Abstract
Polygalacturonate 4-α-galacturonosyltransferase (pectin synthase) was solubilized from pollen tubes of Petunia axillaris and characterized. To accomplish this, an assay method using fluorogenic pyridylaminated-oligogalacturonic acids (PA-OGAs) as acceptor substrates was developed. When the pollen tube enzyme was solubilized with 0.5% (v/v) Triton X-100 and was incubated with PA-OGA and UDP-galacturonic acid (UDP-GalUA), successive transfer activity of more than 10 GalUAs from UDP-GalUA to the nonreducing end of PA-OGA was observed by diethylaminoethyl high-performance liquid chromatography. This activity was time- and enzyme concentration-dependent. The optimum enzyme activity was observed at pH 7.0 and 30°C. Among the PA-OGAs investigated, those with a degree of polymerization of more than 10 were preferred as substrates. The crude pollen tube enzyme had an apparent Km value of 13 μm for the PA-OGA with a degree of polymerization 11 and 170 μm for UDP-GalUA. The characteristics of the P. axillaris pollen tube enzyme and the usefulness of fluorogenic PA-OGAs for the assay of this enzyme are discussed.
Pectin, one of the major components of plant cell walls, is mainly composed of homogalacturonan (HGA), rhamnogalacturonan I, and rhamnogalacturonan II (Ridley et al., 2001). HGA is a homopolymer of α-1,4-linked galacturonic acid (GalUA) partially methyl esterified at C-6 of GalUA (Mort et al., 1993). Pectic HGA- or polygalacturonic acid (PGA)-synthesizing polygalacturonate 4-α-galacturonosyltransferase (PGA-GalUAT; EC 2.4.1.43) is a key enzyme for pectin biosynthesis. However, it has not been purified nor has its gene been cloned. There are reports on the activity of the suspended membrane-bound enzyme in mung bean (Phaseolus aureus; Villemez et al., 1965), tomato (Lycopersicon esculentum), turnip (Brassica rapa; Lin et al., 1966), sycamore (Acer pseudoplatanus; Bolwell et al., 1985), tobacco (Nicotiana tabacum; Doong et al., 1995), and adzuki bean (Vigna angularis; Takeuchi and Tsumuraya, 2001). Solubilization of the tobacco enzyme with detergents has been attempted; however, when it was solubilized with 40 mm CHAPS, the enzyme did not exhibit successive glycosyltransfer activity (consecutive addition of sugar residues) but transferred only a single GalUA residue (Doong and Mohnen, 1998). The solubilized tobacco enzyme has been shown to add GalUA from UDP-GalUA onto the nonreducing end of oligogalacturonic acid (OGA; Scheller et al., 1999).
To study PGA-GalUAT, we selected the growing pollen tube of Petunia axillaris as the enzyme source. This solanaceous petunia bears relatively large flowers (styles of approximately 5 cm) and can be transformed by an exogenous gene using standard techniques. Its pollen tube grows very rapidly through the transmitting tissue in the style; even under the in vitro system used in this study, its growth rate reached 60 μm h−1. Cell wall synthases appear to be highly expressed in P. axillaris pollen tube tissue. Pectin is localized in an outer layer of the cell wall, and its methylesterification is considered to control the mechanical strength and extensibility necessary for the growth of the tube (Li et al., 1994). In addition to pectin methyltransferase and pectin methylesterase, PGA-GalUAT may be involved in this control.
The assay for PGA-GalUAT reported in the literature is based on measurement of the radioactivity of the product incorporating [14C]GalUA from UDP-[14C]GalUA (Villemez et al., 1965). However, because the degradation of acceptor substrates by glycosidases is not detected, the target enzyme may not be correctly evaluated. Polysaccharide-degrading enzymes are considered to be highly expressed where the corresponding polysaccharide synthase is expressed.
Here, we report on a new assay method for PGA-GalUAT. The reducing ends of OGA were modified with 2-aminopyridine, a superior fluorescent-labeling reagent (Hase et al., 1978). The method was used to identify and characterize PGA-GalUAT prepared from the microsomal membrane fraction of the P. axillaris pollen tube. The enzyme showed successive glycosyltransfer activity, which is assumed to be a characteristic of polysaccharide synthase. The characteristics of the enzyme and the advantages of our new assay method are also reported and discussed.
