Abstract
Emerging evidence suggests that transforming growth factor-β (TGF-β) is an important mediator of diabetic nephropathy. We showed previously that short-term treatment with a neutralizing monoclonal anti-TGF-β antibody (αT) in streptozotocin-diabetic mice prevents early changes of renal hypertrophy and increased matrix mRNA. To establish that overactivity of the renal TGF-β system mediates the functional and structural changes of the more advanced stages of nephropathy, we tested whether chronic administration of αT prevents renal insufficiency and glomerulosclerosis in the db/db mouse, a model of type 2 diabetes that develops overt nephropathy. Diabetic db/db mice and nondiabetic db/m littermates were treated intraperitoneally with αT or control IgG, 300 μg three times per week for 8 wk. Treatment with αT, but not with IgG, significantly decreased the plasma TGF-β1 concentration without decreasing the plasma glucose concentration. The IgG-treated db/db mice developed albuminuria, renal insufficiency, and glomerular mesangial matrix expansion associated with increased renal mRNAs encoding α1(IV) collagen and fibronectin. On the other hand, treatment with αT completely prevented the increase in plasma creatinine concentration, the decrease in urinary creatinine clearance, and the expansion of mesangial matrix in db/db mice. The increase in renal matrix mRNAs was substantially attenuated, but the excretion of urinary albumin factored for creatinine clearance was not significantly affected by αT treatment. We conclude that chronic inhibition of the biologic actions of TGF-β with a neutralizing monoclonal antibody in db/db mice prevents the glomerulosclerosis and renal insufficiency resulting from type 2 diabetes.
Diabetic nephropathy, a common complication in patients with either type 1 or type 2 diabetes mellitus, has long been recognized to cause severe morbidity and mortality. The renal structural alterations in susceptible patients are characterized by the early appearance of hypertrophy in glomerular and tubular components, the subsequent development of thickened glomerular and tubular basement membranes (but with enhanced glomerular permeability to albumin), and the progressive accumulation of extracellular matrix components in the glomerular mesangium and tubulointerstitium. Glomerulosclerosis and tubulointerstitial fibrosis are the structural hallmarks of advanced diabetic nephropathy with renal insufficiency (1, 2).
In experimental animal models of diabetic kidney disease, there is increased gene expression and protein synthesis of several extracellular matrix components, such as type IV collagen, laminin, and fibronectin, in renal cortical specimens and isolated glomeruli (reviewed in ref. 3). In vitro studies provide evidence that high ambient glucose increases the synthesis of extracellular matrix in all glomerular cell types (4–7). In general, hyperglycemia exerts its adverse effects in the kidney by activating enzymatic pathways for glucose metabolism (8–11), nonenzymatically glycosylating circulating or tissue proteins (12, 13), and altering the responsiveness to vasoactive hormones or locally generated cytokines or growth factors (3, 14, 15).
Several in vitro and in vivo studies implicate transforming growth factor-β (TGF-β) in the pathogenesis of diabetic kidney disease (reviewed in ref. 16). This cytokine acts in autocrine or paracrine fashion to elicit profound effects on cell growth and extracellular matrix accumulation. Production of the TGF-β isoforms (-1, -2, and -3) and expression of the TGF-β receptors (types I, II, and III) are typically found in renal cell types exposed to diabetic conditions. TGF-β1 mRNA and protein levels are significantly increased in the kidney cortex of type 1 diabetic animals such as the spontaneously diabetic BioBreeding rat, the nonobese diabetic mouse (17), and the streptozotocin (STZ) diabetic rat (18–20) or mouse (21). The type II receptor for TGF-β is concomitantly up-regulated in the kidney of STZ diabetic mice (21) and type 2 diabetic db/db mice (22). Patients with diabetic nephropathy also demonstrate up-regulated TGF-β1 mRNA and protein in the glomerulus (23, 24) and tubulointerstitium (23). In fact, the diabetic kidney is capable of net TGF-β1 synthesis as seen in our study demonstrating significantly greater TGF-β1 levels in the renal vein vs. the artery and increased TGF-β1 protein excreted in the urine of diabetic compared with nondiabetic patients (25).
The biologic effects of the TGF-β system in kidney cells most closely resemble those of hyperglycemia, which include cellular hypertrophy and stimulation of extracellular matrix production (26). We have reported that tubular epithelial cells, glomerular mesangial cells, and interstitial fibroblasts (27–30) significantly increase their TGF-β1 expression and bioactivity when cultured in high ambient glucose. Additionally, neutralizing anti-TGF-β antibodies prevent the stimulation of collagen biosynthesis by high glucose in tissue culture (7, 30). To assess the functional role of the TGF-β system in the early manifestations of diabetic renal disease, we previously evaluated the feasibility and efficacy of administering a neutralizing anti-TGF-β antibody over a 9-day period to STZ diabetic mice (21). We found increased production of TGF-β1 and up-regulation of the TGF-β type II receptor in the diabetic kidney, preceding the onset of renal hypertrophy. Inhibition of TGF-β activity prevented glomerular enlargement and attenuated the increase in the mRNAs encoding α1(IV) collagen and fibronectin (21).
However, none of the cited studies has established a direct causal link between increased activity of the renal TGF-β system and the more advanced clinical manifestations of diabetic nephropathy such as glomerulosclerosis, proteinuria, and renal insufficiency. To address this question, we have resorted to an experimental model of overt diabetic nephropathy that closely resembles the human disease. We therefore tested the effectiveness of long-term administration of neutralizing anti-TGF-β antibodies in preventing glomerulosclerosis and renal insufficiency in diabetic db/db mice. An 8-wk treatment period significantly depressed plasma TGF-β1 levels and prevented renal insufficiency, excess matrix expression, and expansion in the glomerular mesangium. This is, to our knowledge, the first proof-of-concept study to provide strong support for the premise that chronic inhibition of the biologic actions of TGF-β in the kidney effectively prevents renal failure resulting from diabetes.
Methods
Experimental Animals.
