0.3 |
| C22:1 | 2.2 | 32.4 ± 1.2 | 13.0 | 26.4 |
Fatty acid content is given as percent of total fatty acids (w/w, mean ± sd). Early cotyledon embryos (20 d after flowering [DAF]) of B. napus were grown for 14 d in the Suc/amino acid/malate (SMA) medium.
The values are compared with those given by Pomeroy et al. (1991) for greenhouse-grown plants.
Glc Is Metabolized Preferentially over Suc as Carbon Source for Seed Metabolism
When both Glc and Suc were provided with a 20% 13C enrichment (S medium, [U-13C6]Glc, [U-13C12]Suc), after 15 d growth, the 13C enrichment in both fatty acids and amino acids was found to be very close to 20% (data not shown). This result indicates that fatty acids and proteins have been fully labeled to the same isotope abundance as provided by the 13C carbohydrate in the media. This 13C abundance would not have been reached if preexisting or other carbon sources than Glc and Suc were available. This result also indicates that incorporation of atmospheric CO2 does not contribute substantially to fatty acid or protein synthesis despite the obvious ability for photosynthetic CO2 fixation of developing B. napus embryos (King et al., 1998; see below).
In preliminary experiments, when embryos were grown on S medium with uniform 13C-labeled Glc (99% 13C enrichment) and unlabeled Suc in a molar ratio of 20:80 (as related to hexose units), the resulting total 13C enrichment in fatty acids and amino acids was 30% to 35%, which is substantially higher than the expected 20%. Although both Glc and Suc clearly have been used as carbon sources in this labeling experiment, the 13C enrichment of approximately 35% in labeled end products indicates a preferential utilization of Glc over Suc. In addition, as described in “Materials and Methods,” MS analysis of the 13C label pattern in different fatty acids indicated that a small subpopulation of fatty acid molecules was derived mainly from [U-13C6]Glc, whereas the bulk of fatty acid molecules derived from [U-13C6]Glc diluted as described above. This inhomogeneous distribution of labeled carbon in the metabolic products can pose problems for the interpretation of 13C-labeling patterns (Hellerstein and Neese, 1999). As a result of these considerations, further experiments were performed with a carbohydrate mixture in which, in addition to unlabeled Suc, unlabeled Glc was added (10% [U-13C6]Glc, 10% unlabeled Glc, and 80% unlabeled Suc, as related to hexose units). Under these conditions, analysis of the labeled fatty acids (GC/MS, molecular ion clusters) showed that the inhomogeneity of 13C in the fatty acids was greatly reduced such that valid interpretations based on the isotopic steady-state assumption could be made (see “Materials and Methods”).
Glycolytic Breakdown of Glc Dominates in Plastidic Acetyl-CoA Formation
The oxidative pentose phosphate pathway (OPPP) provides an alternative pathway to glycolysis for metabolism of hexose to acetyl-CoA and has been proposed as a source of reductant for fatty acid synthesis in oilseeds (Kang and Rawsthorne, 1996; Eastmond and Rawsthorne, 2000). Earlier studies attempted to assess OPPP activity by measurement of the reduction of label from [1-13C]Glc in metabolic products by Glc 6-phosphate dehydrogenase reaction relative to the label from [6-13C]Glc. However, refixation of the 13CO2 released and/or redistribution of label due to exchange reactions make such experiments difficult to interpret (Flanigan et al., 1993; Roscher et al., 2000).
For a first semiquantitative estimate of carbon flux through the OPPP, we used multiple 13C-labeled Glc ([1,2-13C2]Glc and [U-13C6]Glc). When cells metabolize [13C]Glc containing 13C-13C bonds and in the presence of unlabeled Glc, information on metabolic pathways can be gained from the conservation of 13C-13C bonds (“connectivity”) in metabolites formed from the Glc (Szyperski, 1998). By sole glycolytic breakdown of Fru-6P via triose-P to pyruvate and finally oxidative decarboxylation to acetyl-CoA, the 13C-13C bonds of [U-13C6]Glc or of [1,2-13C2]Glc are 100% retained in pyruvate and in acetyl-CoA. In contrast, during the oxidative pentose phosphate cycle, synthesis of Fru-6P from pentose-P involves the enzyme transfer of two and three carbon units between different intermediate sugar phosphates, which results in the reduction of 13C-13C connectivity, i.e. a characteristic isotopomer pattern in Fru-6P and in its glycolytic products (triose-P, pyruvate, and acetyl-CoA). In addition, the reversibility of transketolase and transaldolase reactions leads to exchange reactions with glycolytic intermediates, producing similar isotopomer patterns.
As shown in Figure 3a, glycolysis of [1,2-13C2]Glc will produce [2,3-13C2]pyruvate. The 13C2 isotopomers will also be retained by the reversible exchange of C1/C2 of Fru-6P by transketolase. However, if Glc enters the OPPP, C-1 of [1,2-13C2]Glc is lost by decarboxylation producing [1-13C]pentose-P. Recycling of Fru-6P from [113C]pentose-P by transketolase and transaldolase reactions will result in different single positional 13C-labeled Fru-6P isotopomers.
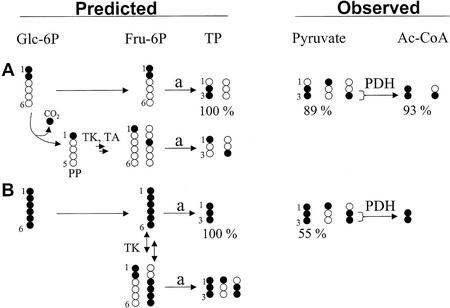
Figure 3.
Predicted and observed isotopomer pattern in triose-P (TP) and derivatives (pyruvate and acetyl-CoA) after feeding of 13C-labeled Glc. Developing embryos were labeled with [1,2-13C2]Glc (A) or [U-13C6]Glc (B) in SMA medium (10% enrichment of labeled Glc). Fragments of Ala and C18:1(1-2) were measured by GC/MS, representing pyruvate and acetyl-CoA, respectively. For the sake of clarity, the figure does not show all expected and found isotopomers. Fru-6P is derived from Glc or by reactions of the OPPP. After glycolytic cleavage of Fru-6P (a), dihydroxyacetone phosphate and glyceraldehyde 3-phosphate are assumed to be in isotopic equilibrium (triose-P [TP]). A, By sole glycolysis, the abundance of 13C2 label from [1,2-13C2]Glc is retained in triose-P, whereas the OPPP reduces the abundance of 13C2-labeled triose-P. Nearly 100% of possible 13C2 abundance was found for pyruvate and acetyl-CoA. B, By sole glycolysis, [U-13C6]Glc is transformed to [U-13C3]pyruvate (100%). By involvement of transketolase (TK) and transaldolase (TA) in synthesis of Fru-6P, the abundance of [U-13C3]pyruvate is reduced and mainly [1-13C]- and [2,3-13C2]pyruvate are formed. Because the contribution of the oxidative part of the OPPP is very low (A), the observed labeling pattern from [U-13C6]Glc can be explained by reversible exchange of C1/C2 of Fru-6P by transketolase. Due to highly reversible reactions of the OPPP, an exact determination of fluxes requires the measurement of fractional labeling in intermediates of the OPPP (see e.g. Roscher et al., 2000). PDH, Pyruvate dehydrogenase complex; PP, pentose-phosphate.
After labeling with [1,2-13C2]Glc, the distribution of 13C in pyruvate was measured for the fragments Ala(1-3), Ala(2-3), and Phe(1-2), representing pyruvate(1-3), pyruvate(2-3), and pyruvate(1-2), respectively. The fractional 13C label of these three pyruvate fragments allows the determination of the abundances of all eight possible 13C isotopomers in pyruvate, as described by Christensen and Nielsen (1999). Our analysis of pyruvate isotopomers revealed that the relative abundance of [2,3-13C2]pyruvate was 89% of the abundance defined by sole glycolysis (Fig. 3A). Similarly, the abundance of 13C2 isotopomer in acetate units of C18:1 [fragment C18:1(1-2)] was 93% of the maximal possible abundance (Fig. 3A). Together, these fatty acid and amino acid analyses indicate that approximately 90% of the [1,2-13C2]Glc molecules were transformed to pyruvate without being subjected to oxidative decarboxylation. Although Ala possibly does not represent the plastidic pyruvate pool, Val is derived from plastidic pyruvate (Singh, 1999). The isotopomer pattern of Val(2-5) was found to be in accordance with the 13C2 abundance found in Ala (data not shown), confirming that approximately 90% of the [1,2-13C2]Glc molecules were transformed to plastidic pyruvate without being subjected to oxidative decarboxylation. In addition, the mass spectrum of His, labeled from [1,2-13C2]Glc, revealed that pentose-P is mainly formed by transketolase, rather than by the oxidative decarboxylation of [1,2-13C2]Glc-6P (data not shown).
