Abstract
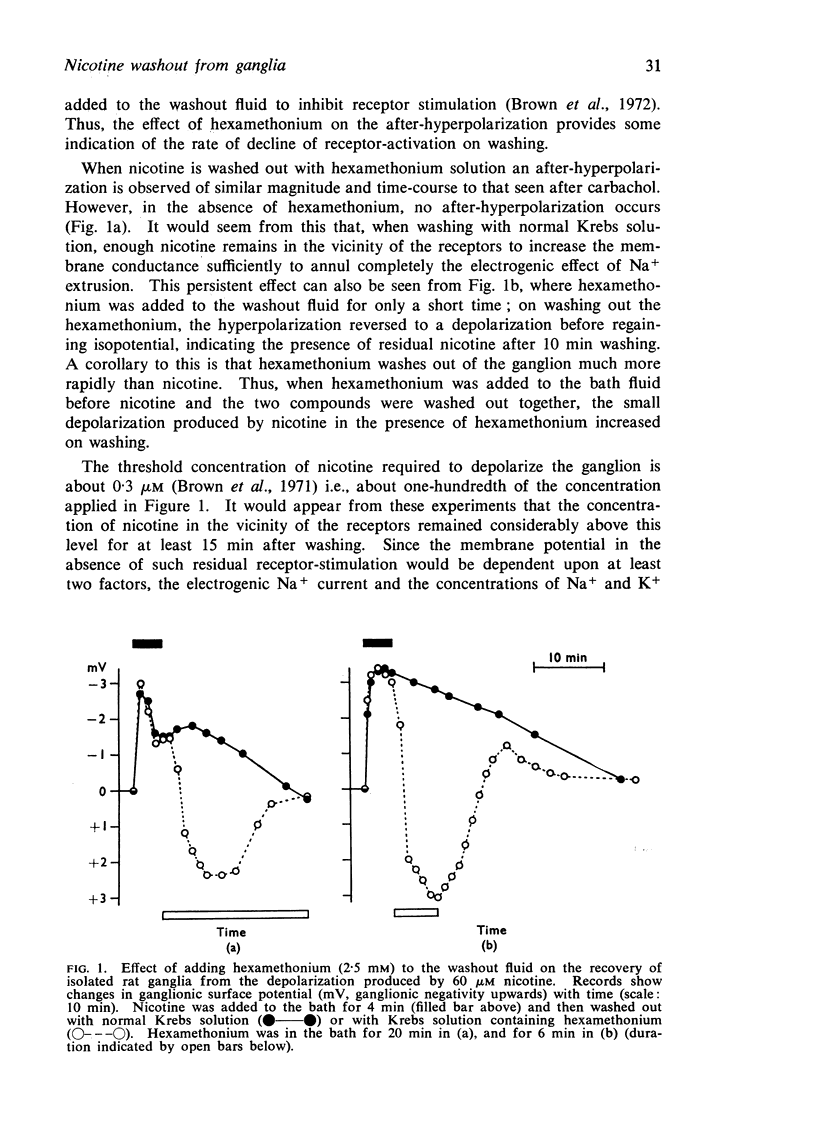
1. Isolated rat superior cervical ganglia recovered more slowly from the depolarizing action of nicotine than from that of carbachol or acetylcholine. This was due to sustained high nicotine concentrations in the vicinity of the receptors, since recovery was hastened by adding hexamethonium to the washout fluid.
