Abstract
Synthesis of proteinase inhibitor I protein in response to wounding in leaves of excised tomato (Lycopersicon esculentum) plants was inhibited by NO donors sodium nitroprusside and S-nitroso-N-acetyl-penicillamine. The inhibition was reversed by supplying the plants with the NO scavenger 2-(4-carboxiphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide. NO also blocked the hydrogen peroxide (H2O2) production and proteinase inhibitor synthesis that was induced by systemin, oligouronides, and jasmonic acid (JA). However, H2O2 generated by glucose oxidase and glucose was not blocked by NO, nor was H2O2-induced proteinase inhibitor synthesis. Although the expression of proteinase inhibitor genes in response to JA was inhibited by NO, the expression of wound signaling-associated genes was not. The inhibition of wound-inducible H2O2 generation and proteinase inhibitor gene expression by NO was not due to an increase in salicylic acid, which is known to inhibit the octadecanoid pathway. Instead, NO appears to be interacting directly with the signaling pathway downstream from JA synthesis, upstream of H2O2 synthesis. The results suggest that NO may have a role in down-regulating the expression of wound-inducible defense genes during pathogenesis.
Nitric oxide (NO) and reactive oxygen species (ROS) have important roles in the activation of defense responses against pathogen attacks (for review, see Bolwell, 1999; Durner and Klessig, 1999; Beligni and Lamattina, 2001). The addition of sodium nitroprusside (SNP), an NO donor, can cause cell death to soybean (Glycine max) suspension cultures at millimolar concentrations, when ROS are present (Delledonne et al., 1998, 2001; Durner and Klessig, 1999).
The molecular mechanism for NO synthesis or action is currently unknown. NO can activate or inhibit certain heme-containing enzymes (Stamler, 1994), and it can stimulate plant defense responses through a cGMP-dependent signaling cascade involving, at least in some cases, the generation of cADPR and the activation of mitogen-activated protein kinases (Durner et al., 1999; Klessig et al., 2000; Kumar and Klessig, 2000).
The generation of ROS in response to pathogen and herbivore attacks has been well documented (for review, see Bi and Felton, 1995; Low and Merida, 1996; Lamb and Dixon, 1997; Bolwell, 1999). One ROS species, hydrogen peroxide (H2O2), appears to be a key signaling molecule as well as a defensive chemical that physically damages attacking organisms (Levine et al., 1994; Alvarez et al., 1998; Orozco-Cárdenas et al., 2001).
Tomato (Lycopersicon esculentum) plants are known to accumulate relatively high levels of H2O2 in response to wounding and elicitors, without apparent toxicity to the plants (Orozco-Cárdenas and Ryan, 1999). Although NO has been associated with ROS and the activation of defense responses against pathogens, its possible role in wound signaling has not been reported. Herein, we show that supplying young excised tomato plants with NO donors strongly inhibited the expression of wound-inducible proteinase inhibitors, but did not inhibit the activation of octadecanoid pathway genes. The inhibitory action of NO did not involve the synthesis of salicylic acid (SA), a potent inhibitor of wound-inducible defense gene signaling (Doares et al., 1995).
RESULTS
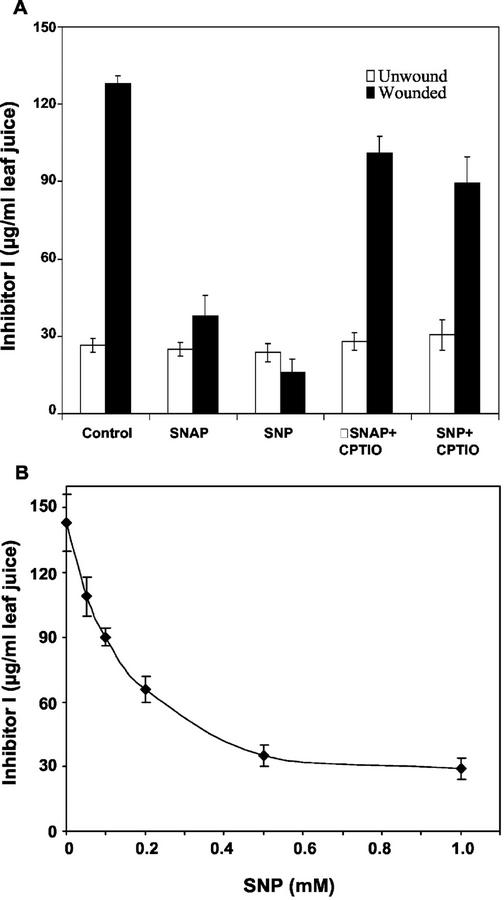
