Abstract
To distinguish their roles in early kernel development and stress, expression of soluble (Ivr2) and insoluble (Incw2) acid invertases was analyzed in young ovaries of maize (Zea mays) from 6 d before (−6 d) to 7 d after pollination (+7 d) and in response to perturbation by drought stress treatments. The Ivr2 soluble invertase mRNA was more abundant than the Incw2 mRNA throughout pre- and early post-pollination development (peaking at +3 d). In contrast, Incw2 mRNAs increased only after pollination. Drought repression of the Ivr2 soluble invertase also preceded changes in Incw2, with soluble activity responding before pollination (−4 d). Distinct profiles of Ivr2 and Incw2 mRNAs correlated with respective enzyme activities and indicated separate roles for these invertases during ovary development and stress. In addition, the drought-induced decrease and developmental changes of ovary hexose to sucrose ratio correlated with activity of soluble but not insoluble invertase. Ovary abscisic acid levels were increased by severe drought only at −6 d and did not appear to directly affect Ivr2 expression. In situ analysis showed localized activity and Ivr2 mRNA for soluble invertase at sites of phloem-unloading and expanding maternal tissues (greatest in terminal vascular zones and nearby cells of pericarp, pedicel, and basal nucellus). This early pattern of maternal invertase localization is clearly distinct from the well-characterized association of insoluble invertase with the basal endosperm later in development. This localization, the shifts in endogenous hexose to sucrose environment, and the distinct timing of soluble and insoluble invertase expression during development and stress collectively indicate a key role and critical sensitivity of the Ivr2 soluble invertase gene during the early, abortion-susceptible phase of development.
In field-grown maize (Zea mays), losses in grain yield are maximal when drought occurs during the flowering stage (Denmead and Shaw, 1960; Doorenbos and Kassam, 1979). The magnitude of yield reduction is greater than can be explained by decreases in current photosynthesis alone, and analyses of yield components generally reveal large reductions in seed number and harvest index (e.g. Edmeades et al., 1999). Reproductive development at the time of flowering is apparently especially sensitive to drought stress (Zinselmeier et al., 1995, 1999), and therefore an understanding of how the involved processes are affected is of particular interest for improving drought tolerance (Boyer, 1996).
Drought stress immediately before or after anthesis can affect gynoecium development in several ways. First, the expansion rate of the style is typically decreased, causing asynchrony between pollen shedding and silk emergence (Herrero and Johnson, 1981). The slow silk emergence can also reduce female receptivity because of its decrease with age (Bassetti and Westgate, 1993). This may result in failure of the pollination process (Herrero and Johnson, 1981; Westgate and Boyer, 1985; Bassetti and Westgate, 1994), despite little or no reduction in pollen viability (Westgate and Boyer, 1986). Second, newly formed zygotes are especially sensitive to drought stress, so that even if pollination does take place, reproductive failure can still occur in response to only a few days of water deficit. Third, abortion can even occur if dehydration stress is relieved before pollination (Westgate and Boyer, 1986). For these zygotes, growth and development cease about 1 to 2 d after fertilization, but may partially resume if rescued by feeding Suc to the plant stem (Boyle et al., 1991; Zinselmeier et al., 1999).
However, in addition to Suc availability per se, capacity for Suc use and hexose to Suc balance may be critically important to zygote development under drought conditions. In general, Suc levels of stressed ovaries are higher or at least similar to those of non-stressed ovaries (Schussler and Westgate, 1991; Schussler and Westgate, 1995; Zinselmeier et al., 1995), indicating that the capacity to use Suc may be impaired by drought. A central role has also been implicated for hexose to Suc balance in regulating key aspects of ovary and seed development (Weber et al., 1996, 1998; Wobus and Weber, 1999; Weschke et al., 2000). Imported Suc can be cleaved by either invertase or the reversible Suc synthase reaction (e.g. Sturm and Tang, 1999). In maize, activity of vacuolar and cell wall-bound acid invertases predominate during ovary and early kernel development, whereas Suc synthase also becomes important during the storage phase of grain-fill (Zinselmeier et al., 1995; Weschke et al., 2000). Drought stress decreases activities of both vacuolar and cell wall-bound acid invertase during kernel development (Zinselmeier et al., 1995), with parallel reductions in ovary growth and concentration of hexoses. In addition, metabolic pools downstream of Suc in the starch formation pathway are depleted as well as starch deposits in cells of the inner pericarp and ovary base (Zinselmeier et al., 1999). Together, these observations designate the very early ovary growth (especially before pollination) as an extremely important, yet vulnerable, stage of reproduction, when drought stress may compromise metabolic and assimilate transfer processes necessary for successful kernel development.
The objectives of the present study were thus (a) to resolve temporal and spatial differences in contributions by vacuolar and cell wall-bound invertase genes during critical stages of early kernel development, and (b) to appraise the involvement of these genes in the drought sensitivity of ovaries before and immediately after pollination. This work extends previous advances by targeting early developmental stages of young ovaries that have been difficult to address in detail at the cellular and molecular levels. It also resolves previous questions about different invertase genes during kernel development and indicates a role for soluble invertase in the hexose to Suc balance implicated elsewhere as central to signaling during seed development (Wobus and Weber, 1999). In addition, analyses were applied to developmental perturbations imposed in field-grown plants using a series of carefully controlled, increasingly severe drought stress treatments. Expression of genes encoding vacuolar invertases (Ivr1 and Ivr2; Koch et al., 1995; Xu et al., 1995) and cell wall-bound invertases (Incw1 and Incw2; Shanker et al., 1995; Cheng et al., 1996; Taliercio et al., 1999) was quantified in young developing ovaries pre- and post-pollination, mRNA was localized in situ, and results were compared with crude extract enzymatic activity, sugar composition, abscisic acid (ABA) levels, and in situ localized enzymatic activity. The Ivr2 gene for vacuolar invertase was of particular interest in these studies because of its responsiveness to drought stress in vegetative tissues (Kim et al., 2000), its sugar regulation (Xu et al., 1996), and the potential role of its hexose products as substrates for kernel growth, osmotic and turgor adjustment, and sugar signaling during development (Koch et al., 1996; Weber et al., 1996, 1998; Wobus and Weber, 1999; Fisher and Cash-Clark, 2000b; Weschke et al., 2000). The following research presents evidence for a central role of the Ivr2 vacuolar invertase during early ovary development in maize and in the particular sensitivity of these structures to drought-induced abortion before and immediately after pollination.
RESULTS
Drought Conditions and Plant Growth
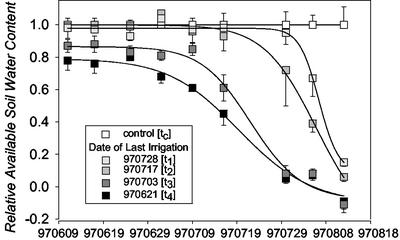
The changes in available soil water content during the drought periods are shown in Figure 1 for the five treatments spanning a well-watered control (tc) and drought stress treatments of increasing severity and duration (t1-t4). All plots were rewatered to field capacity 7 d after pollination (+7 d), when young-ear sampling was complete, and held at this level for the remainder of the growing season. Plant water use continued during the sampling period (July 29–August 12, 1997) from −6 d to +7 d under moderate stress (t1 and t2) but ceased before this time under severe stress (t3 and t4), where soil water was already depleted. Predawn leaf water potentials decreased accordingly, and severe stress significantly reduced grain yield, aboveground biomass, harvest index, and kernel number per ear (Table I). Although severe stress increased the anthesis-silking interval from about 3 to 7 d, this delay in pollination is not likely to have significantly reduced fertilization in the present study (Bassetti and Westgate, 1993, 1994).
Figure 1.
Development of relative available soil moisture over time for well-watered control plots and four drought treatments. The loamy sand soil had a capacity for available soil water of 100 to 130 mm. se shown (n = 3–4).
Table I.
Grain and dry matter yield, harvest index, kernel no. per ear, and predawn leaf water potential (ψw) in maize in treatments ranging from well-watered to severe drought stress
| Treatment | Grain Yield | Dry Matter Yield | Harvest Index | Kernel No. per Ear | Predawn ψw |
|---|---|---|---|---|---|
| g m−2 | MPa | ||||
| [tc] | 723 a | 1415 b | 0.51 a | 299 a | −0.10 a |
| [t1] | 844 a | 1509 b | 0.56 a | 295 a | −0.11 ab |
| [t2] | 953 a | 1865 a | 0.51 a | 177 ab | −0.14 b |
| [t3] | 426 b | 899 c | 0.48 a | 191 ab | −0.23 c |
| [t4] | 218 b | 815 c | 0.27 b | 101 b | −0.39 d |
Yield characters were measured at kernel maturity, and pressure chamber values of ψw of the last fully developed leaf obtained on the last day of the drought period. The depletion of available soil water content characterizing the five treatments is shown in Figure 1. Statistically significant differences between treatments were assessed by F-tests using the analysis of variance procedure PROC GLM (SAS Institute, 1988). Provided that the F-test was significant at the 5% level or less, comparison of means was performed with the Gabriel-algorithm of PROC GLM, and results are shown by letters after the values. Mean values that are significantly different at α ≤ 5% have no letters in common. No. of observations for each treatment (n = 3–4).
