Abstract
Certain plant and animal introns increase expression of protein-coding sequences when placed in the 5′ region of the transcription unit. The mechanisms of intron-mediated enhancement have not been defined, but are generally accepted to be post- or cotranscriptional in character. One of the most effective plant introns in stimulating gene expression is the 1,028-bp first intron of the Sh1 gene that encodes maize (Zea mays) sucrose synthase. To address the mechanisms of intron-mediated enhancement, we used reporter gene fusions to identify features of the Sh1 first intron required for enhancement in cultured maize cells. A 145-bp derivative conferred approximately the same 20- to 50-fold stimulation typical for the full-length intron in this transient expression system. A 35-bp motif contained within the intron is required for maximum levels of enhancement but not for efficient transcript splicing. The important feature of this redundant 35-bp motif is T-richness rather than the specific sequence. When transcript splicing was abolished by mutations at the intron borders, enhancement was reduced to about 2-fold. The requirement of splicing for enhancement was not because of upstream translation initiation codons contained in unspliced transcripts. On the basis of our current findings, we conclude that splicing of the Sh1 intron is integral to enhancement, and we hypothesize that transcript modifications triggered by the T-rich motif and splicing may link the mRNA with the trafficking system of the cell.
Most plant genes contain intervening sequences (introns) that are transcribed into pre-mRNA and later removed by splicing. Introns separate gene segments (exons) that hold protein-coding information or are non-coding but retained in the mature transcript. The observation that some introns stimulate gene expression was first made in animal systems and extended to plants when Callis et al. (1987) demonstrated that the maize (Zea mays) Adh1 first intron increased expression of several genes. Other maize introns that have been shown to increase expression include the Bz1 intron (Callis et al., 1987), Hsp82 first intron (Silva et al., 1988), Sh1 first intron (Vasil et al., 1989), Adh1 introns 2 and 6 (Mascarenhas et al., 1990), actin third intron (Luehrsen and Walbot, 1991), GapA1 first intron (Donath et al., 1995), Ubi1 first intron (Vain et al., 1996), and the RpoT fourth intron (Bourdon et al., 2001).
Plant introns that stimulate expression have been documented in petunia (Petunia hybrida; Dean et al., 1989; Vain et al., 1996), oat (Avena sativa; Bruce and Quail, 1990), rice (Oryza sativa; McElroy et al., 1990; Snowden et al., 1996; Rethmeier et al., 1997), castor bean (Ricinus communis; Tanaka et al., 1990), potato (Solanum tuberosum; Leon et al., 1991; Fu et al., 1995a, 1995b), Arabidopsis (Rose and Last, 1997; Chaubet-Gigot et al., 2001), soybean (Glycine max; Kato et al., 1998), and tobacco (Nicotiana tabacum; Plesse et al., 2001). The approximately 2- to 10-fold range of intron-mediated enhancement usually seen in dicots is much less than increases observed in monocots, which can be more than 100-fold (for review, see Simpson and Filipowicz, 1996). Not all plant introns enhance gene expression. Three dicot introns do not enhance gene expression in transgenic plants(Chee et al., 1986; Kuhlemeier et al., 1988; Vancanneyt et al., 1990) and two maize introns are nonenhancing in transient assays: Adh1 intron 9 (Mascarenhas et al., 1990) and Hsp81 intron 1 (Sinibaldi et al., 1992).
Intron-mediated enhancement of gene expression has been studied in plants for about 15 years, and although the underlying mechanisms have not been identified, several features are known. Positioning of the intron in the natural orientation within the 5′ portion of the transcription unit is typically required for enhancement (Callis et al., 1987; Mascarenhas et al., 1990; Maas et al., 1991; Clancy et al., 1994; Snowden et al., 1996; Bourdon et al., 2001; Chaubet-Gigot et al., 2001), establishing that introns do not function as traditional transcriptional enhancers. Length (Sinibaldi and Mettler, 1992) and composition of flanking sequences influence the degree of stimulation (Luehrsen and Walbot, 1991; Maas et al., 1991; Clancy et al., 1994). The degree of enhancement is usually greater for relatively weak promoters (Callis et al., 1987; Vasil et el., 1987; Mascarenhas et al., 1990; Luehrsen et al., 1994). The magnitude of stimulation also depends on the coding sequences (Sinibaldi and Mettler, 1992; Rethmeier et al., 1997, 1998), the tissue of expression, and physiological conditions (Tanaka et al., 1990; Sinibaldi and Mettler, 1992; Gallie and Young, 1994; Fu and Park, 1995a, 1995b; Chaubet-Gigot et al., 2001; Plesse et al., 2001).
Potential mechanisms for intron-mediated enhancement include increased transcription, splicing-facilitated transcript maturation, stabilization or export, and targeting of spliced transcripts for protein synthesis. Introns do not increase the rate of transcription in two-animal systems (Hamer et al., 1979; Lai and Khoury, 1979), and we know that plant introns do not generally function as position-independent transcriptional enhancing elements. Rose and Last (1997) determined that transcription rates were comparable for constructs containing or lacking Arabidopsis PAT1 introns, but either intron resulted in increased levels of mRNA and enzyme activity. Chaubet-Gigot et al. (2001) recently reported increases in β-glucuronidase enzyme activity of up to 70-fold for the first introns of Arabidopsis replacement histone H3 genes, a particularly high level of stimulation for a dicot. The magnitude of the increases for these introns varied with the tissue type and promoter and was greatest for the weakest promoters. Transcription rates were not measured, but the authors argue that promoter activation as well as transcript stabilization must be responsible for the increases in enzyme activity. Transcript splicing, capping, and polyadenylation are cotranscriptional processes in animals (for reviews, see Bentley, 1999; Cramer et al., 2001). Most observations to date in plant systems indicate that intron-mediated enhancement occurs by a cotranscriptional or posttranscriptional mechanism. However, if the Arabidopsis replacement histone H3 introns are proven to affect the rate of transcription, then there must be multiple modes of action for intron-mediated enhancement of gene expression.
Intron-mediated enhancement is generally correlated with increased steady-state mRNA levels (for examples, see Callis et al., 1987; Luehrsen and Walbot, 1991; Rose and Last, 1997). However, mRNA amounts are not always correlated with increased enzyme activity (Mascarenhas et al., 1990; Tanaka et al., 1990; Bourdon et al., 2001). Tanaka et al.(1990) found that decreased splicing efficiency was associated with reduced enzyme activity. There is evidence from animal studies that transcript polyadenylation and transport may be linked to intron splicing (Collis et al., 1990; Huang and Gorman, 1990; Damert et al., 1996). Intron splicing could conceivably stabilize the mature transcript. However, Nash and Walbot (1992) reported that the half-lives of spliced and unspliced maize Bz2 transcripts are identical, indicating that extended mRNA persistence is not the defining characteristic of intron-mediated enhancement.
Deletions of 5′-flanking exon sequences, mutations of splice junctions, and intron deletions that block splicing all reduce gene expression (Mascarenhas et al., 1990; Sinibaldi and Mettler, 1992; Luehrsen and Walbot, 1994), indicating that splicing is needed for intron-mediated enhancement. However, these results are not conclusive because the unspliced mRNAs in the first two reports contained translation initiation codons upstream of the reporter gene start site. Retention of modified introns in the third study introduces downstream termination codons in-frame with the AUG. These short open reading frames and termination signals might affect transcript stability or interfere with translation. To avoid these complications, Rose and Beliakoff (2000) employed an in-frame translational fusion of the PAT1 first intron to β-glucuronidase. The approximately 5-fold enhancement of RNA and protein was reduced only slightly when splicing was blocked by mutation of the 5′ splice junction or by large internal deletions. Thus, for at least this particular intron, splicing is not required for expression enhancement. The collective observation that large deletions can be made within various introns without affecting enhancement indicates that if specific sequences are required, they must be present in multiple copies within these introns (Clancy et al., 1994; Luehrsen and Walbot, 1994; Rose and Beliakoff, 2000).
We have continued our study of the maize Sh1 first intron to investigate mechanisms of intron-mediated enhancement. The Sh1 first intron is a highly effective stimulator of gene expression, routinely increasing CAT activity 20- to 50-fold. Deletion analysis has been expanded to define the minimal intron sequences that condition enhancement, and a redundant motif required for maximum expression has been identified. We examined the influence of transcript splicing and observed that whereas efficient splicing is required for stimulation of expression, differences in efficiency are not sufficient to account for differences in the degree of enhancement. Mutations of intron splice sites blocked transcript splicing and dramatically reduced gene expression, demonstrating that splicing of the Sh1 first intron is essential for intron-mediated enhancement. Furthermore, we show that the lack of enhancement in the absence of splicing is not attributable to nonsense-mediated decay of mRNA or other effects resulting from translation start codons contained within unspliced introns.