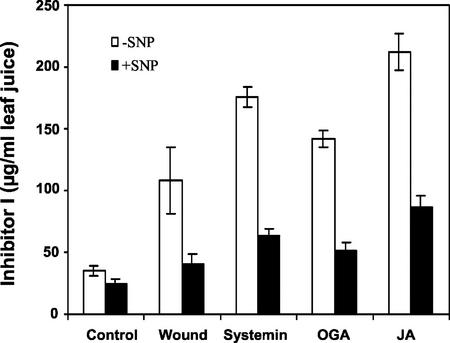
The effect of NO on the synthesis of proteinase inhibitor I (Inh I) in leaves of young tomato plants in response to wounding was initially studied using two NO donors, S-nitroso-N-acetyl-penicillamine (SNAP) and SNP, which are known to elevate levels of NO when supplied to plants (Beligni and Lamattina, 2000). When young tomato plants were supplied through their cut stems with solutions of SNAP or SNP and wounded 1 h later, the synthesis and accumulation of Inh I in response to wounding was severely diminished compared with control plants that were not supplied with NO donors (Fig. 1A). The inhibition of the wound response by both SNAP and SNP was reversed by the simultaneous addition of the NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (CPTIO; Fig. 1A). Wounding tomato leaves does not cause an increase in NO (Fig. 2), and CPTIO and the NO synthase inhibitors S,S′-1,3-phenylene-bis(1,2-ethanediyl)-bis-isothiourea and Nω-nitro-l-Arg had no effect on the wound response (data not shown). SNP inhibited the wound-induced accumulation of proteinase Inh I in a concentration-dependent manner. The concentration of SNP required for half-maximal inhibition was about 0.1 to 0.2 mm (Fig. 1B). SNP similarly inhibited accumulation of proteinase inhibitor I induced in tomato plants by the elicitors systemin, jasmonic acid (JA), and oligogalacturonides (OGA) (Fig. 3). Treatment of plants with SNAP under the same conditions as SNP resulted in similar effects (data not shown).
Figure 1.
NO inhibits the wound-induced accumulation of proteinase Inh I in tomato leaves. A, Effect of NO donors on the accumulation of Inh I. B, Concentration dependence of SNP inhibition of proteinase Inh I accumulation induced by wounding. Fourteen-day-old plants having two expanded leaves and a small apical leaf were excised at the base of the stems and supplied through the stems with a solution of phosphate buffer alone, pH 6.5, for 1 h (control) or a buffer containing 1.0 mm SNAP, 1.0 mm SNP, 1.0 mm SNAP + 1.0 mm CPTIO, or 1.0 mm SNP + 1.0 mm CPTIO for 1 h. All experiments were carried out under light (300 μEm−2 s−1). Plants, except controls, were wounded twice at the middle of each expanded leaf, perpendicular to the main petiole and were incubated in water under light as described in “Materials and Methods.” Proteinase Inh I levels in leaf extracts were assayed immunologically in leaf juice 24 h later. Data are means ± sd; n = 6.
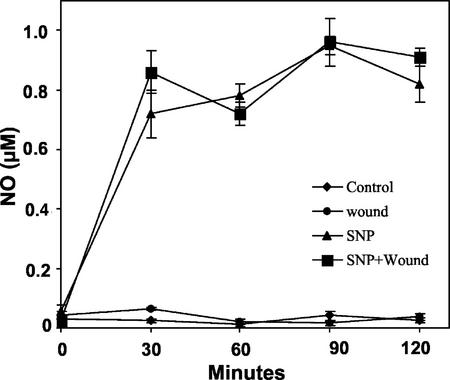
Figure 2.
Effect of SNP on NO accumulation in leaves of young excised tomato plants. The plants were supplied with phosphate buffer alone (control) or 1.0 mm SNP in buffer for 1 h. Leaf extracts of wounded and unwounded plants were assayed for NO accumulation at the times indicated. Data are means ± sd; n = 4.
Figure 3.
Inhibition of elicitor-induced accumulation of proteinase Inh I by SNP. Excised tomato plants were supplied through the stem with phosphate buffer alone (control) or with 1.0 mm SNP for 1 h and were transferred for 30 min to 25 nm systemin, 250 μg mL−1 OGA, and 100 μm JA in phosphate buffer as described in “Materials and Methods.” After each treatment, plants were incubated in water for 24 h and were then immunologically assayed for proteinase Inh I content in leaf juice. Data are means ± sd; n = 6.