ABA, Growth, and Carbohydrate Content of Ovaries
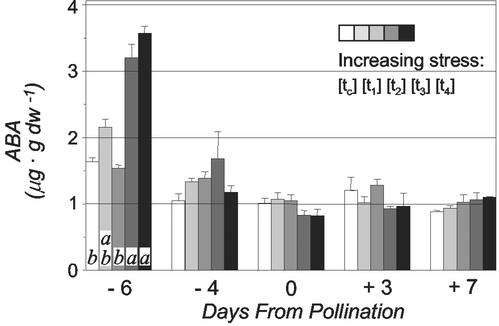
To test possible contributions of ABA to ovary invertase expression under varying degrees of drought stress, we analyzed the levels of endogenous ABA concentrations in ovary samples from 6 d before (−6 d) to 7 d after (+7 d) pollination (Fig. 2). Under control conditions (tc), ovary ABA levels were about 1.5 μg g−1 dry mass at −6 d, decreasing to about 1.0 μg g−1 dry mass at pollination and thereafter. Drought had relatively little effect on these values except when stress was most severe (t3 and t4), and ABA levels transiently rose in the youngest ovaries (−6 d). Such differences were no longer evident by −4 d, however, and under less severe drought (t1 and t2), ovary ABA concentrations remained at control levels throughout development.
Figure 2.
The effect of increasing drought stress on ovary ABA concentrations at five different stages of ear development. Legend as for Figure 1. F-tests and comparison of means for each stage was performed with the Gabriel-algorithm as described in caption for Table I (n = 3–4).
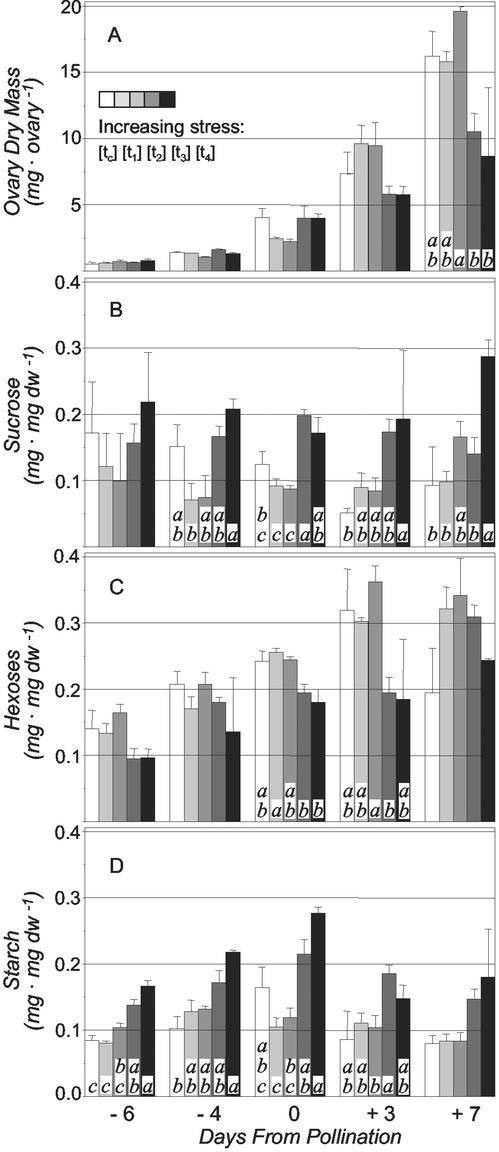
Drought stress perturbed growth and sugar content of very young ovaries in different ways depending on severity of the treatment (Fig. 3, A–C). In well-watered plants (tc), ovary dry mass increased from about 1 to 17 mg during the 2-week sampling period. Growth rate was maintained at or slightly above control levels under modest stress (t1 and t2), but severe stress (t3 and t4) decreased post-pollination growth, and by +7 d, a 2-fold difference in dry mass was evident (Fig. 3A). Ovary Suc concentration decreased overall in well-watered plants (tc; Fig. 3B), showed little change under moderate stress (t1 and t2), and increased with severe stress (t3 and particularly t4). These differences were consistent with probable changes in capacity for Suc use. The dynamics in levels of hexoses (Fig. 3C) were opposite to those of Suc and increased overall during early ovary development regardless of stress (Fig. 3B). This rise was much slower under severe drought (t3 and t4), however, with hexose levels at pollination significantly less than under moderate stress (t1). Starch accumulated in unfertilized ovaries and to consistently higher levels under severe stress (t3 and t4) but decreased rapidly after pollination in all treatments (Fig. 3D). The sharpness of this drop between 0 d and +3 d indicated a net mobilization of starch within ovaries (compare Fig. 3, A and D on 0 d and +3 d). A similar pattern is observed for well-watered control plants grown in containers (Zinselmeier et al., 1999), but total starch levels tend to be lower under these conditions and were markedly reduced by rapid onset of drought stress (Zinselmeier et al., 1995, 1999).
Figure 3.
The effect of increasing drought stress on ovary dry mass (A), Suc (B), reducing sugars (C), and starch concentrations (D) at five different stages of ear development. Legend as for Figure 1. F-tests and comparison of means for each stage was performed with the Gabriel-algorithm as described in caption for Table I (n = 3–4). Aggregate statistical analyses for the entire period, where effects of dates were removed, showed that Suc concentration (B) was significantly higher under severe stress (t3 and t4) than in control (tc) and moderate stress (t1 and t2); reducing sugars (C) were significantly lower under severe stress (t3 and t4) than under moderate stress (t1 and t2); and starch (D) was significantly higher under severe stress (t3 and t4) than in control (tc) and moderate stress (t1 and t2).
Invertase Activities and Hexose/Suc Balance
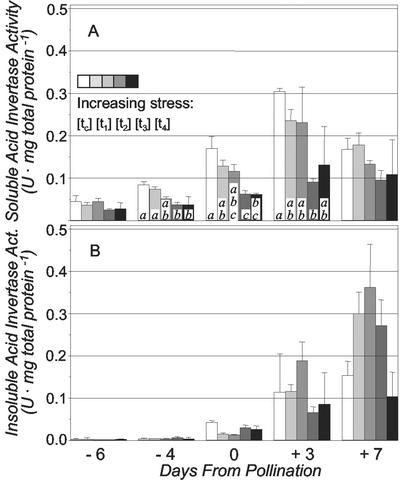
Because drought stress increased the concentration of Suc, decreased that of hexoses, and decreased the hexose to Suc ratio, it might be expected that activities of Suc-cleaving enzymes had been reduced, especially those of the acid invertases that predominate in young ovaries. However, differences were evident between soluble and insoluble acid invertase activities in ovaries during development and in response to drought stress (Fig. 4, A and B). Soluble activity rose early and strongly in ovaries of the well-watered control (tc) from −6 d to +3 d and declined thereafter (Fig. 4A). Severe drought (t3 and t4) significantly reduced this peak in soluble invertase activity beginning very early in development (from −4 d up to +3 d). In contrast, activity of insoluble acid invertase was barely detectable before pollination, increased strongly thereafter, and reached or surpassed soluble invertase activity by +7 d. Xu et al. (1996) and Zinselmeier et al. (1999) reported similar developmental patterns of activities, with higher levels of soluble than insoluble invertase immediately after pollination, followed by later rises in activity of the insoluble forms.
Figure 4.
The effect of increasing drought stress on soluble acid invertase activity (A) and insoluble acid invertase activity (B) at five different stages of ear development. A unit of enzymatic activity (U) equals 1 μmol of Suc hydrolyzed per minute under the assay conditions described in “Materials and Methods.” Legend as for Figure 1. F-tests and comparison of means for each stage were performed with the Gabriel-algorithm as described in caption for Table I (n = 3–4). Aggregate statistical analyses for the entire period showed that soluble acid invertase activity (A) was significantly lower under severe stress (t3 and t4) than in control (tc) and moderate stress (t1 and t2). Paired t-tests showed that soluble acid invertase activity (A) was significantly higher (α ≤ 0.05) than insoluble acid invertase activity (B) on −6 d, −4 d, 0 d, and +3 d and significantly lower on +7 d.
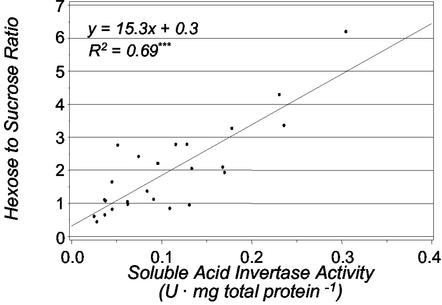
Figure 5 shows that the hexose to Suc ratio (from Fig. 3, B and C) was closely related to the soluble invertase activity. A correlation of r2 = 0.69*** was observed despite import and metabolism of sugars in the ovary that would prevent a true equilibrium from being established. The hexose to Suc ratio did not correlate significantly to insoluble invertase activity (r2 = 0.11), and correlation to the sum of activities (r2 = 0.41***) was lower than to soluble invertase activity alone.
Figure 5.
The correlation between the hexose to Suc ratio (w/w) and soluble acid invertase activity. A unit of enzymatic activity (U) equals 1 μmol of Suc hydrolyzed per minute under the assay conditions described in “Materials and Methods.” Regression analysis was performed with the PROC REG procedure (SAS Institute Inc., 1988). *** denotes that the correlation is significant at the 0.1% level. Each point is the mean of three to four observations.
