Abstract
1. A method is described for the detection and assay of picogramme quantities of noradrenaline. This involves transferring Krebs solution containing noradrenaline to a cascade system where the catecholamine may be bioassayed on superfused preparations of the rabbit aorta and iliac artery.
2. Electrical field stimulation of the rabbit vas deferens and portal vein caused the release, into the bathing medium, of a material which was identified by pharmacological and chemical tests as noradrenaline.
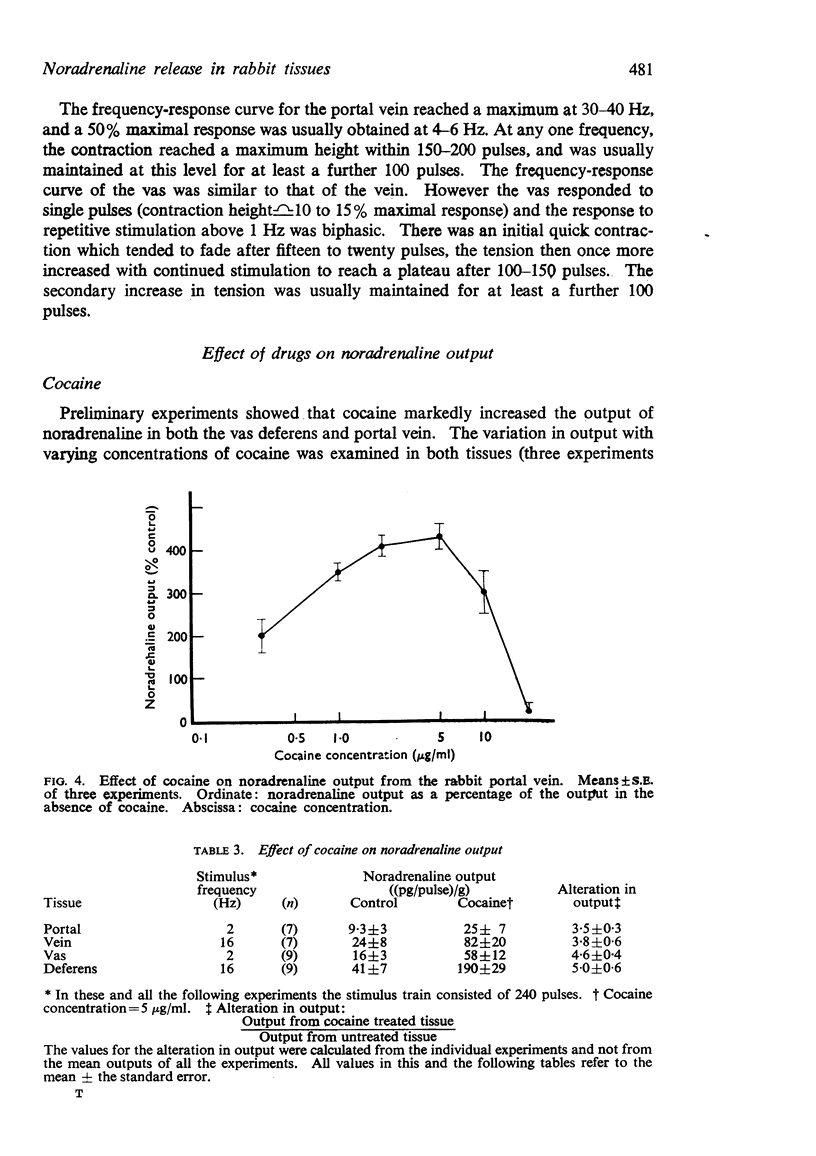
3. Cocaine (0·3-5 μg/ml) caused a marked increase in noradrenaline output after electrical stimulation of the portal vein and vas deferens. This effect appeared to be maximal at a concentration of 2·4 μg/ml; when the cocaine concentration was increased above 10 μg/ml the noradrenaline output was greatly reduced.
4. Phenoxybenzamine (5 μg/ml) caused a 4-8 times greater increase in noradrenaline output than cocaine; however, the increase in output due to phenoxybenzamine was much smaller in tissues pretreated with cocaine.
5. Corticosterone (20 μg/ml) increased noradrenaline output by 30-40% in untreated vas deferentia, but caused a 300% increase in output in tissues pretreated with cocaine. Cocaine also caused a much greater increase in output in tissues pretreated with corticosterone than in untreated tissues.
