Abstract
By using immunofluorescence microscopy, we observed rapidly altered distribution patterns of cell wall pectins in meristematic cells of maize (Zea mays) and wheat (Triticum aestivum) root apices. This response was shown for homogalacturonan pectins characterized by a low level (up to 40%) of methylesterification and for rhamnogalacturonan II pectins cross-linked by a borate diol diester. Under boron deprivation, abundance of these pectins rapidly increased in cell walls, whereas their internalization was inhibited, as evidenced by a reduced and even blocked accumulation of these cell wall pectins within brefeldin A-induced compartments. In contrast, root cells of species sensitive to the boron deprivation, like zucchini (Cucurbita pepo) and alfalfa (Medicago sativa), do not internalize cell wall pectins into brefeldin A compartments and do not show accumulation of pectins in their cell walls under boron deprivation. For maize and wheat root apices, we favor an apoplastic target for the primary action of boron deprivation, which signals deeper into the cell via endocytosis-mediated pectin signaling along putative cell wall-plasma membrane-cytoskeleton continuum.
Boron is an essential microelement for plant cells. Boron deficiency causes defects in assembly and mechanical properties of cell walls, in structural and functional integrity of the plasma membrane, and in several metabolic and physiological processes (for reviews, see Loomis and Durst, 1991; Goldbach, 1997; Blevins and Lukaszewski, 1998; Goldbach et al., 2001; Brown et al., 2002). Nevertheless, it is still not clear which of these responses are of primary and which of secondary nature. Recent research has revealed several rapid reactions to boron removal (within 5–20 min), including altered cell wall physics, with a transitory decrease of the elasticity modulus ε followed by a secondary rehardening, and a reduction of inducible plasma membrane-bound reductase activity (for review, see Goldbach et al., 2001). Although rhamnogalacturonan II (RGII) pectins were identified as the major boron-binding fraction in plant cell walls (Kobayashi et al., 1996; Matoh, 1997; Matoh et al., 1998), it is unclear how these rapid structural alterations at the cell wall-plasma membrane interface are related to the RGII-borate complex.
In addition to boron cross-linked RGII pectins (e.g. Höfte, 2001), the pectin network of calcium cross-linked de-esterified homogalacturonan pectins (e.g. Jarvis, 1984) is also important for the regulation of mechanical properties of cell walls. In tip-growing pollen tube apices, for instance, pectin epitopes with a relatively high level of methylesterification are abundant and responsible for the loosened nature of cell walls (Li et al., 1997; Franklin-Tong, 1999). In muro de-esterification of homogalacturonan pectins in the subapical region of pollen tubes makes them proned for cross-linking by calcium, which increases mechanical strength of cell wall (Li et al., 1997; Franklin-Tong, 1999). Intriguingly, boron deprivation caused an immediate halt of the tip growth, and apices of pollen tubes became enriched with JIM5-reactive de-esterified pectins (Yang et al., 1999). This implies that boron may affect the extensibility of cell walls not only via cross-linking of RGII pectins (O'Neill et al., 2001) but also by affecting the distribution of de-esterified homogalacturonan pectins cross-linked with calcium (Jarvis, 1984; Matoh and Kobayashi, 1998).
Increasing evidence supports the hypothesis that the cytoskeleton-plasma membrane-extracellular matrix continuum represents an essential structural assembly directing the growth and morphogenesis of higher plants (Wyatt and Carpita, 1993; Kohorn, 2000). Structural alterations of the pectic matrix, attributable to the removal of boron, are expected to interfere directly and/or indirectly with the cell wall-plasma membrane-cytoskeleton continuum. The extracellular part (apoplast) of this structural continuum has been suggested to represent the essential site of aluminum toxicity (Horst et al., 1999). Using anti-actin and anti-tubulin monoclonal antibodies, we revealed increased levels of actin and tubulin proteins upon short-term boron deprivation in roots of hydroponically grown Arabidopsis (Yu et al., 2001). This response is also associated with changes of cytoskeletal polymerization patterns in cells of maize (Zea mays) root apices (Yu et al., 2002). A possible explanation of these findings could be that these rapid responses of the root cytoskeleton are related to changes in cell wall structure mediated via alterations of cell wall pectins. The aim of this study was to investigate the rapid responses of cell wall pectins to boron deprivation from the emerging perspective of putative cytoskeleton-plasma membrane-extracellular matrix continuum of plant cells.
