Abstract
The function of hydrogen peroxide (H2O2) as a signal molecule regulating gene expression and cell death induced by external stresses was studied in birch (Betula pendula). Ozone (O3), Pseudomonas syringae pv syringae (Pss), and wounding all induced cell death of various extents in birch leaves. This was temporally preceded and closely accompanied by H2O2 accumulation at, and especially surrounding, the lesion sites. O3 and Pss, along with an artificial H2O2 producing system glucose (Glc)/Glc oxidase, elicited elevated mRNA levels corresponding to genes encoding reactive oxygen species detoxifying enzymes, Pal, Ypr10, and mitochondrial phosphate translocator 1. In addition to the regulation of gene expression, Glc/Glc oxidase also induced endogenous H2O2 production in birch leaves, accompanied by cell death that resembled O3 and Pss damage. Wound-induced gene expression differed from that induced by O3 and Pss. Thus, it appears that at least two separate defense pathways can be activated in birch leaves by stress factors, even though the early H2O2 accumulation response is common among them all.
Reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), superoxide (O2−•), hydroxyl radicals (OH−), and singlet oxygen are involved as early messenger molecules in signaling cascades activated by several external and developmental stimuli (Lamb and Dixon, 1997; Bolwell, 1999). H2O2 serves as a signal molecule under various abiotic stresses (Chamnongpol et al., 1998), in acclimation to photooxidative stress (Karpinski et al., 1999), and in plant-pathogen interactions (Levine et al., 1994). In plant-pathogen interactions ROS are centrally involved in the induction of pathogen defense genes, such as genes encoding pathogenesis-related (PR) proteins, genes regulating accumulation of phenylpropanoid compounds, and genes encoding ROS detoxifying enzymes (Levine et al., 1994; Mittler et al., 1999; Schenk et al., 2000). In addition, extracellular ROS formation, the oxidative burst, has a predominant role in the regulation and execution of the hypersensitive cell death program (Bolwell and Wojtaszek, 1997).
Ozone (O3) is a gaseous air pollutant that enters plant leaves through stomata. It degrades immediately (Laisk et al., 1989) in the cell wall forming ROS (Langebartels et al., 2002). These ROS can react further with the components of the cell wall and plasma membrane (Kangasjärvi et al., 1994). It has previously been thought that these degradation products directly affect the loss of membrane integrity and thus are responsible for the subsequent cell death, manifested as necrotic lesions. However, it has been shown in several recent studies that, in addition to this short-lived primary radical attack (Heath and Taylor, 1997), O3 induces an active ROS production in the cells affected (Schraudner et al., 1998; Pellinen et al., 1999; Rao and Davis, 1999; Overmyer et al., 2000), similar to the oxidative burst that is elicited in plant tissues by incompatible pathogens (Bestwick et al., 1997). O3-exposed plants display similar responses as those that take place during the hypersensitive response (HR) to pathogens: cell death and activation of several pathogen defense-related genes. This seems to relate to the signaling-function of the apoplastic oxygen radical formation in the tissues affected (Kangasjärvi et al., 1994; Sandermann, 1996; Rao and Davis, 2001). Thus, O3 is an ideal tool to study the function and role of extracellular ROS in regulating plant responses and cell death (Rao et al., 2000a; Rao and Davis, 2001; Langebartels et al., 2002).
The activation of phenylpropanoid pathway and PR genes as a response to a wide diversity of stress-factors has lead to their use as genetic markers for the induction of plant defense responses. PAL catalyzes the first step in phenylpropanoid biosynthetic pathway (Jones, 1984). Derivatives of the pathway have an important role in several aspects of plant growth and development and possess significant function in the inducible plant defenses against both biotic and abiotic stresses (Hahlbrock and Scheel, 1989).
PR proteins sharing sequence similarity with the parsley (Petroselinum crispum) PR1 are classified as PR-10 (van Loon et al., 1994). Birch (Betula pendula) Ypr10 genes encode proteins of the PR-10 class that are structurally, but most probably not functionally, similar to the major birch pollen allergens. Even though the birch pollen allergens have been proposed to possess ribonuclease activity (Bufe et al., 1996; Swoboda et al., 1996), no actual role or function or RNase activity has been shown for the PR-10 class of PR proteins (Draper, 1997). Unlike the pollen allergen genes, birch genes encoding proteins of the PR-10 family are induced by fungal elicitors (Swoboda et al., 1995), O3 (Pääkkönen et al., 1998), and copper (Utriainen et al., 1998), all treatments known to induce ROS production in the tissues affected.
Because of their involvement in regulating ROS accumulation, expression of genes encoding antioxidant enzymes has been studied in plants as a response to environmental factors causing oxidative stress (Schenk et al., 2000). Ascorbate peroxidase (Apx), copper/zinc superoxide dismutase (Cu/ZnSod), and catalase (Cat) delimit excess ROS accumulation, and glutathione-S-transferases (Gst) detoxify hydrophobic oxidation products when plants have been challenged with various biotic (Schenk et al., 2000) or abiotic stresses (Sharma and Davis, 1994; Willekens et al., 1994; Örvar et al., 1997). Gst and Apx transcript levels increased even as a response to ROS as such (Desikan et al., 1998; Mittler et al., 1999), suggesting that their regulation is directly ROS dependent. Thus, they are good indicators for the action of ROS as signal molecules eliciting direct plant responses during oxidative stress.
We have previously shown that O3 induces regulated cell death and several genes of diverse functions in tomato (Lycopersicon esculentum; Tuomainen et al., 1997), Arabidopsis (Overmyer et al., 2000), and birch. In birch, for example, Pal, Ypr10 (Tuomainen et al., 1996; Pääkkönen et al., 1998), and mitochondrial phosphate translocator 1 (Mpt1; Kiiskinen et al., 1997), a transmembrane protein of the inner mitochondrial membrane that imports inorganic phosphate for ATP synthesis, are activated by O3. O3 triggers also an active ROS production that continues in the absence of O3 in birch (Pellinen et al., 1999), Arabidopsis (Overmyer et al., 2000), and tobacco (Nicotiana tabacum; Schraudner et al., 1998). Here, we have examined the role of H2O2 as a connection between O3 and subsequent defense gene activation and cell death in birch. We show that both O3 and H2O2 induce further H2O2 accumulation and necrotic lesion formation within birch leaf tissue preferably around the growing lesion. A similar pattern of H2O2 accumulation and cell death was also seen in non-host pathogen-infected and wounded leaf tissues. We also show that these stress factors, including in planta H2O2 production, induce expression of stress-related genes, placing H2O2 as one of the earliest factors involved in the transcriptional activation of defense-related genes in birch.