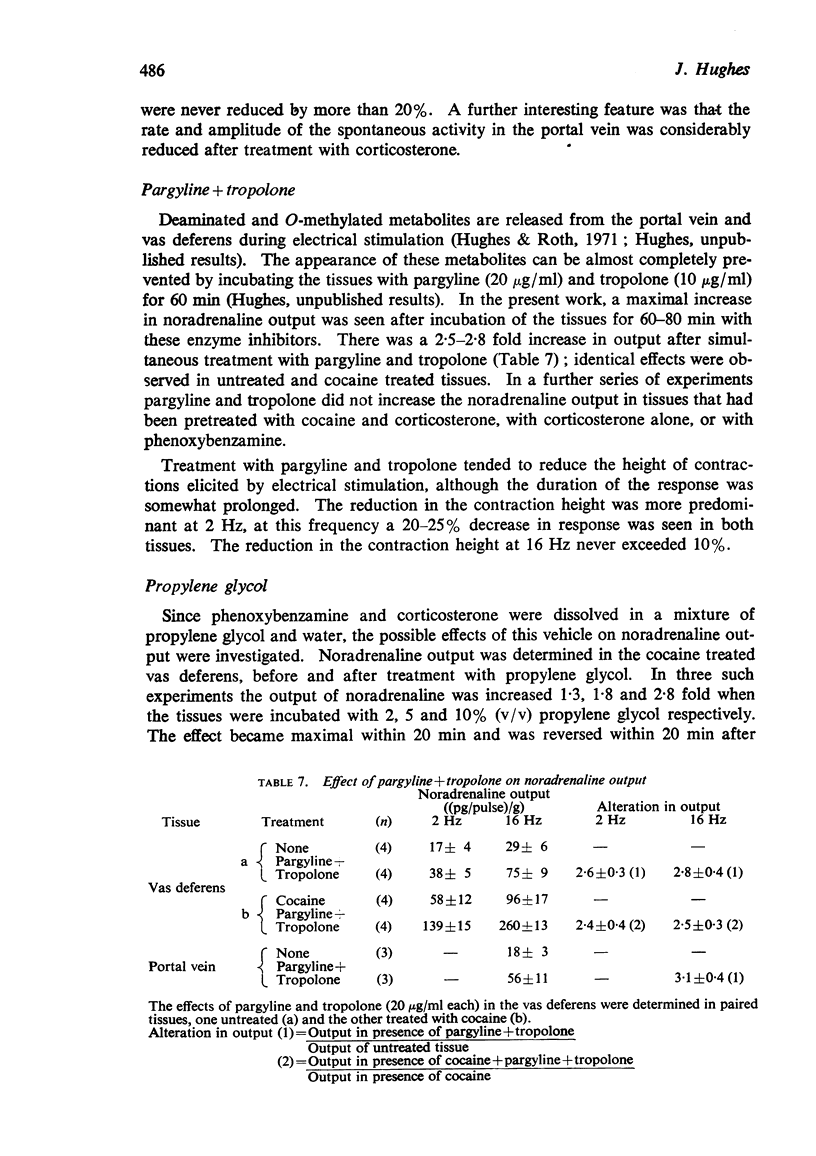
6. Treatment with pargyline plus tropolone caused a 100-200% increase in noradrenaline output; this effect was not modified by cocaine, but was abolished when the tissues were pretreated with either phenoxybenzamine or corticosterone.
