Abstract
l-Ascorbic acid (AsA) was found to be loaded into phloem of source leaves and transported to sink tissues. When l-[14C]AsA was applied to leaves of intact plants of three different species, autoradiographs and HPLC analysis demonstrated that AsA was accumulated into phloem and transported to root tips, shoots, and floral organs, but not to mature leaves. AsA was also directly detected in Arabidopsis sieve tube sap collected from an English green aphid (Sitobion avenae) stylet. Feeding a single leaf of intact Arabidopsis or Medicago sativa with 10 or 20 mm l-galactono-1,4-lactone (GAL-l), the immediate precursor of AsA, lead to a 7- to 8-fold increase in AsA in the treated leaf and a 2- to 3-fold increase of AsA in untreated sink tissues of the same plant. The amount of AsA produced in treated leaves and accumulated in sink tissues was proportional to the amount of GAL-l applied. Studies of the ability of organs to produce AsA from GAL-l showed mature leaves have a 3- to 10-fold higher biosynthetic capacity and much lower AsA turnover rate than sink tissues. The results indicate AsA transporters reside in the phloem, and that AsA translocation is likely required to meet AsA demands of rapidly growing non-photosynthetic tissues. This study also demonstrates that source leaf AsA biosynthesis is limited by substrate availability rather than biosynthetic capacity, and sink AsA levels may be limited to some extent by source production. Phloem translocation of AsA may be one factor regulating sink development because AsA is critical to cell division/growth.
l-Ascorbic acid (AsA; vitamin C) is found in all plants in practically every compartment of the cell (Loewus and Loewus, 1987; Smirnoff, 1996; Noctor and Foyer, 1998; Loewus, 1999; Arrigoni and DeTullio, 2000; Davey et al., 2000; Horemans et al., 2000; Smirnoff, 2000a; Smirnoff and Wheeler, 2000; Conklin, 2001; Smirnoff et al., 2001). There has been a resurgence of interest in AsA due to the increasing evidence of the importance of AsA in both redox-associated and developmental processes and also to the recent determination of a complete biosynthetic pathway in plants (Wheeler et al., 1998). The broad distribution of AsA implicates a role of this compound in a wide range of physiological phenomena. Best studied among these functions is its role in redox processes during photosynthesis, environment-induced oxidative stress (ozone, UV, high light, SO2, etc.), and during wound- and pathogen-induced oxidative processes (Noctor and Foyer, 1998; Davey et al., 2000; Horemans et al., 2000; Smirnoff, 2000b). This antioxidant property is also one of the major functions of AsA in humans (Homo sapiens), who are unable to synthesize their own AsA and, in most of the world, rely primarily on plants as their vitamin C source (Griffiths and Lunec, 2001; Kaur and Kapoor, 2001).
There is emerging evidence that AsA is involved in growth and development by mechanisms that have yet to be determined (Alcain and Buron, 1994; Arrigoni, 1994; Cordoba and Gonzales-Reyes, 1994; De Gara and Tommasi, 1999; Navas and Gomes-Diaz, 1995; Davey et al., 2000; Horemans et al., 2000). Experiments where AsA levels are manipulated indicates AsA can modulate cell cycle and/or cell division (Liso et al., 1984; De Cabo et al., 1993; Citterio et al., 1994; de Pinto et al., 1999; Kato and Esaka, 1999) and cell elongation (Hidalgo et al., 1989; Hidalgo et al., 1991; Gonzales-Reyes et al., 1995; Kato and Esaka, 1999) in plants. Mutant and transgenic plants with decreased endogenous AsA levels show reduced shoot growth rates (Veljovic-Jovanovic et al., 2001) and reduced cell division and cell growth (Tabata et al., 2001). The effect on cell growth suggests that AsA levels may affect cell wall properties. In support of this, transgenic plants with lowered AsA levels have been shown to have cell wall changes, including reduction of Man (Keller et al., 1999) and decrease in cellulose content (Lukowitz et al., 2001).
Considering the potential role of AsA in growth of developing organs, it is important to know how AsA levels are maintained in these rapidly growing areas, known as “sinks” for assimilates, versus mature leaves, which are known as a “source” of assimilates. The possibilities include regulating rate of AsA synthesis, rate of AsA turnover, or, as we propose, import of AsA from source regions. Photosynthetic organs and certain storage organs and meristems are known to have high concentrations of AsA (Loewus and Loewus, 1987) and there is a consistent theme in the literature of highest AsA levels in non-photosynthetic organs during their most rapid phase of growth. This includes such diverse fruits as mango (Mangifera indica; Kudachikar et al., 2001) and tomato (Lycopersicon esculentum) and peppers (Capsicum annuum; Yahia et al., 2001), and other organs such as stolon tips (Viola et al., 1998), developing lateral roots (Innocenti et al., 1993), and germinating embryo axis (Pallanca and Smirnoff, 2000). Unfortunately, there is no comprehensive data on both AsA concentrations and AsA synthesis rates of different organs and tissues of an individual plant during its developmental and physiological phases. It is probable that AsA can be synthesized and maintained more readily in photosynthetic organs, which have high levels of assimilated carbon, reductant, and biosynthetic capacity compared with rapidly growing sink organs.