Invertase mRNA Levels and Activity
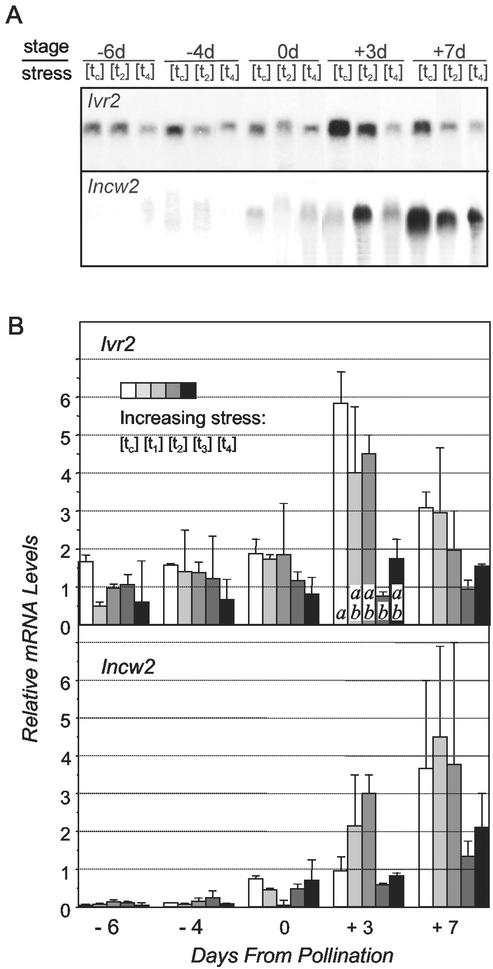
The relatively large developmental- and drought-induced changes in soluble and insoluble-acid invertase activity (at least 5- to 10-fold; Fig. 4) were also evident at the mRNA level (Fig. 6) based on quantification using probes for two vacuolar invertases (Ivr1 and Ivr2) and two cell wall-bound invertases (Incw1 and Incw2). Signal was not detectable for either Ivr1 or Incw1. This is consistent with other studies showing Ivr2 mRNAs predominate strongly over those of Ivr1 in young ovaries (Wu, 2000; P.D. Commuri, K.E. Koch, and R.J. Jones, unpublished data), with little or no detection of either Ivr1 or Ivr2 transcripts later in kernel development (Xu et al., 1996; Carlson and Chourey, 1999; P.D. Coummuri, K.E. Koch, and R.J. Jones, unpublished data). The Incw1 is apparently expressed only at later stages of seed development (Taliercio et al., 1999). In the present work, Ivr2 provided a strong signal from bands about 2.1 kb, whereas Incw2 gave a weak signal, subsequently improved by application of a [32P]UTP-RNA probe (Fig. 6A).
Figure 6.
A, Gel blots showing the effect of increasingly severe drought stress treatments on accumulation of Ivr2 and Incw2 mRNA in maize ovaries from −6 d before to +7 d after pollination ([tc] = control, [t2] = moderate stress, and [t4] = severe stress, as described below). The apparent size of both Ivr2 and Incw2 mRNA was about 2.1 kb. B, Relative levels of Ivr2 and Incw2 mRNA quantified from maize ovaries between −6 d before to +7 d after pollination based on two replicates (n = 2) of the following treatments: [tc], control; [t1], last irrigated July 28; [t2], last irrigated July 17; [t3], last irrigated July 03; and [t4], last irrigated June 21). Shading of bars (left to right) indicates increasing severity of drought stress treatment. F-tests and comparison of means for each stage were performed with the Gabriel-algorithm as described for Table I. The statistical analyses showed that Ivr2 mRNA level was significantly lower under severe stress (t3) than in control (tc), both on +3 d and for the entire period as a whole. For RNA gel blots, 20 μg of total RNA was loaded into each lane, and density of ribosomal RNA bands was used as a loading control. These were uniform in all instances.
Changes in relative abundance of Ivr2 and Incw2 mRNAs differed clearly from one another and exhibited patterns that paralleled those of soluble and insoluble invertase activities, respectively. This was evident over time and in different treatments (compare Fig. 4, A and B, with Fig. 6B). The relative level of Ivr2 mRNA (Fig. 6B) was significantly lowered by severe stress (t3) compared with well-watered controls (tc), both for the entire period and at +3 d when apparent expression was maximal. Although mean values for relative Incw2 levels of mRNA (Fig. 6B) tended to be lower under severe stress (t3 and t4), these did not differ significantly from the well-watered controls (tc).
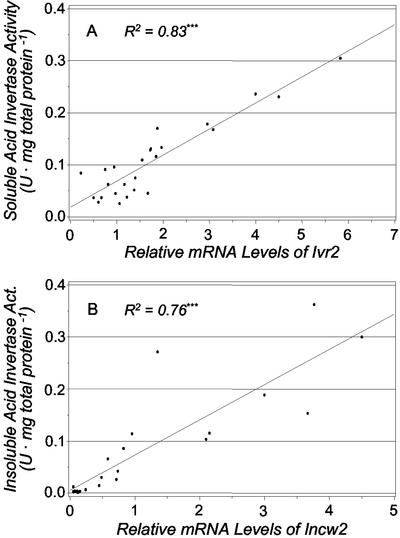
The regression analysis in Figure 7 confirms a close correspondence between differences in soluble invertase activity (Fig. 4A) and changes in relative levels of Ivr2 mRNA (Fig. 6B) for all developmental stages and treatments (Fig. 7A). A significant correlation was also observed between insoluble invertase activity and Incw2 mRNA (Fig. 7B). These data thus indicate that transcript abundance makes a significant regulatory contribution to activities of both invertase forms. Although a nearly full compliment of maize kernel invertase genes was tested, the possibility remains that some soluble or insoluble invertase activity might have been derived from other coregulated sources.
Figure 7.
A, The correlation between soluble acid invertase activity and relative mRNA levels of Ivr2. B, The correlation between insoluble acid invertase and relative mRNA levels of Incw2. A unit of enzymatic activity (U) equals 1 μmol of Suc hydrolyzed per minute under the assay conditions described in “Materials and Methods.” Regression analysis was performed with the PROC REG procedure (SAS Institute Inc., 1988). *** denotes that the correlation is significant at the 0.1% level. Each point is the mean of two observations.
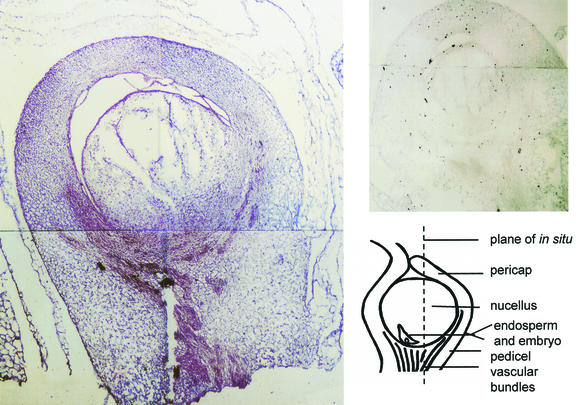
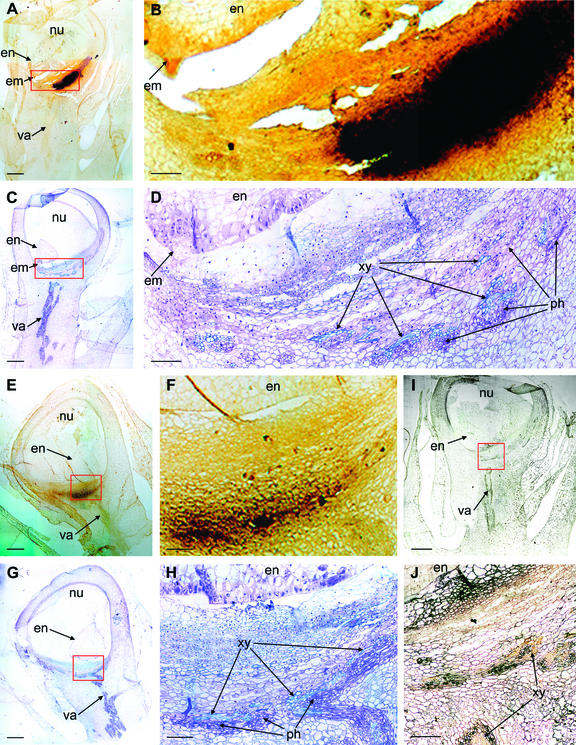
To further define the functional role of soluble invertase during early ovary development, sites of Ivr2 mRNA were localized in situ (Fig. 8). Kernels were examined at +6 d because Ivr2 expression remained high at this stage and pollination status could be visually verified for individual ovaries. The maternal tissues included the pericarp, pedicel, and basal region of the nucellus, all of which grow markedly in the young kernel. They compose the predominant sink tissues during the first third of kernel development (about 10 d), later giving way to growth of the endosperm and embryo. In these recently pollinated kernels, Ivr2 mRNAs were evident throughout most maternal tissues (including integuments and basal parts of nucellus), but especially in vascular zones and in the region between vascular tissue and the newly developing embryo and endosperm.
Figure 8.