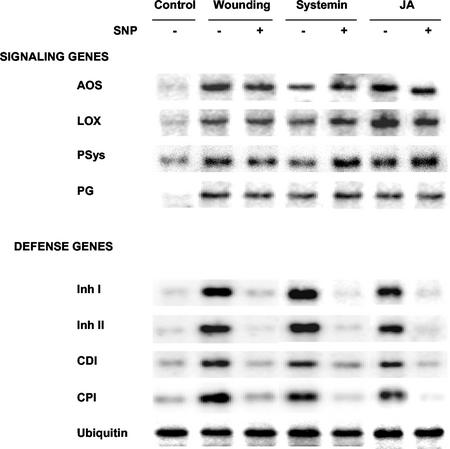
In leaves of young tomato plants, the genes that code for components of the octadecanoid signaling pathway are activated within 0.5 to 1 h after wounding (Ryan, 2000; Orozco-Cárdenas et al., 2001). This is in contrast with proteinase inhibitor genes that are activated about 4 to 12 h after wounding (Ryan, 2000; Orozco-Cárdenas et al., 2001). Gel-blot analyses were carried out to determine whether SNP inhibited the early (0.5–1 h) and/or late (4–12 h) genes induced by wounding, systemin, or JA. The early-associated signaling pathway mRNAs included prosystemin, lipoxygenase, allene oxide synthase, and polygalacturonase catalytic subunit. The late-associated mRNAs included Inh I, Inh II, cathepsin D inhibitor, and metallocarboxypeptidase inhibitor (Orozco-Cárdenas et al., 2001). The levels of mRNAs coding for the signaling pathway-related proteins induced by wounding, systemin, or JA were not inhibited by SNP, whereas levels of mRNAs encoding the defensive genes were all strongly reduced when SNP was present (Fig. 4). Therefore, SNP was not blocking the activation of wound-inducible signaling pathway genes, but was inhibiting the pathway downstream from JA.
Figure 4.
Effects of SNP on the expression of genes induced by wounding, systemin, and JA. Young excised tomato plants were supplied with phosphate buffer alone (control) or 1.0 mm SNP for 1 h. Plants, except controls, were wounded, transferred to water, and assayed by RNA gel blotting after 2 h for allene oxide synthase, lipoxygenase, prosystemin, and polygalacturonase catalytic subunit (and after 8 h for proteinase Inh), proteinase Inh II, cathepsin D inhibitor, and metallocarboxypeptidase inhibitor. Equal amounts of RNA were loaded as confirmed by probing with an ubiquitin cDNA.
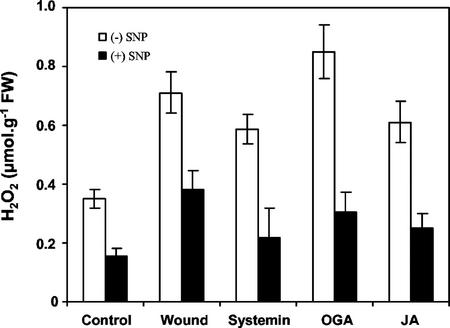
Tomato leaves had previously been shown to maximally accumulate H2O2 between 4 and 6 h following wounding, decreasing thereafter (Orozco-Cárdenas and Ryan, 1999). Here, we report the direct quantification of wound-inducible H2O2 that accumulated in wounded and unwounded leaves of young tomato plants 6 h after they had been supplied with SNP. SNP reduced the accumulation of H2O2 to less than 50% of levels caused by excision alone (unwounded control), and by wounding, systemin OGA, and JA (Fig. 5).
Figure 5.
Effect of SNP on wound- and elicitor-induced accumulation of H2O2. Young tomato plants were treated as described in Figure 3. H2O2 concentration was measured 6 h after elicitor treatment as described in “Materials and Methods.”