Although a number of studies have indicated AsA or its oxidation product dehydroascorbic acid (DHA) can be transported across the plasma membrane of plant cells, the possibility of AsA translocation from source to sink tissues has never been studied before. The only work we could find that had any bearing on potential long-distance transport of AsA was by Mozafar and Oertli (1993), who found that addition of 14C-AsA to the medium gave rise to incorporation of 14C but not an increase in AsA levels in the treated or distant organs, suggesting AsA was rapidly degraded in the application zone. However, the experiments were done over a number of days, which likely gave rise to complete turnover of AsA. During our recent work on AsA metabolism to oxalic acid (Keates et al., 2000), we noticed that AsA appeared to be transported from source to sink via the phloem (V.R. Franceschi, unpublished data). This holds important implications with respect to regulation of AsA levels in sink tissues. The AsA transport studies conducted have looked at transport across the plasma membrane of intact organs, protoplasts, and isolated membranes and organelles, but not phloem uptake and long-distance translocation (Horemans et al., 2000). Transport of AsA or its oxidation product, DHA, via facilitated diffusion, proton symport, and AsA-DHA antiport (or exchange) have been proposed (Horemans et al., 2000), and investigations of a potential role of Glc porters in AsA/DHA transport, such as seen in animal cells, are inconclusive (Foyer and Lelandais, 1996; Horemans et al., 1996, 1998, 2000). Kollist et al. (2001) recently demonstrated that both DHA and AsA are transported from the apoplast to symplast of intact leaf tissue; however, the Km for transport was 12.8 mm, while the observed level of AsA/DHA in the apoplast was 0.73 mm. Measured Km for AsA or DHA transport have ranged from 0.09 to 12.8 mm as reported by Kollist et al. (2001), and it is possible that specific tissues, such as the phloem, may have localized conditions of high apoplastic AsA or low-Km transporters. These studies, taken collectively, indicate that protein-based transporters exist for AsA and/or DHA transport across the plasma membrane of plant cells, although the distribution of this activity among cell types is not known.
The purpose of this study was to test the hypothesis that AsA is loaded into the phloem and exported to sink tissues, as indicated by our initial observations. We used three species for these studies to ensure the phenomenon was generalized and not species specific. The results are the first to demonstrate long-distance transport of AsA in plants, which is important to our understanding of basic plant physiology and to the potential to manipulate AsA levels.
RESULTS AND DISCUSSION
Exogenously Applied AsA Is Loaded into Phloem
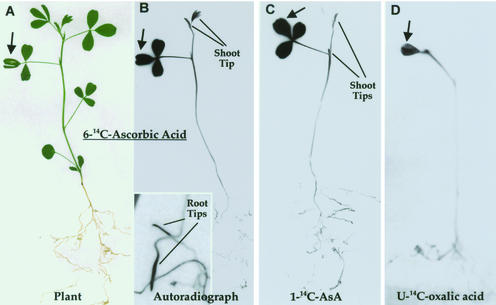
Autoradiographs of whole leaves exposed to [14C]AsA for 12 or 24 h showed that the veins of all three species accumulated label. Due to the flat, thin nature of the Impatiens walleriana leaf, both major veins and minor veins could be seen clearly to accumulate label (Fig. 1A), whereas in Arabidopsis and M. sativa, similar patterns were seen but it was more difficult to resolve the finer veins (Fig. 1, B and C). [1-14C] and [6-14C] AsA gave the same results, indicating AsA, and not a breakdown product such as OxA (1-14C) or threonic/tartaric acid (6-14C), was giving rise to the phloem labeling (not shown). Micro-autoradiography showed that the label was concentrated in the phloem and absent from xylem (Fig. 1D). These results demonstrate that exogenously supplied AsA is actively loaded into the phloem. All three species are apoplastic phloem loaders; thus, the results of our exogenous application may reflect a real process occurring in the intact plant.
Figure 1.
Autoradiograph showing phloem loading of l-[1-14C]AsA. A, Major and minor vein (arrows) loading in I. walleriana. B, Arabidopsis vein loading, including minor veins (arrows). C, Medicago sativa phloem loading. Major veins can be seen but the minor veins cannot be resolved. D, Micro-autoradiograph of cross section through a larger M. sativa vein showing label (black dots) in the phloem but not the xylem.