RESULTS
O3 and Pseudomonas syringae pv syringae (Pss) Induce Rapid Cell Death and H2O2 Accumulation, But No O2−• Accumulation in Birch Leaves
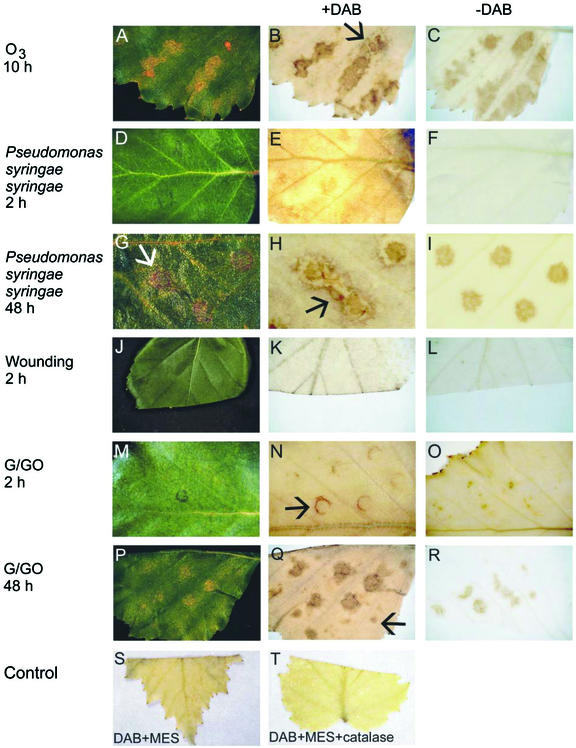
Exposure of 4-week-old birch leaves to 150 nL L−1 O3 for 8 h caused typical (Tuomainen et al., 1996) O3 damage (Fig. 1A), first visible at 10 h. H2O2 accumulation, detected by 3′3-diaminobenzidine (DAB) staining, showed strong spatial correlation with lesion formation and was visible as dark-brown coloration only at and in near vicinity of the lesions (Fig. 1B), which were apparent in cleared leaves as light-brown distinct areas (Fig. 1C). During the progression of lesion formation, H2O2 was visible also in areas that did not show detectable damage (Fig. 1B, arrow).
Figure 1.
Lesion development and H2O2 accumulation in birch leaves. A, Birch leaf with lesions 10 h after the beginning of an 8-h ozone (150 nL L−1) exposure. B, DAB staining reveals H2O2 accumulation at and around lesion sites (arrow) in a cleared leaf 10 h after the beginning of the O3 exposure. C, A leaf treated similarly as in B but without DAB shows the location and shape of the necrotic lesions. D, Pss-infiltrated leaf at 2 h after the infiltration, showing no dead necrotic lesions. E, DAB-stained and -cleared Pss-infiltrated leaf at 2 h shows H2O2 accumulation at the infiltration sites. F, A leaf treated similarly as in E, but without inclusion of DAB. G, Pss-infiltrated leaf at 48 h after the infiltration showing dead necrotic lesions at injection sites (arrow). H, DAB-stained and -cleared Pss-infiltrated leaf at 48 h shows H2O2 accumulation around lesions (arrow). I, A leaf treated similarly as in H, but without inclusion of DAB. J, Wounded leaf at 2 h. K, DAB staining shows H2O2 accumulation at the wound surface 2 h after wounding in a cleared leaf. L, A leaf treated similarly as in K, but without inclusion of DAB. M, G/GO-treated leaf at 2 h. N, DAB-stained G/GO-treated leaf at 2 h showing H2O2 accumulation surrounding injection site (arrow). O, A leaf at 2 h, treated similarly as in N, but without inclusion of DAB. P, G/GO-treated leaf at 48 h showing lesions resembling those caused by O3 treatment (Fig. 1A). Q, DAB-stained G/GO-treated leaf at 48 h showing H2O2 accumulation around lesions and in sites not showing visible damage (arrow). R, A leaf at 48 h treated similarly as in Q, but without inclusion of DAB. S, DAB staining shows H2O2 accumulation at the wound surface 2 h after wounding in a cleared leaf. T, A leaf treated similarly as in K and S, but with inclusion of CAT in the infiltration buffer. Removal of H2O2 by CAT indicates the specificity of the DAB staining for H2O2.
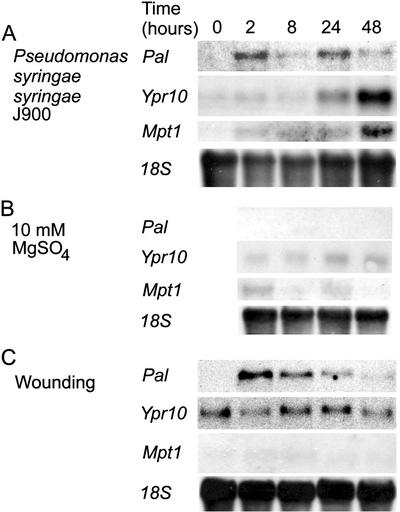
From the three different Pss strains, J900, R32, Cit7, and one Pseudomonas fluorescens strain used, infiltration with Pss J900 caused rapid development of dark-brown HR lesions at 24 h (data not shown). H2O2 accumulation was evident at infiltration sites already 2 h after the infiltration (Fig. 1E), when at the same time no cell death was visible either in the intact (Fig. 1D) or cleared (Fig. 1F) leaf, thus, the H2O2 accumulation clearly preceded the later-occurring cell death. By 48 h, lesions in Pss-infiltrated leaves had reached a state where all the cells within the infiltrated area were dead, appearing as dried brown lesions (Fig. 1G, arrow). H2O2 accumulation, visualized with DAB staining, was still detectable surrounding the already necrotic areas (Fig. 1, compare H, arrow, and I). Mock infiltration with MgSO4 did not cause any wide spread cell death or H2O2 accumulation (not shown). H2O2 production was also induced 2 h after wounding at the wound site (Fig. 1, K and S, cut edge of the leaf) but did not occur at later time points (data not shown).
To elucidate the mechanistic connection between cell death and H2O2 accumulation induced by O3, pathogen challenge, and mechanical injury, we infiltrated birch leaves with an artificial H2O2 generating system Glc/Glc oxidase (G/GO). H2O2 accumulation from G/GO around the edges of injection sites at 2 h was clearly visualized by DAB staining (Fig. 1N, arrow) when no cell death was yet visible (Fig. 1, M and O). By 48 h, the infiltrated areas turned necrotic (Fig. 1, P and R). Furthermore, there was still H2O2 accumulation detectable in the G/GO-infiltrated leaves within and especially around the lesions (Fig. 1Q). Because H2O2 production by G/GO lasted only about 4 to 7 h in test tube, the H2O2 accumulation visible in the infiltrated leaves at the later time points must thus represent the active H2O2 production by the leaf cells (Pellinen et al., 1999), which was induced by the G/GO-produced H2O2. Furthermore, H2O2 accumulation and cell death had spread from the G/GO-injected areas to the adjoining leaf tissue and surrounding the lesions in a spot like manner (Fig. 1Q, arrow). No lesion formation or H2O2 accumulation was detected at 48 h in leaves injected with Glc (G) alone (data not shown). Glc oxidase (GO) alone caused minor, hardly detectable damage on the leaves, along with weak DAB staining (data not shown). It is probable that small amounts of H2O2 are also formed in reactions between the endogenous Glc and the GO injected. The specificity of the DAB staining for H2O2 is demonstrated by co-infiltration of DAB with CAT, which prevented the DAB precipitation at the wounded surface (Fig. 1, compare S and T).
All the leaf samples described above were also stained for superoxide accumulation with the nitroblue tetrazolium (NBT) staining. No NBT precipitation was detected in any of the leaves (not shown), when O3-exposed Arabidopsis mutant rcd1 (Overmyer et al., 2000) leaves stained in the same tubes as a positive control showed clear NBT precipitation as a result of O2−• accumulation.
