Abstract
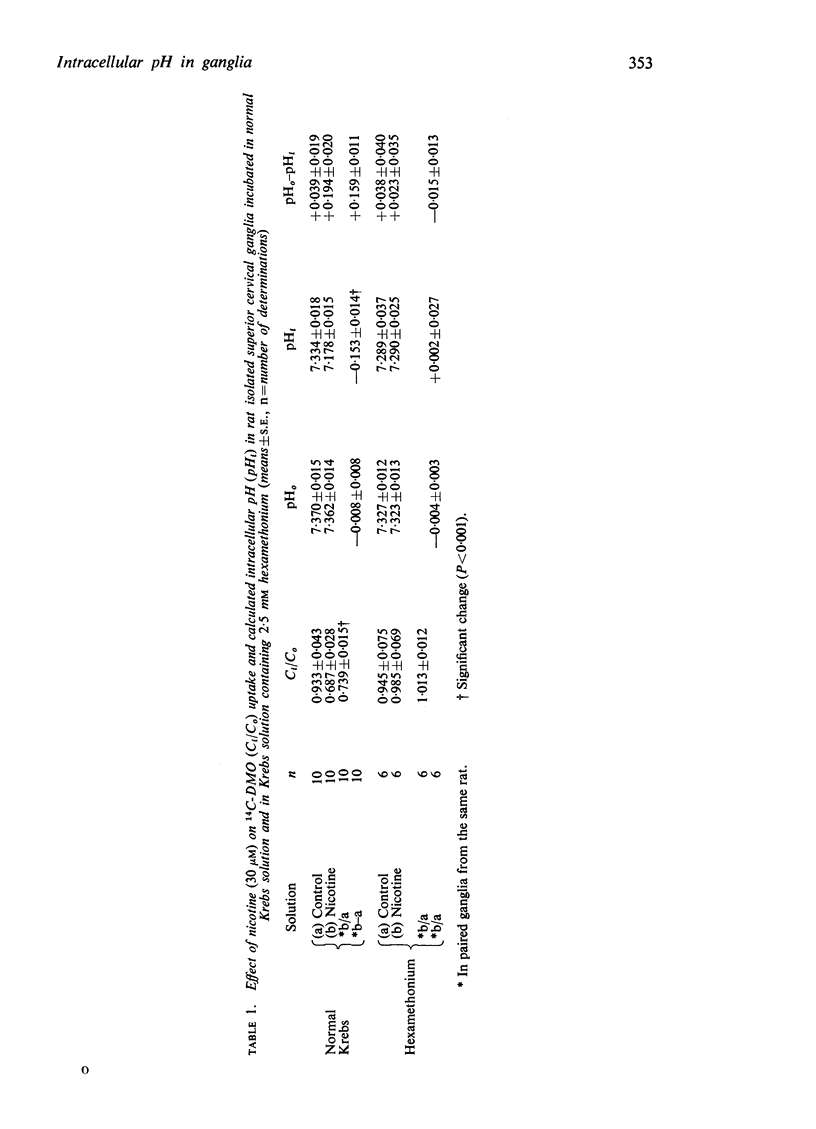
1. The intracellular pH (pHi) of rat isolated superior cervical ganglia incubated in normal Krebs solution (pHo=7·37) was estimated to be 7·33 from the uptake of a weak acid, 14C-5,5-dimethyloxazolidine-2,4-dione (DMO). Addition of 30 μM nicotine for 30 min reduced the DMO-estimated pHi by 0·15 units to 7·18. This effect was prevented by hexamethonium (2·5 mM) or by depolarizing the ganglion with K+ (124 mM).
