Abstract
Oligogalacturonic acid (OGA) affects plant growth and development in an antagonistic manner to that of the auxin indole-3-acetic acid (IAA), the mechanism by which remains to be determined. This study describes the relationship between IAA and OGA activity in intact cucumber (Cucumis sativus) seedlings. Both OGA and IAA induced rapid and transient extracellular alkalinization; however, the characteristics of the OGA and IAA responses differed in their kinetics, magnitude, calcium dependence, and region of the root in which they induced their maximal response. IAA (1 μm) induced a saturating alkalinization response of approximately 0.2 pH unit and a rapid reduction (approximately 80%) in root growth that only partially recovered over 20 h. OGAs, specifically those with a degree of polymerization of 10 to 13, induced a maximal alkalinization response of 0.48 pH unit, but OGA treatment did not alter root growth. Saturating concentrations of OGA did not block IAA-induced alkalinization or the initial IAA-induced inhibition of root growth but allowed IAA-treated roots to recover their initial growth rate within 270 min. IAA-induced alkalinization occurs primarily in the growing apical region of the root, whereas OGA induced its maximal response in the basal region of the root. This study demonstrates that OGA and IAA act by distinct mechanisms and that OGA does not simply act by inhibition of IAA action. These results also suggest that IAA-induced extracellular alkalinization is not sufficient to account for the mechanism by which IAA inhibits root growth.
Oligogalacturonic acid (1→4-linked α-d-oligo-Gal-UA; OGA) is a biologically active oligosaccharide produced by hydrolysis of de-esterified pectin (poly-GalUA). OGA has been studied extensively in plant tissue cultures and dissected plants and has been shown to alter growth and development, including inhibition of auxin-induced elongation and ethylene accumulation in pea (Pisum sativum) stems (Branca et al., 1988) and regulation of organogenesis in tobacco (Nicotiana tabacum) explants (Eberhard et al., 1989; Bellincampi et al., 1993), to elicit a number of defense responses, including accumulation of phytoalexins (Hahn et al., 1981) and proteinase inhibitors (Bishop et al., 1984), and to induce rapid responses at the plasma membrane, such as trans-membrane ion flux (Mathieu et al., 1991) and phosphorylation of plasma membrane proteins (Farmer et al., 1991). Most of these responses are elicited maximally by OGAs with a degree of polymerization (DP) of 10 to 14 in the concentration range of 10−6 to 10−9 m; OGAs outside this size range are typically at least 10-fold less active. This indicates that OGA acts by a specific high-affinity mechanism in a manner similar to the classic plant hormones. However, it has not been demonstrated that biologically active OGA of the appropriates size and concentration are present in plant tissues.
It has recently been shown that wounding and the wound-induced signal, systemin, induce the systemic accumulation of polygalacturonase, which may result in the production of endogenous OGA (Bergey et al., 1999). In addition, systemin has been shown to reduce the lag time and to increase greatly the magnitude of the OGA-induced oxidative burst in tomato (Lycopersicon esculentum) cells (Stennis et al., 1998). This indicates that the mechanism of OGA action in intact tissues may differ from that of wounded plants or tissue cultures. To date, all studies of OGA activity have been carried out in dissected plants or tissue cultures. This paper presents the first description of an OGA-induced response in intact unwounded plants, OGA-induced alkalinization in the roots of whole cucumber (Cucumis sativus) seedlings.
The ability of OGA to alter plant growth and development has been proposed to rely on its ability to inhibit auxin action (Branca et al., 1988; Bellincampi et al., 1996). OGA inhibits auxin-induced pea stem elongation and root formation in tobacco explants in a competitive manner with IAA (Branca et al., 1988; Bellincampi et al., 1993). OGAs competitively inhibit auxin-induced rolB expression and root formation in tobacco explants carrying the rolB gene of Agrobacterium rhizogenes. IAA activates transcription of the rolB gene, which results in the formation of up to 25 adventitious roots per explant. OGAs, with a DP of 9 to 18, block rolB expression and root formation but do not affect the metabolism or uptake of IAA in these explants. Therefore, OGA has been proposed to rapidly block the signal transduction pathway between auxin perception and transcriptional activation of rolB (Bellincampi et al., 1996, 2000). It has been suggested that OGA inhibits IAA-induced transcription of rolB very rapidly; however, the kinetics of this OGA effect could not be properly established with the methods used, because rolB gene activity in IAA-treated explants was measured between 6 to 24 h after OGA addition (Bellincampi et al., 2000). The kinetics and the mechanism of OGA inhibition of IAA action remain to be determined. This paper characterizes the interaction between OGA and IAA in the induction of extracellular alkalinization and regulation of root growth in intact cucumber seedlings. We have demonstrated that IAA and OGA act by distinct mechanisms and that OGA is not simply an inhibitor of auxin action.