Exogenously Applied AsA Is Transported from Source to Sink Tissues
Whole-plant autoradiographs demonstrated that over 12 or 24 h, label from source leaves was specifically translocated to sink regions. In young M. sativa plants, label was transported to the developing shoot tip and to the root tips but not mature leaves (Fig. 2, A and B). The same pattern was seen for [1-14C]- and [6-14C]-labeled AsA (Fig. 2, B and C), indicating that the transported compound was primarily AsA. [14C]oxalic acid, which is derived from carbons 1 and 2 of AsA, was only weakly transported out of the application leaf (Fig. 2D), and could not be seen in shoot or root tips. When label was applied to mature M. sativa leaves adjacent to developing inflorescences, label was transported to the very young floral buds and flowers but not to adjacent source leaves (Fig. 3). A similar partitioning pattern was seen with Arabidopsis (Fig. 4), where the flowers (Fig. 4C), siliques (including ovules, Fig. 4D), root tips (Fig. 4E), and sink leaves accumulated label from [1-14C] and [6-14C] AsA but not [14C]OxA. A 12-h application gave the same distribution pattern as 24 h, though the intensity of labeling was overall lower. It was interesting to note that some side sinks showed little label, which is consistent with individual leaves and certain vascular bundles transporting to certain sinks. HPLC analysis demonstrated that after 12 h, 75% to 80% of the label in the application leaf and 50% to 70% of the label in sinks is still present as AsA, although some turnover is apparent (Table I). The data prove that exogenous application of AsA results in AsA accumulation in the phloem; this AsA is present in the phloem translocation stream (stems), and the veins that are accumulating the labeled AsA are active in translocation of AsA to sink tissues.
Figure 2.
AsA translocation in M. sativa. A source leaf (arrow) was exposed to [14C]AsA for 24 h. A plant (A) and its autoradiograph (B) showing distribution of label ([6-14C]AsA). Enlargement shows root tips. C, Patterns for [1-14C]AsA distribution. D, [U-14C]oxalic acid (product of carbons 1 and 2 of AsA).
Figure 3.
AsA translocation in flowering M. sativa. A source leaf (arrow) below flower clusters was exposed to [1-14C]AsA for 24 h. The pressed plant and its autoradiograph are shown. Label is transported to the developing flowers but not source leaves.
Figure 4.
Translocation of AsA in Arabidopsis. A whole plant (A) fed [1-14C]AsA to a source leaf (arrow) and its autoradiograph (B). Label is clearly accumulated in floral parts as well as young leaves that are still expanding. C, Autoradiograph of flowers and young siliques. D, Part of silique showing heavy label in ovules (arrows). E, Root with heavily labeled tip (arrow).
Table I.
Percent label remaining as ascorbic acid in application leaf and sinks
| Hours
|
||||||
|---|---|---|---|---|---|---|
| 6 | 12 | 6 | 12 | 6 | 12 | |
| M. sativa | Leaf | Shoot | Root | |||
| AsA | 74 | 79 | 70 | 66 | 59 | 68 |
| Pre-AsA | 2 | 1 | 4 | 7 | 13 | 7 |
| Post-AsA | 24 | 20 | 26 | 27 | 28 | 25 |
| Arabidopsis | Leaf | Flowers | Siliques | |||
| AsA | 76 | 74 | 35 | 51 | 45 | 59 |
| Pre-AsA | 4 | 2 | 33 | 20 | 25 | 14 |
| Post-AsA | 20 | 24 | 32 | 29 | 30 | 27 |
l-[14C]AsA was applied to a source leaf of an intact plant and application leaf, shoot tips, and roots were analyzed by HPLC and scintillation counting for percent of label present as AsA and as a pre- or post-AsA fraction. The data indicate AsA is transported intact but is metabolized to various degrees in sink tissues, especially in Arabidopsis.
Endogenously Produced AsA Is Translocated to Sink Tissues
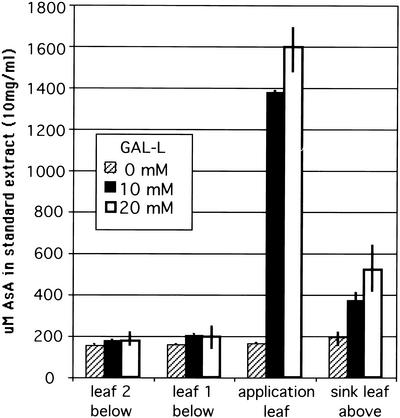
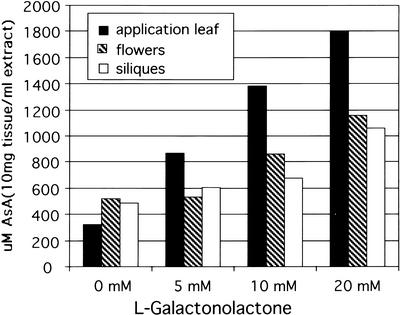
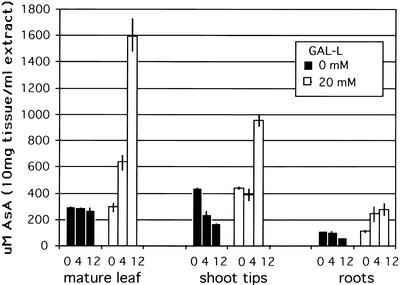
When a source leaf is fed l-galactono-1,4-lactone (GAL-l), the immediate precursor of AsA, by the flap method, it must first enter cells before being converted to AsA because the enzyme for this conversion is on the mitochondrial membrane (Siendones et al., 1999). Exposure of a mature M. sativa leaf to 10 or 20 mm GAL-l for 24 h results in a 7- to 8-fold increase in AsA levels in the leaf (Fig. 5). The shoot tip above the application leaf showed a 2- to 2.5-fold increase in AsA level, whereas the two mature leaves below the application leaf showed no significant increase in AsA (Fig. 5). GAL-l was not detected in the stems or sink tissue and thus is not transported, so the results show endogenously produced AsA enters the phloem in source leaves and is translocated after normal source-sink dynamics of phloem transport. A similar experiment with flowering Arabidopsis plants showed that as GAL-l concentration is increased, the level of AsA in the application leaf increased incrementally up to 6-fold at the highest level of GAL-l (Fig. 6). The amount of AsA in flowers and siliques above the treated leaf also increased in proportion to the level in the leaf, reaching a 2- to 3-fold increase at the highest GAL-l application (Fig. 6). Studies with l-Gal gave similar results (not shown). It is important to point out that these large AsA increases in sink tissues are only from a single treated leaf, and if multiple leaves were treated, we expect that the level could increase even further. These data indicate that AsA transport by phloem is a normal process in plants. In addition, it suggests that: (a) AsA production in source leaves is limited by substrate availability and not biosynthetic capacity, as also indicated in previous work by DeGara et al. (1997) and Davey et al. (2000); (b) the level of AsA transported depends on endogenous AsA level; and (c) sink tissue AsA levels may be partly limited by source processes.