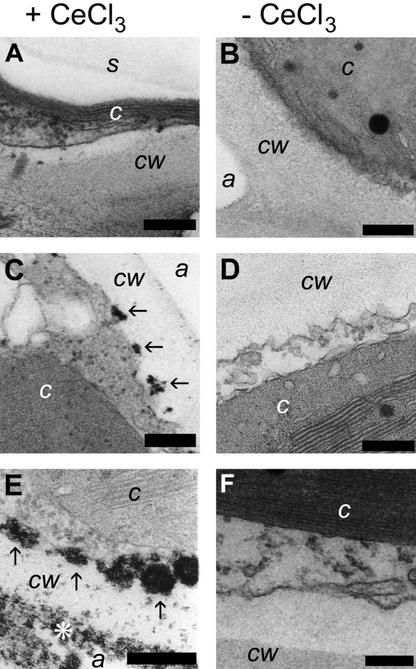
Because the sensitivity of DAB staining in birch during the early time points (2 h) is not good enough to reliably detect H2O2 accumulation, a more sensitive detection method, staining of hydrogen peroxide with CeCl3 and detection with transmission electron microscopy (Bestwick et al., 1997; Pellinen et al., 1999), was used to confirm that already 2 h after the beginning of ozone exposure there was endogenous H2O2 accumulation (Fig. 2). Before the beginning of the exposure, no H2O2 accumulation was visible (Fig. 2A), whereas 2 h after the beginning of the treatment (Fig. 2C, arrows), there was clearly endogenous H2O2 production at the surface of the plasma membrane, which had increased considerably by 10 h (Fig. 2E) and took place in both the surface of plasma membrane (arrows in Fig. 2E) and in and the surface of the cell wall (asterisk). The difference in the magnitude between 2 h (Figs. 1E and 2C) and 10 h (Figs. 1B and 2E) lie between the clear detection limit of H2O2 accumulation with DAB staining in birch. The specificity of the CeCl3 staining has been shown before (Pellinen et al., 1999) and is also illustrated in Figure 2, B, D, and F, which do not show any dark precipitates that might be a result of fixation or staining artifacts. Thus, as a conclusion, endogenous H2O2 accumulation induced by the treatments is clearly visible before the onset of visible cell death in the experimental material.
Figure 2.
Subcellular localization of O3-induced H2O2 accumulation in birch leaf cells with CeCl3 staining and TEM. H2O2 precipitates CeCl3 forming electron-dense cerium perhydroxide, visible as black spots. Individual precipitates are indicated by arrows in C and E. Samples were exposed to 150 nL L−1 of O3 for 8 h and kept in clean air for 15 min before infiltration with CeCl3 essentially to visualize only H2O2 that is produced by the plant cells. Samples were collected before the beginning of treatment (A and B), 2 h after the beginning of the treatment (C and D), and 10 h after the beginning of the treatment (E and F). Samples are shown both with (+CeCl3; A, C, and E) and without (−CeCl3; B, D, and F) cerium chloride staining. cw, Cell wall; c, chloroplast; s, starch grain in chloroplast; a, (intercellular) air space. Scale bar = 200 nm.
It can be concluded that exogenously introduced H2O2 was sufficient to induce endogenous H2O2 production and subsequent cell death in the leaves. Furthermore, the endogenous H2O2 accumulation, without visible superoxide accumulation, was both necessary and sufficient for inducing cell death in birch leaves.
Gene Expression in O3-Exposed Leaves
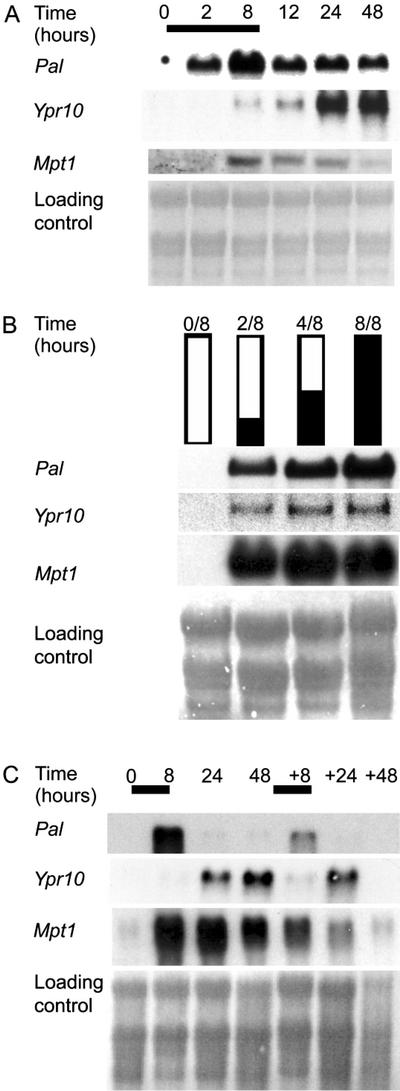
A variety of different genes are induced in plants by O3 (Kangasjärvi et al., 1994; Langebartels et al., 2002). However, the regulatory circuitries responsible for the gene induction in O3-exposed plants are still mostly unidentified. We have earlier demonstrated that birch Mpt1, Pal, and Ypr10 were induced by O3 (Tuomainen et al., 1996; Kiiskinen et al., 1997; Pääkkönen et al., 1998). To elucidate the regulatory circuitries, and the role of H2O2 in the induction of Pal, Mpt1, and Ypr10 by O3 in a similar way as has previously been shown for the birch Mpt1 (Kiiskinen et al., 1997), transcript levels were first analyzed with RNA gel-blot analysis from O3-stressed birch leaves (Fig. 3A). mRNAs corresponding to the genes assayed were not detectable in the clean air-grown leaves (0 h). In O3-exposed plants, Pal mRNA levels increased at 2 h, and highest level was seen at 8 h. Mpt1 mRNA accumulation was slower than Pal; mRNA accumulation was clearly visible at 8 h and elevated, but decreasing levels were seen up to 48 h. O3 exposure also induced the accumulation of Ypr10 transcripts in the treated leaves. mRNA levels of Ypr10 began to increase at 8 h, continuing up to 48 h.
Figure 3.
O3 induction of birch Pal, Mpt1, and Ypr10 at the transcriptional level. A, Plants were exposed to 150 nL L−1 O3 for 8 h, leaves were collected at the indicated time points after the beginning of the exposure, and transcript steady-state levels of Pal, Mpt1, and Ypr10 were determined. Bar represents O3 exposure. B, To determine the duration of O3 exposure required for changes in mRNA levels at 8 h, plants were exposed to 0, 2, 4, or 8 h of O3 followed by 8, 6, 4, or 0 h of clean air, respectively (0/8, 2/6, 4/4, and 8/0), after which leaf samples were collected and transcript steady-state levels were determined. Black and white bars represent the duration of O3 exposure and clean air, respectively. C, To study the re-inducibility of the genes, plants were exposed to two O3 peaks, the second one taking place 48 h after the beginning of the first one, and samples were collected at indicated time points. Bars represent the duration of O3 exposure. Methylene blue-stained RNA is shown as loading control.