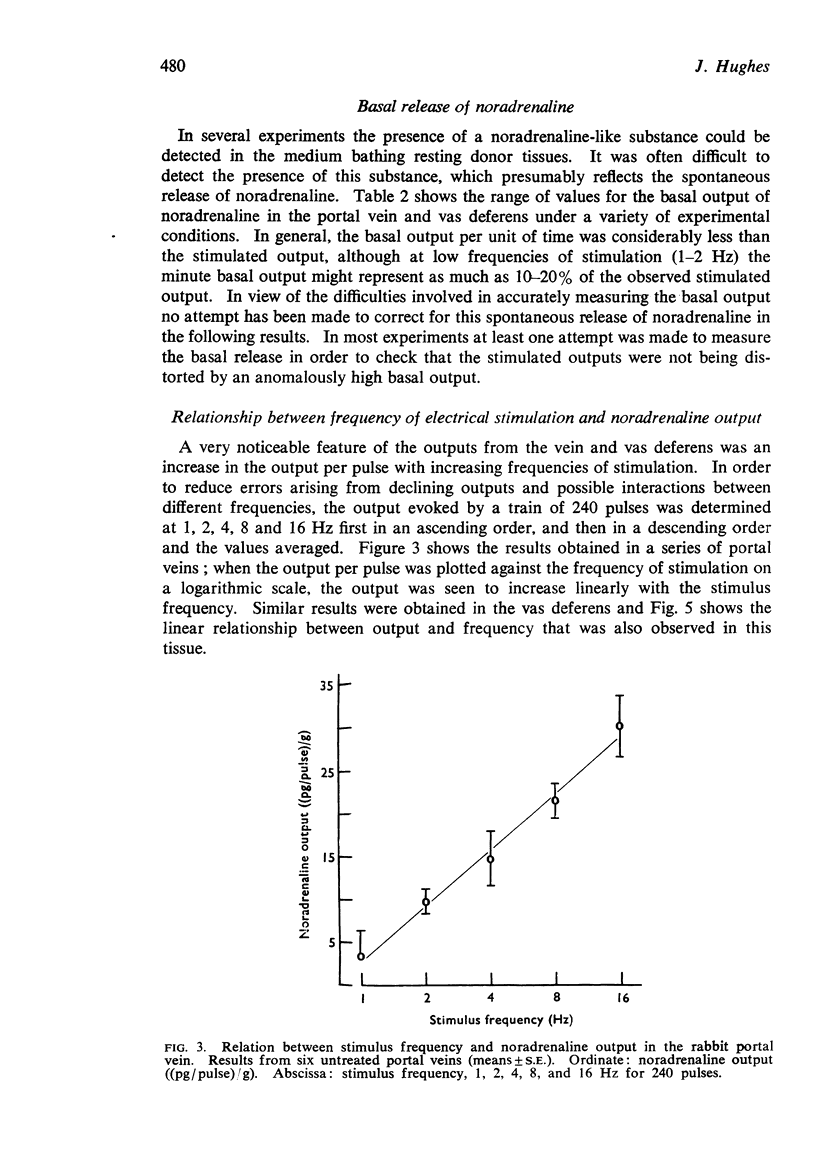
7. When tissues were stimulated for 240 pulses at 1-16 Hz, the output per pulse of noradrenaline increased linearly with the logarithm of the frequency of stimulation. This relationship between frequency and output was seen in both untreated tissues, and in tissues treated with cocaine, phenoxybenzamine, corticosterone or pargyline plus tropolone.
8. It is concluded that cocaine enhances output by blocking the neuronal reuptake of noradrenaline, and corticosterone by blocking the extraneuronal uptake and subsequent metabolism of noradrenaline. Phenoxybenzamine acts by blocking both neuronal and extraneuronal uptake mechanisms. There appears to be a dynamic balance in the distribution of noradrenaline between the two uptake mechanisms after the release of the transmission from the nerve endings.
9. It is calculated that more than 90% of the noradrenaline released by nerve stimulation (240 pulses at 2-16 Hz) is inactivated by neuronal and extra neuronal uptake mechanisms.
10. It is calculated that the fraction of the total noradrenaline store that is released by one pulse at 2 Hz is 6·6 × 10-5 in the portal vein and 5·6 × 10-5 in the vas deferens; the corresponding values at 16 Hz were 15·9 × 10-5 and 16·2 × 10-5.