Figure 5.
Twenty-four-hour application of GAL-l to a source leaf of an intact M. sativa plant gives a 7- to 8-fold increase of AsA in the leaf. This results in transport to and significant increase (2–3-fold) of AsA in sink tissues but not mature source leaves below the application leaf. Average from two pools of replicates of six plants for each point. Values are concentration ± sd in an extract of 10 mg dry weight in 1 mL of buffer.
Figure 6.
Twenty-four-hour application of GAL-l to a source leaf of an intact Arabidopsis plant. AsA increases in treated leaf as level of GAL-l is increased. The increase gives rise to AsA translocation to and an increase of AsA in flowers and siliques, especially at the two higher GAL-l concentrations. Average of bulked pools of organs from six plants for each point. Values are concentration in an extract of 10 mg dry weight in 1 mL of buffer.
Mature Leaves Have a Higher Capacity for AsA Synthesis Than Sink Tissues
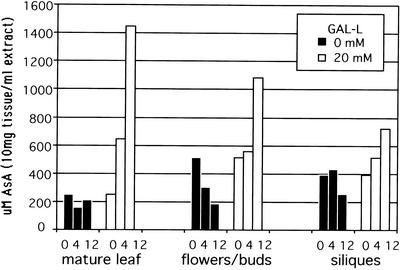
Mature excised Arabidopsis leaves showed an AsA increase of 3-fold after 4 h and 7-fold after 12 h of exposure to 20 mm GAL-l (Fig. 7). In contrast, in excised sink organs upon exposure to GAL-l, the increase of AsA in flowers and siliques after 4 h was minimal, and reached a maximum of about 2-fold after 12 h. This indicates leaves had an approximately 3 times greater capacity for AsA synthesis from the immediate precursor, GAL-l. A similar result was seen in M. sativa, where in excised leaf, AsA increased 2-fold after 4 h and 5-fold after 12 h of exposure to GAL-l (Fig. 8). Excised shoot tips had no increase after 4 h, and a 2-fold increase after 12 h, whereas excised roots had a total increase of about 2-fold. These experiments also showed that during incubation in buffer alone, AsA decreased significantly in sink tissue versus source leaf (50%–70% versus 5%–10% after 12 h). These results support the whole-plant GAL-l studies and further demonstrate that source leaves have a higher biosynthetic capacity and lower rate of AsA loss or utilization compared with sink tissues. This difference between source and sink may be behind the requirement for AsA translocation from source to sink: to support the rapid growth and high demand for AsA that cannot be entirely met by sink processes due to the lower synthesis and higher turnover rates.
Figure 7.
Synthesis of AsA from GAL-l in Arabidopsis organs. Numbers on x axis are hours of exposure to 0 or 20 mm GAL-l. Mature leaves have highest rates of synthesis, whereas even with high substrate levels, floral organs/fruits have very low synthesis rates and significant degradation rates. Tissue (bulked pools of each organ and time from six plants) was incubated at 22°C and 200 μE m−2 s−1. Values are concentration in an extract of 10 mg dry weight in 1 mL of buffer.
Figure 8.
Synthesis of AsA from GAL-l in M. sativa organs. Even with high GAL-l, AsA synthesis is low in shoot tips over 4 h, whereas it is high in leaves. In the absence of GAL-l, AsA is significantly degraded in sink tissues but only slightly decreases in mature leaves. Tissue (two replicate pools for each organ and time) was incubated at 22°C and 200 μE m−2 s−1. Values are concentration in an extract of 10 mg dry weight in 1 mL of buffer.