In situ localization of Ivr2 soluble invertase mRNAs in tissues of young maize kernels (NK-508) at +6 d post-pollination. Labeled Ivr2 mRNAs are evident in several areas of the maternal tissues that make up the maize kernel at this stage of development, with strongest signal in cells surrounding the tiny, newly fertilized embryo + endosperm (minute and off the field of view; see drawing to lower right) and in cells near vascular bundles of the pedicel. Signal proximal to the embryo + endosperm is localized in basal regions of the nucellus (maternal tissue filling the central kernel during early development), plus cells of the lower pericarp (ovary wall). Individual kernels were sampled as soon as pollinated ovaries could be visually distinguished from non-pollinated neighbors. Fixed and sectioned samples were probed with sense (control, top right) and antisense (in situ localization) RNA probes synthesized with digoxygenin-labeled UTP from a 576-bp Ivr2 fragment as described in the text.
To confirm acid invertase activity in the regions of mRNA expression and further distinguish tissue specific differences, activity was localized in situ on longitudinal methacrylate thin sections of +7-d ovaries using a Glc oxidase/peroxidase-diaminobenzidine (DAB)-staining method (Fig. 9). Modest activity (gold in Fig. 9) was evident throughout most maternal tissues, in integuments and basal parts of nucellus, endosperm, and embryo. Strong activity (black in Fig. 9) was localized around terminal regions of the vascular strands and as a declining gradient across the placental parenchyma toward the nucellus and filial tissues. Overall, this pattern is consistent with the mRNA localization and the prominent Ivr2 expression in basal regions of maternal tissues in the young ovary. As observed for quantified, whole-ovary assays (refer back to Fig. 4), a decrease in activity from well-watered control (tc) to severely drought-stressed ovaries (t4) can also be seen in the in situ analysis of activity when comparing Figure 9, A and B, with Figure 9, E and F. Severe drought stress reduced both the activity and the size of the placental region in which the invertase activity was most strongly detected. Modest activity in basal endosperm cells (gold in top left of Fig. 9B) may have reflected a prelude to the later differentiation of transfer cells and expression of insoluble invertase at these sites (Cheng et al., 1996). On one hand, this evidence that acid invertase activity is localized to both maternal and filial tissues of young ovaries is consistent with data from later in development (Doehlert and Felker, 1987; Miller and Chourey, 1992). However, the dominant localization to maternal tissue in the young, developing ovary contrasts to that of older stages, where activity in filial tissue predominates.
Figure 9.
In situ assay of acid invertase activity in kernel tissues during the first phase of development (+7 d post pollination) under well-watered conditions (A and B) or severe drought-stress (E and F), with each image paired to a parallel, serial section (C, D, G, and H) stained with toluidine blue for structural clarity. A, Whole-ovary view during the first phase of kernel development under well-watered conditions, with invertase activity shown as increasing from light-gold (throughout young ovary) to black (placental region immediately adjacent to nucellus [contiguous maternal tissues]). B, Detail of basal kernel regions, with filial generation to the top left (endosperm and tiny embryo), maternal tissues to the bottom right (nucellus and placental zone), and parallel structural information in subsequent panels. C and D, Toluidine blue staining of structural features for comparison to parallel sections in A and B, showing tissues in the zone of greatest invertase activity for very young kernels to be anastomosing vascular bundles with contiguous maternal parenchyma (phloem, nuclei, and cell walls are stained indigo, and xylem, turquoise). E, Whole-ovary view during the first phase of kernel development under severe drought stress, with invertase activity shown as gradations of gold (as for A). F, Detail of E. G and H, Toluidine blue staining of structural features for comparison with parallel sections in E and F, showing tissues in the zone of greatest invertase activity for very young kernels under drought stress to be much reduced areas of anastomosing vascular bundles with contiguous maternal parenchyma (phloem, nuclei, and cell walls are stained indigo, and xylem, turquoise). I, Negative control stained for acid invertase activity but without substrate (Suc) addition, well-watered treatment. J, Detail of I; artifactual staining of xylem may be a result of endogenous peroxidases involved in lignin biosynthesis. Longitudinal 5-μm methacrylate sections were obtained within 20 μm and stained alternatively by the two methods. Acid invertase activity was visualized using a coupled enzymatic reaction with Suc as substrate. Hydrogen peroxide produced by Glc-oxidase was used to oxidize DAB in a reaction catalyzed by horseradish-peroxidase, which formed a dark-brown/yellow DAB deposit at sites of acid invertase activity. Counterstaining with silveroxide and staining of adjacent sections with toluidine blue is described in the text. em, Embryo; en, endosperm; nu, nucellus; ph, phloem; va, vascular strand; and xy, xylem. Bars in A, C, E, G, and I = 500 μm. Bars in B, D, F, H, and J = 100 μm.
The magnitude of insoluble activity assayed in ovaries at +7 d indicated that additional maternal sites of insoluble invertase activity developed before those of the basal endosperm transfer cells. At least some vascular activity may have arisen from insoluble invertase once post-pollination increases began, because Cheng et al. (1996) immunologically detected a cell wall-bound form in vascular tissue of older kernels. However, if so, it did not significantly influence the hexose to Suc ratio.
DISCUSSION
Research presented here provides significant insight into our understanding of early stages in grain development, into their marked sensitivity to stress, and also into the regulation and roles of different invertase genes during these formative periods. The work was initiated to resolve contributions by genes for soluble and insoluble acid invertases during key phases in early development of maize ovaries. In addition to thorough analysis of very early stages, the approach included perturbation of early reproductive development by a controlled series of increasingly severe water-deficit treatments (Fig. 1). Comparative analysis of responses extended from the whole-plant and yield levels (Table I; Asch et al., 2001) to temporal changes in ovary ABA levels (Fig. 2), carbohydrate composition (Fig. 3), and the activity and expression of specific invertases (Figs. 4–7). Changes in the latter over time were supplemented with analysis of spatial distribution in situ (Figs. 8 and 9).
Soluble Invertase and Hexose to Suc Balance in Young Ovaries
Drought stress during early ovary development, especially before pollination, may lead to zygotic abortion followed by a complete inhibition of ovary growth (Westgate and Boyer, 1986; Zinselmeier et al., 1995). However, mechanisms underlying this stress-induced abortion remain unclear. Few studies have addressed metabolic processes and their role in ovary development at this stage, when maternal tissue still constitutes the major part of the kernel. Drought stress has been found to consistently affect sugar metabolism and decrease activities of soluble and insoluble invertases (Zinselmeier et al., 1995, 1999).
In the present study, the hexose to Suc ratio was correlated to activity of soluble invertase (Fig. 5), but not to activity of insoluble invertase in young ovaries. Relationships between acid invertase activity and concentrations of Suc, Glc, and Fru are common among many plant organs (e.g. Nielsen et al., 1991) and appear to involve product inhibition by Fru and Glc that effectively prevents complete hydrolysis of Suc by acid invertase in vacuoles (Isla et al., 1998). The young, unfertilized ovary receives Suc through the phloem of the vascular system that ends in the pedicel region (Felker and Shannon, 1980). Subsequent post-phloem transport through maternal tissue of the ovary encounters little or no barrier to symplastic transfer via plasmodesmata. Recent studies of other species have indicated this to be the case for assimilates entering maternal tissues of many seeds. In Arabidopsis, green fluorescent protein produced in the vascular system is symplastically transported through the maternal seed coat tissues of the developing ovules (Imlau et al., 1999). In wheat (Triticum aestivum), fluorescent tracers of different size have shown that radii of plasmodesmata in maternal portions of the developing grain are appreciably enlarged and can allow a high rate of assimilate transport (Fisher and Cash-Clark, 2000a). In developing grains, like many other fruits, Suc transport and metabolism thus occur largely in the symplast of maternal tissues that predominate in the young ovaries. As such, Suc transfer is likely to be mediated by soluble rather than insoluble invertase during the period when drought stress can most critically influence the survival of zygotes. The correlation between hexose to Suc ratio and activity of soluble invertase observed here is compatible with a predominantly symplastic path for sugar movement in young maize ovaries.
The agreement between levels of Ivr2 mRNA and soluble invertase activity (Fig. 7), together with contrasting developmental profiles for Ivr2 and Incw2 mRNA (Fig. 6), clearly support distinct temporal roles that were not readily resolvable by concurrent extraction of both enzymes in more mature kernels (Carlson and Chourey, 1999). The role of the Incw2 cell wall-bound invertase activity in Suc hydrolysis and assimilate transfer to developing endosperm at a later stage of kernel development has been detailed previously (Cheng et al., 1996). The cell wall-bound invertase in the cell wall space can facilitate assimilate transfer from maternal tissues to the developing endosperm because lack of symplastic connections between these tissues necessitates an apoplastic cell wall step (Felker and Shannon, 1980). This is consistent with the suggestion that Incw2 is expressed primarily in filial cells (Carlson and Chourey, 1999). However, during early phases of growth, the kernel is almost entirely maternal tissue. In addition, critical effects of drought on early kernels are less likely to involve cell wall-bound invertase because more than 90% of this activity is dispensable for normal seed development, and kernels of the miniature mutant are small but not aborted (Cheng et al., 1996).