Additional conclusions on the extent of reversibility of transketolase were derived after labeling with [U-13C6]Glc. This analysis revealed that the transketolase reaction largely reduced the abundance of the [U-13C3]pyruvate isotopomer to 55% of its abundance defined by sole glycolysis (Fig. 3B). By carbon transfer reactions, mainly [1-13C]pyruvate and [2,3-13C2]pyruvate were produced, which is interpreted as a signature of the transketolase reaction on [U-13C6]Fru-6P (Fig. 3B).
In summary, labeling with both [1,2-13C2]Glc and with [U-13C6]Glc with subsequent analysis of isotopomer abundance in pyruvate and C18:1(1-2) demonstrated that during Glc breakdown, about 90% of 13C-13C connectivity from [1,2-13C2]Glc is retained in pyruvate and acetyl-CoA, indicating low OPPP activity relative to the total glycolytic flux. Furthermore, labeling with [U-13C6]Glc showed that the precursors of pyruvate and acetyl-CoA (Fru-6P and Glc-6P) are subjected to the reactions of the nonoxidative part of the pentose phosphate pathway. Because only the isotopomer pattern of the oxidative part of the OPPP is missing, we consider unlikely the possibility of a “sequestered” OPPP in which [1-13C]pentose-P could be largely produced by oxidative decarboxylation of [1,2-13C2]Glc-6P, but the recycled single 13C-labeled Fru-6P would not enter the glycolytic route leading to fatty acids—and, therefore, would not contribute to the measured isotopomer pattern (Hartwell et al., 1996). This kind of “sequestered” cyclic OPPP is also not supported by the labeling pattern of His, which is derived from plastidic pentose-P. We conclude that the OPPP has a low contribution to Glc breakdown, and is unlikely to provide the major source of NADPH for fatty acid synthesis in B. napus embryos cultured as described. However, more exact measurements of relative fluxes through glycolysis and OPPP will require labeling experiments specifically designed for the sensitive measurement of small fluxes through OPPP. Also, modeling of the central carbon metabolism, including compartmentation effects and the effect of highly reversible reactions of the nonoxidative part of the OPPP, are necessary to further confirm our findings.
Amino Acids Do Not Provide Carbon for Plastidic Fatty Acid Synthesis
From the data given in Table I, it can be calculated that amino acids and malate constitute up to 25% of the carbon in endosperm liquid. To assess the contribution of these compounds to protein and fatty acid synthesis, embryo labeling experiments with the basic growth medium containing [U-13C6]Glc were compared with experiments where unlabeled carbon sources (amino acids and/or malate) were also added. In such experiments, a reduction in 13C label in the end product indicates incorporation of carbon from the unlabeled carbon source. As shown in Figure 4A, the 13C enrichment of fatty acids is not significantly reduced by addition of either unlabeled amino acids or malate as carbon sources. In contrast, as shown in Figure 4B, 13C levels of several amino acids of proteins were strongly reduced by added unlabeled amino acids. Thus, amino acids provided to the embryos are readily incorporated into proteins but do not serve as a carbon source for de novo fatty acid synthesis.
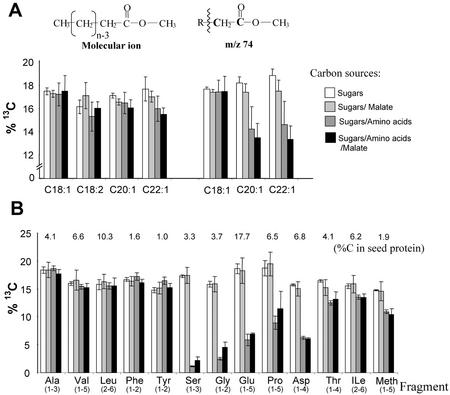
Figure 4.
Incorporation of [U-13C6]Glc into seed oil and storage protein of B. napus embryos and dilution of label by additional unlabeled carbon sources. Amino acids (Glu, Asp, and Ser) and/or malic acid were added to the growth medium. Isotopic enrichment was measured by GC/MS of fatty acid methyl esters and TBDMS amino acids. A, The fatty acids from seed oil show no isotopic dilution, except for the terminal acetate units of C20:1 and C22:1. B, Several amino acids from seed protein show high isotopic dilution by unlabeled amino acids in the medium. The percentage of carbon contributed from each amino acid (or fragment) to seed protein is given. Due to acidic conditions during hydrolysis of seed protein, Asn and Gln are found as Asp and Glu, respectively. Not measured were Lys, His, Arg, Cys, and Trp. For the calculation of the isotopic dilution in total seed protein, the 13C enrichment of these amino acids was derived from other amino acids according to the closest biosynthetic relations. The seed protein was assumed to consist of 60% (w/w) cruciferin, 20% (w/w) napin, and 20% (w/w) oleosin (Norton, 1989). For these proteins, the amino acid compositions were taken from Norton (1989) and Murphy et al. (1991). For five independent experiments, the average 13C label ± se is given.
Carbon from Amino Acids Contributes to the Cytosolic Acetyl-CoA Pool
B. napus cv Reston produces 38% C20 and C22 fatty acids in addition to 62% C16 and C18 fatty acids (Table II). Whereas de novo fatty acid synthesis of C16 and C18 fatty acids occurs in the plastid from acetyl-CoA, elongation of oleic acid outside the plastids is responsible for the addition of the terminal two or four carbons of C20:1 and C22:1, respectively (Ohlrogge et al., 1978; von Wettstein-Knowles, 1982). Therefore, these very long-chain fatty acids offer an opportunity to compare the labeling of plastid versus cytosolic pools of acetyl-CoA. Label in the terminal acetate unit of fatty acids was examined from the abundant McLafferty fragment (m/z 74, see “Materials and Methods”). For oleic acid (Fig. 4A) and palmitic acid (data not shown), no dilution of label in the terminal acetate carbon was observed upon addition of unlabeled amino acids to the growth medium (Fig. 4A). In contrast, 13C labeling of the terminal acetate units of both 20:1 and 22:1 were significantly reduced by added amino acids. The observation that the label of the cytosolic acetyl-CoA pool is significantly diluted by unlabeled amino acids, whereas plastidic acetyl-CoA is not, suggests involvement of different pathways to produce the two pools. Thus, not only is the plastid pool of acetyl-CoA clearly spatially distinct from the cytosolic pool (as previously observed in numerous labeling studies), but these results further demonstrate a different metabolic origin of acetyl-CoA.
Source of Cytosolic Acetyl-CoA
Acetyl-CoA apparently does not cross membranes. Based on a lack of cytosolic pyruvate dehydrogenase, direct synthesis of acetyl-CoA from pyruvate in the cytosolic compartment and the formation of cytosolic acetyl-CoA in plants is still not clearly understood (Ohlrogge and Browse, 1995; Fatland et al., 2000). The results in Figure 4A indicate a reduction of 13C label in cytosolic acetyl-CoA by carbon from unlabeled Asp and Gln. This result also occurred with experiments using only Gln as unlabeled amino acid (data not shown). This dilution of 13C by Asp and Gln points to a possible relation of cytosolic acetyl-CoA to mitochondrial metabolism because both amino acids can be metabolized to tricarboxylic acid (TCA) cycle intermediates. From known metabolic pathways in plants, and from the results in Figure 4A, the following possible route can be proposed: (a) Unlabeled Glu and Asp are transformed into TCA cycle intermediates 2-ketoglutarate, oxaloacetate, succinate, fumarate, and malate. (b) Mitochondrial pyruvate, imported from the cytosol and derived from sugars, would have maximal 13C enrichment as found in plastid-derived fatty acids and amino acids (Val and Leu). A reduction of 13C enrichment is most likely due to the activity of mitochondrial malic enzyme (Douce and Neuburger, 1989) because malate is in part derived from unlabeled Asp and Glu. (c) By pyruvate dehydrogenase complex, mitochondrial acetyl-CoA is formed and transformed to citrate by citrate synthase. After export of citrate to the cytosol, acetyl-CoA is formed by cytosolic ATP:citrate lyase (EC 4.1.3.8; Ratledge et al., 1997; Fatland et al., 2000). In particular, due to the Pro-3S specific cleavage of citrate by ATP:citrate lyase, only carbons of mitochondrial pyruvate are the source of cytosolic acetyl-CoA and no carbons from mitochondrial oxaloacetate end up in acetyl-CoA. In summary, given the influx of unlabeled carbon into the TCA cycle via oxaloacetate and 2-oxoglutarate and the export of mitochondrial acetyl-CoA into the cytosol via citrate, the isotopic dilution in mitochondrial pyruvate and in cytosolic acetyl-CoA can best be explained by the action of mitochondrial malic enzyme.