The db/db mouse, lacking the hypothalamic leptin receptor (31), is a model of type 2 (noninsulin-dependent) diabetes mellitus that exhibits hyperglycemia, hyperinsulinemia, and hyperleptinemia associated with hyperphagia and obesity manifesting around 4–7 wk after birth (32). The therapeutic intervention was started at 8 wk of age because 100% of the db/db genotype become frankly hyperglycemic (12). One group of db/db mice (n = 9, db/db-αT) and one group of nondiabetic db/m littermates (n = 9, db/m-αT) were treated with a murine monoclonal antibody (αT) that neutralizes all three mammalian TGF-β isoforms (-β1, -β2, and -β3) (33). As control, a group of db/db mice (n = 9, db/db-IgG) and another group of db/m mice (n = 9, db/m-IgG) received isotype-matched irrelevant murine IgG. Antibody was administered intraperitoneally (300 μg each injection) three times per week over an 8-wk period, and the animals were killed after 16 wk of age. The antibody treatment regimen was selected on the basis of previous experiments (12, 34) that involved chronic administration of monoclonal antibodies and our experiment, which showed that circulating TGF-β1 levels were significantly reduced 48 h after i.p. antibody administration (21). Individual mice were placed in metabolic cages to obtain 24-h urine collections. Plasma glucose and creatinine and urinary creatinine were measured by colorimetric assays (Sigma).
Antibody Preparation.
The hybridoma 2G7 cell line, which produces αT (33), was kindly provided by Brian Fendly (Genentech, South San Francisco, CA). Large amounts of antibody were purified by affinity chromatography on Protein G (Charles River Breeding Laboratories) from the ascitic fluid of naive mice injected with 2G7 cells. Reactivity of αT was evaluated by using a mink lung bioassay as previously described (29, 35). Antibody preparations were sterile filtered and administered intraperitoneally in buffered saline. The control isotype-matched IgG, which does not react with TGF-β, was subjected to the same procedure.
TGF-β1 ELISA.
Plasma was obtained as previously described (36). Retroorbital blood (200 μl) was collected in a siliconized microfuge tube containing 20 μl of anticoagulant (3.2% sodium citrate/15 mM theophylline/3.7 mM adenosine/0.2 mM dipyridamole; American BioProducts, Parsippany, NJ). Blood was stored on ice and centrifuged within 30 min at 1,000 × g at 4°C. Half the supernatant was transferred to another microfuge tube by using a siliconized pipette tip, avoiding the interface where platelets are present, and spun for 2 min at maximum speed to sediment the platelets. The supernatant was frozen at −20°C until assayed by a sandwich ELISA kit (Genzyme). Acid activation of plasma was required to convert latent into active TGF-β1 that can be recognized by the ELISA antibody. Total (latent + active) TGF-β1 in a sample was compared with known standards and read as nanograms/milliliters. The αT administered to mice did not interfere with the ELISA antibody (i.e., ELISA readings were not affected by the addition of αT to control plasma at dilutions of 1:10, 1:100, and 1:1,000 as compared with vehicle).
Urine Albumin Assay.
Albumin concentrations in 24-h urine samples were measured with a competitive ELISA (12) in which mouse albumin in the soluble phase competes with albumin immobilized onto microtiter wells (250 ng/well) for binding to horseradish peroxidase-conjugated antialbumin antibody (Exocell, Philadelphia, PA). To avoid errors from an incomplete urine collection, albumin excretion was normalized to urine creatinine.
Northern Analysis.
Kidney RNA (20 μg) was prepared, electrophoresed, transferred to nylon membranes, UV crosslinked, and prehybridized as described (17). Murine TGF-β type II receptor, α1(IV) collagen, and fibronectin cDNA probes were synthesized by PCR by using mouse kidney cDNA as template followed by cloning in pCRII TA (Invitrogen). Nucleotide sequencing of the probes confirmed their identities. The cDNA inserts were labeled with 32P-deoxycytidine 5′-triphosphate (3,000 Ci/mmol, Amersham Pharmacia) by using a DNA labeling kit (Amersham). Procedures for membrane hybridization, high-stringency washing, and autoradiography were as described (12, 17). Blots were stripped and rehybridized with a probe encoding mouse ribosomal protein L32 (mrpL32) to account for loading and transfer variations. Exposed films were scanned with a laser densitometer, and RNA levels relative to those of mrpL32 were calculated.
Immunoblot Analysis.
Frozen kidney was homogenized in a lysis buffer containing 50 mM Tris⋅HCl (pH 8.0), 150 mM NaCl, 1% Nonidet P-40, 0.1% SDS, 1 mM EDTA, 0.5 mM DTT, 1 mM PMSF, and 5 μg/ml each of aprotinin and leupeptin. Protein was quantitated by an assay kit (Bio-Rad), electrophoresed (30 μg) with SDS/12% PAGE, transferred to nitrocellulose membrane, and blocked with 5% nonfat milk. The membranes were incubated with rabbit antibody directed against TGF-β type II receptor (Santa Cruz Biotechnology) at room temperature for 3 h. A horseradish peroxidase-conjugated anti-rabbit IgG was used to detect bands (72 kDa) by using the enhanced chemiluminescence detection system (Amersham). Equal loading and transfer were assessed by staining with Ponceau S. Specificity was determined by a blocking peptide (Santa Cruz Biotechnology). Densitometric analysis was performed as described above.
TGF-β1 Riboprobe Preparation and in Situ Hybridization.