RESULTS
OGA Induces a Rapid and Transient Extracellular Alkalinization Response in Intact Cucumber Seedlings
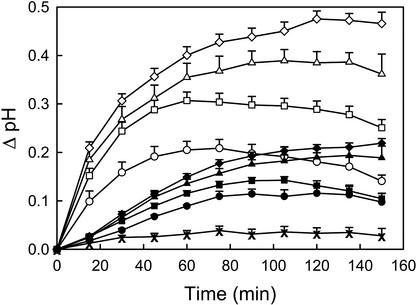
OGA pool induced a rapid and transient alkalinization of the incubation medium of intact cucumber seedlings. A saturating response of approximately 0.48 pH units was induced by 50 μg mL−1 OGA pool, and an approximately half-maximal response was induced by 7.5 μg mL−1 (Fig. 1). The first measurable alkalinization was detected within 3 min of treatment (data not shown), and the most rapid rate of alkalinization occurred within the first 15 min. The incubation medium reached its maximal pH after approximately 60 min for seedlings treated with 7.5 μg mL−1 OGA pool, but only after roughly 120 min when treated with 50 μg mL−1. The medium returned to its original pH after approximately 4 h when seedlings were treated with low concentrations of OGA pool, but only after more than 5 h when treated with saturating concentrations.
Figure 1.
Time courses of the alkalinization response of the incubation medium of intact cucumber seedlings induced by OGA pool or IAA. Data represents the mean + se of at least three separate experiments (some error bars are smaller than symbols). The following treatments were added at time zero to 50 2-d-old seedlings in 10 mL of incubation medium: 50 μg mL−1 OGA pool (⋄), 30 μg mL−1 OGA pool (▵), 15 μg mL−1 OGA pool (□), 7.5 μg mL−1 OGA pool (○), 1 μm IAA (♦), 0.2 μm IAA (▴), 0.05 μm IAA (▪), 0.02 μm IAA (●), and no addition (X).
OGA with a DP of 10 to 13 Have the Highest Activity for Induction of Alkalinization
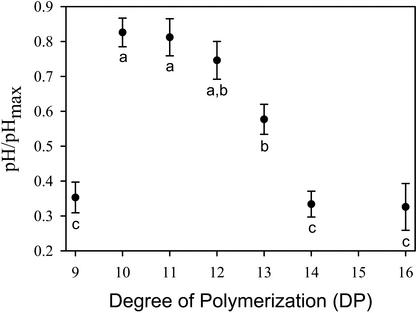
The size requirement for OGA to induce alkalinization in cucumber roots was investigated using fractions containing size-specific OGAs of at least 80% purity. Each of the biologically active OGA fractions was tested at several concentrations in at least three independent experiments. The maximal alkalinization response differed slightly from one experiment to the next, so to compare data between experiments, the pH increase induced by each treatment was divided by the mean alkalinization response induced by saturating concentrations of OGA pool within that experiment (pH/pHmax). Analysis of covariance indicated that the DP of the OGA has a highly significant effect on its biological activity (F7, 97 = 33.8, P < 0.0005). According to a post hoc Bonferroni multiple comparison test, OGAs of DP of 10 to 12 had the highest activity, and the OGA of DP of 13 was significantly lower than that of DP of 10 and 11 (P < 0.05) but not significantly different from DP of 12 (Fig. 2). The OGAs of DP 9, 14, and 16 had significantly lower activity than DP of 10 to 12 (P < 0.0005) and DP of 13 (P < 0.02). The concentration of each OGA required to induce a half-maximal alkalinization response (EC50) was calculated from the model; the OGAs of DP of 10 to 12 had an EC50 of 1.25 μm or lower, the OGA of DP of 13 had an EC50 of approximately 2 μm, whereas the OGAs of DP of 9, 14, or 16 had an EC50 higher than 8 μm, the highest concentration tested. A narrow size range of DP 10 to 13 was required for maximal activity; OGAs differing in size by only a single residue (i.e. DP 9 or 14) were more than 4-fold less active. OGAs with a DP 3, 5, and 7 did not induce any measurable alkalinization response at the highest concentration tested (8 μm). This indicates that OGA-induced alkalinization response relies on recognition of a specific chemical structure that is active at low concentrations, similar to the characteristics of a hormone-induced response.
Figure 2.
Effect of the DP of OGA on their ability to induce alkalinization in the incubation medium of intact cucumber seedlings. Each OGA fraction was tested at four concentrations in at least three separate experiments. The activity of each OGA treatment was calculated by the ratio of the pH increase induced by that treatment divided by the average alkalinization response induced by saturating concentrations of OGA pool in the same experiment (pH/pHmax). The data presented represent the mean ± se of pH/pHmax at a concentration of 2.84 μm as determined by an analysis of covariance model. The letters represent groups that are significantly different based on Bonferroni adjusted multiple comparisons (see text for P values).
IAA Induces an Alkalinization Response in Cucumber Seedlings with Different Kinetics and Magnitude Than That of the OGA-Induced Alkalinization
Several OGA-induced developmental responses have been proposed to be attributable to competitive inhibition of IAA action (Branca et al., 1988; Bellincampi et al., 1993, 1996). We compared the characteristics of IAA- and OGA-induced alkalinization in cucumber seedlings to determine whether these responses involve common or distinct mechanisms. The magnitude and kinetics of the IAA-induced extracellular alkalinization are clearly different from that induced by OGA (Fig. 1). Treatment with saturating concentrations of IAA (1 μm) resulted in an alkalinization of approximately 0.2 pH units, less than half the maximal effect of OGA. The maximum rate of alkalinization occurred only after a lag phase of 15 to 30 min. The incubation medium reached its maximal pH 120 to 150 min after treatment with saturating concentrations (1 μm) and after roughly 90 min for concentrations that induced an approximately half-saturating response (0.02 μm). The medium returned to its original pH after approximately 5 h when treated with low concentrations of IAA and after 6 h or more for saturating concentrations. These data suggest that IAA and OGA alkalinization responses take place via distinct mechanisms.
