Abstract
1. A new smooth muscle preparation, the rat anococcygeus muscle, is described. The muscle is paired, thin, consists of smooth muscle only and the muscle cells are organized in parallel bundles. It has a dense adrenergic innervation distributed throughout the muscle but apparently no cholinergic innervation. The muscles are easily isolated.
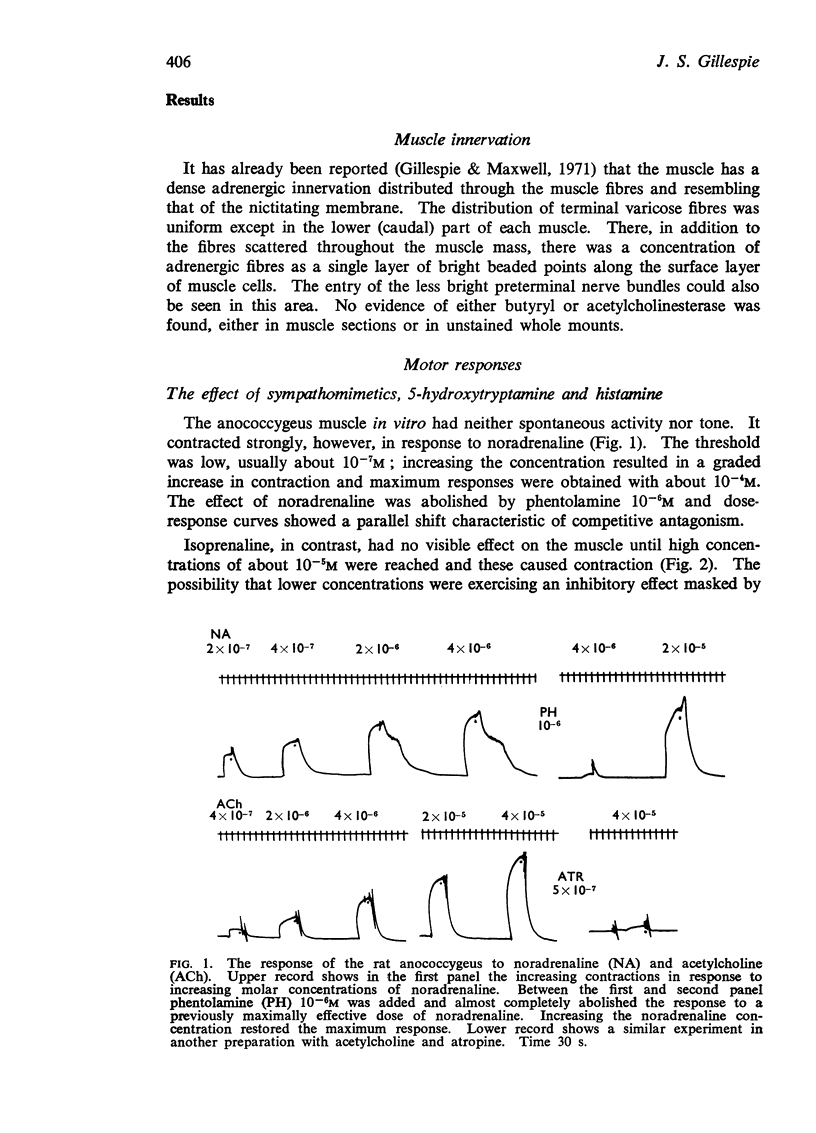