RESULTS
14% of the Sh1 First Intron Conditions Full Enhancement of Gene Expression
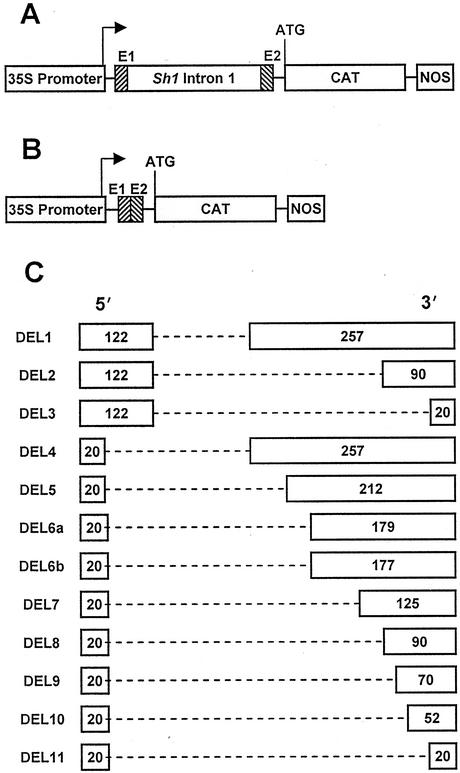
The Sh1 intron 1 cassette consists of the 1,028-bp intron flanked by 12 bp of 5′ exon sequence and 18 bp of 3′ exon sequence. This cassette was placed in the 5′-untranslated region (5′-UTR) between a modified cauliflower mosaic virus (CaMV) 35S promoter and CAT-coding sequence followed by the NOS polyadenylation region, to generate the construct 35SIfSCN (Fig. 1A). We previously reported that a 379-bp derivative of the Sh1 first intron, produced by removing 649 bp from the interior of the Sh1 first intron, conditioned enhancement of CAT reporter gene activity equal to that of the full-length intron (Clancy et el., 1994). Ten additional deletions within the intron were synthesized (Fig. 1C). The DEL series of constructs expands deletions of intron sequences into the 122 bp from the 5′ end of the intron and the 257 bp of the 3′ end of the intron retained in the 379-bp intron of construct DEL1. With the exception of DEL11, all constructs contain sequences matching the splicing branchpoint consensus (C/U) UNAN found in over 80% of plant introns (Brown et al., 1996; Liu and Filipowicz, 1996; Simpson et al., 1996). Potential Sh1 intron 1 branchpoints are located at typical positions about 20 to 60 bp upstream of the 3′ splice site.
Figure 1.
Diagrams of chimeric gene constructions for transient expression. A, Construct 35SIfSCN contains the 1,028-bp Sh1 first intron flanked by short portions of Sh1 exons 1 and 2 (E1 and E2). The 35S promoter is from CaMV, CAT encodes chloramphenicol acetyltransferase, and the termination region is the 3′ part of the nopaline synthase gene. The transcription start site is designated by the arrow, and the CAT translation initiation codon is indicated as ATG. B, The control construct 35SECN lacks the Sh1 first intron but is otherwise identical to construct 35SIfSCN. C, Deletions of internal portions of the Sh1 first intron were made while retaining all other elements of construct 35SIfSCN. Diagrams of the deletion derivatives indicate the basepairs retained from the 5′ and 3′ ends of the intron.
The constructs were tested in a transient gene expression system using particle bombardment to transfect cultured maize cells. CAT enzyme activity was standardized to expression of a cotransfected luciferase (LUX) gene construct. Reverse transcription (RT)-PCR was used to amplify transcripts and to determine splicing efficiencies. Construct 35SIfSCN increased CAT activity 29-fold relative to 35SECN, which lacks the intron but is otherwise identical (Table I). As in our previous findings using electroporation of maize protoplasts, the 379-bp intron of DEL1 increased CAT activity to approximately the same extent as the complete intron of 35SIfSCN.
Table I.
Up to 86% of the Sh1 first intron can be deleted without affecting the enhancement of gene expression
| Construct | CAT Activity
|
Intron
|
Splicing Efficiencya
|
||
|---|---|---|---|---|---|
| Mean | Range | AT | Mean | Range | |
| % | % | ||||
| DEL1 | 134.4 | 94.0–174.7 | 57.5 | 71.2 | 64.4–77.0 |
| DEL2 | 40.8 | 30.8–50.8 | 51.9 | 20.8b | 16.9–27.5 |
| DEL3 | 24.3 | 13.6–35.0 | 48.6 | 17.7b | 11.4–26.8 |
| DEL4 | 87.7 | 82.6–92.8 | 60.6 | 65.1 | 41.3–77.0 |
| DEL5 | 89.4 | 57.6–121.3 | 61.2 | 47.9 | 38.7–59.2 |
| DEL6a | 168.5 | 98.7–238.2 | 61.8 | 69.0 | 64.4–75.4 |
| DEL7 | 82.7 | 59.9–105.5 | 58.6 | 53.1 | 46.0–70.5 |
| DEL8 | 32.5 | 27.6–37.4 | 54.5 | 50.9 | 44.5–55.4 |
| DEL9 | 40.7 | 40.4–41.0 | 53.3 | 55.3 | 52.5–57.6 |
| DEL10 | 11.3 | 10.1–12.5 | 51.4 | 42.3 | 34.4–42.5 |
| DEL11 | 4.6 | 2.3–6.8 | 47.5 | 0c | – |
Enzyme extracts were prepared 45 h post-transfection in one experiment and at 24 h in a second experiment. CAT activity is given relative to construct 35SIfSCN, which contains the entire Sh1 first intron, and is the result of the two determinations. In the same two experiments, RNA was prepared at both 1 and 2 d post-bombardment. Transcript splicing efficiency is the result of the four measurements.
Splicing efficiency = percentage of transcripts correctly spliced.
Alternatively spliced transcripts also produced.
Only unspliced transcripts were detected.
Constructs DEL2 and DEL3 contain 90 and 20 bp from the 3′ end of the Sh1 first intron, respectively, and 122 bp from the 5′ end. Compared with 35SIfSCN, CAT activity was 41% and 24%, respectively. The placement of the 122 bp from the 5′ end adjacent to very 3′ parts of the intron is unique to DEL2 and DEL3 and was accompanied by alternatively spliced transcripts not seen for any of the other constructs. The percentage of total transcripts correctly spliced (splicing efficiency) was reduced to 21% for DEL2 and 18% for DEL3, compared with 71% for DEL1.
All other DEL constructs retain 20 bp of the intron's 5′ end, and from 257 to 20 bp of the 3′ end. Enzyme activity was elevated about 1.7-fold for DEL6a compared with the complete Sh1 first intron. DEL4, DEL5, and DEL7 produced levels of CAT activity similar to 35SIfSCN. The 145-bp intron of DEL7, which makes up just 14% of the total sequence of the Sh1 first intron, is the minimal intron that exhibits about the same level of expression enhancement as the full-length intron. DEL8 and DEL9 contain 110- and 90-bp introns, respectively. CAT activity generated by these constructs was reduced by more than one-half relative to DEL7, but without a similar decrease in splicing efficiency. Construct DEL10 has a 72-bp intron and showed a substantial decrease in CAT activity and a lesser reduction in splicing efficiency. CAT activity from DEL10 was only 14% of DEL7, whereas their splicing efficiencies were 42% and 53%, respectively. In general, more efficient splicing correlates with higher expression. However, some constructs with comparable splicing efficiencies have less than one-half the enzyme activity (DEL8 and DEL9 versus DEL5 and DEL7).
The 40-bp intron of DEL11 was not spliced, and CAT activity was at the level of the intronless construct. The lack of splicing is not surprising, because very few plant introns are as small as the intron in DEL11. Using synthetic introns in a maize transient expression system, Goodall and Filipowicz (1990) established that the minimum length for efficient intron splicing was 70 to 73 bp, depending on nucleotide composition. Luehrsen et al.(1994) reported that only approximately 3% of maize introns are less than 73 bp. Also, the DEL11 intron lacks a sequence matching the putative plant branchpoint consensus. The low level of enzyme activity generated by DEL11 might result from a translational fusion, because the single ATG within this intron is in frame with the CAT translation start site. Alternatively, translation might be correctly initiated or reinitiated at the reporter gene start codon.
35-bp Elements of the Sh1 First Intron Are Needed for Maximum Enhancement
The 145-bp intron of construct DEL7 is the minimal intron that conditions enhancement of gene expression to approximately the level of the complete Sh1 first intron. Deletion of a further 35 bp within DEL7 to generate DEL8 resulted in a 2.5-fold reduction in CAT activity without a corresponding reduction in transcript splicing efficiency (Table I). Thus, this region is required for maximum enhancement of gene expression, but not for efficient splicing. This 35-bp sequence, termed T1, has 71.4% AT content. To further investigate the effect of the T1 element on gene expression, we selected other 35-bp portions of the Sh1 first intron (Table II). The sequence T2 is a more 5′ fragment of the intron and is also 71.4% AT. The 60.0% AT content of a third region, termed T3, is more representative of overall base composition of the full-length intron, 56.9% AT. The T3 sequence is present in DEL5 but deleted from DEL6a.