OGA-Induced, But Not IAA-Induced, Alkalinization Requires the Addition of Calcium for Maximal Response
To further distinguish between the OGA- and IAA-induced alkalinization responses, the requirement for the addition of extracellular calcium was examined. OGA has been shown to require extracellular calcium for induction of extracellular alkalinization and regulation of stomatal aperture (Mathieu et al., 1991; Lee et al., 1999). OGA induces a rapid and transient influx of extracellular calcium associated with hydrogen peroxide accumulation and extracellular alkalinization in tobacco cells (Chandra and Low, 1997; Mathieu et al., 1991). Before addition of OGA pool or IAA, cucumber seedlings were equilibrated either in normal incubation media (0.5 mm Ca2+) or in media to which no calcium was added. The magnitude of the OGA-induced alkalinization was 2.2-fold higher in the presence than in the absence of added calcium for both concentrations of OGA pool tested (Table I). However, the addition of calcium did not significantly affect the IAA-induced alkalinization. These data indicate that the addition of calcium is necessary for maximal OGA-induced alkalinization, but is not necessary for IAA-induced alkalinization. This is further evidence that OGA and IAA alkalinization involve distinct mechanisms.
Table I.
Comparison of the alkalinization response of cucumber seedlings to IAA and OGA pool in the presence and absence of added Ca2+
| Treatment | ΔpH
|
Ratio
|
|
|---|---|---|---|
| +Ca2+ | −Ca2+ | Response ± | |
| None | 0.017 ± 0.008 | 0.044 ± 0.017 | N.A. |
| 0.05 μm IAA | 0.14 ± 0.011 | 0.15 ± 0.01 | 0.93 |
| 1 μm IAA | 0.25 ± 0.009 | 0.23 ± 0.01 | 1.1 |
| 15 μg mL−1 OGA pool | 0.33 ± 0.02 | 0.15 ± 0.02 | 2.2 |
| 50 μg mL−1 OGA pool | 0.56 ± 0.013 | 0.26 ± 0.03 | 2.2 |
Fifty 2-d-old seedlings were equilibrated in incubation medium containing either 0.5 mm (+Ca2+) or no added Ca2+ (−Ca2+). Nos. represent the mean ± se for three separate experiments. The ratio of the +Ca2+ average response divided by the −Ca2+ average response is shown. N.A., Not applicable.
IAA, But Not OGA, Inhibits Root Growth in Cucumber Seedlings
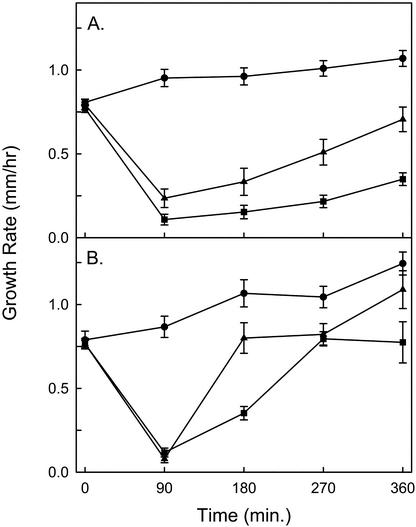
Evans and others demonstrated that the addition of 2 μm IAA reduces the growth of corn root segments by approximately 90% with a lag period similar to the IAA-induced alkalinization; the authors proposed that the growth reduction was attributable to an inhibition of acid-induced growth (Evans et al., 1980). We compared the effects of IAA and OGA on root growth rate in cucumber seedlings. We predicted that OGA treatment would alter root growth rate by inhibition of auxin-mediated signal transduction or simply by an inhibition of acid-induced growth because of increased extracellular pH. However, we found that OGA-induced alkalinization was not accompanied by an alteration in the growth rate of cucumber roots (Fig. 3). Under the conditions used for the growth assays, the pH change induced by OGA pool or IAA had a slightly lower magnitude and slower kinetics than in the alkalinization assays (Fig. 4). This may be because of the fact that there was a larger volume of incubation medium per seedling in the growth assays than in conditions of the alkalinization assays (1.8 versus 0.2 mL). After an equilibration period of 2 to 3 h, the initial root growth rate was measured for 3 h before the addition of OGA pool or IAA. Roots treated with 1 μm IAA exhibited an alkalinization response of 0.18 ± 0.04 pH units (n = 6) accompanied by a rapid and sustained reduction in their growth rate (Fig. 3A). During the initial 90 min after the addition of 1 μm IAA, the roots grew at an average of 17% ± 4.1% of their pretreatment growth rate. Untreated roots grew at 120% ± 7.2% of their initial rate during the same period. The growth rate of roots treated with 1 μm IAA slowly recovered to approximately 50% of their initial growth rate after 20 h (data not shown). Roots treated with 0.2 μm IAA demonstrated an alkalinization response of 0.2 ± 0.028 pH units (n = 4) and, during the first 90 min after treatment, grew at 30% ± 7.4% of their pretreatment growth rate (Fig. 3A). Roots treated with 0.2 μm IAA recovered to 90% ± 8.2% of their initial growth rate within 6 h, a more rapid recovery than observed in roots treated with 1 μm IAA.
Figure 3.