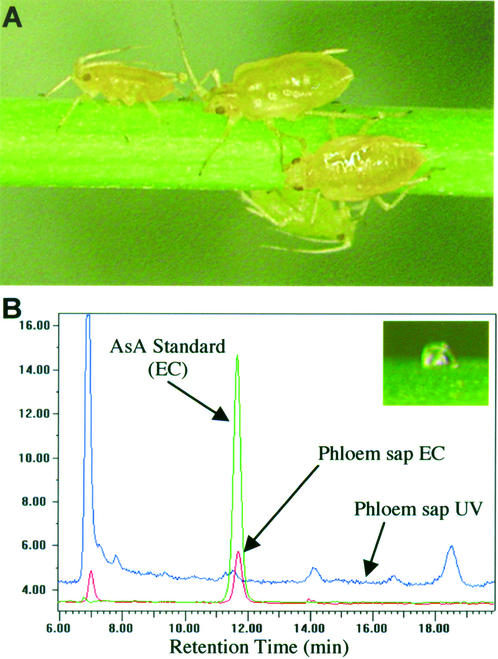
AsA Is Present in Sieve Tube Sap
English green aphid (Sitobion avenae) stylets allow for direct sampling of sieve tube sap because these insects feed only on the sieve tube contents. The English green aphids could be easily encouraged to settle onto Arabidopsis stems (control plants only were used for this study) and feed on the phloem (Fig. 9A). After severing the stylet from the insect with the microcautery device, the stylets exuded sieve tube sap to a volume of about 5 to 12 nL (Fig. 9B), which could be collected for analysis. Figure 9B shows the HPLC analysis of a single drop (approximately 5 nL) of sieve tube sap using an EC detector set at a voltage selective for AsA as well as a UV detector that will show UV-absorbing substances (including AsA, though not at the low levels present in the small samples tested). AsA could be detected in the sieve tube stylet exudate collected from Arabidopsis, as seen from a distinct peak with the EC detector and the alignment of the peak retention time with an AsA standard (Fig. 9B). We also found AsA in sieve tube sap collected from wheat stems (data not shown). Although we have not done enough replications to get a reliable quantitative value for the AsA concentration, this qualitative observation supports the other data presented here on AsA phloem transport and is the first direct demonstration of the presence of AsA in sieve tube sap.
Figure 9.
Aphid stylet sampling of Arabidopsis sieve tube sap. A, English green aphids feeding on an Arabidopsis stem. B, HPLC analysis of an approximately 5-nL droplet of stylet exudate (inset). A distinct AsA peak can be seen by electrochemical (EC) detection in the phloem sap (red trace). Other peaks can be detected by UV210, which does not pick up AsA.
CONCLUSIONS
Long-distance transport of AsA by the phloem is demonstrated for the first time, to our knowledge, and holds significant implications with respect to sink development. Although no one seems to have considered the prospect of phloem loading and transport of AsA, the potential systems for energizing transport (pH gradients and K+ exchange) are present in phloem. The sieve tube sap is also a good environment for the retention and stability of AsA: It has a slightly alkaline pH (which will keep AsA in a charged state and, thus, membrane impermeant), plenty of counter ion (K+, 40–100 mm), and is a highly reducing environment (high thioredoxin and glutaredoxin; Hayashi et al., 2000). Further studies on the mechanism of AsA transport are fundamental to our understanding of basic plant physiology with respect to our ability to manipulate these processes to enhance resistance of plants to various oxidative stresses, and to improve AsA-related nutritional and processing qualities of plants or plant products.
MATERIALS AND METHODS
Plant Material
Plants used were Arabidopsis ecotype Columbia, Medicago sativa cv Champ, and Impatiens walleriana cv Sultani. I. walleriana was used because it has well-developed transfer cells in the phloem, and the other species were used because of their compact form. Plants were grown at 22°C in 14 h of light/10 h of dark, and a photon flux density of approximately 475 μE m−2 s−1.
AsA Uptake and Transport
To determine if exogenous AsA can be accumulated by phloem and transported to sink tissues, l-[1-14C] and l-[6-14C]AsA (5 mm, 0.5 μCi/M. sativa and I. walleriana plants; and 0.5 mm, 1 μCi/Arabidopsis plant), and [U-14C]oxalic acid (2 mm, 5 μCi/plant) was applied to mature leaves of the three species. Arabidopsis was particularly sensitive (wilting) to exogenous AsA, so we kept the total concentration low for these small plants. We used a “flap” application technique, which we found gives minimal damage and wound response as compared with abrasion. The tip of a leaf (leaflet) was sliced 5 mm longitudinally on either side of the midvein and the resulting attached “flap” was inserted into a tube containing the labeled compound. Plants were sampled after 6, 12, and 24 h. Some plants were prepared for whole-plant autoradiography to show patterns of label transport throughout the plant. Small samples were taken for micro-autoradiography to determine the cellular sites of AsA accumulation. Plants were also sampled for HPLC analysis to confirm that the label seen is in AsA. AsA has only a few metabolic products, which is why we ran experiments with 1- and 6-labeled AsA. Carbon 1 and 2 of AsA can give rise to oxalic acid, so we used [14C]oxalic acid as a control.
Whole-Plant Autoradiography
Plants were removed from pots, spread on sheets of chromatography paper, placed in a plant press, and dried at 25°C. Dried plants were glued to paper, which was placed into a cassette with x-ray film, and the film was developed after 1 to 7 d exposure at −80°C. The plants and their autoradiographs were scanned into a computer.