As an alternative to the export of acetyl-CoA via citrate, free acetate export from mitochondria, after acetyl-CoA hydrolysis (Liedvogel and Stumpf, 1982), cannot be ruled out because direct acetate export would lead to the same conclusions on isotope dilution as made with the export of citrate. However, some in vitro biochemical data suggest a lack of cytosolic acetyl-CoA synthase (Ke et al., 2000).
Is Malate an Intermediate in B. napus Embryo Fatty Acid Biosynthesis?
Unlabeled malic acid did not significantly reduce the incorporation of [U-13C6]Glc into fatty acids or amino acids (Fig. 4B). Even Asp, which can be formed from malate via malate dehydrogenase and Asp aminotransferase, was not significantly reduced in 13C label (Fig. 4B, SM medium). This indicates that externally supplied malic acid is not used as a major carbon source during embryo development, although it was found in considerable concentration in the endosperm liquid (Table I). This, however, does not exclude the existence of malate metabolism into fatty acids inside the embryo from internally generated pools and in this regard, malate was proposed to be a precursor for plastidic acetyl-CoA in developing rapeseed embryos (Singal et al., 1995). Based on in vitro enzyme activity analysis, Singal et al. (1995) proposed that cytosolic phosphoenolpyruvate (PEP) is transformed to oxaloacetate (by PEP carboxylase) and reduced to malate (NAD-malate dehydrogenase), which is imported into the plastid and transformed to pyruvate by malic enzyme. We consider this scenario unlikely because the incorporation of [U-13C6]Glc into plastidic fatty acids was not reduced by unlabeled Asp, although carbon from this amino acid was readily incorporated into seed protein and cytosolic acetyl-CoA, most likely via malate as described above.
Amino Acid Incorporation into Seed Storage Proteins
On a dry weight basis, mature B. napus embryos contain about 50% oil and 30% protein (Murphy and Cummis, 1989). Based on the concentrations of amino acids in the endosperm liquid (Table I) and on studies on nitrogen metabolism in rapeseed plants (Rossato et al., 2001), we reasoned that the embryos could meet their high demand for amino acids at least in part by uptake from the endosperm liquid rather than by de novo synthesis from sugars and nitrate. As a consequence, in vivo there may be a reduced demand for precursors of amino acids (e.g. oxaloacetate and α-ketoglutarate) and cofactors for amino acid biosynthesis as compared with growth where no amino acids are supplied.
When embryos were grown on [U-13C6]Glc with unlabeled Gln, Asn, and Ser, 13C enrichment in several seed protein amino acids was highly reduced as compared with the control experiment without amino acids (Fig. 4B). This reduction in 13C label in particular amino acids indicates incorporation of the unlabeled amino acid or its derivative. Asn and Gln are readily converted to Asp and Glu and can be assumed to deliver amide-bound nitrogen for the synthesis of other amino acids. The incorporation of Gln, Asn, and Ser into different amino acids of the seed protein reflects expected biogenetic relations as follows:
Addition of amino acids to the medium reduced 13C label in Ser of seed protein 90% to 95% (Fig. 4B), indicating that it was nearly completely incorporated from the medium rather than de novo synthesized from carbohydrates. Gly was similarly reduced in 13C label (Fig. 4B), which can be explained by a synthesis from Ser by Ser transhydroxymethylase (Hanson and Roje, 2001).
An additional related conclusion can be made. If photorespiration was highly active in developing B. napus embryos, Ser and Gly would be a part of the carbon flux for recycling of phosphoglycerate from phosphoglycolate, produced by the Rubisco oxygenase reaction. Unlabeled Ser would be expected to reduce 13C label in plastidic phosphoglycerate and in derived intermediates and end products as well as the 13C label of plastidic glycolate should end up in Ser. Because the plastid-derived fatty acids showed no dilution of 13C label and Ser was very low in 13C label, these results indicate that a major flux through photorespiration pathways is unlikely.
Glu and Pro were also strongly reduced in 13C content (Fig. 4B) as expected because both Glu and Pro are derived from Gln. Asp, Thr, Ile, and Met were also reduced in 13C label, whereas Thr, Ile, and Met were less reduced in 13C label than Asp (Fig. 4B). Thr and most of the carbon skeleton of Ile and Met are derived from Asp (via Asp semialdehyde as common precursor). Their biosynthesis is localized in the plastid (Galili, 1995) and the difference in 13C enrichment between Asp and Thr, Ile, and Met (Fig. 4B) most likely reflects the compartmentation of different Asp pools.
Amino acids related to plastidic pyruvate (Val and Leu) or to plastidic PEP [Phe(1-2)] were not reduced in label by the supply of Ser, Gln, and Asn (Fig. 4B). This is in further agreement with the finding that Ser, Gln, and Asn are not incorporated into the plastid-derived fatty acids.
How Much Carbon in Seed Protein Is Derived from Preformed Amino Acids?
To assess the amount of carbon that is contributed from the exogenously supplied unlabeled amino acids to seed protein formation, we calculated the reduction of 13C label (from [U-13C6]Glc) in protein relative to experiments without added amino acids. By considering the percentage of carbon each amino acid contributes to seed protein (see legend of Fig. 4) and the isotopic dilution from the unlabeled amino acids, we calculated that the seed proteins reached approximately 70% of the maximum possible label. Thus, 30% of the carbon of the seed protein is derived from the unlabeled amino acids in the growth medium and 70% from de novo biosynthesis from sugars. This substantial contribution of exogenous amino acids to total carbon in seed protein is in contrast to the seed oil, which did not experience dilution in 13C abundance by carbon from amino acids.
This statement is valid, although it was found that the terminal acetate units of C20 and C22 fatty acids are diminished in 13C by unlabeled amino acids (Fig. 4A). Because these terminal acetate units only contribute about 8% of total carbon in seed oil, the contribution of exogenous amino acids to oil synthesis is minimal.
The fact that exogenous amino acids are used as a nitrogen and carbon source for protein synthesis, but not as a carbon source for the plastidic fatty acid synthesis, clearly emphasizes that although many intermediates of metabolism can be considered on paper to connect amino acid and fatty acid metabolism, in B. napus embryos, compartmentation or other barriers prevent these connections. This result also emphasizes the independence of protein and oil biosynthesis and may in part explain why decreasing the amount of protein or oil in seeds of Arabidopsis mutants does not generally lead to a balancing increase in the other major storage component. For example, in Arabidopsis abi/aba mutants (Finkelstein and Somerville, 1990), storage protein content is strongly reduced but oil content does not change significantly, and in the wri1 mutant (Focks and Benning, 1998), oil is reduced 80% but storage protein content remains similar to wild-type seeds.
SUMMARY AND CONCLUSIONS
To better understand the flow of carbon in developing embryos of B. napus during storage product accumulation, we initiated 13C-labeling experiments with embryos growing in culture. Because developing embryos take up nutrients from a liquid environment, there is the opportunity for steady-state stable isotope labeling experiments to closely mimic in planta seed metabolism and the transport of nutrients from the mother plant to the embryo. By proffering nutrients in similar concentrations to those found in the endosperm liquid, labeling experiments on cultured embryos can reveal, therefore, fluxes through central carbon metabolism very close to the in planta conditions and much more reliable conclusions can be made than from studies using extracts, organelles, or pulses of radioactive precursors. The initial studies using this approach have led to the following conclusions:
In addition to Glc, Fru, and Suc, endosperm liquid of developing seeds of B. napus contains considerable amounts of amino acids (mainly Gln) and malate as organic constituents (Table I). Therefore, a labeling approach with multiple carbon sources is a precondition to quantitatively reflect the fluxes of central carbon metabolism that occur in planta in developing embryos.
We found that in addition to hexoses and Suc, amino acids are used to deliver both carbon and nitrogen to the growing embryo. The amino acids of the growth medium are incorporated primarily into storage proteins and did not provide carbon for plastid fatty acid synthesis.
Amino acids of the growth medium provide an additional carbon source for cytosolic acetyl-CoA used for fatty acid elongation. The involvement of mitochondrial malic enzyme in the formation of cytosolic acetyl-CoA was postulated as an explanation for incorporation of carbon from Asn and Gln into the terminal carbons of C20:1 and C22:1 fatty acids.