Growth rates of the roots of intact cucumber seedlings treated with IAA and/or OGA. The roots of ten 2-d-old cucumber seedlings were bathed in 18 mL of incubation medium in hydroponic growth chambers. Each point represents the mean ± se of the growth rate measured at 90′ intervals in at least four separate experiments. The growth rate at time zero represents the average growth rate measured over the preceding 3 h. A, Growth rate of roots treated with 1 μm IAA (▪; n = 6), 0.2 μm IAA (▴; n = 4), and untreated roots (●; n = 6). B, Growth rate of roots treated with 50 μg mL−1 OGA pool at −60 min followed by addition at 0 min of 1 μm IAA (▪; n = 6), 0.2 μm IAA (▴; n = 4), or no additional treatment (●; n = 4).
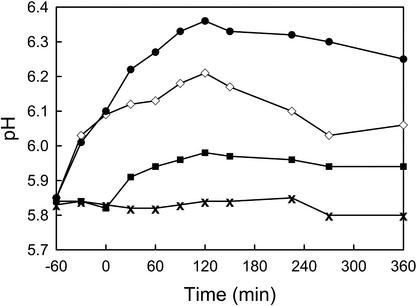
Figure 4.
Representative time courses of OGA- and IAA-induced alkalinization in hydroponic growth chambers under the same conditions described for Figure 3. OGA pool (50 μg mL−1) was added at −60 min and 1 μm IAA was added at 0 min. The treatments are: OGA pool followed by IAA (●), OGA pool alone (⋄), IAA alone (▪), and no addition (X).
The alkalinization response induced by 50 μg mL−1 OGA pool (0.36 ± 0.03 pH units, n = 4) was consistently greater than that induced by 1 μm IAA; however, roots treated with OGA grew in a manner similar to untreated roots, demonstrating a gradual increase in their growth rate over the 6-h measurement period (Fig. 3B). Ninety minutes after the addition of 50 μg mL−1 OGA pool, the roots grew at an average of 116% ± 12% of their pretreatment growth rate. IAA-induced, but not OGA-induced, alkalinization is accompanied by a reduction in root growth. This demonstrates that OGA and IAA initiate distinct developmental pathways in cucumber roots and indicates that extracellular alkalinization is not sufficient to inhibit root growth.
OGA Does Not Block IAA-Induced Extracellular Alkalinization or the Initial IAA-Induced Root Growth Inhibition, But OGA Allows for a More Rapid Recovery from IAA-Induced Root Growth Inhibition
We characterized the ability of OGA-treated roots to undergo IAA-induced alkalinization and root growth inhibition. OGA did not block the action of IAA; roots treated with OGA followed by IAA demonstrated an increased rate of alkalinization upon addition of IAA and a greater alkalinization response than induced by OGA alone (Fig. 4). Roots treated with 50 μg mL−1 OGA pool, followed after 60 min by addition of 0.2 or 1 μm IAA, demonstrated alkalinization responses of 0.38 ± 0.024 (n = 4) and 0.42 ± 0.028 (n = 6) pH units, respectively. Roots treated with OGA 60 min before the addition of IAA demonstrated an equivalent reduction in their growth rate, during the first 90 min after IAA treatment, as roots treated with the same concentration of IAA alone (Fig. 3B). However, the growth rate of roots treated with OGA and IAA recovered their growth rate much more quickly than roots treated with IAA alone. In the first 90 min, the growth rates for roots treated with 50 μg mL−1 OGA pool plus 0.2 or 1 μm IAA were 10.4% ± 2% and 16.6% ± 4.1% of their initial growth rate respectively. However, roots treated with 50 μg mL−1 OGA pool and 0.2 μm IAA regained their initial growth rate (106% ± 12.4%) by 180 min after IAA treatment, whereas at the same time point, roots treated with 0.2 μm IAA alone grew at only 41% ± 9.9%. Roots treated with 50 μg mL−1 OGA pool and 1 μm IAA regained their initial growth rate (106% ± 12.4%) by 270 min after IAA treatment, whereas at the same time point, roots treated with 1 μm IAA alone grew at only 30% ± 5.2%. These data demonstrate that the IAA-induced alkalinization and initial reduction in root growth are not blocked by OGA, but that OGA allows for a more rapid recovery of root growth in IAA-treated roots. This indicates that the OGA do not act solely by inhibition of auxin. The OGA-mediated recovery of IAA-induced root growth inhibition took place without causing reacidification of the incubation medium, further evidence that extracellular pH is not directly correlated to growth rate in this system, even in the presence of IAA.
The concentration of OGA pool required to block IAA inhibition of growth was lower than that required for induction of alkalinization. In two independent experiments, roots were treated with a range of concentrations of OGA pool from 7.5 μg mL−1 to 75 μg mL−1 60 min before the addition of 1 μm IAA. Each demonstrated very similar root growth kinetics, with an approximately full recovery of their initial growth rate by 270 min of IAA treatment. This indicates that the concentration of OGA pool required to induce a maximal effect in blocking IAA inhibition of root growth is lower than the concentration required to induce a maximal alkalinization of the root incubation medium.