Regulation of Vacuolar Invertase in Young Ovaries
Transcript abundance appeared to be a prominent contributor to regulation of both invertase activities (Fig. 7), and expression of the Ivr2 vacuolar invertase was significantly reduced by drought (Fig. 6B). In the present work, stress-induced changes in ABA-levels of young ovaries did not seem to be directly linked to regulation of vacuolar invertases (Figs. 2, 4A, and 6B). Only the most severe stress increased ovary ABA levels, and only during very early development. Although endogenous ABA levels in these ovaries were initially 2-fold greater than those of the control, this was no longer evident after −6 d (Fig. 2). Drought-stress reductions in the soluble invertase activity (Fig. 4A) and Ivr2 mRNA levels (Fig. 6B) did become apparent later, but although the increase in ABA concentration preceded the decrease in soluble invertase activity, no immediate relationship could be discerned. Fairly rapid ABA responses might have been expected (e.g. Jensen et al., 1996) but were not evident. Nonetheless, a more indirect regulatory role of ABA could be involved (Kim et al., 2000), possibly including overlap between ethylene, sugar, and ABA at the level of signal transduction (Rolland et al., 2002).
Soluble invertase expression and activity may also be regulated by signals of sugar availability under drought stress. Ivr2 expression can be induced in maize root tips by exogenous sugars (Xu et al., 1996) at concentrations reported here for endogenous sugars of maize ovaries. Levels of Ivr2 mRNA correlated negatively to Suc levels, which increased under severe stress. However, reducing sugar content was decreased, resulting in a positive correlation between Ivr2 transcript abundance and the molar sum of hexoses plus Suc on a dry weight basis. Both the quality and quantity of available sugar may therefore contribute to the drought stress signals acting here. In leaves, Kim et al. (2000) recently reported an inductive effect of drought on Ivr2 expression in vegetative organs. Although the opposite response was observed for young ovaries in the present study, sugar status and/or a number of other endogenous effectors could alter regulation in different organs.
Role of Vacuolar Invertase in Young Ovaries
Drought-responsiveness of vacuolar invertase could contribute to maternal mechanisms for adjustment of ovary sink strength and kernel number, and it could do so in several ways (Fig. 4; Table I; Zinselmeier et al., 1995, 1999). These include direct effects of a vacuolar cleavage site on Suc import, use, and post-phloem transfer, as well as changes in the endogenous sugar environment and osmotic contributions to growth or stress acclimation.
A vacuolar invertase path for Suc hydrolysis would be especially useful in a symplastically continuous system of phloem-unloading and post-phloem transport where internal Suc cleavage could sustain Suc gradients across plasmodesmata (Duke et al., 1991; Sturm et al., 1995; Fisher and Cash-Clark, 2000b; Kim et al., 2000). The abundant activity and mRNA localization in parenchyma surrounding terminal regions of vascular bundles in maize ovaries (Figs. 8 and 9) are compatible with a role for Ivr2 vacuolar invertase in post-phloem transport. Impairment of this process could also contribute to the Suc accumulation and decreased Suc use observed for young, drought-stressed ovaries by Zinselmeier et al. (1995, 1999). In fact, if phloem unloading in grains occurs by mass flow and a descending turgor gradient (Fisher and Cash-Clark, 2000a), then drought-induced declines in vacuolar invertase could have a greater effect on post-phloem transfer than on import per se. Regardless of mechanisms or points of control, a key feature of symplastic sinks appears to be Suc gradients across plasmodesmata (Patrick and Offler, 1995; Fisher and Cash-Clark, 2000a, 2000b), which could be readily modulated by endogenous vacuolar invertase.
In addition to a drop in Suc delivery, capacity of endogenous Suc use could be critically reduced in young ovaries by the observed decreases in soluble invertase. The importance of this process was initially suggested by Zinselmeier et al. (1995, 1999), when drought treatments applied to container-grown plants led to an almost complete depletion of ovary starch at pollination. Because invertase activities and intermediates in starch biosynthesis were also depleted, the authors suggested that low activity of acid invertases might contribute to the observed reductions of starch biosynthesis. They further noted that depletion of these starch reserves, together with the reduced Suc supply, could be lethal for the newly formed zygote.
However, a marked reduction in capacity for Suc cleavage via invertase may affect kernel development independently of ovary starch. First, ovary starch synthesis may be more directly related to Suc synthase activity (Herbers and Sonnewald, 1998; Sturm and Tang, 1999, and refs. therein), and maize ovaries at the time of pollination show a colocalization of Suc synthase activity (Wittich and Vreugdenhil, 1998) with sites of starch deposition (Zinselmeier et al., 1999). Second, reported increases in wheat kernel number of transgenic plants with a more active endosperm ADP-Glc pyrophosphorylase (elevating endosperm starch via insensitivity to Pi inhibition) would be expected to act at later stages of development after filial endosperm had formed (Smidansky et al., 2002). This is compatible with different avenues contributing to kernel number adjustment at different stages of development. Third, under field conditions in the present study, ovary starch levels were significantly elevated rather than reduced by drought. This was evident as early as −6 d (Fig. 3D), and ovary Suc concentrations were generally also higher during severe drought (Fig. 3B). The longer, slower drying of soil under field conditions in the present work may have allowed more time for stress acclimation, and differences in root system size, varieties, and irradiation may also have been involved. Nonetheless, kernel abortion did occur under conditions of the present work, when starch and Suc were apparently abundant, although it may have been the ovaries with least starch that were affected. The reduced kernel number per ear at harvest (Table I; Asch et al., 2001) probably was due in part to the initiation of fewer flowers, as indicated by shorter ears and reduction of kernel number in the rows. However, the decrease in individual ovary dry mass (Fig. 3A), visual observation of nondeveloping kernels at +7 d, and pattern of kernel distribution in ears at harvest indicate that drought-induced abortion can occur without detectable previous reductions in overall starch or Suc levels (also observed for other aborting kernels by Setter et al. [2001]).
Drought-induced inhibition of vacuolar invertase could also affect generation of hexose-based turgor pressure for cell expansion during growth and development. The distribution of at least some activity and Ivr2 mRNA throughout most of the ovary tissues (Figs. 8 and 9) is consistent with such a function. A role for vacuolar invertase has been implicated in a broad range of expanding young sinks such as elongating silks (Xu et al., 1996), tulip (Tulipa spp.) stalks (Balk and de Boer, 1999), tobacco (Nicotiana tabacum) leaves (Hoffmann et al., 1997), carnation (Dianthus caryophyllus) petals (Woodson and Wang, 1987), and carrot (Daucus carota) tap roots (Tang et al., 1999). Zinselmeier et al. (1999) found that drought stress decreased ovary water potential and turgor unless plant stems were infused with Suc. In the present work, severe stress decreased the molar sum of Suc and hexoses; thus, ovary sugars did not appear to contribute to osmotic acclimation. This lack could have been detrimental to normal cell expansion, which in turn would have broad secondary effects including turgor or osmolyte sensing.
A final possibility is that further metabolism of the invertase products, Glc and Fru, mediated by hexokinase, could initiate a path of signal transduction of central importance to normal zygote development (Koch et al., 1996; Wobus and Weber, 1999; Weschke et al., 2000; Rolland et al., 2002). Invertase-derived hexoses were found to be critical morphogenic factors for development of normal carrot embryos (Sturm and Tang, 1999; Tang et al., 1999). In addition, extensive studies of seed development have shown hexoses to be closely associated with cell division and expansion, with Suc favoring storage and maturation (Weber et al., 1996, 1998; Wobus and Weber, 1999; Weschke et al., 2000). Despite multiple mechanisms for sugar sensing (Koch, 1996; Koch et al., 2000; Rolland et al., 2002), it seems clear that shifts in hexose to Suc balance could have far-reaching effects.
In conclusion, drought-induced expression of Ivr2 in vegetative tissues may restrict Suc export (Kim et al., 2000), and together with a concomitant repression in young ovaries, may limit Suc transport and use and result in partial zygote abortion. This, in turn, could confer a critical survival advantage for few versus many seeds in terminal drought environments by reducing sink numbers at a key point in development and thus secure a sufficient Suc supply for maturation of a few remaining seeds.
This work extends our current understanding of maize invertases and grain development to include the physiologically distinct phases of initial growth and their marked sensitivity to drought-induced abortion. Evidence supports the following advances: (a) Sequential expression of first soluble, then insoluble invertases, at both the mRNA and activity levels, indicates distinct contributions by these invertases to young ovaries during normal development and under stress. (b) Soluble invertase expression in young ovaries is an early target of drought stress, and the response is localized to sites of import and expansion by maternal tissues. Hence, soluble invertase may contribute to a maternal mechanism for control of kernel number under stress before or immediately after pollination. (c) A strong link was also evident between changes in expression of specifically the soluble invertase and shifts in hexose to Suc balance, the latter being recently implicated as an effector of seed development both in dicots and monocots.
MATERIALS AND METHODS
Plant Material and Sampling
Maize (Zea mays L. cv Loft) was grown during the summer of 1997 under field conditions at the Royal Veterinary and Agricultural University experimental station Höjbakkegaard outside Copenhagen (55°40′ N; 12°18′ E; 28 m above sea level), as detailed by Asch et al. (2001). In short, 18 plots were established in concrete lysimeter tanks, each with a surface area of 4 m2, filled to 1 m deep with loamy sand soil holding 100 to 130 mm of plant available water. A mobile glass roof automatically covered the plants during rainfall, and all water was supplied through a trickle irrigation system in each tank. Plants were grown using otherwise normal husbandry, i.e. plant spacing, fertilizer, etc. (Asch et al., 2001). Five treatments were imposed in a fully randomized design. Control plots were irrigated to field capacity twice weekly. In addition, four drought treatments were imposed by withholding irrigation from plots belonging to the drought treatments beginning at 38, 50, 64, or 75 d after seeding. Different stress levels and durations were thus achieved during the sampling before and after pollination. After the last sampling, all plots were watered to field capacity and held at this level until crop maturity. Yield components were measured at this time.