Previous experiments with birch, parsley, pine, Arabidopsis, and tobacco have shown that pathogen defense-related genes are induced by a 5- to 10-h O3 exposure (Langebartels et al., 2002). These experiments have not, however, shown whether continuous stimulation of the cells by O3 is required for the increase in mRNA levels or whether only a short O3 pulse is sufficient to trigger an increase in transcript levels that continues without further presence of external stimulus. Birch Mpt1 gene was induced by a short, 2-h O3 exposure (Kiiskinen et al., 1997). The time of O3 exposure required for changes in Pal, Mpt1, and Ypr10 transcript levels was elucidated similarly as described previously (Kiiskinen et al., 1997) by exposing birch to clean air, O3 for 2 or 4 h followed by clean air or 8 h of O3. Leaf samples from all treatments were collected at 8 h (Fig. 3B). Two hours of O3 was sufficient to induce all three genes in such way that mRNA levels were elevated at 8 h, whereas the strongest induction was seen with an 8-h exposure (Fig. 3B). To study whether the genes are responsive to repeated O3 exposures, birch saplings were exposed to a second 8-h O3 peak 48 h after the beginning of the first one. Pal was, again, induced 8 h after the second O3 peak (Fig. 3C), but mRNA levels were lower this time. Ypr10 was also induced by the second O3 peak (Fig. 3C), and the increase in mRNA levels was as high as after the first peak. However, Ypr10 transcript levels did not remain elevated for a prolonged time, because no mRNA hybridizing with the Ypr10 probe was present at 48+48 h. As in Kiiskinen et al. (1997), only the first of two consecutive O3 exposures induced birch Mpt1. Hence, it can be concluded that Mpt1 is most probably under different regulation than Pal and Ypr10, which are responsive to consecutive oxidative attacks within 48 h.
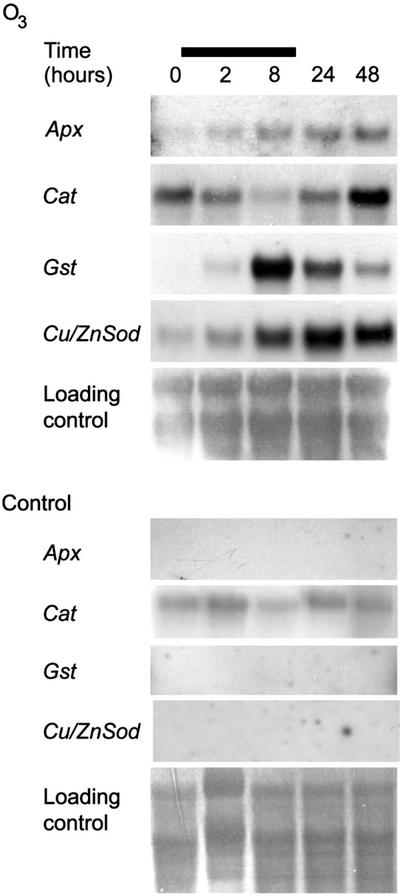
Expression of genes encoding antioxidant enzymes is a useful marker for detecting the presence of ROS in the tissue. We have earlier shown that enzyme activities of ROS detoxifying enzymes increased in O3-exposed birch clones (Tuomainen et al., 1996). Expression of genes encoding ROS detoxifying enzymes was studied here in O3-exposed birch leaves. Figure 4 shows that the transcript levels of Apx (AJ279686) and CuZnSod (AJ279694) increased slightly after the beginning of the treatment and kept increasing further after the O3 exposure. The Gst (AJ279691) mRNA level showed a significant increase as a response to O3, but after peaking at 8 h, the mRNA level returned almost to the level seen at 2 h by the end of the experiment. Cat (AJ302710), which is similar to the Cat1 of Arabidopsis CATs, on the other hand, was not affected by O3. Its mRNA levels decreased during the afternoon according to the circadian rhythm and at 24 h were at the same level as before the beginning of the exposure. However, at 48 h when the lesioned tissue had turned necrotic and the spreading of the lesions had ceased, Cat transcript levels were slightly elevated when compared with 0 and 24 h levels. It can be concluded that O3 exposure caused in birch ROS formation and oxidative stress that continued past the O3 exposure.
Figure 4.
O3 induces birch Apx, Cat, Cu/ZnSod, and Gst at the transcriptional level. Saplings were exposed to 150 nL L−1 O3 for 8 h, and samples were collected at 0, 2, 8, 24, and 48 h. Bar represents O3 exposure. Methylene blue-stained RNA is shown as loading control.
Gene Expression upon Pathogen Attack and Wounding
Infiltration of birch leaves with the Pss strain J900 induced Pal, Mpt1, and Ypr10 with different temporal patterns (Fig. 5). Pss J900 induced a rapid increase in Pal transcript levels at 2 h, after which the mRNA levels decreased, followed by an increase again at 24 h (Fig. 5A). Ypr10 transcript levels began to increase at 24 h and continued until 48 h, when increase in Mpt1 mRNA level was already visible at 2 h in pathogen-treated leaves, with a maximum at 48 h (Fig. 5A). Mock-injection with 10 mm MgSO4 did not affect mRNA levels of Pal or Ypr10, but slightly elevated the Mpt1 transcript level at 2 and 24 h (Fig. 5B).
Figure 5.
Pss and wound induction of gene expression in birch leaves. A, Leaves were infiltrated with an avirulent Pss strain J900 and collected at indicated time points, and the transcript accumulation of Pal, Ypr10, and Mpt1 was determined. B, Control infiltration with 10 mm MgSO4 had no effect. C, Leaves were wounded throughout the leaf area and leaves were collected at indicated time points. Pal mRNA levels, but not Ypr10 or Mpt1, peak at 2 h and decline thereafter. Equal loading of RNA is visualized with hybridization with 18S rRNA.
Wounding induces numerous defense-related genes in several species (Reymond et al., 2000). In birch, Pal was induced 2 h after wounding (Fig. 5C). However, after that mRNA levels began to decrease. Birch Mpt1 and Ypr10 mRNA levels were not affected by wounding (Fig. 5C). Thus, when expression patterns of these three genes are concerned, wounding induced in birch leaves a distinct response when compared with Pss and O3. A low constitutive basal transcript level for Ypr10 is apparent in Figure 5C, but not in Figures 3 and 6. This is attributable to the use of slightly older leaves for wounding and longer exposure of the blot. Ypr10 transcript levels increase in leaves that are past their full expansion and highest photosynthetic capacity (Valjakka et al., 1999), but this does not affect their responsiveness to stresses.
Figure 6.
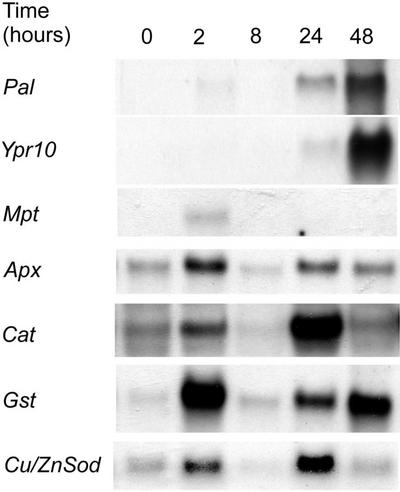
H2O2 generating system G/GO induces birch Pal, Ypr10, Apx, Cat, Cu/ZnSod, Gst, and Mpt1 at the transcript level with differential timing patterns.