OGA- and IAA-Induced Alkalinization Occur Maximally in Different Regions of the Root
To identify the region of the cucumber seedlings that is responsible for the OGA- and IAA-induced alkalinization, seedlings with roots of approximately 2 cm length were dissected into three segments: the root apical 1 cm, the root basal 1 cm, and the remaining segment containing the hypocotyl and cotyledons. Fifty segments were placed in separate tubes containing 10 mL of incubation medium and assayed, in three independent experiments, for their ability to respond to OGA pool (50 μg mL−1) or IAA (1 μm) compared with 50 intact seedlings in 10 mL of incubation medium. In these experiments, the untreated apical segments equilibrated at a lower average pH (5.48) than the other segments (approximately 5.7). The basal segments demonstrated the greatest average OGA-induced alkalinization; the response was 1.4-fold higher than that of whole seedlings, 1.6-fold higher than that of the apical segments, and 3.1-fold higher than that of the hypocotyl/cotyledon segments (Table II). In contrast, the apical segments and whole seedlings demonstrated a nearly equal IAA-induced alkalinization that was approximately 3.3-fold higher than the response of basal segments (Table II). The hypocotyl/cotyledon segments showed no pH change in response to IAA. These results may explain why IAA-induced, but not OGA-induced, alkalinization alters root growth. A kinematic study, performed on cucumber seedlings, demonstrated that elongation occurred within the region 1.5 to 9 mm from the root tip and that the fastest rate of growth was in the region 4 to 7 mm from the root tip (data not shown). This indicates that IAA alters the extracellular pH of the growing portion of the root as has been previously shown in corn (Zea mays) roots (Peters and Felle, 1999), whereas OGA induces its maximal alkalinization outside of the zone of elongation.
Table II.
Comparison of the alkalinization response of whole and dissected 2-d-old cucumber seedlings treated with 50 μg mL−1 OGA pool or 1 μm IAA
| ΔpH
|
||||
|---|---|---|---|---|
| Treatment | Whole | Apical Root | Basal Root | Hypocotyl/ cotyledons |
| 50 μg mL−1 OGA pool | 0.49 ± 0.043 | 0.43 ± 0.041 | 0.69 ± 0.077 | 0.22 ± 0.022 |
| 1 μm IAA | 0.18 ± 0.012 | 0.19 ± 0.042 | 0.057 ± 0.014 | 0 |
Seedlings with roots of approximately 2-cm length were dissected into three segments: the root apical 1 cm, the root basal 1 cm, and the remaining segment containing the hypocotyl and cotyledons. Fifty of each segment or whole seedlings were assayed individually in 10 mL of incubation medium. Nos. represent the mean ± se of the maximal pH change corrected for the pH change in the absence of treatment in three separate experiments.
DISCUSSION
We have shown that OGA induces two separate effects in the roots of cucumber seedlings: extracellular alkalinization and recovery from auxin-mediated root growth inhibition. Although OGA has previously been shown to induce rapid membrane responses, such as ion flux, and to alter growth and development in dissected plants and plant tissue cultures (for review, see Côté and Hahn, 1994), this is the first study, to our knowledge, of OGA-induced effects in intact unwounded plants. A growing body of evidence suggests that signals arising from wounded plant tissues alter the sensitivity and kinetics of OGA-induced responses (Bergey et al., 1999; Stennis et al., 1998). For this reason, we were interested in developing a system for the investigation of hormonal action in intact plants. The root of seedlings provides an ideal system for such studies; it is a rapidly growing organ in which the cells are not covered by a waxy cuticle, but are in direct contact with the external solution. The roots of cucumber seedlings appear to readily perceive even large hydrophilic molecules, because OGAs with a DP of 10 to 13, induce a measurable alkalinization response within 3 min of addition.
All OGA responses that have been characterized have been shown to require OGAs of a specific size range for maximal activity, most commonly those with a DP of 10 to 15. Our results show that OGAs with a DP of 10 to 13 induce a half-maximal alkalinization response in cucumber roots at concentrations of 2 μm or less, whereas OGAs outside this size range are more than 4-fold less active. These size and concentration requirements are similar to those observed in several other OGA responses (for review, see Côté and Hahn, 1994). This strengthens the theory that OGA action relies on recognition by a specific high-affinity membrane-bound receptor. The existence of such a protein is strongly supported by the observation that micromolar concentrations of OGA DP ≥ 13 induce protein phosphorylation in purified plasma membranes of tomato, potato (Solanum tuberosum), and soybean (Glycine max; Farmer et al., 1991; Reymond et al., 1995).
OGA-induced extracellular alkalinization has also been reported in suspension-cultured tobacco cells (Mathieu et al., 1991; Spiro et al., 1998). The OGA-induced alkalinization responses in cucumber roots and tobacco cells differ in their magnitude, kinetics, and response to varying concentrations of OGAs. Saturating concentrations of OGA induce an approximately 2-fold higher alkalinization response in tobacco cells than in cucumber seedlings (Spiro et al., 1998). The kinetics of alkalinization and reacidification in tobacco cells are independent of OGA concentration. OGA treatment of tobacco cells leads to a maximal alkalinization response in as little as 14 min and the pH returns to its original value within 120 min of treatment, regardless of the concentration of OGA used (Mathieu et al., 1991; Spiro et al., 1998). In contrast, the kinetics of alkalinization and reacidification in cucumber roots are dependent upon the OGA concentration and are much slower. Half-saturating concentrations of OGA pool induced a maximal alkalinization within 60 min of treatment and allowed for reacidification within 4 h, whereas saturating concentrations induced a maximal alkalinization only after 120 to 150 min and required more than 5 h for reacidification. It appears that OGA-induced alkalinization in cucumber roots and tobacco cells occur by distinct mechanisms; however, it is not clear whether this is a result of genetic factors or of differences between intact and cultured plant tissues.