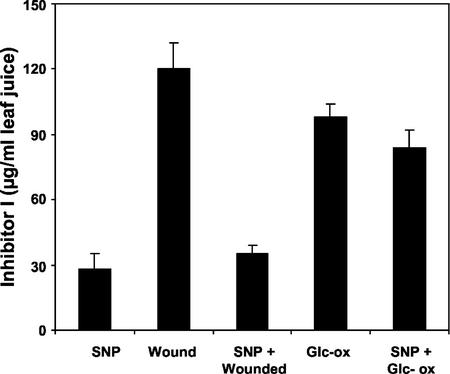
Previous research has shown that H2O2 can act as a second messenger for the expression of the late-associated defensive genes during the wound response (Orozco-Cárdenas et al., 2001). An H2O2-generating system composed of Glc oxidase plus Glc was employed to generate enough H2O2 to cause the induction and accumulation of defensive proteinase inhibitor proteins in excised tomato plants (Orozco-Cárdenas et al., 2001), and was used to investigate whether NO can inhibit H2O2-mediated synthesis of Inh I. Young excised tomato plants were supplied with SNP for 1 h and then with Glc and Glc oxidase. The results shown in Figure 6 indicate that SNP did not block the synthesis of Inh I induced by H2O2, suggesting that the site of inhibition of late genes was at a step or steps between JA and H2O2 generation.
Figure 6.
Inhibition of H2O2-mediated accumulation of proteinase Inh I by SNP. Excised tomato plants were supplied through the stem with phosphate buffer alone (control) or 1.0 mm SNP for 1 h and were transferred for 1 h to a buffer containing 50 μm Glc plus 2.5 units mL−1 Glc oxidase. Plants were then incubated in water for 24 h and were immunologically assayed for proteinase Inh I content in leaf juice. Data are means ± sd; n = 6.
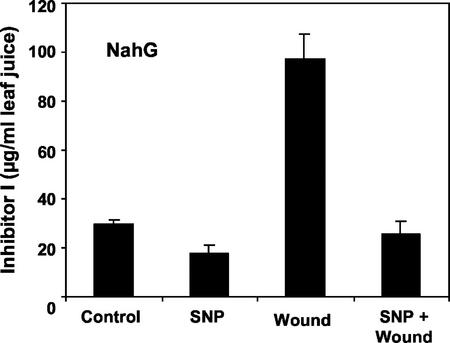
Because NO had previously been reported to induce the synthesis of SA in tobacco (Nicotiana tabacum) leaves (Durner et al., 1999; Klessig et al., 2000), the possibility that NO may be inducing the accumulation of SA was considered as a possible cause of the inhibitory action of NO on wound signaling. To evaluate whether NO is able to inhibit wound-defense gene expression in the absence of SA, NahG transgenic tomato plants overexpressing the bacterial salicylate hydroxylase enzyme, which removes endogenous SA by converting it to catechol (Brading et al., 2000), were assayed to see if SNP had the same inhibitory effect on the wound inducibility of Inh I protein in the wild-type and the transgenic NahG plants. NO had the same inhibitory effect in the NahG plants as in wild-type plants (Fig. 7). In addition, direct quantification of SA in leaves of unwounded and wounded wild-type tomato plants that were pretreated with SNP showed no differences compared with SA found in leaves of untreated plants within 8 h after SNP treatment (data not shown). It is within this time period that wound signaling takes place (Ryan, 2000), indicating that SA was likely not to have a significant effect on wound signaling.
Figure 7.
NO-mediated inhibition of wound-inducible accumulation of proteinase Inh I in SA-deficient (NahG) transgenic tomato plants. Plants were treated and assayed as described in Figure 1. Data are means ± sd; n = 6.
DISCUSSION
Pathogen-induced production of H2O2 and NO in plant cells has been shown to regulate the hypersensitive response and cell death (Delledonne et al.; 1998; Durner et al., 1998; Klessig et al., 2000). H2O2 is also generated in response to mechanical wounding, and acts as a second messenger that regulates the expression of wound-inducible defense-associated genes (Ryan, 2000; Orozco-Cárdenas et al., 2001). Whether NO has a role in the wound-inducible signaling pathway has not been assessed. Therefore, a possible role for NO in the wound-inducible signaling pathway was investigated in tomato plants.
Supplying young excised tomato plants with the NO generators SNP and SNAP before wounding caused a nearly complete inhibition of the induction of synthesis of proteinase Inh I, one of several wound-inducible proteinase inhibitor proteins in tomato leaves (Fig. 1A). The inhibition of wound-inducible Inh I by SNP was concentration dependent (Fig. 1B) and could be reversed by supplying the NO scavenger CPTIO (Fig. 1A). When wounded and unwounded excised tomato plants were supplied with 1 mm SNP, NO levels from 0.7 to 1.0 mm were detected in both set of plants within 0.5 h. The levels remained essentially constant for the next 1.5 h, and by 4 h, the levels had declined to 0.5 μm (Fig. 2). However, in the absence of the NO donors, NO levels remained unchanged in the leaves of wounded and unwounded plants, indicating that NO synthesis was not regulated by the wounding. NO inhibited Inh I synthesis in young, excised tomato plants induced by systemin, OGA, and JA (Fig. 3), indicating that the inhibition of the signaling pathway likely occurred downstream from JA synthesis.