Gene Expression upon H2O2
Both O3 and Pss-infiltration caused a build-up of H2O2 in the tissues affected (Fig. 1). Earlier we have shown that this H2O2 accumulation involves an active H2O2 production, first in cell walls and on the plasma membrane (2–12 h), followed by intracellular H2O2 accumulation at 24 h (Pellinen et al., 1999). To test whether this primary, O3-induced apoplastic burst of H2O2 plays a role in the subsequent increase in transcript levels of defense-related genes, we injected birch leaves with an H2O2 producing system G/GO (Alvarez et al., 1998). mRNA levels of all the genes studied increased as a response to H2O2 produced by G/GO with differences in timing and magnitude of the response (Fig. 6). Pal mRNA increased first slightly at 2 h and again at 24 to 48 h; the maximum level was detected at 48 h. Ypr10 transcript increase was first detectable 24-h postinjection and peaked at 48 h (Fig. 6). The other genes studied had different expression patterns: Mpt1 mRNA abundance increased at 2 h and disappeared at the later time points, and Apx, Cat, Gst, and Cu/ZnSod transcripts were first elevated at 2 h and again at 24 h (Fig. 6).
Biphasic transcript accumulation of the genes studied is likely to result from the biphasic H2O2 accumulation caused by G/GO treatment. The first H2O2 peak comes quickly from G/GO. The second one is produced later by the cells, induced by the first, G/GO-produced H2O2. Mpt1 and Ypr10 were induced only once, consistent with their O3-induction patterns. Ypr10, however, was slower in its induction, and its transcripts always started to accumulate as late as 24 h also in the O3- (Fig. 3) and pathogen-treated material (Fig. 5).
DISCUSSION
Oxidative Stress, H2O2 Accumulation, and Lesion Formation
Active ROS production is a central component in the regulation of induced defense responses in plants exposed to various stresses. Antioxidative enzymes, peroxidases, CAT, and superoxide dismutases (SODs), on the other hand, control the extent and the duration of oxidative stress and ROS accumulation. The central role, for example for CAT as a sink for the H2O2 produced in stress responses has been demonstrated with transgenic tobaccos that have reduced CAT activities and thus higher H2O2 accumulation (Willekens et al., 1994; Chamnongpol et al., 1998). Genes encoding these enzymes are also widely used as indicators of ROS responsive, oxidative stress-specific signaling. We have shown earlier that O3 exposure increased ROS detoxifying enzyme activities in birch (Tuomainen et al., 1996). However, increases in the activities of the antioxidative enzymes were evident only during the lesion formation. The interesting question is whether the function of antioxidative enzymes is to protect cells from the primary ROS attack or to remove the actively produced ROS that also can act as signaling intermediates. Antioxidative genes were induced by O3 in birch with differences in timing and magnitude of the response but customarily only after the O3 exposure (Fig. 4), in a manner similar to the antioxidative enzyme activities (Tuomainen et al., 1996). Thus, the increase takes place only after the onset of the active cellular ROS production in the affected tissue. O3 induction of Apx in a similar manner has been shown in tobacco (Örvar et al., 1997) and O3 induction of Gst and Sod in Arabidopsis, whereas Cat did not show O3 induction (Sharma and Davis, 1994). In birch, the Cat mRNA level analogously was not affected until late, at 48 h, when there was a slight increase. It thus seems that the genes encoding antioxidative enzymes respond to the oxidative challenge of the O3 exposure only after the primary attack is over and mainly regulate the extent of the active ROS accumulation in the cells affected.
It has been proposed that regulated ROS production and accumulation is required for cell death that protects wounded tissue or is visible as HR- or O3-lesion formation (Rao and Davis, 1999; Overmyer et al., 2000; Rao et al., 2000b). In all treatments that were inflicted to birch leaves, H2O2 burst was accordingly localized to the sites undergoing cell death (Fig. 1, B, E, H, and Q). Furthermore, in both O3-exposed and pathogen-infiltrated leaves, H2O2 accumulation was evident already 2 h after the treatment and had similar spatial location, which resembled the later-appearing lesions. Mechanistic connection with H2O2 accumulation and cell death was demonstrated by infiltration of an artificial H2O2-producing system, G/GO, which also induced cell death at the infiltration sites. Furthermore, H2O2 accumulation was induced in G/GO-infiltrated leaves in a spot-like manner surrounding the lesions and also further apart. This suggests that an internal signal that is positively regulated by H2O2 induces systemic oxidative bursts, which are in turn involved in rapid lesion formation. This resembles the oxidative bursts that are required for induction of defense-related genes in leaves of pathogen-infected plants during the HR (Alvarez et al., 1998).
Birch Shows H2O2, But Not O2−•, Accumulation Preceding Cell Death
Earlier (Pellinen et al., 1999) and here, we have shown that H2O2 accumulates in the cell walls of O3- and pathogen-treated birch during the lesion formation. H2O2 accumulation has been similarly detected, for example, in tobacco (Schraudner et al., 1998), soybean (Glycine max; Levine et al., 1994), and barley (Hordeum vulgare; Thordal-Christensen et al., 1997) during HR lesion formation, and in the transgenic CAT-antisense tobaccos, a central role for H2O2 has been shown in the regulation of cell death (Willekens et al., 1994; Chamnongpol et al., 1998). In Arabidopsis, however, superoxide accumulation has been shown as the ROS responsible for cell death (Jabs et al., 1996; Rao and Davis, 1999; Overmyer et al., 2000). We did not detect any visible O2−• accumulation by NBT precipitation in birch leaves, which raises the question on the key ROS molecule required for cell death in different species.
In a survey where tobacco, seven tomato cultivars, 12 Arabidopsis accessions, two Rumex spp., and one Malva sp. (Wohlgemuth et al., 2002) were assayed for H2O2 and O2−• accumulation and for their spatial correlation with O3-lesions, a clear spatial and quantitative correlation was seen with either H2O2 accumulation or O2−• accumulation and O3 damage, depending on the species. In tobacco and tomato, there was a clear correlation with H2O2 and the later-appearing lesions with no O2−• accumulation detectable, even though diphenyl iodonium (DPI), an inhibitor of O2−• production, decreased considerably both H2O2 accumulation and lesion formation. In Arabidopsis, Rumex, and Malva, on the contrary, there was a clear correlation with O2−• accumulation and lesion formation. Furthermore, those Arabidopsis accessions that showed the highest O2−• accumulation after a short ozone exposure and before visible lesion formation were also the most sensitive to O3. Birch, based on the results here (Figs. 1 and 2) and before (Pellinen et al., 1999), accumulates H2O2 around the growing lesions in a similar way as tobacco and tomato, though the previous inhibitor studies (Pellinen et al., 1999) suggested that, at least in part, the primary source of the H2O2 is most likely O2−• produced by the NADPH oxidase complex. In tobacco, which accumulates H2O2, ozone exposure similarly up-regulates two homologs of the NADPH oxidase (Wohlgemuth et al., 2002) and H2O2 accumulation, and lesion formation is inhibited by DPI, an inhibitor of flavin-containing oxidases. Overall, the results with birch (Pellinen et al., 1999; this work) agree well with the role of H2O2 as a regulatory signal molecule of defense gene expression and cell death as shown with several model systems (Levine et al., 1994; Willekens et al., 1994; Bestwick et al., 1997; Thordal-Christensen et al., 1997; Alvarez et al., 1998; Chamnongpol et al., 1998).