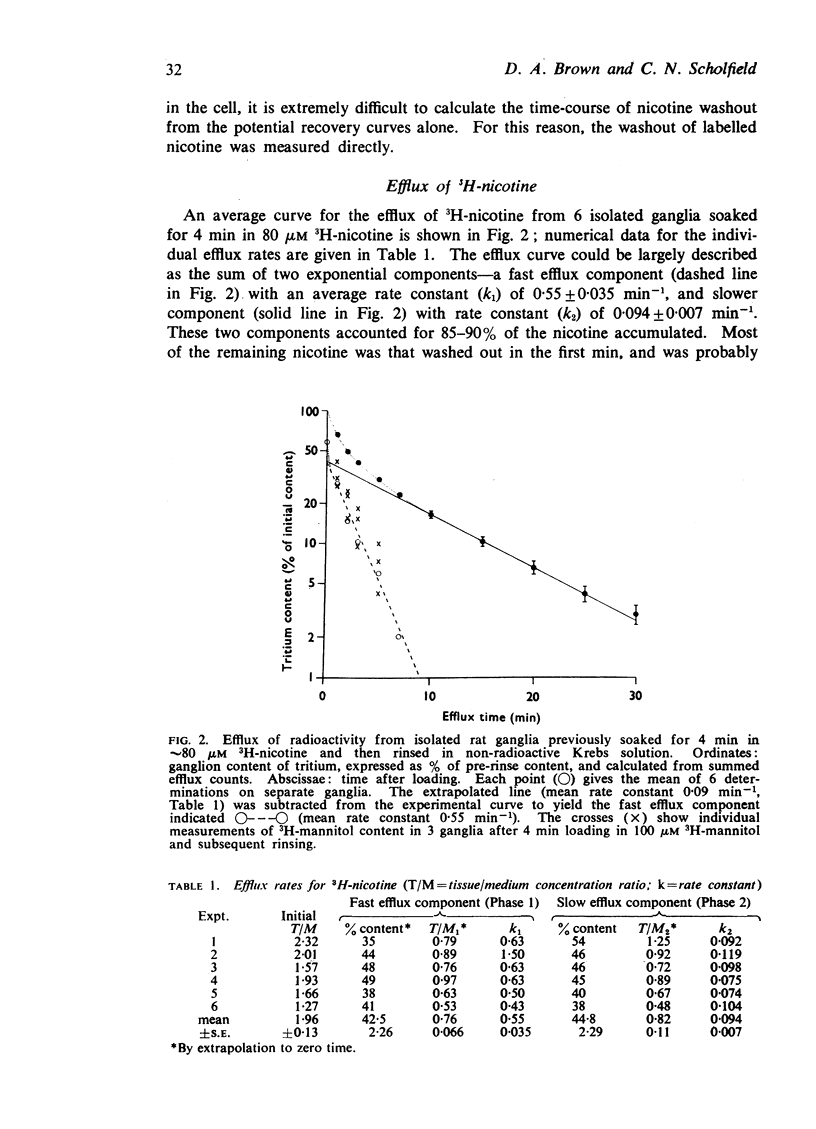
2. Ganglia incubated for 4 min in 80 μM 3H-nicotine accumulated nicotine to a level exceeding the extracellular space, as judged from the uptake of 3H-mannitol.
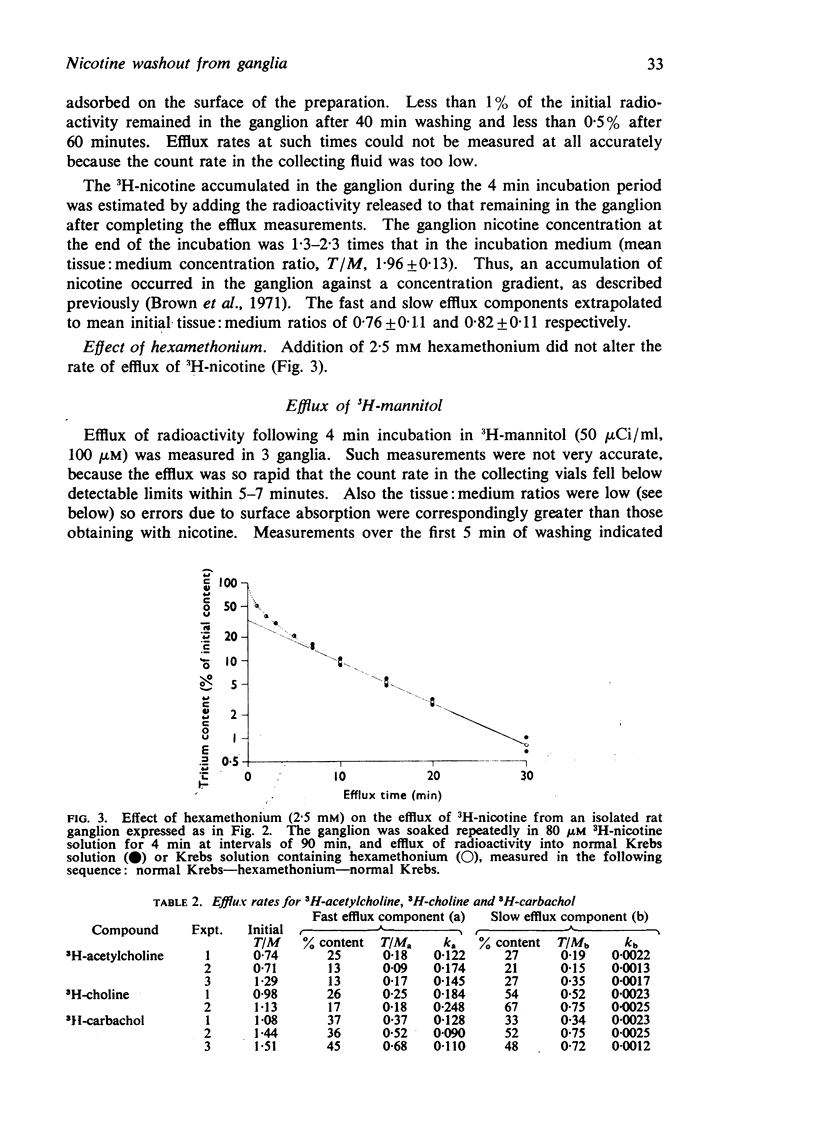
3. The subsequent efflux of 3H-nicotine into non-radioactive solution could be largely resolved into two exponential components, with rate constants of 0·55±0·04 and 0·094±0·007 min-1. The former was similar to that for total mannitol efflux, and so might be largely ascribed to clearance of extracellular nicotine. The slower efflux might be due to clearance from intracellular compartments. Nicotine efflux rates were not affected by hexamethonium indicating that receptor-activation did not modify the slow efflux.
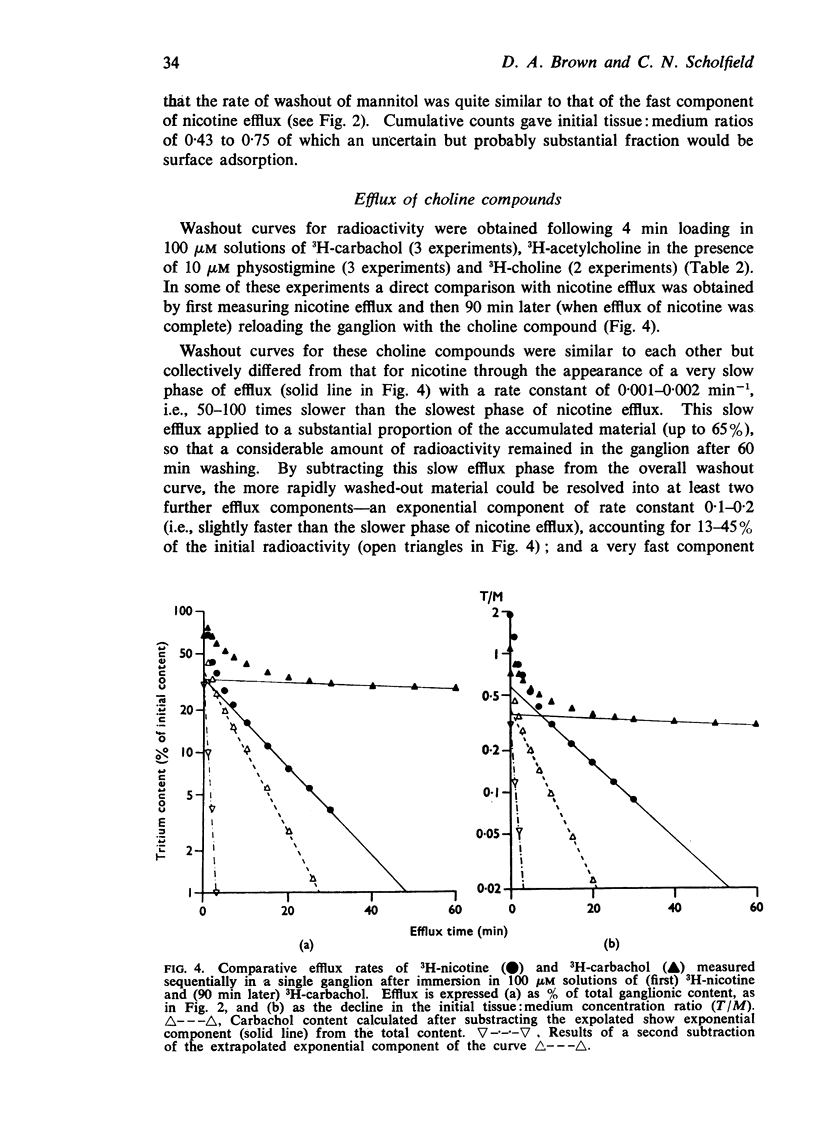
4. Efflux of choline compounds (3H-acetylcholine, 3H-choline and 3H-carbachol) showed an additional, very slow component (rate constant 0·001 to 0·002 min-1).
5. It was suggested that slow efflux of intracellular nicotine might sustain depolarization on washing by maintaining high perineuronal concentrations of nicotine. With choline compounds the efflux rate from such sources may be too slow to affect perineuronal concentrations.