Abstract
1. Cat spleens were perfused with Krebs-bicarbonate solution, using a constant-flow pump at a rate of about 7 ml/min at 33-35° C. Noradrenaline (NA) overflow by nerve stimulation at 10 Hz for 20 s was determined with or without flow-stop before and after treatment with cocaine, phentolamine or phenoxybenzamine. In order to determine the effect of flow-stop on overflow, the arterial and the venous flows were occluded by clamping the inflow and outflow tubes during the period of stimulation plus 30, 60 or 120 seconds.
2. Without flow-stop, NA output was 0·93±0·25 ng/stimulus, which was significantly increased after cocaine (123±6·6%), phentolamine (415±93%) and phenoxybenzamine (578±107%). Phentolamine and phenoxybenzamine were much more effective than cocaine in enhancing overflow.
3. Before treatment with drugs, flow-stops of 30, 60 and 120 s reduced NA outputs to 70±6·6, 27·5±2 and 7%, respectively, of the control outputs without flow-stop. None of the drugs significantly influenced the percentage reductions in NA outputs during a 30 s flow-stop. However, the percentage outputs after cocaine or phenoxybenzamine treatment during a 60 s flow-stop significantly increased to 45±2·5% and 57±6%, respectively, as compared to the percentage output of 27·5±2% from untreated spleens during a corresponding flow-stop period. During flow-stop, there was no appreciable metabolism of the released transmitter.
4. Diffusion of the released transmitter from the site of liberation plays only a minor role in the removal of the released NA.