OGA acts in an antagonistic manner with IAA in its ability to induce several developmental responses. The most thoroughly studied of these effects is the ability of OGA to block IAA-induced transcription of rolB and the ensuing root formation in leaf explants of transgenic tobacco carrying the rolB gene of A. rhizogenes (Bellincampi et al., 1996, 2000). OGA acts in a competitive manner with IAA in these effects, but does not alter the rate of IAA degradation or uptake; therefore, it has been proposed that OGA blocks the IAA-induced signal transduction leading to the activation of rolB (Bellincampi et al., 1996). It remains to be determined whether the ability of OGA to alter growth and development is solely due to its capacity to block IAA action and at what point this inhibition takes place. The timing and degree of interaction between the IAA- and OGA-initiated signal transduction pathways are not well characterized. We have investigated the ability of OGA and IAA, individually and when added together, to alter extracellular pH and to regulate growth in roots of cucumber seedlings. Our data suggests that OGA and IAA induce these responses through independent mechanisms and that OGA does not act as a general auxin inhibitor.
The OGA- and IAA-induced extracellular alkalinization responses differ in their magnitude, kinetics, calcium requirement, and region of maximal effect within the root. Each of these criteria indicate that OGA and IAA operate by distinct mechanisms. The absence of added calcium in the incubation medium reduces OGA-induced extracellular alkalinization by more than 2-fold but does not significantly alter IAA-induced alkalinization. This may indicate an involvement of a calcium flux in OGA-induced signal transduction (Chandra and Low, 1997) that is not required for IAA-induced alkalinization. As an alternative, the lack of calcium may alter the conformation of the OGA. OGAs of DP ≥ 10 undergo a conformational change in the presence of calcium (Kohn, 1975, 1987; Powell et al., 1982) that may be necessary for biological activity. An anionic isoperoxidase from zucchini hypocotyls has recently been shown to specifically bind to biologically active OGA in a calcium-dependent manner. Fifty micromolar calcium was found to be sufficient for this binding (Penel et al., 1999). Because we did not chelate calcium arising from plant tissues, it is very likely that the calcium concentration was greater than 50 μm, especially at the cell surface where OGA has its effect, and would have allowed OGA to take on its biologically active conformation. This suggests that the observed effects of calcium are attributable to a specific role for calcium in OGA-induced, but not IAA-induced, signal transduction leading to extracellular alkalinization.
The interaction between IAA and OGA signal transduction was further characterized in experiments in which roots were treated with both compounds. We found that OGA does not block either IAA-induced alkalinization or IAA-mediated inhibition of root growth, but does allow for a more rapid recovery of growth in IAA-treated roots. Growth recovery does not begin until after a lag period of at least 90 min after IAA inhibition. This lag period is not attributable to a delay in OGA-mediated signal transduction, because OGA was added 60 min before IAA treatment and OGA induces alkalinization within 3 min of treatment. IAA-induced alkalinization continued to increase even as OGA was facilitating the recovery of root growth in IAA-treated roots. This suggests that OGA does not broadly inhibit IAA action but targets certain IAA-mediated processes.
The acid-growth theory states that auxin-induced extracellular acidification allows for loosening of the cell wall during cell growth and predicts that an increase in the pH of the apoplast would lead to an inhibition of auxin-induced growth in stems (for review, see Rayle and Cleland, 1992; Cosgrove, 2001). The observation that IAA inhibition of maize root growth is accompanied by an alkalinization of the root incubation medium is consistent with the acid-growth theory (Evans et al., 1980). We observed a similar IAA-induced alkalinization and a sustained reduction in the growth rate of the roots of intact cucumber seedlings. However, there is no correlation between root growth and alkalinization in roots treated with OGA alone or in combination with IAA. OGA pool (50 μg mL−1) induced an alkalinization approximately 2-fold higher than that induced by 1 μm IAA, but did not inhibit root growth. Cucumber roots treated with 50 μg mL−1 OGA pool, followed by 0.2 or 1 μm IAA, responded with a greater alkalinization than induced by 50 μg mL−1 OGA pool alone. The growth of these roots was inhibited in the first 90 min to the same extent as roots treated with IAA alone, but these roots regained their initial growth rate much more quickly than roots treated with IAA alone. This growth recovery did not coincide with reacidification of the growth media; indeed, IAA continued to induce a pH increase during the growth recovery period. This demonstrates that extracellular alkalinization, generally, and IAA-induced alkalinization, specifically, are not sufficient to inhibit root growth.
Peters and Felle (1999) found a tight correlation between the surface pH of the root and the relative elemental growth rate (REGR) in the apical 14 mm of maize roots. A region of acidification occurring 4 to 5 mm from the root tip, the proximal acidification zone, was found to have the highest REGR. Treatment with IAA (10−5 m) eliminated the acidification of this zone and reduced the REGR to zero. The authors concluded that there is a functional relationship between extracellular pH and growth within the proximal acidification zone but that the localized pH of this region is not necessarily correlated to the pH of the growth medium. A possible explanation of why OGA-induced alkalinization does not reduce root growth is that OGA primarily affects the pH in a region outside of the zone of elongation. However, this does not explain why roots treated simultaneously with IAA and OGA recover their growth rate even as IAA-induced alkalinization is taking place. An analysis of the changes in the surface pH and the REGR in the presence of OGA alone and in combination with IAA may provide important information relating to the relationship between extracellular pH and root growth.