RESULTS
Pyridylaminated-OGA (PA-OGA) Preparation
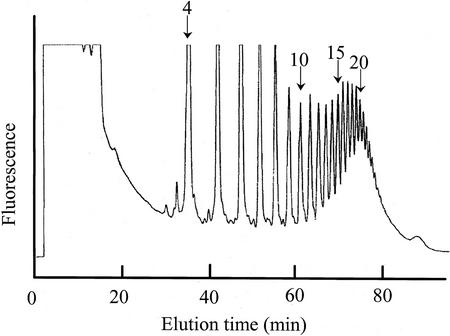
OGAs obtained as partial hydrolyzates of PGA were pyridylaminated. PA-OGAs with a degree of polymerization (DP) ranging from 4 to 27 were separated by DEAE anion-exchange HPLC (Fig. 1). Each fraction was rechromatographed. The purity of each PA-OGA used for the enzyme assay was checked by ensuring that it gave a single peak on DEAE HPLC and reversed-phase HPLC and that the mass of each PA-OGA corresponded with the value calculated using MALDI-TOF MS (data not shown). The relative fluorescence intensities of the PA-OGAs were almost the same except for that of the PA-OGA of DP 1 (Table I), as was also found for pyridylaminated neutral oligosaccharides (Hase, 1993). The peak area ratios for PA-OGAs with a DP > 2 are thus considered as molar ratios.
Figure 1.
DEAE anion-exchange HPLC of PA-OGAs. A mixture of PA-OGAs was analyzed by DEAE HPLC. The peak eluted at 35 min was identified as PA-OGA of DP 4 by matrix-assisted laser-desorption ionization time of flight mass spectrometry (MALDI-TOF MS). Arrows and numbers indicate the PA-OGA elution position and DP of the PA-OGA eluted, respectively.
Table I.
Fluorescence intensity of PA-OGAs
| PA-OGA | Peak Area (n = 2) |
|---|---|
| PA-GalUA | 1.30 ± 0.04 |
| DP 3 | 1.00 |
| DP 7 | 1.00 ± 0.03 |
| DP 10 | 0.98 ± 0.03 |
| DP 12 | 1.06 ± 0.04 |
| DP 14 | 0.94 ± 0.01 |
Peak areas are shown relative to that of the PA-OGA of DP 3 on a molar basis. The amount of each PA-OGA was quantified as described in “Materials and Methods.”
Assay Procedure for PGA-GalUAT Using PA-OGAs
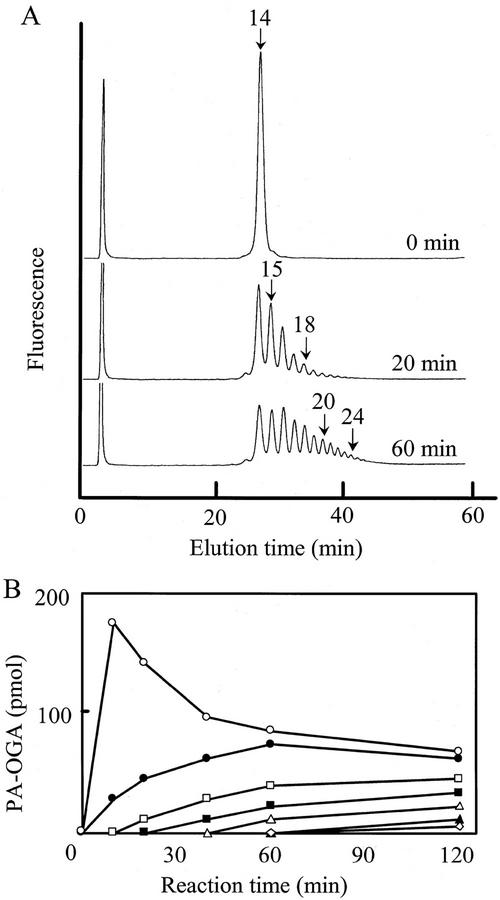
The assay for PGA-GalUAT was performed by incubating PA-OGA and UDP-GalUA with the crude enzyme (solubilized with 0.5% [v/v] Triton X-100). The chromatogram for the assay with 15 μm of the PA-OGA of DP 14 and 1 mm UDP-GalUA is shown in Figure 2A. The elution times of the products corresponded with those of standard PA-OGAs, showing that GalUA was successively transferred to PA-OGA. More than 10 GalUAs were transferred to the PA-OGA of DP 14 in 1 h. PA-OGAs with a DP up to 27 were produced in 2 h (Fig. 2B). PA-OGAs in the DP range of 15 to 17 initially increased but then decreased because they were also used as acceptor substrates. All PA-OGAs of DP > 18 gradually increased during the 2-h period. No glycosyltransfer activity was detected in the soluble fraction of the pollen tube extract (data not shown).
Figure 2.
DEAE HPLC of products obtained with PGA-GalUAT using PA-OGA of DP 14. A, The enzyme reaction was conducted in the presence of 15 μm PA-OGA of DP 14 and 8.7 microunits of the crude enzyme for 0 to 60 min. Arrows and numbers indicate the PA-OGA elution position and DP of the PA-OGA eluted, respectively. B, Amounts of products plotted against incubation time. PA-OGAs of DP 15 (○), 17 (●), 19 (□), 21 (▪), 23 (▵), 25 (▴), and 27 (⋄) are shown.