RESULTS
Cell Wall Pectins Become More Abundant in Maize Roots under Boron Deprivation
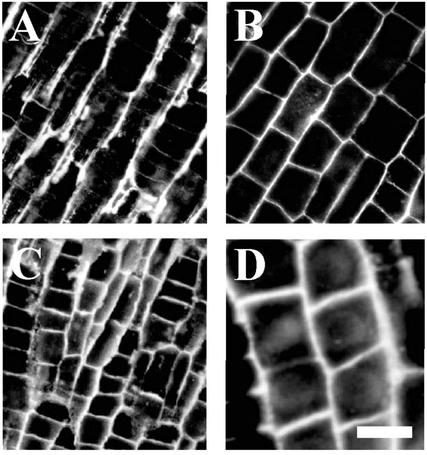
JIM5 antibody recognizes partially (up to 40%) esterified homogalacturonan pectins (Willats et al., 2000). These were localized in cell walls of all root cells with the exception of epidermis and root cap. RGII antibody, which recognizes RGII cross-linked by a borate diol diester (Matoh et al., 1998), was reactive in cell walls throughout root apices in all plant species under investigation. In the maize root apex, JIM5-reactive pectins accumulated in cell walls facing intracellular spaces, and only a weak signal was associated with cross-walls (Fig. 1A). On the other hand, RGII-borate pectins were distributed in all cell walls (Fig. 1B) throughout the maize root apex. Importantly, both RGII- and JIM5-reactive pectins were excluded from the cytoplasm (see also Baluška et al., 2002), which corresponds well with the fact that their epitopes are formed in muro. Deprivation of maize root apices of boron for 1 h was associated with an enhancement of cell wall signal for both of these pectin epitopes (Fig. 1, C and D). Intriguingly, JIM5-reactive pectins accumulated abundantly also at cross-walls of boron-deprived root apices (Fig. 1C), which were almost devoid of these pectins in control root apices (Fig. 1A).
Figure 1.
Localization of cell wall pectins in control and boron-deprived maize root apices. Immunofluorescence microscopy revealed that boron deprivation (1 h) induced alterations of cell wall pectins, reactive to JIM5 (A and C) and to RGII (B and D) antibodies, in cells of maize root apices. A and B, Control images taken from the cortex document that the signal is preferentially associated with cell walls. Especially longitudinal cell walls are strongly labeled with JIM5 antibody (A), whereas all cell walls are labeled with RGII antibody (B). Distribution patterns of de-esterified homogalacturonan pectins (responsive to JIM5 antibody) change under boron deprivation (1 h). C, Besides slightly stronger signal throughout root apices, cross walls of boron-deprived cells are also strongly labeled with JIM5 antibody. Similarly, the RGII-reactive signal is enhanced in cells deprived of boron (D) when compared with the control (B). Bars = 25 μm in A and C, 22 μm in B, and 15 μm in D.
Boron Deprivation Inhibits Internalization of Cell Wall Pectins in Maize and Wheat (Triticum aestivum) Roots
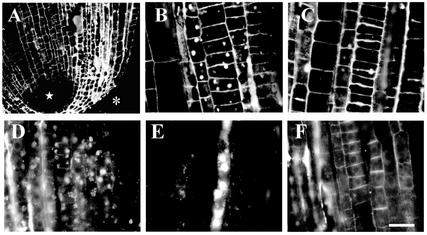
Brefeldin A (BFA) is very useful drug for visualization of internalizing and recycling molecules because it blocks exocytosis but still allows endocytotic internalization of extracellular molecules such as RGII- and JIM5-reactive cell wall pectins (Baluška et al., 2002). The internalization of JIM5-reactive cell wall pectins occurred mainly in cortical cells of the meristem (Fig. 2, A and B). On the other hand, stele cells formed only few and smaller BFA compartments (Fig. 2A). Note that stele cells above quiescent center are devoid (Fig. 2A, star), similar to the epidermis and root cap cells (Fig. 2A, asterisk), of any signal in response to JIM5 antibody.
Figure 2.