Experiments with DPI have suggested that the plasma membrane NADPH oxidase is one possible source for the H2O2 accumulation in the cell wall during the oxidative burst (Alvarez et al., 1998; Pellinen et al., 1999; Rao and Davis, 1999). This suggests that H2O2 is formed from O2−• as a result of spontaneous or enzyme-catalyzed dismutation. Thus, the dismutation rate on one hand and the O2−• production rate on the other hand could determine which of these two ROS is accumulating: The difference between O2−• and H2O2 accumulation detectable with NBT and DAB staining could just be in the extent and balance of up-regulation of O2−• production and the rate of O2−• dismutation to H2O2. When the dismutation rate remains high, no O2−• accumulation would be detected even when the production rate at the cell level is increased; however, at the cell level this could make the difference resulting in cell death. It has been shown that LSD1 is involved in the salicylic acid-dependent regulation of CuZnSOD (Kliebenstein et al., 1999); in the lsd1 mutant, O2−• accumulates resulting in runaway cell death (Jabs et al., 1996). Accordingly, SA is an important regulatory molecule in O3-induced cell death (Rao and Davis, 1999, 2001; K. Overmyer, H. Tuominen, and J. Kangasjärvi, unpublished data).
Stress-Induced H2O2 Accumulation Is Involved in Gene Activation
H2O2 is involved in the regulation of several stress-related genes (Bi et al., 1995; Chamnongpol et al., 1998; Desikan et al., 1998). Pal transcript levels in birch leaves were increased by O3 (Fig. 3), Pss infiltration (Fig. 5A), and wounding (Fig. 5C), which all also induced H2O2 accumulation in the tissues affected (Fig. 1). In O3-exposed leaves, mRNA levels of Ypr10 began to increase at 8 h. This increase continued until 48 h (Fig. 3), coinciding with visible damage. Swoboda et al. (1995) have shown that Ypr10 transcript levels were increased by fungal elicitors in birch cell suspensions. Infiltration of leaves with Pss similarly induced Ypr10 mRNA levels with comparable timing (Fig. 5A). Mpt1 transcript levels were similarly induced by O3 exposure (Fig. 3; Kiiskinen et al., 1997) and Pss infiltration (Fig. 5A), both treatments that also induced H2O2 accumulation in the leaves. Direct H2O2 response of these genes was shown by G/GO infiltration. This suggests that H2O2 is a part of the signaling cascade that leads to induction of a wide array of defense-related genes.
Albeit G/GO infiltration induced both spreading H2O2 accumulation and cell death, lesion formation was not the primary reason for the activation of defense genes in the tissue affected. Short-term O3 exposures induced both Pal and Ypr10 in birch leaves without visible damage. Thus, activation of defense gene expression and cell death in birch are likely to be independent from each other, although both seem to be H2O2 dependent. Differences between signaling leading to cell death and defense gene expression have also been detected in Arabidopsis mutant defense no death1, where defense-related genes were up-regulated in pathogen challenged plants without visible HR cell death (Yu et al., 1998). However, this does not completely exclude the requirement of cell death for the activation of defense genes. It has been shown, for example in Arabidopsis that death of a few individual cells per leaf, micro HR, is required for the development of systemic acquired resistance (Alvarez et al., 1998). In an analogous fashion, death of a few individual cells or small cell clusters were detected in O3-resistant birch clones, where O3 did not cause any visible lesions, but up-regulated defense gene expression (Tuomainen et al., 1996).
Induction of the antioxidative genes and Pal by the G/GO was biphasic, peaking at 2 and at 24 to 48 h, when Ypr10 and Mpt1 transcript levels increased only once; Mpt1 at 2 h and Ypr10 at 24 to 48 h. Schraudner et al. (1998) showed that in tobacco, a biphasic H2O2 accumulation was required for the onset of cell death in the O3-sensitive tobacco cv Bel W3. In accordance, the biphasic expression pattern of the birch genes may be a result from two separate oxidative challenges. The gene induction by G/GO-produced H2O2 can be divided into to phases: The first H2O2 burst is produced by the G/GO. The second phase is active, cellular H2O2 accumulation induced by the G/GO-produced H2O2. Ypr10 was only induced at 24 and 48 h (Fig. 6), and its O3 responsiveness was generally slower than that of Pal. Because of the slower response pattern of Ypr10, it remains unclear whether its expression would also be biphasic.
Birch Mpt1 was also induced by G/GO, but increased expression was detected only at 2 h (Fig. 6). Mpt1 thus reacted to the first H2O2 challenge produced by G/GO. At the later time points, when H2O2 was produced by the plant, Mpt1 did not respond anymore. Mpt1 similarly was not induced by consecutive O3 exposures (Kiiskinen et al., 1997), unlike Pal and Ypr10 (Fig. 3C). This suggests that Mpt1 induction is likely to be mediated by H2O2, but that it may become less sensitive by the first challenge of ROS. Becoming less sensitive may serve as a mechanism to avoid overloading signals leading to mitochondrial stress responses. The primary up-regulation of Mpt1 could be related to these processes and controlled by mitochondrial electron transport- and ATP-synthesis-related signaling cascades that regulate mitochondrial redox and energy homeostasis. H2O2 accumulation and structural alterations in mitochondria after the primary apoplastic H2O2 production have been seen during the progression of oxidative cell death in birch (Pellinen et al., 1999), which could represent a part of these responses.
Both O3 and Pss-infection caused spreading cellular H2O2 accumulation and induced birch Pal, Mpt1, and Ypr10. Thus, H2O2 could be the common inductive factor regulating these genes through still unidentified signaling chain(s) and transcription factors.
MATERIALS AND METHODS
Plant Material
One-year-old birch (Betula pendula Roth) saplings were used in all experiments. For O3 and wounding experiments, saplings were grown in growth chambers as described earlier (Kiiskinen et al., 1997). For pathogen and G/GO treatments saplings were grown in a greenhouse under 20/4-h photoperiod (day/night) with 18°C/11°C temperature, respectively.
Treatments
Plants were exposed to a single 8-h O3 pulse of 150 nL L−1 (±5 nL L−1) 4 weeks after bud burst as described earlier (Kiiskinen et al., 1997). All time points for sample analysis are expressed as hours after the beginning of exposure or treatment. Leaf samples from O3-exposed plants were collected 0, 2, 8, 12 (only in Fig. 3), 24, and 48 h. Fully expanded leaves from four individuals were collected at every time point, frozen in liquid nitrogen, and stored at −70°C. To determine the duration of O3 exposure required for gene induction, plants were exposed to O3 for 2 or 4 h, followed by transfer to clean air, or for 8 h. Leaf samples were collected at 8 h. To study whether the genes are repeatedly induced by consecutive O3 treatments, birch saplings were exposed to two 150 nL L−1 O3 peaks for 8 h so that the second one begun 48 h after the first one. Samples were collected at 0, 8, 24, 48, 48+8, 48+24, and 48+48 h.
In wounding experiments, the whole-leaf areas of four individual birch saplings per time point were wounded by piercing the leaf with needles and leaves were collected at 0, 2, 8, 24, and 48 h. For pathogen treatments Pss strains J900, R32, Cit7, and P. fluorescens were grown overnight at 28°C in King's B medium, washed three times with 10 mm MgSO4, and kept on ice until infiltration. The bacteria (1–20 × 107 cfu mL−1) resuspended in 10 mm MgSO4 were injected into leaves of 16 birch saplings per time point with a syringe. In each tree, 12 middle-aged leaves were infiltrated in six separate spots on each leaf. In two control treatments, leaves were injected either with 10 mm MgSO4 or were left untreated. Leaves were collected at 0, 2, 8, 24, and 48 h.
An artificial H2O2 production system, G/GO (10 mm:220 units mL−1 [1:1, w/v]; Calbiochem, San Diego; Alvarez et al., 1998) was applied with syringe to fully expanded birch leaves into 14 individual spots per leaf. G and GO alone were injected into plants similarly. Three individual plants per time point were treated, and leaves were collected at 0, 2, 8, 24, and 48 h.