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPELGREN L. E., HANSSON E., SCHMITERLOEW C. G. LOCALIZATION OF RADIOACTIVITY IN THE SUPERIOR CERVICAL GANGLION OF CATS FOLLOWING INJECTION OF C14-LABELLED NICOTINE. Acta Physiol Scand. 1963 Dec;59:330–336. doi: 10.1111/j.1748-1716.1963.tb02748.x. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Brownstein M. J., Scholfield C. N. Origin of the after-hyperpolarization that follows removal of depolarizing agents from the isolated superior cervical ganglion of the rat. Br J Pharmacol. 1972 Apr;44(4):651–671. doi: 10.1111/j.1476-5381.1972.tb07305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Halliwell J. V., Scholfield C. N. Uptake of nicotine and extracellular space markers by isolated rat ganglia in relation to receptor activation. Br J Pharmacol. 1971 May;42(1):100–113. doi: 10.1111/j.1476-5381.1971.tb07090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Hoffmann P. C., Roth L. J. 3H-Nicotine in cat superior cervical and nodose ganglia after close-arterial injection in vivo. Br J Pharmacol. 1969 Mar;35(3):406–417. doi: 10.1111/j.1476-5381.1969.tb08282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Active transport of cations in giant axons from Sepia and Loligo. J Physiol. 