Effects of BFA on localization of cell wall pectins in maize root apices. Effects of BFA on distributions of JIM5- and RGII-reactive pectins in cells of maize root apices. A, JIM5-reactive pectins are absent from stele cells above quiescent center (star) and epidermis/root cap cells (asterisk). A and B, Cortical cells internalize JIM5-reactive pectins as documented by large and clear BFA compartments. C, The internalization of JIM5-reactive pectins was reduced as a result of 5 h of boron deprivation. D, Active internalization of RGII-reactive pectins in cortical and particularly in stele cells, as evidenced by intracellular aggregates in the presence of BFA. E, Note that especially phloem cells are active in internalization of RGII-reactive pectins. F, Internalization of RGII-reactive pectins was completely blocked upon 5 h of boron deprivation. Bars = 35 μm in A, 16 μm in B and C, and 25 μm in D through F.
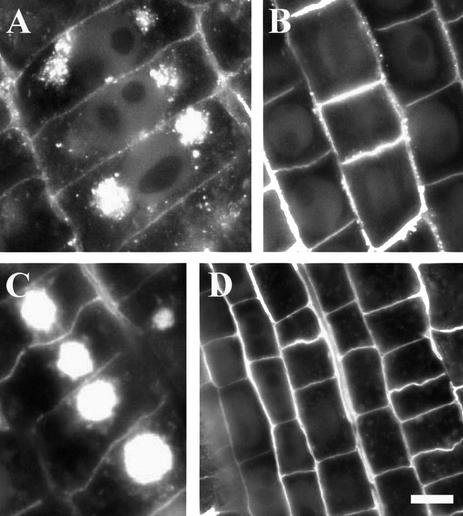
In contrast, RGII-borate pectins were internalized in all meristematic cells exposed to BFA (Fig. 2D). Dramatic accumulation of RGII-borate pectins within BFA compartments was observed especially in phloem cells (Fig. 2E). The internalization of RGII-borate pectins in maize was completely blocked after 5 h of boron deprivation (Fig. 2F). In contrast to RGII pectins, internalization of JIM5 pectins in maize was not blocked completely under boron deprivation, although its magnitude was highly reduced (Fig. 2, C versus B). In cells of wheat root apices, internalization of both JIM5-reactive (Fig. 3, A and B) and RGII-reactive (Fig. 3, C and D) pectins was blocked completely under boron deprivation (Fig. 3, A–D).
Figure 3.
Localization of cell wall pectins in control and boron-deprived wheat root apices. Distributions of JIM5-reactive (A and B) and RGII-reactive (C and D) pectins in BFA-treated root apices of wheat. Note that boron deprivation (B and D) blocks completely cytoplasmic internalization of these cell wall pectins, which accumulate abundantly within prominent BFA compartments in the presence of boron (A and C). Bars = 5 μm in A, 15 μm in B, 12 μm in C, and 22 μm in D.
Cell Wall Pectins Do Not Internalize in Cells of Zucchini (Cucurbita pepo) and Alfalfa (Medicago sativa) Roots
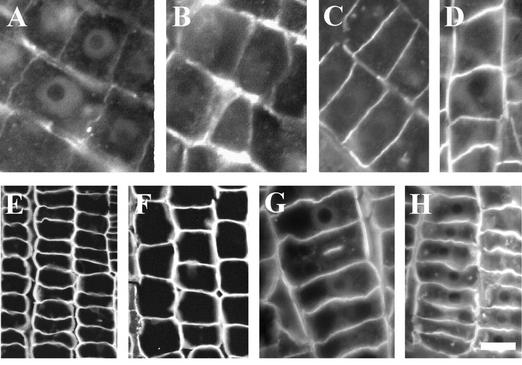
Intriguingly, this rapid internalization of cell wall pectins reactive to JIM5 (Fig. 4, A, B, E, and F) and RGII (Fig. 4, C, D, G, and H) antibodies was not detectable in BFA-treated alfalfa (Fig. 4, A–D) and zucchini (Fig. 4, E–H) root apices. Some pectin-positive BFA compartments were occasionally found in epidermis cells of both alfalfa and zucchini root apices (data not shown). Importantly, we did not detect strongly increased amounts of these pectins in their control cell walls (Fig. 4, A, C, E, and G) when boron was deprived (Fig. 4, B, D, F, and H), although a slight increase was visible in alfalfa root apices (Fig. 4, B and D).
Figure 4.
