Abstract
Using a transgenic line that overexpresses a fusion of the KNAT2 (KNOTTED-like Arabidopsis) homeodomain protein and the hormone-binding domain of the glucocorticoid receptor (GR), we have investigated the possible relations between KNAT2 and various hormones. Upon activation of the KNAT2-GR fusion, we observed a delayed senescence of the leaves and a higher rate of shoot initiation, two processes that are also induced by cytokinins and inhibited by ethylene. Furthermore, the activation of the KNAT2-GR fusion induced lobing of the leaves. This feature was partially suppressed by treatment with the ethylene precursor 1-aminocyclopropane-1-carboxylic acid, or by the constitutive ethylene response ctr1 mutation. Conversely, some phenotypic traits of the ctr1 mutant were suppressed by the activation of the KNAT2-GR fusion. These data suggest that KNAT2 acts synergistically with cytokinins and antagonistically with ethylene. In the shoot apical meristem, the KNAT2 gene is expressed in the L3 layer and the rib zone. 1-Aminocyclopropane-1-carboxylic acid treatment restricted the KNAT2 expression domain in the shoot apical meristem and reduced the number of cells in the L3. The latter effect was suppressed by the activation of the KNAT2-GR construct. Conversely, the KNAT2 gene expression domain was enlarged in the ethylene-resistant etr1-1 mutant or in response to cytokinin treatment. These data suggest that ethylene and cytokinins act antagonistically in the meristem via KNAT2 to regulate the meristem activity.
Plant organs are formed continuously during postembryonic development from groups of indeterminate meristematic cells. In Angiosperms like Arabidopsis, the cells within the shoot apical meristem (SAM) are distributed in three layers: L1, L2, and L3 (Clark, 1997; Barton, 1998). The cells derived from the L1 will preferentially form the epidermis, whereas L2 and L3 layers will give rise to the inner parts of the organs. The SAM is furthermore organized in three distinct zones: the peripheral zone, the central zone, and the rib zone. The central zone maintains a population of founder cells. Cells are recruited to the peripheral zone and the rib zone from the central zone, and generate the lateral organs and the inner parts of the stem, respectively. The family of KNOX (KNOTTED homeobox) genes plays a crucial role in the SAM: in Arabidopsis, the KNAT (KNOTTED-like in Arabidopsis) family comprises eight members (Serikawa et al., 1996; Reiser et al., 2000; Semiarti et al., 2001). Of these, four have been associated with meristem function. The best characterized is the STM (SHOOTMERISTEMLESS) gene, which is absolutely required for meristem maintenance (Long et al., 1996). More recently, loss-of-function mutants have been described for KNAT1 (Douglas et al., 2002; Venglat et al., 2002). The molecular characterization of the bp (brevipedicellus) mutant of Arabidopsis has revealed that BP encodes the KNAT1 protein. In addition to its role in meristem maintenance in redundancy with STM, BP plays a key role in regulating the inflorescence architecture (Byrne et al., 2002; Douglas et al., 2002; Venglat et al., 2002). Although two other members, KNAT2 and KNAT6, are also expressed in the meristem, their function is not clearly established (Lincoln et al., 1994; Dockx et al., 1995; Semiarti et al., 2001).
Cell-cell interactions and signaling are known to be crucial for the ordered development of multicellular organisms. Several lines of evidence suggest a link between the KNOX genes and hormone signaling pathways (Tamaoki et al., 1997; Rupp et al., 1999). In particular, the relation with cytokinins has been quite well documented. For example, overexpression of the maize (Zea mays) KNOTTED 1 gene KN1, the rice (Oryza sativa) KNOX gene OSH1, the tobacco (Nicotiana tabacum) KNOX gene NTH15, and the Arabidopsis KNAT1 gene in lettuce (Lactuca sativa) leads to higher cytokinin levels (Tamaoki et al., 1997; Kusaba et al., 1998a; Hewelt et al., 2000; Frugis et al., 2001). Conversely, plants overproducing cytokinins exhibit higher levels of KNAT1 and STM mRNA (Rupp et al., 1999). In addition to cytokinin changes, modifications of endogenous levels of auxin and GA were observed after overexpression of KNOX genes (Tamaoki et al., 1997; Kusaba et al., 1998b). Recently, the repression of a GA biosynthesis gene by the NTH15 KNOX protein has been demonstrated in tobacco (Sakamoto et al., 2001).
Here, we analyzed the link between hormone signaling pathways and a member of the KNAT family: KNAT2. This gene is expressed in the inner parts of the vegetative SAM and is down-regulated when leaf primordia are initiated (Dockx et al., 1995; Laufs et al., 1998). It is also expressed during flower development in carpels (Dockx et al., 1995; Pautot et al., 2001). In a previous paper, we used a transgenic line that expresses a KNAT2-glucocorticoid receptor (GR) fusion under the control of the cauliflower mosaic virus 35S promoter (Pautot et al., 2001). In the absence of dexamethasone (DEX), the fusion protein is maintained in the cytoplasm and is inactive, whereas in the presence of DEX, the fusion protein moves to the nucleus and transactivates target genes (for review, see Picard, 2000). The phenotype of the KNAT2-GR line was indistinguishable from the wild type in the absence of DEX and the phenotype of the wild-type control was not altered in the presence of DEX in all the conditions we tested. The activation of the KNAT2-GR fusion leads to plants with a reduced size, epinastic cotyledons, and curled, lobed leaves with ectopic stipules on their margins. In addition to the alterations of leaf development, the DEX-induced KNAT2-GR plants show a homeotic conversion of ovules into carpel-like structures (Pautot et al., 2001). In this study, we used this line to examine the link between KNAT2 and cytokinins. We showed that both factors are synergistic and we described an antagonistic relationship between KNAT2 and ethylene. Based on these results, we discussed the potential role of these interactions in the regulation of meristem function.
RESULTS
The DEX-Induced KNAT2-GR Line Exhibited Some Features of a Cytokinin Overproducer
The lobed leaf phenotype of the DEX-induced KNAT2-GR line was reminiscent of plants that overproduce cytokinins (Estruch et al., 1993). Therefore, we tested whether the DEX-induced KNAT2-GR line exhibits characteristics of plants overproducing cytokinins.
