Abstract
The natural occurrence of 22-hydroxylated steroids in cultured Catharanthus roseus cells and in Arabidopsis seedlings was investigated. Using full-scan gas chromatography-mass spectrometry analysis, (22S)-22-hydroxycampesterol (22-OHCR), (22S,24R)-22-hydroxyergost-4-en-3-one (22-OH-4-en-3-one), (22S,24R)-22-hydroxy-5α-ergostan-3-one (22-OH-3-one), 6-deoxocathasterone (6-deoxoCT), 3-epi-6-deoxoCT, 28-nor-22-OHCR, 28-nor-22-OH-4-en-3-one, 28-nor-22-OH-3-one, 28-nor-6-deoxoCT, and 3-epi-28-nor-6-deoxoCT were identified. Metabolic experiments with deuterium-labeled 22-OHCR were performed in cultured C. roseus cells and Arabidopsis seedlings (wild type and det2), and the metabolites were analyzed by gas chromatography-mass spectrometry. In both C. roseus cells and wild-type Arabidopsis seedlings, [2H6]22-OH-4-en-3-one, [2H6]22-OH-3-one, [2H6]6-deoxoCT, and [2H6]3-epi-6-deoxoCT were identified as metabolites of [2H6]22-OHCR, whereas the major metabolite in det2 seedlings was [2H6]22-OH-4-en-3-one. Analysis of endogenous levels of these brassinosteroids revealed that det2 accumulates 22-OH-4-en-3-one. The levels of downstream compounds were remarkably reduced compared with the wild type. Exogenously applied 22-OH-3-one and 6-deoxoCT were found to rescue det2 mutant phenotypes, whereas 22-OHCR and 22-OH-4-en-3-one did not. These results substantiate the existence of a new subpathway (22-OHCR → 22-OH-4-en-3-one → 22-OH-3-one → 6-deoxoCT) and reveal that the det2 mutant is defective in the conversion of 22-OH-4-en-3-one to 22-OH-3-one, which leads to brassinolide biosynthesis.
A biosynthetic pathway for brassinolide (C28 brassinosteroid [BR]) was elucidated by feeding labeled brassinolide intermediates to cultured cells of Catharanthus roseus, followed by analyzing the metabolites by gas chromatography-mass spectrometry (GC-MS). Parallel branched pathways, namely early and late C-6 oxidation pathways, have been proposed (Fujioka and Sakurai, 1997a, 1997b; Fujioka et al., 2000). We recently reported evidence of these pathways in Arabidopsis seedlings (Noguchi et al., 2000). Most of the steps have been demonstrated using stepwise metabolic experiments, but some steps remain uncharacterized and other possible intermediates have yet to be placed in the pathways. Furthermore, a recent biosynthesis study revealed a cross-linked pathway, the conversion of 6-deoxotyphasterol to typhasterol (Noguchi et al., 2000), and yeast functional assays also support the presence of cross-linked pathways (Shimada et al., 2001). Therefore, BR biosynthetic pathways may consist of a metabolic grid rather than two parallel branched pathways, and as yet uncharacterized pathways may function in the plant kingdom. Biosynthetic pathways of C27 BRs and C29 BRs remain undetermined. To better understand the biosynthesis of BRs in higher plants, we are attempting to elucidate the entire BR biosynthetic pathway.
We previously reported the syntheses of (22S)-22-hydroxycampesterol (22-OHCR), 6-deoxocathasterone (6-deoxoCT) and some other related compounds (Takatsuto et al., 1997; 1998). C-22-hydroxylated steroids such as 22-OHCR and 6-deoxoCT have been useful for analyzing BR biosynthesis mutants such as dwf4 and sax1 (Choe et al., 1998; 1999; Ephritikhine et al., 1999). In the past, however, most C-22 hydroxylated steroids were synthetic compounds. Later studies showed that some of these compounds occur naturally in plants. Several BRs with one hydroxyl group in the side chain have been found in plants. The first compound identified was cathasterone, which was found in cultured cells of C. roseus (Fujioka et al., 1995), and later 6-deoxoCT and 3-epi-6-deoxoCT were found in the same plant source (Fujioka et al., 2000). Naturally occurring 6-deoxoCT was also found in tomato (Lycopersicon esculentum), pea (Pisum sativum), and Arabidopsis (Bishop et al., 1999; Koka et al., 2000; Nomura et al., 2001), and some other plant species (S. Fujioka and S. Takatsuto, unpublished data). Naturally occurring 22-OHCR was found very recently in Arabidopsis (Choe et al., 2001), and naturally occurring 28-nor-6-deoxoCT was found in tomato (Yokota et al., 2001).
We originally proposed that the first step of brassinolide biosynthesis was the conversion of campesterol to campestanol (Suzuki et al., 1995). However, our later studies refined the pathway. Using Arabidopsis seedlings and cultured cells from C. roseus, we provided evidence for the biosynthetic sequence: campesterol → (24R)-ergost-4-en-3β-ol (4-en-3β-ol) → (24R)-ergost-4-en-3-one (4-en-3-one) → (24R)-5α-ergostan-3-one (3-one) → campestanol (Fujioka et al., 1997; Noguchi et al., 1999). Because there is evidence that 22-OHCR and 6-deoxoCT are present, extrapolation of the refined pathway suggests that 22-OHCR is converted to 6-deoxoCT via intermediates such as (22S,24R)-22-hydroxyergost-4-en-3-one (22-OH-4-en-3-one) and (22S,24R)-22-hydroxy-5α-ergostan-3-one (22-OH-3-one). Therefore, it is important to determine whether the C-22 oxidation subpathway exists. To test our hypothesis, we first investigated the natural occurrence of 22-hydroxylated intermediate steroids in cultured C. roseus cells and Arabidopsis seedlings. We then examined [2H6]22-OHCR metabolism in wild-type (Columbia) and det2 mutant Arabidopsis seedlings and in cultured C. roseus cells. Furthermore, we examined the rescue effects of 22-hydroxylated steroids on the det2 mutant. Here, we provide several lines of evidence for a new subpathway via early C-22 oxidation, and for a new blocked step in the det2 mutant.