Localization of cell wall pectins in alfalfa and zucchini root apices. Distributions of JIM5-reactive (A, B, E, and F) and RGII-reactive (C, D, G, and H) pectins in BFA-treated root apices of alfalfa (A–D) and zucchini (E–H) root apices. Note that there are no BFA compartments formed in these dicot species. In contrast to maize and wheat, boron deprivation (B, D, F, and H) does not prominently change the abundance of these pectins in cell walls of alfalfa and zucchini root apices. Bars = 10 μm in A and B, 15 μm in C and D, 20 μm in E and F, 15 μm in G, and 10 μm in H.
DISCUSSION
Eukaryotic cells perform continuous recycling of the plasma membrane proteins and extracellular matrix molecules from the cell surface back to the cytoplasm (for plant cells, see Low and Chandra, 1994; Robinson et al., 1998). Our recent study (Baluška et al., 2002) provides experimental evidence that cell wall pectins are internalized after in muro de-esterification (Micheli, 2001) and cross-linking with calcium and boron (Matoh and Kobayashi, 1998; Kobayashi et al., 1999). These almost exclusive cell wall pectin epitopes, reactive to JIM5 and RGII antibodies, accumulate abundantly within intracellular BFA-induced compartments, which are obviously formed through aggregation of trans-Golgi networks and putative plant endosomes (Baluška et al., 2002). Here, we report that intracellular BFA compartments accumulate these cell wall pectins in meristematic cells of maize and wheat, but not of zucchini and alfalfa, root apices. Intriguingly, boron deprivation inhibits endocytosis of cell wall pectins. These findings implicate differences between pectin turnover in cell walls of graminaceous monocots and dicots. Interestingly in this respect, root apices of gramineae are relatively insensitive to boron deprivation.
Internalization of these cell wall pectins must have profound effects on wall structure and porosity. For instance, the RGII-borate dimer, being the antigen of RGII antibody (Matoh et al., 1998), has been convincingly shown to play a crucial role in both plant growth and development (Höfte, 2001; O'Neill et al., 2001). Comparative analysis of mur1 and mur2 mutants of Arabidopsis implies that pectins, instead of xyloglucan, are the most important partner of cellulose microfibrils in rendering strong mechanical properties of plant cell walls (Höfte, 2001; O'Neill et al., 2001; Vanzin et al., 2002).
Tip-growing pollen tubes, which have high-pectin content in their apical cell walls, might be expected to rely on cross-linkings of pectins with boron and calcium (Li et al., 1997; Franklin-Tong, 1999). In accordance with this concept, tip-growing pollen tubes burst at their apices under boron deficiency (e.g. Geitmann, 1997). Interestingly, tip-growing root hairs show a high capacity to bind fluorescein isothiocyanate (FITC)-phenyl boronic acid, indicative of high boron-binding capabilities, at their expanding apices (Glüsenkamp et al., 1997). In extending root hair apices, initiation of Rhizobium sp. bacteria versus legume plants interactions starts with formation of infection threads growing inwardly into root hairs. Growth of infection threads through root tissues bears a histological similarity to pollen tubes growing through pistil tissues. Interestingly, growth of infection threads is arrested under boron deficiency (Redondo-Nieto et al., 2001). Later in the Rhizobium sp. host plant interactions, boron obviously plays an important role in mediating surface interactions between Rhizobium sp. bacteria and root cortex cells that lead to endocytosis-like internalization of bacteria by host cells during the nodule development (Bolaños et al., 1996; Redondo-Nieto et al., 2001).
Yang et al. (1999) demonstrated a dramatic increase of homogalacturonan cell wall pectins, reactive to JIM5 antibody (Willats et al., 2000), at tips of pollen tubes suffering from boron deficiency. A similar response was demonstrated in pollen tubes exposed to Yariv reagent (Roy et al., 1998, 1999), a compound that cross-links plasma membrane-associated arabinogalactan proteins as evidenced by altering lateral diffusion of plasma membrane proteins (Serpe and Nothnagel, 1994). These findings would indicate that, as with the absence of boron, the exposure to Yariv reagent also inhibits internalization of JIM5-reactive cell wall pectins.