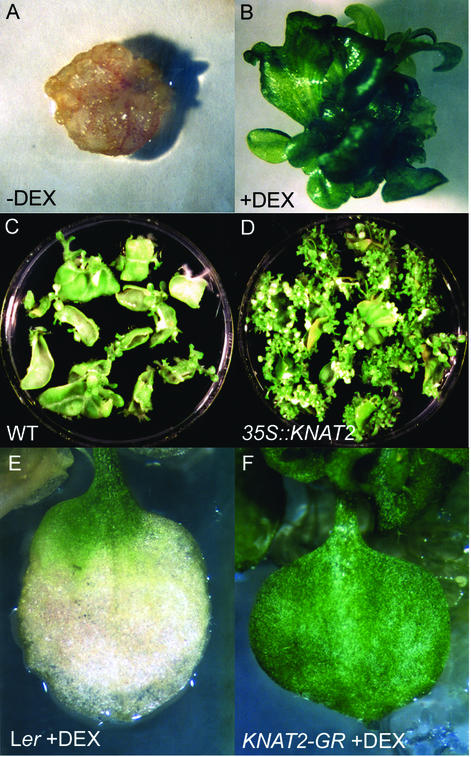
First, we examined the capacity to regenerate, a characteristic enhanced by cytokinins (Skoog and Miller, 1957). Seeds of the KNAT2-GR line were sown in vitro on medium containing 0 or 10 μm DEX. Leaves from 1-month-old grown plants were excised and transferred to the induction medium for 3 d and then transferred to the regeneration medium. After 3 weeks, calli developed on the wounded margin of noninduced KNAT2-GR leaves (Fig. 1A), whereas numerous shoots were present on all DEX-induced KNAT2-GR leaves (Fig. 1B). The same result was obtained with transgenic tobacco lines overexpressing the KNAT2 gene (Fig. 1, C and D).
Figure 1.
Effect of KNAT2 overexpression on bud induction and senescence. Leaf discs from 4-week-old KNAT2-GR Arabidopsis plants grown in vitro in the absence (A) or in the presence (B) of 10 μm DEX were transferred to the regeneration medium in the absence (A) or in the presence (B) of 10 μm DEX for 3 weeks. Leaf discs from 8-week-old non-transgenic (C) and 35S::KNAT2 (D) tobacco plants were transferred to the regeneration medium for 1 month. Landsberg erecta ecotype (Ler; E) and KNAT2-GR (F) plants were grown for 30 d in the absence of DEX, and for 30 more d in the presence of 10 μm DEX.
Another process we tested was leaf senescence, which is usually delayed by cytokinins (van Staden et al., 1988). To examine the senescence of the DEX-induced KNAT2-GR line, Ler and KNAT2-GR plants were grown in vitro for 1 month in the absence of DEX and were transferred to medium containing 10 μm DEX. Two months after germination, the oldest leaves of the Ler and noninduced KNAT2-GR plants were white and senescent, whereas the DEX-induced KNAT2-GR leaves were still green (Fig. 1, E and F).
Third, cytokinins inhibit hypocotyl elongation (Cary et al., 1995) and cause a slight epinasty of the cotyledons on light-grown seedlings (O. Hamant and V. Pautot, unpublished data). In the presence of DEX, the KNAT2-GR plants exhibited an inhibition of hypocotyl elongation and epinastic cotyledons (Fig. 2A). To quantify the inhibitory effect on growth, the hypocotyl length of 7- and 14-d-old seedlings, grown in the presence and in the absence of DEX in the light, was measured (Table I). Thirty plants were analyzed in each assay. The inhibition of hypocotyl elongation was due to the inhibition of hypocotyl cell growth because the number of cells in a file on hypocotyl epidermis of DEX-induced and noninduced KNAT2-GR plants was on average 22.8 and 22.2, respectively.
Figure 2.
Phenotype of noninduced and DEX-induced seedlings grown in vitro. A, Seven-day-old noninduced and DEX (10 μm)-induced KNAT2-GR seedling grown with a 16-h-light photoperiod. B, Seven-day-old noninduced and DEX (10 μm)-induced KNAT2-GR seedlings grown in the dark. Bar = 1 cm.
Table I.
Hypocotyl and primary root length of noninduced and DEX (10 μm)-induced KNAT2-GR seedlings
| −DEX | +DEX | |
|---|---|---|
| mm | ||
| Hypocotyl length | ||
| d 7 (Light) | 2 ± 0.2 | 1.3 ± 0.2 |
| d 7 (Dark) | 14.6 ± 2.2 | 14.9 ± 2.3 |
| Root length (mm) | ||
| d 7 (Light) | 7.6 ± 1.1 | 8.8 ± 1.1 |
| d 14 (Light) | 36 ± 3 | 32 ± 3 |
Thirty seedlings were grown in vitro with a 16-h-light photoperiod or in the dark, in the presence or in the absence of 10 μm DEX from the start of germination. Hypocotyl and root lengths were measured using Optimas software (Optimas Corporation, Bothell, WA).
In conclusion, the DEX-induced KNAT2-GR line displayed three features of cytokinins: increased shoot regeneration in tissue culture, delayed leaf senescence, and inhibition of hypocotyl elongation in the light.
In contrast, other cytokinin-specific responses such as the inhibition of root elongation and deetiolation were not observed in the DEX-induced KNAT2-GR line (Chory et al., 1991; Cary et al., 1995). The DEX-induced KNAT2-GR line grown in the dark did not show any abnormalities (Fig. 2B). The length of the hypocotyl of the DEX-induced seedlings grown in the presence and in the absence of DEX in the dark was the same (Table I). Root elongation was also quantified in 7- and 14-d-old seedlings, grown in the presence and in the absence of DEX in the light. The length of the root of the DEX-induced seedlings was normal (Table I). Thus, the activation of the KNAT2-GR fusion did not affect root development and etiolation.
KNAT2 and Ethylene Are Mutually Antagonistic
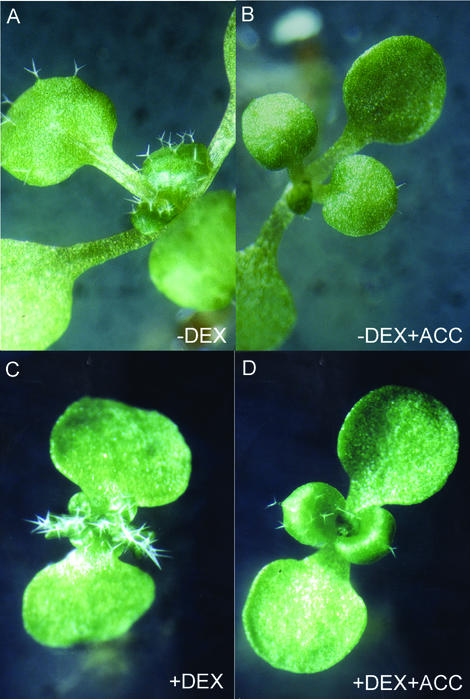
Cytokinins have often been associated with ethylene (Cary et al., 1995; Vogel et al., 1998). Therefore, we tested the interactions between KNAT2 and this hormone. First, we examined the phenotype of the DEX-induced KNAT2-GR line in the presence of the immediate ethylene precursor, 1-aminocyclopropane-1-carboxylic acid (ACC). When noninduced KNAT2-GR seedlings were continuously grown in the presence of ACC, leaves were small, dark green, and glabrous (Fig. 3, A and B). These features were similar to the morphological characteristics displayed by the constitutive ethylene response ctr1 mutant (Kieber et al., 1993). Without ACC and in the presence of 0.2 μm DEX, the KNAT2-GR seedlings exhibited an inhibition of hypocotyl elongation, epinastic cotyledons, and lobed leaves (Fig. 3C). These features were suppressed by the application of 10 μm ACC (Fig. 3D). In the presence of 10 μm DEX and 10 μm ACC, the epinasty of the cotyledons was reversed, and the lobes and the curling of the leaves were significantly less marked (data not shown). These data suggested that ethylene partially reversed the DEX-induced KNAT2-GR phenotype.