1955 Apr 28;128(1):28–60. doi: 10.1113/jphysiol.1955.sp005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosterlitz H. W., Lees G. M., Wallis D. I. Further evidence for an electrogenic sodium pump in a mammalian sympathetic ganglion. Br J Pharmacol. 1970 Feb;38(2):464P–465P. [PMC free article] [PubMed] [Google Scholar]
- Kosterlitz H. W., Lees G. M., Wallis D. I. Resting and action potentials recorded by the sucrose-gap method in the superior cervical ganglion of the rabbit. J Physiol. 1968 Mar;195(1):39–53. doi: 10.1113/jphysiol.1968.sp008445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter L. T., Murphy W. Electrophoresis of acetylcholine, choline and related compounds. Biochem Pharmacol. 1967 Jul 7;16(7):1386–1388. doi: 10.1016/0006-2952(67)90174-8. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr, Borzelleca J. F. On the mechanisms of 14C-nicotine distribution in rat submaxillary gland in vitro. J Pharmacol Exp Ther. 1971 Jul;178(1):180–191. [PubMed] [Google Scholar]
- Weiss G. B. Dependence of nicotine-C14 distribution and movements upon pH in frog sartorius muscle. J Pharmacol Exp Ther. 1968 Mar;160(1):135–147. [PubMed] [Google Scholar]