Full text
PDF

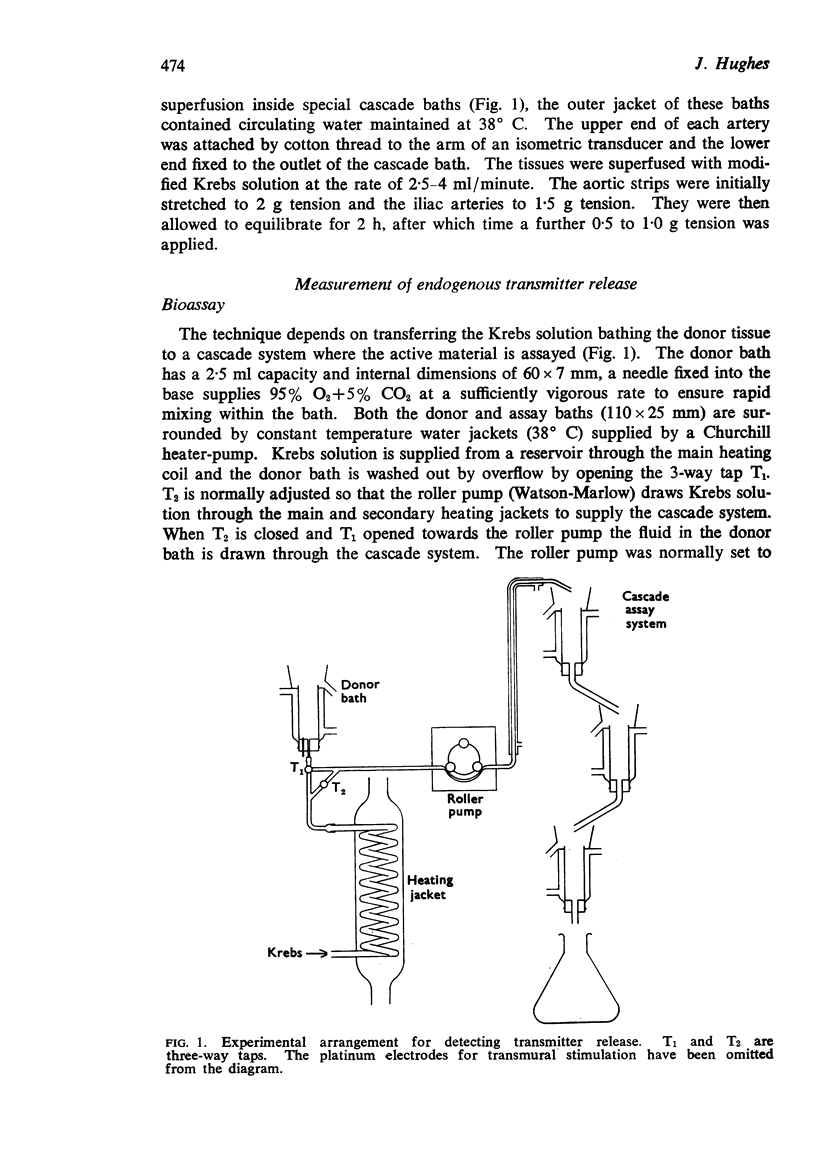













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avakian O. V., Gillespie J. S. Uptake of noradrenaline by adrenergic nerves, smooth muscle and connective tissue in isolated perfused arteries and its correlation with the vasoconstrictor response. Br J Pharmacol Chemother. 1968 Jan;32(1):168–184. doi: 10.1111/j.1476-5381.1968.tb00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN G. L., GILLESPIE J. S. The output of sympathetic transmitter from the spleen of the cat. J Physiol. 1957 Aug 29;138(1):81–102. doi: 10.1113/jphysiol.1957.sp005839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagat B., Zeidman H. Increased retention of norepinephrine-3H in vas deferens during nerve stimulation. Am J Physiol. 1970 Sep;219(3):691–696. doi: 10.1152/ajplegacy.1970.219.3.691. [DOI] [PubMed] [Google Scholar]
- Boadle-Biber M. C., Hughes J., Roth R. H. Acceleration of noradrenaline biosynthesis in the guinea-pig vas deferens by potassium. Br J Pharmacol. 1970 Dec;40(4):702–720. doi: 10.1111/j.1476-5381.1970.tb10648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAIRNIE A. B., KOSTERLITZ H. W., TAYLOR D. W. Effect of morphine on some sympathetically innervated effectors. Br J Pharmacol Chemother. 1961 Dec;17:539–551. doi: 10.1111/j.1476-5381.1961.tb01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies B. N., Withrington P. G. The release of noradrenaline by the sympathetic post-ganglionic nerves to the spleen of the cat in response to low frequency stimulation. Arch Int Pharmacodyn Ther. 1968 Jan;171(1):185–197. [PubMed] [Google Scholar]
- Folkow B., Häggendal J., Lisander B. Extent of release and elimination of noradrenaline at peripheral adrenergic nerve terminals. Acta Physiol Scand Suppl. 1967;307:1–38. [PubMed] [Google Scholar]
- Gillis C. N., Schneider F. H. Frequency-dependent potentiation by various drugs of the chronotropic response of isolated cat atria to sympathetic nerve stimulation. Br J Pharmacol Chemother. 1967 Aug;30(3):541–553. doi: 10.1111/j.1476-5381.1967.tb02160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis C. N., Schneider F. H., Van Orden L. S., Giarman N. J. Biochemical and microfluorometric studies of norepinephrine redistribution accompanying sympathetic nerve stimulation. J Pharmacol Exp Ther. 1966 Jan;151(1):46–54. [PubMed] [Google Scholar]
- HAEFELY W., HUERLIMANN A., THOENEN H. A QUANTITATIVE STUDY OF THE EFFECT OF COCAINE ON THE RESPONSE OF THE CAT NICTITATING MEMBRANE TO NERVE STIMULATION AND TO INJECTED NORADRENALINE. Br J Pharmacol Chemother. 1964 Feb;22:5–21. doi: 10.1111/j.1476-5381.1964.tb01539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haefely W., Hürlimann A., Thoenen H. Relation between the rate of stimulation and the quantity of noradrenaline liberated from sympathetic nerve endings in the isolated perfused spleen of the cat. J Physiol. 