5. It is suggested that the NA released by nerve stimulation acts on the presynaptic α sites to inhibit its own release by a negative feedback mechanism. Adrenoceptor blocking agents enhance the NA overflow from spleen because they remove this autoinhibition by blocking the presynaptic α sites.
Full text
PDF


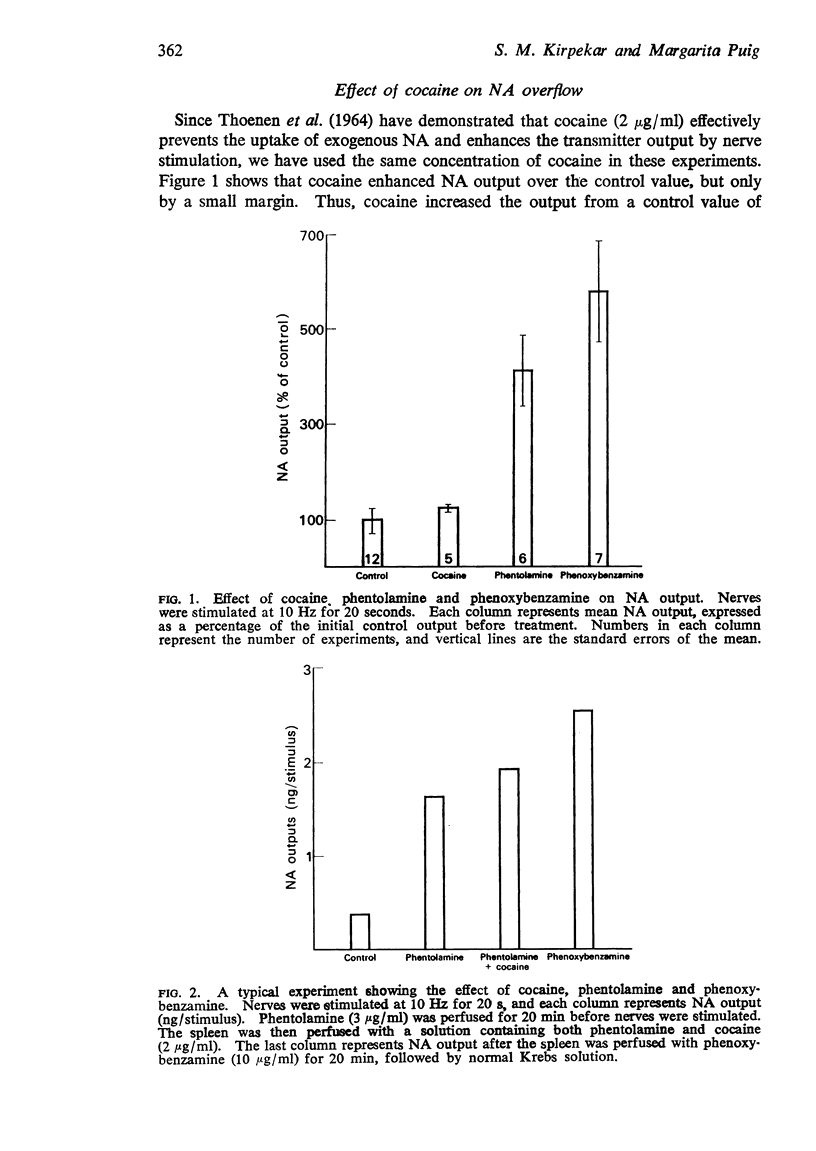

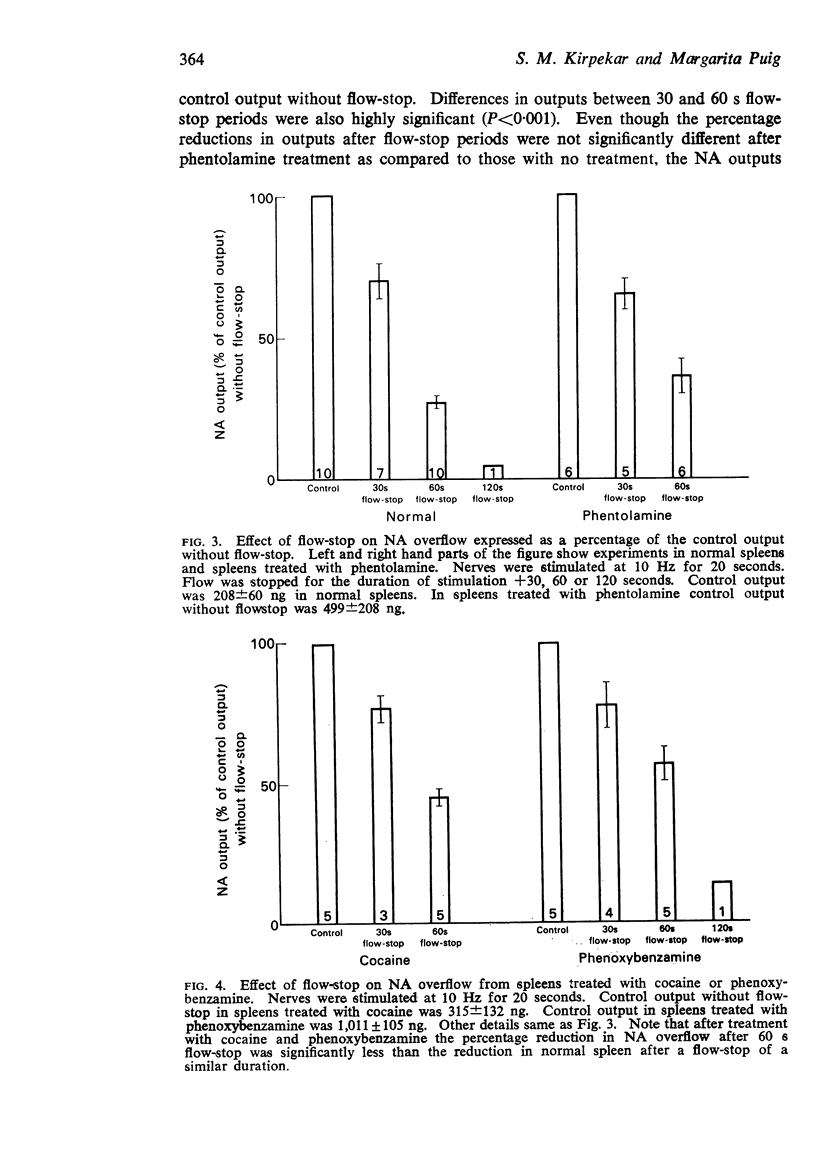

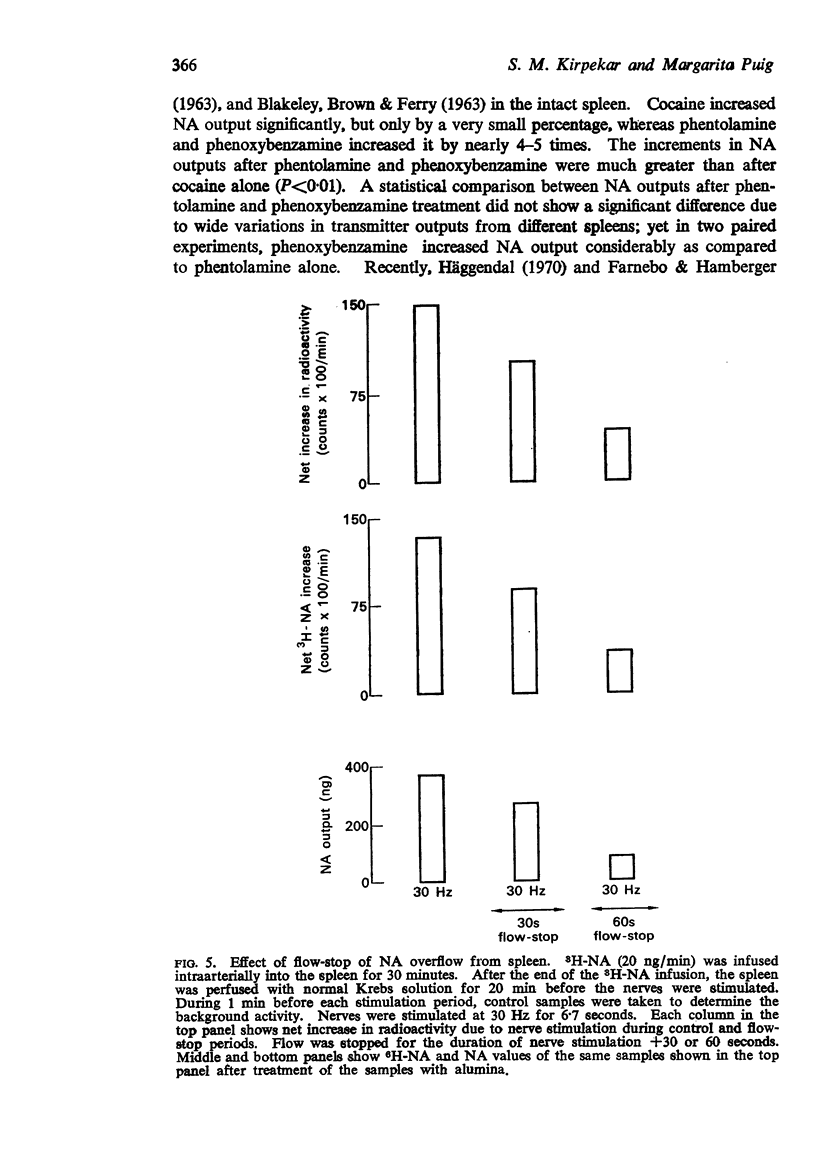



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., SAYRE D. F. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962 Dec;138:360–375. [PubMed] [Google Scholar]
- BLAKELEY A. G., BROWN G. L., FERRY C. B. Pharmacological experiments on the release of the sympathetic transmitter. J Physiol. 1963 Jul;167:505–514. doi: 10.1113/jphysiol.1963.sp007165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN G. L., GILLESPIE J. S. The output of sympathetic transmitter from the spleen of the cat. J Physiol. 1957 Aug 29;138(1):81–102. doi: 10.1113/jphysiol.1957.sp005839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnebo L. O., Hamberger B. Effects of desipramine, phentolamine and phenoxybenzamine on the release of noradrenaline from isolated tissues. J Pharm Pharmacol. 1970 Nov;22(11):855–857. doi: 10.1111/j.2042-7158.1970.tb08455.x. [DOI] [PubMed] [Google Scholar]
- Gillespie J. S., Kirpekar S. M. The uptake and release of radioactive noradrenaline by the splenic nerves of cats. J Physiol. 1966 Nov;187(1):51–68. doi: 10.1113/jphysiol.1966.sp008075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERTTING G., AXELROD J. Fate of tritiated noradrenaline at the sympathetic nerve-endings. Nature. 1961 Oct 14;192:172–173. doi: 10.1038/192172a0. [DOI] [PubMed] [Google Scholar]