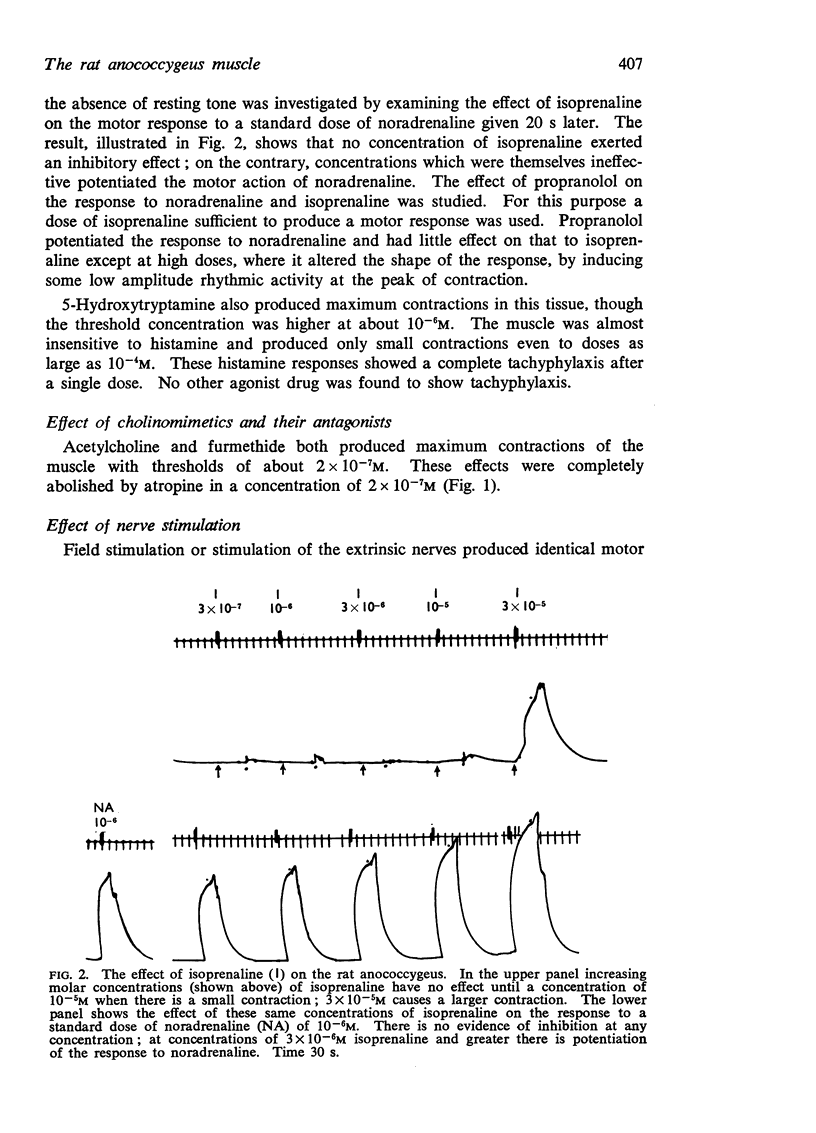
2. The muscle contracts to noradrenaline, acetylcholine, furmethide, 5-hydroxytryptamine, but not to histamine. Isoprenaline produces contraction at high concentrations. The effects of noradrenaline and acetylcholine are blocked by phentolamine and atropine respectively. The response to isoprenaline is little affected by propranolol.