Table II.
Composition of 35-bp sequences
| Name | Nucleotide Sequence | % A | % T | % G | % C |
|---|---|---|---|---|---|
| T1 | CAAATTTAGGTTGCTTTGGCATGATCTATTTTTTT | 20.0 | 51.4 | 17.2 | 11.4 |
| T1a | CAAATTTAGGTTGCTTTGGCAAGATCTATTTTTTT | 22.8 | 48.6 | 17.2 | 11.4 |
| T2 | TTTTTTGTTCTTTTACTACGAAAAGCATCTTCTTG | 20.0 | 51.4 | 11.4 | 17.1 |
| T3 | GGTGGCAACTGTTTTGCTATAAGATTCCATGTGTT | 20.0 | 40.0 | 25.7 | 14.3 |
T1, T2, and T3 are 35-bp sequences contained within the Sh1 first intron. T1a is a derivative of T1 and has a single base change indicated by the underline.
T1 in DEL7 was replaced with T2 and T3, generating constructs DEL7-T2 and DEL7-T3. The T2 sequence could functionally substitute for T1, giving rise to elevated levels of CAT activity and comparable splicing efficiency (Table III). In contrast, the less AT-rich T3 decreased both CAT activity and splicing efficiency. T1 and T2 were each placed in the intronless constructs 35SCN and 35SECN in the region corresponding to the mRNA 5′-UTR to test whether the elements could influence gene expression when not part of an intron. To avoid introducing an upstream translation initiation codon, the ATG in T1 was mutated to AAG, yielding the 35-bp sequence T1a (Table II). Neither T1a nor T2 enhanced expression in the absence of an intron (Table IV, constructs 35ST1aCN, 35ST2CN, 35ST1aECN, and 35ST2ECN). T1a or T2 positioned upstream of the intron of DEL8 reduced CAT activity. Splicing efficiency was also reduced from 64.1% for DEL8 to 47.4% for T1a-DEL8 and 56.4% for T2-DEL8. This placement of either T1a or T2 increases the AU composition of the 71 nucleotides immediately upstream of the intron in the pre-mRNA to 56%, from 44% for DEL8. McCullough and Schuler (1997a) reported a significant decrease in splicing efficiency when AU-content was increased 5′ to a dicot intron.
Table III.
The AT-content of the 35-bp sequences affects CAT activity and splicing efficiency
| Construct | AT
|
CAT Activity
|
Splicing Efficiencya
|
|||
|---|---|---|---|---|---|---|
| 35-bp Sequence | Intron | Mean | Range | Mean | Range | |
| % | % | |||||
| DEL7 | 71.4 | 58.6 | 76.3 | 71.2–81.4 | 67.2 | 63.8–70.5 |
| DEL7-T2 | 71.4 | 58.6 | 126.2 | 103.5–148.9 | 80.2 | 77.7–82.4 |
| DEL7-T3 | 60.0 | 55.8 | 26.1 | 24.2–28.0 | 30.5 | 27.7–33.3 |
Construct DEL7 contains the T1 sequence within the 145-bp intron. Constructs DEL7-T2 and DEL7-T3 were created by replacing T1 with sequences T2 and T3. RNA and enzyme extracts were prepared at approximately 24 h post-transfection in two experiments. CAT activity is given relative to construct 35SIfSCN, which contains the entire Sh1 first intron.
Splicing efficiency = percentage of transcripts correctly spliced.
Table IV.
Sequences T1 and T2 must be within the intron to enhance CAT activity
| Construct | Placement of Test Sequence | CAT Activity
|
|
|---|---|---|---|
| Mean | Range | ||
| % | |||
| 35SCN | None | 2.6 | 1.6–3.5 |
| 35ST1aCN | T1a in 5'-UTR of 35SCN | 3.1 | 1.8–4.4 |
| 35ST2CN | T2 in 5'-UTR of 35SCN | 2.9 | 1.6–4.2 |
| 35SECN | None | 4.1 | 3.5–4.7 |
| 35ST1aECN | T1a in 5'-UTR of 35SECN | 4.0 | 2.0–6.0 |
| 35ST2ECN | T2 in 5'-UTR of 35SECN | 4.2 | 3.6–4.9 |
| DEL8 | None | 57.2 | 48.0–66.3 |
| T1a-DEL8 | T1a in 5'-UTR of DEL8 | 26.7 | 25.0–28.4 |
| T2-DEL8 | T2 in 5'-UTR of DEL8 | 39.0 | 31.3–46.6 |
| DEL7 | Contains T1 within intron | 104.7 | 99.6–109.8 |
| DEL7-T2 | T1 replaced by T2 within intron of DEL7 | 120.8 | 119.0–122.7 |
Enzyme extracts were prepared 24 h post-transfection in two separate experiments. CAT activities are shown relative to construct 35SIfSCN, which contains the entire Sh1 first intron.
We conclude that the 35-bp T1 element is required for maximum enhancement of gene expression but not for efficient splicing. Nucleotide composition, rather than the actual sequence, is the important factor for enhancement, because the T2 fragment can substitute for T1. The T1-like element must be located within the intron to enhance gene expression. There are other short regions of the intron that affect gene expression. Removal of 45 bp from DEL4 to produce DEL5 reduced splicing efficiency, but CAT activity was not altered. Deletion of the 35-bp T3 sequence from DEL5 increased both CAT activity and splicing efficiency (Table I, DEL5 versus DEL6a). T3 similarly decreased splicing and enzyme activity when substituted for the T1 sequence in DEL7. Sequences present in DEL6a but not DEL7 increased CAT activity substantially and splicing efficiency to a lesser extent. The T-rich sequences T1 and T2 are unique in increasing enzyme activity without affecting transcript splicing.
Intron Splicing Is Necessary for Increased Enzyme Activity
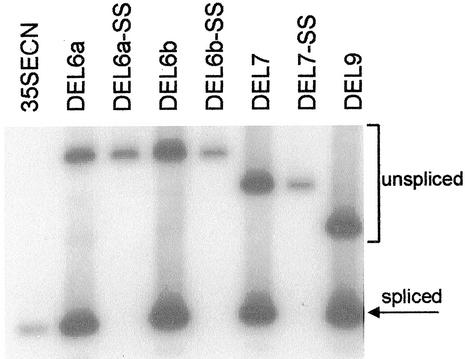
To assess the role of transcript splicing in intron-mediated enhancement of gene expression, we mutated the terminal dinucleotides of the introns in three DEL constructs. The 5′ GT was changed to AC, and the 3′ AG was altered to CT in derivatives of DEL6a, DEL6b, and DEL7. RT-PCR products from RNA are shown in Figure 2, and enzyme data and transcript splicing efficiencies are presented in Table V. Constructs with mutated splice sites have the suffix SS. Splicing efficiencies and CAT activities for DEL6a and DEL7 were similar to those observed in previous experiments (Tables I, III, and IV). Construct DEL6b is identical to DEL6a except for elimination of an AT dinucleotide duplication in the intron. The 2-bp duplication in DEL6a does not impact gene expression, because DEL6b has comparable levels of splicing efficiency and enzyme activity. DEL6a-SS, DEL6b-SS, and DEL7-SS produced only unspliced transcripts, indicating that the intron splice site mutations abolished transcript splicing. Cryptic splice sites were not activated (Fig. 2). CAT activities for the three constructs were approximately 2-fold those produced by the intronless control construct 35SECN, in contrast to the 25- to 44-fold levels of enhancement conferred by the constructs with intact splice junctions. Although this indicates that a low level of enhancement of gene expression may be conferred by an unspliced intron, it is clear that intron splicing is required for maximal enhancement.
Figure 2.
Mutation of Sh1 first intron termini abolishes transcript splicing. Total RNA (1.0 μg) from cultured cells transfected with each construct was amplified by RT-PCR. The products were analyzed by gel-blot hybridization using a 32P-labeled bp probe composed of 5′-UTR and CAT sequences. The 230-bp probe is identical to the RT-PCR products from spliced transcripts.
Table V.