Characterization of the P. axillaris Pollen Tube Enzyme
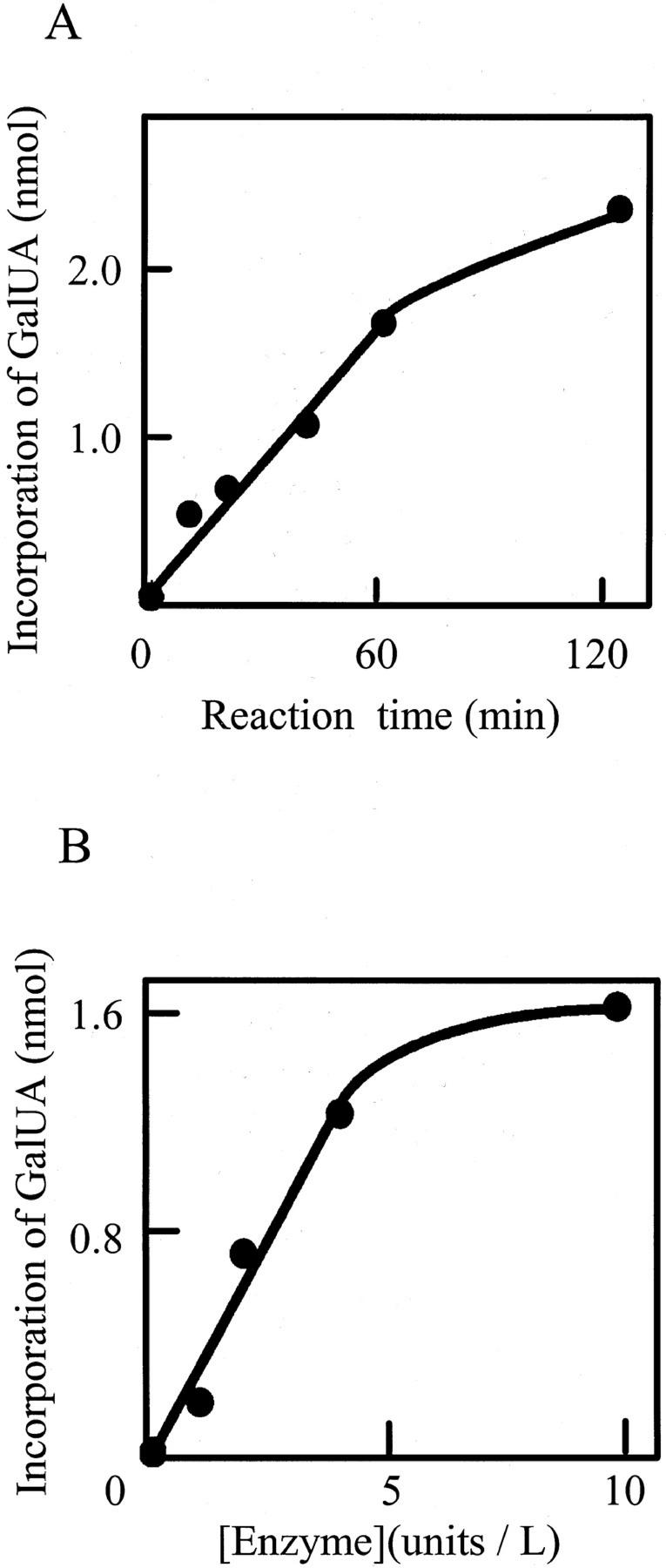
The time course of the reaction (Fig. 3A) shows that incorporation of GalUA increased linearly during the first 60 min. Linearity between the enzyme concentration and GalUA incorporation was observed for 0 to 5 units L−1 of the crude enzyme in a 30-min reaction (Fig. 3B).
Figure 3.
Time- and enzyme concentration-dependence of the enzyme reaction. A, Time course of GalUA incorporation into PA-OGA of DP 14. B, Relationship between enzyme concentration and incorporation of GalUA into PA-OGA. The enzyme reaction was continued for 30 min.