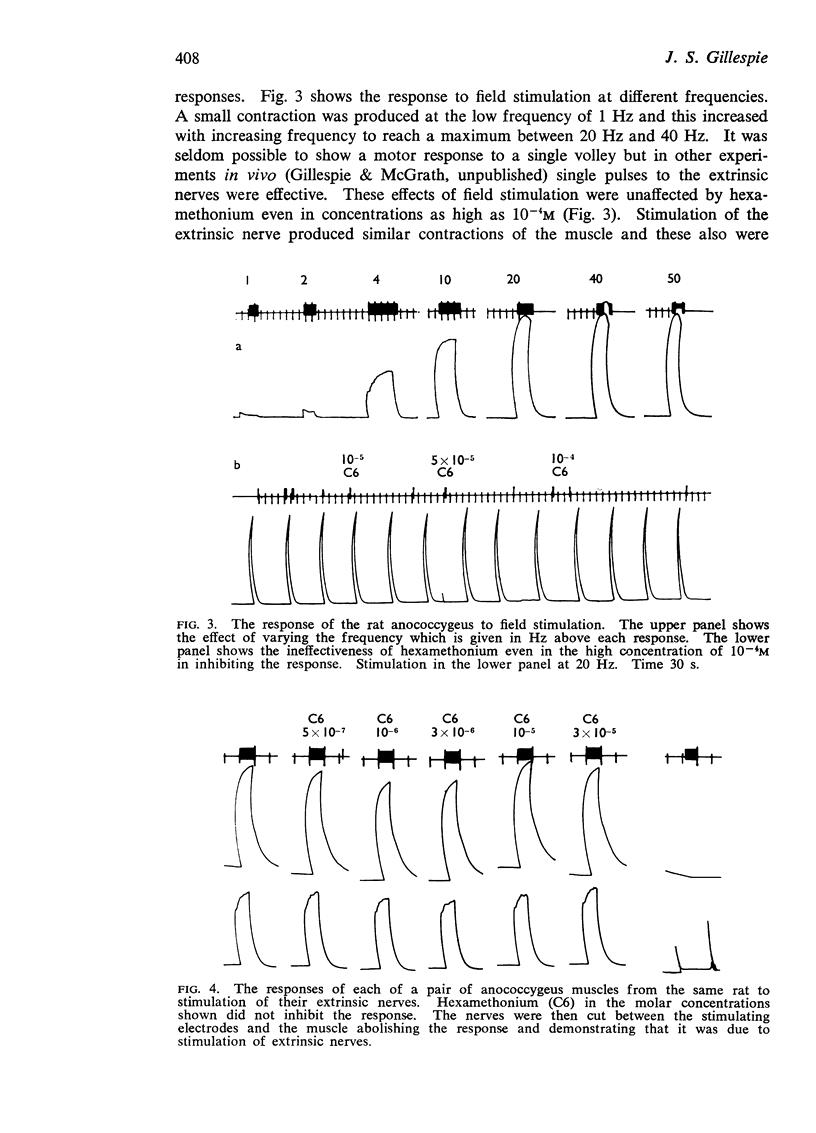
3. The muscle contracts in response to field stimulation or stimulation of extrinsic nerves. This response is completely blocked by phentolamine but unaffected by hexamethonium or atropine.