Ear development was staged according to silk length and appearance, and whole ears were sampled at the same stages of development, i.e. −6, −4, 0, +3, and +7 d from pollination, in the different treatments. The ears were immediately frozen in liquid N2 and stored at −80°C until further processing. Ovaries were excised from the central part of the ears under liquid N2. These ovary samples included pericarp, internal tissues, upper part of the pedicel, and parts of glumes and lemmae, whereas silks were detached (see also Fig. 9). These tissues were ground coarsely in a mortar with liquid N2, and the frozen material was used in subsequent analyses. Dry matter percentage and ovary dry weight were determined by freeze-drying 50 ovaries per ear until constant weight for 3 to 4 d.
For in situ localization of Ivr2 mRNAs, kernels were sampled from maize (hybrid NK 508) plants grown under standard field conditions in north Florida from April to July, 1998. Ovules were pollinated 1 d after silking. Samples were harvested 6 d later when pollinated and unpollinated kernels could be visually distinguished from one another. Individual kernels including pedicels and other subtending tissues were excised and fixed in cold formaldehyde-acetic acid (10% [w/v] formalin, 5% [v/v] acetic acid, and 45% [v/v] ethanol) at 4°C overnight.
Metabolite Analyses
For determination of ABA content, 50 mg of lyophilized material was boiled for 2 min in 1 mL of H2O and extracted on a shaker at 4°C for 24 h. After centrifugation, the supernatant was analyzed for ABA by indirect ELISA as described by Weiler (1982) and Hansen (1996). No cross-reaction was found when tested as described by Quarrie et al. (1988).
Carbohydrate content was measured after grinding approximately 5 mg of frozen samples in Eppendorf vials with acid-washed sand and 500 μL of 80% (v/v) ethanol. Material was subsequently heated to 80°C for 15 min and centrifuged for 5 min at 20,000g to pellet insoluble material. Extraction was repeated twice with 500 μL of 80% (v/v) ethanol, and supernatants were pooled for evaporation to dryness in a vacuum centrifuge. Carbohydrates in this fraction were resolubilized in 900 μL of water. Reducing sugars were quantified in an aliquot of 100 μL according to Nelson (1944) with Glc as a standard. Suc was quantified by subtraction using the same methods after Suc inversion by β-fructosidase. Complete Suc hydrolysis was achieved by adding 20 units of β-fructosidase (Roche Diagnostics, Basel) per sample and incubating for 10 min at 30°C in 50 mm sodium acetate buffer with 15 mm magnesium chloride (pH 4.6). The Nelson reducing sugar assay (Nelson, 1944) was also used to estimate starch content after digestion of insoluble material. These fractions were dried in a vacuum centrifuge and boiled for 30 min with a thermostable amylase (Termamyl, Novo Nordisk, Glostrup, Denmark) in 1 mL of 5 mm sodium dihydrogen phosphate buffer (pH 6.0). Starch was further hydrolyzed in a 100-μL aliquot with 2.5 units of amyloglucosidase (Roche Diagnostics) in 50 mm sodium acetate buffer with 15 mm magnesium chloride (pH 4.6) at 65°C. Sample blanks, reagent blanks, and samples with known starch content were included. Three samples from each date and plot were analyzed.
Quantification and in Situ Localization of Enzymatic Activity
Crude enzyme extracts from approximately 40 mg of frozen ovary material were further ground in Eppendorf vials with sand and 300 μL of extraction buffer consisting of 50 mm HEPES-NaOH, 1 mm EDTA, and 2.5 mm dithiothreitol, pH 7.0. Samples were centrifuged for 10 min at 20,000g to pellet insoluble material. The soluble protein extract was removed, and the remaining pellet was washed three times with extraction buffer. Insoluble proteins were then extracted with buffer containing 1 m NaCl (Doehlert and Felker, 1987). Soluble protein extract (200 μL) was dialyzed against extraction buffer for 16 h at 0°C on a 10,000 molecular weight cutoff dialysis membrane (Pierce, Rockford, IL) to remove endogenous soluble carbohydrates. The concentration of total protein was measured in the extract as described by Bradford (1976) using a bovine serum albumin (BSA) standard.
Activities of soluble acid invertase and insoluble acid invertase were measured as described by Tsai et al. (1970) with minor modifications. Invertase extracts (10 or 20 μL) were assayed in a total volume of 300 μL, with an assay buffer containing 50 mm sodium acetate, 15 mm magnesium chloride, and 100 mm Suc (pH 5.0). Assays were incubated for 0.5 to 2 h at 30°C, with blanks terminated immediately after addition of protein extracts. All reactions were terminated by adding 300 μL of Nelson's no. 1 reagent. Reducing sugars were quantified by spectrometry according to the Nelson-Somogyi method (Nelson, 1944) with a Glc standard. Two samples were assayed from each date and plot, with duplicate quantification for each.
For in situ localization of acid invertase activity, slightly frozen ovaries were cut longitudinally in two halves with a scalpel and briefly fixed for 30 min in phosphate buffer (pH 7) with 4% (w/v) formaldehyde. Fixed material was dehydrated in increasing concentrations of ethanol and infiltrated and embedded in Technovit 7100 (Heraeus Kulzer, Wehrheim, Germany) according to manufacturer's instructions. All steps were conducted at 4°C, and resulting solidified methacrylate blocks were stored at −20°C until sectioning. Sections were cut at 5 μm with a steel knife on a microtome, floated on extraction buffer (noted above), mounted on glass slides, and briefly dried at 40°C. Acid invertase activity was visualized by deposition of oxidized DAB in a coupled enzymatic reaction. Slides were immersed in assay buffer (noted above) amended with 25 units mL−1 Glc oxidase (Roche Diagnostics), 25 units mL−1 horseradish peroxidase (Sigma, St. Louis), 0.3 mg mL−1 DAB (Sigma), and 1 mg mL−1 NiCl2, 6H2O, and the reaction was allowed to proceed for 24 h at 30°C. Negative controls without Suc were incubated likewise. Slides were washed two times in 1% (w/v) sodium acetate, 3H2O for 1 min and counterstained with silver nitrate solution as per Merchenthaler et al. (1989). This approach was originally developed to intensify the oxidized DAB precipitate, but in our procedure black-gray silver oxide alone was deposited preferentially on cell walls rather than on DAB, thereby enhancing the contrast to the yellow-brown DAB-deposits and providing a good outline of general tissue anatomy. This presents, to our knowledge, the first example of acid invertase activity detection in thin sections from methacrylate embedded tissue. Sections adjacent to those used for activity localization were stained for 10 s in 0.5% (w/v) toluidine blue O (Sigma) in a 1% (w/v) disodiumtetraborate solution and briefly washed in water and 0.1% (v/v) acetic acid.
Quantification and in Situ Localization of mRNA
Total RNA was extracted (using a modified method of Dong and Dunstan, 1996) from approximately 200 mg of frozen samples that were ground thoroughly using a mortar and pestle with sand. Samples were suspended in 4.5 mL of an RNA extraction buffer of 50 mm Tris (pH 8.0), 300 mm NaCl, 5 mm EDTA, 2% (w/v) SDS, 14 mm mercaptoethanol, and 1 mm aurintricarboxylic acid, and 0.7 mL of 3 m KCl was added. After 15 min on ice, extracts were centrifuged for 10 min at 4°C and 10,500g followed by the addition of 2 mL of 8 m LiCl to the supernatant. After overnight precipitation at 4°C, extracts were centrifuged for 20 min at 4°C and 10,500g. The pellet was resuspended in 2 mL of water, and proteins were removed by extracting twice with phenol/chloroform/isoamylalcohol. Total RNA was precipitated with 200 μL of 3 m NaAc and 5 mL of absolute ethanol for 2 h at −20°C and was pelleted by centrifuging for 20 min at 4°C and 10,500g. This RNA was washed by resuspending in 2 mL of 80% (v/v) ethanol and centrifuging for 10 min at 4°C and 10,500g. The final RNA pellet was dried and resuspended in 100 μL of H2O.