In contrast to cytosolic acetyl-CoA, the plastidic fatty acid biosynthesis pathway is clearly independent and fed only by the sugars that are metabolized primarily by the glycolytic pathway and without substantial contribution of the OPPP. This finding underlines the independence of cytosolic amino acid and protein biosynthesis from plastid fatty acid synthesis in B. napus despite the potential exchange of common intermediates between the two pathways.
Although developing B. napus embryos are green and have substantial Rubisco and photosynthetic capacity (King et al., 1998), 13C incorporation from hexose into fatty acids and amino acids showed no evidence of dilution from the fixation of atmospheric CO2. Also, as discussed above, photorespiration is not a substantial part of embryo metabolism.
In addition to the above conclusions, our considerations of carbon economy in developing oil seeds raise some new questions. During the formation of fatty acids, it is notable that one-third of the carbon of precursors is released as CO2 when pyruvate is transformed to acetyl-CoA by the pyruvate dehydrogenase complex. Thus, without refixation, a substantial fraction of the carbon entering oilseeds as carbohydrate would be lost. Based on maximal rates of oil synthesis in developing rapeseed embryos (Bao et al., 1998), CO2 produced would saturate the embryo cells within 10 min. Because the seed coat surrounding the developing embryo is a major barrier for gas diffusion (King et al., 1998), the produced CO2 cannot simply escape as fast as it is produced. As a consequence, it may not only be efficient but also essential for the developing oilseed embryo to conduct refixation or export of CO2. B. napus embryos have green chloroplasts with the capability of photosynthetic carbon fixation (Eastmond et al., 1996; Asokanthan et al., 1997; Eastmond and Rawsthorne, 1998; King et al., 1998). Measured rates of oxygen evolution of developing B. napus embryos (Eastmond et al., 1996; King et al., 1998) as well as Rubisco activity (King et al., 1998) are at least theoretically sufficient to refix the CO2 that is produced by maximal oil synthesis. In addition to Rubisco, high activities of PEP carboxylase and malic enzyme are found in Brassica campestris in the developing seeds (Singal et al., 1987, 1995; King et al., 1998). The latter enzyme activities are essential to the CO2 concentration mechanism in C4 photosynthesis. By analogy, we speculate that PEP carboxylase in the embryo could refix a part of the CO2 into oxaloacetate and malate, which could be exported to the seed coat or the pod wall, where more light is available for photosynthesis. Thus, the CO2 evolved in oil-accumulating embryos could be refixed by PEP carboxylase into oxaloacetate and malate, which could be exported into seed coat or pod wall. In fact, we have found high malate levels in the liquid endosperm.
The use of a complex medium for stable isotope labeling (labeled Glc and unlabeled amino acids) provided additional insights, such as the involvement of amino acids in biosynthesis of cytosolic acetyl-CoA. However, for the formation of acetyl-CoA in plants, a multitude of possible combinations of existing reactions might be proposed. The actual in vivo fluxes may be best found by more detailed “metabolic flux analysis” using in vivo stable isotope labeling approaches. These approaches will involve introduction of 13C-labeled Glc, amino acids, and other precursors labeled in specific positions, followed by detailed isotopomer analysis. Such experiments offer the possibility to further dissect several aspects of central carbon metabolism, including the questions outlined above and the contributions of alternative sources for reducing equivalents [NAD(P) H] for fatty acid biosynthesis in developing seeds.
MATERIALS AND METHODS
Chemicals
d-[U-13C6]Glc, d-[1,2-13C2]Glc, and [U-13C12]Suc (99% 13C-enrichment) were purchased from Isotec (Miamisburg, OH).
Analysis of the Endosperm Liquid
Collection of Endosperm Liquid
Endosperm liquid was collected from seeds, directly after harvest of siliques, with a thin pipette (1–3 μL per seed). After centrifugation (10,000g for 1 min), the supernatant was heated to 100°C for 1 min and centrifuged again at 10,000g for 5 min. This was done to ensure the absence of any enzymic activities that could interfere with metabolite assays. The resulting supernatant was assayed as described below.
Sugars in the Endosperm Liquid
A semiquantitative determination of sugars was performed by TLC of 0.5 to 1 μL of endosperm liquid on cellulose (cellulose MN 300, Machery-Nagel, Dueren, Germany) with t-butanol:ethyl methyl ketone:formic acid:water (40:30:15:15 [v/v]). The sugars and reference standards were visualized by staining with a solution containing 0.2 g of aniline, 0.2 g of diphenylamine, and 1 mL of H3PO4 in 10 mL of ethanol and heating to 80°C. Using 0.2 μL of endosperm liquid, the Glc concentration was determined enzymatically with a hexokinase/Glc-6-phosphate dehydrogenase test (Sigma, St. Louis) as described by Stitt et al. (1989). With prior addition of invertase to an endosperm sample, the concentration of the sum of Glc and Suc could be determined.
Fractionation of Endosperm Liquid into Neutral/Acidic and Basic Fractions
HCl was added to endosperm liquid to a final concentration of 0.1 m. The endosperm liquid was then applied on a short column of DOWEX 50 W kation exchange resin (H+; Sigma, St. Louis). Neutral and acidic substances were eluted with water and amino acids were eluted with 2 n NH4OH.
Malate in Endosperm Liquid
One to 5 μL of endosperm liquid was separated by TLC on cellulose (cellulose MN 300, Machery-Nagel) with 1-butanol:acetic acid:water (80:20:20 [v/v]). Acidic spots were visualized with bromphenol blue. An acidic fraction of endosperm liquid was derivatized with ethyl chloroformate as O-ethoxycarbonyl ethyl esters (Husek, 1991) and separated by GC/MS (see below). The content of malic acid in endosperm liquid was measured with malic enzyme (EC 1.1.1.40, Sigma). To 1 mL of reaction buffer (25 mm HEPES/NaOH, pH 7.5; 10 mm MgCl2; and 2 mm NADP), 2 μL of endosperm liquid and 0.5 units of malic enzyme (Sigma) were added. The increase in A340 was measured for several minutes at room temperature in a spectrophotometer (DU640, Beckman Instruments, Fullerton, CA). One millimolar NADPH formed equals 1 mm malic acid and an increase in A340 of 6.22.
Amino Acids in Endosperm Liquid
Endosperm liquid from different developmental stages was separated by TLC on silica gel with n-butanol:acetic acid:water (80:20:20 [v/v]) and with CHCl3:methanol:NH4 (40:40:20 [v/v]). Amino acids were visualized with ninhydrin (0.2% [w/v] in ethanol) and heating to 100°C. For quantification of amino acids, a solution of uniformly 13C-labeled amino acids was made by hydrolysis of uniformly 13C-labeled algal crude protein extract (Isotec) in 6 n HCl for 24 h at 110°C. After removing HCl, the concentration of labeled amino acids in this standard solution was determined by adding known amounts of unlabeled amino acids, derivatization to the TBDMS derivatives (see below), and analysis of the mass isotopomers of selected fragments by GC/MS (selective ion monitoring). To a small volume (10–30 μL) of endosperm liquid, an aliquot of the uniformly 13C-labeled amino acid standard was added and the amino acids were purified on an anion-exchange column (see above). After derivatization to the TBDMS derivatives (see below) and analysis of the mass isotopomers of selected fragments by GC/MS (selective ion monitoring), the concentrations of 15 amino acids were determined. Gln, Asn, Trp, Cys, and Arg were not recovered in the uniformly labeled protein hydrolysate and therefore could not be determined. Using 5–10 μL of endosperm liquid, Glu and Gln were determined enzymatically using glutaminase and l-Gln dehydrogenase as described by Lund (1986). Assuming the same efficiency of derivatization for Gln and Asn, the concentration of Asn was determined by comparison of the respective peak intensities in the total ion chromatogram. For the analysis by GC/MS, amino acid fractions were derivatized to their TBDMS derivatives (Das Neves and Vasconcelos, 1987).
Growth Medium for Embryos
Inorganic constituents of all growth media were based on the medium used by Monnier (1976) for growth of embryos of Capsella bursa-pastoris. A total osmotic pressure of –14 atm (see below) was confirmed to be optimal for growth of Brassica napus embryos. To define the osmotic pressure of the medium, the partial osmotic pressure of PEG in solutions was calculated according to Mexal et al. (1975). For the calculation of the partial osmotic pressure caused by Suc and Glc, values were taken from Wolf et al. (1972). For all other media components, the osmotic pressure was calculated from the molar concentration (c) by the Van't Hoff equation: P = cRT (R = 8.14 J mol−1 K−1, T = absolute temperature in K). The total osmotic pressure was assumed to be the sum of all partial osmotic pressures. The concentration of K+ is important for embryo growth (Monnier, 1976). If KNO3 was omitted (SA and SMA media), the total concentration of K+ of the S medium was maintained by addition of KCl. The amount of KOH added for titration of pH was also considered.
