Abstract
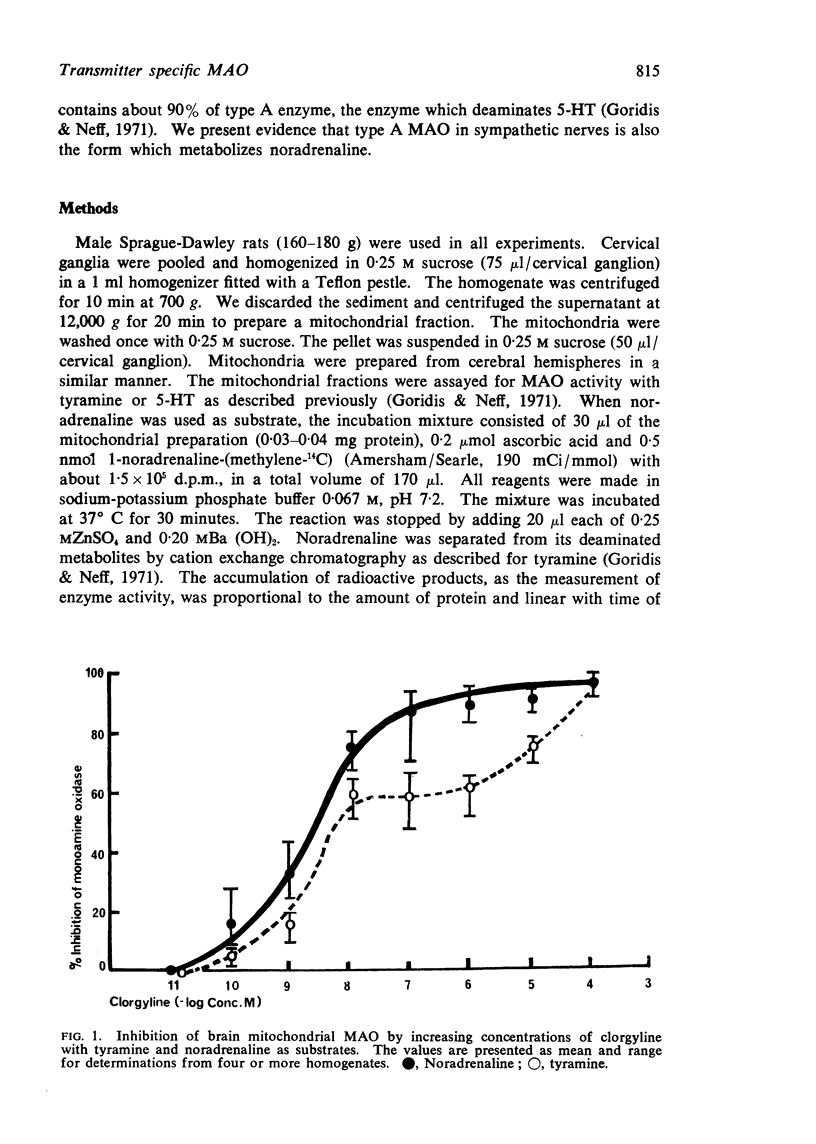
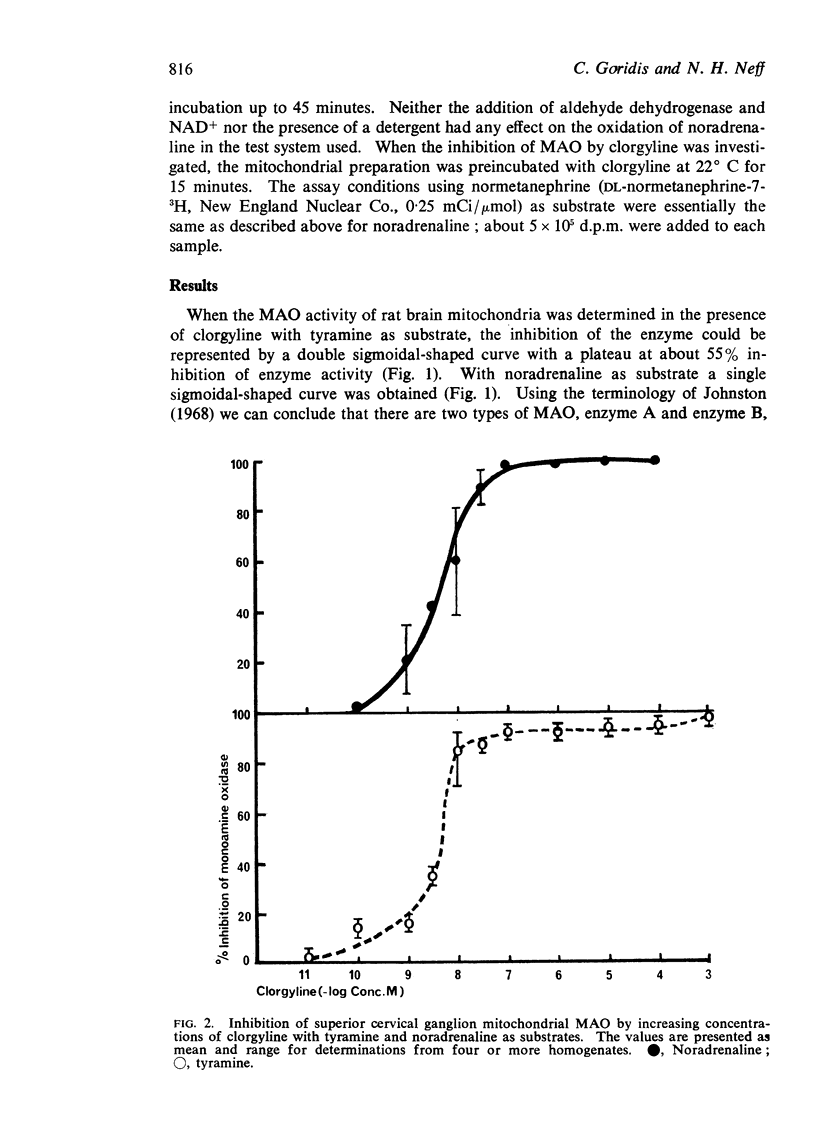
1. When rat brain or superior cervical ganglion monoamine oxidase was incubated with increasing concentrations of clorgyline, using tyramine as substrate, the inhibition of the enzyme could be represented by a pair of sigmoidal curves joined by a horizontal region where inhibition was constant. Tyramine appeared to be metabolized by two enzymes, one of which was highly sensitive to clorgyline, designated A, whereas the other enzyme, designated B, was less sensitive to clorgyline.
2. The ratio of A/B activity for brain was 6/4 while in the ganglion it was 9/1.
3. When the experiments were repeated using noradrenaline as the substrate, the inhibition of the enzyme followed a simple sigmoidal curve where deamination was inhibited by low concentrations of clorgyline as observed with enzyme A.
4. We conclude that tyramine is deaminated by both A and B enzymes whereas noradrenaline is deaminated only by enzyme A, the enzyme which is most active in the ganglion. Our observations are consistent with the hypothesis that a specific intraneuronal monoamine oxidase plays an important role in the catabolism of noradrenaline in sympathetic nerves.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CARLSSON A., HILLARP N. A. Formation of phenolic acids in brain after administration of 3,4-dihydroxyphenylalanine. Acta Physiol Scand. 1962 May;55:95–100. doi: 10.1111/j.1748-1716.1962.tb02422.x. [DOI] [PubMed] [Google Scholar]
- Collins G. G.S., Youdim M. B.H., Sandler M. Isoenzymes of human and rat liver monoamine oxidase. FEBS Lett. 1968 Sep;1(4):215–218. doi: 10.1016/0014-5793(68)80065-1. [DOI] [PubMed] [Google Scholar]