The mouse TGF-β1 cDNA (17) was subcloned into pcDNA3 vector (Invitrogen) and linearized with NotI for antisense and HindIII for sense orientation. The linearized plasmids were gel isolated, and the transcription reaction for nonisotopic labeling of riboprobe was performed according to the manufacturer's instruction (Boehringer Mannheim). The mixture of digoxigenin labeling (50 μl) included 1 μg template cDNA, 2 μl NTP labeling mixture, 2 μl transcription buffer, 1 μl RNase inhibitor, and 2 μl T7 or SP6 polymerase. Transcription was performed for 2 h at 37°C followed by digestion with 2 μl RNase-free DNase for 15 min at 37°C. The riboprobe was purified with spin columns. For in situ hybridization, frozen kidney sections (5 μm) were overlaid with 30 μl of hybridization buffer containing the labeled RNA probe and incubated at 58°C overnight in a humid chamber. After hybridization, sections were washed once in 2 × SSC at room temperature, once in 2 × SSC at 65°C, and once in 0.1 × SSC at 65°C. Slides were equilibrated with buffer I (100 mM Tris⋅HCl/150 mM NaCl, pH 7.5) for 5 min. Antidigoxigenin antibody conjugated to alkaline phosphatase (1:5,000) was applied to the slides and incubated in a humid chamber for 2 h at room temperature. After two washes in buffer I for 15 min each, the sections were equilibrated with developing buffer (100 mM Tris/100 mM NaCl/50 mM MgCl2, pH 9.5) for 5 min. The color reaction was developed by NBT/BCIP (Boehringer) according to the manufacturer's instructions. Quantitative evaluation of the hybridization signal was performed by computer program, image-pro plus 3.0 (Media Cybernetics, Silver Spring, MD).
Glomerular Histology and Morphometry.
Portions of the renal cortex were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned (3 μm), and stained with periodic acid/Schiff reagent (PAS). Coded sections were read by an observer unaware of the experimental protocol. Thirty glomeruli were randomly selected from each animal, and the extent of extracellular mesangial matrix was identified by PAS-positive material in the mesangium by using a digital planimeter and factored by the glomerular tuft area (12).
Statistical Analysis.
Data are presented as mean ± SE, with n the number of animals. Groups were compared by ANOVA, and individual groups were compared by the Mann–Whitney test for unpaired analysis; P < 0.05 was considered significant.
Results
Glomerular TGF-β1 mRNA Localization.
To address the question of whether TGF-β1 expression is preferentially up-regulated within the glomerular compartment of the diabetic db/db mouse, in conjunction with the appearance of glomerulosclerosis, we resorted to nucleic acid in situ hybridization studies. Fig. 1 shows a selective increase in TGF-β1 mRNA by around 5-fold within the glomerular tuft (consistent with a mesangial pattern) in db/db mice compared with control db/m mice. In our previous study using Northern analysis of whole-kidney RNA (22), we demonstrated a slight decrease in total TGF-β1 mRNA, but this represented a predominant contribution by the tubular epithelium because glomeruli comprise less than 10% of the kidney parenchyma. On the other hand, expression of the TGF-β type II receptor in the db/db mouse kidney is up-regulated in both tubules and glomeruli (not shown). By Northern analysis and immunoblotting (Fig. 2), the diabetic state stimulates TGF-β type II receptor mRNA and protein production in whole-kidney specimens by more than 2-fold, confirming our previous study (22). Thus the kidney in the db/db mouse is characterized by a selective increase in the glomerular mesangial TGF-β1 mRNA and a generalized (tubular and glomerular) increase in the TGF-β type II receptor mRNA.
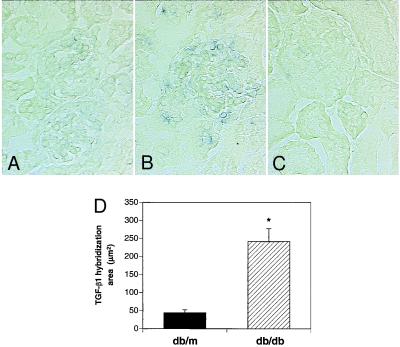
Figure 1.
Increased glomerular TGF-β1 mRNA in diabetic db/db mice. In situ hybridization of kidney sections: (A) antisense TGF-β1 riboprobe in 16-wk-old control db/m mouse showing minimal hybridization signal; (B) antisense TGF-β1 riboprobe in 16-wk-old diabetic db/db mouse showing increased hybridization signal in the mesangial area; (C) sense TGF-β1 riboprobe used as negative control in diabetic db/db mouse showing absence of hybridization signal; and (D) quantitative analysis of area of TGF-β1 hybridization signal in db/db vs. db/m mice. Twenty glomeruli were studied in each group, and the values presented are the mean ± SE for four mice in each group. *, P < 0.05 vs. db/m.
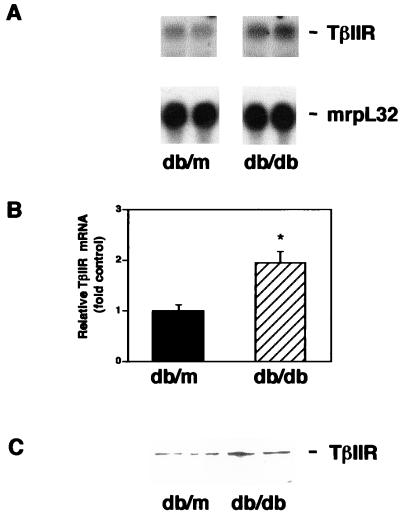
Figure 2.
Up-regulation of renal TGF-β type II receptor in db/db mice. (A) Representative Northern blot showing increased TGF-β type II receptor mRNA in db/db mouse kidney compared with db/m mouse kidney. (B) Summary of densitometric analyses of receptor/mrpL32 mRNA ratios (mean ± SE, n = 5 for each group). The relative ratio in the db/m group is assigned a value of 1. *, P < 0.05 vs. db/m. (C) Representative immunoblot showing increased TGF-β type II receptor protein in db/db mouse kidney compared with db/m mouse kidney.
Clinical Characteristics.