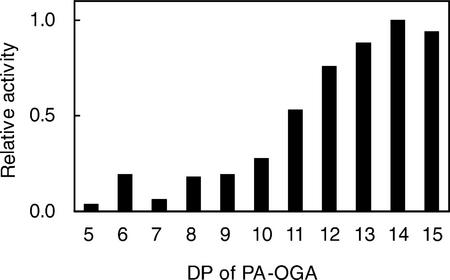
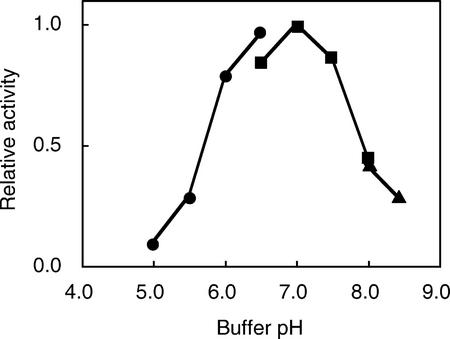
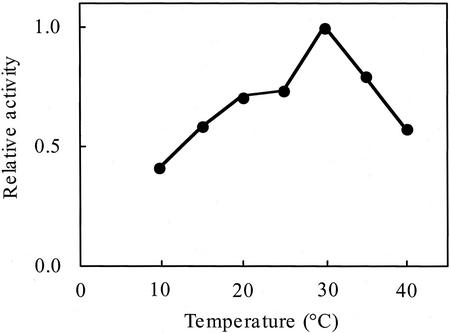
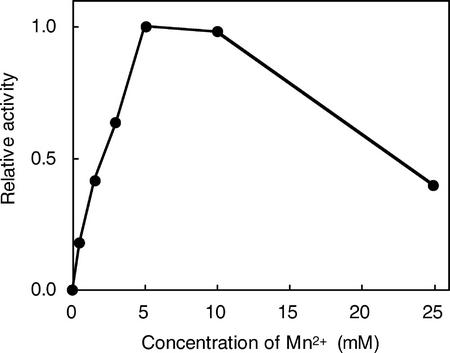
The influence of the PA-OGA size on PGA-GalUAT activity was investigated (Fig. 4). The smaller PA-OGAs (DP 5–10) functioned as acceptors, although the enzyme activity was low, whereas PA-OGAs of DP > 12 worked as better acceptor substrates. The optimum pH for the enzyme activity was around 7 (Fig. 5). The optimum temperature of the enzyme was about 30°C (Fig. 6). The enzyme was active over a wide temperature range and retained about 40% of its maximum activity even at 10°C. PGA-GalUAT had no activity without divalent cations and was most activated by the addition of Mn2+ (Table II). Mg2+ and Ca2+ ions activated 20% of the activity of the Mn2+-treated enzyme. The optimum Mn2+ concentration for the enzyme activity was 5 to 10 mm (Fig. 7). The apparent Km values for the PA-OGAs of DP 7, 11, and 14 were 44, 13, and 11 μm, respectively; for UDP-GalUA, the value was 170 μm. The apparent Vmax values for the PA-OGAs of DP 7, 11, and 14 were 120, 240, and 640 pmol min−1 mg−1 protein; for UDP-GalUA, the value was 480 pmol min−1 mg−1 protein. The enzyme solubilized with other detergents (1% [v/v] digitonin or 40 mm CHAPS) also had an activity of successive transfer of GalUA. The activity for unit weight of protein in a crude enzyme solubilized with these detergents were on a level with 0.5% (v/v) Triton X-100 (data not shown).
Figure 4.
Effects of DP of acceptors on PGA-GalUAT activity. The reaction was conducted with 2.6 microunits of the crude enzyme and various PA-OGAs of the following DP: 5 (10.8 μm), 6 (14.2 μm), 7 (13.2 μm), 8 (12.3 μm), 9 (13.4 μm), 10 (13.2 μm), 11 (11.7 μm), 12 (14.2 μm), 13 (13.4 μm), 14 (12.1 μm), and 15 (14.0 μm). The initial transfer activities are shown relative to that of the PA-OGA of DP 14.
Figure 5.
Effects of pH on PGA-GalUAT activity. The crude enzyme (3.3 microunits) was incubated with 7.9 μm PA-OGA of DP 12. The buffers used were 100 mm MES-NaOH (–●–), HEPES-NaOH (–▪–), and Tris-HCl (–▴–). The enzyme activity at pH 7.0 was taken as 1.0.
Figure 6.
Effect of temperature on PGA-GalUAT activity. The crude enzyme (4.3 microunits) and 6.8 μm PA-OGA of DP 15 were incubated for 30 min. The activities are shown relative to that at 30°C.
Table II.
Effects of cations on PGA-GalUAT activity
| Cation | Relative Activity |
|---|---|
| None | <0.01 |
| Mn2+ | 1.00 |
| Mg2+ | 0.20 |
| Cu2+ | 0.07 |
| Ca2+ | 0.20 |
| Co2+ | 0.14 |
| Zn2+ | 0.06 |
The reaction was performed with 2.6 microunits of the crude enzyme, 28 μm of the PA-OGA of DP 12, and 5 mm of each cation at 28°C for 30 min. The activities relative to that of Mn2+ are shown. The enzyme solution contained 0.13 mm EDTA.
Figure 7.
Effect of Mn2+ concentration on PGA-GalUAT activity. The crude enzyme (1.6 microunits) was incubated with 7.9 μm PA-OGA of DP 12. Each reaction mixture contained 0.13 mm EDTA derived from the extraction buffer. The activities relative to 5 mm Mn2+ are shown.