RESULTS
Identification of Novel BRs in Cultured Catharanthus roseus Cells and Arabidopsis Seedlings
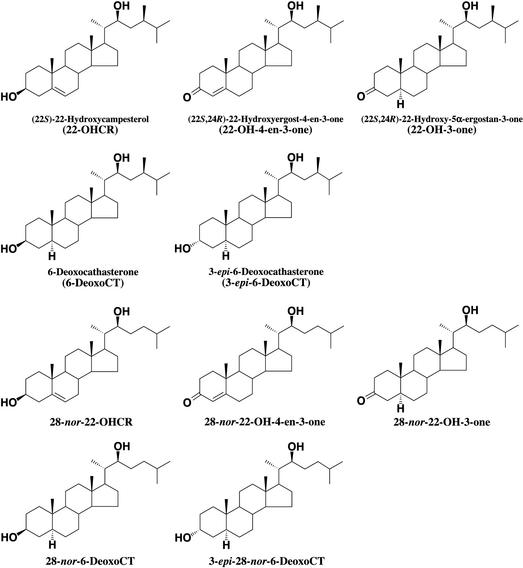
Cultured C. roseus cells (V208) in log phase were used to identify 22-hydroxylated steroids. The cells were extracted with methanol, subjected to solvent partitioning and several chromatographic steps, and finally purified by HPLC. Purified fractions were analyzed by GC-MS after conversion to a trimethylsilyl derivative. By direct comparison with authentic specimens, the natural occurrence of many 22-hydroxylated steroids was definitely established (Table I; Fig. 1). These steroids were 6-deoxoCT, 3-epi-6-deoxoCT, 22-OHCR, 22-OH-4-en-3-one, and 22-OH-3-one. In addition, naturally occurring 28-nor-6-deoxoCT, 28-nor-22-OHCR, and 28-nor-22-OH-3-one were also found in the C. roseus cells. Although authentic 3-epi-28-nor-6-deoxoCT was not available, 3-epi-28-nor-6-deoxoCT was identified in cultured C. roseus cells, based on a comparison with mass spectral data and the retention times of closely related compounds such as 6-deoxoCT, 3-epi-6-deoxoCT, and 28-nor-6-deoxoCT. Relative intensities of the fragment ions of putative 3-epi-28-nor-6-deoxoCT (C27 BR) were in good accordance with those of the corresponding fragment ions of 3-epi-6-deoxoCT (C28 BR; Table I). Relative retention times of 6-deoxoCT, 3-epi-6-deoxoCT, 28-nor-6-deoxoCT, and putative 3-epi-28-nor-6-deoxoCT also supported the conclusion.
Table I.
GC-MS data indicating the occurrence of 22-hydroxylated steroids in C. roseus and Arabidopsis
| Compound | Retention Time in GC | Characteristic Ions |
|---|---|---|
| min | m/z (relative intensity %) | |
| 6-DeoxoCT | 9.72 | 547 [M+ -15] (1.1), 477 (0.8), 387 (0.7), 345 (1), 297 (2.5), 187 (100), 97 (87) |
| 3-epi-6-DeoxoCT | 9.37 | 547 [M+ -15] (0.1), 477 (0.4), 387 (0.3), 345 (0.4), 297 (2.5), 187 (100), 97 (85) |
| 22-OHCR | 9.68 | 545 [M+ -15] (0.4), 475 (0.6), 385 (0.7), 343 (0.8), 295 (2), 187 (100), 97 (86) |
| 22-OH-4-en-3-one | 10.02 | 401 (4), 372 (14), 317 (1), 300 (2), 269 (3), 196 (77), 187 (66), 97 (100) |
| 22-OH-3-one | 9.77 | 560 [M+] (0.4), 403 (2), 374 (21), 345 (2), 313 (3), 271 (3), 187 (79), 97 (100) |
| 28-nor-6-DeoxoCT | 9.48 | 533 [M+ -15] (1.5), 477 (1), 387 (0.7), 345 (1.5), 297 (3), 173 (100), 83 (71) |
| 3-epi-28-nor-6-DeoxoCT | 9.17 | 533 [M+ -15] (0.1), 477 (0.4), 387 (0.3), 345 (0.4), 297 (2), 173 (100), 83 (61) |
| 28-nor-22-OHCR | 9.47 | 531 [M+ -15] (0.2), 475 (0.4), 385 (0.5), 343 (0.5), 295 (2), 173 (100), 83 (55) |
| 28-nor-22-OH-4-en-3-one | 9.75 | 401 (5), 372 (16), 317 (2), 300 (6), 269 (7), 196 (52), 173 (72), 83 (100) |
| 28-nor-22-OH-3-one | 9.53 | 546 [M+] (3), 531 (1), 455 (1), 374 (4), 345 (1), 271 (1), 173 (100), 83 (67) |
Figure 1.
Structures of BRs identified in cultured C. roseus cells and Arabidopsis seedlings.
In wild-type Arabidopsis seedlings, 22-OHCR, 22-OH-3-one, 6-deoxoCT, 3-epi-6-deoxoCT, 28-nor-22-OHCR, 28-nor-22-OH-3-one, 28-nor-6-deoxoCT, and 3-epi-28-nor-6-deoxoCT were identified by GC-MS, but their endogenous levels were lower in wild-type Arabidopsis seedlings than in cultured C. roseus cells (data not shown). In the det2 mutant, 22-OHCR, 22-OH-4-en-3-one, 28-nor-22-OHCR, and 28-nor-22-OH-4-en-3-one were identified by GC-MS. Among the BRs identified in this study, 22-OH-4-en-3-one, 22-OH-3-one, 28-nor-22-OHCR, 3-epi-28-nor-6-deoxoCT, 28-nor-22-OH-4-en-3-one, and 28-nor-22-OH-3-one were identified for the first time, to our knowledge, in the plant kingdom.