Micro-Autoradiography
Micro-autoradiography was done as previously described for water-soluble compounds (Lansing and Franceschi, 2000). Samples were frozen in isopentane:methylcyclohexane (9:1 [v/v]) cooled to −170°C with liquid nitrogen. Tissue was transferred to, and freeze substituted in, acetone at −80°C, and infiltrated with Spurr epoxy resin under a dry atmosphere. Sections were cut 1 μm thick, transferred to gelatin-coated slides, counter-stained with Safranin O, and coated with Ilford L4 nuclear track emulsion. After development, micro-autoradiographs were photographed with a light microscope in bright-field mode.
HPLC Analysis
HPLC analysis of AsA in plant samples was as previously described (Tarlyn et al., 1998; Keates et al., 2000). Samples were frozen with liquid nitrogen, lyophilized, and stored at −80°C. Lyophilized tissues were ground in a chilled mortar and suspended in HPLC mobile phase (4 mm H2SO4) containing 5 mm dithiothreitol and 0.01% (w/v) insoluble polyvinyl-polypyrrolidone, at 10 mg mL−1. Extracts were spun at 15,000g for 30 min at 4°C to produce a clear supernatant. Three hundred microliters of supernatant was filtered (0.45 μm) and analyzed for AsA, OxA, and l-glactono-1,4-lactone by HPLC (Aminex HPX-87H ion exclusion column, 300 × 7.8 mm, 0.6 mL min−1, 35°C, 900 psi, Bio-Rad Laboratories, Hercules, CA). HPLC data was integrated and analyzed with Gilson 715 controller software version 1.21 (Gilson USA, Middleton, WI). OxA was measured by UV at 210 nm (SpectraChrom 100, Thermal Separation Products, San Jose, CA) and AsA measured by EC (amperometric) detection (model LC-4C, BioAnalytical Systems, West Lafayette, IN) at 600 mV. External standards were used to prepare calibration plots for AsA and OxA quantitation. Peaks of interest were collected manually and analyzed for 14C by liquid-scintillation counting.
GAL-l Application
Unlabeled GAL-l was used to determine if endogenously produced AsA is accumulated by the phloem and transported to sink tissues. GAL-l was applied to the mature leaf of intact plants by the flap technique at concentrations of 5, 10, and 20 mm in 20 mm MES buffer with 2 mm CaCl2 (pH 5.5). Buffer without GAL-l was applied as control. At varying times, the application leaves and other tissues were sampled and analyzed by HPLC for AsA and GAL-l levels. To test the relative ability of different Arabidopsis and M. sativa organs to synthesize AsA, parts of the plants were excised under water and incubated with GAL-l or buffer for 4 and 12 h. Samples were analyzed by HPLC.
Phloem Sap from English Green Aphids (Sitobion avenae) Stylet
English green aphids were caged onto Arabidopsis floral stems of untreated plants to encourage feeding. An aphid was severed from its stylet using a microcautery device (Fisher and Cash-Clark, 2000). After about 1 h, a droplet of about 5 to 10 nL was exuded from the stylet and collected with a glass microcapillary. The sample was diluted with 0.6 μL water, centrifuged to the bottom of a microfuge tube, and suspended into 20 μL of 5 mm H2SO4/5 mm dithiothreitol for HPLC assay. The sample was analyzed for AsA using the amperometric detector at 600 mV and 1-nA range and the UV detector at 210 nm and 0.001 absorbance units full scale, to see if other peaks could be detected in the very small volume of sap. Amperometric detection is much more sensitive for AsA than UV detection, though it can be detected with both methods.
ACKNOWLEDGMENTS
We thank Dr. Frank Loewus (Washington State University) for the generous gift of l-[6-14C]AsA, and Dr. Ning Wang (DuPont, Wilmington, DE) for helping us with the aphid stylet technique.
Footnotes
This work was supported in part by the National Science Foundation (grant no. MCB–9904562 to V.R.F.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.007062.