MATERIALS AND METHODS
All reagents were obtained from Sigma-Aldrich (St. Louis) unless otherwise stated.
Preparation of OGA
OGA was prepared and characterized as previously described (Spiro et al., 1993). In brief, OGA was generated by partial digestion of poly-GalUA with a homogeneous α-1,4-endopolygalacturonase purified from Fusarium moniliforme. OGAs with a DP of 7 to 25 (OGA pool) were selectively precipitated from the digest in the presence of 50 mm NaOAc and 11% (v/v) ethanol. Fractions containing size-specific OGAs (≥80% homogeneity) were purified from the resolubilized precipitate by Q-Sepharose anion-exchange chromatography. The purity and composition of the Q-Sepharose fractions were determined by high-performance anion-exchange chromatography in comparison with homogeneous OGAs that were characterized by fast atom bombardment mass spectrometry. These fractions were used to determine the size requirement for biological activity of the OGA.
Plant Material
Cucumber (Cucumis sativus L. cv Burpee Pickler) seeds were obtained from Burpee Seed Company (Warminster, PA). Seeds were surface sterilized with 10% (v/v) bleach for 5 min, and placed between several layers of damp paper towels in the dark at 25°C. After 40 h, seedlings with tap roots of 1.5 to 2.5 cm length and hypocotyls of less than 2 mm length were used in bioassays.
Measurement of Alkalinization
Fifty cucumber seedling were placed in 10 mL of incubation medium (15 mm KCl, 0.5 mm CaCl2, and 0.5 mm KH2PO4, pH 5.2) that was aerated by vigorous bubbling of humidified air. The incubation medium was exchanged four times at 30 min intervals followed by an equilibration period of approximately 2 h before treatment of seedlings with OGA or IAA. The pH of the incubation medium was measured using a semimicro pH electrode. The OGA and IAA samples had a pH between 5 and 6, and their addition did not alter the pH of the incubation medium. In experiments testing the effect of extracellular calcium, the same conditions were used except that the incubation medium contained no added CaCl2.
Growth Measurements
The growth rate of the taproot of cucumber seedlings was measured using specially fabricated hydroponic growth chambers. Each growth chamber consisted of a box (55 × 47 × 8 mm) constructed of 3-mm-thick clear Plexiglas filled with 18 mL of incubation media and fitted with two air hoses of 1 mm diameter for aeration of the media. The top edge of each box had ten 5-mm holes that each firmly held a 50-mm-long segment of clear rigid polypropylene tubing. Each piece of tubing had a 5-mm-long notch cut into its top edge to hold the seed coat of a cucumber seedling in place with the root pointing down into the incubation medium and its hypocotyl above the medium. A 3-mm-wide hole was drilled through each piece of tubing 15 mm from its top to allow for circulation of the aerated media. The incubation medium was replaced every 15 min for the 1st h after the seedlings were placed into the chamber. After an additional 2- to 3-h equilibration period, the length of the roots were recorded by marking the Plexiglas wall of the growth chambers with an indelible fine tip marker. The lengths of the roots were recorded again at 90-min intervals, and the growth rate was determined by measuring the distance between the marks. The initial growth rate was recorded over a 3-h period before the addition of OGA or IAA.
In preliminary experiments, it was determined that between light- and dark-grown cucumber seedlings, there were no differences in the growth rate of the roots or in their response to addition of OGA or IAA. Therefore, the growth experiments were carried out in the light.
ACKNOWLEDGMENTS
We thank Dr. Carl Bergmann of the Complex Carbohydrate Research Center at the University of Georgia for kindly supplying us with purified endopolygalacturonase, Dr. Bob Sharp of the University of Missouri for advice on designing root growth chambers, and Dr. Amy Whipple of Bucknell University for assistance with statistical analysis.
Footnotes
This work was supported in part by the Root Biology Training Program at the Pennsylvania State University, a unit of the Department of Energy/National Science Foundation/U.S. Department of Agriculture Collaborative Research Program in Plant Biology, and in part by the Department of Energy (grant no. DE–FG02–84ER13179).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.006064.