Metabolism of [2H6]22-OHCR in Cultured C. roseus Cells
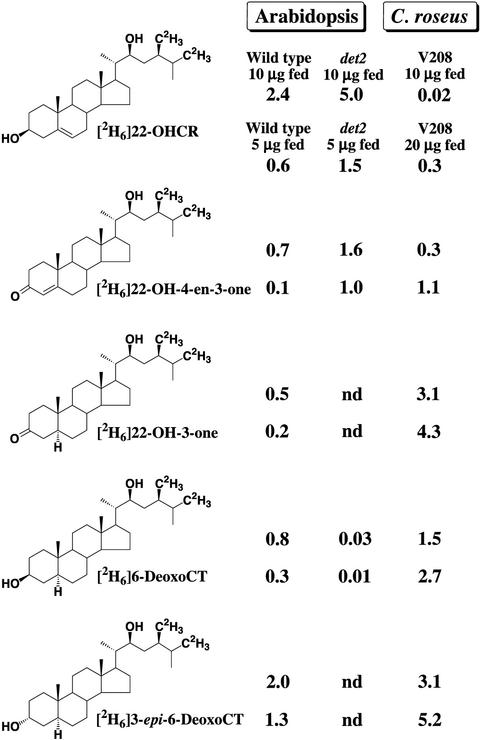
To investigate the metabolic relationship of these BRs, we examined the metabolism of [2H6]22-OHCR in cultured C. roseus cells. Ten micrograms of [2H6]22-OHCR was fed to cultured C. roseus cells, and the cells were incubated for 2 d. The culture was extracted with methanol, and the extract was purified by HPLC. The purified fractions were converted to trimethylsilyl derivatives and analyzed by GC-MS. The results are summarized in Figure 2. [2H6]22- OH-4-en-3-one (0.3 μg), [2H6]22-OH-3-one (3.1 μg), [2H6]6-deoxoCT (1.5 μg), and [2H6]3-epi-6-deoxoCT (3.1 μg) were identified as metabolites of [2H6]22-OHCR. A small amount of the substrate remained unmetabolized (0.02 μg). Conversion ratios (calculated as a percentage of the detected amount of each metabolite versus the amount of the substrate added to the culture) in this feeding were 3% [2H6]22-OH-4-en-3-one, 31% [2H3]22-OH-3-one, 15% [2H6]6-deoxoCT, and 31% [2H6]3-epi-6-deoxoCT. Along with metabolites, corresponding endogenous compounds were also identified. The ratios of endogenous compounds and corresponding metabolites were around 1:1 in all cases (22-OH-4-en-3-one, 22-OH-3-one, 6-deoxoCT, and 3-epi-6-deoxoCT). When we fed 20 μg of [2H6]22-OHCR to the cultured cells, similar results were obtained (Fig. 2). In our previous studies, the conversion of campesterol to campestanol was investigated in detail, revealing the early operating steps of BR biosynthesis in Arabidopsis and C. roseus: campesterol → 4-en-3β-ol → 4-en-3-one → 3-one → campestanol (Fujioka et al., 1997; Noguchi et al., 1999). Although 22-OH-4-en-3β-ol was not identified as an endogenous BR and a metabolite in this study, other expected intermediates such as 22-OH-4-en-3-one and 22-OH-3-one were definitely identified as endogenous BRs and metabolites of 22-OHCR. Therefore, these results strongly suggest that a biosynthetic sequence of 22-OHCR → 22-OH-4-en-3- one → 22-OH-3-one → 6-deoxoCT operates in cultured C. roseus cells.
Figure 2.
Metabolism of [2H6]22-OHCR in cultured C. roseus cells (V208) and Arabidopsis seedlings (wild type and det2 mutant). Five, 10, or 20 μg of [2H6] 22-OHCR was fed to cultured cells or seedlings for 2 d. Each value shows the amounts (μg) of unmetabolized substrate and metabolites detected. nd, Not detected (below detection limit).
Metabolism of [2H6]6-deoxoCT in Cultured C. roseus Cells
The metabolism of [2H6]6-deoxoCT was investigated in cultured C. roseus cells. Ten micrograms of [2H6]6-deoxoCT was fed to the cells, and they were incubated for 2 d. The culture was extracted with methanol, and the extract was purified by HPLC. The purified fractions were converted to trimethylsilyl derivatives and analyzed by GC-MS. [2H6]3-epi-6-deoxoCT (3.5 μg) and [2H6]22-OH-3-one (0.5 μg) were identified as metabolites of [2H6]6-deoxoCT. One-half of the substrate remained unmetabolized (5.0 μg). This study shows that 6-deoxoCT can be converted to 3-epi-6-deoxoCT and 22-OH-3-one under the experimental conditions, probably by reversal of an enzyme that primarily reduces 22-OH-3-one in vivo.
Metabolism of [2H6]22-OHCR and [2H6]6-deoxoCT in Arabidopsis Seedlings
To confirm the findings in C. roseus, we examined the metabolism of [2H6]22-OHCR in wild-type (Columbia) and det2 mutant Arabidopsis seedlings. Ten micrograms of [2H6]22-OHCR was fed to seedlings grown in one-half-strength Murashige-Skoog medium, and they were incubated for 2 d. The culture was extracted with methanol, and the extract was purified by HPLC. The purified fractions were converted to trimethylsilyl derivatives and analyzed by GC-MS. In wild-type seedlings, [2H6]22-OH-4-en-3-one (0.7 μg, 7%), [2H6]22-OH-3-one (0.5 μg, 5%), [2H6]6-deoxoCT (0.8 μg, 8%), and [2H6]3-epi-6-deoxoCT (2.0 μg, 20%) were identified as metabolites of [2H6]22-OHCR (2.4 μg, 24% unmetabolized substrate), whereas [2H6]22-OH-4-en-3-one (1.6 μg, 16%) and a small amount of [2H6]6-deoxoCT (0.03 μg, 0.3%) were identified in det2 seedlings (Fig. 2). Neither [2H6]22-OH-3-one nor [2H6]3-epi-6-deoxoCT was identified. In det2, accumulation of endogenous 22-OH-4-en-3-one was found, together with [2H6]22-OH-4-en-3-one as a major metabolite of [2H6]22-OHCR. When we fed 5 μg of [2H6]22-OHCR to wild-type (Columbia) and det2 seedlings, similar results were obtained (Fig. 2). These results strongly suggest that the biosynthetic sequence: 22-OHCR → 22-OH-4-en-3-one → 22-OH-3-one → 6-deoxoCT also operates in Arabidopsis seedlings. In addition, these metabolic studies indicate that the det2 mutant is defective in the conversion of 22-OH-4-en-3-one to 22-OH-3-one and in the conversion of 4-en-3-one to 3-one that leads to brassinolide biosynthesis (Fujioka et al., 1997; Noguchi et al., 1999).