The baseline and final characteristics of the four groups of mice are presented in Table 1. One mouse from each IgG group died before the completion of the study, and they were not included in the analysis. As expected, the initial (age 8 wk) and final (age 16 wk) body weights of db/db mice were significantly greater than those of db/m controls. The increment in weight over time in the db/db mice was slightly less in the αT-treated group than in the IgG-treated group. The diabetic mice remained hyperglycemic throughout the experimental period, and the plasma glucose concentration was slightly higher in the αT-treated group (Table 1). Because of glycosuria, urine volumes were markedly increased in both db/db groups. Kidney weights were significantly greater in db/db compared with db/m mice. By the end of the study, the αT-treated db/db mice appeared to have less renal hypertrophy than the IgG-treated db/db mice, given the significantly lower kidney weights in the αT-treated group (Table 1). However, the average kidney-to-body weight ratios remained unchanged between the αT- and IgG-treated groups, and the ratio was predictably lower in the db/db vs. the db/m groups because the diabetic animals were much heavier. As expected, the obese db/db mice had considerably greater liver weights than the db/m mice, but the αT-treated db/db mice had less liver weight gain compared with the IgG-treated db/db mice (Table 1). There were no significant differences in heart weight among the four groups (not shown), which may indicate that chronic hypertension did not develop.
Table 1.
Parameters of the experimental groups of mice
| db/m–IgG (n = 8) | db/m–αT (n = 9) | db/db–IgG (n = 8) | db/db–αT (n = 9) | |
|---|---|---|---|---|
| Body wt. initial, g | 20.4 ± 0.39 | 21.2 ± 0.89 | 37.1 ± 0.56* | 37.0 ± 0.71* |
| Body wt. final, g | 23.0 ± 0.30 | 23.8 ± 1.06 | 49.6 ± 0.66* | 46.3 ± 0.97† |
| Plasma glucose, mg/dl | 116 ± 7 | 98 ± 20 | 430 ± 26* | 612 ± 16† |
| Urine volume, ml/day | 0.53 ± 0.13 | 0.41 ± 0.13 | 4.33 ± 0.56* | 3.83 ± 0.81* |
| Kidney wt, mg | 127 ± 2 | 138 ± 4 | 194 ± 6* | 176 ± 4*† |
| Kidney wt/body wt, × 10−3 | 5.5 ± 0.1 | 5.8 ± 0.3 | 3.9 ± 0.2* | 3.7 ± 0.1* |
| Liver wt, mg | 1022 ± 39 | 1057 ± 65 | 2551 ± 75* | 2269 ± 81*† |
| Plasma TGF-β1, % control | 100 ± 5 | 44 ± 9* | 134 ± 14 | 49 ± 9*† |
| Plasma creatinine, mg/dl | 0.34 ± 0.02 | 0.38 ± 0.06 | 0.62 ± 0.06* | 0.34 ± 0.04† |
Mice (nondiabetic db/m or diabetic obese db/db) were treated for 8 wk with either control IgG or neutralizing anti-TGF-β antibody (αT). Unless specified, parameters were recorded at the end of the experimental period (16 wk of age). wt., weights. *, P < 0.05 vs. db/m–IgG;
, P < 0.05 vs. db/db–IgG.
Basal plasma TGF-β1 levels in the IgG-treated groups tended to be higher, but not significantly, in the db/db mice than in the db/m mice (Table 1). Confirming the efficacy of the treatment protocol, the circulating TGF-β1 levels measured 48 h after the last injection of αT were reduced by 70% in the db/db mice and 50% in the db/m mice (Table 1). TGF-β2 and TGF-β3 could not be measured in the plasma, as these isoforms are known to be undetectable in the circulation (36).
Renal Gene Expression of Matrix Molecules.
Renal α1(IV) collagen mRNA was significantly increased in the IgG-treated diabetic group compared with the respective nondiabetic group (Fig. 3A). However, treatment with αT virtually prevented the 8-fold increase in type IV collagen gene expression in the db/db mice (Fig. 3B). Similarly, renal fibronectin mRNA levels were increased more than 4-fold in control db/db mice but were reduced to normal values by treatment with αT (Fig. 3 C and D).
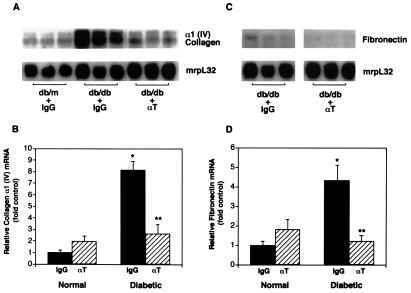
Figure 3.
Kidney matrix gene expression in diabetic mice treated with anti-TGF-β antibody. A representative Northern blot of kidney RNA probed with α1(IV) collagen cDNA (A) and fibronectin cDNA (C) and then with ribosomal mrpL32. Each lane represents RNA from individual mice (db/m or db/db), treated with either IgG or anti-TGF-β antibody (αT). Summary of densitometric analyses of α1(IV) collagen/mrpL32 mRNA ratios (B) and fibronectin/mrpL32 mRNA ratios (D) in the four treatment groups (mean ± SE, n = 8 for each IgG group and n = 9 for each αT group). The relative mRNA ratio in the normal-IgG group is assigned a value of 1. *, P < 0.05 vs. normal-IgG, **, P < 0.05 vs. diabetic-IgG.
Glomerular Histology.
At the end of the study, the glomeruli of db/db mice demonstrated increased accumulation of PAS-positive matrix in the mesangium compared with glomeruli of db/m mice (Fig. 4 C vs. A). Consistent with previous observations in the db/db mouse, there were only slight pathological changes in the tubulointerstitial compartment (not shown). However, the db/db mice treated with αT had relatively normal appearing glomeruli (and tubulointerstitium) with minimal mesangial matrix expansion compared with db/db mice treated with IgG (Fig. 4 D vs. C). Fig. 4E displays the differences in the fraction of glomerular cross-sectional area occupied by mesangial extracellular matrix, quantitated by glomerular morphometry. The mesangial matrix fraction was increased more than 2.4-fold in diabetic vs. nondiabetic mice, but αT treatment prevented the increase in the db/db mice, nearly reaching the normal value seen in the db/m mice (Fig. 4E).