Total RNA samples (20 μg lane−1), together with a Mr marker, were separated by electrophoresis in a denaturing 1.3% (w/v) agarose gel with 6% (v/v) formaldehyde. Equal loading was assessed by ethidium bromide staining. RNA was transferred to a nylon membrane (Zeta-Probe, Bio-Rad, Hercules, CA) by vacuum blotting in 10× SSC and cross-linked by UV irradiation. [32P]ATP probes of cDNAs encoding two vacuolar invertases (Ivr1, accession no. U16123: 1,150-bp cDNA-fragment covering approximately position 400–1,550 of the coding sequence; and Ivr2, accession no. U31451: 0.87-kb 3′-cDNA fragment, including 465-bp 3′ coding and approximately 400-bp 3′-untranslated region [Xu et al., 1996]) and two cell wall-bound invertases (Incw1, a 270-bp PCR-fragment covering position 1,740–2,010 of the coding sequence including 40-bp 3′-coding sequence and 230-bp 3′-untranslated region showing >98% homology to accession no. U17695; Incw2, a 330-bp PCR-fragment covering position 1,614–1,944 of the coding sequence including 165-bp 3′-coding sequence and 165-bp 3′-untranslated region showing >98% homology to accession no. AF050631) were applied successively. Incw1 and Incw2 fragments were isolated by PCR amplification using nested, gene-specific primers from genomic DNA isolated from leaf material of maize cv Loft. Ivr1, Ivr2, and Incw1 [32P]ATP probes were produced with a random primed DNA-labeling kit (Roche Diagnostics) whereas for Incw2, [32P]UTP RNA probes were generated using a T7 phage polymerase RNA-labeling kit (Ambion Inc., Austin, TX). Membranes were hybridized for 20 h under conditions providing probe specific responses (0.25 m sodium phosphate, 7% [w/v] SDS, 1% [w/v] BSA, pH 7.2 at 65°C for the [32P]ATP DNA probes, and 50% [v/v] formamide hybridization solution (Ultrahyb, Ambion Inc.) at 65°C for the [32P]UTP RNA probe) and washed at high stringency (20 mm sodium phosphate, 5% [w/v] SDS, pH 7.2, and 20 mm sodium phosphate, 1% [w/v] SDS, pH 7.2, at 65°C for the [32P]ATP DNA probes, and 2× SSC/0.1% [w/v] SDS, 0.1× SSC/0.1% [w/v] SDS at 68°C for the [32P]UTP RNA probe). Filters were exposed to x-ray film (Eastman Kodak, Rochester, NY) in cassettes with one intensifying screen at −80°C. Bands were quantified by the Imagemaster 1D software (Amersham Pharmacia Biotech AB, Uppsala). Two samples per treatment were analyzed. Values were normalized by comparison with a calibration curve generated by loading 5, 10, 20, 40, and 80 μg of total RNA of some of the samples.
For in situ hybridization a 576-bp, NcoI-NotI fragment of Ivr2 cDNA (the NcoI and NotI sites respectively, lie 64 and 640 bp downstream from the 5′ end of the existing cDNA and 130 bp downstream of the conserved NDPNG consensus sequence common to functional invertases defined thus far) was selected because of its minimal homology to Ivr1 and other invertases. This fragment was subcloned into the NotI and HincII sites of a Bluescript II SK plasmid, which has T3 and T7 promoters at each side of its polylinker. DNA templates were linearized with either NotI or EcoRI for in vitro transcription and were driven by T3 or T7 promoters, respectively. Sense and antisense RNA probes were synthesized with digoxigenin-labeled UTP (Roche Diagnostics) according to manufacturer's instructions. Probes were hydrolyzed with carbonate buffer (pH 12.2) at 60°C for 22 min to yield an average size of approximately 200 bp. For each slide, 25 ng of RNA probe was applied for in situ hybridization.
Tissue fixations proceeded at 4°C overnight (as described above), under vacuum for the first 2 h. Fixed plant tissues were dehydrated in a series of increasing concentrations of ethanol and histoclear before being imbedded into paraplast. Embedded tissues were cut into 10-μm sections with a microtome and mounted on ProbeOn Plus microscope slides (Fischer Scientific, Pittsburgh). In situ hybridization procedures were conducted as per Jackson et al. (1994). Tissue sections were pretreated with 0.2 m HCl for 20 min, 1 μg mL−1 proteinase K for 30 min, 4% (w/v) paraformaldehyde for 10 min, and 0.5% (v/v) acetic anhydride for 10 min before being probed with labeled sense or antisense RNAs.
Hybridization at 55°C for 12 h was followed by 2 h of rinsing in 0.2× SSC at 50°C. Hybridized sections were treated with 20 μg mL−1 RNase A at 37°C for 30 min followed by another hour of stringent rinsing in 0.2× SSC at 50°C. For immunological detection of hybridized RNA probes, sections were incubated with alkaline phosphatase-conjugated anti-dig antibody (diluted 1:1000) for 2 h at room temperature after incubation for 45 min in 1% (w/v) blocking agent (Roche Diagnostics) and another 45 min in 1% (w/v) BSA. Visible product was generated from alkaline phosphatase activity by incubating the sections with 100 mm Tris-HCl (pH 9.5), 100 mm NaCl, and 50 mm MgCl2, containing a 2% (v/v) mixture nitroblue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate (Roche Diagnostics) for 1 to 3 d at room temperature. Sections were dehydrated with increasing concentration of ethanol and histoclear before mounting with Permount (Fischer Scientific).
ACKNOWLEDGMENTS
We thank Jytte Toft for the analyses of carbohydrates and invertase activities and Mohamoud A. Hashi for assistance with in situ detection of invertase activity.
Footnotes
This work was supported by the Danish Institute of Agricultural Sciences, by the Institute for Agricultural Sciences at the University of Florida (journal series no. R–08830), by the Danish Agricultural and Veterinary Research Council, and by the U.S. National Science Foundation.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.005637.
LITERATURE CITED
- Asch F, Andersen MN, Jensen CR, Mogensen VO. Ovary abscisic acid concentration does not induce kernel abortion in field-grown maize subjected to drought. Eur J Agron. 2001;15:119–129. [Google Scholar]
- Balk AB, de Boer AD. Rapid stalk elongation in tulip (Tulipa gesneriana L. cv. Apeldorn) and the combined action of cold-induced invertase and the water-channel protein γTIP. Planta. 1999;209:346–354. doi: 10.1007/s004250050642. [DOI] [PubMed] [Google Scholar]
- Bassetti P, Westgate ME. Water deficit affects receptivity of maize silks. Crop Sci. 1993;33:279–282. [Google Scholar]
- Bassetti P, Westgate ME. Floral asynchrony and kernel set in maize quantified by image analysis. Agron J. 1994;86:699–703. [Google Scholar]
- Boyer JS. Advances in drought tolerance in plants. Adv Agron. 1996;56:187–218. [Google Scholar]
- Boyle MG, Boyer JS, Morgan PW. Stem infusion of liquid culture medium prevents reproductive failure of maize at low water potentials. Crop Sci. 1991;31:1246–1252. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carlson SJ, Chourey PS. A re-evaluation of the relative roles of two invertases, INCW2 and IVR1, in developing maize kernels and other tissues. Plant Physiol. 1999;121:1025–1035. doi: 10.1104/pp.121.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng WH, Taliercio EW, Chourey PS. The miniature 1 seed locus of maize encodes a cell-wall invertase required for normal development of endosperm and maternal cells in the pedicel. Plant Cell. 1996;8:971–983. doi: 10.1105/tpc.8.6.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denmead OT, Shaw RH. The effects of soil moisture stress at different stages of growth on the development and yield of corn. Agron J. 1960;52:272–274. [Google Scholar]
- Doehlert DC, Felker FC. Characterization and distribution of invertase activity in developing maize (Zea mays) kernels. Physiol Plant. 1987;70:51–57. [Google Scholar]
- Dong JZ, Dunstan DI. A reliable method for extraction of RNA from various conifer tissues. Plant Cell Rep. 1996;15:516–521. doi: 10.1007/BF00232985. [DOI] [PubMed] [Google Scholar]
- Doorenbos J, Kassam AH. Yield Response to Water. Food and Agriculture Organization of the United Nations (FAO) Irrigation and Drainage Paper No. 33. Rome: FAO; 1979. [Google Scholar]
- Duke ER, McCarty DR, Koch KE. Organ-specific invertase deficiency in the primary root of an inbred maize line. Plant Physiol. 1991;97:523–527. doi: 10.1104/pp.97.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmeades GO, Bolanos J, Chapman SC, Lafitte HR, Banziger M. Selection improves drought tolerance in tropical maize populations: I. Gains in biomass, grain yield, and harvest index. Crop Sci. 1999;39:1306–1315. [Google Scholar]
- Felker FC, Shannon JC. Movement of 14C-labeled assimilates into kernels of Zea mays L.: III. An anatomical examination and microautoradiographic study of assimilate transfer. Plant Physiol. 1980;65:864–870. doi: 10.1104/pp.65.5.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DB, Cash-Clark CE. Sieve tube unloading and post-phloem transport of fluorescent tracers and proteins injected into sieve tubes via severed aphid stylets. Plant Physiol. 2000a;123:125–137. doi: 10.1104/pp.123.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DB, Cash-Clark CE. Gradients in water potential and turgor pressure along the translocation pathway during grain filling in normally watered and water-stressed wheat plants. Plant Physiol. 2000b;123:139–147. doi: 10.1104/pp.123.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen H. Vorkommen und Bedeutung von Abscisinsäure und Cytokininen sowie deren Metaboliten im Xylemsaft von Helianthus annus unter Dürrebedingungen. Dissertation. Hamburg, Germany: Universität Hamburg; 1996. [Google Scholar]