The metabolism of [2H6]6-deoxoCT (10 μg) was investigated in wild-type seedlings. Although most of the substrate remained unmetabolized (6.3 μg), [2H6]3-epi-6-deoxoCT (0.72 μg) and [2H6]22-OH-3-one (0.36 μg) were identified as metabolites of [2H6]6-deoxoCT. Thus, in both Arabidopsis and C. roseus, 6-deoxoCT was shown to be converted to 3-epi-6-deoxoCT and 22-OH-3-one.
Biological Activity of 22-Hydroxylated Steroids in det2 Assay
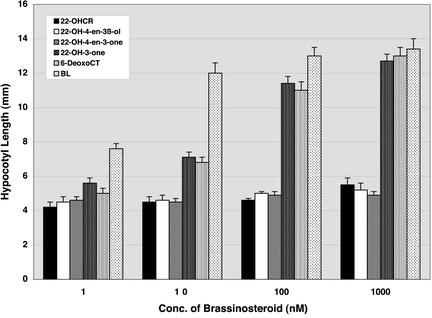
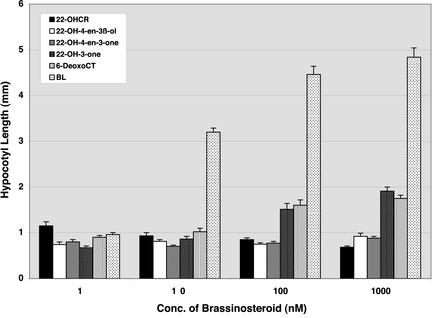
If the pathway proposed in this study operates in Arabidopsis, we would expect 22-OH-3-one and its downstream compounds to rescue the det2 mutant to the wild-type phenotype. To test this idea, we examined the effect of 22-hydroxylated steroids on hypocotyl elongation in det2 in both light and dark conditions. Although 22-OH-4-en-3β-ol was not detected in this study, we also examined the effect of this steroid as a possible precursor of 22-OH-4-en-3-one. In the dark, 22-OHCR, 22-OH-4-en-3β-ol, and 22-OH-4-en-3-one failed to rescue hypocotyl elongation in det2, whereas hypocotyl elongation was rescued by exogenous application of 22-OH-3-one and 6-deoxoCT (Fig. 3). In the light, hypocotyl length was also rescued to that of the wild type by 22-OH-3-one and 6-deoxoCT, whereas 22-OHCR, 22-OH-4-en-3β-ol, or 22-OH-4-en-3-one failed (Fig. 4). This study confirms previous findings (Ephritikhine et al., 1999), and provides new data on the biological activity of 22-hydroxylated steroids. Furthermore, in 3-week-old det2 plants, a single application of 500 ng of 22-OH-3-one or 6-deoxoCT to the shoot apex rescued mutant phenotypes in the light. Two days after application, the petiole length was clearly elongated, and 1 week later the overall morphology of det2 mutant plants was almost identical to that of the wild-type controls (data not shown). In contrast, similar application of 22-OHCR or 22-OH-4-en-3-one failed to rescue the det2 mutant phenotype. Thus, even in later developmental stages, 22-OH-3-one and 6-deoxoCT were found to be effective in rescuing the det2 mutant phenotype.
Figure 3.
Effect of 22-OHCR, (22S,24R)-22-hydroxy-ergost-4-en-3β-ol (22-OH-4-en-3β-ol), (22S,24R)-22-hydroxy-ergost-4-en-3-one (22-OH-4-en-3-one), 22-OH-3-one, 6-deoxoCT, and brassinolide (BL) on hypocotyl elongation in dark-grown det2 mutant seedlings. Each data point represents the mean of 15 replicates ± se. Hypocotyl length of the wild type (Columbia) without BR treatment was 14.4 ± 0.4 mm, whereas that of the det2 mutant was 4.4 ± 0.4 mm.
Figure 4.
Effect of 22-OHCR, (22S,24R)-22-hydroxy-ergost-4-en-3β-ol (22-OH-4-en-3β-ol), 22-OH-4-en-3-one, 22-OH-3-one, 6-deoxoCT, and brassinolide (BL) on hypocotyl elongation in light-grown det2 mutant seedlings. Each data point represents the mean of 15 replicates ± se. Hypocotyl length of the wild type (Columbia) without BR treatment was 1.6 ± 0.1 mm, whereas that of the det2 mutant was 0.8 ± 0.04 mm.
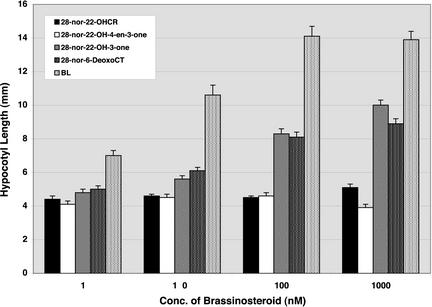
The effect of 28-nor-22-OHCR, 28-nor-22-OH-4-en-3-one, 28-nor-22-OH-3-one, and 28-nor-6-deoxoCT (C27 BRs) was also examined. Both 28-nor-22-OH-3-one and 28-nor-6-deoxoCT partially rescued the det2 mutant phenotype in the dark, whereas 28-nor-22-OHCR and 28-nor-22-OH-4-en-3-one failed to rescue the mutant phenotype (Fig. 5). In the light, the results were similar. Both 28-nor-22-OH-3-one and 28-nor-6-deoxoCT partially rescued the det2 mutant phenotype, but 28-nor-22-OHCR and 28-nor-22-OH-4-en-3-one failed. The activities of both 28-nor-22-OH-3-one and 28-nor-6-deoxoCT were one magnitude lower than corresponding C28 BRs. We also examined the effect of 28-homo-22-OHCR and 28-homo-6-deoxoCT (C29 BRs) on det2. The 28-homo-6-deoxoCT also partially rescued the det2 mutant phenotype, whereas 28-homo-22-OHCR did not. The activity of 28-homo-6-deoxoCT was comparable with that of 28-nor-6-deoxoCT (data not shown). Thus, corresponding C27 BRs and C29 BRs were also shown to be effective in rescuing det2 mutant phenotypes, although they were less active than corresponding C28 BRs. These rescue experiments provide further data to support the presence of an early C-22 oxidation pathway and the blocked step of det2.