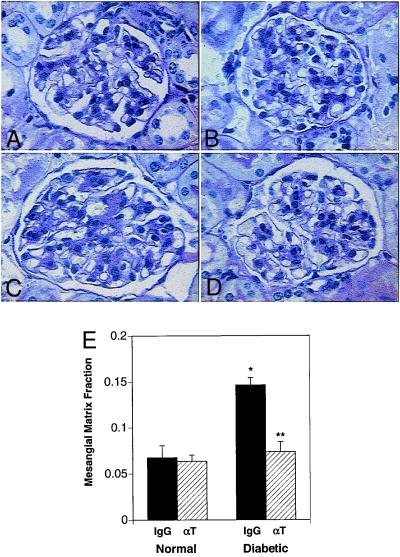
Figure 4.
Anti-TGF-β antibody therapy significantly prevents mesangial matrix expansion in diabetic mice. Representative photomicrographs of PAS-stained kidney sections from: (A) normal db/m mouse treated with control IgG; (B) normal db/m mouse treated with anti-TGF-β antibody (αT); (C) diabetic db/db mouse treated with control IgG; and (D) diabetic db/db mouse treated with αT. Note the diffusely expanded extracellular mesangial matrix in the diabetic mouse treated with IgG and the marked prevention of this expansion in the diabetic mouse treated with αT. Quantitative measurement of extracellular mesangial matrix expansion (E) is expressed as PAS-positive mesangial material per total glomerular tuft cross-sectional area. An average value was obtained from analyses of 30 glomeruli per mouse. Data are mean ± SE, n = 8 for each IgG group and n = 9 for each αT group. *, P < 0.05 vs. normal-IgG, **, P < 0.05 vs. diabetic-IgG.
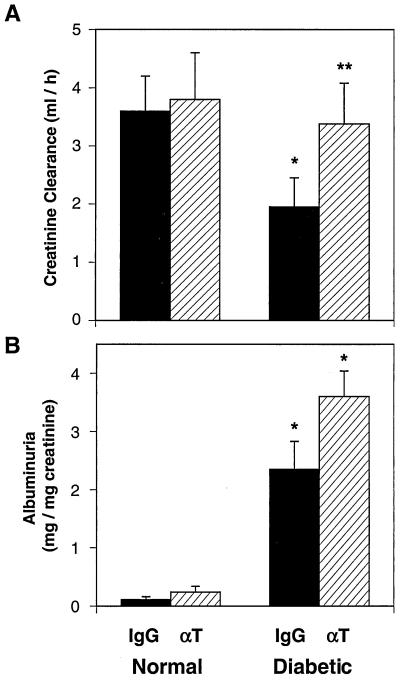
αT Treatment Prevents Renal Insufficiency.
At the onset of intervention (8 wk of age), the plasma creatinine concentration and the creatinine clearance of the diabetic group were identical to those of the nondiabetic group (37). By 16 wk of age, the IgG-treated db/db mice developed renal insufficiency, as measured by the elevated plasma creatinine (Table 1) and the reduced creatinine clearance from 24-h urine collections (Fig. 5A). Treatment with αT completely prevented the increase in plasma creatinine (Table 1) and the decrement in creatinine clearance in db/db mice (Fig. 5A). The urine albumin excretion was calculated relative to creatinine excretion to control for possibly incomplete collections. Albumin excretion rates were significantly increased in db/db compared with db/m mice at the conclusion of the study (Fig. 5B). Unlike the other renal parameters assessed, urine albumin excretion was not lowered by αT treatment in the diabetic mice (Fig. 5B). It should be noted, however, that the urinary excretion of albumin factored for the creatinine clearance was slightly lower in the db/db mice treated with αT vs. IgG (not shown). Thus, αT did not have a significant beneficial effect on the evolution of albuminuria in this mouse model of diabetes.
Figure 5.
Anti-TGF-β antibody therapy normalizes renal function but not albuminuria in diabetic mice. (A) Creatinine clearance in normal db/m and diabetic db/db groups treated with either IgG or anti-TGF-β antibody (αT). Clearance was calculated based on urine volumes and plasma and urine creatinine concentrations. ELISA specific for mouse albumin was used to assess albuminuria in 24-h urine collections, which was standardized per mg creatinine. (B) The increased excretion of albumin in db/db mice treated with IgG (vs. db/m mice) remained persistently elevated in db/db mice treated with αT. Data are mean ± SE, n = 8 for each IgG group and n = 9 for each αT group. *, P < 0.05 vs. normal-IgG or normal-αT, **, P < 0.05 vs. diabetic-IgG.
Discussion
The db/db mouse, which expresses a mutant form of the full-length leptin receptor in the hypothalamus, is a genetic model of type 2 diabetes mellitus that develops hyperglycemia in association with insulin resistance and obesity beginning in the second month of age (31). After 10–20 wk of sustained hyperglycemia, it exhibits significant renal pathobiology, including diffuse glomerulosclerosis (accumulation of mesangial matrix encroaching the normal capillary network), increased expression of mRNAs encoding α1(IV) collagen and fibronectin, progressive worsening of proteinuria, and reduction in the glomerular filtration rate (12, 37). Because the structural and functional abnormalities, in their evolution and nature, resemble those observed in human diabetic nephropathy, the db/db mouse represents a more suitable model than the STZ diabetic rat for studying diabetic glomerulosclerosis and examining pathogenic influences and treatment strategies that may be applicable to the human disease.