LITERATURE CITED
- Bellincampi D, Cardarelli M, Zaghi D, Serino G, Salvi G, Gatz C, Cervone F, Altamura MM, Constantino P, De Lorenzo G. Oligogalacturonides prevent rhizogenesis in rolB-transformed tobacco explants by inhibiting auxin-induced expression of the rolB gene. Plant Cell. 1996;8:477–487. doi: 10.1105/tpc.8.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellincampi D, Dipierro G, Salvi G, Cervone F, De Lorenzo G. Extracellular H2O2 induced by oligogalacturonides is not involved in the inhibition of the auxin-regulated rolB gene expression in tobacco leaf explants. Plant Physiol. 2000;122:1379–1385. doi: 10.1104/pp.122.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellincampi D, Salvi G, De Lorenzo G, Cervone F, Marfà V, Eberhard S, Darvill A, Albersheim P. Oligogalacturonides inhibit the formation of roots on tobacco explants. Plant J. 1993;4:207–213. [Google Scholar]
- Bergey RD, Orozco-Cardenas M, de Moura DS, Ryan CA. A wound- and systemin-inducible polygalacturonase in tomato leaves. Proc Natl Acad Sci USA. 1999;96:1756–1760. doi: 10.1073/pnas.96.4.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop PD, Pearce G, Bryant JE, Ryan CA. Isolation and characterization of the proteinase inhibitor-inducing factor from tomato leaves: identity and activity of poly- and oligogalacturonide fragments. J Biol Chem. 1984;259:13172–13177. [PubMed] [Google Scholar]
- Branca C, De Lorenzo G, Cervone F. Competitive inhibition of the auxin-induced elongation by α-d-oligogalacturonides in pea stem segments. Physiol Plant. 1988;72:499–504. [Google Scholar]
- Chandra S, Low PS. Measurement of Ca2+ fluxes during elicitation of the oxidative burst in aequorin-transformed tobacco cells. J Biol Chem. 1997;272:28274–28280. doi: 10.1074/jbc.272.45.28274. [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. Wall structure and wall loosening: a look backwards and forwards. Plant Physiol. 2001;125:131–134. doi: 10.1104/pp.125.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté F, Hahn MG. Oligosaccharins: structures and signal transduction. Plant Mol Biol. 1994;26:1375–1411. doi: 10.1007/BF00016481. [DOI] [PubMed] [Google Scholar]
- Eberhard S, Doubrava N, Marfà V, Mohnen D, Southwick A, Darvill A, Albersheim P. Pectic cell wall fragments regulate tobacco thin-cell-layer explant morphogenesis. Plant Cell. 1989;1:747–755. doi: 10.1105/tpc.1.8.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans ML, Mulkey TJ, Vesper MJ. Auxin action on proton influx in corn roots and its correlation with growth. Planta. 1980;148:510–512. doi: 10.1007/BF00552667. [DOI] [PubMed] [Google Scholar]
- Farmer EE, Moloshok TD, Saxton MJ, Ryan CA. Oligosaccharide signaling in plants: specificity of oligouronide-enhanced plasma membrane protein phosphorylation. J Biol Chem. 1991;266:3140–3145. [PubMed] [Google Scholar]
- Hahn MG, Darvill AG, Albersheim P. Host-pathogen interactions: XIX. The endogenous elicitor, a fragment of a plant cell wall polysaccharide that elicits phytoalexin accumulation in soybeans. Plant Physiol. 1981;68:1161–1169. doi: 10.1104/pp.68.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn R. Ion binding on polyuronates: alginate and pectin. Pure Appl Chem. 1975;42:371–397. [Google Scholar]
- Kohn R. Binding of divalent cations to oligomeric fragments of pectin. Carbohydr Res. 1987;160:343–353. [Google Scholar]
- Lee S, Choi H, Suh S, In-Suk D, Ki-Young O, Choi EJ, Schroeder Taylor AT, Low PS, Lee Y. Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reactive oxygen species from guard cells of tomato and Commelina communis. Plant Physiol. 1999;121:147–152. doi: 10.1104/pp.121.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu Y, Kurkjian A, Xia H, Guern J, Koller A, Spiro MD, O'Neill M, Albersheim P, Darvill A. Membrane responses induced by oligogalacturonides in suspension-cultured tobacco cells. Plant J. 1991;1:333–343. doi: 10.1046/j.1365-313X.1991.t01-10-00999.x. [DOI] [PubMed] [Google Scholar]
- Penel C, Van Cutsem P, Greppin H. Interactions of a plant peroxidase with oligogalacturonides in the presence of calcium ions. Phytochemistry. 1999;51:193–198. [Google Scholar]
- Peters WS, Felle HH. The correlation of profiles of surface pH and elongation growth in maize roots. Plant Physiol. 1999;121:905–912. doi: 10.1104/pp.121.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DA, Morris ER, Gidley MJ, Rees DA. Conformations and interactions of pectins: II. Influence of residue sequence on chain association in calcium pectate gels. J Mol Biol. 1982;155:517–531. doi: 10.1016/0022-2836(82)90485-5. [DOI] [PubMed] [Google Scholar]
- Rayle DL, Cleland RE. The acid growth theory of auxin-induced cell elongation is alive and well. Plant Physiol. 1992;99:1271–1274. doi: 10.1104/pp.99.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond P, Grünberger S, Paul K, Müller M, Farmer EE. Oligogalacturonide defense signals in plants: large fragments interact with the plasma membrane in vitro. Proc Natl Acad Sci USA. 1995;92:4145–4149. doi: 10.1073/pnas.92.10.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro MD, Kates KA, Koller AL, O'Neill MA, Albersheim P, Darvill A. Purification and characterization of biologically active 1,4-linked α-d-oligogalacturonides after partial digestion of polygalacturonic acid with endopolygalacturonase. Carbohydr Res. 1993;247:9–20. [Google Scholar]
- Spiro MD, Ridley BL, Eberhard S, Kates KA, Mathieu Y, O'Neill MA, Mohnen D, Guern J, Darvill A, Albersheim P. Biological activity of reducing-end-derivatized oligogalacturonides in tobacco tissue cultures. Plant Physiol. 1998;116:1289–1298. doi: 10.1104/pp.116.4.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stennis MJ, Chandra S, Ryan CA, Low PS. Systemin potentiates the oxidative burst in cultured tomato cells. Plant Physiol. 1998;117:1031–1036. doi: 10.1104/pp.117.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]