Localization of H2O2 and O2−•
For localizing H2O2 produced by birch cells, treated whole leaves were vacuum infiltrated with DAB in MES (Thordal-Christensen et al., 1997) and cleared by boiling in alcohol:lactophenol (2:1, v/v) for 3 to 5 min. Detection of superoxide with NBT was performed essentially according to Jabs et al. (1996) and Overmyer et al. (2000). Subcellular detection of H2O2 accumulation with CeCl3 staining and transmission electron microscopy was performed as described by Pellinen et al. (1999).
RNA Gel-Blot Analysis
RNA was isolated from approximately 1 g of frozen leaves with the procedure of Chang et al. (1993). For RNA gel-blot analysis, 10 μg of total RNA was fractionated and transferred onto positively charged nylon membrane (Roche Diagnostics, Indianapolis). Equal loading on each lane was verified by staining the membranes with methylene blue. Membranes were baked and prehybridized in DIG EasyHyb hybridization buffer (Roche Diagnostics) for 2 to 6 h at 50°C and hybridized overnight.
Double-stranded DNA probes for birchYpr10 (X77601; Swoboda et al., 1995), Pal (X76077; Tuomainen et al., 1996), Mpt1 (Y08499; Kiiskinen et al., 1997), Apx (AJ279686), Gst (AJ279691), Cu/ZnSod (AJ 279694), and Cat (AJ302710) were generated by PCR using 1 unit of DNA-polymerase (DynaZyme, Finnzymes, or Taq-polymerase, Promega, Madison, WI), 5.0 μL of dNTP labeling mix (0.1 mm digoxigenin-labeled dUTP, Roche Diagnostics), 2.5 mm MgCl2, 30 pmol of degenerate Pal or Cat primers, 30 pmol of polylinker specific oligonucleotide primers (Ypr10, Mpt1, Apx, Gst, and CuZnSod probes) and 0.1 μg of corresponding cDNA or PCR-fragment template. PCR conditions were: 95°C for 1 min, 55°C to 59°C for 1 min, and 72°C for 1 min, for 30 cycles. Membranes were washed under high stringency conditions. Digoxigenin labeling and detection kit (Roche Diagnostics) was used for detection with the exception of doubled blocking time and an additional washing step. Chemiluminescent detection was performed by pipetting disodium 3-(4-methoxyspiro {1,2-dioxetane-3,2′-(5-chloro)tricyclo[3.3.1.13,7]decan}-4-yl) phenyl phosphate substrate directly onto the membrane. Membranes were incubated at 37°C for 15 to 60 min and autoradiographed at room temperature.
ACKNOWLEDGMENTS
We thank Timo Oksanen (University of Kuopio, Finland) for the construction of O3-exposure facilities. The Ypr10 cDNA was a kind gift from Dr. Oscar Vicente (Institute of Microbiology and Genetics, University of Vienna). Pss strains were a gift from Dr. Martin Romantschuk (University of Helsinki).
Footnotes
This work was supported by the Scientific Council of Research of Environment and Natural Resources in Finland (grant no. 43327), by the Finnish Centre of Excellence Program (2000–2005), Biocentrum Helsinki, by the graduate programs of the University of Kuopio and Åbo Academi, and by the Teknologian kehittämiskeskus biodiversity program.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.003954.
LITERATURE CITED
- Alvarez ME, Pennell RI, Meijer P-J, Ishikawa A, Dixon RA, Lamb C. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell. 1998;92:773–784. doi: 10.1016/s0092-8674(00)81405-1. [DOI] [PubMed] [Google Scholar]
- Bestwick CS, Brown IR, Benneth MHR, Mansfield JW. Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv phaseolicola. Plant Cell. 1997;9:209–221. doi: 10.1105/tpc.9.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Y-M, Kenton P, Mur L, Darby R, Draper J. Hydrogen peroxide does not function downstream of salicylic acid in the induction of PR protein expression. Plant J. 1995;8:235–245. doi: 10.1046/j.1365-313x.1995.08020235.x. [DOI] [PubMed] [Google Scholar]
- Bolwell GP. Role of active oxygen species and NO in plant defense responses. Curr Opin Plant Biol. 1999;2:287–294. doi: 10.1016/S1369-5266(99)80051-X. [DOI] [PubMed] [Google Scholar]
- Bolwell GP, Wojtaszek P. Mechanisms for the generation of reactive oxygen species in plant defence: a broad perspective. Physiol Mol Plant Pathol. 1997;51:347–366. [Google Scholar]
- Bufe A, Spangfort MD, Kahlert H, Schlaak M, Becker W-M. The major birch pollen allergen, Bet v 1, shows ribonuclease activity. Planta. 1996;199:413–415. doi: 10.1007/BF00195733. [DOI] [PubMed] [Google Scholar]
- Chamnongpol S, Willekens H, Moeder W, Langebartels C, Sandermann H, Jr, Van Montagu M, Inzé D, van Camp W. Defense activation and enhanced pathogen tolerance induced by H2O2 in transgenic tobacco. Proc Natl Acad Sci USA. 1998;95:5818–5823. doi: 10.1073/pnas.95.10.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney C. A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep. 1993;11:113–116. [Google Scholar]
- Desikan R, Reynolds A, Hancock JT, Neill SJ. Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on defense gene expression in Arabidopsis suspension cultures. Biochem J. 1998;330:115–120. doi: 10.1042/bj3300115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper J. Salicylate, superoxide synthesis and cell suicide in plant defence. Trends Plant Sci. 1997;2:162–165. [Google Scholar]
- Hahlbrock K, Scheel D. Physiology and molecular biology of phenylpropanoid metabolism. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:347–369. [Google Scholar]
- Heath RL, Taylor GE., Jr . Physiological processes and plant responses to ozone exposure. In: Sandermann H, Wellburn AR, Heath RL, editors. Ecological Studies, 127: Forest Decline and Ozone. Berlin: Springer-Verlag; 1997. pp. 317–368. [Google Scholar]
- Jabs T, Dietrich RA, Dangl JL. Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science. 1996;273:1853–1856. doi: 10.1126/science.273.5283.1853. [DOI] [PubMed] [Google Scholar]
- Jones DH. Phenylalanine ammonia-lyase: regulation of its induction, and its role in plant development. Phytochemistry. 1984;23:1349–1359. [Google Scholar]
- Kangasjärvi J, Talvinen J, Utriainen M, Karjalainen R. Plant defense systems induced by ozone. Plant Cell Environ. 1994;17:783–794. [Google Scholar]
- Karpinski S, Reynolds H, Karpinska B, Wingsle G, Creissen G, Mullineaux P. Systemic signaling and acclimation in response to excess excitation energy in Arabidopsis. Science. 1999;284:654–657. doi: 10.1126/science.284.5414.654. [DOI] [PubMed] [Google Scholar]
- Kiiskinen M, Korhonen M, Kangasjärvi J. Isolation and characterization of cDNA for a plant mitochondrial phosphate translocator (Mpt1): Ozone stress induces Mpt1 mRNA accumulation in birch (Betula pendula Roth) Plant Mol Biol. 1997;35:271–279. doi: 10.1023/a:1005868715571. [DOI] [PubMed] [Google Scholar]