Figure 3.
Effect of ACC on the phenotype of DEX-induced KNAT2-GR in vitro seedlings. A, Two-week-old noninduced KNAT2-GR seedling. B, Two-week-old noninduced KNAT2-GR seedling grown in the presence of 10 μm ACC. C, Two-week-old KNAT2-GR seedling grown in the presence of 0.2 μm DEX. D, Two-week-old KNAT2-GR seedling grown in the presence of 0.2 μm DEX and 10 μm ACC.
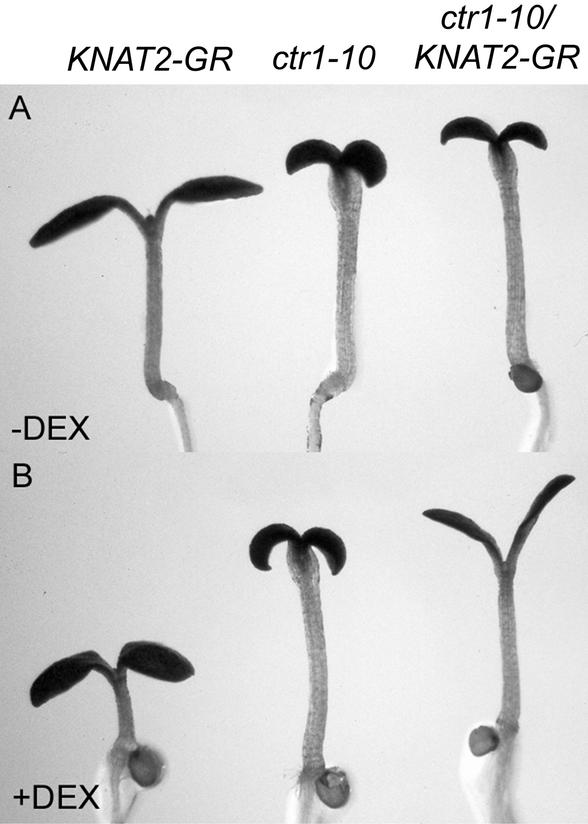
To confirm these data, we next examined the effect of KNAT2-GR activation in a ctr1-10 background (Kieber et al., 1993; Beaudoin et al., 2000). For this purpose, plants homozygous for both the ctr1-10 mutation and the KNAT2-GR construct were obtained. In the light and in the absence of DEX, the ctr1-10/KNAT2-GR line displayed the characteristic ctr1 phenotype (Goeschl et al., 1966; Kieber et al., 1993; Smalle et al., 1997): inhibition of root elongation, stimulation of hypocotyl elongation, radial swelling of the hypocotyl, delayed opening, and expansion of the cotyledons (Fig. 4A). Moreover, in the absence of DEX, the ctr1-10/KNAT2-GR line exhibited small, dark-green, and glabrous leaves, as did the ctr1-10 mutant (Kieber et al., 1993; Fig. 5, B and C). Application of DEX did not affect the ctr1-10 phenotype (Figs. 4, A and B, and 5, B and E). When ctr1-10/KNAT2-GR seedlings were grown in the presence of 0.2 μm DEX, the cotyledons were not epinastic (Fig. 4B) and the leaves were unlobed (Fig. 5F) in contrast to the DEX-induced KNAT2-GR line (Fig. 5D). However, the elongation of the petioles was still inhibited (Fig. 5F). These data are similar to the results obtained with the KNAT2-GR line grown in the presence of DEX and ACC (Fig. 3D).
Figure 4.
Phenotype of 7-d-old noninduced and DEX-induced ctr1-10/KNAT2-GR seedlings grown in vitro. A, KNAT2-GR, ctr1-10, and ctr1-10/KNAT2-GR seedlings grown in the absence of DEX. B, KNAT2-GR, ctr1-10, and ctr1-10/KNAT2-GR seedlings grown in the presence of 0.2 μm DEX. The root is out of focus but was unaffected by the DEX treatment.
Figure 5.
Phenotype of the 3-week-old noninduced and DEX-induced ctr1-10/KNAT2-GR plants grown in vitro. A, Noninduced KNAT2-GR plant. B, Ctr1-10 plant in the absence of DEX. C, Noninduced ctr1-10/KNAT2-GR plant. D, KNAT2-GR plant in the presence of 0.2 μm DEX. E, Ctr1-10 plant in the presence of 0.2 μm DEX. F, Ctr1-10/KNAT2-GR plant in the presence of 0.2 μm DEX. Bar = 4 mm.
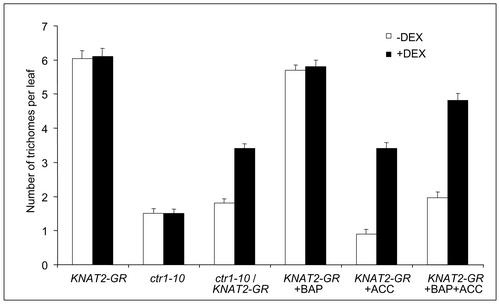
Next, we examined the effect of the activation of the KNAT2-GR fusion on specific features of the ctr1-10 phenotype. When ctr1-10/KNAT2-GR seedlings were grown in the presence of 0.2 μm DEX, the delayed opening of the cotyledons was suppressed (Fig. 4B) and the leaves exhibited a phenotype close to that of wild-type plants (Fig. 5F). In particular, the leaves of the DEX-induced ctr1-10/KNAT2-GR plants were not dark green and the number of trichomes was increased compared with the noninduced ctr1-10/KNAT2-GR plants (Figs. 5, C and F, and 6). In contrast, the inhibition of root elongation of the ctr1-10 mutant was not corrected by the activation of the KNAT2-GR fusion. Thus, ethylene partially corrected the phenotype of the DEX-induced KNAT2-GR line, and the activation of the KNAT2-GR fusion counterbalanced some of the effects of ethylene.
To further investigate the antagonism between KNAT2/cytokinin and ethylene, we monitored the number of trichomes in response to ACC and BAP on the first leaves of noninduced and DEX-induced KNAT2-GR plants. The number of trichomes was not altered when the noninduced KNAT2-GR plants were grown in the presence of 0.1 μm BAP, whereas it was reduced in the presence of 10 μm ACC (Fig. 6). When the noninduced KNAT2-GR plants were grown in the presence of both 10 μm ACC and 0.1 μm BAP, the number of trichomes was 2-fold greater than that observed with ACC alone. Thus, the application of cytokinin can antagonize the effect of ACC on trichome development. In the presence of 0.1 μm DEX, the KNAT2-GR leaves exhibited the same number of trichomes than the noninduced KNAT2-GR plants. When the KNAT2-GR plants were grown in the presence of both DEX and 10 μm ACC, the number of trichomes was 3-fold greater than that observed with ACC and without DEX (Fig. 6). These data confirmed not only the result obtained with the DEX-induced ctr1-10/KNAT2-GR line, but also revealed that the activation of the KNAT2-GR construct can mimic a cytokinin treatment. The increase in the number of trichomes was even higher when the ACC DEX-induced plants were grown in the presence of BAP (Fig. 6).