LITERATURE CITED
- Alcain FJ, Buron MI. Effect of ascorbate on cell growth and differentiation. J Bioenerg Biomembr. 1994;26:393–398. doi: 10.1007/BF00762780. [DOI] [PubMed] [Google Scholar]
- Arrigoni O. Ascorbate system in plant development. J Bioenerg Biomembr. 1994;26:407–419. doi: 10.1007/BF00762782. [DOI] [PubMed] [Google Scholar]
- Arrigoni O, DeTullio MC. The role of ascorbic acid in cell metabolism: between gene-directed functions and unpredictable chemical reactions. J Plant Physiol. 2000;157:481–488. [Google Scholar]
- Citterio S, Sgorbati S, Scippa S, Sparvoli E. Ascorbic acid effect on the onset of cell proliferation in pea root. Physiol Plant. 1994;92:601–607. [Google Scholar]
- Conklin PL. Recent advances in the role and biosynthesis of ascorbic acid in plants. Plant Cell Environ. 2001;24:383–394. [Google Scholar]
- Cordoba F, Gonzales-Reyes JA. Ascorbate and plant cell growth. J Bioenerg Biomembr. 1994;26:399–405. doi: 10.1007/BF00762781. [DOI] [PubMed] [Google Scholar]
- Davey MW, Van Monatgu M, Sanmatin M, Kanellis A, Smirnoff N, Benzie IJJ, Strain JJ, Favell D, Fletcher J. Plant l-ascorbic acid: chemistry, function, metabolism, bioavailability and effects of processing. J Sci Food Agric. 2000;80:825–860. [Google Scholar]
- De Cabo RC, Gonzalez-Reyes JA, Nava P. The onset of cell proliferation is stimulated by ascorbate free radical in onion primordia. Biol Cell. 1993;77:231–233. [Google Scholar]
- De Gara L, de Pinto MC, Arrigoni O. Ascorbate synthesis and ascorbate peroxidase activity during the early stages of wheat germination. Physiol Plant. 1997;100:894–900. [Google Scholar]
- De Gara L, Tommasi F. Ascorbate redox enzymes: a network of reactions involved in plant development. Rec Dev Phytochem. 1999;3:1–15. [Google Scholar]
- de Pinto MC, Francis D, De Gara L. The redox state of the ascorbate-dehydroascorbate pair as a specific sensor of cell division in tobacco BY-2 cells. Protoplasma. 1999;209:90–97. doi: 10.1007/BF01415704. [DOI] [PubMed] [Google Scholar]
- Fisher DB, Cash-Clark CE. Sieve tube unloading and post-phloem transport of fluorescent tracers and proteins injected into sieve tubes via severed aphid stylets. Plant Physiol. 2000;123:125–137. doi: 10.1104/pp.123.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Lelandais M. A comparison of the relative rates of transport of ascorbate and glucose across the thylakoid, chloroplast and plasmalemma membranes of pea leaf mesophyll cells. J Plant Physiol. 1996;148:391–398. [Google Scholar]
- Gonzales-Reyes JA, Alcain FJ, Caler JA, Serrano A, Cordoba F, Navas P. Stimulation of onion root elongation by ascorbate and ascorbate free radical in Allium cepa. Protoplasma. 1995;184:31–35. [Google Scholar]
- Griffiths HR, Lunec J. Ascorbic acid in the 21st century: more than a simple antioxidant. Environ Toxicol Pharmacol. 2001;10:173–182. doi: 10.1016/s1382-6689(01)00081-3. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Fukuda A, Suzui N, Fujimaki S. Proteins in the sieve-element cell complexes: their detection, localization and possible functions. Aust J Plant Physiol. 2000;27:489–496. [Google Scholar]
- Hidalgo A, Garcia-Herdugo G, Gonzales-Reyes JA, Morre DJ, Navas P. Ascorbate free radical stimulates onion root growth by increasing cell elongation. Bot Gaz. 1991;152:282–288. [Google Scholar]
- Hidalgo A, Gonzales-Reyes JA, Navas P. Ascorbate free radical enhances vacuolization in onion root meristems. Plant Cell Environ. 1989;12:455–460. [Google Scholar]
- Horemans N, Asard H, Caubergs RJ. Transport of ascorbate into plasma membrane vesicles of Phaseolus vulgaris L. Protoplasma. 1996;194:177–185. [Google Scholar]
- Horemans N, Asard H, Caubergs RJ. Carrier mediated uptake of dehydroascorbate into higher plant plasma membrane vesicles shows trans-stimulation. FEBS Lett. 1998;421:41–44. doi: 10.1016/s0014-5793(97)01534-2. [DOI] [PubMed] [Google Scholar]
- Horemans N, Foyer CH, Asard H. Transport and action of ascorbate at the plant plasma membrane. Trends Plant Sci. 2000;5:263–267. doi: 10.1016/s1360-1385(00)01649-6. [DOI] [PubMed] [Google Scholar]
- Innocenti AM, Bitonti MB, Mazzuca A, Liso R, Arrigoni O. Histochemical localization of exogenous ascorbic acid in the pericycle nuclei of Vicia faba. Caryologia. 1993;46:1–4. [Google Scholar]
- Kato N, Esaka M. Changes in ascorbate oxidase gene expression and ascorbate levels in cell division and cell elongation in tobacco cells. Physiol Plant. 1999;105:321–329. [Google Scholar]
- Kaur C, Kapoor HC. Antioxidants in fruits and vegetables: the millennium's health. Int J Food Sci Technol. 2001;36:703–725. [Google Scholar]
- Keates SE, Tarlyn NM, Loewus FA, Franceschi VR. l-Ascorbic acid and l-galactose are sources for oxalic acid and calcium oxalate in Pistia stratiotes. Phytochemistry. 2000;53:433–440. doi: 10.1016/s0031-9422(99)00448-3. [DOI] [PubMed] [Google Scholar]