- Kliebenstein DJ, Dietrich RA, Martin AC, Last RL, Dangl JL. LSD1 regulates salicylic acid induction of copper zinc superoxide dismutase in Arabidopsis thaliana. Mol Plant-Microbe Interact. 1999;12:1022–1026. doi: 10.1094/MPMI.1999.12.11.1022. [DOI] [PubMed] [Google Scholar]
- Laisk A, Kull O, Moldau H. Ozone concentration in leaf intercellular air spaces is close to zero. Plant Physiol. 1989;90:1163–1167. doi: 10.1104/pp.90.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- Langebartels C, Schraudner M, Heller W, Ernst D, Sandermann H. Oxidative stress and defense reactions in plants exposed to air pollutants and UV-B radiation. In: Inzé D, Van Montagu M, editors. Oxidative Stress in Plants. London: Taylor and Francis; 2002. pp. 105–135. [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Mittler R, Lam E, Shulaev V, Cohen M. Signals controlling the expression of cytosolic ascorbate peroxidase during pathogen-induced programmed cell death in tobacco. Plant Mol Biol. 1999;39:1025–1035. doi: 10.1023/a:1006110223774. [DOI] [PubMed] [Google Scholar]
- Örvar BL, McPherson J, Ellis BE. Pre-activating wounding response in tobacco prior to high-level ozone exposure prevents necrotic injury. Plant J. 1997;11:203–212. doi: 10.1046/j.1365-313x.1997.11020203.x. [DOI] [PubMed] [Google Scholar]
- Overmyer K, Tuominen H, Kettunen R, Betz C, Langebartels C, Sandermann H, Jr, Kangasjärvi J. The ozone-sensitive Arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxide-dependent cell death. Plant Cell. 2000;12:1849–1862. doi: 10.1105/tpc.12.10.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pääkkönen E, Seppänen S, Holopainen T, Kokko H, Kärenlampi S, Kärenlampi L, Kangasjärvi J. Induction of genes for the stress proteins PR-10 and PAL in relation to growth, visible injuries and stomatal conductance in birch (Betula pendula) clones exposed to ozone and/or drought. New Phytol. 1998;138:295–305. doi: 10.1046/j.1469-8137.1998.00898.x. [DOI] [PubMed] [Google Scholar]
- Pellinen R, Palva T, Kangasjärvi J. Subcellular localization of ozone-induced hydrogen peroxide production in birch (Betula pendula) leaf cells. Plant J. 1999;20:349–356. doi: 10.1046/j.1365-313x.1999.00613.x. [DOI] [PubMed] [Google Scholar]
- Rao MV, Davis KR. Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: the role of salicylic acid. Plant J. 1999;17:603–614. doi: 10.1046/j.1365-313x.1999.00400.x. [DOI] [PubMed] [Google Scholar]
- Rao MV, Davis KR. The physiology of ozone-induced cell death. Planta. 2001;213:682–690. doi: 10.1007/s004250100618. [DOI] [PubMed] [Google Scholar]
- Rao MV, Koch JR, Davis KR. Ozone: a tool for probing programmed cell death in plants. Plant Mol Biol. 2000a;44:345–358. doi: 10.1023/a:1026548726807. [DOI] [PubMed] [Google Scholar]
- Rao MV, Lee HI, Creelman RA, Mullet JA, Davis KR. Jasmonic acid signaling modulates ozone-induced hypersensitive cell death. Plant Cell. 2000b;12:1633–1646. doi: 10.1105/tpc.12.9.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond P, Weber H, Damond M, Farmer EE. Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell. 2000;12:707–719. doi: 10.1105/tpc.12.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandermann H., Jr Ozone and plant health. Annu Rev Phytopathol. 1996;34:347–366. doi: 10.1146/annurev.phyto.34.1.347. [DOI] [PubMed] [Google Scholar]
- Schenk PM, Kazan K, Wilson I, Anderson JP, Richmond T, Somerville SC, Manners JM. Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc Natl Acad Sci USA. 2000;97:11655–11660. doi: 10.1073/pnas.97.21.11655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraudner M, Moeder W, Wiese C, van Camp W, Inzé D, Langebartels C, Sandermann H., Jr Ozone-induced oxidative burst in the ozone biomonitor plant, tobacco Bel W3. Plant J. 1998;16:235–245. doi: 10.1046/j.1365-313x.1998.00294.x. [DOI] [PubMed] [Google Scholar]
- Sharma YK, Davis K. Ozone-induced expression of stress-related genes in Arabidopsis thaliana. Plant Physiol. 1994;105:1089–1096. doi: 10.1104/pp.105.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swoboda I, Hoffmann-Sommergruber K, O'Riordáin G, Scheiner O, Heberle-Bors E, Vicente O. Bet v 1 proteins, the major birch pollen allergens and members of a family of conserved pathogenesis-related proteins, show ribonuclease activity in vitro. Physiol Plant. 1996;96:433–438. [Google Scholar]
- Swoboda I, Scheiner O, Heberle-Bors E, Vicente O. cDNA cloning and characterization of three genes in the Bet v 1 gene family that encode pathogenesis-related proteins. Plant Cell Environ. 1995;18:865–874. [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB. Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997;11:1187–1194. [Google Scholar]
- Tuomainen J, Betz C, Kangasjärvi J, Ernst D, Yin ZH, Langebartels C, Sandermann H., Jr Ozone induction of ethylene emission in tomato plants: regulation by differential transcript accumulation for the biosynthetic enzymes. Plant J. 1997;12:1151–1162. [Google Scholar]
- Tuomainen J, Pellinen R, Roy S, Kiiskinen M, Eloranta T, Karjalainen R, Kangasjärvi J. Ozone affects birch (Betula pendula Roth) phenylpropanoid, polyamine and active oxygen detoxifying pathways at biochemical and gene expression level. J Plant Physiol. 1996;148:179–188. [Google Scholar]
- Utriainen M, Kokko H, Auriola S, Sarrazin O, Kärenlampi S. PR-10 protein is induced by copper stress in roots and leaves of a Cu/Zn tolerant clone of birch, Betula pendula. Plant Cell Environ. 1998;21:821–828. [Google Scholar]
- Valjakka M, Tuhkanen E, Kangasjärvi J, Vapaavuori E. Expression of photosynthesis and senescence-related genes during leaf development and senescence in silver birch (Betula pendula Roth) seedlings. Physiol Plant. 1999;106:302–310. [Google Scholar]
- van Loon LC, Pierpoint WS, Boller T, Conejero V. Recommendations for naming plant pathogenesis-related proteins. Plant Mol Biol Rep. 1994;12:245–264. [Google Scholar]
- Willekens H, Langebartels C, Tiré C, van Montagu M, Inzé D, Van Camp W. Differential expression of catalase genes in Nicotiana plumbaginifolia (L.) Proc Natl Acad Sci USA. 1994;91:10450–10454. doi: 10.1073/pnas.91.22.10450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlgemuth H, Mittelstrass K, Kschieschan S, Bender J, Weigel HJ, Overmyer K, Kangasjärvi J, Langebartels C, Sandermann H. Activation of an oxidative burst is a general feature of sensitive plants exposed to the air pollutant ozone. Plant Cell Environ. 2002;25:717–726. [Google Scholar]
- Yu I-C, Parker J, Bent AF. Gene-for-gene disease resistance without the hypersensitive response in Arabidopsis dnd1 mutant. Proc Natl Acad Sci USA. 1998;95:7819–7824. doi: 10.1073/pnas.95.13.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]