Figure 5.
Effect of 28-nor-22-OHCR, 28-nor-22-OH-4-en-3-one, 28-nor-22-OH-3-one, 28-nor-6-deoxoCT, and brassinolide (BL) on hypocotyl elongation in dark-grown det2 mutant seedlings. Each data point represents the mean of 15 replicates ± se. Hypocotyl length of the wild type (Columbia) without BR treatment was 12.3 ± 0.4 mm, whereas that of the det2 mutant was 4.4 ± 0.1 mm.
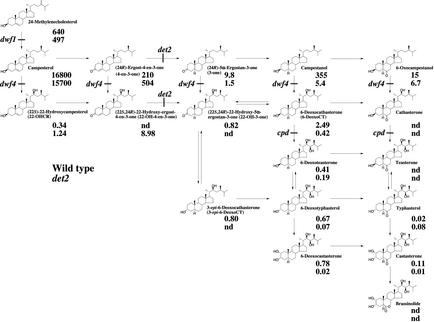
Quantification of BRs in Wild-Type and det2 Seedlings
Rescue data strongly suggested that det2 is defective in the conversion of 22-OH-4-en-3-one to 22-OH-3-one. To verify the presence of this defect, endogenous sterol and BR levels were determined by GC-MS in wild-type and det2 seedlings that were cultured in liquid medium (under the same conditions as the metabolic experiments). The results are summarized in Figure 6. Compared with the wild type, det2 mutants accumulated 22-OH-4-en-3-one, whereas endogenous levels of 22-OH-3-one and 6-deoxoCT in det2 were greatly reduced. Downstream BRs were also reduced in this mutant. Therefore, the present study provides firm evidence that the det2 mutant is defective in the conversion of 22-OH-4-en-3-one to 22-OH-3-one. Because we have already shown that the conversion of 4-en-3-one to 3-one, leading to brassinolide biosynthesis, is defective in the det2 mutant (Fujioka et al., 1997; Noguchi et al., 1999), this study expands our knowledge of blocked steps in the det2 mutant.
Figure 6.
Proposed biosynthetic pathway for brassinolide and endogenous levels of BRs in wild-type and det2 Arabidopsis seedlings. The endogenous levels are shown under the names of each compound (ng g−1 fresh weight). nd, Not detected (below detection limit).
DISCUSSION
An Early C-22 Oxidation in BR Biosynthesis
In this study, the natural occurrence of 22-OHCR, 22-OH-4-en-3-one, 22-OH-3-one, 6-deoxoCT, and 3-epi-6-deoxoCT was established by full-scan GC-MS analysis in both cultured C. roseus cells and Arabidopsis seedlings. Metabolic studies revealed that 22-OHCR is converted to 22-OH-4-en-3-one, 22-OH-3-one, 6-deoxoCT, and 3-epi-6-deoxoCT in both Arabidopsis and C. roseus, indicating that 22-OHCR is the biosynthetic origin of these compounds. In our previous studies, stepwise metabolic experiments definitely established the biosynthetic sequence of campesterol → 4-en-3β-ol → 4-en-3-one → 3-one → campestanol in both Arabidopsis and C. roseus (Noguchi et al., 1999). Therefore, it is most likely that the novel biosynthetic pathway: 22-OHCR → 22-OH-4-en-3-one → 22-OH-3-one → 6-deoxoCT operates in both Arabidopsis and C. roseus (Fig. 6). The analysis of the det2 mutant, and evaluation of the biological activity of these BRs also support this prediction. The present study also revealed the natural occurrence of the corresponding C27 22-hydroxylated steroids. This finding also predicts the in vivo operation of the biosynthetic pathway: 28-nor-22-OHCR → 28-nor-22-OH-4-en-3-one → 28-nor-22-OH-3-one → 28-nor-6-deoxoCT. Because these novel BRs are also found in several other plant species (S. Fujioka and S. Takatsuto, unpublished data), these subpathways may operate widely in the plant kingdom. Further stepwise metabolic experiments will provide conclusive evidence for these pathways.
Conversion of 6-deoxoCT to 3-epi-6-deoxoCT
In this study, the biosynthetic origin of 3-epi-6-deoxoCT was established using metabolic experiments with [2H6]6-deoxoCT, and the 6-deoxoCT to 3-epi-6-deoxoCT path is functional in both C. roseus and Arabidopsis. At the moment, it remains unknown whether 3-epi-6-deoxoCT is linked to the known BR pathway. However, from the chemical structure, it is possible to predict that 3-epi-6-deoxoCT can be converted to 6-deoxotyphasterol via C-23 hydroxylation. Metabolic experiments with labeled 3-epi-6-deoxoCT will be necessary to verify our prediction. In addition, metabolic experiments with [2H6]6-deoxoCT revealed that 6-deoxoCT is converted to 22-OH-3-one. This finding is reminiscent of the reversible conversions between teasterone and typhasterol and between 6-deoxoteasterone and 6-deoxotyphasterol. 3-Dehydroteasterone and 3-dehydro-6-deoxoteasterone were shown to be involved in the reversible conversions between teasterone and typhasterol, and between 6-deoxoteasterone and 6-deoxotyphasterol, respectively (Abe et al., 1994; Suzuki et al., 1994; Noguchi et al., 2000). Therefore, it is likely that 22-OH-3-one is involved in the conversion of 6-deoxoCT to 3-epi-6-deoxoCT, and the conversion may be reversible via 22-OH-3-one. The biosynthetic genes involved in these steps remain unknown. The molecular and enzymatic characterization of these processes in BR biosynthesis are interesting subjects for future research.