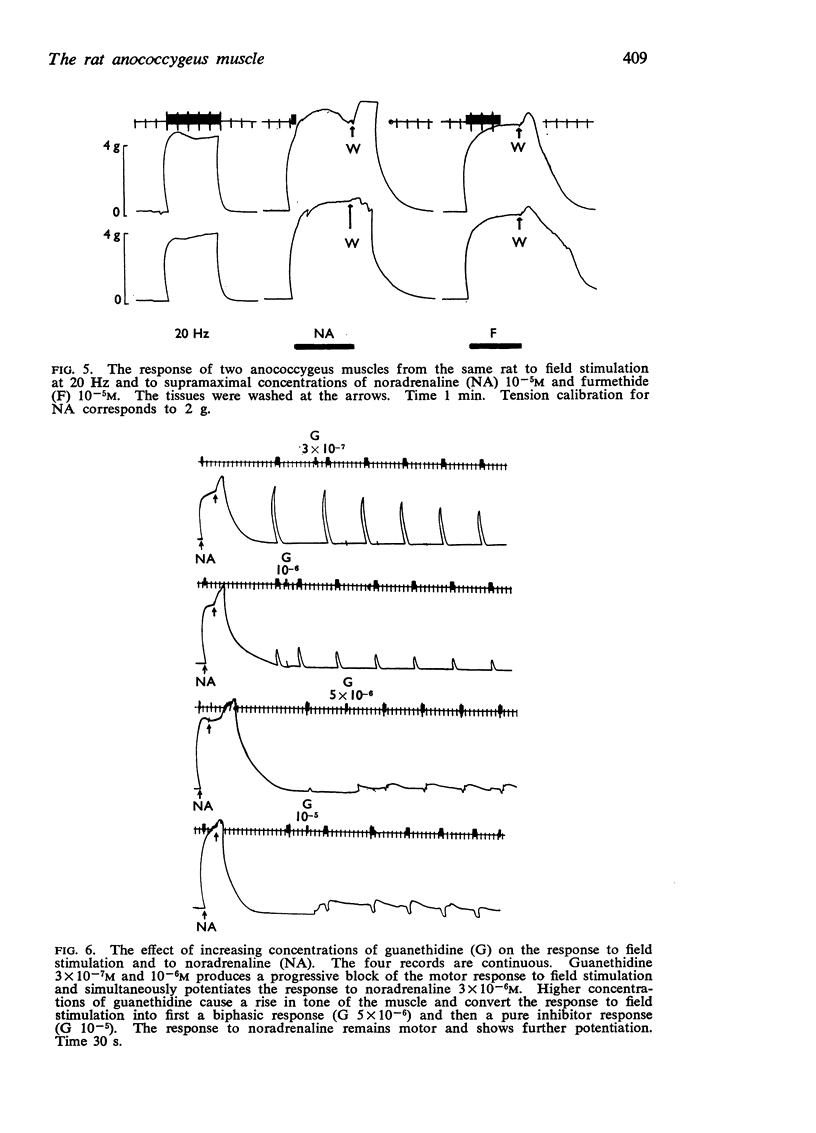
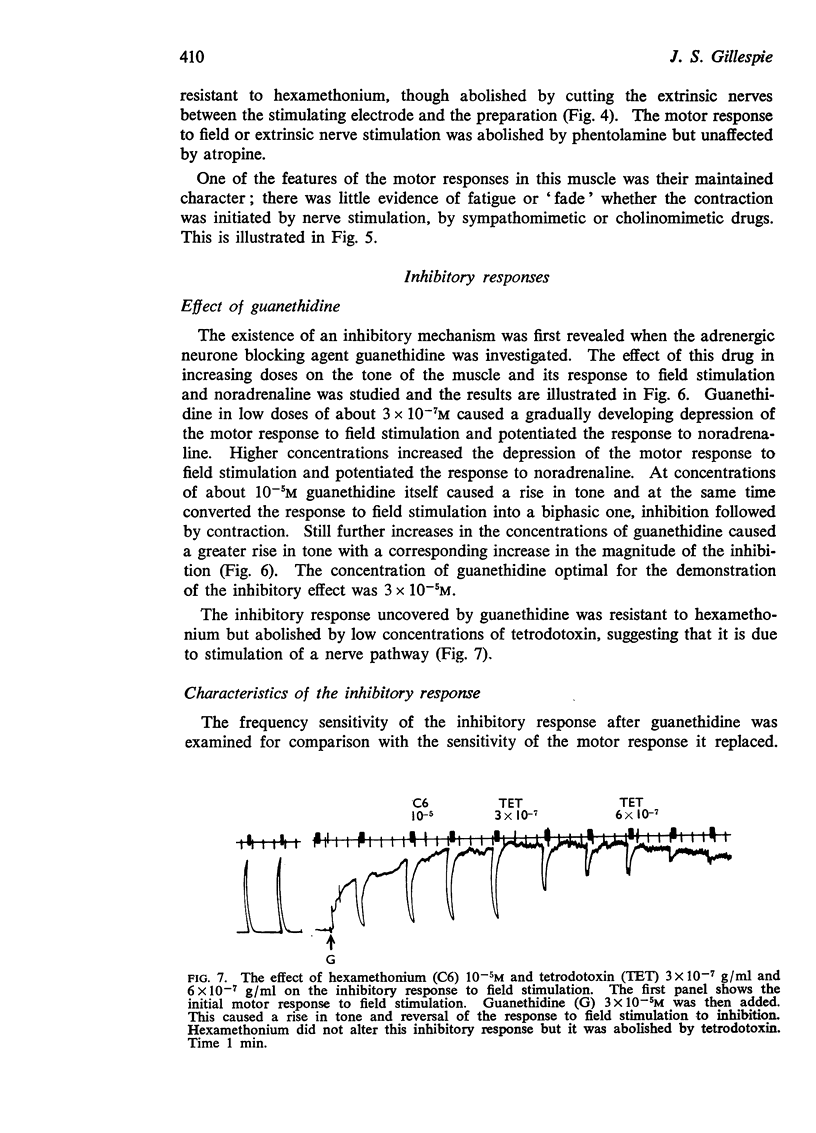
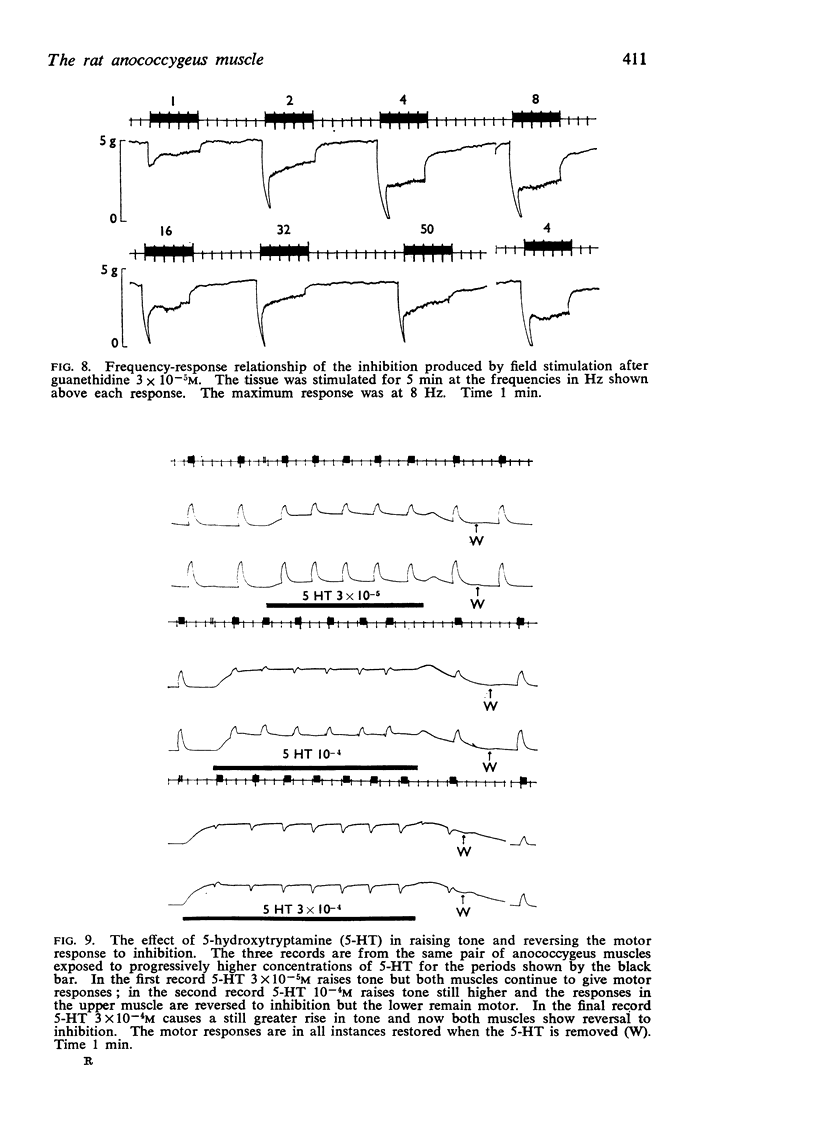
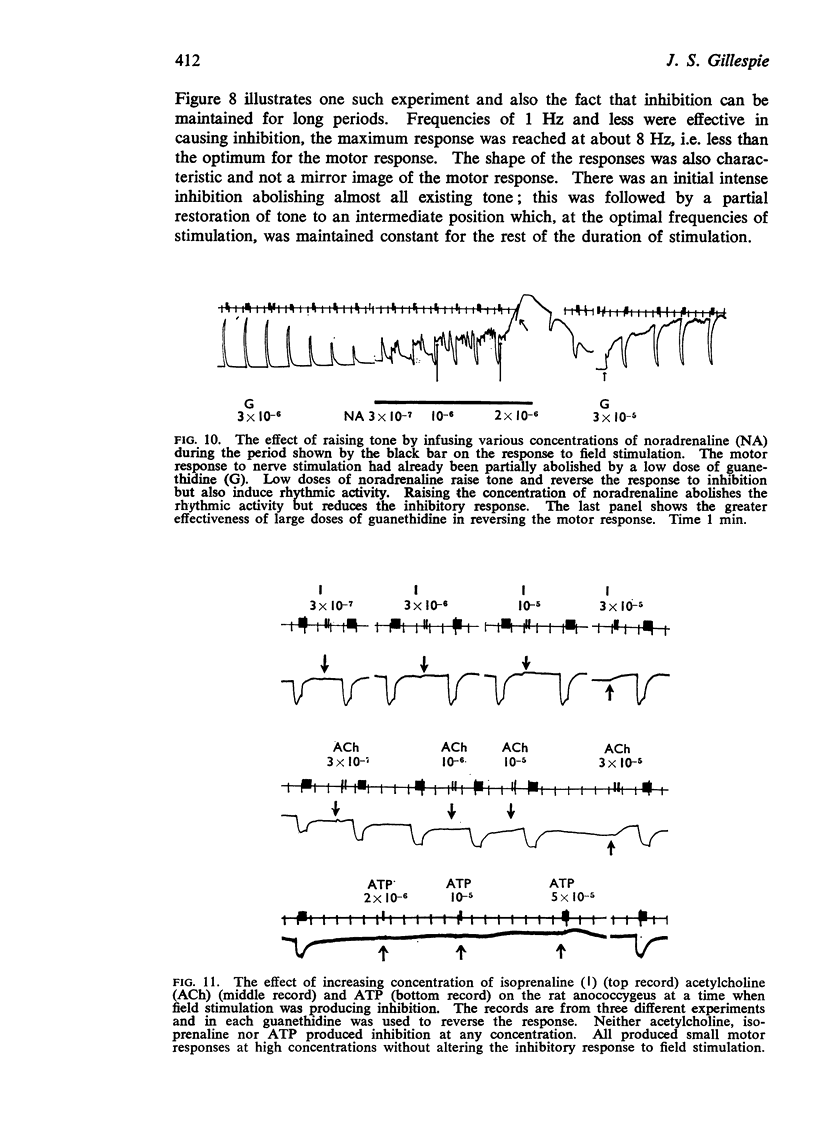
4. Guanethidine 10-6-5 × 10-6M blocks the motor response to nerve stimulation and potentiates that to noradrenaline. Higher concentrations of guanethidine raise tone. In the presence of raised tone, field stimulation produces an inhibitory response insensitive to hexamethonium but abolished by tetrodotoxin 2 × 10-7 g/ml. This inhibitory response to stimulation can also be shown after other drugs which raise tone.