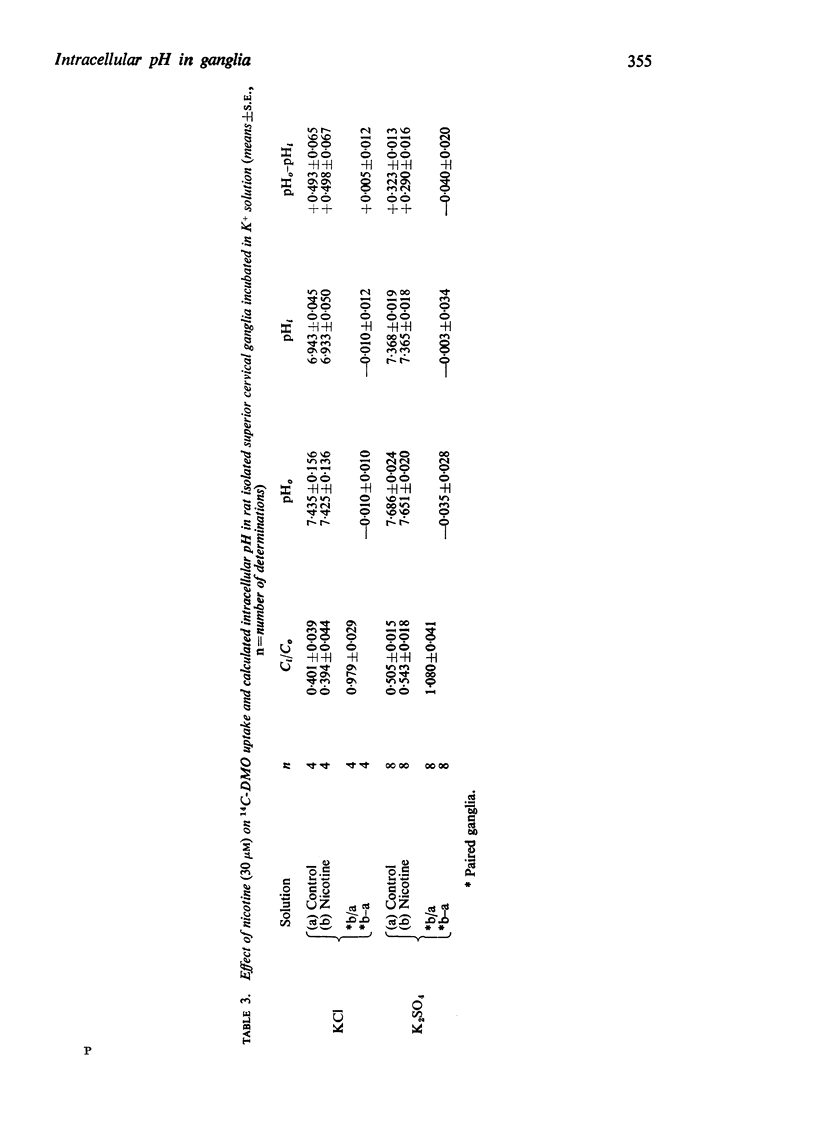
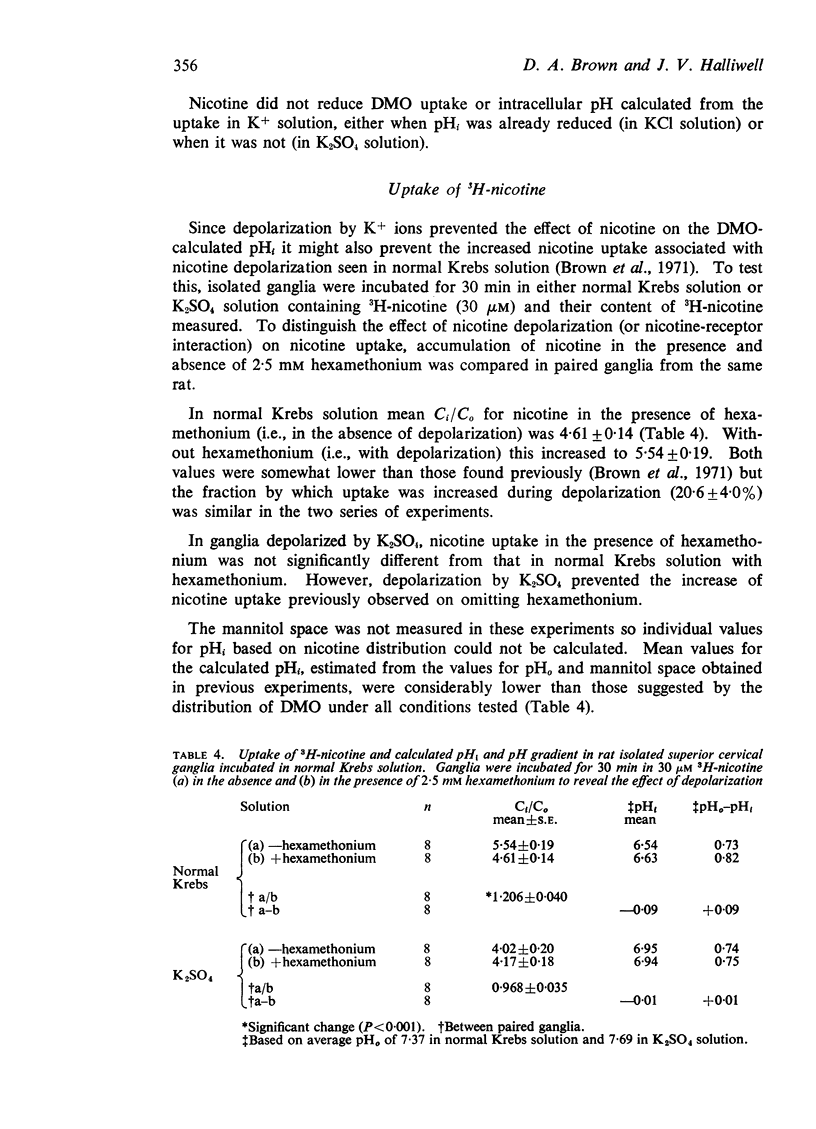
2. 3H-Nicotine (30 μM) was concentrated within the ganglia to an intracellular/extracellular concentration ratio (Ci/Co) of 5·54 in normal Krebs solution and 4·61 in 2·5 mM hexamethonium. This would suggest an intracellular pH of 6·54 and 6·63 respectively. In ganglia previously depolarized by K+ the corresponding values for Ci/Co were 4·02 (minus hexamethonium, estimated pHi 6·95) and 4·17 (plus hexamethonium, estimated pHi 6·94).