- Goridis C., Neff N. H. Evidence for a specific monoamine oxidase associated with sympathetic nerves. Neuropharmacology. 1971 Sep;10(5):557–564. doi: 10.1016/0028-3908(71)90021-9. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Glowinski J., Axelrod J. The physiologic disposition and metabolism of norepinephrine in immunosympathectomized animals. J Pharmacol Exp Ther. 1966 Feb;151(2):273–284. [PubMed] [Google Scholar]
- Jarrott B. Occurrence and properties of monoamine oxidase in adrenergic neurons. J Neurochem. 1971 Jan;18(1):7–16. doi: 10.1111/j.1471-4159.1971.tb00162.x. [DOI] [PubMed] [Google Scholar]
- Johnston J. P. Some observations upon a new inhibitor of monoamine oxidase in brain tissue. Biochem Pharmacol. 1968 Jul;17(7):1285–1297. doi: 10.1016/0006-2952(68)90066-x. [DOI] [PubMed] [Google Scholar]
- KOPIN I. J., AXELROD J. The role of monoamine oxidase in the release and metabolism of norepinephrine. Ann N Y Acad Sci. 1963 Jul 8;107:848–855. doi: 10.1111/j.1749-6632.1963.tb13328.x. [DOI] [PubMed] [Google Scholar]
- Sierens L., D'Iorio A. Multiple monoamine oxidases in rat liver mitochondria. Can J Biochem. 1970 Jun;48(6):659–663. doi: 10.1139/o70-105. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Axelrod J., Zweig M. A sensitive and specific fluorescence assay for tissue serotonin. Biochem Pharmacol. 1965 May;14(5):831–835. doi: 10.1016/0006-2952(65)90102-4. [DOI] [PubMed] [Google Scholar]
- Youdim M. B., Collins G. G., Sandler M. Multiple forms of rat brain monoamine oxidase. Nature. 1969 Aug 9;223(5206):626–628. doi: 10.1038/223626a0. [DOI] [PubMed] [Google Scholar]