Splice site mutations abolish splicing and the enhancement of gene expression
| Construct | CAT Activity
|
Splicing Efficiencya
|
||
|---|---|---|---|---|
| Mean | Range | Mean | Range | |
| % | ||||
| DEL6a | 193.6 | 120.4–287.6 | 75.1 | 70.8–79.4 |
| DEL6a-SS | 7.8 | 3.3–12.3 | 0b | |
| DEL6b | 163.2 | 141.3–183.8 | 78.4 | 70.0–86.7 |
| DEL6b-SS | 9.3 | 6.6–12.1 | 0b | |
| DEL7 | 111.2 | 68.0–184.2 | 67.6 | 64.6–70.5 |
| DEL7-SS | 8.6 | 5.7–12.4 | 0b | |
Gene constructs with both splice sites mutated are designated by the suffix SS. RNA and enzyme extracts were prepared approximately 24 h post-transfection. CAT activity is shown relative to construct 35SIfSCN, which contains the entire Sh1 first intron, and is the result of three experiments. Transcript splicing efficiency is the result of two determinations.
Splicing efficiency = percentage of transcripts correctly spliced.
Only unspliced transcripts detected.
Upstream AUGs in Unspliced Transcripts Do Not Reduce Synthesis of the CAT Protein
Our series of intron deletion constructs with intact splice junctions exhibits a range of splicing efficiencies. Depending on the particular intron derivative, unspliced transcripts contain one or more translation initiation codons upstream of the CAT translation start site. For example, the unspliced transcript from DEL7 contains two upstream AUG codons, whereas that produced by DEL7-T2 has only the more 5′ of these two intron AUGs. The 5′ AUG is in frame with the reporter gene AUG, but there are multiple intervening translation stop codons. The 3′ AUG of the intron is out of frame and is also closely followed by multiple stop codons. To investigate whether translation initiation at these upstream sites in unspliced transcripts affects CAT activity, the ATGs in the introns of DEL7 and DEL7-T2 were altered. The first ATG is part of the 5′ splice signal. It was mutated to ACG to preserve the same degree of agreement with the plant splice site sequence, because modifying the match to the consensus can alter splicing efficiency (for review, see Simpson and Filipowicz, 1996). The second ATG was changed to AAG. Constructs with the suffix ATG1 have the first ATG mutated, and ATG2 and ATG1,2 denote mutation of the second ATG and both ATGs, respectively. Splicing efficiencies and CAT activities were essentially unchanged compared with the parental constructs (Table VI), although relative to DEL7-T2, the CAT activity produced by DEL7-T2-ATG was somewhat reduced.
Table VI.
Upstream translation start codons do not affect enzyme activity or splicing efficiency
| Construct | CAT Activity
|
Splicing Efficiencya
|
||
|---|---|---|---|---|
| Mean | Range | Mean | Range | |
| % | ||||
| DEL6a | 184.1 | 172.7–193.6 | 76.1 | 72.8–79.4 |
| DEL7 | 87.4 | 71.2–109.7 | 67.2 | 63.8–70.5 |
| DEL7-ATG1 | 95.8 | 84.6–116.0 | 66.2 | 65.3–67.2 |
| DEL7-ATG2 | 99.8 | 48.2–114.2 | 64.6 | 64.0–65.2 |
| DEL7-ATG1,2 | 71.6 | 49.7–100.7 | 63.3 | 61.6–65.0 |
| DEL7-T2 | 158.9 | 103.5–224.3 | 80.2 | 77.7–82.4 |
| DEL7-T2-ATG1 | 71.6 | 61.2–71.0 | 72.2 | 67.6–76.7 |
| DEL9 | 43.9 | 37.6–53.6 | 62.0 | 53.0–71.1 |
Altered ATGs contained in the intron are indicated by the suffix ATG. RNA and enzyme extracts were prepared at approximately 24 h post-transfection. CAT activity is given relative to construct 35SIfSCN, which contains the entire Sh1 first intron, and is the result of three experiments. Transcript splicing efficiency is the result of two determinations.
Splicing efficiency = percentage of transcripts correctly spliced.
Enzyme activity was not increased by alteration of the translation initiation codons contained within the introns. If the upstream open reading frames in unspliced transcripts inhibited initiation or reinitiation of translation at the reporter gene AUG, then their disruption would be expected to increase CAT activity by allowing translation of functional CAT enzyme from unspliced transcripts. For example, approximately 30% of the transcripts detected from expression of DEL7 are not spliced (Table VI). If these unspliced transcripts were available for translation to the same extent as are spliced transcripts, CAT activity would be expected to increase by approximately 30% in the constructs with ATG mutations. This does not occur, indicating that splicing is an indispensable component of the mechanism of intron-mediated enhancement of gene expression.
To further test the contribution of unspliced transcripts to CAT activity, a derivative of DEL7 was generated with altered 5′ and 3′ intron splice sites and mutations of the two intron ATGs. This construct, termed DEL7-SS-ATG1,2, would give rise to transcripts with a longer 5′-UTR because the intron is not spliced and there are no AUGs upstream of the CAT translation start codon. Results from expression of DEL7-SS-ATG1,2 and related constructs are shown in Table VII. Consistent with our earlier observations, transcript splicing efficiency is not affected by the ATG mutations (construct DEL7-ATG1,2), whereas the splice site mutations (constructs DEL7-SS and DEL7-SS-ATG1,2) abolished intron splicing and reduced CAT activity to about the level of the intronless control. These results confirm that whereas a low level of CAT expression occurs when transcripts contain an unspliced intron, the splicing process is integral to the mechanism of intron-mediated enhancement of gene expression.
Table VII.
Splicing is required for intron-mediated enhancement of gene expression
| Construct | CAT Activity
|
Splicing Efficiencya
|
||
|---|---|---|---|---|
| Mean | Range | Mean | Range | |
| % | ||||
| DEL6a | 202.8 | 202.7–202.8 | 80.5 | 80.1–80.9 |
| DEL7 | 107.8 | 104.6–111.1 | 67.3 | 62.6–72.0 |
| DEL7-ATG1,2 | 45.4 | 36.7–54.1 | 62.6 | 56.0–69.3 |
| DEL7-SS | 4.3 | 4.1–4.5 | 0b | |
| DEL7-SS-ATG1,2 | 2.8 | 2.7–2.9 | 0b | |
Construct DEL7-SS has mutations of the intron splice sites, DEL7-ATG1,2 has alterations in the ATGs contained within the intron of DEL7, and DEL7-SS-ATG1,2 has both the intron splice sites and ATGs mutated. RNA and enzyme extracts were prepared at approximately 24 h post-transfection in two experiments. CAT activity is given relative to construct 35SIfSCN, which contains the entire Sh1 first intron.
Splicing efficiency = percentage of transcripts correctly spliced.
Only unspliced transcripts detected.
DISCUSSION
A Redundant T-Rich Motif Is Required for Maximum Enhancement
We have defined the features of the maize Sh1 first intron that are required for maximal enhancement of gene expression. Sequences from the termini that together make up only 14% of the full-length intron are sufficient for high levels of enhancement. This finding is an extension of our previous work (Clancy et al., 1994) and is in agreement with other reports that large deletions can be made within introns without decreasing the enhancement effect or splicing efficiency (Luehrsen and Walbot, 1994; Rose and Beliakoff, 2000). The latter study demonstrated that every part of the Arabidopsis PAT1 first intron is individually dispensable, consistent with the conclusion of Luehrsen and Walbot that if internal sequences or elements are required for intron-mediated enhancement, then these elements must be redundant. We have identified such an element within the Sh1 first intron. Deletion of the T-rich 35-bp region, which we called T1, caused a substantial reduction in reporter enzyme activity. We also showed that another T-rich portion of the intron could efficiently substitute for T1, but a third segment with reduced T content could not. However, the third segment had a negative impact on transcript splicing efficiency correlated with the decreased enzyme activity. AU-richness is an important determinant of intron recognition and processing in pre-mRNA (Goodall and Filipowicz, 1989, 1991; Lou et al., 1993; McCullough et al., 1993), presumably functioning via interactions with AU-binding proteins. AU-rich elements can improve the splicing efficiency of impaired introns (Luehrsen and Walbot, 1994) and influence 3′ splice site selection (Baynton et al., 1996; McCullough and Schuler, 1997b). Removal of T1 caused a minor decrease in splicing efficiency that is not sufficient to account for the 60% reduction in enzyme activity.
Our results show that maximum enhancement of expression by deletion derivatives of the Sh1 first intron requires at least one copy of a redundant T-rich motif and the impact of the element is not at the level of transcript splicing efficiency. To our knowledge, this is the first report of an intron sequence that contributes to enhanced gene expression but does not affect splicing. The T1 motif does not increase gene expression in the absence of an intron or when positioned 5′ to an intron and thus does not function as a classic transcriptional enhancer. Because the element must be within an intron for enhancement and therefore not present in the mature transcript, it must function before or concurrent with intron splicing. There is a great deal of evidence in animals and insects that transcript maturation processes such as splicing, capping, and polyadenylation are coupled with transcription rather than following it (for review, see Bentley, 1999; Cramer et al., 2001). Some RNA-binding proteins remain associated with mRNA after splicing and may subsequently interact with other binding proteins. The formation of these protein/mRNA complexes affects transcript translation, transport, and stability (Matsumoto et al., 1998; Luo and Reed, 1999; Le Hir et al., 2000). These findings suggest pathways by which the T-rich Sh1 intron1 elements could increase gene expression without altering the rate of transcription or efficiency of transcript splicing. These elements could potentially participate in recruitment to the spliceosome of RNA-binding proteins that promote transcript processing, export, localization, or translatability. In the future, we plan to use deletions to determine the core portion of the elements that is required for the enhancement effect.