Previous reports had demonstrated that wounding, systemin and JA all induced the expression of signal pathway genes leading to JA synthesis within 0.5 to 2 h after wounding, whereas defensive proteinase inhibitor genes were synthesized much later, from 4 to 12 h after wounding (Orozco-Cárdenas et al., 2001). Further studies indicated that JA was not only activating the early signal pathway genes, but was activating the expression of a polygalacturonase gene and the production of H2O2 (Bergey et al., 1999; Orozco-Cárdenas and Ryan, 1999), with H2O2 acting as a second messenger for proteinase inhibitor gene expression (Orozco-Cárdenas et al., 2001). Therefore, the effects of NO on the expression of early and late genes induced by wounding, systemin, and JA were examined. NO did not inhibit signal pathway gene expression, but strongly inhibited the expression of several proteinase inhibitor genes (Fig. 4). Supplying the excised tomato plants with an H2O2-generating system of Glc oxidase plus Glc is known to induce the expression of the proteinase inhibitor genes, but not the early-inducible signal pathway-associated genes (Orozco-Cárdenas et al., 2001). In this study, the levels of H2O2 detected in the leaves of plants supplied with this H2O2-generating system were not affected by the presence of SNP (data not shown), and the H2O2-induced synthesis of proteinase Inh I was reduced by only 12% (Fig. 7). H2O2 levels generated in the plants by Glc/Glc oxidase were approximately 13 μm, which is near the levels induced by wounding in the wild-type tomato plants (Orozco-Cárdenas et al., 2001). NO also inhibited the accumulation of H2O2, which occurs in planta in response to wounding or treatment with chemical elicitors, and JA (Fig. 5). Together, these results suggest that NO is inhibiting signaling downstream from JA, but before the steps that generate H2O2.
The wound signaling pathway in tomato plants was shown previously to be inhibited by SA (Doares et al., 1995), indicating that “crosstalk” between the pathogen-inducible defense signaling pathway and the herbivore (wound)-inducible defense signaling pathways can be mediated by SA (Raskin, 1992; Felton and Korth, 2000). However, SNP inhibited the wound-induced accumulation of proteinase inh I in SA-deficient tomato plants (Fig. 7), and SA levels remained unaffected in the wild-type plants during the first 8 h after wounding (data not shown). Thus, the effects of NO were likely not due to the induction of synthesis of SA.
During plant defense against pathogens, NO potentiates the hypersensitive cell death in soybean cell cultures (Delledonne et al., 1998), and inhibition of NO synthesis compromises hypersensitive disease resistance in Arabidopsis and tobacco plants (Huang and Knopp, 1997; Delledonne et al., 1998). Moreover, NO at 0.5 to 1.0 mm mediates plant defense gene activation, triggering the expression of pathogenesis-related proteins and Phe ammonia lyase, and the synthesis of protective natural products (Beligni et al., 1997; Durner et al., 1998; Klessig et al., 2000). However, in this study of the tomato wound-defense response, NO at 1.0 mm inhibited the activation of antiherbivory proteinase inhibitor genes, which is mediated by the accumulation of nonlethal levels of about 10 μm H2O2 (Orozco-Cárdenas et al., 2001). In this latter system, NO might be acting as an antioxidant agent (Laxalt et al., 1997; Beligni and Lamattina, 1999), protecting the plant cells and tissues from ROS damage. It is interesting that after 24 h, no symptoms of necrosis or hypersensitive cell death were observed in the leaves of plants treated with SNP and exposed at the same time to the H2O2-generating system of Glc plus Glc oxidase. Therefore, in addition to H2O2 and NO, SA may be required for the onset of pathogen-induced programmed cell death in tomato (for review, see Bolwell, 1999; Durner and Klessig, 1999; Beligni and Lamattina, 2001).
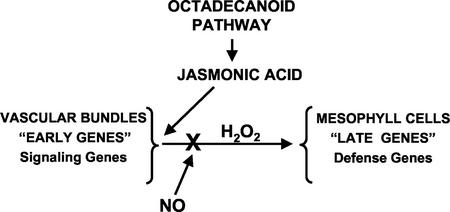
Although there is no evidence to support a specific mechanism of inhibition by NO, NO appears to be specifically inhibiting a step between JA synthesis and H2O2 production (Fig. 8). Although NO does not seem to be a component of the wound defense response in tomato plants, it might act antagonistically to inhibit the expression of antiherbivory defense genes during the plant defense response against pathogens. In this regard, it has been reported that NO can antagonize ethylene biosynthesis and action in plants (Leshem and Pinchasov, 2000). Because ethylene is required for the wound-defense response in tomato plants (O'Donnell et al., 1996), NO may be interfering with the participation of ethylene in the wound signaling pathway. However, at present, the specific molecular target for NO inhibition of wound signaling remains to be identified.
