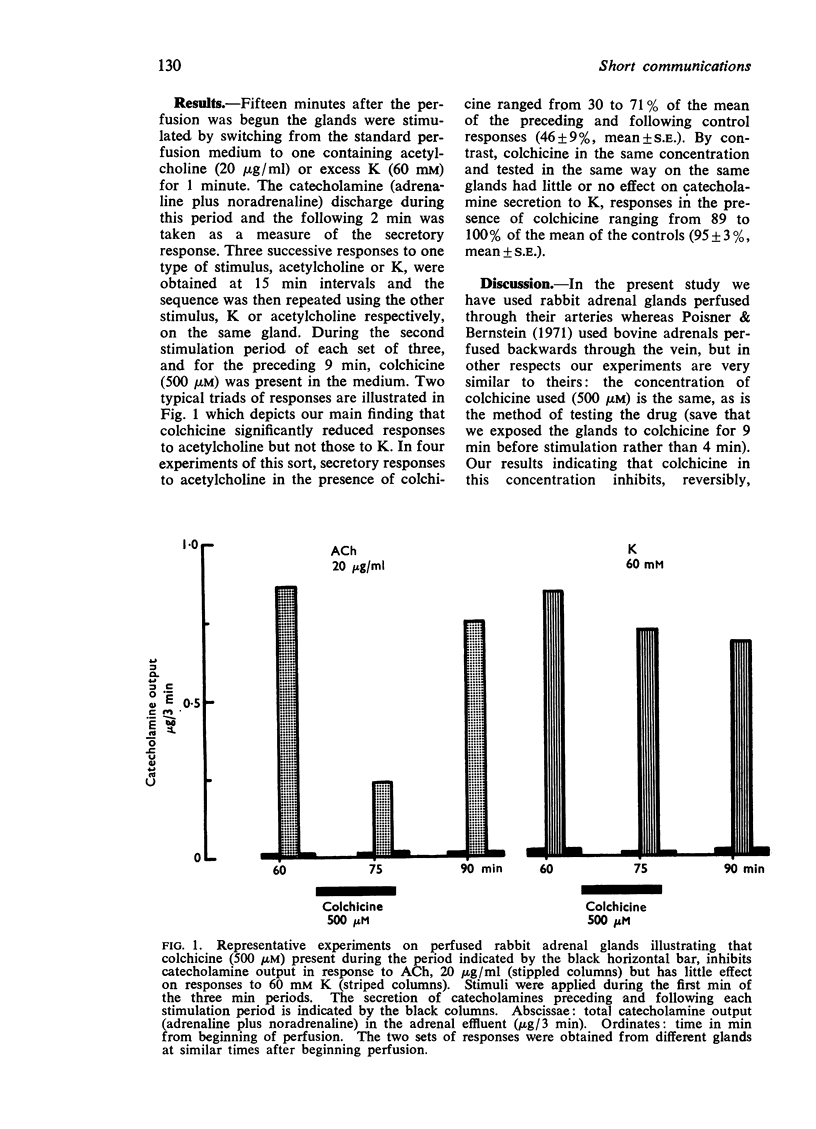
Abstract
In perfused rabbit adrenal glands, colchicine (500 μM) inhibited the catecholamine secretion evoked by acetylcholine (20 μg/ml) but not that evoked by excess potassium (60 mM). Since both stimuli are believed to release catecholamines ultimately by the same secretory process, exocytosis, it is concluded that these inhibitory effects of colchicine exerted against acetylcholine result from an action early in the process of stimulus-secretion coupling, possibly at the level of the acetylcholine receptor, and that it is inappropriate to use such inhibitory effects to support the view that secretion proper (exocytosis) in chromaffin cells involves colchicine-sensitive elements such as microtubules.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTON A. H., SAYRE D. F. A study of the factors affecting the aluminum oxide-trihydroxyindole procedure for the analysis of catecholamines. J Pharmacol Exp Ther. 1962 Dec;138:360–375. [PubMed] [Google Scholar]
- Borisy G. G., Taylor E. W. The mechanism of action of colchicine. Binding of colchincine-3H to cellular protein. J Cell Biol. 1967 Aug;34(2):525–533. doi: 10.1083/jcb.34.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. On the mode of action of acetylcholine in evoking adrenal medullary secretion: increased uptake of calcium during the secretory response. J Physiol. 1962 Aug;162:385–392. doi: 10.1113/jphysiol.1962.sp006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. The role of calcium in the secretory response of the adrenal medulla to acetylcholine. J Physiol. 1961 Nov;159:40–57. doi: 10.1113/jphysiol.1961.sp006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Kanno T., Sampson S. R. Effects of acetylcholine and other medullary secretagogues and antagonists on the membrane potential of adrenal chromaffin cells: an analysis employing techniques of tissue culture. J Physiol. 1967 Jan;188(1):107–120. doi: 10.1113/jphysiol.1967.sp008127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Kanno T., Sampson S. R. Influence of the ionic environment on the membrane potential of adrenal chromaffin cells and on the depolarizing effect of acetylcholine. J Physiol. 1967 Jul;191(1):107–121. doi: 10.1113/jphysiol.1967.sp008239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Poisner A. M. Preferential release of adrenaline from the adrenal medulla by muscarine and pilocarpine. Nature. 1965 Dec 11;208(5015):1102–1103. doi: 10.1038/2081102a0. [DOI] [PubMed] [Google Scholar]