Galacturonidase Digestion of PGA-GalUAT Products
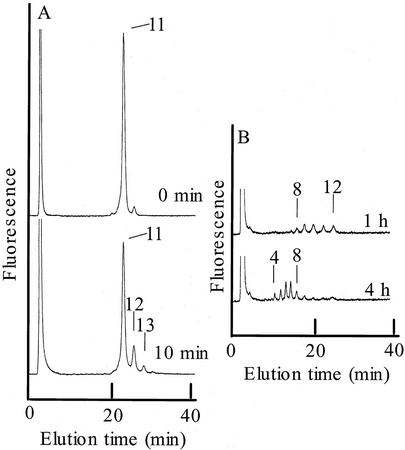
The enzyme products, PA-OGAs of DP 12 and 13 prepared from the PA-OGA of DP 11 with PGA-GalUAT (Fig. 8A), were hydrolyzed with the P. axillaris pollen tube soluble fraction that contained galacturonan α-1,4-galacturonidase (EC 3.2.1.67) activity (Fig. 8B). The digest products were eluted at the same retention times as those for the PA-OGAs of DP 11, 10, 9, 8, 7, 6, 5, and 4, substantiating the evidence that GalUA was transferred onto the nonreducing end of PA-OGA through α-1,4-linkages.
Figure 8.
Galacturonidase digestion of products obtained with PGA-GalUAT. A, PA-OGA of DPs 12 and 13 were prepared from the reaction with 2.3 microunits of the crude enzyme, and 26.3 μm PA-OGA of DP 11. B, The enzymatic products (PA-OGAs of DP 12 and 13) shown in A were collected and treated with the soluble fraction containing galacturonidase activity from P. axillaris pollen tubes for 1 and 4 h at 28°C. Arrows and numbers indicate the PA-OGA elution position and DP of the PA-OGA eluted, respectively.
DISCUSSION
The P. axillaris pollen tube enzyme of the microsomal fraction solubilized with a buffer containing 0.5% (v/v) Triton X-100 has similar enzymatic characteristics to those of the solubilized enzyme obtained from a tobacco-cell suspension culture (Doong and Mohnen, 1998) with respect to optimum pH, requirement of Mn2+, and the Km value for a donor substrate. The P. axillaris enzyme, thus, appears to be in the same family as the solubilized tobacco enzyme. As novel information on this enzyme family, the Km values for the acceptor substrates (PA-OGAs) were determined and were found to be on the order of 10−5 m. The enzyme exhibited successive GalUA transfer activity using the assay developed in this study (Fig. 2). This is the first report, to our knowledge, of successive glycosyltransfer activity of solubilized PGA-GalUAT, although similar successive glycosyltransfer of other polysaccharide synthases has been observed (DeAngelis, 1999; Kuroyama and Tsumuraya, 2001). Our finding of successive transfer is inconsistent with previous reports that the CHAPS-solubilized tobacco cell enzyme transferred only one GalUA residue to OGA (Doong and Mohnen, 1998; Ridley et al., 2001).
Because galacturonan α-1,4-galacturonidase activity is known to occur in pollen (Pressey, 1991) and pollen tube (Dearnaley and Daggard, 2001), the synthesis and degradation of pectin in the growing pollen tube must constitute a dynamic equilibrium. No degradation products were detected, that is, only synthase activity was observed under the assay conditions used in this study (Fig. 2A). However, when the concentration of UDP-GalUA in the reaction mixture was reduced to less than 100 μm, not only PGA-GalUAT activity but also galacturonidase activity against PA-OGA were observed (data not shown). This means that the microsomal fraction of the P. axillaris pollen tube contains both PGA-GalUAT and galacturonidase activity. The galacturonidase activity in the microsomal fraction has already been reported elsewhere (Takeuchi and Tsumuraya, 2001). The glycosyltransfer reaction observed in this study did not derive from transglycosylation by α-1,4-galacturonidase because no glycosyltransfer was observed in the absence of UDP-GalUA and in the presence of 1 or 5 mm GalUAα1–4GalUA or GalUAα1–4GalUAα1–4GalUA (data not shown).
In nature, HGA has some modifications, including methylesterification at C-6 of the GalUA residue, but it has been unclear whether this modification is needed for PGA synthesis. The fact that PGA-GalUAT successively transferred GalUA to PA-OGAs, which do not have any such modification, indicates that this enzyme does not necessarily require this particular HGA modification for its activity.
A model for the synthesis of a flip-flop polysaccharide has been proposed based on the idea that every GalUA appears to flip approximately 180° in HGA, giving rise to the notion that PGA is constructed of disaccharide repeating units (Albersheim et al., 1997). The formation of a dimer of GalUA before polymerization in this model conflicts with the successive transfer of every GalUA observed in the present study (Fig. 2). The solubilized PGA-GalUAT from the pollen tube of P. axillaris transferred a single GalUA to the nonreducing end of PA-OGA in a non-processive fashion (Fig. 2).