Figure 8.
Differential regulation of signal pathway genes and defensive genes in leaves of tomato plants in response to wounding and pathogens. In this model, wounding or systemin produces JA, which up-regulates the signal pathway genes (early genes). NO, produced after pathogen attacks, inhibits only the wound-inducible (late) genes.
MATERIALS AND METHODS
Plant Material and Bioassays
Two-week-old tomato (Lycopersicon esculentum cv Castlemart and Moneymaker) plants and the transgenic tomato line cv Moneymaker containing the salicylate hydroxylase (nahG) gene (Brading et al., 2000) were grown from seeds in growth chambers having 18-h days of 300 μEm−2 s−1 of light at 28°C and 6-h nights at 18°C. The plants had two expanding leaves and a small developing apical leaf when used for experimentation.
SNAP and CPTIO were from Molecular Probes (Eugene, OR). All other reagents were from Sigma.
To investigate the effects of NO on the induction of proteinase Inh I synthesis and accumulation in response to wounding and chemical elicitors, 14-d-old tomato plants were excised at the base of the stem and were supplied for 1 h with 10−3 m potassium phosphate buffer, pH 6.5, or buffer containing NO-related compounds. Thereafter, the plants were wounded across the main vein of each terminal leaflet or were incubated for another 0.5 to 1.0 h in buffer solutions alone or a buffer solution containing systemin (25 nm), OGA (250 μg mL−1), JA (100 μm), or Glc (50 μm) plus Glc oxidase (2.5 units mL−1), as previously described (Orozco-Cárdenas et al., 2001). The plants were transferred to glass vials containing water, placed within closed Plexiglas boxes, and incubated for 24 h in light (300 mEm−2 s−1) at 28°C. Levels of wound- and elicitor-inducible proteinase Inh I protein was quantified in juice expressed from the leaves by use of radial immunodiffusion assays (Ryan, 1967; Trautman et al., 1971), or the Inh I and II mRNAs were analyzed by gel-blot analyses.
RNA Gel-Blot Analyses
Leaves of treated and control tomato plants were removed and immersed in liquid nitrogen, ground to a fine powder, and stored at −80°C to isolate total RNA. Total RNA was extracted, fractionated by electrophoresis in 1.4% (w/v) agarose-formaldehyde gels, blotted onto nylon membranes, and hybridized with radioactive 32P-dCTP-labeled probes as described previously (Orozco-Cárdenas et al., 2001). An 18S ribosomal RNA gene probe was used as a loading control. Membranes were washed once with 2× SSPE for 20 min at room temperature, two to three times with 2× SSPE and 1% (w/v) SDS for 15 to 30 min at 65°C, and then exposed for 15 to 32 h to x-ray film or to a PhosphorImager (Bio-Rad, Hercules, CA).
Quantification of NO
Leaves of young excised tomato plants that had been pretreated with water or SNP for 1 h and then wounded or not wounded were assayed for NO concentration. In brief, 200 mg of frozen leaves of young tomato plants were grounded and homogenized in 1 mL of cooled buffer (0.1 m sodium acetate, 1 m NaCl, and 1% [w/v] ascorbic acid, pH 6.0). The homogenates were centrifuged at 10,000g for 20 min at 4°C and the supernatants were passed through 0.8- × 4-cm columns in 1-X8 resin (Bio-Rad). NO was quantified in cleared extracts spectrophotometrically measuring the conversion of oxyhemoglobin to methemoglobin (Murphy and Noack, 1994).
Quantification of H2O2
The quantification of H2O2 in extracts from tomato leaves was according to Rao et al. (2000). Leaves were frozen and ground to a powder under liquid nitrogen and stored at −80°C. Leaf powder (500 mg) was extracted with 1 mL of 0.2 m HClO 4, incubated on ice for 5 min, and pelleted by centrifugation at 10,000g for 10 min at 4°C. The supernatant was neutralized to pH 7.0 to 8.0 with 0.2 m NH 4OH, pH 9.5, and was briefly centrifuged at 3,000g for 2 min to sediment the insoluble material. The extracts were passed through 0.8- × 4-cm columns of AG 1X-8 resin (ionic-form chloride; Bio-Rad) and were eluted with double-distilled water (Rao et al., 2000).
