Abstract
1. Rats lesioned unilaterally in the substantia nigra show no obvious abnormalities after recovery from the operation but rotate towards the lesioned side after administration of drugs of the amphetamine and ephedrine groups.
2. (+)-Amphetamine and (-)-amphetamine are equally potent in producing turning behaviour but (+)-methylamphetamine is considerably more effective.
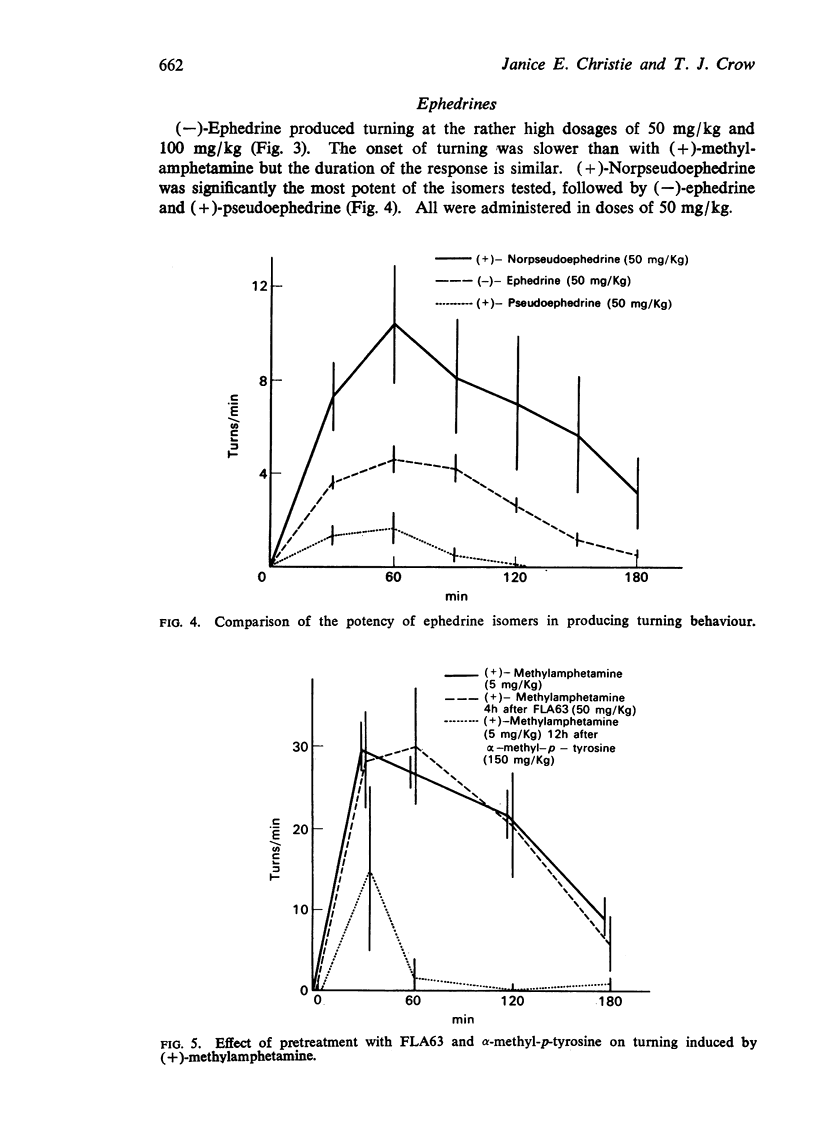
3. (-)-Ephedrine (with a β-OH group on the ethylamine side chain) induces turning but only in doses approximately 20 times greater than (+)-methylamphetamine.
4. (+)-Norpseudoephedrine was the most effective of the ephedrine isomers tested followed by (-)-ephedrine and (+)-pseudoephedrine.