Figure 6.
Effect of ethylene, cytokinin, and KNAT2-GR activation on trichome number. The number of trichomes was quantified on the first leaves of 3-week-old KNAT2-GR, ctr1-10, and ctr1-10/KNAT2-GR in the absence of DEX (white) or in the presence of 0.1 μm DEX (black). The number of trichomes was also quantified on the first leaves of 3-week-old KNAT2-GR plants grown in the presence of 10 μm ACC, or 0.1 μm 6-benzylaminopurine (BAP), or 10 μm ACC and 0.1 μm BAP, in the absence of DEX (white) or in the presence of 0.1 μm DEX (black).
The Effect of Ethylene on Meristem Structure Was Antagonized by the Overexpression of KNAT2
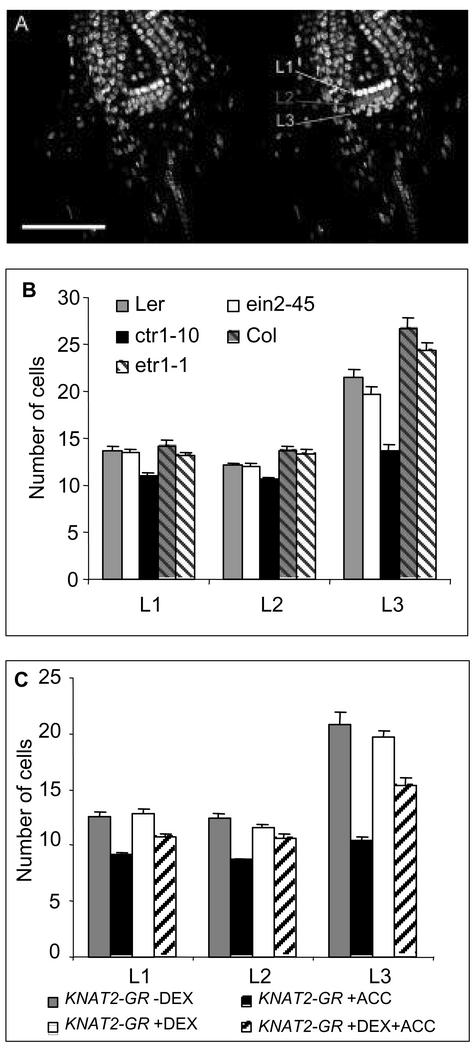
The antagonism with ethylene described above was inferred from the activation of the KNAT2-GR fusion, i.e. outside the meristem. To study the relevance for SAM function, we next examined the effect of ethylene on meristem structure in the ctr1 background. For this purpose, 7-d-old Ler and ctr1-10 plantlets were optically sectioned using confocal scanning laser microscopy and the number of cells in each layer of the SAM in cross sections was determined as described in Figure 7A. For each genotype, 30 plants were analyzed. The ctr1-10 meristem showed a reduction in the number of cells in the SAM when compared with Ler. Although the number of cells in the L1 and the L2 layers was only slightly reduced, Ler and ctr1-10 exhibited 21.5 ± 0.8 and 13.7 ± 0.7 cells in the L3, respectively (Fig. 7B). Thus, the ctr1-10 mutation affects the number of cells in the L3 layer. The same experiment was conducted in the insensitive ethylene mutant backgrounds (ein2-45 and etr1-1) seedlings. In cross sections of Ler and ein2-45, and ColO and etr1-1 seedlings, the number of the cells in the L1, L2, and L3 was similar (Fig. 7B). Thus, endogenous ethylene does not seem to play a major role in controlling the meristem structure.
Figure 7.
Number of cells in the SAM of ethylene mutants and KNAT2-GR line. A, Seven-day-old seedlings were stained with the nuclear stain propidium iodide and viewed directly in a TCS-NT confocal laser scanning microscope (left; [Leica Microsystems, Heidelberg]). The number of cells in each layer of the SAM was determined on cross sections in the median plan (right). B, Number of cells in L1, L2, and L3 layers determined from 30 7-d-old optical views of Ler, ein2-45, ctr1-10, ColO, and etr1-1 seedlings grown and stained as described in A. C, Number of cells in L1, L2, and L3 layers determined from 30 7-d-old optical views of KNAT2-GR seedlings grown and stained as described in A.
Next, we examined the effect of ethylene on the meristem after the activation of the KNAT2-GR fusion. Thirty noninduced and 30 DEX-induced plantlets were treated as above. The number of cells in the three layers of the SAM was similar in noninduced and DEX-induced KNAT2-GR plants (Fig. 7C). In the presence of 10 μm ACC, noninduced KNAT2-GR plants exhibited a reduced number of cells in the SAM, and phenocopied the ctr1-10 mutant (Fig. 7, B and C). When KNAT2-GR plants were grown in the presence of 10 μm ACC and 10 μm DEX, the number of cells in the SAM was increased compared with the noninduced KNAT2-GR plants grown in the presence of ACC (Fig. 7C). Thus, the activation of the KNAT2-GR fusion can antagonize the effect of ACC in the meristem by increasing the number of cells in the L3.