The quantification of H2O2 in the cleared extracts was carried out using an Amplex Red Hydrogen Peroxide Assay kit (Molecular Probes), following the manufacturer's recommendations. In brief, 50 to 100 μL of extract was mixed with an equal volume of a solution containing 1 U mL−1 horseradish peroxidase in 50 mm sodium phosphate buffer, pH 7.4, and was incubated for 1 h at room temperature. Fluorescence was measured with a fluorescence microplate reader (Perkin-Elmer, Beaconsfield, Buckinghamshire, UK) using excitation at 560 ± 5 nm and fluorescence detection at 590 ± 5 nm. The concentration of H2O2 in each sample was calculated using a standard curve obtained with known concentrations of pure H2O2.
Quantification of SA
Leaves of young excised tomato plants that had been pretreated with water or SNP for 1 h and then wounded or not wounded were assayed for SA. SA was extracted from leaves with methanol and was quantified by using HPLC as described by (Pearce et al., 1998).
ACKNOWLEDGMENTS
We thank Sue Vogtman for growing the plants for this research, Gregory Pearce, Dr. Javier Narvaez-Vasquez, and Dr. Justin Scheer for their scientific advice and helpful discussions, and Dr. Greg Martin for the gift of NahG tomato seeds.
Footnotes
This work was supported by Project 1791 of the College of Agriculture and Home Economics and by the National Science Foundation (grant no. IBN 0090766).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.008375.
LITERATURE CITED
- Alvarez ME, Penell RI, Meijer PJ, Ishikawa A, Dixon RA, Lamb C. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell. 1998;92:773–784. doi: 10.1016/s0092-8674(00)81405-1. [DOI] [PubMed] [Google Scholar]
- Beligni MV, Lamattina L. Nitric oxide counteracts cytotoxic processes mediated by reactive oxygen species in plant tissues. Planta. 1999;208:337–344. [Google Scholar]
- Beligni MV, Lamattina L. Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light-inducible responses in plants. Planta. 2000;210:215–221. doi: 10.1007/PL00008128. [DOI] [PubMed] [Google Scholar]
- Beligni MV, Lamattina L. Nitric oxide in plants: The history is just beginning. Plant Cell Environ. 2001;24:267–278. [Google Scholar]
- Beligni MV, Laxalt A, Lamattina L. Moncada S, Toda N, Higgs EA, eds, The Biology of Nitric Oxide, Part 6. Portland Press, Kyoto. 1997. Putative role of nitric oxide in plant-pathogen interactions. [Google Scholar]
- Bergey DR, Orozco-Cárdenas ML, de Moura DS, Ryan CA. A wound- and systemic-inducible polygalacturonase in tomato leaves. Proc Natl Acad Sci USA. 1999;96:1756–1760. doi: 10.1073/pnas.96.4.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi JL, Felton GW. Foliar oxidative stress and insect herbivory: primary compounds, secondary metabolites, and reactive oxygen species as components of induced resistance. J Chem Ecol. 1995;21:1511–1530. doi: 10.1007/BF02035149. [DOI] [PubMed] [Google Scholar]
- Bolwell GP. Role of active oxygen species and NO in plant defense responses. Curr Opin Plant Biol. 1999;2:287–294. doi: 10.1016/S1369-5266(99)80051-X. [DOI] [PubMed] [Google Scholar]
- Brading PA, Hammond-Kosack KE, Parr A, Jones JDG. Salicylic acid is not required for Cf-2 and Cf-9-dependent resistant of tomato to Cladosporium fulvum. Plant J. 2000;23:305–318. doi: 10.1046/j.1365-313x.2000.00778.x. [DOI] [PubMed] [Google Scholar]
- Delledonne M, Xia Y, Dixon RA, Lamb C. Nitric oxide functions as a signal in plant disease resistance. Nature. 1998;394:585–588. doi: 10.1038/29087. [DOI] [PubMed] [Google Scholar]
- Delledonne M, Zeier J, Marocco A, Lamb C. Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA. 2001;98:13454–13459. doi: 10.1073/pnas.231178298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doares SH, Narváez-Vásquez J, Conconi A, Ryan CA. Salicylic acid inhibits synthesis of proteinase inhibitors in tomato leaves by systemin and jasmonic acid. Plant Physiol. 1995;108:1741–1746. doi: 10.1104/pp.108.4.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durner J, Klessig D. Nitric oxide as a signal in plants. Curr Opin Plant Biol. 1999;2:369–374. doi: 10.1016/s1369-5266(99)00007-2. [DOI] [PubMed] [Google Scholar]