What are further physiological consequences of reduced or even completely blocked internalization of cell wall pectins in boron-deprived root apices? Boron obviously affects size of pores within cell walls (Fleischer et al., 1999). Moreover, it is known that a high-plasma membrane tension, which may rapidly build up under boron deficiency because of the initial weakness of the cell wall challenged with a high turgor pressure (Goldbach et al., 2001), generally inhibits endocytosis (Fricke et al., 2000; Morris and Homann, 2001). This lead us to speculate that cell wall boron might regulate internalization of RGII- and JIM5-reactive acidic pectins through interference with receptor-mediated endocytosis. One characteristic hallmark of receptor-mediated endocytosis in eukaryotic cells is that hypertonic media inhibit effectively this process by blocking clathrin-coated pit formation (Heuser and Anderson, 1989). Further hallmarks of receptor-mediated endocytosis are its dependence on temperatures above 4°C (for plants, see Horn et al., 1989; Low et al., 1993) and on an intact F-actin cytoskeleton (Lamaze et al., 1997). In our accompanying paper (Baluška et al., 2002), we showed that endocytosis of cell wall pectins is blocked upon cold treatment and after depolymerization of F-actin. Importantly, pectin-derived elicitors were reported to be taken up into plant cells via receptor-mediated endocytosis (Horn et al., 1989; Low et al., 1993). In fact, endocytosis of cell wall pectins might be part of pectin-mediated signaling between the plasma membrane and nucleus (Messiaen et al., 1993; Messiaen and Van Cutsem, 1994, 1999). Interestingly in this respect, boron was suggested to function at “membrane rafts” (Brown et al., 2002), which are known to act as platforms for signaling and endocytosis in cells of multicellular eukaryotic organisms (Simons and Ikonen, 1997; Brown and London, 1998; for plants, see Sherrier et al., 1999). All of this gives us a new fresh look on still enigmatic (Läuchli, 2002) roles of boron in plant cells.
MATERIALS AND METHODS
Plant Growth
Maize (Zea mays), wheat (Triticum aestivum), alfalfa (Medicago sativa), and zucchini (Cucurbita pepo) seeds were soaked in distilled water for 8 h with aeration and germinated in moistened rolls of filter paper for 2 d in dark at 24°C (maize) or 28°C (zucchini). Uniform seedlings with straight primary roots were transferred to solution culture, which was maintained in a phytotrone at 24°C under 14 h of light (400 μmol m−2 s−1) and 70% humidity. The nutrient solution contained 2,000 μm Ca(NO3)2, 3,000 μm KNO3, 1,000 μm KH2PO3, 500 μm Mg(NO3)2, 100 μm NaCl, 1,000 μm MgSO4, 44.8 μm FeEDDHA, 18.2 μm MnSO4, 3.1 μm CuCl2, 6.1 μm ZnSO4, 0.2 μm (NH4)6Mo7O24, 0.016 μm CoCl2, 0.017 μm NiCl2, and 40 μm H3BO3. All the nutrient stock solutions were prepared with Milli-Q ultrapure water that was deprived of boron with B-specific ion-exchange resin Amberlite IRA-743 (Sigma, St. Louis). The pH of the nutrient solution was adjusted to 6.0 by addition of 1 n NaOH. Seedlings were allowed to grow for 2 d in a one-tenth-strength nutrient solution with 15 min of aeration every 45 min and then in a full-strength nutrition solution for a further 2 d.
Boron Deprivation Treatment
After transplanting, seedlings were transferred to a fresh nutrient solution with (+B) or without boron (−B). In the −B treatment, an ample amount of the borate-specific exchange resin Amberlite IRA 743 was also placed within the nutrient container, and the nutrient solution was constantly stirred to adsorb virtually all possible B contaminations. The roots were rinsed in a 5-L −B nutrient solution for a few seconds and then allowed to grow in −B solution for 10, 30, and 60 min and 5 h. When the internalization of cell wall pectins was studied, seedlings were grown further for 2 h in the ±B nutrition solution containing 100 μm BFA.
Indirect Fluorescence Microscopy
Indirect fluorescence microscopy was performed essentially according to Baluška et al. (1992) with a few modifications. Apical segments of primary root from +B and −B-treated seedlings were vacuum infiltrated for 10 min with 3.7% (w/v) formaldehyde made up in stabilizing buffer (SB; 50 mm PIPES, 5 mm EGTA, and 5 mm MgSO4, pH 6.9) and then fixed at room temperature for 1 h. After a brief rinse in SB, they were dehydrated in a graded ethanol series diluted with phosphate-buffered saline (PBS; pH 7.3) and embedded in Steedman's wax (for further details, see Baluška et al., 1992). Eight-micrometer-thick longitudinal sections were prepared from the embedded materials, and the most median sections were allowed to expand on a small drop of distilled water onto slides coated with glycerol-albumen (Serva, Heidelberg).