- Herbers K, Sonnewald U. Molecular determinants of sink strength. Curr Opin Plant Biol. 1998;1:207–216. doi: 10.1016/s1369-5266(98)80106-4. [DOI] [PubMed] [Google Scholar]
- Herrero MP, Johnson RR. Drought stress and its effect on maize reproductive systems. Crop Sci. 1981;21:105–110. [Google Scholar]
- Hoffmann BS, Willmitzer L, Fisahn J. Analysis of growth, composition and thickness of the cell walls of transgenic tobacco plants expressing a yeast-derived invertase. Protoplasma. 1997;200:146–153. [Google Scholar]
- Imlau A, Truernit E, Sauer N. Cell-to-cell and long-distance trafficking of the green fluorescent protein in the phloem and symplastic unloading of the protein into sink tissues. Plant Cell. 1999;11:309–322. doi: 10.1105/tpc.11.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isla MI, Vattuone MA, Sampeitro AR. Hydrolysis of sucrose within isolated vacuoles from Solanum tuberosum L. tubers. Planta. 1998;205:601–605. doi: 10.1007/s004250050362. [DOI] [PubMed] [Google Scholar]
- Jackson D, Veit B, Hake S. Expression of maize KNOTTED1 related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot. Development. 1994;120:405–413. [Google Scholar]
- Jensen AB, Mundy J, Pages M, Leone A. Expression of the maize rab17 gene in response to abscisic acid and osmotic stress. In: Grillo S, editor. Physical stresses in plants: genes and their products for tolerance. Proceedings of the Workshop Maratea, 24–27 September 1995, Italy. Berlin: Springer-Verlag; 1996. pp. 123–130. [Google Scholar]
- Kim JY, Mahé A, Brangeon J, Prioul JL. A maize vacuolar invertase, IVR2, is induced by water stress: organ/tissue specificity and diurnal modulation of expression. Plant Physiol. 2000;124:71–84. doi: 10.1104/pp.124.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KE. Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:509–540. doi: 10.1146/annurev.arplant.47.1.509. [DOI] [PubMed] [Google Scholar]
- Koch KE, Wu Y, Xu J. Sugar and metabolic regulation of genes for sucrose metabolism: potential influence of maize sucrose synthase and soluble invertase responses on carbon partitioning and sugar sensing. J Exp Bot. 1996;47:1179–1185. doi: 10.1093/jxb/47.Special_Issue.1179. [DOI] [PubMed] [Google Scholar]
- Koch KE, Xu J, Duke ER, McCarty DR, Yuan CX, Tan BC, Avigne WT. Sucrose provides a long distance signal for coarse control of genes affecting its metabolism. In: Pontis HG, Salerno GL, Echeverria EJ, editors. Sucrose Metabolism, Biochemistry, Physiology and Molecular Biology. Rockville, MD: American Society of Plant Physiologists; 1995. pp. 266–277. [Google Scholar]
- Koch KE, Zeng Y, Wu Y, Avigne WT. Multiple paths of sugar-sensing and a sugar/oxygen overlap for genes of sucrose and ethanol metabolism. J Exp Bot. 2000;51:417–427. doi: 10.1093/jexbot/51.suppl_1.417. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Stankovics J, Gallyas F. A highly sensitive one-step method for silver intensification of the nickel-diaminobenzidine endproduct of peroxidase reaction. J Histochem Cytochem. 1989;37:1563–1565. doi: 10.1177/37.10.2674275. [DOI] [PubMed] [Google Scholar]
- Miller ME, Chourey PS. The maize invertase-deficient miniature-1 seed mutation is associated with aberrant pedicel and endosperm development. Plant Cell. 1992;4:197–305. doi: 10.1105/tpc.4.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N. A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem. 1944;153:375–380. [Google Scholar]
- Nielsen TH, Skjaerbaek HC, Carlsen P. Carbohydrate metabolism during fruit development in sweet pepper (Capsicum annuum) plants. Physiol Plant. 1991;82:311–319. [Google Scholar]
- Patrick JW, Offler CE. Post-sieve element transport of sucrose in developing seeds. Aust J Plant Physiol. 1995;22:681–702. [Google Scholar]
- Quarrie SA, Whitford PN, Appleford NEJ, Wang TL, Cook SK, Henson IE, Loveys BR. A monoclonal antibody to (S)-abscisic acid: its characterisation and use in radioimmunoassay for measuring abscisic acid in crude abstracts of cereal and lupin leaves. Planta. 1988;173:330–339. doi: 10.1007/BF00401020. [DOI] [PubMed] [Google Scholar]
- Rolland F, Moore B, Sheen J. Sugar sensing and signaling in plants. Plant Cell. 2002;14:S185–205. doi: 10.1105/tpc.010455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute. SAS/STAT Users Guide. Release 6.03 Ed. Cary, NC: SAS Institute; 1988. [Google Scholar]
- Schussler JR, Westgate ME. Maize kernel set at low water potential: II. Sensitivity to reduced assimilates at pollination. Crop Sci. 1991;31:1196–1203. [Google Scholar]
- Schussler JR, Westgate ME. Assimilate flux determines kernel set at low water potential in maize. Crop Sci. 1995;35:1074–1080. [Google Scholar]
- Setter TL, Flannigan BA, Melkonian J. Loss of kernel set due to water deficit and shade in maize: carbohydrate supplies, abscisic acid, and cytokinins. Crop Sci. 2001;41:1530–1540. [Google Scholar]
- Shanker S, Salazar RW, Taliercio EW, Chourey PS. Cloning and characterization of full-length cDNA encoding cell-wall invertase from maize. Plant Physiol. 1995;108:873–874. doi: 10.1104/pp.108.2.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smidansky ED, Clancy M, Meyer FD, Lanning SP, Blake NK, Talbert LE, Giroux MJ. Enhanced ADP-glucose pyrophosphorylase activity in wheat endosperm increases seed yield. Proc Natl Acad Sci USA. 2002;99:1724–1729. doi: 10.1073/pnas.022635299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm A, Sebkova V, Lorenz K, Hardegger M, Lienhard S, Unger C. Development- and organ-specific expression of the genes for sucrose synthase and three isoenzymes of acid β-fructofuranosidase in carrot. Planta. 1995;195:601–610. [Google Scholar]
- Sturm A, Tang GQ. The sucrose-cleaving enzymes of plants are crucial for development, growth and carbon partitioning. Trends Plant Sci. 1999;4:401–407. doi: 10.1016/s1360-1385(99)01470-3. [DOI] [PubMed] [Google Scholar]
- Taliercio EW, Kim JY, Mahé A, Shanker S, Choi J, Cheng WH, Prioul JL, Chourey PS. Isolation, characterization and expression analyses of two cell wall invertase genes in maize. J Plant Physiol. 1999;155:197–204. [Google Scholar]
- Tang GQ, Lüscher M, Sturm A. Antisense repression of vacuolar and cell wall invertase in transgenic carrot alters early plant development and sucrose partitioning. Plant Cell. 1999;11:177–189. doi: 10.1105/tpc.11.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CY, Salamini F, Nelson OE. Enzymes of carbohydrate metabolism in the developing endosperm of maize. Plant Physiol. 1970;46:299–306. doi: 10.1104/pp.46.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H, Borisjuk L, Wobus U. Controlling seed development and seed size in Vicia faba: a role for seed coat-associated invertases and carbohydrate state. Plant J. 1996;10:823–830. [Google Scholar]
- Weber H, Heim U, Golombek S, Borisjuk L, Manteuffel R, Wobus U. Expression of a yeast-derived invertase in developing cotyledons of Vicia narbonensis alters the carbohydrate state and affects storage functions. Plant J. 1998;16:163–172. doi: 10.1046/j.1365-313x.1998.00282.x. [DOI] [PubMed] [Google Scholar]
- Weiler EW. An enzyme immunoassay for cis-(+)-abscisic acid. Physiol Plant. 1982;54:510–514. [Google Scholar]
- Weschke W, Panitz R, Sauer N, Wang Q, Neubohn B, Weber H, Wobus U. Sucrose transport in barley seeds: molecular characterization of two transporters and implications for seed development and starch accumulation. Plant J. 2000;21:455–467. doi: 10.1046/j.1365-313x.2000.00695.x. [DOI] [PubMed] [Google Scholar]
- Westgate ME, Boyer JS. Carbohydrate reserves and reproductive development at low leaf water potentials in maize. Crop Sci. 1985;25:762–769. [Google Scholar]
- Westgate ME, Boyer JS. Reproduction at low silk and pollen water potentials in maize. Crop Sci. 1986;26:951–956. [Google Scholar]
- Wittich PE, Vreugdenhil D. Localization of sucrose synthase activity in developing maize kernels by in situ enzyme histochemistry. J Exp Bot. 1998;49:1163–1171. [Google Scholar]
- Wobus U, Weber H. Sugars as signal molecules in plant seed development. Biol Chem. 1999;380:937–944. doi: 10.1515/BC.1999.116. [DOI] [PubMed] [Google Scholar]
- Woodson WR, Wang H. Invertases of carnation petals: partial purification, characterization and changes in activity during petal growth. Physiol Plant. 1987;71:224–228. [Google Scholar]
- Wu Y. Molecular and physiological analysis of soluble acid invertases in maize. PhD thesis. Gainesville: University of Florida; 2000. [Google Scholar]
- Xu J, Avigne WT, McCarty DR, Koch KE. A similar dichotomy of sugar modulation and developmental expression affects both paths of sucrose metabolism: evidence from a maize invertase gene family. Plant Cell. 1996;8:1209–1220. doi: 10.1105/tpc.8.7.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Pemberton GH, Almira EC, McCarty DR, Koch KE. The Ivr1 gene for invertase in maize. Plant Physiol. 1995;108:1293–1294. doi: 10.1104/pp.108.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinselmeier C, Jeong BR, Boyer JS. Starch and the control of kernel number in maize at low water potentials. Plant Physiol. 1999;121:25–35. doi: 10.1104/pp.121.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinselmeier C, Westgate ME, Schussler JR, Jones RJ. Low water potential disrupts carbohydrate metabolism in maize (Zea mays L.) ovaries. Plant Physiol. 1995;107:385–391. doi: 10.1104/pp.107.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]