The current study evaluated the role of the renal TGF-β system in the development of chronic structural and functional changes of diabetic nephropathy by assessing the response of db/db mice to chronic treatment with neutralizing anti-TGF-β antibodies. In this model, there is a selective increase in glomerular mesangial TGF-β1 and a generalized (tubular and glomerular) up-regulation of the TGF-β type II receptor. Chronic administration of an antibody that neutralizes the activity of all three mammalian isoforms of TGF-β resulted in marked beneficial effects on renal function and structure. Despite persistent hyperglycemia, mesangial matrix expansion was prevented, and the glomerular filtration rate, assessed by plasma creatinine concentration and creatinine clearance, was preserved. These findings strongly suggest that overt nephropathy was prevented. Progressive expansion of the glomerular mesangial matrix resulting in diffuse intercapillary sclerosis is the most important structural lesion of diabetic glomerulopathy, because it correlates closely with the progressive decline in the glomerular capillary surface area available for filtration and hence with the glomerular filtration rate (38, 39). It is noteworthy that the anti-TGF-β antibody regimen protected the kidney without causing any noticeable systemic side effects. Perhaps the circulating TGF-β1 level was sufficiently (see Table 1) but not excessively reduced to the point that immune responses would be impaired (40).
The beneficial effects of anti-TGF-β antibody treatment on reducing mesangial matrix expansion are likely related to neutralization of excess TGF-β1 expression in the glomerular mesangium and subsequent prevention of extracellular matrix accumulation (41). In experimental glomerulonephritis, an anti-TGF-β antibody has been used to demonstrate that the TGF-β system plays an important role in matrix production (42). In the current study, the antibody effectively prevented increases in the renal expression of matrix genes including type IV collagen and fibronectin and may have also stimulated matrix degradative pathways, because TGF-β suppresses the activity of metalloproteinases and increases the expression of protease inhibitors such as plasminogen activator inhibitor-1 (43). Future studies will be needed to determine whether anti-TGF-β antibody therapy can be used to reverse established diabetic nephropathy. The possible mediators of mesangial matrix expansion in the db/db mouse include hyperglycemia and increased concentrations of Amadori glucose adducts of circulating albumin (7, 12). Correcting hyperglycemia or antagonizing the effects of Amadori-glycated albumin prevents the pathologic changes of diabetes in the kidney (12, 44). Moreover, hyperglycemia and Amadori-glycated albumin activate mesangial cell protein kinase C (10, 11, 45, 46), up-regulating the TGF-β system and subsequently stimulating matrix synthesis in these cells (7, 47). These investigations further prove that increased intrarenal activity of the TGF-β/TGF-β receptor system mediates the fibrogenic effects of diabetic metabolic changes such as hyperglycemia and increased glycated albumin.
Our previous study (21), using STZ diabetic mice, has shown that TGF-β plays an important role in renal hypertrophy, an early manifestation of diabetes. Neutralization of TGF-β after only 9 days of treatment prevented glomerular hypertrophy and markedly reduced total kidney hypertrophy. The current study using db/db mice reveals that TGF-β also plays a central role in mesangial matrix expansion and loss of renal function, the late manifestations of diabetes. Thus, we conclude that blocking the activity of the renal TGF-β system significantly benefits the initial as well as the progressive stages of diabetic kidney disease. Further, TGF-β neutralization therapy can be applied to both types of diabetes mellitus. These conclusions are supported by the human findings (23, 24) demonstrating that patients with either type 1 or type 2 diabetes have up-regulated renal TGF-β expression before and after the development of nephropathy.
It is important to note that treatment with the anti-TGF-β antibody did not attenuate the degree of albuminuria in the db/db mice despite its beneficial effects on glomerular matrix expansion and renal function. At first glance, this might suggest that TGF-β does not play a role in the disturbances that regulate macromolecular permselectivity in diabetes. Albumin permeability is increased across the glomerular basement membrane in diabetic nephropathy, likely because of hemodynamic stress (48, 49), an increase in the membrane pore size, a reduction in the anionic charge (50, 51), or perhaps an increase in the activity of permeability factors such as vascular endothelial growth factor (VEGF) (52, 53). In this study, VEGF mRNA was increased 2-fold in db/db kidneys but anti-TGF-β antibodies only slightly prevented this increment (not shown). Studies in cell culture suggest that TGF-β increases VEGF expression but down-regulates VEGF receptors (52, 53) and does not reduce the sulfation of glomerular proteoglycans (54). However, transgenic mice expressing high levels of circulating TGF-β1 still develop glomerular basement membrane thickening and proteinuria (36). Therefore, a longer duration of therapy or more complete blockade of glomerular TGF-β activity may still reduce albuminuria. It should be noted that αT treatment preserves the glomerular filtration rate, and this may need to be accounted for when assessing the degree of albuminuria. In fact, the urinary albumin excretion divided by the creatinine clearance was found to be slightly decreased in the db/db mice treated with αT vs. IgG.
That αT effectively maintains renal function without decreasing albuminuria seems at first to contradict the prevailing theory that albuminuria itself promotes progressive renal dysfunction (55). However, the disparities can be reconciled if one considers that proteinuria contributes to tubulointerstitial fibrosis via chemoattractant and prosclerotic mechanisms that mostly involve activation of the TGF-β pathway. Inhibiting the tubulointerstitial TGF-β system with αT thus minimizes the damaging effects of albuminuria even if it persists.
Diabetic nephropathy is the leading cause of end-stage renal disease in the industrialized world. Almost 40% of all new patients with renal failure admitted to renal replacement programs in the United States have diabetic kidney disease. Adherence to the therapeutic recommendations, such as strict glycemic and blood pressure control, significantly decreases the morbidity and mortality associated with this disease. However, conventional therapies, even when optimally provided, do not totally halt the progression of diabetic nephropathy. The data we have provided here strongly support the hypothesis that elevated intrarenal production or activity of the TGF-β/TGF-β receptor system mediates the functional and structural lesions of overt diabetic renal disease. The design of new strategies to block the production or the activity of the renal TGF-β system may provide a valuable adjunctive therapy for those patients destined to develop diabetic nephropathy.
Acknowledgments
This work was supported in part by the Juvenile Diabetes Foundation International (F.N.Z., M.I., S.C.), the National Kidney Foundation (K.S., T.A.M.), and the National Institutes of Health (grants DK-44513, DK-45191, and DK-54608 to F.N.Z. and training grant DK-07006). D.C.H. and S.W.H. are visiting scholars at the University of Pennsylvania and are supported by the Hyonam Kidney Laboratory and Yonsei University, respectively, Seoul, South Korea. M.C.I.C. is a visiting scholar at the University of Pennsylvania and is supported by the Ministerio de Educación y Cultura, Spain.