After drying at room temperature overnight, the mounted sections were dewaxed in ethanol, rehydrated in a PBS-diluted ethanol series, and then left in SB for 30 min before being rinsed with methanol at −20°C for 20 min. The sections were transferred to SB for 30 min and incubated with the following primary antibodies for 1 h at room temperature: the monoclonal anti-acidic pectin antibody (JIM5) raised against rat (Willats et al., 2000) and the polyclonal anti-RGII-boron-complex antibody raised against rabbit (Matoh et al., 1998). All the primary antibodies were diluted in PBS supplemented with 0.1% (w/v) bovine serum albumin. After a rinse in SB, the sections were incubated with FITC-conjugated anti-mouse IgG (Sigma) and diluted 1:100 (w/v) in PBS containing 0.1% (w/v) bovine serum albumin. The labeled sections, after being rinsed with PBS and further stained with 0.01% (w/v) toluidine blue to diminish autofluorescence from root tissue, were mounted using an antifade mountant containing p-phenylenediamine (Baluška et al., 1992). Fluorescence was detected with an Axiovert 405M inverted microscope (Zeiss, Oberkochen, Germany) equipped with epifluorescence and standard FITC exciter and barrier filters (BP 450–490, LP520). Photos were taken on T-Max films rated at 400 ASA (Eastman Kodak, Rochester, NY).
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
ACKNOWLEDGMENTS
We thank Keith Roberts and Paul Knox for the generous gifts of the JIM5 monoclonal antibody.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. Go 415/14–3, to H.E.G.), by the Alexander von Humboldt Foundation (to Q.Y.), and by the Deutsches Zentrum für Luft- und Raumfahrt (Bonn; to F.B. and D.V.). In addition, F.B. receives partial support from the Slovak Academy of Sciences, Grant Agency Vega (Bratislava, Slovakia; project no. 2031).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.006163.
LITERATURE CITED
- Baluška F, Hlavacka A, Šamaj J, Palme K, Robinson DG, Matoh T, McCurdy DW, Menzel D, Volkmann D. F-actin-dependent endocytosis of cell wall pectins in meristematic root cells. Insights from brefeldin A-induced compartments. Plant Physiol. 2002;130:422–431. doi: 10.1104/pp.007526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluška F, Parker JS, Barlow PW. Specific patterns of cortical and endoplasmic microtubules associated with cell growth and tissue differentiation in roots of maize (Zea mays L.) J Cell Sci. 1992;103:191–200. [Google Scholar]
- Blevins DG, Lukaszewski KM. Boron in plant structure and function. Annu Rev Plant Physiol Mol Biol. 1998;49:481–500. doi: 10.1146/annurev.arplant.49.1.481. [DOI] [PubMed] [Google Scholar]
- Bolaños L, Brevin NJ, Bonilla I. Effects of boron on Rhizobium-legume cell-surface interactions and nodule development. Plant Physiol. 1996;110:1249–1256. doi: 10.1104/pp.110.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- Brown PH, Bellaloui N, Wimmer MA, Bassil ES, Ruiz J, Hu H, Pfeffer H, Dannel F, Römheld V. Boron in plant biology. Plant Biol. 2002;4:205–223. [Google Scholar]
- Fleischer A, O'Neill MA, Ehwald R. The pore size of non-graminaceous plant cell walls is rapidly decreased by borate ester cross-linking of the pectic polysaccharide rhamnogalacturonan II. Plant Physiol. 1999;121:829–838. doi: 10.1104/pp.121.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin-Tong VE. Signalling and the modulation of pollen tube growth. Plant Cell. 1999;11:727–738. doi: 10.1105/tpc.11.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke W, Jarvis MC, Brett CT. Turgor pressure, membrane tension and the control of exocytosis in higher plants. Plant Cell Environ. 2000;23:999–1003. [Google Scholar]
- Geitmann A. Growth and formation of the cell wall in pollen tubes of Nicotiana tabacum and Petunia hybrida. PhD thesis. Egelsbach, Frankfurt: Hänsel-Hohenhausen Verlag; 1997. [Google Scholar]