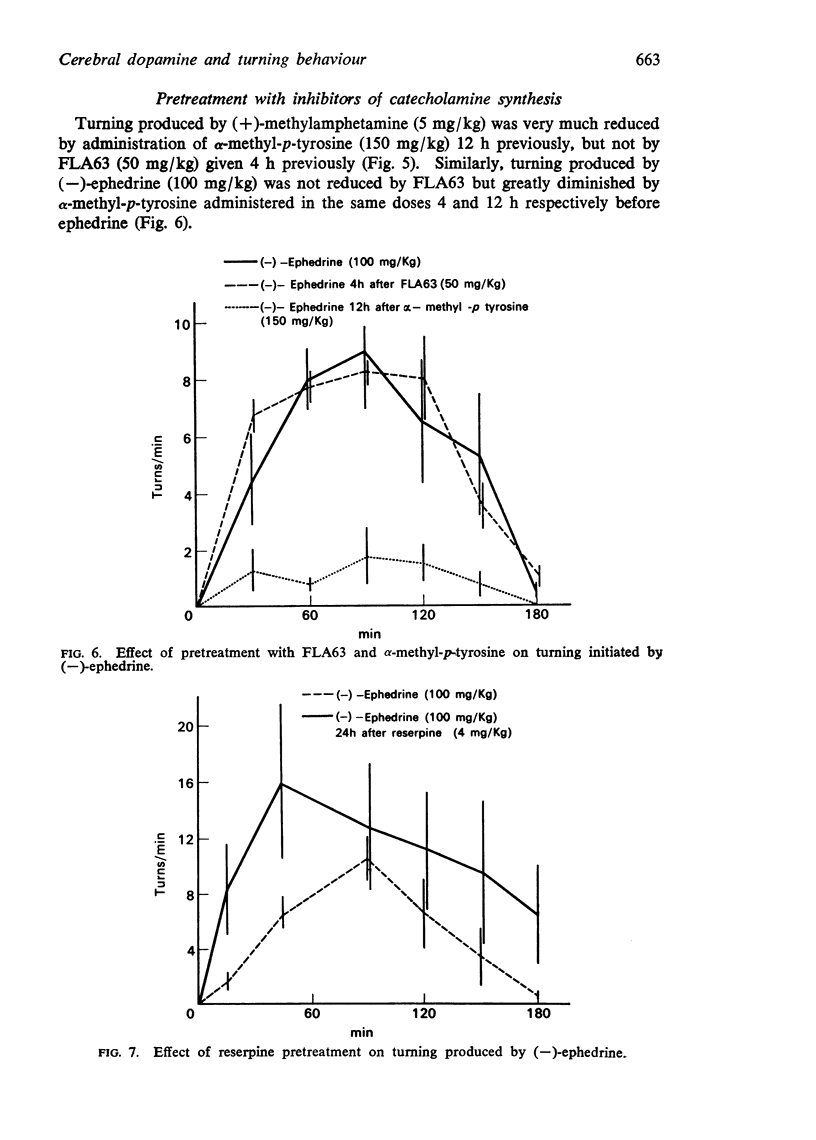
5. Turning produced by (-)-ephedrine and (+)-methylamphetamine is not reduced by FLA63 (50 mg/kg) 4 h previously, but is almost completely inhibited by α-methyl-p-tyrosine (150 mg/kg) 12 h previously.