Abbreviations
- TGF-β
transforming growth factor-β
- STZ
streptozotocin
- PAS
periodic acid/Schiff reagent
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.120055097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.120055097
References
- 1.Ziyadeh F N. Am J Kidney Dis. 1993;22:736–744. doi: 10.1016/s0272-6386(12)80440-9. [DOI] [PubMed] [Google Scholar]
- 2.Ziyadeh F N. Kidney Int Suppl. 1996;54:S10–S13. [PubMed] [Google Scholar]
- 3.Ziyadeh F N. Miner Electrolyte Metab. 1995;21:292–302. [PubMed] [Google Scholar]
- 4.Ayo S H, Radnik R A, Glass W F, II, Garoni J A, Rampt E R, Appling D R, Kreisberg J I. Am J Physiol. 1991;260:F185–F191. doi: 10.1152/ajprenal.1991.260.2.F185. [DOI] [PubMed] [Google Scholar]
- 5.Danne T, Spiro M J, Spiro R G. Diabetes. 1993;42:170–177. doi: 10.2337/diab.42.1.170. [DOI] [PubMed] [Google Scholar]
- 6.Haneda M, Kikkawa R, Horide N, Togawa M, Koya D, Kajiwara N, Ooshima A, Shigeta Y. Diabetologia. 1991;34:198–200. doi: 10.1007/BF00418276. [DOI] [PubMed] [Google Scholar]
- 7.Ziyadeh F N, Sharma K, Ericksen M, Wolf G. J Clin Invest. 1994;93:536–542. doi: 10.1172/JCI117004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bleyer A J, Fumo P, Snipes E R, Goldfarb S, Simmons D A, Ziyadeh F N. Kidney Int. 1994;45:659–666. doi: 10.1038/ki.1994.88. [DOI] [PubMed] [Google Scholar]
- 9.Goldfarb S, Ziyadeh F N, Kern E F O, Simmons D A. Diabetes. 1991;40:465–471. doi: 10.2337/diab.40.4.465. [DOI] [PubMed] [Google Scholar]
- 10.Studer R K, Craven P A, DeRubertis F R. Diabetes. 1993;42:118–126. doi: 10.2337/diab.42.1.118. [DOI] [PubMed] [Google Scholar]
- 11.Fumo P, Kuncio G S, Ziyadeh F N. Am J Physiol. 1994;267:F632–F638. doi: 10.1152/ajprenal.1994.267.4.F632. [DOI] [PubMed] [Google Scholar]
- 12.Cohen M P, Sharma K, Jin Y, Hud E, Wu V Y, Tomaszewski J, Ziyadeh F N. J Clin Invest. 1995;95:2338–2345. doi: 10.1172/JCI117926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brownlee M. Annu Rev Med. 1995;46:223–234. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- 14.Wolf G, Ziyadeh F N. Am J Kidney Dis. 1997;29:153–163. doi: 10.1016/s0272-6386(97)90023-8. [DOI] [PubMed] [Google Scholar]
- 15.Wolf G, Ziyadeh F N. Kidney Int. 1999;56:393–405. doi: 10.1046/j.1523-1755.1999.00590.x. [DOI] [PubMed] [Google Scholar]
- 16.Ziyadeh F N. Curr Pract Med. 1998;1:87–89. [Google Scholar]
- 17.Sharma K, Ziyadeh F N. Am J Physiol. 1994;267:F1094–F1101. doi: 10.1152/ajprenal.1994.267.6.F1094. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto T, Nakamura T, Noble N A, Ruoslahti E, Border W A. Proc Natl Acad Sci USA. 1993;90:1814–1818. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakamura T, Fukui M, Ebihara I, Osada S, Nagaoka I, Tomino Y, Koide H. Diabetes. 1993;42:450–456. doi: 10.2337/diab.42.3.450. [DOI] [PubMed] [Google Scholar]
- 20.Shankland S J, Scholey J W, Ly H, Thai K. Kidney Int. 1994;46:430–442. doi: 10.1038/ki.1994.291. [DOI] [PubMed] [Google Scholar]
- 21.Sharma K, Jin Y, Guo J, Ziyadeh F N. Diabetes. 1996;45:522–530. doi: 10.2337/diab.45.4.522. [DOI] [PubMed] [Google Scholar]
- 22.Cohen M P, Sharma K, Guo J, Eltayeb B O, Ziyadeh F N. Exp Nephrol. 1998;6:226–233. doi: 10.1159/000020527. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto T, Noble N A, Cohen A H, Nast C C, Hishida A, Gold L I, Border W A. Kidney Int. 1996;49:461–469. doi: 10.1038/ki.1996.65. [DOI] [PubMed] [Google Scholar]
- 24.Iwano M, Kubo A, Nishino T, Sato H, Nishioka H, Akai Y, Kurioka H, Fujii Y, Kanauchi M, Shiiki H, Dohi K. Kidney Int. 1996;49:1120–1126. doi: 10.1038/ki.1996.162. [DOI] [PubMed] [Google Scholar]
- 25.Sharma K, Ziyadeh F N, Alzahabi B, McGowan T A, Kapoor S, Kurnik B R C, Kurnik P B, Weisberg L S. Diabetes. 1997;46:854–859. doi: 10.2337/diab.46.5.854. [DOI] [PubMed] [Google Scholar]
- 26.Sharma K, Ziyadeh F N. Semin Nephrol. 1997;17:80–92. [PubMed] [Google Scholar]
- 27.Rocco M V, Chen Y, Goldfarb S, Ziyadeh F N. Kidney Int. 1992;41:107–114. doi: 10.1038/ki.1992.14. [DOI] [PubMed] [Google Scholar]
- 28.Wolf G, Sharma K, Chen Y, Ericksen M, Ziyadeh F N. Kidney Int. 1992;42:647–656. doi: 10.1038/ki.1992.330. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman B B, Sharma K, Zhu Y, Ziyadeh F N. Kidney Int. 1998;54:1107–1116. doi: 10.1046/j.1523-1755.1998.00119.x. [DOI] [PubMed] [Google Scholar]