Intron Splicing Is Required for Enhancement
The 5′ GT and 3′ AG of introns are highly conserved in plants, and mutations of these dinucleotides generally results in failure of splicing at these sites (for review, see Simpson and Filipowicz, 1996). We have shown that mutations of the Sh1 first intron 5′ and 3′ splice sites block transcript splicing and dramatically reduce CAT activity. The 25- to 44-fold enhancement relative to the intronless control drops to about 2-fold in the absence of splicing. With the exception of DEL11, the unspliced transcripts generated by expression of the Sh1 intron constructs would contain translation initiation codons that are out of frame with the downstream CAT start codon and/or are followed by intervening stop codons. Transcripts retaining introns may remain in the nucleus, as is the case for yeast (Rutz and Seraphin, 2000). If exported to the cytoplasm, initiation at the upstream codons could reduce enzyme activity produced and thus mask intron-mediated enhancement if it occurred at the level of RNA accumulation. Also, nonsense-mediated decay could reduce transcript abundance. To address these possibilities, we altered the intron ATGs in constructs with intact splice junctions and found no change in enzyme activity or splicing. Because CAT activity was not increased by the removal of the intron translation initiation signals, we conclude that they have no deleterious effects on gene expression. When upstream ATGs were altered in a construct in which splicing was blocked, CAT activity was again not increased and was at a similar level to the intronless control. This result confirms that these intron AUGs do not cause instability of unspliced transcripts or interfere with CAT translation and verifies that Sh1 intron splicing is essential for more than 90% of intron-mediated enhancement.
Fundamentally Different Mechanisms Are Likely Involved in Intron-Enhanced Gene Expression
The demonstration that the maize Sh1 first intron must be spliced for significant enhancement to occur contrasts with recent findings of Rose and Beliakoff (2000) for the Arabidopsis PAT1 first intron. A translational fusion of the PAT1 promoter and modified first intron conferred about a 5-fold increase in expression that was only moderately reduced when the intron was rendered unspliceable by large internal deletions or mutation of the first nucleotide of the 5′ splice junction. The authors hypothesize that the single-base change at the 5′ splice site allows the PAT1 intron to retain enough intron identity to permit base pairing of transcripts with the U1 small nuclear RNA, followed by association with the spliceosome. This putative association with the spliceosome results in some form of transcript stabilization, even though transcript splicing cannot be completed. According to this model, intron splicing is not required for the major portion of intron-mediated enhancement, although the completed act of splicing results in a further degree of stimulation. This model contrasts with our observations for the maize Sh1 intron DEL11 construct. One might expect that pre-mRNA from our constructs DEL6a-SS, DEL6b-SS and DEL7-SS would not be recognized by the U1 small nuclear RNA because there is a 2-bp mismatch at the 5′ splice site. These transcripts, which we have shown to be unspliceable, would not associate with the spliceosome and not undergo stabilization. The DEL11 construct contains a 40-bp derivative of the maize Sh1 first intron that is not spliced, presumably because of its suboptimal length and lack of a good match to the branchpoint consensus sequence. However, this small intron retains other features important for intron recognition, including native Sh1 first intron splice junctions. The transition in nucleotide composition at the 5′ exon/intron border is the same as for the full-length Sh1 intron. Like the Arabidopsis PAT1 introns, the intron sequence in DEL11 is an in-frame translational fusion to the reporter enzyme-coding sequence. The almost total loss of enhancement that accompanies the lack of splicing of the DEL11 intron indicates that completed transcript splicing is required for the major component of enhancement conditioned by the Sh1 intron.
Our results with the Sh1 intron taken together with the observations for the Arabidopsis PAT1 first intron lead us to suggest that there are multiple mechanisms by which different introns influence gene expression. This is consistent with the observations that some introns fail to enhance and the wide range in the extent of stimulation among enhancing introns. The Sh1 first intron stimulates expression in excess of 18-fold more than the Adh1 first intron in otherwise identical constructs (Clancy et al., 1994).
In those cases where intron splicing is integral to expression enhancement, the splicing process must influence the accumulation, stabilization, localization, or translatability of mRNA. Intron-mediated enhancement is usually associated with elevated levels of steady-state mRNA, but the correlation is not absolute. Studies in maize cells using introns of the Adh1 gene (Callis et al., 1987; Luehrsen and Walbot, 1991) and salT gene (Rose and Last, 1997; Rose and Beliakoff, 2000) showed that increases in mRNA were about the same as increases in enzyme activity. However, the fourth intron of the maize RpoT gene boosted protein activity about 2.4-fold without any increase in the steady-state level of mRNA (Bourdon et al., 2001). Also, expression of the castor bean catalase first intron in rice calli (Tanaka et al., 1990) and the maize Adh1 second intron in maize protoplasts (Mascarenhas et al., 1990) produced increases in enzyme activity that were substantially greater than the increases in mRNA. Preliminary results using several of our intron deletion constructs indicate that Sh1 intron derivatives condition elevations in reporter enzyme activity that are 5- to 10-fold higher than the increases in transcript levels (not shown).
When Mascarenhas et al. (1990) reported that maize Adh1 introns 2 and 6 increased CAT activity more than mRNA levels, they suggested that splicing must alter some quality of the mature transcript as well as the amount. Subsequent studies suggest pathways by which this could take place. Matsumoto et al. (1998) determined that intron splicing in Xenopus laevis oocytes increased translational efficiency without affecting the amount of mRNA in the cytoplasm. The position of the intron in the gene is important. The same level of mRNA was synthesized from constructs with or without the intron and with the intron in a 5′ or 3′ position, but only the 5′ intron resulted in enhanced translational efficiency. X. laevis RNA-binding proteins that remain associated with the mature transcript after splicing and mark exon/exon junctions facilitate export from the nucleus and participate in nonsense-mediated decay in the cytoplasm (Le Hir et al., 2001). Splicing may also be required for nonsense-mediated decay in plants (Isshiki et al., 2001). The process of intron splicing clearly can function not only to remove non-coding regions from RNA but also to tag or package transcripts in ways that affect transport to and activity within the cytoplasm.
CONCLUSION
Splicing of the Sh1 first intron is necessary for intron-mediated enhancement of gene expression. Maximum enhancement requires at least one copy of a redundant T-rich intron motif that does not affect splicing. We suggest that enhancement of gene expression by the maize Sh1 first intron depends on modifications of the mature mRNA resulting from the T-rich motif and the process of intron splicing. These modifications distinguish spliced transcripts from those that retain or never possessed an intron and may increase transport, maturation, stability, and/or translatability of the mRNA. Matsumoto et al. (1998) reported a case where increased translational efficiency was the major consequence of splicing. Transcripts from intronless genes accumulated in the cytoplasm to the same level as spliced transcripts from identical but intron-containing genes. Other studies have shown that proteins left near exon/exon borders after splicing subsequently bind factors that promote transcript export from the nucleus (Luo and Reed, 1999; Le Hir et al., 2001). In plant systems, increased expression is sometimes but not always correlated with increased steady-state RNA levels.
These observations, coupled with the fact that enhancing introns vary widely in their ability to stimulate expression, strongly suggest that different mechanisms of intron-enhanced expression occur in plants. Individual introns likely facilitate one or more of the processes of transcript transport, stabilization, or translation to differing degrees. A complete understanding of intron-mediated enhancement of gene expression must account for these specific findings and for the intriguing observation that the magnitude of enhancement is generally greater for weaker promoters. The evolution of different mechanisms for intron-enhanced gene expression in plants points to the critical importance of this phenomenon in plant gene expression.
MATERIALS AND METHODS
Gene Constructions
The plasmid 35SCN has been previously described (Vasil et al., 1989) and contains a modified CaMV 35S promoter, CAT-coding region, and nopaline synthase 3′ polyadenylation region in pUC19. A cassette consisting of the maize (Zea mays) Sh1 first intron and portions of Sh1 exons 1 and 2 was generated from a genomic clone as detailed in Clancy et al. (1994). This cassette contains a cloning-derived CC dinucleotide at the 5′ end, 10 bp of exon 1, the 1,028-bp intron, 17 bp of exon 2, and an additional C nucleotide at the 3′ end. Construct 35SIfSCN was created by placing the Sh1 intron 1 cassette in the 5′-UTR of 35SCN in the forward orientation relative to the direction of transcription. The Sh1 exon sequences that flank the intron in the cassette were isolated from a cDNA clone as previously described (Clancy et al., 1994). These Sh1 exonic sequences were cloned in 35SCN to yield 35SECN, which lacks the intron but is otherwise identical to 35SIfSCN.