- Keller R, Springer F, Renz A, Kossmann J. Antisense inhibition of the GDP-mannose pyrophosphorylase reduces the ascorbate content in transgenic plants leading to developmental changes during senescence. Plant J. 1999;19:131–141. doi: 10.1046/j.1365-313x.1999.00507.x. [DOI] [PubMed] [Google Scholar]
- Kollist H, Moldau H, Oksanen E, Vapaavuori E. Ascorbate transport from the apoplast to the symplast in intact leaves. Physiol Plant. 2001;113:377–383. doi: 10.1034/j.1399-3054.2001.1130311.x. [DOI] [PubMed] [Google Scholar]
- Kudachikar VB, Kulkarni SG, Prakash MNK, Vasantha MS, Prasad BA, Ramana KVR. Physio-chemical changes during maturity of mango (Mangifera indica L.) variety “Neelum.”. J Food Sci Technol. 2001;38:540–542. [Google Scholar]
- Lansing AJ, Franceschi VR. Paraveinal mesophyll: a specialized path for intermediary transfer of assimilates in legume leaves. Aust J Plant Physiol. 2000;27:757–767. [Google Scholar]
- Liso R, Calabrese G, Bitonti BM, Arrigoni O. Relationship between ascorbic acid and cell division. Exp Cell Res. 1984;150:314–320. doi: 10.1016/0014-4827(84)90574-3. [DOI] [PubMed] [Google Scholar]
- Loewus FA. Biosynthesis and metabolism of ascorbic acid in plants and of analogs of ascorbic acid in fungi. Phytochemistry. 1999;52:193–210. [Google Scholar]
- Loewus FA, Loewus MW. Biosynthesis and metabolism of ascorbic acid in plants. CRC Crit Rev Plant Sci. 1987;5:101–119. [Google Scholar]
- Lukowitz W, Nickle TC, Meinke DW, Last DW, Conklin PL, Somerville CR. Arabidopsis cyt1 mutants are deficient in a mannose-1-phosphate guanylyltransferase and point to a requirement of N-linked glycosylation for cellulose biosynthesis. Proc Natl Acad Sci USA. 2001;98:2262–2267. doi: 10.1073/pnas.051625798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozafar A, Oertli JJ. Vitamin-C (ascorbic-acid): uptake and metabolism by soybean. J Plant Physiol. 1993;141:316–321. [Google Scholar]
- Navas P, Gomes-Diaz P. Ascorbate free radical and its role in growth control. Protoplasma. 1995;184:8–13. [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Mol Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Pallanca JE, Smirnoff N. The control of ascorbic acid synthesis and turnover in pea seedlings. J Exp Bot. 2000;51:669–674. [PubMed] [Google Scholar]
- Siendones E, González-Reyes JA, Santos-Ocaña, Navas P, Córdoba F. Biosynthesis of ascorbic acid in kidney bean: l-galactono-γ-lactone dehydrogenase is an intrinsic protein located at the mitochondrial inner membrane. Plant Physiol. 1999;120:907–912. doi: 10.1104/pp.120.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N. The function and metabolism of ascorbic acid in plants. Ann Bot. 1996;78:661–669. [Google Scholar]
- Smirnoff N. Ascorbic acid: metabolism and functions of a multi-facetted molecule. Curr Opin Plant Biol. 2000a;3:229–235. [PubMed] [Google Scholar]
- Smirnoff N. Ascorbate biosynthesis and function in photoprotection. Phil Trans R Soc Lond Ser B Biol Sci. 2000b;355:1455–1464. doi: 10.1098/rstb.2000.0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N, Wheeler GL. Ascorbic acid in plants: biosynthesis and function. Crit Rev Biochem Mol Bio. 2000;35:291–314. doi: 10.1080/10409230008984166. [DOI] [PubMed] [Google Scholar]
- Smirnoff N, Conklin PL, Loewus FA. Biosynthesis of ascorbic acid in plants: a renaissance. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:437. doi: 10.1146/annurev.arplant.52.1.437. [DOI] [PubMed] [Google Scholar]
- Tabata K, Oba K, Suzuki K, Esaka M. Generation and properties of ascorbic acid-deficient transgenic tobacco cells expressing antisense RNA for l-galactono-1,4-lactone dehydrogenase. Plant J. 2001;27:139–148. doi: 10.1046/j.1365-313x.2001.01074.x. [DOI] [PubMed] [Google Scholar]
- Tarlyn NM, Kostman TA, Nakata PA, Keates SE, Franceschi VR. Axenic culture of Pistia stratiotes for use in plant biochemical studies. Aquatic Bot. 1998;60:161–168. [Google Scholar]
- Veljovic-Jovanovic SD, Pignocchi C, Noctor G, Foyer CH. Low ascorbic acid in vtc-1 mutant of Arabidopsis is associated with decreased growth and intracellular redistribution of the antioxidant system. Plant Physiol. 2001;127:426–435. [PMC free article] [PubMed] [Google Scholar]
- Viola R, Vreugdenhil D, Davies HV, Sommerville L. Accumulation of l-ascorbic acid in tuberising stolon tips of potato (Solanum tuberosum L.) J Plant Physiol. 1998;152:58–63. [Google Scholar]
- Wheeler GL, Jones MA, Smirnoff N. The biosynthetic pathway of vitamin C in higher plants. Nature. 1998;393:365–369. doi: 10.1038/30728. [DOI] [PubMed] [Google Scholar]
- Yahia EM, Contreras-Padilla M, Gonzalez-Aguilar G. Ascorbic acid content in relation to ascorbic acid oxidase activity and polyamine content in tomato and bell pepper fruits during development, maturation and senescence. Food Sci Technol. 2001;34:452–457. [Google Scholar]