3. A multicompartment cell interior comprising an acid cytoplasm (pH∼6·6) and more alkaline nucleus and mitochondria is proposed to explain the difference between the values of pHi estimated from the uptake of DMO and nicotine. It is suggested that the fall in pHi during nicotine-depolarization results from metabolic stimulation following Na+ entry.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPELGREN L. E., HANSSON E., SCHMITERLOEW C. G. LOCALIZATION OF RADIOACTIVITY IN THE SUPERIOR CERVICAL GANGLION OF CATS FOLLOWING INJECTION OF C14-LABELLED NICOTINE. Acta Physiol Scand. 1963 Dec;59:330–336. doi: 10.1111/j.1748-1716.1963.tb02748.x. [DOI] [PubMed] [Google Scholar]
- Addanki A., Cahill F. D., Sotos J. F. Determination of intramitochondrial pH and intramitochondrial-extramitochondrial pH gradient of isolated heart mitochondria by the use of 5,5-dimethyl-2,4-oxazolidinedione. I. Changes during respiration and adenosine triphosphate-dependent transport of Ca++, Mg++, and Zn++. J Biol Chem. 1968 May 10;243(9):2337–2348. [PubMed] [Google Scholar]
- Addanki S., Cahill F. D., Sotos J. F. Reliability of the quantitation of intramitochondrial pH and pH gradient of heart mitochondria. Anal Biochem. 1968 Oct 24;25(1):17–29. doi: 10.1016/0003-2697(68)90076-6. [DOI] [PubMed] [Google Scholar]
- Boyle P. J., Conway E. J. Potassium accumulation in muscle and associated changes. J Physiol. 1941 Aug 11;100(1):1–63. doi: 10.1113/jphysiol.1941.sp003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Halliwell J. V., Scholfield C. N. Uptake of nicotine and extracellular space markers by isolated rat ganglia in relation to receptor activation. Br J Pharmacol. 1971 May;42(1):100–113. doi: 10.1111/j.1476-5381.1971.tb07090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Hoffmann P. C., Roth L. J. 3H-Nicotine in cat superior cervical and nodose ganglia after close-arterial injection in vivo. Br J Pharmacol. 1969 Mar;35(3):406–417. doi: 10.1111/j.1476-5381.1969.tb08282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Stumpf W. E., Roth L. J. Location of radioactively labelled extracellular fluid indicators in nervous tissue by autoradiography. J Cell Sci. 1969 Jan;4(1):265–288. doi: 10.1242/jcs.4.1.265. [DOI] [PubMed] [Google Scholar]
- Butler T. C., Waddell W. J., Poole D. T. Intracellular pH based on the distribution of weak electrolytes. Fed Proc. 1967 Sep;26(5):1327–1332. [PubMed] [Google Scholar]
- CALDWELL P. C. Studies on the internal pH of large muscle and nerve fibres. J Physiol. 1958 Jun 18;142(1):22–62. doi: 10.1113/jphysiol.1958.sp005998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLIVO M., LARRABEE M. G. Metabolism of glucose and oxygen in a mammalian sympathetic ganglion at reduced temperature and varied pH. J Neurochem. 1958 Oct;3(1):72–88. doi: 10.1111/j.1471-4159.1958.tb12611.x. [DOI] [PubMed] [Google Scholar]
- IRVINE R. O., SAUNDERS S. J., MILNE M. D., CRAWFORD M. A. Gradients of potassium and hydrogen ion in potassiumdeficient voluntary muscle. Clin Sci. 1961 Feb;20:1–18. [PubMed] [Google Scholar]
- Putney J. W., Jr, Borzelleca J. F. On the mechanisms of 14C-nicotine distribution in rat submaxillary gland in vitro. J Pharmacol Exp Ther. 1971 Jul;178(1):180–191. [PubMed] [Google Scholar]
- SPYROPOULOS C. S. Cytoplasmic pH of nerve fibres. J Neurochem. 1960 Feb;5:185–194. doi: 10.1111/j.1471-4159.1960.tb13352.x. [DOI] [PubMed] [Google Scholar]
- WADDELL W. J., BUTLER T. C. Calculation of intracellular pH from the distribution of 5,5-dimethyl-2,4-oxazolidinedione (DMO); application to skeletal muscle of the dog. J Clin Invest. 1959 May;38(5):720–729. doi: 10.1172/JCI103852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell W. J., Bates R. G. Intracellular pH. Physiol Rev. 1969 Apr;49(2):285–329. doi: 10.1152/physrev.1969.49.2.285. [DOI] [PubMed] [Google Scholar]
- Weiss G. B. Dependence of nicotine-C14 distribution and movements upon pH in frog sartorius muscle. J Pharmacol Exp Ther. 1968 Mar;160(1):135–147. [PubMed] [Google Scholar]
- Weiss G. B. The effect of pH on nicotine-induced contracture and Ca45 movements in frog sartorius muscle. J Pharmacol Exp Ther. 1966 Dec;154(3):605–612. [PubMed] [Google Scholar]