Internal deletions within the intron in 35SIfSCN were created in two ways. An initial deletion of 649 bp was generated by removing an SstI to ScaI fragment (Clancy et al., 1994) to yield the construct here termed DEL1. The intron in DEL1 retains 122 bp from the 5′ end of the Sh1 first intron and 257 bp from the 3′ end. Ten more constructs with deletions within the intron were produced by PCR methodology using primer pairs flanking each desired deletion. After PCR amplification with Vent DNA polymerase (New England Biolabs, Beverly, MA) for 25 cycles, parental DNA was digested with the restriction enzyme DpnI. The PCR products were subsequently purified, ligated, and transformed into Escherichia coli. The deletions in constructs DEL2 through DEL11 were confirmed by DNA sequencing. In one case, an AT dinucleotide was duplicated in the primer used for PCR, and the resulting construct is termed DEL6a. This duplication was subsequently removed to produce construct DEL6b.
The PCR method described above was employed to introduce nucleotide changes within constructs of the DEL series. Primers containing nucleotide substitutions were used to alter the intron splice sites as follows: The intron 5′ GT was changed to AC, and the intron 3′ AG was changed to CT. Also, two translation initiation codons contained within Sh1 intron deletion derivatives were altered by PCR. The first ATG, which comprises bases +3 to +5 of the intron 5′ splice signal, was altered to ACG. This substitution was chosen to maintain the degree of conformity to the plant splice site consensus sequence (Hanley and Schuler, 1988; Goodall and Filipowicz, 1991). The ATG that begins 105 bp from the 3′ end of the intron was converted to AAG.
Transient expression experiments with the DEL series of constructs identified an interesting 35-bp region, here termed T1, that begins 125 bp from the 3′ end of the Sh1 first intron. T1 is present in construct DEL7 and is precisely deleted in DEL8. T2 is a 35-bp sequence of nucleotide composition similar to T1, and starts 520 bp from the 3′ end of the Sh1 first intron. The two sequences were synthesized by annealing 35-bp oligonucleotides and placed in the 5′-UTR of DEL8 between the nucleotides 32 and 31 bp upstream of the intron, and also at the identical position in the 5′-UTR of the intronless constructs 35SCN and 35SECN. To avoid introducing a translation initiation codon upstream of the reporter gene start site, an ATG contained in T1 was changed to AAG and the altered sequence was termed T1a. The resulting constructs are designated by the prefixes T1a and T2. A third 35-bp intron fragment was chosen for its lower A+T content relative to T1 and T2. This sequence, termed T3, is contained in construct DEL5 and deleted in constructs DEL6a and DEL6b. The T1 sequence contained within the intron of DEL7 was replaced by T2 and T3 to produce constructs DEL8–7-T2 and DEL7-T3, respectively.
Cell Line and Transfection Procedure
The mesocotyl-derived maize cell line described by Chourey and Zurawski (1981) was the gift of Prem Chourey. Cell suspensions were grown in the dark with shaking at 130 rpm in Murashige and Skoog complete medium (Invitrogen, Carlsbad, CA) supplemented with 2 mg L−1 2,4-dichlorophenoxyacetic acid and subcultured at 7-d intervals. Cells were harvested 3 to 4 d after subculture for transfection by particle bombardment (Klein et al., 1987). For each transfection, approximately 2 mL of suspension cells were spread on a 3MM paper circle (Whatman, Clifton, NJ) and placed in a petri dish containing culture medium solidified with 1.5% (w/v) agar. Gold particles (1.0 μm average diameter, Bio-Rad, Hercules, CA) were coated with plasmid DNA as described by Taylor and Vasil (1991) and introduced into maize cells using the Bio-Rad PDS-1000/He biolistic particle delivery system. All constructs to be compared were tested together in the same experiments. Each transfection included a LUX expression construct (Christensen and Quail, 1996) in addition to the test construct. After particle bombardment, 2 mL of liquid culture medium were added to the petri dish. Each dish was sealed with laboratory film (Parafilm, American National Can, Greenwich, CT) and incubated in the dark.
Determination of Transient Expression
In initial experiments, cells were collected at approximately 24 and 45 h post-bombardment. These first experiments established that RNA and enzyme expression patterns were comparable for the two harvest times. In subsequent experiments, cells were collected at approximately 22 h post-bombardment. Enzyme extracts were prepared immediately, and cell samples for RNA analyses were frozen in liquid N2 and stored at −80°C.
CAT and LUX Enzyme Assays
Enzyme extracts were prepared by grinding approximately 500 μL of cells with 250 μL of CAT/LUX extraction buffer (0.1 m potassium phosphate, pH 7.8, 2 mm dithiothreitol) using a mortar and pestle. Extracts were centrifuged at 16,000g for 10 min at 4°C. Each supernatant was divided into two tubes. One tube was frozen for use in the LUX assay, and the other tube was heated at 60°C for 10 min to inactivate inhibitors of CAT activity. Extracts were stored at −80°C until enzyme assays were performed.
CAT activity was measured by the phase-extraction method of Seed and Sheen (1988). [3H]Chloramphenicol (PerkinElmer Life Sciences, Boston) was diluted to 20 Ci mol−1 with unlabeled chloramphenicol (Sigma-Aldrich, St. Louis) and pre-extracted twice with mixed xylene isomers (Aldrich Chemical Co., Milwaukee) to reduce assay background. CAT assays were performed in duplicate, and each 100-μL reaction contained 5 μL of heat-treated enzyme extract, 250 mm Tris buffer, pH 8.0, 0.2 μCi of [3H]chloramphenicol, and 0.25 mg mL−1 n-butyryl-CoA. Reactions were incubated at 37°C for 60 min and terminated by extraction with 200 μL of a 2:1 mixture (v/v) of tetramethylpentadecane and mixed xylenes (Aldrich Chemical Co.). A portion of the organic phase containing the acylated chloramphenicol was mixed with 5 mL of scintillation fluid and counted. With these parameters, the assay was linear over greater than 2 orders of magnitude (data not shown), covering the range of CAT activity expressed by our constructs.
For determination of LUX activity, 25 μL of unheated enzyme extract was added to 200 μL of LUX assay buffer (25 mm Tricine, pH 7.8, 15 mm MgCl2, 5 mm ATP, and 0.5 mg mL−1 bovine serum albumin), and 100 μL of 0.5 mm luciferin was added by injection in a luminometer (Monolight 2010, Analytical Luminescence Laboratory, Ann Arbor, MI). The LUX assay was performed in triplicate for each sample, and the results were used to standardize CAT activity expressed by the cotransfected CAT construct.
RNA Isolation and Analysis
All reagents for RNA isolation and RT-PCR amplification were from Invitrogen and were used according to the manufacturer's protocols. Total RNA was prepared from approximately 300 μL of cells using TRIzol Reagent and treated with RNase-free DNase I before amplification. First-strand cDNA synthesis was performed with 1.0 μg of RNA, oligo(dT)12–18 primers, and the Superscript Preamplification System. The primers for PCR flank the Sh1 intron and its derivatives, and generate a 230-bp product from correctly spliced transcripts. Because of their design, the primers yield PCR products only from the CAT gene constructs and not from the Sh1 first intron endogenous to the tissue culture cells. The forward primer is composed of upstream leader and Sh1 exon 1 sequence. This primer begins 30 bp upstream of the intron and has the sequence: 5′-CTAGAGTCGAGATCCGTCCCGAC. The reverse primer begins 200 bp downstream of the intron within the CAT-coding region and has the sequence: 5′-AGGCCGTAATATCCAGCTGAACGG. PCR was performed with 10% of the first-strand cDNA reaction and 1.25 units of Tax DNA polymerase, using a GeneAmp PCR System 2400 (PerkinElmer Life Sciences) for 30 cycles. Each PCR cycle consisted of denaturation at 94°C for 45 s, annealing at 55°C for 30 s, and extension at 72°C for 1.5 min.