Abundance of C27, C28, and C29 BRs Is Regulated by C-22 Hydroxylation
The present study provides evidence for the natural occurrence of C28 22-hydroxylated steroids, and corresponding C27 steroids (Table I). The natural occurrence of corresponding C29 steroids was suggested by GC-MS analysis, but their levels were very low, if they were detected at all (data not shown). DWF4, which catalyzes C-22 hydroxylation (Choe et al., 1998; 2001), seems to prefer C27 and C28 steroids as substrates, but not C29 steroids. Some of the possible DWF4 substrates are the C27 steroids, cholesterol, cholest-4-en-3-one, cholestan-3-one, cholestanol, and 6-oxocholestanol, the C28 steroids, campesterol, 4-en-3-one, 3-one, campestanol, and 6-oxocampestanol, and the C29 steroids, sitosterol, sito-4-en-3-one, sito-3-one, sitostanol, and 6-oxositostanol. In general, sitosterol (C29) is the most abundant steroid in the plant kingdom, constituting 50% to 80% of the total steroids in most plant species. Of the other steroids mentioned above, all C29 steroids are predominant quantitatively, followed by C28 steroids, whereas C27 steroids are minor in several plant species including Arabidopsis (Takatsuto et al., 1999; Narumi et al., 2000; S. Fujioka and S. Takatsuto, unpublished data). In contrast, C28 BRs are major plant BRs, followed by C27 BRs, whereas C29 BRs are minor (Fujioka, 1999). In the present study, the status of 22-hydroxy steroids was found to be similar to other BRs; C28-22-hydroxy steroids were found predominantly, followed by C27-22-hydroxy steroids, whereas C29-22-hydroxy steroids were found in trace amounts or were below the detection limit. Therefore, the relative abundance of C27, C28, and C29 BRs seems to be strictly regulated by C-22 hydroxylation. A more detailed functional analysis of DWF4 would provide conclusive evidence for the above prediction.
The det2 Mutant Is Defective in the Conversion of 22-OH-4-en-3-one to 22-OH-3-one, Which Leads to Brassinolide Biosynthesis
In our previous study, we demonstrated that the det2 mutant is blocked early in BR biosynthesis (Fujioka et al., 1997). Our later study defined the blocked step as 4-en-3-one to 3-one (Noguchi et al., 1999). In this study, metabolic experiments with [2H6]22-OHCR (Fig. 2), rescue experiments with intermediates (Figs. 3 and 4), and quantification of endogenous BRs (Fig. 6) revealed that det2 is defective in the conversion of 22-OH-4-en-3-one to 22-OH-3-one. This study expanded our knowledge of the substrate specificity of plant 5α-reductase. This enzyme can catalyze multiple 5α-reductions of 3-oxo-Δ4 steroids, including 4-en-3-one and 22-OH-4-en-3-one. Because we found 28-nor-22-OH-4-en-3-one accumulation in det2 mutants, it should also be a substrate for DET2. Although metabolic experiments with labeled C27 BRs have not yet been performed, the natural occurrence of these C27 BRs suggests an in vivo biosynthetic sequence of 28-nor-22-OHCR → 28-nor-22-OH-4-en-3-one → 28-nor-22-OH-3-one → 28-nor-6-deoxoCT. Rescue experiments (Fig. 5) also support the above prediction and suggest that det2 is defective in the conversion of 28-nor-22-OH-4-en-3-one to 28-nor-22-OH-3-one.
CONCLUSIONS
This paper identifies novel BRs in C. roseus and Arabidopsis and expands our knowledge of naturally occurring BRs in the plant kingdom. In addition, we provided evidence for a novel subpathway via early C-22 oxidation, namely 22-OHCR → 22-OH-4-en-3-one → 22-OH-3-one → 6-deoxoCT. Furthermore, in addition to our previously established step, this study demonstrated that the det2 mutant is defective in the conversion of 22-OH-4-en-3-one to 22-OH-3-one.
MATERIALS AND METHODS
GC-MS Analysis
GC-MS analysis was carried out on a mass spectrometer (JMS-AM SUN200, JEOL, Tokyo) connected to a gas chromatograph (6890A, Agilent Technologies, Wilmington, DE) with a capillary column DB-5 (0.25 mm × 15 m, 0.25-μm film thickness; J&W Scientific, Folsom, CA). The analytical conditions were the same as previously described (Noguchi et al., 1999).
Plant Material
The allele of det2 used in this study is det2-1. The det2-1 allele has a nonconservative substitution of Lys for Glu at position 204 (Li et al., 1996). In this study, det2-1 is referred to as det2.
Identification of 22-Hydroxylated Compounds in Cultured C. roseus Cells and Arabidopsis Seedlings
To identify 22-hydroxylated compounds, 20 g (fresh weight) of cultured C. roseus cells (log phase, V208) and 5-week-old Arabidopsis seedlings (wild type and det2 mutant) was used. The plant materials were extracted twice with 300 mL of MeOH, and the MeOH extract was partitioned between CHCl3 and H2O. The CHCl3-soluble fraction was purified with a silica gel cartridge column (Sep-Pak Vac 2 g, Waters, Milford, MA), which was eluted with 40 mL of CHCl3. The eluent was purified with an octadecyl silane (ODS) cartridge column (Sep-Pak Plus C18, Waters), which was eluted with 20 mL of MeOH, and subjected to ODS-HPLC (Senshu Pak ODS 1151-D, 4.6 × 150 mm, Senshu Scientific, Tokyo) at a flow rate of 1 mL min−1 with 100% (v/v) MeOH. The fractions were collected at 30-s intervals (Rt of 2–6 min). Each fraction was subjected to GC-MS analysis after derivatization with N-methyl-N-trimethylsilyltrifluoroacetamide at 80°C for 30 min.
Metabolism of [2H6]22-OHCR and [2H6]6-deoxoCT in Cultured C. roseus Cells
[2H6]22-OHCR and [2H6]6-deoxoCT were chemically synthesized from [2H6]crinosterol (T. Watanabe, T. Noguchi, S. Fujioka, and S. Takatsuto, unpublished data). Cultured C. roseus cells (V208) were grown in Murashige-Skoog medium supplemented with 3% (w/v) Suc at 27°C on a shaker at 100 rpm in the dark. A MeOH solution of [2H6]22-OHCR (1 μg μL−1) or [2H6]6-deoxoCT (1 μg μL−1) was added to a 200-mL flask containing cultured cells, which were grown for 8 d (log phase) in 60 mL of Murashige-Skoog medium. After a 2-d incubation, the cultures were extracted twice with 200 mL of MeOH. The MeOH extract was partitioned between CHCl3 and H2O, and the CHCl3-soluble fraction was purified and analyzed using the method described above. The metabolite content was roughly estimated by comparing the peak areas of predominant ions with those of authentic samples.