Constituents of the growth medium (pH 5.7) were: KNO3 (19 mm), NH4NO3 (10 mm), CaCl2 (5.99 mm), MgSO4 (1.5 mm), KCl (4.69 mm), KH2PO4(1.25 mm), PEG 4000 (220 g L−1), Na2EDTA (14.9 mg L−1), FeSO4 7H2O (11.1 mg L−1), H3BO3 (12.4 mg L−1), MnSO4 H2O (33.6 mg L−1), ZnSO4 7H2O (21 mg L−1), KI (1.66 mg L−1), Na2MoO4 2H2O (0.5 mg L−1), CuSO4 5H2O (0.05 mg L−1), CoCl2 6H2O (0.05 mg L−1), inositol (100 mg L−1), nicotinic acid (5 mg L−1), pyridoxine HCl (0.5 mg L−1), thiamine HCl (0.5 mg L−1), folic acid (0.5 mg L−1), and biotin (0.05 mg L−1).
S Medium
The medium contained Suc (60 mm) and d-Glc (40 mm) as carbon sources.
SM Medium
Ten millimolar malic acid was added into S medium.
SA Medium
The medium was derived from the S medium: KNO3 and NH4NO4 were omitted. Gln (10 mm), Asn 5 (mm), and Ser 5 (mm) were included.
SMA Medium
KNO3 and NH4NO4 were omitted. Ten millimolar malic acid was added and Gln (10 mm), Asn 5 (mm), and Ser 5 (mm) were included.
Influence of Osmotic Pressure on Growth
In addition to the nutrient composition, the total osmotic pressure of the growth medium is an important factor influencing embryo development (Johnson et al., 1997). High osmotic pressure must be maintained to prevent precocious germination (Finkelstein and Crouch, 1986; Johnson et al., 1997). Johnson et al. (1997) defined a minimum osmotic pressure of −14 atm, which will inhibit precocious germination in 100% of B. napus embryos and enabled continuous growth in embryonic mode. To maintain such a high osmotic pressure in liquid growth media, polyols (without nutritional effect) such as sorbitol or mannitol have been used in liquid culture of B. napus embryos. However, Ilic-Grubor et al. (1998) observed an overall better development of microspore-induced embryos if sorbitol was replaced by PEG.
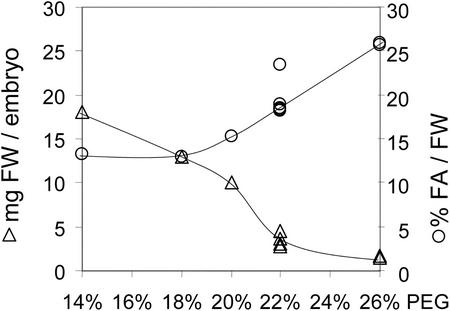
To establish optimum water potential for culture, the effect of PEG as osmoticum on the gain of fresh weight and oil content of zygotic B. napus embryos was tested with constant concentrations of Suc and Glc of 80 and 40 mm, respectively. PEG 4000 was included between 14% and 26% (w/v; Fig. 5). Growth in culture was initiated with embryos in the early cotyledon stage (20 ± 1 DAF, average 0.3 mg fresh weight). Precocious germination was not observed in any of the tested PEG concentrations. As shown in Figure 5, with increasing PEG concentration, the final fresh weight decreased over 10-fold from an average 17-mg embryo−1 (14% [w/v] PEG) to 1.5-mg embryo−1 (26% [w/v] PEG). In addition, the relative oil content increased (Fig. 5), confirming that the osmotic pressure is a major factor that influences growth and development of the embryo. With 22% (w/v) PEG, an osmotic pressure of −14 atm is maintained in the liquid medium and the embryos grew to about 4 mg fresh weight (Fig. 5), which compares well with the 3.7 mg fresh weight for late cotyledon stage (26 DAF) embryos developed in planta (Pomeroy et al., 1991). Therefore, for this study the total osmotic pressure was adjusted to −14 atm by adding PEG 4000 to the medium.
Figure 5.
Dependence of growth and oil content on osmotic pressure of the culture medium. Early cotyledon embryos were cultured for 15 d in liquid medium with different concentration of polyethylene glycol (PEG) 4000.
Growth of B. napus Plants
Seeds of B. napus cv Reston were planted in 30-cm plastic pots in a 2:1 (v/v) mixture of peat moss:vermiculite (Therm-O-Rock Inc., New Eagle, PA) and grown under constant conditions (15°C nights, 20°C days; 16-h day, 600 μE m−2 s−1).
Growth of Isolated Embryos
Embryos 20 DAF were defined by their fresh weight of 0.3 to 0.5 mg. Siliques with developing seeds were treated for 5 min with diluted commercial bleach (about 1% [w/v] NaOCl as active ingredient) and rinsed several times in sterile water. Seeds were removed from the siliques and embryos were dissected aseptically and placed immediately into growth medium.
Each embryo was grown under aseptic conditions in an Erlenmeyer vessel (250 mL) in 6 mL of filter-sterilized growth medium, sealed with a cotton plug. Under these conditions, the embryo was only 1 to 2 mm under the liquid surface to ensure gas exchange. The vessels were not shaken to avoid mechanical stresses. The vessels were kept in a growth chamber under a temperature of 20°C and fluorescent light (50–100 μE m−2 s−1) in a 16-h-light, 8-h-dark cycle. The silique wall absorbs ∼80% of incident light (Eastmond and Rawsthorne, 1996; King et al., 1998). Therefore, this light level in cultures approximates average light intensities reaching embryos in planta. Embryos were harvested after 15 d of culture for analysis as described below.
Analytical Methods
Extraction of Proteins and Lipids
Each embryo was homogenized in a tissue grinder in 2 mL of an ice-cold buffer containing Na-phosphate, pH 7.5 (10 mm), and NaCl (500 mm). Fifty micrograms of triheptadecanoin was added as internal standard for triacylglycerols. The lipids were extracted three times with 1 mL of hexane. After centrifugation (10,000g for 20 min), the hexane phase was evaporated to dryness in a stream of nitrogen. Measurement of protein concentration in the aqueous phase was according to Bradford (1976), with bovine serum albumin as the standard.
Fatty acid methyl esters were prepared by transmethylation of the extracted lipids in a mixture of 0.7 mL of toluene and 1.3 mL of 10% (w/v) BCl3 in methanol for 1 h at 95°C. After subsequent addition of 0.5 mL of water and extraction three times into 1 mL of hexane, fatty acid methyl esters were measured by GC/MS.
Protein Hydrolysis and Amino Acid Derivatives
Soluble proteins were precipitated by addition of 10% volume of 50% (w/v) trichloroacetic acid. After 1 h of incubation on ice and centrifugation (10,000g for 10 min), the protein pellet was washed twice with diethylether:ethanol (1:1 [v/v]). Proteins were dissolved in a small amount of water and then hydrolyzed in 6 n HCl for 24 h at 100°C. HCl was removed under reduced pressure, amino acids were dried under vacuum, and then derivatized to their TBDMS derivatives (Das Neves and Vasconcelos, 1987).
GC/MS Analysis of Amino Acid Derivatives and Fatty Acid Derivatives
All GC/MS analyses were performed using an HP 5890 II gas chromatograph with an HP 5972 mass spectrometer (Hewlett-Packard, Palo Alto, CA). Carrier gas was helium at 1 mL min−1. For fatty acid methyl esters and N,O(S)-ethoxycarbonyl ethyl esters of carboxylic acids, a 30-m × 0.25-mm DB23 column was used (J&W Scientific, Folsom, CA). For TBDMS derivatives of amino acids, a 30-m × 0.25-mm DB1 column was used (J&W Scientific).
The GC conditions were as follows for fatty acid methyl esters: The injector was set to 250°C, and the detector was set to 300°C. The oven temperature programming was: 90°C for 3 min, increased to 160°C at 30°C min−1, increased to 190°C at 10°C min−1, and 190°C for 7 min. Finally, temperature was raised to 240°C at 20 min.
For N,O(S)-ethoxycarbonyl ethyl esters of carboxylic acids, the injector was set to 250°C, and the detector was set to 250°C. The oven temperature programming was: The initial temperature was 110°C for 2 min, and was increased to 255°C at 10°C min−1. The final temperature was 255°C for 20 min.