- Douglas W. W., Poisner A. M., Rubin R. P. Efflux of adenine nucleotides from perfused adrenal glands exposed to nicotine and other chromaffin cell stimulants. J Physiol. 1965 Jul;179(1):130–137. doi: 10.1113/jphysiol.1965.sp007652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Rubin R. P. The mechanism of catecholamine release from the adrenal medulla and the role of calcium in stimulus-secretion coupling. J Physiol. 1963 Jul;167(2):288–310. doi: 10.1113/jphysiol.1963.sp007150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Sorimachi M. Effects of cytochalasin B and colchicine on secretion of posterior pituitary and adrenal medullary hormones. Br J Pharmacol. 1972 May;45(1):143P–144P. [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie E., Levine R. J., Malawista S. E. Histamine release from rat peritoneal mast cells: inhibition by colchicine and potentiation by deuterium oxide. J Pharmacol Exp Ther. 1968 Nov;164(1):158–165. [PubMed] [Google Scholar]
- Kraicer J., Milligan J. V. Effect of colchicine on in vitro ACTH release induced by HIGH K+ and by hypothalamus-stalk-median eminance extract. Endocrinology. 1971 Aug;89(2):408–412. doi: 10.1210/endo-89-2-408. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Howell S. L., Young D. A., Fink C. J. New hypothesis of insulin secretion. Nature. 1968 Sep 14;219(5159):1177–1179. doi: 10.1038/2191177a0. [DOI] [PubMed] [Google Scholar]
- Levy D. A., Carlton J. A. Influence of temperature on the inhibition by colchicine of allergic histamine release. Proc Soc Exp Biol Med. 1969 Apr;130(4):1333–1336. doi: 10.3181/00379727-130-33786. [DOI] [PubMed] [Google Scholar]
- Poisner A. M., Bernstein J. A possible role of microtubules in catecholamine release from the adrenal medulla: effect of colchicine, vinca alkaloids and deuterium oxide. J Pharmacol Exp Ther. 1971 Apr;177(1):102–108. [PubMed] [Google Scholar]
- Sorimachi M. Effects of alkali metal and other monovalent ions on the adrenomedullary secretion. Eur J Pharmacol. 1968 Jun;3(3):235–241. doi: 10.1016/0014-2999(68)90136-2. [DOI] [PubMed] [Google Scholar]
- Spoor R. P., Ferguson F. C., Jr Colchicine. IV. Neuromuscular transmission in isolated frog and rat tissues. J Pharm Sci. 1965 May;54(5):779–780. doi: 10.1002/jps.2600540524. [DOI] [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ash J. F., Bradley M. O., Luduena M. A., Taylor E. L., Wrenn J. T., Yamada K. Microfilaments in cellular and developmental processes. Science. 1971 Jan 15;171(3967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]