Metabolism of [2H6]22-OHCR and [2H6]6-deoxoCT in Arabidopsis Seedlings
Before the feeding experiments, 7-d-old Arabidopsis seedlings (20 wild type and 50 det2) were transferred to a 200-mL flask containing 30 mL of one-half-strength Murashige-Skoog medium supplemented with 1% (w/v) Suc. Seven days after the transfer, MeOH solution containing [2H6]22-OHCR (1 μg μL−1) or [2H6]6-deoxoCT (1 μg μL−1) was added. The seedlings were incubated for 2 d at 22°C in the light on a shaker (120 rpm) and then extracted with MeOH. Metabolites were purified and analyzed by the method described above.
Quantification of Sterols and BRs in Arabidopsis Seedlings
Seven-day-old Arabidopsis seedlings were transferred to a 200-mL flask containing 30 mL of one-half-strength Murashige-Skoog medium supplemented with 1% (w/v) Suc at 22°C in the light on a shaker (120 rpm). After a 9-d culture under the same growth conditions, the seedlings were harvested. The plants (20 g fresh weight equivalent) were extracted twice with 200 mL of MeOH, and [2H6]brassinolide, [2H6]castasterone, [2H6]typhasterol, [2H6]teasterone, [2H6]cathasterone, [2H6]6-deoxocastasterone, [2H6]6-deoxotyphasterol, and [2H6]6-deoxoteasterone (each 1 ng g−1 fresh weight) were added to the extract as internal standards. BR purification and quantification were carried out according to the method described by Noguchi et al. (1999).
To analyze 6-deoxoCT, other 22-hydroxylated steroids, and sterols, plants (1 g fresh weight equivalent) were extracted twice with 40 mL of MeOH-CHCl3 (4:1). [2H7]24-methylenecholesterol (1 μg), [2H6]campesterol (20 μg), [2H6]4-en-3-one (500 ng), [2H6]3-one (50 ng), [2H6]campestanol (500 ng), [2H6]6-oxocampestanol (50 ng), and [2H6]6-deoxoCT (5 ng) were added to the extract as internal standards, and the extract was partitioned three times between CHCl3 and water. The CHCl3-soluble fraction was purified by a silica gel cartridge (Sep-Pak Vac Silica, 2 g, Waters) with 40 mL of CHCl3. The eluent was subjected to ODS-HPLC (Senshu Pak ODS 1151-D, 4.6 × 150 mm, Senshu Scientific) at a flow rate of 1 mL min−1 with 100% (v/v) MeOH. Fractions were collected every 0.5 min (Rt, 2.5–18 min). Each fraction was trimethylsilylated and analyzed by GC-MS. The endogenous levels of 24-methylenecholesterol, campesterol, 4-en-3-one, 3-one, campestanol, and 6-oxocampestanol were calculated from the peak area ratios of molecular ions of the internal standard and the endogenous sterol. The endogenous level of 6-deoxoCT was calculated from the peak area ratios of m/z 193 for the internal standard and m/z 187 for the endogenous one. The endogenous levels of other 22-hydroxylated compounds were calculated from the peak area ratios of m/z 193 of [2H6]6-deoxoCT and m/z 187 for the endogenous ones.
Rescue Experiments with Various 22-Hydroxylated Steroids and Brassinolide
Various 22-hydroxylated steroids and brassinolide were used for rescue experiments with the det2 mutant. Twenty-five seeds were sown on one-half-strength Murashige-Skoog agar medium with or without BRs, supplemented with 1% (w/v) Suc (8 mL of medium per petri dish, 50 × 9 mm). They were allowed to grow under continuous light or in the dark at 22°C for 8 d. Fifteen seedlings were chosen randomly and their hypocotyl lengths were measured. The wild type (Columbia) was used as a control. In a different series of experiments, det2 and wild-type plants were grown on soil for 3 weeks under continuous light at 22°C. Five hundred nanograms per 1 μL MeOH solution (22-OHCR, 22-OH-4-en-3β-ol, 22-OH-4-en-3-one, 22-OH-3-one, and 6-deoxoCT) was applied to the shoot apex of each plant, and they were allowed to grow under the same conditions.
ACKNOWLEDGMENTS
We thank Makoto Kobayashi and Masayo Sekimoto for their excellent technical assistance.
Footnotes
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.008722.
LITERATURE CITED
- Abe H, Honjo C, Kyokawa Y, Asakawa S, Natsume M, Narushima M. 3-Oxoteasterone and the epimerization of teasterone: identification of lily anthers and Distylium-racemosum leaves and its biotransformation into typhasterol. Biosci Biotechnol Biochem. 1994;58:986–989. [Google Scholar]
- Bishop GJ, Nomura T, Yokota T, Harrison K, Noguchi T, Fujioka S, Takatsuto S, Jones JDG, Kamiya Y. The tomato DWARF enzyme catalyses C-6 oxidation in brassinosteroid biosynthesis. Proc Natl Acad Sci USA. 1999;96:1761–1766. doi: 10.1073/pnas.96.4.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S, Dilkes BP, Fujioka S, Takatsuto S, Sakurai A, Feldmann KA. The DWF4 gene of Arabidopsis encodes a cytochrome P450 that mediates multiple 22α-hydroxylation steps in brassinosteroid biosynthesis. Plant Cell. 1998;10:231–243. doi: 10.1105/tpc.10.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S, Dilkes BP, Gregory BD, Ross AS, Yuan H, Noguchi T, Fujioka S, Takatsuto S, Tanaka A, Yoshida S et al. The Arabidopsis dwarf1 mutant is defective in the conversion of 24-methylenecholesterol to campesterol in brassinosteroid biosynthesis. Plant Physiol. 1999;119:897–907. doi: 10.1104/pp.119.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S, Fujioka S, Noguchi T, Takatsuto S, Yoshida S, Feldmann KA. Overexpression of DWARF4 in the brassinosteroid biosynthetic pathway results in increased vegetative growth and seed yield in Arabidopsis. Plant J. 2001;26:573–582. doi: 10.1046/j.1365-313x.2001.01055.x. [DOI] [PubMed] [Google Scholar]