RT-PCR products were separated on 3% (w/v) agarose gels (NuSieve 3:1 agarose, FMC Bioproducts, Rockland, ME), transferred to nylon membranes (Hybond-N+, Amersham Biosciences AB, Uppsala) and hybridized with a 230-bp [32P]dCTP probe identical to the spliced transcripts. A PhosphorImager (Molecular Dynamics, Sunnyvale, CA) was used to quantify the signals. Transcript splicing efficiency is the ratio of correctly spliced transcripts to total transcripts for a given expression construct. To verify that spliced and unspliced transcripts co-amplify with equal efficiency under the PCR conditions, DNA mixing experiments were performed. The plasmid 35SECN, which yields a PCR product corresponding to the correctly spliced transcript, was mixed with DNA of the DEL series constructs. The PCR products corresponding to unspliced transcripts range from 609 bp for DEL1 to 270 bp for DEL11, and all amplified equally well whether or not 35SECN template was included in the reaction (data not shown). RT-PCR was accordingly used to measure the relative abundance of spliced and unspliced transcripts arising from these constructs. Accumulation of the 1,258-bp PCR product from 35SIfSCN was reduced in the presence of 35SECN, and so transcript splicing efficiency could not be measured by RT-PCR for the full-length intron.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
ACKNOWLEDGMENT
We thank Dr. Carla Lyerly Linebarger for many helpful comments on the manuscript.
Footnotes
This work was supported by the National Science Foundation (grant nos. IBN–9316887, IBN–960416, IBN–9982626, and MCB–9420422), by the U.S. Department of Agriculture Competitive Grants Program (grant nos. 94–37300–453, 9500836, 95–37301–2080, 9701964, 97–36306–4461, 98–01006, and 2000–01488), and by the Florida Agricultural Experiment Station (journal series no. R–08673).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.008235.
LITERATURE CITED
- Baynton CE, Potthoff SJ, McCullough AJ, Schuler MA. U-rich tracts enhance 3′ splice site recognition in plant nuclei. Plant J. 1996;10:703–711. doi: 10.1046/j.1365-313x.1996.10040703.x. [DOI] [PubMed] [Google Scholar]
- Bentley D. Coupling RNA polymerase transcription with pre-mRNA processing. Curr Opin Cell Biol. 1999;11:347–351. doi: 10.1016/S0955-0674(99)80048-9. [DOI] [PubMed] [Google Scholar]
- Bourdon V, Harvey A, Lonsdale DM. Introns and their positions affect the translational activity of mRNA in plant cells. EMBO Rep. 2001;2:394–398. doi: 10.1093/embo-reports/kve090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JWS, Smith P, Simpson CG. Arabidopsis consensus intron sequences. Plant Mol Biol. 1996;32:531–535. doi: 10.1007/BF00019105. [DOI] [PubMed] [Google Scholar]
- Bruce WB, Quail PH. cis-Acting elements involved in photoregulation of an oat phytochrome promoter in rice. Plant Cell. 1990;2:1081–1089. doi: 10.1105/tpc.2.11.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callis J, Fromm M, Walbot V. Introns increase gene expression in cultured maize cells. Genes Dev. 1987;1:1183–1200. doi: 10.1101/gad.1.10.1183. [DOI] [PubMed] [Google Scholar]
- Chaubet-Gigot N, Kapros T, Flenet M, Kahn K, Gigot C, Waterborg JH. Tissue-specific enhancement of transgene expression by introns of replacement histone H3 genes of Arabidopsis. Plant Mol Biol. 2001;45:17–30. doi: 10.1023/a:1006487023926. [DOI] [PubMed] [Google Scholar]
- Chee PP, Klassy RC, Slightom JL. Expression of a bean storage protein phaseolin “minigene” in foreign plant tissues. Gene. 1986;41:47–57. doi: 10.1016/0378-1119(86)90266-0. [DOI] [PubMed] [Google Scholar]
- Chourey PS, Zurawski DB. Callus formation from protoplasts of a maize cell line. Theor Appl Genet. 1981;59:341–344. doi: 10.1007/BF00276446. [DOI] [PubMed] [Google Scholar]
- Christensen AH, Quail PH. Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgenic Res. 1996;5:213–218. doi: 10.1007/BF01969712. [DOI] [PubMed] [Google Scholar]
- Clancy M, Vasil V, Hannah LC, Vasil IK. Maize Shrunken-1 intron and exon regions increase gene expression in maize protoplasts. Plant Sci. 1994;98:151–161. [Google Scholar]
- Collis P, Antoniou M, Grosveld F. Definition of the minimal requirements within the human β-globin gene and the dominant control region for high level expression. EMBO J. 1990;9:233–240. doi: 10.1002/j.1460-2075.1990.tb08100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer P, Srebrow A, Kadener S, Werbajh, de la Mata M, Melen G, Nogues G, Kornblihtt AR. Coordination between transcription and pre-mRNA processing. FEBS Lett. 2001;498:179–182. doi: 10.1016/s0014-5793(01)02485-1. [DOI] [PubMed] [Google Scholar]
- Damert A, Leibiger B, Leibiger IB. Dual function of the intron of the rat insulin I gene in regulation of gene expression. Diabetologia. 1996;39:1165–1172. doi: 10.1007/BF02658502. [DOI] [PubMed] [Google Scholar]
- Dean C, Favreau M, Bond-Nutter D, Bedbrook J, Dunsmuir P. Sequences downstream of translation start regulate quantitative expression of two petunia rbcS genes. Plant Cell. 1989;1:201–208. doi: 10.1105/tpc.1.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donath M, Mendel R, Cerff R, Martin W. Intron-dependent transient expression of the maize GapA1 gene. Plant Mol Biol. 1995;28:667–676. doi: 10.1007/BF00021192. [DOI] [PubMed] [Google Scholar]
- Fu H, Kim SY, Park WD. High level tuber expression and sucrose inducibility of a potato Sus4 sucrose synthase gene require 5′ and 3′ flanking sequences and the leader intron. Plant Cell. 1995a;7:1387–1394. doi: 10.1105/tpc.7.9.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Kim SY, Park WD. A potato Sus3 sucrose synthase gene contains a context-dependent 3′ element and a leader intron with both positive and negative tissue-specific effects. Plant Cell. 1995b;7:1395–1403. doi: 10.1105/tpc.7.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie DR, Young TE. The regulation of gene expression in transformed maize aleurone and endosperm protoplasts. Plant Physiol. 1994;106:929–939. doi: 10.1104/pp.106.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall GJ, Filipowicz W. The AU-rich sequences present in the introns of plant nuclear pre-mRNAs are required for splicing. Cell. 1989;58:473–483. doi: 10.1016/0092-8674(89)90428-5. [DOI] [PubMed] [Google Scholar]
- Goodall GJ, Filipowicz W. The minimum functional length of pre-mRNA introns in monocots and dicots. Plant Mol Biol. 1990;14:727–733. doi: 10.1007/BF00016505. [DOI] [PubMed] [Google Scholar]
- Goodall GJ, Filipowicz W. Different effects of intron nucleotide composition and secondary structure on pre-mRNA splicing in monocot plants. EMBO J. 1991;10:2635–2644. doi: 10.1002/j.1460-2075.1991.tb07806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer DH, Smith KD, Boyer SH, Leder P. SV40 recombinants carrying rabbit β-globin gene coding sequences. Cell. 1979;17:725–735. doi: 10.1016/0092-8674(79)90279-4. [DOI] [PubMed] [Google Scholar]
- Hanley BA, Schuler MA. Plant intron sequences: evidence for distinct groups of introns. Nucleic Acids Res. 1988;16:7159–7176. doi: 10.1093/nar/16.14.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MTF, Gorman CM. Intervening sequences increase efficiency of RNA 3′ processing and accumulation of cytoplasmic RNA. Nucleic Acids Res. 1990;18:937–947. doi: 10.1093/nar/18.4.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isshiki M, Yamamoto Y, Satoh H, Shimamoto K. Nonsense-mediated decay of mutant waxy mRNA in rice. Plant Physiol. 2001;125:1388–1395. doi: 10.1104/pp.125.3.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Whittier RF, Shibata D. Increase of foreign gene expression in monocot and dicot cells by an intron in the 5′ untranslated region of a soybean phosphoenolpyruvate carboxylase gene. Biosci Biotechnol Biochem. 1998;62:151–153. doi: 10.1271/bbb.62.151. [DOI] [PubMed] [Google Scholar]
- Klein TM, Wolf ED, Wu R, Sanford JC. High velocity microprojectiles for delivering nucleic acids into living cells. Nature. 1987;327:70–73. [PubMed] [Google Scholar]
- Kuhlemeier C, Fluhr R, Chua NH. Upstream sequences determine the difference in transcript abundance of pea rbcS genes. Mol Gen Genet. 1988;212:405–411. [Google Scholar]