- Glüsenkamp K-H, Kosegarten H, Mengel K, Grolig F, Esch A, Goldbach HE. A fluorescein boronic acid conjugate as a marker for borate binding sites in the apoplast of growing roots of Zea mays L. and Helianthus annuus L. In: Bell RW, Rerkasem B, editors. Boron in Soil and Plants. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1997. pp. 229–235. [Google Scholar]
- Goldbach HE. A critical review on current hypotheses concerning the role of boron in higher plants: suggestions for further research and methodological requirements. J Trace Microprobe Tech. 1997;15:51–91. [Google Scholar]
- Goldbach HE, Yu Q, Wingender R, Schulz M, Wimmer M, Finderklee P, Baluška F. Rapid response reactions of roots to boron deprivation. J Plant Nutr Soil Sci. 2001;161:173–181. [Google Scholar]
- Heuser JE, Anderson RGW. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J Cell Biol. 1989;108:389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfte H. A baroque residue in red wine. Science. 2001;294:795–797. doi: 10.1126/science.1066242. [DOI] [PubMed] [Google Scholar]
- Horn MA, Heinstein PF, Low PS. Receptor-mediated endocytosis in plant cells. Plant Cell. 1989;1:1003–1009. doi: 10.1105/tpc.1.10.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst WJ, Schmohl N, Kollmeier M, Baluška F, Sivaguru M. Does aluminium affect root growth of maize through interaction with the cell wall-plasma membrane-cytoskeleton continuum? Plant Soil. 1999;215:163–174. [Google Scholar]
- Jarvis MC. Structure and properties of pectin gels in plant cell walls. Plant Cell Environ. 1984;7:153–164. [Google Scholar]
- Kobayashi M, Matoh T, Azuma J-I. Two chains of rhamnogalacturonan II are cross-linked by borate-diol ester bonds in higher plant cell walls. Plant Physiol. 1996;110:1017–1020. doi: 10.1104/pp.110.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Nakagawa H, Asaka T, Matoh T. Borate-rhamnogalacturonan II bonding reinforced by Ca2+ retains pectic polysaccharides in higher-plant cell walls. Plant Physiol. 1999;119:199–203. doi: 10.1104/pp.119.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohorn BD. Plasma membrane-cell wall contacts. Plant Physiol. 2000;124:31–38. doi: 10.1104/pp.124.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamaze C, Fujimoto LM, Yin HL, Schmid SL. The actin cytoskeleton is required for receptor-mediated endocytosis in mammalian cells. J Biol Chem. 1997;272:20332–20335. doi: 10.1074/jbc.272.33.20332. [DOI] [PubMed] [Google Scholar]
- Läuchli A. Functions of boron in higher plants: recent advances and open questions. Plant Biol. 2002;4:190–192. [Google Scholar]
- Li YQ, Cai G, Mascatelli A, Cresti M. Functional interaction among cytoskeleton, membranes and cell wall in the pollen tubes of flowering plants. Int Rev Cytol. 1997;176:133–199. doi: 10.1016/s0074-7696(08)61610-1. [DOI] [PubMed] [Google Scholar]
- Loomis WD, Durst RW. Boron and cell walls. Curr Top Plant Biochem Physiol. 1991;10:149–178. [Google Scholar]
- Low PS, Chandra S. Endocytosis in plants. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:609–631. [Google Scholar]
- Low PS, Legendre L, Heinstein PF, Horn MA. Comparison of elicitor and vitamin receptor-mediated endocytosis in cultured soybean cells. J Exp Bot. 1993;44:269–274. [Google Scholar]
- Matoh T. Boron in plant cell walls. Plant Soil. 1997;193:59–70. [Google Scholar]
- Matoh T, Kobayashi M. Boron and calcium, essential inorganic constituents of pectic polysaccharides in higher plant cell walls. J Plant Res. 1998;111:179–190. [Google Scholar]
- Matoh T, Takasaki M, Takabe K, Kobayashi M. Immunocytochemistry of rhamnogalactouronan II in cell walls of higher plants. Plant Cell Physiol. 1998;39:483–491. [Google Scholar]
- Messiaen J, Read ND, Van Cutsem P, Trewavas AJ. Cell wall oligogalacturonides increase cytosolic free calcium in carrot protoplasts. J Cell Sci. 1993;104:365–371. [Google Scholar]