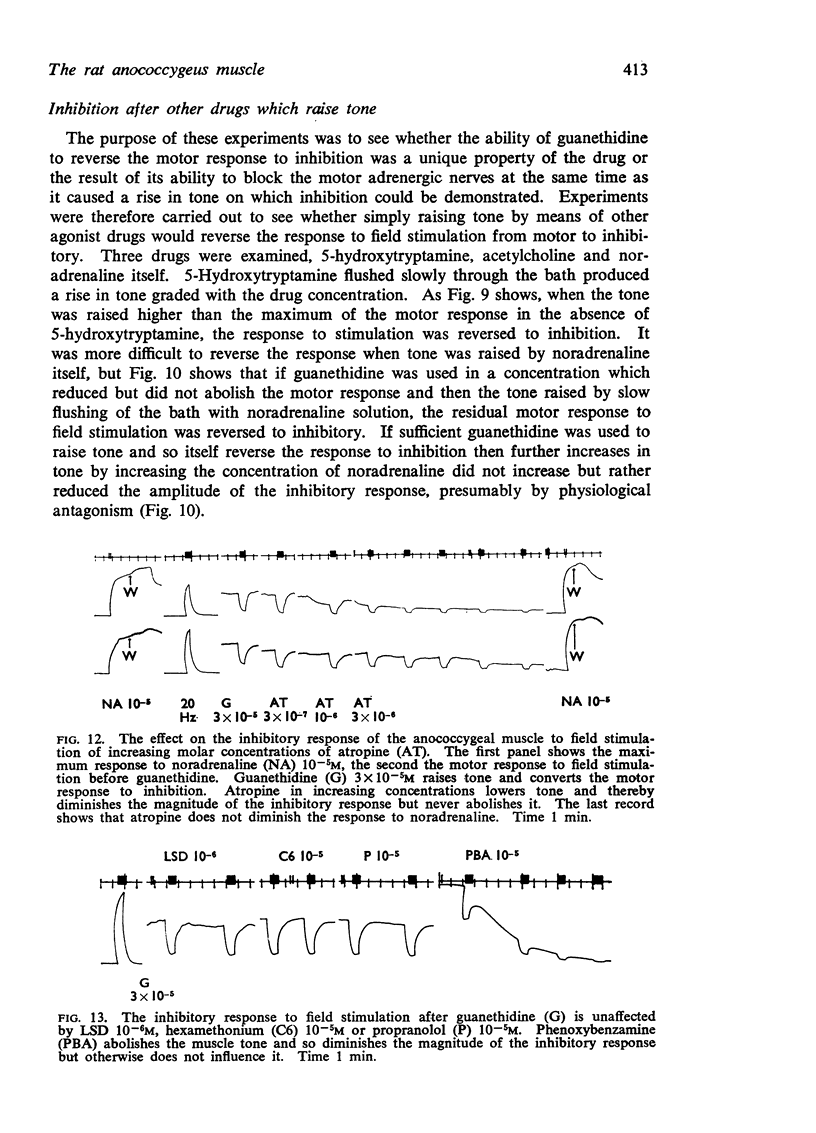
5. The inhibitory response to nerve stimulation is not mimicked by acetylcholine, isoprenaline or ATP, nor blocked by atropine, phentolamine, phenoxybenzamine, propranolol, hexamethonium or lysergic acid diethylamide.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambache N., Dunk L. P., Miall P., Zar M. A. Unexplained inhibitory action of D-lysergic acid diethylamide (LSD) on postganglionic motor transmission in the guinea-pig vas deferens. Br J Pharmacol. 1971 Aug;42(4):659P–660P. [PMC free article] [PubMed] [Google Scholar]
- BURN J. H., RAND M. J. The relation of circulating noradrenaline to the effect of sympathetic stimulation. J Physiol. 1960 Feb;150:295–305. doi: 10.1113/jphysiol.1960.sp006388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Campbell G., Satchell D., Smythe A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol. 1970 Dec;40(4):668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Kirpekar S. M. The histological localization of noradrenaline in the cat spleen. J Physiol. 1966 Nov;187(1):69–79. doi: 10.1113/jphysiol.1966.sp008076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Maxwell J. D. Adrenergic innervation of sphincteric and nonsphincteric smooth muscle in the rat intestine. J Histochem Cytochem. 1971 Nov;19(11):676–681. doi: 10.1177/19.11.676. [DOI] [PubMed] [Google Scholar]
- KOELLE G. B. The histochemical identification of acetylcholinesterase in cholinergic, adrenergic and sensory neurons. J Pharmacol Exp Ther. 1955 Jun;114(2):167–184. [PubMed] [Google Scholar]