- Lai CJ, Khoury G. Deletion mutants of simian virus 40 defective in biosynthesis of late viral mRNA. Proc Natl Acad Sci USA. 1979;76:71–75. doi: 10.1073/pnas.76.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Hir H, Gatfield D, Izaurralde E, Moore MJ. The exon-exon junction complex provides a binding platform for factors involved in mRNA export and nonsense-mediated mRNA decay. EMBO J. 2001;20:4987–4997. doi: 10.1093/emboj/20.17.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Hir H, Izaurralde E, Maquat LE, Moore MJ. The spliceosome deposits multiple proteins 20–24 nucleotides upstream of mRNA exon-exon junctions. EMBO J. 2000;19:6860–6869. doi: 10.1093/emboj/19.24.6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon P, Planckaert F, Walbot V. Transient gene expression in protoplasts of Phaseolus vulgaris isolated from a cell suspension culture. Plant Physiol. 1991;95:968–972. doi: 10.1104/pp.95.3.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HX, Filipowicz W. Mapping of branchpoint nucleotides in mutant pre-mRNAs expressed in plant cells. Plant J. 1996;9:381–389. doi: 10.1046/j.1365-313x.1996.09030381.x. [DOI] [PubMed] [Google Scholar]
- Lou H, McCullough AJ, Schuler MA. Expression of maize Adh1 intron mutants in tobacco nuclei. Plant J. 1993;3:393–403. doi: 10.1046/j.1365-313x.1993.t01-22-00999.x. [DOI] [PubMed] [Google Scholar]
- Luo M, Reed R. Splicing is required for rapid and efficient mRNA export in metazoans. Proc Natl Acad Sci USA. 1999;96:14937–14942. doi: 10.1073/pnas.96.26.14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luehrsen KR, Taha S, Walbot V. Nuclear pre-mRNA processing in higher plants. Prog Nucleic Acid Res Mol Biol. 1994;47:149–193. doi: 10.1016/s0079-6603(08)60252-4. [DOI] [PubMed] [Google Scholar]
- Luehrsen KR, Walbot V. Intron enhancement of gene expression and the splicing efficiency of introns in maize cells. Mol Gen Genet. 1991;225:81–93. doi: 10.1007/BF00282645. [DOI] [PubMed] [Google Scholar]
- Luehrsen KR, Walbot V. Addition of A- and U-rich sequence increases the splicing efficiency of a deleted form of a maize intron. Plant Mol Biol. 1994;24:449–463. doi: 10.1007/BF00024113. [DOI] [PubMed] [Google Scholar]
- Maas C, Laufs S, Grant S, Korfhage C, Werr W. The combination of a novel stimulatory element in the first exon of the maize Shrunken-1 gene with the following intron 1 enhances reporter gene expression up to 1000-fold. Plant Mol Biol. 1991;16:199–207. doi: 10.1007/BF00020552. [DOI] [PubMed] [Google Scholar]
- Mascarenhas D, Mettler IJ, Pierce DA, Lowe HW. Intron-mediated enhancement of heterologous gene expression in maize. Plant Mol Biol. 1990;15:913–920. doi: 10.1007/BF00039430. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Wassarman KM, Wolffe AP. Nuclear history of a pre-mRNA determines the translational activity of cytoplasmic mRNA. EMBO J. 1998;7:2107–2121. doi: 10.1093/emboj/17.7.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough AJ, Lou H, Schuler MA. Factors affecting authentic 5′ splice site selection in plant nuclei. Mol Cell Biol. 1993;13:1323–1331. doi: 10.1128/mcb.13.3.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough AJ, Schuler MA. Intronic and exonic sequences modulate 5′ splice site selection in plant nuclei. Nucleic Acids Res. 1997a;25:1071–1077. doi: 10.1093/nar/25.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough AJ, Schuler MA. Internal AU-rich elements modulate activity of two competing 3′ splice sites in plant nuclei. Plant J. 1997b;12:937–943. doi: 10.1046/j.1365-313x.1997.12040937.x. [DOI] [PubMed] [Google Scholar]
- McElroy D, Zhang W, Cao J, Wu R. Isolation of an efficient actin promoter for use in rice transformation. Plant Cell. 1990;2:163–171. doi: 10.1105/tpc.2.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash J, Walbot V. Bronze-2 gene expression and intron splicing patterns in cells and tissues of Zea mays L. Plant Physiol. 1992;100:464–471. doi: 10.1104/pp.100.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesse B, Criqui M-C, Durr A, Parmentier Y, Fleck J, Genschik P. Effects of the polyubiquitin gene Ubi.U4 leader intron and first ubiquitin monomer on reporter gene expression in Nicotiana tabacum. Plant Mol Biol. 2001;45:655–667. doi: 10.1023/a:1010671405594. [DOI] [PubMed] [Google Scholar]
- Rethmeier N, Kramer E, Van Montagu M, Cornelissen M. Identification of cat sequences required for intron-dependent gene expression in maize cells. Plant J. 1998;13:831–835. [Google Scholar]
- Rethmeier N, Seurinck J, Van Montagu M, Cornelissen M. Intron-mediated enhancement of transgene expression in maize is a nuclear, gene-dependent process. Plant J. 1997;12:895–899. doi: 10.1046/j.1365-313x.1997.12040895.x. [DOI] [PubMed] [Google Scholar]
- Rose AB, Beliakoff JA. Intron-mediated enhancement of gene expression independent of unique intron sequence and splicing. Plant Physiol. 2000;122:535–542. doi: 10.1104/pp.122.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AB, Last RL. Introns act post-transcriptionally to increase expression of the Arabidopsis thaliana tryptophan pathway gene PAT1. Plant J. 1997;11:455–464. doi: 10.1046/j.1365-313x.1997.11030455.x. [DOI] [PubMed] [Google Scholar]
- Rutz B, Seraphin B. A dual role for BBP/ScSF1 in nuclear pre-mRNA retention and splicing. EMBO J. 2000;19:1873–1876. doi: 10.1093/emboj/19.8.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed B, Sheen J-Y. A simple phase-extraction assay for chloramphenicol acyltransferase activity. Gene. 1988;67:271–277. doi: 10.1016/0378-1119(88)90403-9. [DOI] [PubMed] [Google Scholar]
- Silva EM, Mettler IJ, Dietrich PS, Sinibaldi RM. Enhanced transient expression in maize protoplasts. Genome (Suppl 1) 1988;30:72. [Google Scholar]
- Simpson CG, Clark G, Davidson D, Smith P, Brown JWS. Mutation of putative branchpoint consensus sequences in plant introns reduces splicing efficiency. Plant J. 1996;9:369–380. doi: 10.1046/j.1365-313x.1996.09030369.x. [DOI] [PubMed] [Google Scholar]
- Simpson CG, Filipowicz W. Splicing of precursors to mRNA in higher plants: mechanism, regulation and sub-nuclear organisation of the spliceosomal machinery. Plant Mol Biol. 1996;32:1–41. doi: 10.1007/BF00039375. [DOI] [PubMed] [Google Scholar]
- Sinibaldi RM, Mettler IJ. Intron splicing and intron-mediated enhanced expression in monocots. In: Cohn WE, Moldave K, editors. Progress in Nucleic Acid Research and Molecular Biology. Vol. 42. New York: Academic Press; 1992. pp. 229–257. [DOI] [PubMed] [Google Scholar]
- Snowden KC, Buchholz, Hall TC. Intron position affects expression from the tpi promoter in rice. Plant Mol Biol. 1996;31:689–692. doi: 10.1007/BF00042241. [DOI] [PubMed] [Google Scholar]
- Tanaka A, Mita S, Ohta S, Kyozuka J, Shimamoto K, Nakamura K. Enhancement of foreign gene expression by a dicot intron in rice but not in tobacco is correlated with an increased level of mRNA and an efficient splicing of the intron. Nucleic Acids Res. 1990;18:6767–6770. doi: 10.1093/nar/18.23.6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MG, Vasil IK. Histology of, and physical factors affecting, transient GUS expression in plant millet (Pennisetum glaucum (Li) R. Rv.) embryos following microprojectile bombardment. Plant Cell Rep. 1991;10:120–125. doi: 10.1007/BF00232041. [DOI] [PubMed] [Google Scholar]
- Vain P, Finer KM, Engler DE, Pratt RC, Finer JJ. Intron-mediated enhancement of gene expression in maize (Zea mays L.) and bluegrass (Poa pratensis L.) Plant Cell Rep. 1996;15:489–494. doi: 10.1007/BF00232980. [DOI] [PubMed] [Google Scholar]
- Vancanneyt G, Schmidt R, O'Connor-Sanchez A, Willmitzer L, Rocha-Sosa M. Construction of an intron-containing marker gene: splicing of the intron in transgenic plants and its use in monitoring early events in Agrobacterium-mediated plant transformation. Mol Gen Genet. 1990;220:245–250. doi: 10.1007/BF00260489. [DOI] [PubMed] [Google Scholar]
- Vasil V, Clancy M, Ferl RJ, Vasil IK, Hannah LC. Increased gene expression by the first intron of maize Shrunken-1 locus in grass species. Plant Physiol. 1989;91:1575–1579. doi: 10.1104/pp.91.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]