- Messiaen J, Van Cutsem P. Pectic signal transduction in carrot cells: membrane, cytosolic and nuclear responses induced by oligogalacturonides. Plant Cell Physiol. 1994;35:677–689. [Google Scholar]
- Messiaen J, Van Cutsem P. Polyamines and pectins: II. Modulation of pectic-signal transduction. Planta. 1999;208:247–256. doi: 10.1007/s004250050556. [DOI] [PubMed] [Google Scholar]
- Micheli F. Pectin methylesterases: cell wall enzymes with important roles in plant physiology. Trends Plant Sci. 2001;6:414–419. doi: 10.1016/s1360-1385(01)02045-3. [DOI] [PubMed] [Google Scholar]
- Morris C, Homann U. Cell surface regulation and membrane tension. J Membr Biol. 2001;179:79–102. doi: 10.1007/s002320010040. [DOI] [PubMed] [Google Scholar]
- O'Neill MA, Eberhard S, Albersheim P, Darvill AG. Requirement of borate cross-linking of cell wall rhamnogalacturonan II for Arabidopsis growth. Science. 2001;294:846–849. doi: 10.1126/science.1062319. [DOI] [PubMed] [Google Scholar]
- Redondo-Nieto M, Rivilla R, El-Hamdaoui A, Bonilla I, Bolaños L. Boron deficiency affects early infection events in the pea-Rhizobium symbiotic interactions. Aust J Plant Physiol. 2001;28:819–823. [Google Scholar]
- Robinson DG, Hinz G, Holstein SEH. The molecular characterization of transport vesicles. Plant Mol Biol. 1998;38:49–76. [PubMed] [Google Scholar]
- Roy SJ, Holdaway-Clarke TL, Hackett GR, Kunkel JG, Lord EM, Hepler PK. Uncoupling secretion and tip growth in lily pollen tubes: evidence for the role of calcium in exocytosis. Plant J. 1999;19:379–386. doi: 10.1046/j.1365-313x.1999.00515.x. [DOI] [PubMed] [Google Scholar]
- Roy SJ, Jauh GY, Hepler PK, Lord EM. Effects of Yariv phenylglycoside on cell wall assembly in the lily pollen tube. Planta. 1998;204:450–458. doi: 10.1007/s004250050279. [DOI] [PubMed] [Google Scholar]
- Serpe MD, Nothnagel EA. Effects of Yariv phenylglycosides on Rosa cell suspensions: evidence for the involvement of arabinogalactan-proteins in cell proliferation. Planta. 1994;193:542–550. [Google Scholar]
- Sherrier JD, Prime TA, Dupree P. Glycosylphosphatidylinositol-anchored cell-surface proteins from Arabidopsis. Electrophoresis. 1999;20:2027–2035. doi: 10.1002/(SICI)1522-2683(19990701)20:10<2027::AID-ELPS2027>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Vanzin GF, Madson M, Carpita NC, Raikhel NV, Keegstra K, Reiter W-D. The mur2 mutants of Arabidopsis thaliana lacks fucosylated xyloglucan because of a lesion in fucosyltransferase AtFUT1. Proc Natl Acad Sci USA. 2002;99:3340–3345. doi: 10.1073/pnas.052450699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willats WGT, Limberg G, Buchholt HC, van Alabeek G-J, Benen J, Christensen TMIE, Visser J, Voragen A, Mikkelsen JD, Knox JP. Analysis of pectic epitopes recognised by hybridoma and phage display monoclonal antibodies using defined oligosaccharides, polysaccharides, and enzymatic degradation. Carbohydr Res. 2000;327:309–320. doi: 10.1016/s0008-6215(00)00039-2. [DOI] [PubMed] [Google Scholar]
- Wyatt SE, Carpita NC. The plant cytoskeleton: cell wall continuum. Trends Cell Biol. 1993;3:413–417. doi: 10.1016/0962-8924(93)90022-s. [DOI] [PubMed] [Google Scholar]
- Yang XD, Sun SQ, Li YQ. Boron deficiency causes changes in the distribution of major polysaccharides of pollen tube wall. Acta Bot Sin. 1999;41:1169–1176. [Google Scholar]
- Yu Q, Baluška F, Jasper F, Menzel D, Goldbach HE (2002) Short-term boron deprivation enhances levels of cytoskeletal proteins in maize, but not zucchini, root spices. Physiol Plant (in press) [DOI] [PMC free article] [PubMed]
- Yu Q, Wingender R, Schulz M, Baluška F, Goldbach H. Short-term boron deprivation induces increased levels of cytoskeletal proteins in Arabidopsis roots. Plant Biol. 2001;6:1–6. [Google Scholar]