The assay for PGA-GalUAT using fluorogenic acceptor substrates has several advantages over the assay method using a radiolabeled donor substrate. First, the products are easily identified by DEAE HPLC. Second, elongation of oligosaccharides is observed with higher resolution than the method that relies on radiolabeled nucleotide sugars because a fluorescence detector was connected directly with a HPLC. The process of the enzyme reaction can accordingly be followed more quantitatively. Third, activity for both synthesis and degradation of an acceptor substrate can be observed. In several cases, tissues that highly express a polysaccharide synthase also highly express a polysaccharide-degrading enzyme. The activity for substrate hydrolysis must influence the apparent activity for polysaccharide synthase. Fourth, products present in very small amounts can be detected, because PA-OGA offers highly sensitive fluorescence detection. Less than 10 fmol of PA-OGA as the enzyme product was quantified in this study. Fifth, PA-oligosaccharides are chemically stable and do not require a hot laboratory. In addition to these advantages, this method is applicable to assay for other polysaccharide synthases and analysis of the mechanism for elongation of polysaccharides. For example, PA-cello-oligosaccharides can be an acceptor substrate for cellulose synthase. This method is also applicable to assay for the branching enzymes, which transfer the side chain because PA-oligosaccharides can be separated from one another by HPLC with high resolution (Omichi and Hase, 1998).
Our success in detecting the successive glycosyltransfer activity of PGA-GalUAT in the crude pollen tube enzyme of P. axillaris solubilized with detergents and using the assay method developed in this study opens the way to the purification of the enzyme and the cloning of its gene.
MATERIALS AND METHODS
Materials
PGA was purchased from ICN Biomedicals Inc. (Costa Mesa, CA). 2-Aminopyridine supplied by Wako Pure Chemicals (Osaka) was recrystallized from 1-hexane. Dimethylamine-borane complex and a Wakosil-II 5C30 AR column (4.6 × 250 mm) were obtained from Wako, UDP-GalUA from Sigma (St. Louis), and TSK gel DEAE-5PW (7.5 × 75 mm) from Tosoh (Tokyo).
PA-OGA Preparation
PGA (500 mg) dissolved in 50 mL of H2O was titrated to pH 4.2 with NaOH. This solution was autoclaved at 121°C for 40 min (Robertsen, 1986) and then adjusted to pH 2.0 with HCl. The supernatant containing OGA was lyophilized. OGA was pyridylaminated by the method of Hase (1994) with slight modifications. In brief, after dissolving lyophilized OGA (50 mg) in 500 μL of 20 mm ammonium acetate buffer, pH 4.5, 500 μL of a coupling reagent (prepared by mixing 552 mg of 2-aminopyridine and 200 μL of acetic acid) was added, and the resultant solution was heated at 90°C for 60 min. The Schiff base obtained was reduced with 1,750 μL of a reducing reagent (freshly prepared by mixing 200 mg of dimethylamine-borane complex, 50 μL of water, and 80 μL of acetic acid) at 80°C for 35 min. Excess reagents were extracted twice with 2 mL of water-saturated phenol:chloroform (1:1, v/v) and twice with 2 mL of chloroform, after which the aqueous phase was concentrated. Each PA-OGA was separated with TSK gel DEAE-5PW using a linear gradient of ammonium acetate buffer, pH 4.8, from 60 to 800 mm over 60 min at a flow rate of 1.0 mL min−1. Reversed-phase HPLC was performed on a Wakosil-II 5C30 AR column with isocratic elution of 0.1% (v/v) trifluoroacetic acid at a flow rate of 1 mL min−1. The fluorescence intensity of PA-OGAs relative to that of the PA-OGA with a DP of 3 was determined by quantifying the PA-GalUA in the hydrolyzate of each PA-OGA after hydrolysis with 4 m trifluoroacetic acid at 130°C for 4 h. PA-OGAs were detected by fluorescence (excitation wavelength, 310 nm; emission wavelength, 380 nm). For MALDI-TOF MS, a PA-OGA sample was cocrystallized in a matrix of 2,5-dihydroxybenzoic acid and analyzed with a Voyager-DE RP biospectrometry workstation (PerSeptive Biosystems, Framingham, MA), using delayed extraction technology and operated in the reflector mode.