The Synergism between KNAT2 and Cytokinins and the Antagonism between KNAT2 and Ethylene Were Also Observed at the Level of KNAT2::GUS Expression
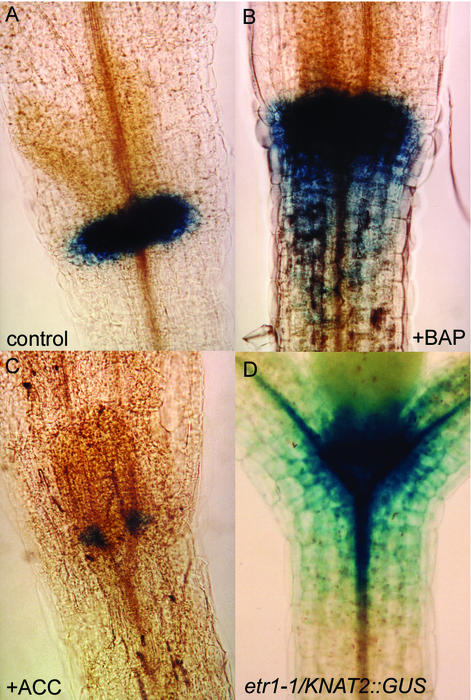
The KNAT2 gene is expressed in the L3 layer and in the rib zone of the vegetative meristem (Dockx et al., 1995). To investigate the regulation of KNAT2 by cytokinins and ethylene, the KNAT2::GUS line was used (Dockx et al., 1995). Seeds from the KNAT2::GUS line were plated on medium containing 0 or 0.1 μm BAP. After 7 d, seedlings were collected and β-glucuronidase (GUS) expression was assayed. Figure 8A shows the typical distribution of GUS activity detected in vegetative tissues of 7-d-old seedlings. When plants were grown in the presence of BAP, the pattern of expression was enlarged in the SAM and reached the upper part of the hypocotyl (Fig. 8B). A strong GUS staining was also detected in the root (data not shown). The same result was obtained with the natural cytokinin zeatin and with N(2Chloro-4pyridyl) N′phenylurea, a urea-type cytokinin (data not shown). In contrast, in the presence of ACC, the KNAT2::GUS expression in the SAM was limited to spots at the base of the leaves (Fig. 8C). No GUS staining was revealed in the root (data not shown). To further examine the interaction between ethylene and KNAT2 expression, the KNAT2::GUS line was crossed to the ethylene-resistant mutant etr1-1 (Chang et al., 1993) and a line homozygous for both the KNAT2::GUS construct and the etr1-1 allele was obtained. The domain of GUS staining was strongly enlarged in the SAM and in the hypocotyl of the homozygous etr1-1/KNAT2::GUS line (Fig. 8D). Thus, the KNAT2 gene expression was inhibited by ethylene, and induced by cytokinins.
Figure 8.
Effect of cytokinin and ethylene on KNAT2::GUS expression. A, Seven-day-old seedling grown on control medium. B, Seven-day-old seedling grown in the presence of 0.1 μm BAP. C, Seven-day-old seedling grown in the presence of 10 μm ACC. D, Seven-day-old etr1-1/KNAT2::GUS seedling grown on control medium.
DISCUSSION
Previous studies have indicated a link between KNOX genes and cytokinins in meristem regulation in different species. Our observations show that KNAT2 is not an exception. First, the lobed leaf phenotype is reminiscent of plants that overproduce cytokinins (Estruch et al., 1993). Likewise, the higher rate of regeneration observed in DEX-induced KNAT2-GR explants is a typical cytokinin feature that was also observed in transgenic lettuce that overexpressed KNAT1 (Frugis et al., 1999, 2001). Third, the KNAT2-GR plants grown in the presence DEX exhibited an inhibition of hypocotyl elongation and epinastic cotyledons, much like the KNAT2-GR plant grown in the presence of BAP (Cary et al., 1995; personal observations). Fourth, the KNAT2-GR activation led to a delay in leaf senescence, a phenotype induced by both cytokinins and overexpression of KN1 in tobacco and KNAT1 in lettuce (Gan and Amasino, 1995; Ori et al., 1999; Frugis et al., 2001). Finally, preliminary differential screening indicated common targets between cytokinin and KNAT2 (O. Hamant, F. Nogré, J. Traas, and V. Pautot, unpublished data). Although, these results pointed to a positive effect of KNAT2 on the cytokinin pathway, the reciprocal was apparently also true because the expression of the KNAT2 gene was induced in the presence of cytokinins. Similar observations were made for KNAT1 and STM (Rupp et al., 1999). It will now be important to elucidate the molecular basis of the interaction between cytokinins and the KNOX genes. In particular, the hierarchy between KNAT2 and cytokinins remains unclear. As supposed for the other members of the KNAT family, cytokinins could act downstream of KNAT2. In this respect, the positive effect of cytokinins on KNAT2 expression could represent a positive feedback. Recent progress, such as the identification of some of the effectors of the cytokinin transduction pathway or the identification of target genes of KNOX genes, should give more insight in this model (Hwang and Sheen, 2001; Inoue et al., 2001). In particular, the analysis of the phenotype of the DEX-induced KNAT2-GR line in a cytokinin-resistant mutant background could be useful.
Our results clearly indicate a positive link between cytokinins and KNAT2. However, in contrast to overproducers of cytokinins, the phenotype of the DEX-induced KNAT2-GR line in the dark was not altered and the elongation of the root was normal. This shows that the link with cytokinins is restricted to the aerial part of the light-grown plants. It could be that KNAT2 is not active in other tissues, for example, due to lack of partners.
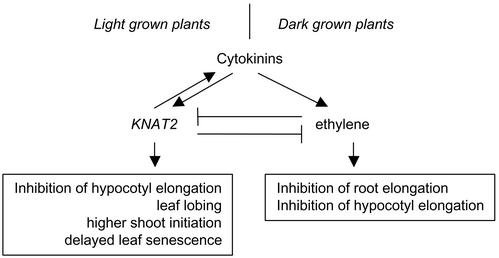
Cytokinins have often been associated with ethylene (Cary et al., 1995). In some cases, this relation is synergistic. More precisely, cytokinins can induce ethylene synthesis via the posttranslational modification of a key enzyme, ACS5 (Vogel et al., 1998). In contrast, in other processes such as senescence, regeneration, and hypocotyl elongation in the light, both hormones act antagonistically (Gan and Amasino, 1995; Grbic and Bleecker, 1995; Smalle et al., 1997; Kumar et al., 1998). In view of the link between both hormone pathways, we asked whether KNAT2 also interfered with the ethylene pathway. We show that ACC attenuated the phenotype of DEX-induced KNAT2-GR plants and restricted the expression pattern of the KNAT2 gene within the SAM. Conversely, KNAT2 was ectopically expressed in the roots and hypocotyl in an ethylene-insensitive background. This would suggest that ethylene could regulate KNAT2, but the reverse seems also to be true: Overexpression of KNAT2 antagonized some of the effects of ACC treatment and some features of the ctr1-10 phenotype. Interestingly, several characteristics of the KNAT2 overexpressers, such as a short hypocotyl and epinastic cotyledons in the light, are also found in ethylene-insensitive backgrounds (Guzman and Ecker, 1990; Beaudoin et al., 2000; for review, see Smalle and van der Straeten, 1997; Bleecker and Kende, 2000). Therefore, our results suggest an antagonistic relation between KNAT2 and ethylene. It remains to be seen to what extent this interaction involves cytokinin signaling. We suggest that the cytokinin signaling pathway could be divided in an ethylene-dependent pathway, which would control the response of cytokinins in the root and in the dark, and a KNAT-dependent pathway. This latter pathway would control the response of cytokinins that are induced in the light and are antagonistic to ethylene, such as cotyledon epinasty, leaf lobing, delayed senescence, and an enhanced capacity to regenerate (Fig. 9). At this stage, we cannot exclude that KNAT2 interacts with the cytokinin pathway, directly via its antagonistic effect on ethylene.
Figure 9.