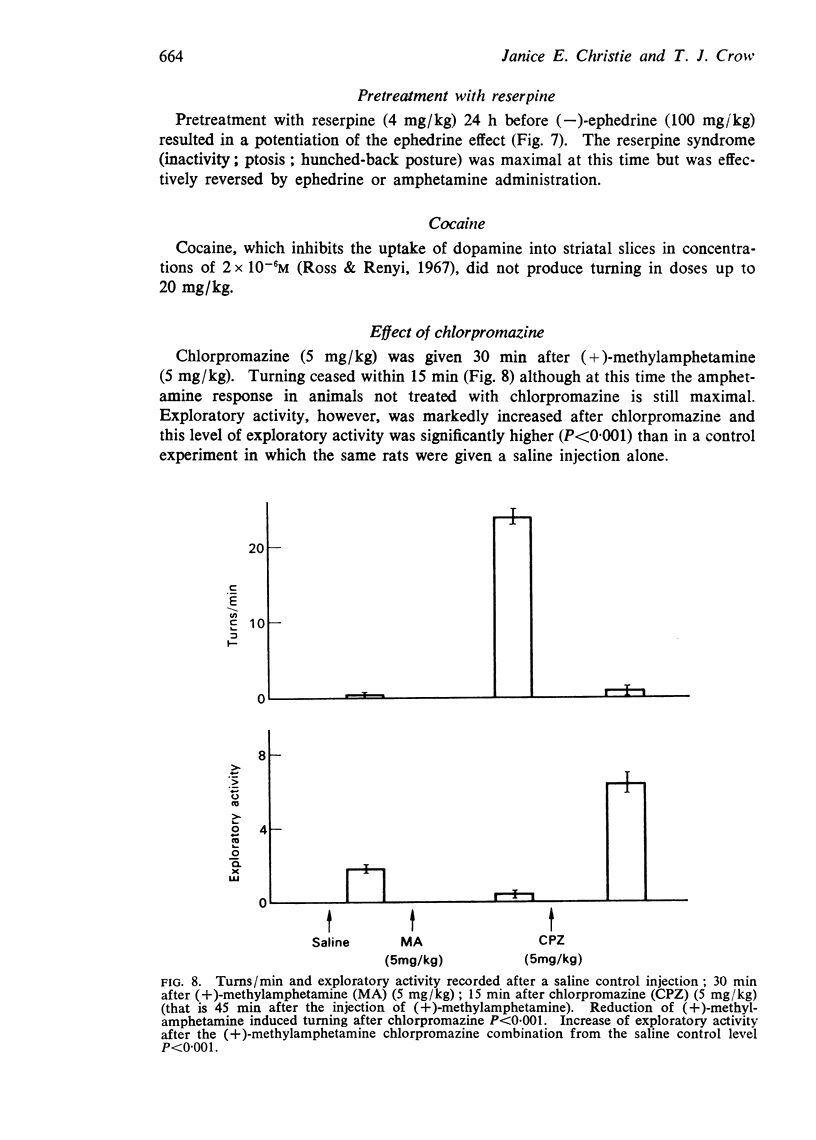
6. Reserpine pretreatment potentiates turning produced by (-)-ephedrine.
7. Chlorpromazine (5 mg/kg) completely blocks turning induced by (+)-methylamphetamine although it concurrently increases exploratory activity. The level of exploratory activity after the (+)-methylamphetamine-chlorpromazine combination is more than 3 times that attained after saline alone.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDEN N. E., CARLSSON A., DAHLSTROEM A., FUXE K., HILLARP N. A., LARSSON K. DEMONSTRATION AND MAPPING OUT OF NIGRO-NEOSTRIATAL DOPAMINE NEURONS. Life Sci. 1964 Jun;3:523–530. doi: 10.1016/0024-3205(64)90161-4. [DOI] [PubMed] [Google Scholar]
- Andén N. E., Dahlström A., Fuxe K., Larsson K. Functional role of the nigro-neostriatal dopamine neurons. Acta Pharmacol Toxicol (Copenh) 1966;24(2):263–274. doi: 10.1111/j.1600-0773.1966.tb00389.x. [DOI] [PubMed] [Google Scholar]
- Andén N. E., Rubenson A., Fuxe K., Hökfelt T. Evidence for dopamine receptor stimulation by apomorphine. J Pharm Pharmacol. 1967 Sep;19(9):627–629. doi: 10.1111/j.2042-7158.1967.tb09604.x. [DOI] [PubMed] [Google Scholar]
- Arbuthnott G. W., Crow T. J., Fuxe K., Olson L., Ungerstedt U. Depletion of catecholamines in vivo induced by electrical stimulation of central monoamine pathways. Brain Res. 1970 Dec 18;24(3):471–483. doi: 10.1016/0006-8993(70)90186-1. [DOI] [PubMed] [Google Scholar]
- Arbuthnott G. W., Crow T. J. Relation of contraversive turning to unilateral release of dopamine from the nigrostriatal pathway in rats. Exp Neurol. 1971 Mar;30(3):484–491. doi: 10.1016/0014-4886(71)90149-x. [DOI] [PubMed] [Google Scholar]
- BERTLER A., ROSENGREN E. Occurrence and distribution of dopamine in brain and other tissues. Experientia. 1959 Jan 15;15(1):10–11. doi: 10.1007/BF02157069. [DOI] [PubMed] [Google Scholar]
- BURN J. H., RAND M. J. The action of sympathomimetic amines in animals treated with reserpine. J Physiol. 1958 Dec 4;144(2):314–336. doi: 10.1113/jphysiol.1958.sp006104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow T. J., Gillbe C. Methamphetamine-protryptiline interaction in rotating rats. Br J Pharmacol. 1970 Feb;38(2):458P–458P. [PMC free article] [PubMed] [Google Scholar]
- Crow T. J. The relationship between lesion site, dopamine neurons, and turning behavior in the rat. Exp Neurol. 1971 Aug;32(2):247–255. doi: 10.1016/0014-4886(71)90068-9. [DOI] [PubMed] [Google Scholar]
- Del Rio J., Fuentes J. A. Further studies on the antagonism of stereotyped behaviour induced by amphetamine. Eur J Pharmacol. 1969 Oct;8(1):73–78. doi: 10.1016/0014-2999(69)90130-7. [DOI] [PubMed] [Google Scholar]
- Fairchild M. D., Alles G. A. The central locomotor stimulatory acitivity and acute toxicity of the ephedrine and norephedrine isomers in mice. J Pharmacol Exp Ther. 1967 Oct;158(1):135–139. [PubMed] [Google Scholar]
- GLOWINSKI J., AXELROD J. EFFECT OF DRUGS ON THE UPTAKE, RELEASE, AND METABOLISM OF H3-NOREPINEPHRINE IN THE RAT BRAIN. J Pharmacol Exp Ther. 1965 Jul;149:43–49. [PubMed] [Google Scholar]
- Hökfelt T., Ungerstedt U. Electron and fluorescence microscopical studies on the nucleus caudatus putamen of the rat after unilateral lesions of ascending nigro-neostriatal dopamine neurons. Acta Physiol Scand. 1969 Aug;76(4):415–426. doi: 10.1111/j.1748-1716.1969.tb04489.x. [DOI] [PubMed] [Google Scholar]