For TBDMS derivatives of amino acids, the injector was set to 300°C, and the detector was set to 300°C. The oven temperature programming was: 150°C for 14 min, increased to 210°C at 5°C min−1, and then increased to 295°C at 10°C min−1. The final temperature was 295°C for 10 min.
Measurement of Isotopomers in Mass Spectra
Mass isotopomers are molar fractions of m0, m1, m2, etc., according to the number of labeled carbons in the molecule. The sum of all mass isotopomers of the molecules (Σmi) is 100%. Single ion monitoring was generally used for the measurement of mass isotopomers. The mass spectra of the entire chromatographic peak were integrated to avoid the influence of possible isotope fractionation during GC separation. Background correction was performed with MS spectra directly preceding the chromatographic peak. The isotope ratios were reproducible over the concentration range of at least 2 orders of magnitude. A matrix-based method was applied for correction of natural isotope content in heteroatoms and in derivative residues, as well as in the labeled molecule, as described by Lee et al. (1991).
The identity of different fragments of the TBDMS amino acid derivatives was derived from literature (Das Neves and Vasconcelos, 1987; Dauner and Sauer, 2000). Derivatives of amino acids with natural isotope enrichment were separated by GC/MS and the mass isotopomer distribution of different fragments in the MS spectra was measured and monitored for their deviations from the expected mass isotopomer pattern, if calculated from the element composition of the fragment. Fragments that were in good agreement with the calculated mass isotopomer distribution (<1% deviation of individual peaks) were used for measurements with labeled amino acids.
Mass Spectra of Fatty Acids
The molecular ion of fatty acids was measured to determine the average 13C content of fatty acids. A matrix-based method was applied for correction of natural isotope content as described by Lee et al. (1991). The 13C enrichment is the weighted sum of labeled mass isotopomer species (Σ mi i/n; mi = fractional molar abundance of the mass isotopomer containing i 13C-atoms, n = total number of carbons).
McLafferty Ion (m/z 74) in Fatty Acids
The molecular ion of fatty acids represents a polymer of acetate units and information on mass isotopomers in acetate units cannot exactly be derived from this ion. However, in mass spectra of fatty acid methyl ester, the ion m/z 74 is a highly abundant ion, and in saturated fatty acid methyl esters m/z 74 is the base peak. This peak represents a rearrangement reaction of the fatty acid methyl ester (McLafferty rearrangement) involving the transfer of γ-H and the break of the C-2/C-3 bond yields an ion of the elemental composition C3H6O2, comprising C-1, C-2, and the O-methyl group of the fatty acid methyl ester (Fig. 1). The fragment comprises C-1 and C-2 of a fatty acid, i.e. the terminal acetate unit (Murphy, 1993). The identity of the fragment m/z 74 was confirmed using specific deuterium-labeled isomers (Murphy, 1993). Mass 75 has a much higher abundance than expected from natural isotopes of C3H6O2 (m/z 74), due to a second proton transfer to the same fragment resulting in C3H7O2 m/z 75 (Murphy, 1993). For saturated fatty acid methyl esters of different chain lengths, this fragment was used to measure incorporation of stable isotopes into the terminal acetate unit (Schmid et al., 1988; Pollard and Ohlrogge, 1999).
The mass isotopomers m0, m1, and m2 (relative abundance of 12C2, 13C1, and 13C2) of the terminal biosynthetic acetate unit can be derived from the ions C3H6O2+ and C3H7O2+ by measuring the intensity of the masses 74, 75, and 76 of the labeled sample. Correction for natural isotope content as well as for the ion C3H7O2+ (m/z 75) was made using the relative intensities of masses 74 to 76 from an unlabeled reference.
To further confirm the suitability of m/z 74 for measurement of stable isotope label, possible superposition by other ions was investigated for different saturated and mono-unsaturated fatty acid methyl esters. Methylation with CD3OD showed that the total intensity of the peaks m/z 74 and 75 is shifted by three mass units, i.e. contains the O-methyl group of the fatty acid methyl esters. By high-resolution MS of methyl oleate, we confirmed again the identity of the following fragments: C3H6O2 (m/z 74) and C3H7O2 (m/z 75). In unlabeled fatty acid methylesters, the ion m/z 76 has a very low abundance (<1% of m/z 74) and it was determined by high-resolution MS to be C4H6. The error contributed by this fragment will be lower than the accuracy of the MS measurements. In methyl oleate, the fragment m/z 73 has 20% intensity of m/z 74 and its elemental composition was determined as C4H9O. Mass isotopomers of this ion will contribute to the masses 74 and 75. Methylation of oleic acid with CD3OD showed that m/z 73 includes the O-methyl group. By assuming that m/z 73 represents the carbon fragment “C3-C2-C1-O-methyl,” this contribution can be corrected for, if the labeling pattern of acetate units is first approached from the molecular ion of the spectrum and the isotopomer composition of C4H9O (m/z 73) is calculated. In addition to m/z 73, the possible superposition of other relatively abundant ions with m/z 74 was tested. In methyl oleate, m/z 69 and 70 are nearly as abundant as m/z 74. If the fatty acid is highly 13C labeled, isotopomers of m/z 69 and 70 may shift to mass 74. By high-resolution MS of methyl oleate, m/z 69, 70, and 71 were identified as C5H9, C5H11, and C5H11, respectively. By knowing the composition of the fragments, the possible superposition with m/z 74 by 13C label can be calculated. With an average 13C label lower than 10%, the superposition of fragments m/z 69, 70, and 71 will not be significant.
In summary, the abundances of m/z 74, 75, and 76 in mass spectra of labeled fatty acids can be used to accurately determine the 13C labeling of the terminal acetate unit. The masses 74 to 76 must be measured with an unlabeled reference to correct for natural isotopes in C3H6O2+ and for the ion C3H7O2+. Ions that are not derived from the terminal acetate unit and that might interfere with the McLafferty ion m/z 74 are only of significant abundance in unsaturated fatty acids C18:1, C20:1, and C22:1 and their contribution to ion abundance can be corrected for. In the saturated fatty acids, the McLafferty ion m/z 74 is much more dominant and has no significant overlap from m/z 69, 70, 71, and 73. Therefore, to achieve the best accuracy of measurements, labeled unsaturated fatty acid methyl esters were isolated by TLC (AgNO3/silica gel, developed three times with toluene at −20°C), reduced (dissolved in methanol; 2 h under oxygen-free H2/Pt catalyst), and measured again by GC/MS.
Measurement of Corrected Mass Isotopomers in the Ion Cluster m/z 74 to 76
After measurement of the ion cluster m/z 74 to 76, a correction method for the contribution of H transfer and natural isotopes to ion abundance was applied, based on correction matrices described by Lee et al. (1991). For different fatty acids, correction matrices were constructed using the relative abundances of the ion clusters m/z 74 to 76 of reference substance with natural isotope abundance. For each fatty acid, the ratio of intensities of m/z 75 and m/z 74 was very constant over a concentration range of fatty acids of 102. However, it was susceptible for changes in tuning of the mass spectrometer.
Inhomogeneous Distribution of 13C Label in Fatty Acids and the Steady-State Assumption
Labeling experiments with a complex medium (multiple carbon sources) seems to be at first sight an offense against a dogma for metabolic flux analysis. However, because we are interested in the status of metabolic fluxes in vivo, it seems not to make much sense to grow developing embryos on one carbon source because in vivo multiple carbon sources are involved. To properly interpret the fractional abundance of different isotopomers in 13C-labeled products, an assumption of metabolic and isotopic steady state is required (Hellerstein and Neese, 1999). This means that the labeling pattern in the end products can be interpreted in terms of metabolic fluxes only if the metabolic fluxes and the distribution of labeled molecule species in the metabolic network are constant during the labeling experiment. To justify this assumption, the influx of labeled Glc and unlabeled Suc into the metabolic network must be constant. However, during development, the embryo may change its preferences for carbon sources. This is suggested by the fact that during embryo development of B. napus embryos, the Suc to hexose ratio in the endosperm liquid increases (see Table I; King et al., 1997). With the increase of Suc concentration in the endosperm liquid and the onset of storage product accumulation, induction of Suc synthase is reported in seeds of B. napus (King et al., 1997) and induction of Suc synthase mRNA and a Suc transporter mRNA are observed in seeds of Arabidopsis (Ruuska et al., 2002). Thus, the relative ratio of uptake of [U-13C6]Glc and Suc may change during embryo development. In fact, we observed in mass spectra of all labeled fatty acids that a small fraction of the fatty acid molecules was very highly labeled from [U-13C6]Glc, whereas the bulk of fatty acids were formed from labeled Glc and unlabeled Suc, which were incorporated at a ratio close to that provided (see Fig. 6). One explanation for these populations of highly labeled fatty acid molecules is that at the beginning of the labeling experiment, the embryos of mid-cotyledon stage are adapted to hexose as the main carbon source and, thus, the initial fatty acid and amino acid synthesis may mainly consume [U-13C6]Glc. An alternative explanation, which we cannot exclude, is that the different fatty acid labeling patterns arise from fatty acids produced in two different types of cells.