- Durner J, Wendehenne D, Klessig D. Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc Natl Acad Sci USA. 1998;95:10328–10333. doi: 10.1073/pnas.95.17.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felton GW, Korth KL. Trade-offs between pathogen and herbivore resistance. Curr Opin Plant Biol. 2000;3:309–314. doi: 10.1016/s1369-5266(00)00086-8. [DOI] [PubMed] [Google Scholar]
- Huang JS, Knopp JA. Involvement of nitric oxide in Ralstonia-induced hypersensitive reaction in tobacco. In: Prior P, Elphinstone J, Allen C, editors. Proceedings of the Second International Wilt Symposium. Versailles, France: Institut National de la Recherche Agronomique; 1997. [Google Scholar]
- Klessig DF, Durner J, Noad R, Navarre DA, Wendehenne D, Kumar D, Zhou JM, Shah J, Zhang S, Kachroo P et al. Nitric oxide and salicylic acid signaling in plant defense. Proc Natl Acad Sci USA. 2000;97:8849–8855. doi: 10.1073/pnas.97.16.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Klessig DF. Differential induction of tobacco MAP kinase by the defense signals nitric oxide, salicylic acid, ethylene, and jasmonic acid. Mol Plant-Microbe Interact. 2000;13:347–351. doi: 10.1094/MPMI.2000.13.3.347. [DOI] [PubMed] [Google Scholar]
- Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- Laxalt A, Beligni MV, Lamattina L. Nitric oxide preserves the level of chlorophyll in potato leaves infected by Phytophora infestans. Eur J Plant Pathol. 1997;73:643–651. [Google Scholar]
- Leshem YY, Haramaty E. The characterization and contrasting effects of the nitric oxide free radical in vegetative stress and senescence of Pisum sativum Linn. Foliage J Plant Physiol. 1996;148:258–263. [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Low PS, Merida JR. The oxidative burst in plant defense: function and signal transduction. Physiol Plant. 1996;96:533–542. [Google Scholar]
- Murphy ME, Noack E. Nitric oxide assay using hemoglobin method. Methods Enzymol. 1994;233:241–250. doi: 10.1016/s0076-6879(94)33027-1. [DOI] [PubMed] [Google Scholar]
- O'Donnell PJ, Calvert C, Atzorn R, Wasternack C, Leyser HMO, Bowles DJ. Ethylene as a signal mediating the wound response of tomato plants. Science. 1996;274:1914–1917. doi: 10.1126/science.274.5294.1914. [DOI] [PubMed] [Google Scholar]
- Orozco-Cárdenas ML, Nárvaez-Vásquez J, Ryan CA. Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin and methyl jasmonate. Plant Cell. 2001;13:179–191. [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cárdenas ML, Ryan C. Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc Natl Acad Sci USA. 1999;96:6553–6557. doi: 10.1073/pnas.96.11.6553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce G, Marchand PA, Griswold J, Lewis NG, Ryan CA. Accumulation of feruloyltyramine and p-coumaroyltyramine in tomato leaves in response to wounding. Phytochemistry. 1998;47:659–664. [Google Scholar]
- Rao MV, Lee H, Creelman RA, Mullet JE, Davis KR. Jasmonic acid signaling modulates ozone-induced hypersensitive cell death. Plant Cell. 2000;12:1633–1646. doi: 10.1105/tpc.12.9.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin I. Role of salicylic acid in plants. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:439–463. [Google Scholar]
- Ryan CA. Quantitative determination of soluble cellular proteins by radial diffusion in agar gels containing antibodies. Anal Biochem. 1967;19:434–440. doi: 10.1016/0003-2697(67)90233-3. [DOI] [PubMed] [Google Scholar]
- Ryan CA. The systemin signaling pathway: differential activation of plant defensive genes. Biochim Biophys Acta. 2000;1477:112–121. doi: 10.1016/s0167-4838(99)00269-1. [DOI] [PubMed] [Google Scholar]
- Stamler JS. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell. 1994;78:931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Trautman R, Cowan KM, Wagner GG. Data processing for radial immunodiffusion. Immunochemistry. 1971;8:901–916. doi: 10.1016/0019-2791(71)90429-0. [DOI] [PubMed] [Google Scholar]