Putative position of KNAT2 in the cytokinin and ethylene network. The order of the elements in the pathway is based on data from “Results,” Cary et al. (1995), and Smalle and Van der Straeten (1997). In the dark, the cytokinin responses shown are some of those examined in wild-type plants in the presence of either BAP or ACC. In the light, the cytokinin responses shown are some of those examined either in cytokinin overproducers or in the DEX-induced KNAT2-GR line.
The interaction with ethylene was observed in a context where KNAT2 was ectopically activated. Therefore, we wondered if these findings were relevant for the SAM itself. So far, the potential role of ethylene in the meristem has been poorly investigated. Ethylene is involved in the control of meristem identity because etr1, ein2, ain1, and ctr1 have a late-flowering phenotype (Guzman and Ecker, 1990; Kieber et al., 1993; for review, see Smalle and Van der Straeten, 1997). In addition, hls1 (hookless 1), which is deficient in apical hook formation, a particular ethylene response, has an increased rate of leaf initiation during the first days of development (Lehman et al., 1996). Moreover, hls1 displays a loss of apical dominance and precocious flowering, suggesting that HLS1 is involved in meristem activity and identity (Lehman et al., 1996). These features are also observed in mutants altered in meristem function. However, it still remains unclear whether HLS1 is involved in ethylene signaling solely or in combination with auxin. Future prospects on the hls1 mutant may bring new evidence on the putative role of ethylene in the meristem. According to our results, ethylene leads to a significant reduction in the number of cells in the SAM. Remarkably, this effect essentially concerns the domain where the KNAT2 gene is expressed (Dockx et al., 1995).
How should the link between ethylene and the SAM be interpreted? One possibility is that the hormone limits meristem size; for instance, by promoting cell differentiation and inactivating meristem identity. However, the structure of the meristem in ein2-45 and etr1-1 was not altered, which would argue against a major role of ethylene in the SAM. At this stage, it cannot be excluded that endogenous ethylene modulates the rate of cell proliferation. More interestingly, we show that the expression domain of KNAT2 is dramatically reduced in the meristem in response to ACC. This suggests that ethylene could control the transition from the meristem into the primordia via the repression of KNAT2 and possibly other meristematic regulators. For instance, ethylene could repress KNAT genes together with AS1 (ASYMETRIC LEAVES 1) and AS2 (ASYMETRIC LEAVES 2) in the emerging leaves (Byrne et al., 2000; Semiarti et al., 2001). Ethylene could also act as a relay of AS1 and AS2 in repressing meristematic regulators in mature and senescent leaves. Finally, under normal conditions, the effect of ethylene as a negative regulator could usually be compensated by a meristem-stimulating factor such as cytokinins.
MATERIALS AND METHODS
Plant Material and Genetic Analysis
The transgenic line, KNAT2-GR, carrying the 35S::KNAT2-GR construct in Ler, has been described (Pautot et al., 2001). 35S::KNAT2 tobacco (Nicotiana tabacum) plants were also generated (Dockx, 1995). The transgenic line carrying a KNAT2::GUS fusion is in the C24 ecotype and has been described (Dockx et al., 1995). We demonstrated previously that the KNAT2::GUS staining pattern matched the expression pattern of KNAT2 obtained by radioactive in situ hybridizations (Pautot et al., 2001). The ctr1-10 (Ler) and ein2-45 (Ler) mutant lines were kindly supplied by Jérôme Giraudat (Institut des Sciences Végétales, Centre de la Recherche Scientifique, GIF sur Yvette, France). The ctr1-10 mutant displays a constitutive ethylene response comparable with the ctr1-1 mutant (Kieber et al., 1993; Beaudoin et al., 2000). The ein2-45 mutant exhibits an ethylene insensitivity comparable with the ein2-1 mutant (Guzman and Ecker, 1990; Alonso et al., 1999; Beaudoin et al., 2000). etr1-1 (Columbia ecotype) was supplied by the Nottingham Arabidopsis Stock Center (University of Nottingham, Loughborough, UK). To introduce the 35S-KNAT2-GR construct in the ctr1-10 mutant, ctr1-10 homozygous plants were crossed to the KNAT2-GR line and a plant homozygous for ctr1-10 and KNAT2-GR construct was selected on the basis of ctr1-10 phenotype and kanamycin resistance.
Growth Conditions and Treatments
Plants were grown in vitro on culture medium adapted from Estelle and Summerville (1987), without Suc [5 mm KNO3, 2.5 mm KH2PO4, 2 mm MgSO4(7H2O), 2 mm Ca(NO3)2, 100 mg L−1 myo-inositol, 1 mg L−1 calcium panthotenate, 0.01 mg L−1 biotin, 1 mg L−1 niacin, 1 mg L−1 pyridoxine, 1 mg L−1 thiamine, 50 mg L−1 ferric ammonium citrate, 70 μm H3BO3, 14 μm MnCl2(4H2O), 0.5 μm CuSO4(5H2O), 0.2 μm Na2MoO4(2H2O), 10 μm NaCl, 1 μm ZnSO4(7H2O), 0.01 μm CoCl2(6H2O), 8 mg L−1 bromocresol purple, 0.7 g L−1 MES, and 0.7% (w/v) agar]. Unless stated otherwise, seedlings were grown under a 16-h-light/8-h-dark photoperiod. DEX (Sigma, St. Louis) was dissolved in ethanol at 10 mm. Experiments were carried out using 0.1 to 10 μm DEX. Unless otherwise stated, plants were continuously grown in the presence of DEX from germination. BAP (Sigma) and ACC (Research Organics Inc., Cleveland) were dissolved in dimethyl sulfoxide. In the medium, the concentration of BAP was 0.1 μm, and the concentration range of ACC was 0.1 to 10 μm. For the regeneration experiments, the culture medium was modified: The induction medium was supplemented with 1% (w/v) Suc and 2.25 mg L−1 BAP (Sigma), and the regeneration medium was supplemented with 1% (w/v) Suc, 0.15 mg L−1 indole-3-acetic acid (Sigma), and 5 mg L−1 N6-[2-isopentenyl]adenosine (Sigma). Leaf discs from 1-month-old KNAT2-GR plants grown in vitro in the absence or in the presence of DEX (10 μm) were used as explants. Leaf discs from noninduced and DEX-induced plantlets were plated on the induction medium for 3 d in the absence or in the presence of DEX (10 μm), respectively. They were then transferred onto the regeneration medium and cultured for 3 weeks in the absence or in the presence of DEX (10 μm). Regeneration in tobacco was conducted as described by Crepy et al. (1982).
Morphometric and Histological Analysis
Thirty KNAT2-GR plantlets grown in vitro were fixed in a 4% (w/v) paraformaldehyde solution, and visualized using a CCD camera (XC-77, Sony, Tokyo). Hypocotyl and root length were measured using the Optimas version 6.1 software on 7- and 14-d-old plants. For the statistical analysis, sds are indicated.