Preparation of Pollen Tubes
Plants of Petunia axillaris (Lam.) Britton, Sterns & Poggenb. subsp. axillaris (Solanaceae) were grown in a greenhouse, and the pollen was separated from the anthers. Dried pollen was used immediately or kept at −70°C. Pollen (2 g) was germinated in a culture medium (8 mg pollen mL−1) containing 0.07% (w/v) Ca(NO3)2·4H2O, 0.02% (w/v) MgSO4·7H2O, 0.01% (w/v) KNO3, 0.01% (w/v) H3BO3, 25 mm MES-KOH (pH 6.0), 5% (w/v) Suc, and 20% (w/v) PEG4000 according to Jahnen et al. (1989). Pollen tubes germinated for 7 h at 25°C were collected and separated from ungerminated pollen using a steel sieve (180-μm mesh).
Preparation of Crude Enzyme from P. axillaris Pollen Tubes
Pollen tubes were ground with a mortar and a pestle under liquid nitrogen and homogenized with 10 mL of a grinding buffer (50 mm HEPES-NaOH, pH 7.3, containing 50% [v/v] glycerol, 25 mm KCl, 0.25 mm MnCl2, and 0.1% [v/v] 2-mercaptoethanol; Doong and Mohnen, 1998) at 4°C for 15 min. The homogenate was centrifuged at 8,500g at 4°C for 20 min. The supernatant was centrifuged at 103,000g at 4°C for 1 h, and the pellet was washed with the grinding buffer and recentrifuged at 103,000g at 4°C for 1 h to yield a membrane pellet. The supernatant was used as a soluble fraction containing α-1,4-galacturonidase. The pellet was solubilized in 300 μL of a solubilization buffer (50 mm HEPES–NaOH, pH 6.8, containing 25% [v/v] glycerol, 25 mm KCl, 0.25 mm MnCl2, 2 mm EDTA, and 0.5% [v/v] Triton X-100) with a hand-held pellet mixer for 15 min in a 1.5-mL microfuge tube polyallomer (Beckman Coulter, Fullerton, CA). The supernatant obtained by centrifugation in a Beckman Coulter ultracentrifuge rotor TLA 100.3 at 103,000g at 4°C for 1 h was used as the crude enzyme.
Assay Procedure for PGA-GalUAT
PGA-GalUAT activity was measured in a reaction mixture (total volume, 30 μL) containing the crude enzyme, 10 μL of a reaction buffer (100 mm HEPES-NaOH, pH 7.3, containing 25 mm KCl, 0.4 m Suc, 0.1% [v/v] bovine serum albumin, and 0.5% [v/v] Triton X-100), 5 mm MnCl2, 1 mm UDP-GalUA, and 1 to 30 μm PA-OGA at 28°C for 30 min unless otherwise specified. The reaction was terminated by heating at 100°C for 4 min. The reaction mixture was centrifuged, and the supernatant was analyzed by DEAE anion-exchange HPLC with a linear gradient of ammonium acetate buffer, pH 4.8 (60 mm for 3 min, to 130 mm in 2 min, to 280 mm in 5 min, and then to 470 mm in 40 min), at a flow rate of 1.0 mL min−1. The products were detected by fluorescence as described above. One unit of enzyme activity was defined as the amount of enzyme that transferred 1 μmol of GalUA from UDP-GalUA to PA-OGA with a DP of 14 (5 μm) per minute under the conditions described above. The apparent Km and Vmax values of PGA-GalUAT as the crude enzyme for the PA-OGAs of DP 7, DP 11, and DP 14 were determined by assay with various concentrations of the PA-OGA of DP 7 (8–80.0 μm), DP 11 (3–59.5 μm), and DP 14 (1.8–22 μm) in the presence of 1 mm UDP-GalUA and 5.1 (for DP 7), 1.3 (for DP 11), and 0.2 (for DP 14) microunits of the crude enzyme, respectively. The apparent Km and Vmax values for UDP-GalUA were determined with various concentrations of UDP-GalUA (10.8–1100 μm) in the presence of 212 μm PA-OGA of DP 11 and 1.3 microunits of the crude enzyme. The reaction mixture was incubated at 28°C for 20 min. The production of the PA-OGA of DP 12 was quantified under conditions in which the production of the PA-OGA of DP 13 was negligible.
Protein Assay
Protein was determined using a BCA protein assay reagent kit (Pierce, Rockford, IL) according to the manufacturer's instructions.
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.005587.