Figure 6.
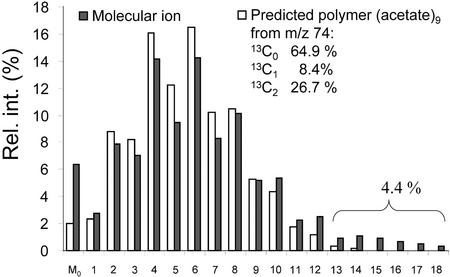
Predicted and measured relative abundance of mass isotopomers of C18:1 methyl ester, after growth on [U-13C6]Glc and unlabeled Suc. From the fragment C18:1(1-2), representing the terminal biosynthetic acetate unit, the distribution of mass isotopomers was predicted. The polymerization of nine acetate units to a C18 fatty acid molecule can be described by a binominal distribution (compare with Lee et al., 1992): (p + (q1 + q2))9. q1, Fraction of (13C1) acetate; q2, fraction of (13C2)-acetate; and p = 1 − q1 − q2, the fraction of (12C2) acetate. The expansion of this binominal distribution leads to the relative abundance of mass isotopomers (m0, m1, m2… m2n) of the fatty acid (Lee et al., 1992). After labeling with [U-13C6]Glc (diluted 20:80 with Suc, as related to hexose units), the fractional labeling in C18:1(1-2) was determined to q1 = 0.084, q2 = 0.267, and p = 0.649. This equals an average 13C content of 31%. The predicted distribution (white bars) and the measured molecular ion of C18:1 (black bars) are similar, indicating that the majority of fatty acid molecules was formed from acetate units labeled as measured in the fragment C18:1(1-2). However, a 4% fraction of fatty acid molecules was much higher labeled. Thus, during fatty acid biosynthesis, a small part of fatty acid molecules has been made mainly from labeled Glc, whereas the bulk of fatty acids was made by a homogeneous mixture of about 30% labeled and 60% unlabeled hexose units.
ACKNOWLEDGMENTS
We are thankful to Drs. Sari Ruuska, Mike Pollard, and Yair Shachar-Hill (all of Michigan State University) for helpful discussions and for critical reading of the manuscript. Mike Pollard stimulated our consideration of CO2 economies in developing oilseeds. We thank Beverly Chamberlin (Michigan State University MS Facility) for measurement of high-resolution mass spectra of methyl-octadecanoate.
Footnotes
This work was supported by the Department of Energy (grant no. DE–FG02–87ER13729), by the National Science Foundation (grant no. MCB 98–17882), and by the Michigan Agricultural Experiment Station.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.004275.
LITERATURE CITED
- Asokanthan PS, Johnson RW, Griffith M, Krol M. The photosynthetic potential of canola embryos. Physiol Plant. 1997;101:353–360. [Google Scholar]
- Bao X, Pollard M, Ohlrogge JB. The biosynthesis of erucic acid in developing embryos of Brassica rapa. Plant Physiol. 1998;118:183–190. doi: 10.1104/pp.118.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Christensen B, Nielsen J. Isotope analysis using GC/MS. Metabolic Eng. 1999;1:282–290. doi: 10.1006/mben.1999.0117. [DOI] [PubMed] [Google Scholar]
- Das Neves HJC, Vasconcelos AMP. Capillary gas chromatography of amino acids, including asparagine and glutamine: sensitive gas chromatographic-mass spectrometric detection of N,O(S)-tert-butylsimethylsilyl derivatives. J Chromatogr. 1987;392:249–258. doi: 10.1016/s0021-9673(01)94270-0. [DOI] [PubMed] [Google Scholar]
- Dauner M, Sauer U. GC-MS analysis of amino acids rapidly provides rich information for isotopomer balancing. Biotechnol Prog. 2000;16:642–649. doi: 10.1021/bp000058h. [DOI] [PubMed] [Google Scholar]
- Douce R, Neuburger M. The uniqueness of plant-mitochondria. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:371–414. [Google Scholar]
- Eastmond P, Kolacna L, Rawsthorne S. Photosynthesis by developing embryos of oilseed rape (Brassica napus L.) J Exp Bot. 1996;47:1763–1769. [Google Scholar]
- Eastmond PJ, Rawsthorne S. Comparison of the metabolic properties of plastids isolated from developing leaves or embryos of Brassica napus L. J Exp Bot. 1998;49:1105–1111. [Google Scholar]
- Eastmond PJ, Rawsthorne S. Coordinate changes in carbon partitioning and plastidial metabolism during the development of oilseed rape embryo. Plant Physiol. 2000;122:767–774. doi: 10.1104/pp.122.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenreich W, Bacher A. Elucidation of biosynthetic pathways by retrodictive/predictive comparison of isotopomer patterns determined by NMR spectroscopy. In: Setlow JK, editor. Genetic Engineering, Principles and Methods. Vol. 22. New York: Kluwer Academic/Plenum Publishing; 2000. pp. 121–153. [DOI] [PubMed] [Google Scholar]
- Fatland B, Anderson M, Nikolau BJ, Wurtele ES. Molecular biology of cytosolic acetyl-CoA generation. Biochem Soc Trans. 2000;28:593–595. [PubMed] [Google Scholar]
- Finkelstein R, Somerville C. 3 classes of abscisic-acid(aba)-insensitive mutations of Arabidopsis define genes that control overlapping subsets of aba responses. Plant Physiol. 1990;94:1172–1179. doi: 10.1104/pp.94.3.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR, Crouch M. Rapeseed embryo development in culture on high osmoticum is similar to that in seeds. Plant Physiol. 1986;81:907–912. doi: 10.1104/pp.81.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanigan I, Collins JG, Arora KK, Macleod JK, Williams JF. Exchange-reactions catalyzed by group-transferring enzymes oppose the quantitation and the unraveling of the identity of the pentose pathway. Eur J Biochem. 1993;213:477–485. doi: 10.1111/j.1432-1033.1993.tb17784.x. [DOI] [PubMed] [Google Scholar]
- Focks N, Benning C. Wrinkled1: a novel, low-seed-oil mutant of Arabidopsis with a deficiency in the seed-specific regulation of carbohydrate metabolism. Plant Physiol. 1998;118:91–101. doi: 10.1104/pp.118.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler DB, Downey RK. Lipid and morphological changes in developing rapeseed, Brassica napus. Can J Plant Sci. 1970;50:233–247. [Google Scholar]
- Galili G. Regulation of lysine and threonine synthesis. Plant Cell. 1995;7:899–906. doi: 10.1105/tpc.7.7.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glawischnig E, Gierl A, Tomas A, Bacher A, Eisenreich W. Retrobiosynthetic nuclear magnetic resonance analysis of amino acid biosynthesis and intermediary metabolism. Metabolic flux in developing maize kernels. Plant Physiol. 2001;125:1178–1186. doi: 10.1104/pp.125.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson AD, Roje S. One-carbon metabolism in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:119–137. doi: 10.1146/annurev.arplant.52.1.119. [DOI] [PubMed] [Google Scholar]
- Hartwell J, Bowsher CG, Emes MJ. Recycling of carbon in the oxidative pentose phosphate pathway in non-photosynthetic plastids. Planta. 1996;200:107–112. [Google Scholar]
- Hellerstein MK, Neese RA. Mass isotopomer distribution analysis at eight years: theoretical, analytic, and experimental considerations. Am J Physiol. 1999;276:E1146–E1170. doi: 10.1152/ajpendo.1999.276.6.E1146. [DOI] [PubMed] [Google Scholar]
- Hill LM, Rawsthorne S. Carbon supply for storage-product synthesis in developing seeds of oilseed rape. Biochem Soc Trans. 2000;28:667–669. [PubMed] [Google Scholar]
- Hirner B, Fischer WN, Rentsch D, Kwart M, Frommer WB. Developmental control of h+/amino acid permease gene expression during seed development of Arabidopsis. Plant J. 1998;14:535–544. doi: 10.1046/j.1365-313x.1998.00151.x. [DOI] [PubMed] [Google Scholar]
- Husek P. Amino acid derivatization and analysis in five minutes. FEBS Lett. 1991;280:354–356. doi: 10.1016/0014-5793(91)80330-6. [DOI] [PubMed] [Google Scholar]