Histochemical Localization of KNAT2::GUS Activity
Seven-day-old KNAT2::GUS plantlets were fixed in 80% (v/v) acetone at −20°C for 1 h, and stained for 4 h at 37°C in a solution containing 0.5 mg mL−1 5-bromo-4-chloro-3-indolyl-β-glucuronic acid (Duchefa, Haarlem, The Netherlands), 0.1% (w/v) Triton X-100, 0.5 mm K4 Fe (CN)6, and 50 mm sodium phosphate buffer, pH 7.2. Whole-mount preparations were made to visualize GUS activity.
Confocal Microscopy
Thirty-seven-day-old Ler, ein2-45, ctr1-10, and noninduced and DEX-induced KNAT2-GR seedlings were fixed and stained with propidium iodide to visualize DNA, as described by Couteau et al. (1999). Seedlings were then mounted in a drop of citifluor glycerol/phosphate-buffered saline (Oxford Institute, Orsay, France), and viewed directly in a Leica TCS-NT confocal laser scanning microscope equipped with an argon/krypton laser (Omnichrome, Chino, CA). To visualize the propidium iodide, a BP568 band pass filter for excitation was used in combination with a long pass filter (LP590). Optical sections in the median plane of the apex of the plantlets were generated. The number of cells in the three layers of the SAM was determined by counting the number of nuclei.
ACKNOWLEDGMENTS
We thank Dr. Patrick Laufs and Dr. Trine Juul for critical reading of the manuscript, and Dr. Michel Laloue for useful discussions. We thank Joël Talbotec and Hervé Ferry for help in the greenhouse.
Footnotes
This work was supported by the Ministère de l'Enseignement Supérieur et de la Recherche and by the European Union Regulatory Gene Initiative In Arabidopsis program (to E.B.-B.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.004564.
LITERATURE CITED
- Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science. 1999;284:2148–2152. doi: 10.1126/science.284.5423.2148. [DOI] [PubMed] [Google Scholar]
- Barton MK. Cell type specification and self renewal in the vegetative shoot apical meristem. Curr Opin Plant Biol. 1998;1:37–42. doi: 10.1016/s1369-5266(98)80125-8. [DOI] [PubMed] [Google Scholar]
- Beaudoin N, Serizet C, Gosti F, Giraudat J. Interactions between abscisic acid and ethylene signalling cascades. Plant Cell. 2000;12:1103–1116. doi: 10.1105/tpc.12.7.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker AB, Kende H. Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol. 2000;16:1–18. doi: 10.1146/annurev.cellbio.16.1.1. [DOI] [PubMed] [Google Scholar]
- Byrne ME, Barley R, Curtis M, Arroyo JM, Dunham M, Hudson A, Martienssen RA. Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis. Nature. 2000;408:967–971. doi: 10.1038/35050091. [DOI] [PubMed] [Google Scholar]
- Byrne ME, Simorowski J, Martienssen RA. ASYMMETRIC LEAVES1 reveals knox gene redundancy in Arabidopsis. Development. 2002;129:1957–1965. doi: 10.1242/dev.129.8.1957. [DOI] [PubMed] [Google Scholar]
- Cary AJ, Liu W, Howell SH. Cytokinin action is coupled to ethylene in its effects on the inhibition of root and hypocotyl elongation in Arabidopsis thaliana seedlings. Plant Physiol. 1995;107:1075–1082. doi: 10.1104/pp.107.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science. 1993;262:539–544. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- Chory J, Aguilar N, Peto CA. The phenotype of Arabidopsis thaliana det1 mutants suggests a role for cytokinins in greening. Symp Soc Exp Biol. 1991;45:21–29. [PubMed] [Google Scholar]
- Clark SE. Organ formation at the vegetative shoot meristem. Plant Cell. 1997;9:1067–1076. doi: 10.1105/tpc.9.7.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couteau F, Belzile F, Horlow C, Grandjean O, Vezon D, Doutriaux MP. Random chromosome segregation without meiotic arrest in both male and female meiocytes of a dmc1 mutant of Arabidopsis. Plant Cell. 1999;11:1623–1634. doi: 10.1105/tpc.11.9.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepy L, Chupeau MC, Chupeau Y. The isolation and culture of leaf protein of Cichorium intybus and their regeneration into plants. Z Pflanzenphysiol Bd. 1982;107:123–131. [Google Scholar]
- Dockx J. On homeobox genes and plant development. PhD thesis. The Netherlands: University of Utrecht; 1995. [Google Scholar]
- Dockx J, Quaedvlieg N, Keultjes G, Kock P, Weisbeek P, Smeekens S. The homeobox gene ATK1 of Arabidopsis thaliana is expressed in the shoot apex of the seedling and in flowers and inflorescence stems of mature plants. Plant Mol Biol. 1995;28:723–737. doi: 10.1007/BF00021196. [DOI] [PubMed] [Google Scholar]
- Douglas SJ, Chuck G, Dengler RE, Pelecanda L, Riggs CD. KNAT1 and ERECTA regulate inflorescence architecture in Arabidopsis. Plant Cell. 2002;14:547–558. doi: 10.1105/tpc.010391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estelle MA, Summerville CR. Auxin-resistant mutants of Arabidopsis thaliana with an altered morphology. Mol Gen Genet. 1987;206:200–206. [Google Scholar]
- Estruch JJ, Granell A, Hansen G, Prinsen E, Redig P, Van Onckelen H, Schwarz-Sommer Z, Sommer H, Spena A. Floral development and expression of floral homeotic genes are influenced by cytokinins. Plant J. 1993;4:379–384. doi: 10.1046/j.1365-313x.1993.04020379.x. [DOI] [PubMed] [Google Scholar]
- Frugis G, Giannino D, Mele G, Nicolodi C, Chiappetta A, Bitonti MB, Innocenti AM, Dewitte W, Van Onckelen H, Mariotti D. Overexpression of KNAT1 in lettuce shifts leaf determinate growth to a shoot-like indeterminate growth associated with an accumulation of isopentenyl-type cytokinins. Plant Physiol. 2001;126:1370–1380. doi: 10.1104/pp.126.4.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frugis G, Giannino D, Mele G, Nicolodi C, Innocenti AM, Chiappetta A, Bitonti MB, Dewitte W, Van Onckelen H, Mariotti D. Are homeobox KNOTTED-like genes and cytokinins the leaf architects? Plant Physiol. 1999;119:371–374. doi: 10.1104/pp.119.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan S, Amasino RM. Inhibition of leaf senescence by autoregulated production of cytokinin. Science. 1995;270:1986–1988. doi: 10.1126/science.270.5244.1986. [DOI] [PubMed] [Google Scholar]
- Goeschl JD, Rappaport L, Pratt HK. Ethylene as a factor regulating the growth of pea epicotyls subjected to physical stress. Plant Physiol. 1966;41:877–884. doi: 10.1104/pp.41.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grbic V, Bleecker AB. Ethylene regulates the timing of leaf senescence in Arabidopsis. Plant J. 1995;8:595–602. [Google Scholar]