LITERATURE CITED
- Albersheim P, Darvill A, Roberts K, Staehelin LA, Varner JE. Do the structures of cell wall polysaccharides define their mode of synthesis? Plant Physiol. 1997;113:1–3. doi: 10.1104/pp.113.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell GP, Dalessandro G, Northcote DH. Decrease of polygalacturonic acid synthase during xylem differentiation in sycamore. Phytochemistry. 1985;24:699–702. [Google Scholar]
- DeAngelis PL. Molecular directionality of polysaccharide polymerization by the Pasteurella multocida hyaluronan synthase. J Biol Chem. 1999;274:26557–26562. doi: 10.1074/jbc.274.37.26557. [DOI] [PubMed] [Google Scholar]
- Dearnaley JDW, Daggard GA. Expression of polygalacturonase enzyme in germinating pollen of Brassica napus. Sex Plant Reprod. 2001;13:265–271. [Google Scholar]
- Doong RL, Lilijebjelke K, Fralish G, Kumar A, Mohnen D. Cell-free synthesis of pectin: identification and partial characterization of polygalacturonate 4-α-galacturonosyltransferase and its products from membrane preparations of tobacco (Nicotiana tabacum L. cv Samsun) cell suspension cultures. Plant Physiol. 1995;109:141–152. doi: 10.1104/pp.109.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doong RL, Mohnen D. Solubilization and characterization of a galacturonosyltransferase that synthesizes the pectic polysaccharide homogalacturonan. Plant J. 1998;13:363–374. [Google Scholar]
- Hase S. Analysis of sugar chains by pyridylamination. In: Hounsell EF, editor. Glycoprotein Analysis in Biomedicine. Methods in Molecular Biology. Vol. 14. Totowa, NJ: Humana Press; 1993. pp. 69–80. [DOI] [PubMed] [Google Scholar]
- Hase S. High-performance liquid chromatography of pyridylaminated saccharides. Methods Enzymol. 1994;230:225–237. doi: 10.1016/0076-6879(94)30015-1. [DOI] [PubMed] [Google Scholar]
- Hase S, Ikenaka T, Matsusima Y. Structure analyses of oligosaccharides by tagging of the reducing end sugars with a fluorescent compound. Biochem Biophys Res Commun. 1978;85:257–263. doi: 10.1016/s0006-291x(78)80037-0. [DOI] [PubMed] [Google Scholar]
- Jahnen W, Lush WM, Clarke AE. Inhibition of in vitro pollen tube growth by isolated S-glycoproteins of Nicotiana alata. Plant Cell. 1989;1:501–510. doi: 10.1105/tpc.1.5.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroyama H, Tsumuraya Y. A xylosyltransferase that synthesizes β-(1→4)-xylans in wheat (Triticum aestivum L.) seedlings. Planta. 2001;213:231–240. doi: 10.1007/s004250000499. [DOI] [PubMed] [Google Scholar]
- Li YQ, Chen F, Linskens HF, Cresti M. Distribution of unesterified and esterified pectins in cell walls of pollen tubes of flowering plants. Sex Plant Reprod. 1994;7:145–152. [Google Scholar]
- Lin T-Y, Elbein AD, Su J-C. Substrate specificity in pectin synthesis. Biochem Biophys Res Commun. 1966;22:650–657. doi: 10.1016/0006-291x(66)90196-3. [DOI] [PubMed] [Google Scholar]
- Mort AJ, Qiu F, Maness NO. Determination of the pattern of methyl esterification in pectin: distribution of contiguous nonesterified residues. Carbohydr Res. 1993;247:21–35. doi: 10.1016/0008-6215(93)84238-2. [DOI] [PubMed] [Google Scholar]
- Omichi K, Hase S. An assay method for glycogen debranching enzyme using new fluorogenic substrates and its application to detection of the enzyme in mouse brain. J Biochem. 1998;123:932–936. doi: 10.1093/oxfordjournals.jbchem.a022027. [DOI] [PubMed] [Google Scholar]
- Pressey R. Polygalacturonase in tree pollens. Phytochemistry. 1991;30:1753–1755. [Google Scholar]
- Ridley BL, O'Neill MA, Mohnen D. Pectins: structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry. 2001;57:929–967. doi: 10.1016/s0031-9422(01)00113-3. [DOI] [PubMed] [Google Scholar]
- Robertsen B. Elicitors of the production of lignin-like compounds in cucumber hypocotyls. Physiol Mol Plant Pathol. 1986;28:137–148. [Google Scholar]
- Scheller HV, Doong RL, Ridly BL, Mohnen D. Pectin biosynthesis: a solubilized α-1,4-galacturonosyltransferase from tobacco catalyzes the transfer of galacturonic acid from UDP-galacturonic acid onto the non-reducing end of homogalacturonan. Planta. 1999;207:512–517. [Google Scholar]
- Takeuchi Y, Tsumuraya Y. In vitro biosynthesis of homogalacturonan by a membrane-bound galacturonosyltransferase from epicotyls of azuki bean. Biosci Biotechnol Biochem. 2001;65:1519–1527. doi: 10.1271/bbb.65.1519. [DOI] [PubMed] [Google Scholar]
- Villemez CL, Lin T-Y, Hassid WZ. Biosynthesis of the polygalacturonic acid chain of pectin by a particulate enzyme preparation from Phaseolus aureus seedlings. Proc Natl Acad Sci USA. 1965;54:1626–1632. doi: 10.1073/pnas.54.6.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]