- McNeill J. H., Muschek L. D., Commarato M. A. Ephedrine-adrenergic amine interaction on heart phosphorylase, adenyl cyclase and amine uptake. Eur J Pharmacol. 1970 May;10(2):145–150. doi: 10.1016/0014-2999(70)90267-0. [DOI] [PubMed] [Google Scholar]
- NAGATSU T., LEVITT M., UDENFRIEND S. TYROSINE HYDROXYLASE. THE INITIAL STEP IN NOREPINEPHRINE BIOSYNTHESIS. J Biol Chem. 1964 Sep;239:2910–2917. [PubMed] [Google Scholar]
- O'Keeffe R., Sharman D. F., Vogt M. Effect of drugs used in psychoses on cerebral dopamine metabolism. Br J Pharmacol. 1970 Feb;38(2):287–304. doi: 10.1111/j.1476-5381.1970.tb08517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POIRIER L. J., SOURKES T. L. INFLUENCE OF THE SUBSTANTIA NIGRA ON THE CATECHOLAMINE CONTENT OF THE STRIATUM. Brain. 1965 Mar;88:181–192. doi: 10.1093/brain/88.1.181. [DOI] [PubMed] [Google Scholar]
- Persson T., Waldeck B. Further studies on the possible interaction between dopamine and noradrenaline containing neurons in the brain. Eur J Pharmacol. 1970;11(3):315–320. doi: 10.1016/0014-2999(70)90007-5. [DOI] [PubMed] [Google Scholar]
- Poirier L. J., Singh P., Sourkes T. L., Boucher R. Effect of amine precursors on the concentration of striatal dopamine and serotonin in cats with and without unilateral brain stem lesions. Brain Res. 1967 Dec;6(4):654–666. doi: 10.1016/0006-8993(67)90123-0. [DOI] [PubMed] [Google Scholar]
- Portig P. J., Vogt M. Release to the cerebral ventricles of substances with possible transmitter function in the caudate nucleus. J Physiol. 1969 Oct;204(3):687–715. doi: 10.1113/jphysiol.1969.sp008939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randrup A., Munkvad I. Special antagonism of amphetamine-induced abnormal behaviour. Inhibition of stereotyped activity with increase of some normal activities. Psychopharmacologia. 1965 May 21;7(6):416–422. doi: 10.1007/BF00402364. [DOI] [PubMed] [Google Scholar]
- Ross S. B., Renyi A. L. Inhibition of the uptake of tritiated catecholamines by antidepressant and related agents. Eur J Pharmacol. 1967 Dec;2(3):181–186. doi: 10.1016/0014-2999(67)90084-2. [DOI] [PubMed] [Google Scholar]
- SMITH C. B. ENHANCEMENT BY RESERPINE AND ALPHA-METHYL DOPA OF THE EFFECTS OF D-AMPHETAMINE UPON THE LOCOMOTOR ACTIVITY OF MICE. J Pharmacol Exp Ther. 1963 Dec;142:343–350. [PubMed] [Google Scholar]
- Svensson T. H., Waldeck B. On the significance of central noradrenaline for motor activity: experiments with a new dopamine beta-hydroxylase inhibitor. Eur J Pharmacol. 1969 Sep;7(3):278–282. doi: 10.1016/0014-2999(69)90092-2. [DOI] [PubMed] [Google Scholar]
- Taylor K. M., Snyder S. H. Amphetamine: differentiation by d and l isomers of behavior involving brain norepinephrine or dopamine. Science. 1970 Jun 19;168(3938):1487–1489. doi: 10.1126/science.168.3938.1487. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U., Arbuthnott G. W. Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Res. 1970 Dec 18;24(3):485–493. doi: 10.1016/0006-8993(70)90187-3. [DOI] [PubMed] [Google Scholar]
- Valette G., Cohen Y., Bralet J. Action de l'éphédrine sur l'aorte du rat in vitro: étude à l'aide de noradrenaline 14 C. Biochem Pharmacol. 1966 Feb;15(2):177–185. doi: 10.1016/0006-2952(66)90058-x. [DOI] [PubMed] [Google Scholar]