- Guzman P, Ecker JR. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell. 1990;2:513–523. doi: 10.1105/tpc.2.6.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewelt A, Prinsen E, Thomas M, Onckelen HV, Meins F., Jr Ectopic expression of maize KNOTTED1 results in the cytokinin-autotrophic growth of cultured tobacco tissues. Planta. 2000;210:884–889. doi: 10.1007/s004250050693. [DOI] [PubMed] [Google Scholar]
- Hwang I, Sheen J. Two-component circuitry in Arabidopsis cytokinin signal transduction. Nature. 2001;413:383–389. doi: 10.1038/35096500. [DOI] [PubMed] [Google Scholar]
- Inoue T, Higuchi M, Hashimoto Y, Seki M, Kobayashi M, Kato T, Tabata S, Shinozaki K, Kakimoto T. Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature. 2001;409:1060–1063. doi: 10.1038/35059117. [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- Kumar PP, Lakshmanan P, Thorpe TA. Regulation of morphogenesis in plant tissue culture by ethylene. In Vitro Cell Dev Biol Plant. 1998;34:94–103. [Google Scholar]
- Kusaba S, Fukumoto M, Honda C, Yamaguchi I, Sakamoto T, Kano-Murakami Y. Decreased GA1 content caused by the overexpression of OSH1 is accompanied by suppression of GA 20-oxidase gene expression. Plant Physiol. 1998b;117:1179–1184. doi: 10.1104/pp.117.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaba S, Kano-Murakami Y, Matsuoka M, Tamaoki M, Sakamoto T, Yamaguchi I, Fukumoto M. Alteration of hormone levels in transgenic tobacco plants overexpressing the rice homeobox gene OSH1. Plant Physiol. 1998a;116:471–476. doi: 10.1104/pp.116.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs P, Dockx J, Kronenberger J, Traas J. MGOUN1 and MGOUN2: two genes required for primordium initiation at the shoot apical and floral meristems in Arabidopsis thaliana. Development. 1998;125:1253–1260. doi: 10.1242/dev.125.7.1253. [DOI] [PubMed] [Google Scholar]
- Lehman A, Black R, Ecker JR. HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell. 1996;85:183–194. doi: 10.1016/s0092-8674(00)81095-8. [DOI] [PubMed] [Google Scholar]
- Lincoln C, Long J, Yamaguchi J, Serikawa K, Hake S. A knotted1-like homeobox gene in Arabidopsis is expressed in the vegetative meristem and dramatically alters leaf morphology when overexpressed in transgenic plants. Plant Cell. 1994;6:1859–1876. doi: 10.1105/tpc.6.12.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JA, Moan EI, Medford JI, Barton MK. A member of the KNOTTED class of homeodomain proteins encoded by the STM gene of Arabidopsis. Nature. 1996;379:66–69. doi: 10.1038/379066a0. [DOI] [PubMed] [Google Scholar]
- Ori N, Juarez MT, Jackson D, Yamaguchi J, Banowetz GM, Hake S. Leaf senescence is delayed in tobacco plants expressing the maize homeobox gene knotted1 under the control of a senescence-activated promoter. Plant Cell. 1999;11:1073–1080. [PMC free article] [PubMed] [Google Scholar]
- Pautot V, Dockx J, Hamant O, Kronenberger J, Grandjean O, Jublot D, Traas J. KNAT2: evidence for a link between knotted-like genes and carpel development. Plant Cell. 2001;13:1719–1734. doi: 10.1105/TPC.010184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard D. Posttranslational regulation of proteins by fusions to steroid-binding domains. Methods Enzymol. 2000;327:385–401. doi: 10.1016/s0076-6879(00)27291-1. [DOI] [PubMed] [Google Scholar]
- Reiser L, Sanchez-Baracaldo P, Hake S. Knots in the family tree: evolutionary relationships and functions of knox homeobox genes. Plant Mol Biol. 2000;42:151–166. [PubMed] [Google Scholar]
- Rupp HM, Frank M, Werner T, Strnad M, Schmulling T. Increased steady state mRNA levels of the STM and KNAT1 homeobox genes in cytokinin overproducing Arabidopsis thaliana indicate a role for cytokinins in the shoot apical meristem. Plant J. 1999;18:557–563. doi: 10.1046/j.1365-313x.1999.00472.x. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Kamiya N, Ueguchi-Tanaka M, Iwahori S, Matsuoka M. KNOX homeodomain protein directly suppresses the expression of a gibberellin biosynthetic gene in the tobacco shoot apical meristem. Genes Dev. 2001;15:581–590. doi: 10.1101/gad.867901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semiarti E, Ueno Y, Tsukaya H, Iwakawa H, Machida C, Machida Y. The ASYMMETRIC LEAVES2 gene of Arabidopsis thaliana regulates formation of a symmetric lamina, establishment of venation and repression of meristem-related homeobox genes in leaves. Development. 2001;128:1771–1783. doi: 10.1242/dev.128.10.1771. [DOI] [PubMed] [Google Scholar]
- Serikawa KA, Martinez-Laborda A, Zambryski P. Three knotted1-like homeobox genes in Arabidopsis. Plant Mol Biol. 1996;32:673–683. doi: 10.1007/BF00020208. [DOI] [PubMed] [Google Scholar]
- Skoog E, Miller CO. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol. 1957;11:118–131. [PubMed] [Google Scholar]
- Smalle J, Haegman M, Kurepa J, Van Montagu M, Van der Straeten D. Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proc Natl Acad Sci USA. 1997;94:2756–2761. doi: 10.1073/pnas.94.6.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalle J, Van der Straeten D. Ethylene and vegetative development. Physiol Plant. 1997;100:593–605. [Google Scholar]
- Tamaoki M, Kusaba S, Kano-Murakami Y, Matsuoka M. Ectopic expression of a tobacco homeobox gene NTH15 dramatically alters leaf morphology and hormone levels in transgenic tobacco. Plant Cell Physiol. 1997;38:917–927. doi: 10.1093/oxfordjournals.pcp.a029252. [DOI] [PubMed] [Google Scholar]
- Van Staden J, Cook EL, Nooden LD. Cytokinins and senescence. In: Nooden LD, Leopold AC, editors. Senescence and Aging in Plants. San Diego: Academic Press; 1988. pp. 281–328. [Google Scholar]
- Venglat SP, Dumonceaux T, Rozwadowski K, Parnell L, Babic V, Keller W, Martienssen R, Selvaraj G, Datla R. The homeobox gene BREVIPEDICELLUS is a key regulator of inflorescence architecture in Arabidopsis. Proc Natl Acad Sci USA. 2002;99:4730–4735. doi: 10.1073/pnas.072626099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JP, Woeste KE, Theologis A, Kieber JJ. Recessive and dominant mutations in the ethylene biosynthetic gene ACS5 of Arabidopsis confer cytokinin insensitivity and ethylene overproduction, respectively. Proc Natl Acad Sci USA. 1998;95:4766–4771. doi: 10.1073/pnas.95.8.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]