- 30.Han D C, Isono M, Hoffman B B, Ziyadeh F N. J Am Soc Nephrol. 1999;10:1891–1899. doi: 10.1681/ASN.V1091891. [DOI] [PubMed] [Google Scholar]
- 31.Chen H, Charlat O, Tartaglia L A, Woolf E A, Weng X, Ellis S J, Lakey N D, Culpepper J, Moore K J, Breitbart R E, et al. Cell. 1996;84:491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 32.Like A A, Lavine R L, Poffenbarger P L, Chick W L. Am J Pathol. 1972;66:193–224. [PMC free article] [PubMed] [Google Scholar]
- 33.Lucas C, Bald L N, Fendly B M, Mora-Worms M, Figari I S, Patzer E J, Palladino M A. J Immunol. 1990;145:1415–1422. [PubMed] [Google Scholar]
- 34.Durie F H, Fava R A, Foy T M, Aruffo A, Ledbetter J A, Noelle R J. Science. 1993;261:1328–1330. doi: 10.1126/science.7689748. [DOI] [PubMed] [Google Scholar]
- 35.Abe M, Harpel J G, Metz C N, Nunes I, Loskutoff D J, Rifkin D B. Anal Biochem. 1994;216:276–284. doi: 10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- 36.Kopp J B, Factor V M, Mozes M, Nagy P, Sanderson N, Bottinger E P, Klotman P E, Thorgeirsson S S. Lab Invest. 1996;74:991–1003. [PubMed] [Google Scholar]
- 37.Cohen M P, Clements R S, Hud E, Cohen J A, Ziyadeh F N. Exp Nephrol. 1996;4:166–171. [PubMed] [Google Scholar]
- 38.Mauer S M, Steffes M W, Ellis E N, Sutherland D E R, Brown D M, Goetz F C. J Clin Invest. 1984;74:1143–1155. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osterby R, Parving H H, Nyberg G, Hommel E, Jorgensen H E, Lokkegaard H, Svalander C. Diabetologia. 1988;31:265–270. doi: 10.1007/BF00277406. [DOI] [PubMed] [Google Scholar]
- 40.Kulkarni A B, Huh C-G, Becker D, Geiser A, Lyght M, Flanders K C, Roberts A B, Sporn M B, Ward J M, Karlsson S. Proc Natl Acad Sci USA. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Border W A, Okuda S, Languino L R, Sporn M B, Ruoslahti E. Nature (London) 1990;346:371–374. doi: 10.1038/346371a0. [DOI] [PubMed] [Google Scholar]
- 42.Border W A, Noble N A, Yamamoto T, Harper J R, Yamaguchi Y, Pierschbacher M D, Ruoslahti E. Nature (London) 1992;360:361–364. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- 43.Sawdey M, Podor T J, Loskutoff D J. J Biol Chem. 1989;264:10396–10401. [PubMed] [Google Scholar]
- 44.Fioretto P, Steffes M W, Sutherland D E, Goetz F C, Mauer M. N Engl J Med. 1998;339:69–75. doi: 10.1056/NEJM199807093390202. [DOI] [PubMed] [Google Scholar]
- 45.Kreisberg J I, Radnik R A, Kreisberg S H. Kidney Int. 1996;50:805–810. doi: 10.1038/ki.1996.379. [DOI] [PubMed] [Google Scholar]
- 46.Cohen M P, Ziyadeh F N, Lautenslager G T, Cohen J A, Shearman C W. Am J Physiol. 1999;276:F684–F690. doi: 10.1152/ajprenal.1999.276.5.F684. [DOI] [PubMed] [Google Scholar]
- 47.Ziyadeh F N, Han D C, Cohen J A, Guo J, Cohen M P. Kidney Int. 1998;53:631–638. doi: 10.1046/j.1523-1755.1998.00815.x. [DOI] [PubMed] [Google Scholar]
- 48.Zatz R, Meyer T W, Rennke H G, Brenner B M. Proc Natl Acad Sci USA. 1985;82:5963–5967. doi: 10.1073/pnas.82.17.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Bryan G T, Hostetter T H. Semin Nephrol. 1997;17:93–100. [PubMed] [Google Scholar]
- 50.Myers B D, Nelson R G, Williams G W, Bennett P H, Hardy S A, Berg R L, Loon N, Knowler W C, Mitch W E. J Clin Invest. 1991;88:524–530. doi: 10.1172/JCI115335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scandling J D, Myers B D. Kidney Int. 1992;41:840–846. doi: 10.1038/ki.1992.129. [DOI] [PubMed] [Google Scholar]
- 52.Gruden G, Thomas S, Burt D, Zhou W, Chusney G, Gnudi L, Viberti G. J Am Soc Nephrol. 1999;10:730–737. doi: 10.1681/ASN.V104730. [DOI] [PubMed] [Google Scholar]
- 53.Cooper M E, Vranes D, Youssef S, Stacker S A, Cox A J, Rizkalla B, Casley D J, Bach L A, Kelly D J, Gilbert R E. Diabetes. 1999;48:2229–2239. doi: 10.2337/diabetes.48.11.2229. [DOI] [PubMed] [Google Scholar]
- 54.van Det N F, van den Born J, Tamsma J T, Verhagen N A, Berden J H, Bruijn J A, Daha M R, van der Woude F J. Kidney Int. 1996;49:1079–1089. doi: 10.1038/ki.1996.157. [DOI] [PubMed] [Google Scholar]
- 55.Remuzzi G, Bertani T. N Engl J Med. 1998;339:1448–1456. doi: 10.1056/NEJM199811123392007. [DOI] [PubMed] [Google Scholar]