- Ephritikhine G, Pagant S, Fujioka S, Takatsuto S, Lapous D, Caboche M, Kendrick RE, Barbier-Brygoo H. The sax1 mutation defines a new locus involved in the brassinosteroid biosynthesis pathway in Arabidopsis thaliana. Plant J. 1999;18:315–320. doi: 10.1046/j.1365-313x.1999.00455.x. [DOI] [PubMed] [Google Scholar]
- Fujioka S. Natural occurrence of brassinosteroids in the plant kingdom. In: Sakurai A, Yokota T, Clouse SD, editors. Brassinosteroids: Steroidal Plant Hormones. Tokyo: Springer-Verlag; 1999. pp. 21–45. [Google Scholar]
- Fujioka S, Inoue T, Takatsuto S, Yanagisawa T, Yokota T, Sakurai A. Identification of a new brassinosteroid, cathasterone, in cultured cells of Catharanthus roseus as a biosynthetic precursor of teasterone. Biosci Biotechnol Biochem. 1995;59:1543–1547. [Google Scholar]
- Fujioka S, Li J, Choi Y-H, Seto H, Takatsuto S, Noguchi T, Watanabe T, Kuriyama H, Yokota T, Chory J et al. The Arabidopsis deetiolated2 mutant is blocked early in brassinosteroid biosynthesis. Plant Cell. 1997;9:1951–1962. doi: 10.1105/tpc.9.11.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka S, Noguchi T, Watanabe T, Takatsuto S, Yoshida S. Biosynthesis of brassinosteroids in cultured cells of Catharanthus roseus. Phytochemistry. 2000;53:549–553. doi: 10.1016/s0031-9422(99)00582-8. [DOI] [PubMed] [Google Scholar]
- Fujioka S, Sakurai A. Brassinosteroids. Nat Prod Rep. 1997a;14:1–10. doi: 10.1039/np9971400001. [DOI] [PubMed] [Google Scholar]
- Fujioka S, Sakurai A. Biosynthesis and metabolism of brassinosteroids. Physiol Plant. 1997b;100:710–715. [Google Scholar]
- Koka CV, Cerny RE, Gardner RG, Noguchi T, Fujioka S, Takatsuto S, Yoshida S, Clouse SD. A putative role for the tomato genes DUMPY and CURL-3 in brassinosteroid biosynthesis and response. Plant Physiol. 2000;122:85–98. doi: 10.1104/pp.122.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Nagpal P, Vitart V, McMorris TC, Chory J. A role for brassinosteroids in light-dependent development of Arabidopsis. Science. 1996;272:398–401. doi: 10.1126/science.272.5260.398. [DOI] [PubMed] [Google Scholar]
- Narumi Y, Gotoh C, Noguchi T, Fujioka S, Yokota T, Takatsuto S. Identification of sterols and steroidal 3-ones in the seeds of Echinochloa frumentacea. J Jpn Oil Soc. 2000;49:367–371. [Google Scholar]
- Noguchi T, Fujioka S, Choe S, Takatsuto S, Tax FE, Yoshida S, Feldmann KA. Biosynthetic pathways of brassinolide in Arabidopsis. Plant Physiol. 2000;124:201–209. doi: 10.1104/pp.124.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi T, Fujioka S, Takatsuto S, Sakurai A, Yoshida S, Li J, Chory J. Arabidopsis det2 is defective in the conversion of (24R)-24-methylcholest-4-en-3-one to (24R)-24-methyl-5α-cholestan-3-one in brassinosteroid biosynthesis. Plant Physiol. 1999;120:833–839. doi: 10.1104/pp.120.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Sato T, Bishop GJ, Kamiya Y, Takatsuto S, Yokota T. Accumulation of 6-deoxocathasterone and 6-deoxocastasterone in Arabidopsis, pea and tomato is suggestive of common rate-limiting steps in brassinosteroid biosynthesis. Phytochemistry. 2001;57:171–178. doi: 10.1016/s0031-9422(00)00440-4. [DOI] [PubMed] [Google Scholar]
- Shimada Y, Fujioka S, Miyauchi N, Kushiro M, Takatsuto S, Nomura T, Yokota T, Kamiya Y, Bishop GJ, Yoshida S. Brassinosteroid-6-oxidases from Arabidopsis and tomato catalyze multiple C-6 oxidations in brassinosteroid biosynthesis. Plant Physiol. 2001;126:770–779. doi: 10.1104/pp.126.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Inoue T, Fujioka S, Saito T, Takatsuto S, Yokota T, Murofushi N, Yanagisawa T, Sakurai A. Conversion of 24-methylcholesterol to 6-oxo-24-methylcholestanol, a putative intermediate of the biosynthesis of brassinosteroids, in cultured cells of Catharanthus roseus. Phytochemistry. 1995;40:1391–1397. [Google Scholar]
- Suzuki H, Inoue T, Fujioka S, Takatsuto S, Yanagisawa T, Yokota T, Murofushi N, Sakurai A. Possible involvement of 3-dehydroteasterone in the conversion of teasterone to typhasterol in cultured cells of Catharanthus roseus. Biosci Biotechnol Biochem. 1994;58:1186–1188. [Google Scholar]
- Takatsuto S, Kosuga N, Abe B, Noguchi T, Fujioka S, Yokota T. Occurrence of potential brassinosteroid precursor steroids in seeds of wheat and foxtail millet. J Plant Res. 1999;112:27–33. [Google Scholar]
- Takatsuto S, Kuriyama H, Noguchi T, Suganuma H, Fujioka S, Sakurai A (1997) Synthesis of cathasterone and its related putative intermediates in brassinolide biosynthesis. J Chem Res (S) 418–419
- Takatsuto S, Watanabe T, Gotoh C, Kuriyama H, Noguchi T, Fujioka S (1998) A convenient synthesis of (22S)-22-hydroxycampesterol and some related steroids. J Chem Res (S) 176–177
- Yokota T, Sato T, Takeuchi Y, Nomura T, Uno K, Watanabe T, Takatsuto S. Roots and shoots of tomato produce 6-deoxo-28-norcathasterone, 6-deoxo-28-nortyphasterol and 6-deoxo-28-norcastasterone, possible precursors of 28-norcastasterone. Phytochemistry. 2001;58:233–238. doi: 10.1016/s0031-9422(01)00237-0. [DOI] [PubMed] [Google Scholar]