- KIRPEKAR S. M., CERVONI P. EFFECT OF COCAINE, PHENOXYBENZAMINE AND PHENTOLAMINE ON THE CATECHOLAMINE OUTPUT FROM SPLEEN AND ADRENAL MEDULLA. J Pharmacol Exp Ther. 1963 Oct;142:59–70. [PubMed] [Google Scholar]
- KIRPEKAR S. M., CERVONI P., FURCHGOTT R. F. Catecholamine content of the cat nicitating membrane following procedures sensitizing it to norepinephrine. J Pharmacol Exp Ther. 1962 Feb;135:180–190. [PubMed] [Google Scholar]
- Kirpekar S. M., Misu Y. Release of noradrenaline by splenic nerve stimulation and its dependence on calcium. J Physiol. 1967 Jan;188(2):219–234. doi: 10.1113/jphysiol.1967.sp008135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer S. Z. The metabolism of (3H)noradrenaline released by electrical stimulation from the isolated nictitating membrane of the cat and from the vas deferens of the rat. J Physiol. 1970 Jul;208(3):515–546. doi: 10.1113/jphysiol.1970.sp009135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOENEN H., HUERLIMANN A., HAEFELY W. THE EFFECT OF SYMPATHETIC NERVE STIMULATION ON VOLUME, VASCULAR RESISTANCE, AND NOREPINEPHRINE OUTPUT IN THE ISOLATED PERFUSED SPLEEN OF THE CAT, AND ITS MODIFICATION BY COCAINE. J Pharmacol Exp Ther. 1964 Jan;143:57–63. [PubMed] [Google Scholar]
- TRENDELENBURG U. The supersensitivity caused by cocaine. J Pharmacol Exp Ther. 1959 Jan;125(1):55–65. [PubMed] [Google Scholar]
- Thoenen H., Huerlimann A., Haefely W. Interaction of phenoxybenzamine with guanethidine and bretylium at the sympathetic nerve endings of the isolated perfused spleen of the cat. J Pharmacol Exp Ther. 1966 Feb;151(2):189–195. [PubMed] [Google Scholar]