- Ilic-Grubor K, Attree SM, Fowke LC. Comparative morphological study of zygotic and microspore-derived embryos of Brassica napus L. as revealed by scanning electron microscopy. Ann Bot. 1998;82:157–165. [Google Scholar]
- Johnson RW, Asokanthan PS, Griffith M. Water and sucrose regulate canola embryo development. Physiol Plant. 1997;101:361–366. [Google Scholar]
- Kang F, Rawsthorne S. Starch and fatty-acid synthesis in plastids from developing embryos of oilseed rape (Brassica napus L.) Plant J. 1994;6:795–805. [Google Scholar]
- Kang F, Rawsthorne S. Metabolism of glucose-6-phosphate and utilization of multiple metabolites for fatty acid synthesis by plastids from developing oilseed rape embryos. Planta. 1996;199:321–327. [Google Scholar]
- Ke J, Behal RH, Back SL, Nikolau BJ, Wurtele ES, Oliver DJ. The role of pyruvate dehydrogenase and acetyl-coenzyme a synthase in fatty acid synthesis in developing Arabidopsis seeds. Plant Physiol. 2000;123:497–508. doi: 10.1104/pp.123.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SP, Badger MR, Furbank RT. CO2 refixation characteristics of developing canola seeds and silique wall. Aust J Plant Physiol. 1998;25:377–386. [Google Scholar]
- King SP, Lunn JE, Furbank RT. Carbohydrate content and enzyme metabolism in developing canola siliques. Plant Physiol. 1997;114:153–160. doi: 10.1104/pp.114.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WNP, Bergner EA, Guo ZK. Mass isotopomer pattern and precursor product relationship. Biol Mass Spectrom. 1992;21:114–122. doi: 10.1002/bms.1200210210. [DOI] [PubMed] [Google Scholar]
- Lee WNP, Byerley LO, Bergner EA, Edmond J. Mass isotopomer analysis-theoretical and practical considerations. Biol Mass Spectrom. 1991;20:451–458. doi: 10.1002/bms.1200200804. [DOI] [PubMed] [Google Scholar]
- Liedvogel B, Stumpf PK. Origin of acetate in spinach leaf cell. Plant Physiol. 1982;69:897. doi: 10.1104/pp.69.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohaus G, Moellers C. Phloem transport of amino acids in two Brassica napus L. genotypes and one B. carinata genotype in relation to their seed protein content. Planta. 2000;211:833–840. doi: 10.1007/s004250000349. [DOI] [PubMed] [Google Scholar]
- Lund P. l-glutamine and l-glutamate: UV-method with glutaminase and glutamate dehydrogenase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. Vol. 8. New York: Wiley; 1986. pp. 357–363. [Google Scholar]
- Mexal J, Fisher JT, Osteryoung J, Reid CPP. Oxygen availability in polyethylene glycol solutions and its implications in plant-water relations. Plant Physiol. 1975;55:20–24. doi: 10.1104/pp.55.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier M. Culture in vitro de l'embryon immature de Capsella bursa pastoris (L.) Rev Cytol Biol Veg. 1976;39:1–120. [Google Scholar]
- Murphy CM. Mass Spectrometry of Lipids. In: Snyder F, editor. Handbook of Lipid Research. Vol. 7. New York: Plenum Press; 1993. pp. 71–130. [Google Scholar]
- Murphy DJ, Cummis I. Biosynthesis of seed storage products during embryogenesis in rape seed, Brassica napus. J Plant Physiol. 1989;135:63–69. [Google Scholar]
- Murphy DJ, Keen JN, Osullivan JN, Au DMY, Edwards EW, Jackson PJ, Cummis I, Gibbons T, Shaw CH, Ryan AJ. A class of amphipathic proteins associated with lipid storage bodies in plants and possible similarities with animal serum apolipoproteins. Biochim Biophys Acta. 1991;1088:86–94. doi: 10.1016/0167-4781(91)90156-g. [DOI] [PubMed] [Google Scholar]
- Norton G. Nature and biosynthesis of storage proteins. In: Röbbelen G, Downey RK, Ashri A, editors. Oil Crops of the World. New York: McGraw Hill; 1989. pp. 165–191. [Google Scholar]
- Norton G, Harris JF. Compositional changes in developing rape seed. Planta. 1975;123:163–174. doi: 10.1007/BF00383865. [DOI] [PubMed] [Google Scholar]
- Ohlrogge J, Browse J. Lipid biosynthesis. Plant Cell. 1995;7:957–970. doi: 10.1105/tpc.7.7.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge JB, Pollard MR, Stumpf PK. Studies on biosynthesis of waxes by developing jojoba seed tissue. Lipids. 1978;13:203–210. [Google Scholar]
- Pollard M, Ohlrogge J. Testing models of fatty acid transfer and lipid synthesis in spinach leaf using in vivo oxygen-18 labeling. Plant Physiol. 1999;121:1217–1226. doi: 10.1104/pp.121.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeroy MK, Kramer JKG, Hunt DJ, Keller WA. Fatty acid changes during development of zygotic and microspore-derived embryos of Brassica napus. Physiol Plant. 1991;81:447–454. [Google Scholar]
- Ratledge C, Bowater MDV, Taylor PN. Correlation of ATP/citrate lyase activity with lipid accumulation in developing seeds of Brassica napus L. Lipids. 1997;32:7–12. doi: 10.1007/s11745-997-0002-7. [DOI] [PubMed] [Google Scholar]
- Roscher A, Kruger NJ, Ratcliffe RG. Strategies for metabolic flux analysis in plants using isotope labelling. J Biotechnol. 2000;77:81–102. doi: 10.1016/s0168-1656(99)00209-6. [DOI] [PubMed] [Google Scholar]
- Rossato L, Laine P, Ourry A. Nitrogen storage and remobilization in Brassica napus L. during the growth cycle: nitrogen fluxes within the plant and changes in soluble protein patterns. J Exp Bot. 2001;52:1655–1663. [PubMed] [Google Scholar]
- Ruuska SA, Girke T, Benning C, Ohlrogge JB. Contrapuntal networks of gene expression during Arabidopsis seed filling. Plant Cell. 2002;14:1191–1206. doi: 10.1105/tpc.000877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid PC, Johnson SB, Schmid HHO. Determination of ester carbonyl O-18/O-16 ratios in phospholipids by gas chromatography-mass spectrometry. Chem Phys Lipids. 1988;46:165–170. doi: 10.1016/0009-3084(88)90018-7. [DOI] [PubMed] [Google Scholar]
- Singal HR, Sheoran IS, Singh R. Photosynthetic carbon fixation characteristics of fruiting structures of Brassica campestris L. Plant Physiol. 1987;83:1043–1047. doi: 10.1104/pp.83.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singal HR, Talwar G, Dua A, Singh R. Pod photosynthesis and seed dark CO2 fixation support oil synthesis in developing Brassica seeds. J Biosci. 1995;20:49–58. [Google Scholar]
- Singh BK. Biosynthesis of valine, leucine and isoleucine. In: Singh BK, editor. Plant Amino Acids: Biochemistry and Biotechnology. New York: Marcel Dekker; 1999. pp. 227–247. [Google Scholar]
- Smith JG. Embryo development in Phaseolus vulgaris: II. Analysis of selected inorganic ions, organic acids, amino acids and sugars in the endosperm liquid. Plant Physiol. 1973;51:454–458. doi: 10.1104/pp.51.3.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, Lilley RM, Gerhardt R, Heldt HW. Metabolite levels in specific cells and subcellular compartments of plant leaves. Methods Enzymol. 1989;174:518–552. [Google Scholar]
- Szyperski T. C-13-NMR, MS and metabolic flux balancing in biotechnology research. Q Rev Biophys. 1998;31:41–106. doi: 10.1017/s0033583598003412. [DOI] [PubMed] [Google Scholar]
- von Wettstein-Knowles PM. Elongase and epicuticular wax biosynthesis. Physiol Veg. 1982;20:797–809. [Google Scholar]
- Wobus U, Weber H. Sugars as signal molecules in plant seed development. Biol Chem. 1999;380:937–944. doi: 10.1515/BC.1999.116. [DOI] [PubMed] [Google Scholar]
- Wolf AV, Brown MG, Prentiss PG. Concentrative properties of aqueous solutions. In: Weast RC, editor. Handbook of Chemistry and Physics. Ed 53. Cleveland: CRC Press; 1972. pp. D181–D223. [Google Scholar]