1965 Nov;181(1):48–58. doi: 10.1113/jphysiol.1965.sp007744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman M. E., Kasby C. B., Suthers M. B., Wilson J. A. Some properties of the smooth muscle of rabbit portal vein. J Physiol. 1968 May;196(1):111–132. doi: 10.1113/jphysiol.1968.sp008498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J., Roth R. H. Evidence that angiotensin enhances transmitter release during sympathetic nerve stimulation. Br J Pharmacol. 1971 Feb;41(2):239–255. doi: 10.1111/j.1476-5381.1971.tb08025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häggendal J., Johansson B., Jonason J., Ljung B. Correlation between noradrenaline release and effector response to nerve stimulation in rat portal vein in vitro. Acta Physiol Scand Suppl. 1970;349:17–32. [PubMed] [Google Scholar]
- Iversen L. L., Fischer J. E., Axelrod J. Enhancement of H-3-norepinephrine uptake in rat tissues by O-methylated metabolites of catecholamines. J Pharmacol Exp Ther. 1966 Oct;154(1):56–63. [PubMed] [Google Scholar]
- Iversen L. L. Role of transmitter uptake mechanisms in synaptic neurotransmission. Br J Pharmacol. 1971 Apr;41(4):571–591. doi: 10.1111/j.1476-5381.1971.tb07066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. L., Salt P. J. Inhibition of catecholamine Uptake-2 by steroids in the isolated rat heart. Br J Pharmacol. 1970 Nov;40(3):528–530. doi: 10.1111/j.1476-5381.1970.tb10637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Misu Y. Release of noradrenaline by splenic nerve stimulation and its dependence on calcium. J Physiol. 1967 Jan;188(2):219–234. doi: 10.1113/jphysiol.1967.sp008135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer S. Z. The metabolism of (3H)noradrenaline released by electrical stimulation from the isolated nictitating membrane of the cat and from the vas deferens of the rat. J Physiol. 1970 Jul;208(3):515–546. doi: 10.1113/jphysiol.1970.sp009135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer S. Z., Vogt M. Noradrenaline release from isolated muscles of the nictitating membrane of the cat. J Physiol. 1971 Apr;214(1):159–171. doi: 10.1113/jphysiol.1971.sp009425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightman S. L., Iversen L. L. The role of uptake2 in the extraneuronal metabolism of catecholamines in the isolated rat heart. Br J Pharmacol. 1969 Nov;37(3):638–649. doi: 10.1111/j.1476-5381.1969.tb08502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hanlon J. F., Jr, Campuzano H. C., Horvath S. M. A fluorometric assay for subnanogram concentrations of adrenaline and noradrenaline in plasma. Anal Biochem. 1970 Apr;34(2):568–581. doi: 10.1016/0003-2697(70)90142-9. [DOI] [PubMed] [Google Scholar]
- Stjärne L., Hedqvist P., Bygdeman S. Neurotransmitter quantum released from sympathetic nerves in cat's skeletal muscle. Life Sci. 1969 Feb 1;8(3):189–196. doi: 10.1016/0024-3205(69)90093-9. [DOI] [PubMed] [Google Scholar]


